Abstract
Objective:
To describe the study protocol of the Multimedia Self-Management Intervention (SMI) to prepare patients and family caregivers (FCGs) for lung cancer surgery.
Design:
The study is a five-year, single site, randomized controlled trial of 160 lung cancer surgery FCG and patient dyads (320 total participants), comparing intervention and attention control arms.
Setting:
One National Cancer-Institute (NCI) designated comprehensive cancer center in Southern California.
Participants:
Patients who are scheduled to undergo lung cancer surgery and their FCGs are enrolled as dyads only.
Intervention:
Based on the Chronic Care Self-Management Model (CCM), the intervention is a nurse-led, caregiver-based, multimedia care program for lung cancer surgery. Its primary focus is to help FCGs develop self-management skills related to their caregiving role through goal setting, proactive planning, building problem-solving skills, and accessing family support services. The intervention also supports dyads to prepare for surgery and post-operative recovery at home. It includes videos, print, web-based, and post-discharge telephone support.
Main Outcome Measures:
FCG and patient psychological distress and QOL; FCG burden and preparedness for caregiving; FCG and patient healthcare resource use (in-home nursing care, urgent care/ER visits, readmissions).
Analysis:
Repeated measures ANCOVA statistical design will be used, removing variances prior to examining mean squares for the group by occasion interactions, and co-varying the baseline scores. In addition, structured equation modeling (SEM) will assess whether mediating and moderating factors are associated with outcomes.
Keywords: Lung cancer, surgery, family caregivers, self-management, self-efficacy, quality of life
Introduction
More than 520,000 Americans, most over age 65, are living with a history of non-small cell lung cancer (NSCLC).1 In 2019, an additional 200,000 Americans will be diagnosed with the disease.2 The number of individuals diagnosed with early stage NSCLC is anticipated to increase due to advances in screening and early detection with low dose computed tomography (CT) scans. For patients with localized disease (stages I and II), the primary treatment modality is surgery. The most common resection performed is a lobectomy, which removes up to 25% of the lung.
By its very nature, lung cancer resections can significantly worsen a patient’s quality of life (QOL).3 Patients experience pain, fatigue, loss of respiratory capacity, and decreased physical function after lung cancer surgery.4 Lung cancer patients also report higher levels of distress, anxiety, fatigue, and breathlessness than other cancer patients at baseline.5,6 QOL remains compromised for up to 6 months after lung cancer surgery. Although lobectomy only removes a quarter of a patient’s breathing capacity, pulmonary function declines to less than half of baseline in the immediate postoperative period.7 More extensive resections result in even greater decline in lung function. In addition, the majority of lung cancer patients are older; median age at diagnosis is 70.8 Tobacco-related comorbid conditions, such as chronic obstructive pulmonary disease (COPD) and cardiovascular diseases, are common.9 Lung cancer treatments, including surgery, often exacerbate these conditions.
For family caregivers (FCGs), lung cancer surgery is an episodic and intense experience. Due to changes in the healthcare environment as well as advances in surgical care with minimally-invasive procedures, lung cancer patients are discharged from the hospital earlier after surgery. As such, a greater proportion of the caregiving burden has fallen on FCGs.10 During care transitions such as hospital discharge, only 54% of FCGs report having ever been asked about their caregiving needs. Alarmingly, only 29% of cancer caregivers report addressing their own self-management needs.11 Patients typically experience ongoing needs for care after hospital discharge, resulting in FCGs taking on greater responsibility for recovery at home.
Emerging evidence in the current literature points to a potential interrelationship between patient and FCG outcomes 12. Patients with FCGs that reported fair or poor health were more likely to report fair or poor perceived quality of care 12,13. In a study with 43 lung cancer patient and caregiver dyads, FCG self-rated burden, emotional distress, and QOL were correlated with patient-reported symptom severity 14. Conversely, higher levels of patient depressive symptoms were also correlated with the FCG’s emotional well-being. This potential interrelationship suggests that studying dyads as a unit is important and meaningful, and may lead to novel designs to simultaneously address FCG and patient QOL.
The caregiving role is a major source of stress for FCGs and results in poor QOL.15–17 Our previous research suggests that lung cancer family caregivers experience significant psychological distress and decreased QOL related to their caregiving role.15,16,18 This may be due to increased caregiver burden during recovery after surgery.19 As hospital stays have shortened, so has the time available for preparing patients and FCGs for recovery at home. This can potentially result in significant morbidity, undesirable healthcare resource use, and immense social and economic costs.
FCGs and patients are often expected to take responsibility for self-managing recovery at home while still experiencing the physical and psychological effects of surgery.20,21 Being adequately prepared for surgery and self-management has the potential to empower patients and families, lower distress, and improve QOL.22 This model of care enables and empowers FCGs and patients to achieve their own goals of care.21,23 The Institute of Medicine (IOM) defines self-management support as the “systematic provision of interventions to increase skills and self-efficacy in managing health problems, including regular assessment of problems, goal-setting and problem-solving support.”24 Observational evidence in lung cancer suggests that 1) patients with low self-efficacy who also had a FCG with low self-efficacy reported worse physical and psychological well-being; 2) dyads with high self-efficacy reported better QOL; and 3) low FCG self-efficacy is associated with higher FCG distress and caregiver burden.25 This suggests that interventions targeted at increasing self-efficacy and self-management may be beneficial for this population.
The inclusion of different media provides participants with alternative modes of learning. A recent meta-analysis found that video interventions in cancer were as effective, and in some randomized trials, superior in knowledge transfer to print materials alone.26 Therefore, videos may be especially suitable for participants with low health literacy.26 The current literature offers few family-centered models of care in cancer surgery that harness the advantage of multimedia approaches of knowledge transfer.
The purpose of this paper is to describe the study protocol of an intervention (Multimedia Self-Management – SMI) to prepare caregiver-patient dyads for lung cancer surgery. The primary aim is to determine whether FCG outcomes (psychological distress, burden, preparedness for caregiving, QOL) and patient outcomes (psychological distress, QOL, discharge disposition, in-home nursing care, unscheduled visits, readmissions) will be improved and sustained over time. The secondary aim is to explore the reciprocal relationships between FCG and patient outcomes. The study also uses qualitative methods to determine which components of the SMI Intervention are most beneficial for participants.
Methods
Study Design
The study is a 5-year, single site, randomized controlled trial of 160 lung cancer surgery FCG and patient dyads (320 total participants). The trial is designed to test a dyadic, multimedia self-management intervention (SMI) that aims to improve FCG and patient outcomes, comparing the intervention and attention control arms.
Participant Eligibility Criteria and Recruitment
Participants will be enrolled as dyads only. FCG eligibility criteria include: 1) a family member or friend identified by the patient as being the primary care provider before and after surgery; 2) Have a patient/care recipient enrolled in the study; 3) Age 21 years or older; and 4) Ability to comprehend and speak English. Patient eligibility criteria include: 1) Diagnosis of NSCLC; 2) Scheduled to undergo surgery for treatment; 3) Have an FCG enrolled in the study; 4) Age 21 years or older; and 5) Ability to comprehend and speak English. We recognize the importance of allowing FCGs to access support, in the situation when a patient declines study participation. The rationale for dyadic enrollment inclusion criteria was based on our study design, and our intent to study the interrelationship between FCG and patient outcomes; with this design, there is a need to assess outcomes by pairs.
The study protocol is approved by the Institutional Review Board (IRB). In accordance with federal policies, the study is registered with ClinicalTrials.gov (). Upon completion, the Consolidated Standards of Reporting Trials (CONSORT) statement for randomized trials of nonpharmacological treatments will be used for reporting.
All eligible participants who meet study inclusion criteria are identified through the Division of Thoracic Surgery. Research staff will work closely with the thoracic surgeons on a weekly basis to identify eligible participants. When necessary, existing institutional registry, records and databases will be reviewed to confirm eligibility. Once eligibility is established, the research staff contacts eligible participants and explains the study purpose, answers questions, and ascertains interest for enrollment. If the participant agrees to enroll, informed consent is obtained. Participants complete baseline assessment following informed consent.
Randomization
Once consented, FCG-patient dyads are randomly assigned to either the intervention or attention control arms, using a stratified and blocked randomization with variable block size. Randomizations are kept in sealed envelopes, and blinded to investigators and research staff until assignment. Strata are defined by surgical approach (open or minimally-invasive). Operationally, the research staff will confirm strata (open or minimally-invasive) at the time of enrollment. The research staff communicates the assignments to the participants. If one member of the participating dyad drops from the study after informed consent and randomization, the other remaining dyad will be considered a drop-off as well.
Study Intervention
Conceptual Framework
The framework driving the design of the SMI Intervention is based on elements of the Chronic Care Self-Management Model (CCM).27–29 The CCM transforms a reactive health system into one that improves FCG and patient outcomes through proactive planning and self-management skills building.30 The CCM is based on social cognitive theory and focuses on skills building to empower and engage individuals in their own care.31 A central construct of self-management is self-efficacy, which is defined as confidence to carry out behaviors necessary to achieve a desired goal.32 Self-efficacy is enhanced when patients and FCGs succeed in building confidence in their ability to manage their health. Self-management includes goal-setting, problem-solving, and post-operative recovery/caregiving skills building. Self-management coaching complements traditional education in supporting patients and FCGs to live the best possible QOL before and after surgery. The SMI Intervention combines both traditional (information and technical skills) and self-management education (enhance activation, self-efficacy, and confidence in using the information and technical skills).
FCGs and patients bring personal and health status factors that influence their capacity to integrate self-management strategies into their daily activities (Figure 1). These factors (e.g., age, sex, marital status, FCG relationship to patient, FCG employment status, co-morbidities) are potential moderators based on the current literature that influence intervention uptake.17,33–36 We hypothesize that the SMI Intervention, as guided by the self-management support element of the CCM, will enhance outcome mediators, such as activation (confidence in their ability to manage their health) and self-efficacy (confidence to carry out behaviors necessary to achieve a desired goal), and knowledge.21,32 The mediator effects, based on CCM evidence32, are hypothesized to serve as the mechanisms whereby the SMI Intervention improves FCG and patient outcomes (psychological distress, caregiver burden, preparedness for caregiving, QOL, healthcare resource/cancer support services use). Reciprocal interactions (Exploratory Aim - EA1) between FCG and patient outcomes are hypothesized to be interrelated.15,16,18 Evidence from our work suggests that lung cancer FCGs’ and patients’ psychological distress are correlated; FCG distress is associated with perceived burden, caregiving preparedness, as well as patient physical and psychological well-being.15–17
Figure 1.
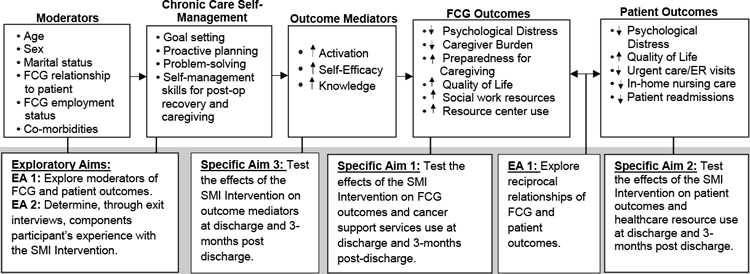
Conceptual Framework
Intervention Design and Content
The SMI Intervention, Preparing for your Lung Cancer Surgery, is a nurse-led, caregiver-based, multimedia intervention. The intervention delivers content that are both dyadic and specific for lung cancer FCGs. FCGs will receive specific coaching on managing their own self-care and QOL needs.37 FCG-patient dyads will both receive self-management coaching knowledge and skills in preparing for surgery and post-operative recovery activities. The current literature provides evidence that FCGs suffer from symptoms such as sleep disturbance and fatigue, forget to manage their own healthcare, and have decreasing ability to cope with the stresses of caregiving.38–41 The intervention uses a multimedia approach (20- minute videos + handbooks) to facilitate delivery of the dyadic content. A 5-minute video and handbook was developed specifically to coach FCGs on self-care. Content in the videos and handbooks are identical. The dyadic videos and handbook reinforce content that involves skills development for postoperative recovery activities. This includes step-by-step instructions on using an incentive spirometer (IS) for deep-breathing exercises. The FCG videos and handbook provides coaching on self-care. The content guides FCGs through classic self-management strategies, including goal-setting, overcoming challenges, and problem-solving.
The SMI Intervention is delivered by a trained nurse (RN). It is divided into five sessions, provided before surgery, before discharge, and at days 2, 7, and 2-months post-discharge (Table 1). Intervention doses are based on current evidence for pre-operative educational support in breast, cardiothoracic, and orthopedic surgery42–46, and established post-operative recovery activities that are associated with outcomes (pulmonary exercise, early ambulation).47,48 Based on our pilot experience, the estimated time to complete sessions 1 and 2 is about 40–60 minutes (video viewing and RN coaching).49 Each telephone call (sessions 3, 4, 5) will last 20–30 minutes.
Table 1.
SMI Intervention Content
| Component | Family Caregiver (FCG) Content | FCG-Patient Content |
|---|---|---|
| Session 1 (before surgery) What to expect before surgery What to expect day of surgery What to expect after surgery |
Importance of FCG self-care Understanding the familiar and unfamiliar Identifying and overcoming challenges Problem-solving coping skills Goal setting Developing self-care plan for emotional and social well-being |
Understanding your surgery Tobacco cessation Breathing exercise (incentive spirometer) Physical activity before surgery Coping with anxiety Day of surgery admissions information |
| Pain assessment and management Chest tube Breathing exercise (incentive spirometer) Early ambulation Daily self-monitoring plan | ||
| Session 2 (before discharge) What to expect after discharge (Healing at home) |
Developing self-care plan for physical and spiritual well-being Identifying and overcoming challenges Problem-solving coping skills Goal setting National and local FCG support services and resources |
Recognizing and managing symptoms and medications at home Pain assessment and management Cough and breathing Nutrition Physical activities, intimacy, return to work Sleep disturbance When to call your doctor |
| Sessions 3, 4, 5(days 2, 7, 2-months post-discharge RN Telephone Support |
Assess FCG QOL needs Re-inforce self-management skills and self-care plan |
Re-inforce self-management skills Assess patient symptoms and QOL needs |
Session 1 focuses on what to expect before surgery and during hospitalization after surgery. Content will be specific to FCGs, as well as for FCG-patient dyads. The information is first presented in an 8-minute video viewed by both FCGs and patients on provided iPads, followed by goal-setting and self-management skills-coaching using the handbooks. Coaching and self-management skills assessment focus on caregiving skills to support patient post-operative recovery as well as skills to manage caregivers’ own QOL needs. FCGs will view a 5-minute video, and be coached on the importance of managing their own physical and psychosocial well-being (anxiety, roles and relationships, social support). FCGs are coached on using problem-solving skills to enhance preparedness in caregiving. Problem-solving skills-coaching involves identification of perceived barriers to caregiving, prior plans or strategies to overcome these barriers, and identification of new strategies to facilitate caregiving responsibilities. Together as a dyad, FCGs and patients learn about what to expect before and after surgery (understanding lung cancer surgery, importance of tobacco cessation, breathing exercises using an IS, physical activity, coping with anxiety, day of surgery information). Dyadic content for what to expect after surgery focuses on promoting postoperative recovery activities (pain assessment and management, use of IS and breathing exercises, early ambulation).
Session 2 focuses on what to expect after discharge and is delivered within 24 hours of planned discharge; the video lasts 7 minutes, and is followed by goal setting and self-management skills coaching using the handbook. FCGs will learn about tending to their own physical well-being and healthcare needs. Physical symptoms of caregiving, such as sleep disturbance and fatigue, will also be addressed. FCGs receive content on managing their own healthcare needs (medications, co-morbid conditions, doctors’ appointments). National and local cancer support services for FCGs are provided. Dyadic content reinforces postoperative recovery activities to be continued at home (recognizing and managing symptoms, medications at home, pain assessment and management, breathing exercises, physical activity). Common postoperative symptoms such as cough, dyspnea, fatigue, and sleep disturbance are reviewed. Content also addresses nutrition, intimacy, return to work, and when to contact the surgical team.
Sessions 3, 4, and 5 involve three RN telephone support sessions after discharge (separate calls for FCGs and patients). During these sessions, the RN reinforces problem-solving approaches and self-management strategies. The RN will reviews goals, assesses the participants’ QOL needs, and facilitates any care coordination and communication with the surgical team as needed. Participants are able to view the videos at home after sessions 1 and 2 through a secure, private website.
Comparison Arm Design
For this trial, an attention control design is the comparison condition. This design is generally used to reduce threats to internal validity in randomized trials.50 In an attention control condition, participants receive some activities that are not the same in intensity and content as the intervention arm activities.50 The attention control condition is administered by a trained Clinical Research Assistant (CRA). Participants randomized to the attention control arm are given information from the American Cancer Society (ACS) on clinical trials. In Session 1, participants view a 3-minute ACS video titled “Why Should I Consider a Clinical Trial?” They receive corresponding ACS print materials on the topic following viewing. In Session 2, participants view a second ACS video titled “How Should I Prepare for Clinical Trial Discussion With My Doctor?” Corresponding ACS materials on the topic are provided and reviewed. In Sessions 3, 4, and 5, the CRA contacts participants by telephone and asks if they have any questions with the clinical trial materials.
Study Procedures
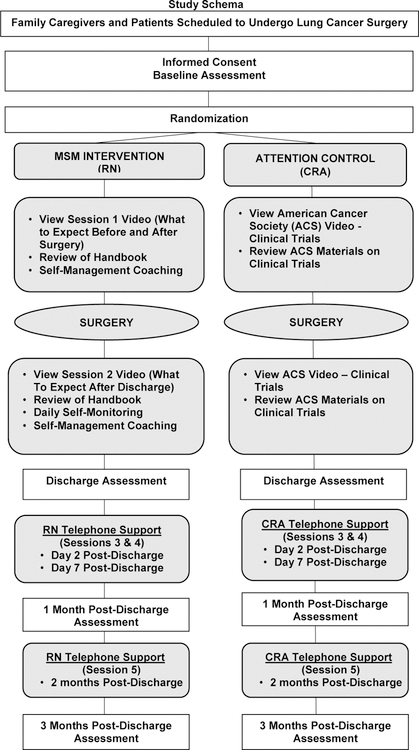
The study schema for this RCT is presented in Figure 2. For the SMI Intervention arm, Session 1 is administered by the RN approximately 3–7 days before surgery during a routine clinic visit. Session 2 is administered within 24 hours of planned hospital discharge after surgery. The RN completes Sessions 3, 4, and 5 of the intervention, by telephone, at day 2, day 7, and 2 months after hospital discharge. FCGs and patients receive separate telephone calls for Sessions 3, 4, and 5.
Figure 2.
Study Schema
For the attention control arm, Session 1 is administered by the CRA approximately 3–7 days before surgery, during a routine clinic visit. After surgery, participants receive Session 2 within 24 hours of planned hospital discharge. The CRA completes Sessions 3, 4, and 5 by telephone, at day 2, day 7, and 2 months after hospital discharge. FCGs and patients receive separate telephone calls for Sessions 3, 4, and 5.
Treatment Fidelity and Quality/Process Evaluation
Several approaches are included to ensure the rigor and validity of the intervention and prevent experimental drift. Design for establishing intervention fidelity is guided by the Technology Model of Intervention Fidelity.51–53 First, a RN interventionist manual was developed to describe intervention goals and strategies to achieve these goals. Second, training and supervision of the RN is undertaken. The RN receives multiple one-on-one training sessions with the PIs, followed by multiple mock training sessions to practice administering the intervention. Third, intervention fidelity is monitored throughout the study using an intervention fidelity measure. All intervention sessions are audio-recorded, and the PIs review each recorded session for the first 6 months of the study. Thereafter, audio-recordings of all sessions for every 5th dyad are reviewed. The investigators discuss fidelity issues and review results with the research staff during weekly team meetings. Additional training is provided when necessary. Other key research staff also received extensive training for their roles and responsibilities on the study. The attention control CRA received multiple one-on-one trainings with the investigators, completed mock training sessions to practice administering the attention control condition, and uses an attention control condition-specific standard operating procedures (SOPs).
As with any randomized design, the intervention can be contaminated either by research staff delivering the intervention and control conditions, or by providers involved in the study and the clinical care of participants. Therefore, several designs and strategies are included in this study to avoid bias and contamination. First, the study has three full-time research staff, each with his/her own role and responsibility on the study. As previously discussed, separate staff deliver the intervention and attention control conditions. In addition, one full-time CRA is responsible for data collection. The research staffs’ responsibilities are separated to minimize participant/responder bias. Second, each study research staff received intensive training on separating intervention, attention control, and data collection activities. Finally, randomization assignments are not discussed during weekly team meetings to avoid potential bias and contamination with the surgeon collaborators.
Outcome Measures
All outcomes are collected and stored in a study-specific REDcap (Research Electronic Data Capture) database. Participants have the option to complete outcome surveys electronically through REDcap, or in paper format. The data collection CRA enters all paper format data into REDcap. For electronic surveys, each participant is assigned his/her own unique survey ID and survey web link at the appropriate assessment time points. When surveys are completed electronically, responses transmit directly to the institutional REDcap server and into the study database. The estimated time to complete the measures is 45 minutes (Table 2). Patients and FCGs receive a separate remuneration of $50 each at completion of baseline and 3-month surveys ($100 per participant).
Table 2.
Outcome Measures by Assessment Time Points
| Measures | Baseline | Discharge | 1 month | 3 months | |
|---|---|---|---|---|---|
| Family Caregivers | Distress Thermometer | x | X | x | x |
| Montgomery Borgatta Caregiver Burden Scale | x | X | x | x | |
| Preparedness for Caregiving Scale | x | X | x | x | |
| City of Hope-Quality of Life-Family | x | X | x | x | |
| Family Caregiver Healthcare Use Survey | X | x | x | ||
| Patients | Distress Thermometer | x | X | x | x |
| Functional Assessment of Cancer Therapy (FACT-L) | x | X | x | x | |
| Patient Healthcare Use Survey | x | x | |||
| Medical Chart Audit Form | x | ||||
| Outcome Mediators | Family Caregiver Activation in Transitions (FCAT) Tool | x | X | x | x |
| Patient Activation Measure (PAM) | x | X | x | x | |
| Self-Efficacy Scale | x | X | x | x | |
| Surgery-Related Knowledge Tool | x | X | x | x | |
| Covariates/Exploratory | Sociodemographic and Health Status | x | |||
| Society of Thoracic Surgeons General Thoracic Surgery Data Collection Form | x | ||||
| Exit Interview Discussion Guide | x | ||||
| Process | Nurse Debriefing Form | After each intervention session | |||
| Intervention Fidelity Checklist | 1st five participants and every 10th session thereafter | ||||
Primary Outcomes
Distress Thermometer (DT).
The DT is an efficient, low subject burden measure to evaluate psychological distress over the past week, based on a scale of 0 (no distress) to 10 (extremely distressed). A cut-off of 3/10 indicates a need for intervention, with acceptable sensitivity (94.1%) and specificity (71.2%) compared to the Hospital Anxiety and Depression Scale (HADS).36 The DT has been validated in FCGs, with receiver operating characteristics (ROC) of 0.88 relative to the HADS.36,54 It is also validated in lung cancer patients.35
Montgomery Borgatta Caregiver Burden Scale (MBCBS).
This 14-item tool was designed to measure the impact of caregiving on three dimensions of burden: objective, subjective demand, and subjective stress. Internal consistency for the three dimensions ranges from .81 to .90.55 The ordinal scale ranges from 1–5 (a lot less to a lot more). The following cutoff scores are interpreted as high burden: 1) a score of 23 or > for objective burden, 2) a score of 15 or > for subjective demand, and 3) a score of 13.5 or > for subjective stress.
Preparedness for Caregiving Scale.
This is an 8-item scale of the Family Care Inventory by Archbold and colleagues.56 Items are scored from 0 to 4, with higher scores indicating better preparedness. A mean score is tabulated for all items. Internal consistency ranges from 0.88 to 0.93.57,58
City of Hope-Quality of Life–Family (COH-QOL-Family).
This is a 37-item instrument that measures the FCG QOL in the physical, psychological, social, and spiritual well-being domains. Ordinal scale ranges from 0–10, with higher scores indicating better QOL. The instrument was revised and tested with 219 FCGs of cancer patients. The test-retest reliability was r=.89 and internal consistency was alpha r=.69.59,60
Family Caregiver Healthcare Use Inventory.
This is a self-report measure of 4 yes/no items on cancer support services use (social work referrals, family resource center use). This inventory was adapted from Given and colleagues61 and Meneses and colleagues, and was used in the pilot study of the intervention.49,62
Functional Assessment of Cancer Therapy-Lung (FACT-L).
The FACT-L is a cancer-specific version of the FACIT System developed specifically for lung cancer. It contains the FACT-G scales with 27 items divided into physical, social/family, emotional, and functional well-being domains. A 10-item disease-specific symptom index (LCS) is included. For each item, the respondent indicates on a 5-point Likert scale (0=not at all; 4=very much) how true each statement is for the past 7 day time period. Reliability was tested in 116 lung cancer patients. Cronbach’s alpha for the FACT-L is 0.89.63 A 2 to 3 point change in scores for the FACT-L subscales is considered to be the minimal clinically important difference (MCID).64
Medical Chart Audit Form.
This form is used to record healthcare resource use provided at the cancer center as documented in electronic medical records (EHR). These include in-home nursing care, urgent care/ER visits, and 30- and 90-day hospital readmissions. The chart review will be completed by the data collection CRA.
Patient Healthcare Use Inventory.
This is a self-report measure of 23 yes/no items on healthcare resource use (hospital admissions, urgent care/ER visits, support services visits) in other institutions that are not captured in the EHR. It was adapted from Given and colleagues61 and Meneses and colleagues.62 McCorkle and colleagues used a similar method for an intervention study, and reported a 95% agreement between patient self-report data and medical chart audits.65
Secondary Outcomes
FCG Activation in Transitions Tool (FCAT).
This 10-item survey was designed to assess FCG’s level of engagement and preparedness to care for patients during care transitions. Psychometric assessment was conducted in two randomly equivalent waves of participants (N=434), including FCGs of patients with cancer and/or chronic lung disease. The estimated person-separation reliability was 0.84.66
Patient Activation Measure (PAM).
This measure assesses patient “activation”, which uses a Guttman-like scale that reflects a patient’s level of engagement and empowerment in their healthcare.67 Cronbach’s alpha from the investigator’s pilot study was 0.92.49
Self-Efficacy Scale.
This tool is a modified version of the Self-Efficacy Scale developed by Lorig and colleagues.68 It contains 8 items that assesses FCG’s and patients’ perceived confidence in self-management. Items are rated on a 4-point Likert scale, with higher scores representing higher confidence. Self-efficacy is assessed for both FCGs and patients. Cronbach’s alpha from the investigator’s pilot study was 0.89.49
Surgery-Related Knowledge Tool.
This brief (10 item) tool was developed by the investigators to assess FCG and patient knowledge on what to expect before and after surgery. Each item addresses specific content within the SMI intervention to assess changes in knowledge. Scoring is based on the number of questions answered correctly.
Covariates/Exploratory
Society of Thoracic Surgeons (STS) General Thoracic Surgery Database Data Collection Form.
This form is used to record clinical and healthcare resource use outcomes. These include date of diagnosis, pre-operative risk factors, pre-operative treatments, pre-operative pulmonary function tests, date of surgery, type of surgical procedures, admission date and status, clinical staging, postoperative events/complications, discharge date and status, discharge disposition, pathological staging, and smoking cessation plans.
Exit Interview Discussion Guide.
Qualitative data is obtained through semi-structured interviews using a discussion guide. The guide contains a series of overarching questions, presented in an open-ended fashion. The questions are designed to solicit FCGs’ and patients’ perspectives on the components of the intervention that were most effective. FCGs and patients are interviewed separately. The interviews are completed at the final data collection time point of 3 months post-discharge, and will only be completed with intervention arm participants.
Data Management and Quality Monitoring
Data management and monitoring is of key importance in this study, as the primary outcomes are captured via participant self-reported surveys and chart reviews. Data obtained through paper format are entered by the data collection CRA into the study REDcap dataset. Paper surveys are retained on file for data auditing purposes. A data quality auditing plan is initiated every six months to address data validity and integrity by early identification of cases of data collection inconsistency, data inaccuracy, and level of data incompleteness. At each of the six-month audits, the study statistician generates a missing data report for all primary and secondary outcomes. Data distribution summaries are generated to assess out-of-range or unexpected data; these are presented in frequency tables for each variable. In addition, random audits of five medical chart reviews are conducted by the PIs to assess quality of data extrapolated from EHRs. Additional training for the data collection CRA are provided as needed and based on audit results.
Power Analysis and Statistical Analysis Plan
The target sample size for this study is 160 caregiver-patient dyads (80 per arm), with a projected 20% attrition. For FCG outcomes, a reduction of the mean score of 3.7 (SD 2.6) by 1.2 points for psychological distress could be detected with 83% power. For caregiver burden, a reduction by 1.8 points (mean 22.0, SD 4.0) could be detected with 81% power. For caregiving preparedness, an increase in mean score from 3.7 to 4.1 (SD 0.72) could be detected with 93% power. For FCG total QOL, an increase of 0.7 (mean 5.93, SD 1.42) could be detected with 87% power. Finally, for FCG healthcare resource use (defined as use of social work services or family resource center), a difference of 20 percentage points for social work referrals and 30 percentage points for family resource center would be detectable with 80 subjects per group. For patient outcomes (psychological distress and QOL), the mean control group value, the root mean square (RMS) standard deviation, and the detectable improvements (Delta) in mean score was calculated for a two-sided 0.05 significance level test using 80 subjects per group. For healthcare resource use, a difference of 22 percentage points would be detectable with 80 subjects per group. For FCG and patient activation, an improvement of the mean by 8.0 points could be detected with 80% power. For self-efficacy, an improvement of 2.0 points could be detected with 80% power. For knowledge, a difference of 5.0 points can be detected with 88% for 80 dyads per group.
Our primary data analysis will be according to intention-to-treat. Participants who are lost to follow-up due to mortality will be included if they have at least one follow-up assessment. Participants with only baseline data will be excluded. The planned analysis will adjust for baseline values and will combine longitudinal assessments, both of which will improve power. Scores for all outcome measures will initially be summarized graphically and numerically by time points for each stratum. Each analysis will be a repeated measures ANCOVA statistical design, removing variances prior to examining mean squares for the group by occasion interactions, and co-varying the baseline scores. The independent variable is group (intervention vs. attention control). For FCG and patient healthcare and resource use, each subject is classified as having an event or not. Data will be tabulated by surgery stratum and tested for homogeneity (interaction) before estimating and testing the overall treatment effect.
For outcome mediators, repeated measures ANCOVA statistical design will be used. In addition, we will use structured equation modeling (SEM) to evaluate whether a mediator accounts for all or part of the effect between treatment and outcome. Potential moderating variables to be examined include FCG age, sex, relationship to patient (spouse vs. others), employment, and co-morbidities. For patients, potential moderators include: age, sex, marital status, and co-morbidities. These will be explored for interactions with treatment in affecting outcomes (psychological distress, caregiver burden, preparedness, QOL at discharge and 3 months), and for interactions with treatment in affecting potential moderators. For reciprocal relationships, FCG and patient outcomes will be pooled and structured so that the family is the unit of analysis; relationships will be tested through structural equation modeling (SEM).
Finally, we will use semi-structured interviews to examine dyadic experiences with the SMI Intervention, including participant engagement based on the number of times the dyads discussed the intervention topics outside of the research sessions. Qualitative data will be analyzed by the PIs using the conventional content analysis approach.69 This approach is used to describe a phenomenon where existing theory or research literature is limited. Data from the tape-recorded interviews will be transcribed. All data will be read repeatedly to achieve immersion and obtain a sense of the whole. Then, data will be read word by word to derive codes. Codes will then be sorted into themes based on links and relationships. Separate investigators with experience in qualitative data analysis will conduct a final validation review of the codes and themes to ensure consistency and clarity across all qualitative data. Data discordantly coded will be discussed for refinement and consensus purposes.
Discussion
FCGs and patients often experience heightened feelings of powerlessness before and after lung cancer surgery.70,71 The transition from postsurgical hospitalization to self-management post-discharge is frequently threatened by unmet discharge needs72, decreased patient functional health status, and increased caregiving burden.73,74 Previous research indicates the need for interventions that jointly examine FCG-patient outcomes. However, dyadic interventions are rarely conducted in surgical populations. To date, the majority of caregiver-patient dyadic interventions are conducted in breast and prostate cancers.
Emerging evidence in lung cancer found that lower levels of self-efficacy (confidence in self-management) in both FCGs and patients are associated with higher levels of anxiety and decreased QOL.25 There is also evidence that FCG educational interventions may improve QOL for lung cancer patients. A telephone-based intervention improved pain, depression, QOL, and self-efficacy for patients as well as improved anxiety and self-efficacy for FCGs. The results suggest that education/support may have been more effective than caregiver-assisted coping skills training for FCGs.25 Additionally, a pulmonary rehabilitation education program targeting FCGs resulted in improved pulmonary function and decreased pain in patients compared with controls.75 While promising, the majority of FCG studies have focused on late stage patients and few interventions were dyadic. The current literature provides few evidence-based, QOL-enhancing interventions that specifically target FCGs and patients as a unit in thoracic surgery.
This study is timely and unique for several reasons. First, few studies use evidence-based theory to design effective self-management strategies in cancer surgery. The goal of explicating intervention mechanisms by testing the relationship between outcome mediators and moderators is novel and necessary to improve care in cancer surgery. The study includes activation, self-efficacy, and knowledge as hypothesized modifiable intervention “active ingredients.” This intervention specifically targets these active ingredients. Understanding these intervention mechanisms is critical for developing efficient and effective self-management models of care in cancer surgery.
Second, the 2015 National Cancer Institute (NCI) and National Institute of Nursing Research (NINR)-sponsored meeting on the science of cancer caregiving identified the lack of explicit application of theoretical frameworks to guide caregiver-patient dyadic interventions.76 The SMI is an evidence-informed intervention built upon the CCM framework, and its application in lung cancer surgery caregiver-patient dyads is novel. Very few FCG interventions have been examined in cancer surgery.
Third, the timing of the intervention is unique, with delivery beginning in the preoperative setting. This is important given the increasing lack of adequate time to intervene with FCGs and patients due to early discharge. There are virtually no studies with FCGs that specifically begin prior to cancer surgery.77 This study comes at a pivotal time as changes in the healthcare system are increasingly 1) mandating value-based cancer care, and 2) recognizing the critical need to make quality cancer care more patient- and family-centered.78 This study is also a response to the recently published National Alliance for Caregiving report, “Cancer Caregiving in the US,” which calls for efforts to address gaps in the healthcare system and social services for FCGs, including specific recommendations to “develop and provide training materials for caregivers” and to “develop and test evidence-based tools to provide clinicians with best practices for training caregivers.”11 Finally, this study addresses two key priorities of the American College of Surgeons (ACS): 1) the critical role of patients and families as integral members of the surgical team, and 2) that the active involvement of patients and families as partners in surgical care can decrease complications through timely identification of risks.79
In summary, this study addresses the unmet need to integrate novel caregiver-patient centered interventions into routine cancer surgical care. Our conceptualization advances the science of cancer caregiving in surgery, and the intervention potentially improves outcomes, including well-being and healthcare resource use for FCGs and patients. Our core research team is interdisciplinary and includes nurse scientists and thoracic surgeons, with expert consultants in the areas of self-management and health services research. Our long-term goal is to transform and improve cancer surgical care through empirically-based QOL-enhancing interventions, and to prove that the CCM can be applied to better prepare FCGs and patients for surgery, improve postoperative outcomes, and optimize healthcare resource/cancer support services use.
Acknowledgements:
Research reported in this publication is supported by the National Cancer Institute of the National Institutes of Health under award number R01CA217841. It also includes work performed in the Biostatistics and Mathematical Modeling Core supported by the National Cancer Institute of the National Institutes of Health under award number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ClinicalTrials.gov Identifier:
References
- 1.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA: a cancer journal for clinicians July 2016;66(4):271–289. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians January 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 3.Raz DJ, Sun V, Kim JY, et al. Long-Term Effect of an Interdisciplinary Supportive Care Intervention for Lung Cancer Survivors After Surgical Procedures. The Annals of thoracic surgery February 2016;101(2):495–502; discussion 502–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Handy JR Jr., Asaph JW, Skokan L, et al. What happens to patients undergoing lung cancer surgery? Outcomes and quality of life before and after surgery. Chest July 2002;122(1):21–30. [DOI] [PubMed] [Google Scholar]
- 5.Dudgeon DJ, Kristjanson L, Sloan JA, et al. Dyspnea in cancer patients: prevalence and associated factors. J Pain Symptom Manage February 2001;21(2):95–102. [DOI] [PubMed] [Google Scholar]
- 6.Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psycho-oncology Jan-Feb 2001;10(1):19–28. [DOI] [PubMed] [Google Scholar]
- 7.Varela G, Brunelli A, Rocco G, et al. Predicted versus observed FEV1 in the immediate postoperative period after pulmonary lobectomy. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery October 2006;30(4):644–648. [DOI] [PubMed] [Google Scholar]
- 8.Howlader NNA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2012 http://seer.cancer.gov/csr/1975_2012/: National Cancer Institute, Bethesda, MD; 2015. [Google Scholar]
- 9.Murray CJ, Lopez AD. Measuring the global burden of disease. The New England journal of medicine August 1 2013;369(5):448–457. [DOI] [PubMed] [Google Scholar]
- 10.Chambers A, Routledge T, Pilling J, et al. In elderly patients with lung cancer is resection justified in terms of morbidity, mortality and residual quality of life? Interactive cardiovascular and thoracic surgery June 2010;10(6):1015–1021. [DOI] [PubMed] [Google Scholar]
- 11.National Alliance for Caregiving. Cancer Caregiving in the U.S 2016.
- 12.Litzelman K, Kent EE, Mollica M, et al. How Does Caregiver Well-Being Relate to Perceived Quality of Care in Patients With Cancer? Exploring Associations and Pathways. Journal of clinical oncology : official journal of the American Society of Clinical Oncology October 10 2016;34(29):3554–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litzelman K, Kent EE, Rowland JH. Interrelationships Between Health Behaviors and Coping Strategies Among Informal Caregivers of Cancer Survivors. Health education & behavior : the official publication of the Society for Public Health Education February 2018;45(1):90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan JY, Molassiotis A, Lloyd-Williams M, et al. Burden, emotional distress and quality of life among informal caregivers of lung cancer patients: An exploratory study. Eur J Cancer Care (Engl) January 2018;27(1):e12691. [DOI] [PubMed] [Google Scholar]
- 15.Fujinami R, Sun V, Zachariah F, et al. Family caregivers’ distress levels related to quality of life, burden, and preparedness. Psycho-oncology January 2015;24(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant M, Sun V, Fujinami R, et al. Family caregiver burden, skills preparedness, and quality of life in non-small cell lung cancer. Oncology nursing forum July 2013;40(4):337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carmack Taylor CL, Badr H, Lee JH, et al. Lung cancer patients and their spouses: psychological and relationship functioning within 1 month of treatment initiation. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine October 2008;36(2):129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Sun V, Raz D, et al. The impact of lung cancer surgery on quality of life trajectories in patients and family caregivers. Lung Cancer 2016;in press. [DOI] [PMC free article] [PubMed]
- 19.Lyons KS, Lee CS, Bennett JA, et al. Symptom incongruence trajectories in lung cancer dyads. J Pain Symptom Manage December 2014;48(6):1031–1040. [DOI] [PubMed] [Google Scholar]
- 20.Lapum J, Angus JE, Peter E, et al. Patients’ discharge experiences: returning home after open-heart surgery. Heart & lung : the journal of critical care May-Jun 2011;40(3):226–235. [DOI] [PubMed] [Google Scholar]
- 21.McCorkle R, Ercolano E, Lazenby M, et al. Self-management: Enabling and empowering patients living with cancer as a chronic illness. CA: a cancer journal for clinicians Jan-Feb 2011;61(1):50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knobf MT. Being prepared: essential to self-care and quality of life for the person with cancer. Clinical journal of oncology nursing June 2013;17(3):255–261. [DOI] [PubMed] [Google Scholar]
- 23.Grey M, Schulman-Green D, Knafl K, et al. A revised Self- and Family Management Framework. Nursing outlook Mar-Apr 2015;63(2):162–170. [DOI] [PubMed] [Google Scholar]
- 24.Institute of Medicine. Priority Areas for National Action: Tranforming Health Care Quality Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 25.Porter LS, Keefe FJ, Garst J, et al. Self-efficacy for managing pain, symptoms, and function in patients with lung cancer and their informal caregivers: associations with symptoms and distress. Pain July 15 2008;137(2):306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gysels M, Higginson IJ. Interactive technologies and videotapes for patient education in cancer care: systematic review and meta-analysis of randomised trials. Support Care Cancer January 2007;15(1):7–20. [DOI] [PubMed] [Google Scholar]
- 27.Coleman K, Austin BT, Brach C, et al. Evidence on the Chronic Care Model in the new millennium. Health affairs Jan-Feb 2009;28(1):75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner EH, Bennett SM, Austin BT, et al. Finding common ground: patient-centeredness and evidence-based chronic illness care. Journal of alternative and complementary medicine 2005;11 Suppl 1:S7–15. [DOI] [PubMed] [Google Scholar]
- 29.Wagner EH, Austin BT, Davis C, et al. Improving chronic illness care: translating evidence into action. Health affairs Nov-Dec 2001;20(6):64–78. [DOI] [PubMed] [Google Scholar]
- 30.Mosen DM, Schmittdiel J, Hibbard J, et al. Is patient activation associated with outcomes of care for adults with chronic conditions? The Journal of ambulatory care management Jan-Mar 2007;30(1):21–29. [DOI] [PubMed] [Google Scholar]
- 31.Bandura A. Social cognitive theory: an agentic perspective. Annual review of psychology 2001;52:1–26. [DOI] [PubMed] [Google Scholar]
- 32.Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine August 2003;26(1):1–7. [DOI] [PubMed] [Google Scholar]
- 33.Oksholm T, Rustoen T, Cooper B, et al. Trajectories of Symptom Occurrence and Severity From Before Through Five Months After Lung Cancer Surgery. J Pain Symptom Manage June 2015;49(6):995–1015. [DOI] [PubMed] [Google Scholar]
- 34.Sterzi S, Cesario A, Cusumano G, et al. How best to assess the quality of life in long-term survivors after surgery for NSCLC? Comparison between clinical predictors and questionnaire scores. Clinical lung cancer January 2013;14(1):78–87. [DOI] [PubMed] [Google Scholar]
- 35.Graves KD, Arnold SM, Love CL, et al. Distress screening in a multidisciplinary lung cancer clinic: prevalence and predictors of clinically significant distress. Lung Cancer February 2007;55(2):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zwahlen D, Hagenbuch N, Carley MI, et al. Screening cancer patients’ families with the distress thermometer (DT): a validation study. Psycho-oncology October 2008;17(10):959–966. [DOI] [PubMed] [Google Scholar]
- 37.Sun V, Kim JY, Raz DJ, et al. Preparing Cancer Patients and Family Caregivers for Lung Surgery: Development of a Multimedia Self-Management Intervention. Journal of cancer education : the official journal of the American Association for Cancer Education June 2018;33(3):557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Litzelman K, Green PA, Yabroff KR. Cancer and quality of life in spousal dyads: spillover in couples with and without cancer-related health problems. Support Care Cancer February 2016;24(2):763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y, Carver CS, Shaffer KM, et al. Cancer caregiving predicts physical impairments: roles of earlier caregiving stress and being a spousal caregiver. Cancer January 15 2015;121(2):302–310. [DOI] [PubMed] [Google Scholar]
- 40.Li Q, Loke AY. A spectrum of hidden morbidities among spousal caregivers for patients with cancer, and differences between the genders: A review of the literature. European Journal of Oncology Nursing 10// 2013;17(5):578–587. [DOI] [PubMed] [Google Scholar]
- 41.Ji J, Zoller B, Sundquist K, et al. Increased risks of coronary heart disease and stroke among spousal caregivers of cancer patients. Circulation April 10 2012;125(14):1742–1747. [DOI] [PubMed] [Google Scholar]
- 42.King J, Chamberland P, Rawji A, et al. Patient educational needs of patients undergoing surgery for lung cancer. Journal of cancer education : the official journal of the American Association for Cancer Education December 2014;29(4):802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ronco M, Iona L, Fabbro C, et al. Patient education outcomes in surgery: a systematic review from 2004 to 2010. International journal of evidence-based healthcare December 2012;10(4):309–323. [DOI] [PubMed] [Google Scholar]
- 44.Preminger BA, Lemaine V, Sulimanoff I, et al. Preoperative patient education for breast reconstruction: a systematic review of the literature. Journal of cancer education : the official journal of the American Association for Cancer Education June 2011;26(2):270–276. [DOI] [PubMed] [Google Scholar]
- 45.Whyte RI, Grant PD. Preoperative patient education in thoracic surgery. Thoracic surgery clinics May 2005;15(2):195–201. [DOI] [PubMed] [Google Scholar]
- 46.Johansson K, Nuutila L, Virtanen H, et al. Preoperative education for orthopaedic patients: systematic review. Journal of advanced nursing April 2005;50(2):212–223. [DOI] [PubMed] [Google Scholar]
- 47.do Nascimento Junior P, Modolo NS, Andrade S, et al. Incentive spirometry for prevention of postoperative pulmonary complications in upper abdominal surgery. The Cochrane database of systematic reviews February 8 2014;2(2):CD006058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agostini PJ, Naidu B, Rajesh P, et al. Potentially modifiable factors contribute to limitation in physical activity following thoracotomy and lung resection: a prospective observational study. Journal of cardiothoracic surgery September 27 2014;9:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun V, Raz DJ, Ruel N, et al. A Multimedia Self-management Intervention to Prepare Cancer Patients and Family Caregivers for Lung Surgery and Postoperative Recovery. Clinical lung cancer May 2017;18(3):e151–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aycock DM, Hayat MJ, Helvig A, et al. Essential considerations in developing attention control groups in behavioral research. Research in nursing & health June 2018;41(3):320–328. [DOI] [PubMed] [Google Scholar]
- 51.Carroll KM, Nich C, Sifry RL, et al. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug Alcohol Depend January 1 2000;57(3):225–238. [DOI] [PubMed] [Google Scholar]
- 52.Santacroce SJ, Maccarelli LM, Grey M. Intervention fidelity. Nurs Res Jan-Feb 2004;53(1):63–66. [DOI] [PubMed] [Google Scholar]
- 53.Stein KF, Sargent JT, Rafaels N. Intervention research: establishing fidelity of the independent variable in nursing clinical trials. Nurs Res Jan-Feb 2007;56(1):54–62. [DOI] [PubMed] [Google Scholar]
- 54.Zwahlen D, Hagenbuch N, Jenewein J, et al. Adopting a family approach to theory and practice: measuring distress in cancer patient–partner dyads with the distress thermometer. Psycho-oncology 2011;20(4):394–403. [DOI] [PubMed] [Google Scholar]
- 55.Montgomery RV, Stull DE, Borgatta EF. Measurement and the analysis of burden. Research on aging March 1985;7(1):137–152. [DOI] [PubMed] [Google Scholar]
- 56.Archbold PG, Stewart BJ, Greenlick MR, et al. Mutuality and preparedness as predictors of caregiver role strain. Research in nursing & health December 1990;13(6):375–384. [DOI] [PubMed] [Google Scholar]
- 57.Schumacher KL, Stewart BJ, Archbold PG. Mutuality and preparedness moderate the effects of caregiving demand on cancer family caregiver outcomes. Nursing research Nov-Dec 2007;56(6):425–433. [DOI] [PubMed] [Google Scholar]
- 58.Scherbring M. Effect of caregiver perception of preparedness on burden in an oncology population. Oncology nursing forum July 2002;29(6):E70–76. [DOI] [PubMed] [Google Scholar]
- 59.Ferrell BR, Grant M, Borneman T, et al. Family caregiving in cancer pain management. Journal of palliative medicine. Summer 1999;2(2):185–195. [DOI] [PubMed] [Google Scholar]
- 60.Ferrell BR, Grant M, Chan J, et al. The impact of cancer pain education on family caregivers of elderly patients. Oncology nursing forum September 1995;22(8):1211–1218. [PubMed] [Google Scholar]
- 61.Given BA, Given CW, Stommel M. Family and out-of-pocket costs for women with breast cancer. Cancer practice May-Jun 1994;2(3):187–193. [PubMed] [Google Scholar]
- 62.Pisu M, Azuero A, Meneses K, et al. Out of pocket cost comparison between Caucasian and minority breast cancer survivors in the Breast Cancer Education Intervention (BCEI). Breast cancer research and treatment June 2011;127(2):521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer June 1995;12(3):199–220. [DOI] [PubMed] [Google Scholar]
- 64.Cella D, Eton DT, Fairclough DL, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) Questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. Journal of clinical epidemiology March 2002;55(3):285–295. [DOI] [PubMed] [Google Scholar]
- 65.McCorkle R, Jeon S, Ercolano E, et al. Healthcare utilization in women after abdominal surgery for ovarian cancer. Nurs Res Jan-Feb 2011;60(1):47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coleman EA, Ground KL, Maul A. The Family Caregiver Activation in Transitions (FCAT) Tool: A New Measure of Family Caregiver Self-Efficacy. Joint Commission journal on quality and patient safety / Joint Commission Resources November 2015;41(11):502–507. [DOI] [PubMed] [Google Scholar]
- 67.Hibbard JH, Stockard J, Mahoney ER, et al. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health services research August 2004;39(4 Pt 1):1005–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lorig K, Chastain RL, Ung E, et al. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis and rheumatism January 1989;32(1):37–44. [DOI] [PubMed] [Google Scholar]
- 69.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qualitative health research November 2005;15(9):1277–1288. [DOI] [PubMed] [Google Scholar]
- 70.Jerofke TA, Weiss M, Yakusheva O. Patient perceptions of patient-empowering nurse behaviors, patient activation and functional health status in postsurgical patients with life-threatening long-term illness. Journal of Advanced Nursing 2013;70(6):1310–1322. [DOI] [PubMed] [Google Scholar]
- 71.Taylor C, Richardson A, Cowley S. Restoring embodied control following surgical treatment for colorectal cancer: a longitudinal qualitative study. Int J Nurs Stud August 2010;47(8):946–956. [DOI] [PubMed] [Google Scholar]
- 72.McMurray A, Johnson P, Wallis M, et al. General surgical patients’ perspectives of the adequacy and appropriateness of discharge planning to facilitate health decision-making at home. Journal of clinical nursing September 2007;16(9):1602–1609. [DOI] [PubMed] [Google Scholar]
- 73.Hodgson NA, Given CW. Determinants of functional recovery in older adults surgically treated for cancer. Cancer Nurs Jan-Feb 2004;27(1):10–16. [DOI] [PubMed] [Google Scholar]
- 74.Elliott D, Lazarus R, Leeder SR. Health outcomes of patients undergoing cardiac surgery: repeated measures using Short Form-36 and 15 Dimensions of Quality of Life questionnaire. Heart & lung : the journal of critical care Jul-Aug 2006;35(4):245–251. [DOI] [PubMed] [Google Scholar]
- 75.Jeong JH, Yoo WG. Effects of pulmonary rehabilitation education for caregivers on pulmonary function and pain in patients with lung cancer following lung resection. Journal of physical therapy science February 2015;27(2):489–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kent EE, Rowland JH, Northouse L, et al. Caring for caregivers and patients: Research and clinical priorities for informal cancer caregiving. Cancer July 1 2016;122(13):1987–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waller A, Forshaw K, Bryant J, et al. Preparatory education for cancer patients undergoing surgery: A systematic review of volume and quality of research output over time. Patient education and counseling May 23 2015. [DOI] [PubMed]
- 78.Birkmeyer JD, Dimick JB, Birkmeyer NJ. Measuring the quality of surgical care: structure, process, or outcomes? Journal of the American College of Surgeons April 2004;198(4):626–632. [DOI] [PubMed] [Google Scholar]
- 79.American College of Surgeons. Statement of Principles of Patient Education Available at: https://www.facs.org/about-acs/statements/54-patient-education#sthash.UCjQ0v3M.dpuf. Accessed April 5, 2016. [PubMed]