Abstract
Introduction:
While trials have demonstrated non-inferiority of direct oral anticoagulant drugs (DOAC) to low-molecular-weight heparins (LMWH) for the treatment of cancer associated thrombosis (CAT), it is unclear if the newer intervention is cost-effective.
Methods:
We performed a cost-utility analysis using a Markov state-transition model over a time horizon of 60 months in a hypothetical cohort of 65-year-old patients with active malignancy and first acute symptomatic CAT who were eligible to receive either rivaroxaban/edoxaban or dalteparin. We obtained transition probability, relative risk, cost, and utility inputs from the literature. We estimated the differential impact on costs and quality-adjusted life years (QALYs) per patient and performed one-way and probabilistic sensitivity analyses to test the robustness of results.
Results:
Using the base-case analysis over 60 months, DOAC versus dalteparin was associated with an incremental cost reduction of $24,129 with an incremental QALY reduction of 0.04. In the one-way sensitivity analysis, the cost of dalteparin contributed the most to the incremental cost difference; relative risk of death related to underlying cancer contributed the most of the incremental QALY difference. The probabilistic sensitivity analysis confirmed the base-case analysis, with a large reduction in cost but small reduction in QALYs.
Conclusion:
Rivaroxaban or edoxaban as compared to dalteparin is cost saving from a payer’s perspective for the treatment of CAT. Professional organizations and healthcare systems may want to consider this analysis in future practice recommendations.
Introduction:
Cancer associated thrombosis (CAT) is associated with increased morbidity and mortality. [1] Low-molecular-weight heparins (LMWHs) such as dalteparin have been the standard of care for the treatment of CAT.[2] LMWHs have been shown to reduce venous thromboembolism (VTE) recurrence rate compared to warfarin in multiple studies.[3,4] However, LMWHs require daily to twice daily subcutaneous injections, are more costly than oral drugs, and are associated with poor long-term adherence. [5] Recently, two randomized controlled trials compared direct oral anticoagulants (DOAC including edoxaban and rivaroxaban) versus LMWH (dalteparin) for the treatment of CAT. [6,7] The results of the two studies were very similar. A meta-analysis comparing the two treatment strategies showed an overall reduced incidence of VTE but an increased risk of bleeding with no significant difference in survival. [8]
Due to the trade-off between the risk of recurrent VTE and bleeding, many medical institutions remain ambivalent to embrace DOAC for the treatment of CAT. Furthermore, whether quality adjusted life years (QALYs) differ and whether it is cost effective from a payer’s perspective remain unclear. In this study, we performed a cost-utility analysis comparing these treatment regimens in adult patients with CAT.
Methods:
Target Population and Model Overview
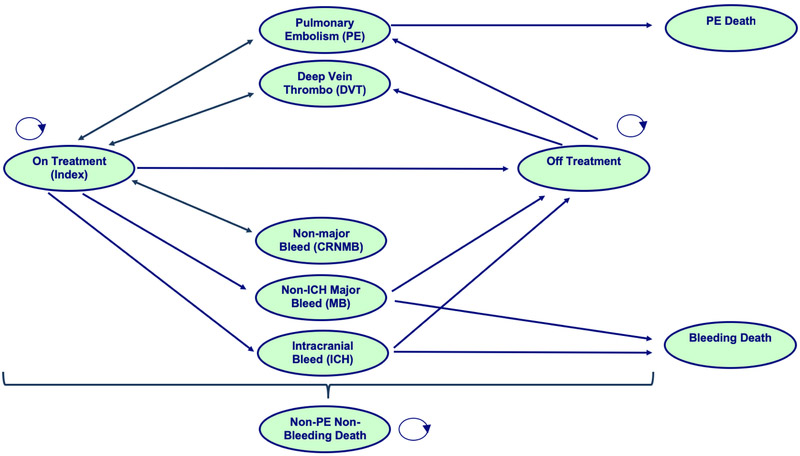
We constructed a Markov state-transition model to evaluate the cost utility of DOAC versus dalteparin for the treatment of CAT over a 60-month time horizon (Figure 1). We used a hypothetical cohort of 65-year-old patients with active malignancy and first acute symptomatic VTE who were eligible to receive either drug. We did not include warfarin in the comparison because it is not considered as current standard of care for CAT treatment in the US. The transition states for the model included on anticoagulant treatment, off anticoagulant treatment, recurrent pulmonary embolism (PE), recurrent deep vein thrombosis (DVT), intracranial hemorrhage (ICH), non-ICH major bleeding (MB), clinically relevant non-major bleeding (CRNMB), PE-related death, MB-related death, and non-PE/non-MB-related death. Among these, PE, DVT, ICH, MB, and CRNMB were temporary states where patients only spent a single cycle. The cycle length was chosen to be 1 month as a clinically meaningful time interval to capture potential transitions. The time horizon of 60 months was chosen as an approximation of “lifelong” time frame because of a high overall mortality of 38% by 12 months. [6] A 3% yearly discount for cost and quality were applied based on an average of the US federal reserve primary and secondary credit interest rates.[9]
Figure 1: Markov transition-state diagram.
This diagram presents all potential health states that a patient may occupy while in the model. Arrows indicate potential health state transitions of patients. Patients are exposed to risks of recurrent PE, recurrent DVT, CRNBM, MB, ICH, PE-related death, MB-related death, and non-PE/non-MB-related death. The states of PE, DVT, ICH, MB, and CRNMB were temporary states where patients only spent a single cycle (1 month) and then return to their originating health state. All death states were self-absorbing.
Our Markov model (Figure 1) made several assumptions: 1) patients existed in mutually exclusive states (i.e. could not have recurrent VTE and bleeding simultaneously), 2) patients who experienced a recurrent VTE event or CRNMB would return to the same anticoagulant “on treatment” state after 1 cycle unless death had occurred, 3) patients who experienced an ICH or MB would transition to an “off treatment” state after 1 cycle unless death had occurred, 4) patients had higher risks for recurrent VTE when “off treatment” than “on treatment” and that risk varied according to the time away from the initial index event, 5) post-thrombotic syndrome (PTS) did not occur at clinically significant rate due to high cancer mortality, 6) cancer mortality occurred at a constant rate in all states, and 7) patients would discontinue anticoagulant at a constant rate according to the rate reported in the clinical trials and not influenced by cancer remission rate.
Model Inputs
Model parameter inputs are shown in Supplemental Table 1. Transition probabilities (TP), risk ratios (RR), and confidence intervals (CI) for the VTE, bleeding, and mortality outcomes were derived from an updated meta-analysis of the Hokusai-VTE and Select-D randomized clinical trials. [8] The TP from “on treatment” to “off treatment” was estimated from the number of patients that permanently discontinued either study drug due to investigator decision, patient decision, patient withdrawal, or other/unknown. Given the differential follow-up lengths for the two studies, only events occurred during the first 6 months were included in the meta-analysis (Table 1). Details regarding the systematic review for study inclusion was described previously. [8] For updated data synthesis, RR estimation was performed with the Mantel-Haenszel random effects model (DerSimonian-Laird analysis). [10] TP and RR for months 7-12 were derived directly from Hokusai-VTE study and the same estimates were extrapolated to time horizon beyond 12 months. The time-varying TP for recurrent VTE when off anticoagulant treatment was estimated from a large population study of cancer patients. [11] Monthly TP was converted from total TP using the formula: TP1=1-(1-TPt)^(1/t).
Table 1.
Meta-analysis of rivaroxaban/edoxaban (DOAC) versus dalteparin (LMWH) during first 6 months of treatment
| DOAC | Dalteparin | RR* | |||
|---|---|---|---|---|---|
| Proportion | Number | Proportion | Number | ||
| Cancer mortality (non-VTE, non-bleed, combined) | 25.0% | 181 | 24.2% | 176 | |
| 1-6 months (edoxaban vs. dalteparin) | 25.9% | 135 | 23.3% | 122 | 1.01 (0.79-1.28) |
| 1-6 months (rivaroxaban vs. dalteparin) | 22.7% | 46 | 26.6% | 54 | |
| Pulmonary embolism (PE +/− DVT, combined) | 3.7% | 27 | 4.5% | 33 | |
| 1-6 months (edoxaban vs. dalteparin) | 4.4% | 23 | 4.6% | 24 | 0.78 (0.40-1.53) |
| 1-6 months (rivaroxaban vs. dalteparin) | 2.0% | 4 | 4.4% | 9 | |
| Deep vein thrombosis only (DVT, combined) | 2.1% | 15 | 4.3% | 31 | |
| 1-6 months (edoxaban vs. dalteparin) | 2.1% | 11 | 4.2% | 22 | 0.49 (0.26-0.89) |
| 1-6 months (rivaroxaban vs. dalteparin) | 2.0% | 4 | 4.4% | 9 | |
| Intracranial hemorrhage (ICH, combined) | 0.3% | 2 | 0.6% | 4 | |
| 1-6 months (edoxaban vs. dalteparin) | 0.4% | 2 | 0.8% | 4 | 0.50 (0.09-2.73) |
| 1-6 months (rivaroxaban vs. dalteparin) | 0.0% | 0 | 0.0% | 0 | |
| Non-ICH major bleeding (MB, combined) | 5.2% | 38 | 2.6% | 19 | |
| 1-6 months (edoxaban vs. dalteparin) | 5.2% | 27 | 2.5% | 13 | 2.00 (1.17-3.44) |
| 1-6 months (rivaroxaban vs. dalteparin) | 5.4% | 11 | 3.0% | 6 | |
| Clinically relevant non-major bleeding (CRNMB, combined) | 12.3% | 89 | 6.9% | 50 | |
| 1-6 months (edoxaban vs. dalteparin) | 12.3% | 64 | 8.2% | 43 | 2.14 (0.92-4.97) |
| 1-6 months (rivaroxaban vs. dalteparin) | 12.3% | 25 | 3.4% | 7 | |
| Drug discontinuation due to choice or withdrawal (combined) | 17.4% | 126 | 28.9% | 210 | |
| Overall (edoxaban vs. dalteparin) | 16.7% | 87 | 29.6% | 155 | 0.58 (0.41-0.82) |
| Overall (rivaroxaban vs. dalteparin) | 19.2% | 39 | 27.1% | 55 | |
| Adverse event mortality (combined) | |||||
| Fatal PE (combined) | 22.6% | 7 | 13.5% | 5 | 1.66 (0.58-4.72) |
| Fatal ICH (combined) | 0.0% | 0 | 25.0% | 1 | 0.56 (0.03-9.73) |
| Fatal non-ICH MB (combined) | 2.3% | 1 | 8.7% | 2 | 0.34 (0.05-2.53) |
A meta-analysis was performed for various outcomes during the first 6 months of treatment using published data from Hokusai-VTE and Select-D randomized controlled trials via the Mantel-Haenszel random effects model.
Cost estimates were derived from published literature and evaluated from a payer’s perspective to include direct medical cost related to drugs and complications. Unit costs for edoxaban 30 mg and 60 mg ($11.22), rivaroxaban 15 mg and 20 mg ($13.97), and dalteparin 10,000 IU and 15,000 IU ($70.90, $106.35) were based on the wholesale acquisition cost (WAC) from the Red Book.[12] For edoxaban, there was a lead-in phase of 5 days of dalteparin. For rivaroxaban, there was a lead-in phase of 21 days of twice daily administration of rivaroxaban. For dalteparin, there was a lead-in phase of 30 days of higher dose at 200 IU/kg. An average of the edoxaban and rivaroxaban total drug costs were used for the DOAC arm. Patients were assumed to weigh an average of 70 kilogram and used 15,000 IU daily of dalteparin during the lead-in phase and 10,000 IU of dalteparin during the subsequent cycles. Adverse event costs for recurrent VTE and bleeding episodes were obtained from Preblick et al where the cost per stay estimates were derived from the Premier Hospital Database and post-hoc analysis of the Hokusai-VTE study. [13] All cost estimates were inflated to 2018 US dollars using the US Consumer Price Index for all urban consumers’ medical care.[14]
Utility weights and CI ranges between 0 and 1 were derived from the literature of general medical patients with VTE.[15] While the previous utility study did not include cancer patients, the utility estimates were very similar to a different study that did include cancer patients.[16] Both studies used time trade-off techniques but the first study interviewed patients who experienced treatment associated complications rather than imagined health states. As such, we used a baseline utility of 0.95 according to the reference study.[15] Both drug treatments were similarly assigned a base utility of 0.95 because several studies showed that LMWH was not associated with significant disutility compared to placebo as measured by EQ-5D-3L.[17,18] We used the average estimates from gastrointestinal and muscular bleeding for the utility of MB.[15]
Base case and sensitivity analyses
For base case analysis, the cumulative cost and quality-adjusted life-years (QALYs) were estimated for each treatment over the time horizon. The incremental cost effectiveness ratio (ICER) was calculated as the difference in cost over the difference in QALYs, if such calculation proved to be meaningful. Half-cycle correction was not performed given the short cycle length of 1 month.
We performed one-way deterministic sensitivity analyses separately for QALY and cost outcomes to assess the impact due to variations from different estimate inputs. The upper and lower bounds of the 95% CI were used if such data were available from literature. Otherwise, the variations were assumed to be +/− 20% from the mean value.
We performed a probabilistic sensitivity analysis (PSA) using Monte Carlo simulation over 1000 times to generate the cost-effectiveness (CE) plane. The distributions assumed for the input parameters were gamma (cost), beta (utility weights and TP), and log-normal (RR). The standard errors were derived from the 95% CI and alpha/beta parameters were estimated using method of moments. We performed additional sensitivity and subgroup analyses based on relevant clinical scenarios. All data analyses were performed in Microsoft Excel for Mac 16.17.
Results:
Base case analysis
The study input parameters, ranges, and references are shown in Supplemental Table 1. The meta-analysis results for the first 6 months are shown in Table 1. The base case analyses for both data-driven (12 months) and extrapolated (60 months) time horizons are shown in Table 2. Over a period of 5 years, DOAC as compared to dalteparin were associated with 14 fewer PEs, 62 fewer DVTs, 2 fewer ICH, 42 more non-ICH MB, and 111 more CRNMB per 1000 patients. Mortality was similarly high in both interventions. DOAC as compared to dalteparin had 5 more PE-related deaths, 2 fewer MB-related deaths, and 5 more non-PE/non-MB related deaths.
Table 2.
Base case analysis for 12- and 60-month time horizons
| DOAC | Dalteparin | DOAC | Dalteparin | |
|---|---|---|---|---|
| Number of events (per 1000 patients) | ||||
| Time horizon | 12-month | 12-month | 60-month | 60-month |
| Recurrent PE | 43 | 54 | 78 | 92 |
| Recurrent DVT | 32 | 62 | 85 | 147 |
| ICH | 2 | 4 | 2 | 4 |
| Non-ICH MB | 54 | 27 | 83 | 41 |
| CRNMB | 177 | 102 | 320 | 209 |
| PE- deaths | 9 | 7 | 17 | 12 |
| MB-deaths | 2 | 3 | 3 | 5 |
| Non-PE/non-MB-deaths | 380 | 375 | 862 | 857 |
| Cost | ||||
| Total cost per patient | $6,239 | $19,337 | $11,452 | $35,581 |
| Health outcomes | ||||
| Life years per patient | 0.76 | 0.76 | 1.86 | 1.90 |
| QALYs per patient | 0.71 | 0.72 | 1.76 | 1.80 |
| Cost effectiveness | ||||
| Δ Cost | −$13,099 | −$24,129 | ||
| Δ QALY | 0.00 | −0.04 | ||
| ICER | $2,861,615 | $623,459 | ||
PE = pulmonary embolism, DVT = deep vein thrombosis, ICH = intracranial bleeding, MB = major bleeding, CRNMB = clinically relevant nonmajor bleeding, QALY = quality adjusted life year
The total cost per patient over 60 months was $11,452 for the DOAC arm and $35,581 for the dalteparin arm (incremental cost difference of −$24,129 per patient). The QALY per patient was 1.76 for DOAC and 1.80 for dalteparin (incremental QALY difference of −0.04). The estimated ICER was $623,459 and represented cost saved per QALY lost.
Sensitivity analysis
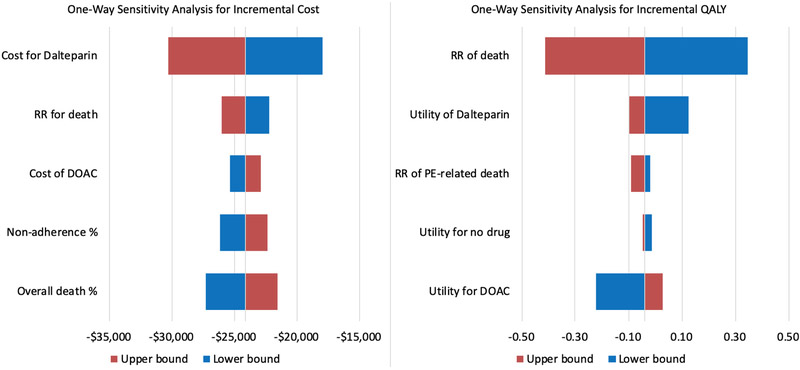
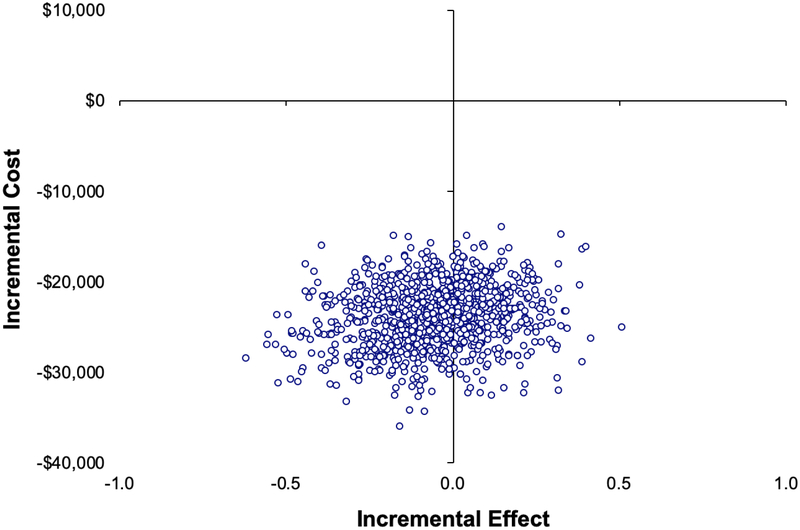
Due to the unstable ICER estimate driven by negligible differences in QALY, we performed separate one-way deterministic sensitivity analyses for incremental cost and incremental QALY differences. By changing one input parameter at a time using both the upper and lower bounds of the estimate (Supplemental Table 1), we were able to detect input parameters associated with significant outcome changes. In this analysis, the only input parameter that would significantly change the incremental cost was the cost of dalteparin (Figure 2 left) and the only ones that would significantly change the incremental QALY were the relative risk of death from cancer and relative utility values of DOAC versus dalteparin (Figure 2 right). Given the lack of mortality difference associated with either treatment, patients would have an overall higher incremental QALY if the oral DOAC contributed to a higher quality of life than subcutaneous dalteparin. In the probabilistic sensitivity analysis (PSA), DOAC versus dalteparin appeared to be cost saving at −$23,892 (95% CI −29,743 to −18,350) with minimal differences in QALY at −0.05 (95% CI −0.36 to 0.23) with an ICER of $439,957 (Figure 3).
Figure 2. One-way deterministic sensitivity analysis for incremental cost and QALY.
The tornado diagrams represent the results of the deterministic sensitivity analyses and depict the key parameters that had the greatest impact on incremental cost difference or quality-adjusted life year (QALY) difference. The boxes to the right and left of the tornado diagram represent the ranges tested for that parameter detailed in the description. While one-way sensitivity analysis was conducted for all input parameters, only the 5 most significant one-way deterministic sensitivity analyses are shown in the above diagram.
Figure 3. Probabilistic sensitivity analysis (PSA) of the cost-effectiveness plane.
The cost-effectiveness plane represents the resulting incremental cost and incremental effect (quality adjusted life year or QALY) differences from 1000 bootstrapped simulations from varying input parameters. The width of the plots represents the 95% confidence interval of the probabilistic estimate. According to the PSA, DOAC was associated with significantly lower cost than dalteparin and was not associated with differences in QALY.
We performed two additional sensitivity analyses based on various clinical scenarios. First, we substituted the cheaper enoxaparin (generic) for dalteparin (trial study drug) in an additional deterministic sensitivity analysis and found that DOAC vs. enoxaparin had a cost difference of −$17 and QALY difference of −0.04 per patient over 60 months (ICER $445). Second, we performed a subgroup analysis based on available bleeding outcomes data from patients with and without gastrointestinal malignancies.[19] In the post-hoc analysis of the Hokusai VTE Cancer study, patients without gastrointestinal malignancy had 18/357 (5.04%) MB in the edoxaban arm and 19/384 (4.95%) MB in the dalteparin arm by 12 months. This would translate to a baseline monthly TP of 0.43% for edoxaban and 0.42% for dalteparin (RR 1.02, 95% CI 0.54-1.91). Our deterministic analysis in this scenario showed a cost reduction of −$24,620 and a QALY decrement of −0.03 over 60 months (ICER $730,183) among patients without gastrointestinal malignancies.
Discussion:
In our cost-utility analysis of different anticoagulant regimens for the treatment of CAT, we found a consistent cost saving with no clinically meaningful differences in QALY for DOAC versus dalteparin. We believe the results of this cost-utility analysis can help policy-makers decide how patients with cancer would benefit from different anticoagulant treatment for CAT. From an efficacy perspective, after accounting for differential adherence of the two interventions, DOAC was associated with fewer VTE and ICH but more non-ICH MB and CRNMB. In a sensitivity analysis, the MB events were lower in the subgroup of patients without gastrointestinal malignancies. Due to the low PE- and MB-related deaths and the similar disutilities assigned to VTE and MB, the small differences in survival or QALYs between DOAC and dalteparin are caused by the high probability of early cancer mortality and the uncertainties associated with the relative risk of death (1.01 (95% CI 0.79-1.28)). Based on the sensitivity analysis, QALY can also be affected by the utility weights assigned to the LMWH treatment. It is well known that adherence to LMWH treatment outside of clinical trials is poor as a result of patient and physician preferences. [5,20] These preferences may be under-captured by the standard domains of the EQ-5D questionnaires. Future studies are needed to directly assess patients’ preferences and values for DOAC versus LWMH as small changes in the utility weight for either intervention could drastically change the QALY difference.
From a cost perspective, DOAC was associated with significantly lower total cost per patient compared to dalteparin. This difference was driven by the price of dalteparin in the US as shown in the sensitivity analysis. We must remember two important facts: 1. The price of dalteparin (non-generic) in the US is likely much higher than many other countries, and 2. Clinicians commonly substitute the cheaper enoxaparin (generic) even if the clinical trials used dalteparin as the drug of choice (enoxaparin WAC is $15 for 100 mg). Based on our sensitivity analysis, DOAC would have very similar benefit and cost as enoxaparin if the latter were extrapolated to have the exact same effect as dalteparin.
We performed our analysis over the assumed life time of an average patient with cancer. Our life-time (60-month) horizon may be unrealistic. Cancer-related mortality tends to be very high upfront and VTE recurrence after initial year can to be variable depending on cancer progression (higher rate) or cure (lower rate). Furthermore, the rate of anticoagulation treatment discontinuation is likely much higher than simulated after the first 12 months. Therefore, it is challenging to predict either the treatment adherence or outcome occurrence due to the heterogeneity of cancer outcomes. Recognizing these limitations, both the 60-month (extrapolated) and the 12-month (data driven) horizons showed a consistent cost saving for DOAC versus dalteparin with no noticeable difference in QALYs in our analysis (Table 2). This finding reassures us that differential time horizons and variabilities in cancer survival rate in real life are likely to impact the magnitude but not consistency of our results.
There are limitations associated with our study. First, we extrapolated the cost and utility values from the general medical patients to cancer patients with VTE. While the associated admission cost would be higher and the baseline utility lower in cancer patients, the relative difference between the two intervention arms were likely be negligible.[21] Standard errors and confidence intervals for some estimates were assumed rather than derived from data; however, as shown in the one-way sensitivity analyses, small variations were unlikely to significantly alter the findings. We did not account for the dis-utilities associated with PTS because we assumed that patients did not live long enough to suffer from it. We also did not assign permanent dis-utilities to ICH given the small number of patients with this particular complication in each arm. We assumed that patients who experienced a recurrent VTE event would return to the same anticoagulant at the same dose for simplicity although it is likely that they would be switched to an alternative treatment based on the physician’s choice. Due to the lack of access to individual patient data, we used the Markov model to assess the population average instead of the patient-level simulation to account for patient heterogeneity. Finally, the probabilities of mortality and adverse events are derived directly from trial data. These are likely to be under-estimates when compared to baseline probabilities obtained from epidemiology studies due to the selection for patients potentially at lower risk of bleeding.
Conclusion:
In conclusion, we found that DOAC (rivaroxaban and edoxaban) was cost saving from a payer’s perspective with a very small decrement in QALYs when compared to dalteparin for the treatment of CAT. Professional organizations and healthcare systems may want to consider this analysis in future practice recommendations.
Supplementary Material
Highlights.
Direct oral anticoagulant (DOAC) is a newer treatment for thrombosis in cancer
Rivaroxaban and edoxaban (DOAC) have been compared to dalteparin in recent studies
We compared the cost and quality adjusted life year (QALY) of DOAC vs dalteparin
DOAC vs dalteparin is associated with a significant incremental cost decrease in US
DOAC vs dalteparin is associated with a non-significant incremental QALY decrease
Acknowledgements:
Research reported in this publication was supported by Conquer Cancer Foundation Young Investigator Award (AL), Hemostasis and Thrombosis Research Society Mentored Research Award supported by an independent medical educational grant from Shire (AL), and National Hemophilia Foundation Shire Clinical Fellowship Award (AL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
The authors have no conflict of interest related to the conduct of the study. DAG received grant funding from Bristol Meyers Squibb, Daiichi Sankyo, Incyte, Bayer, Janssen and personal fees from Alexion, Bristol Meyers Squibb, Boehringer Ingelheim, Incyte, Janssen, Pfizer, Seattle Genetics, outside the submitted work. GHL received grant funding from Amgen and personal fees from Amgen, Agendia, Bristol-Myers Squibb, G1 Therapeutics, Genomic Health, Inc.Halozyme Therapeutics, Helsinn Therapeutics, Hexal, Partners Healthcare, Pfizer, outside the submitted work.
References:
- [1].Prandoni P, a Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins MH, Noventa F, Girolami A, Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis., Blood. 100 (2002) 3484–8. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- [2].Lyman GH, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, Clarke JM, Flowers CR, Francis CW, Gates LE, Kakkar AK, Key NS, Levine MN, Liebman HA, Tempero MA, Wong SL, Prestrud AA, Falanga A, American Society of Clinical Oncology Clinical Practice, Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update., J. Clin. Oncol 31 (2013)2189–204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- [3].Lee AYY, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF, Khorana AA, CATCH Investigators, Tinzaparin vs Warfarin for Treatment of Acute Venous Thromboembolism in Patients With Active Cancer: A Randomized Clinical Trial., JAMA. 314 (2015) 677–686. doi: 10.1001/jama.2015.9243. [DOI] [PubMed] [Google Scholar]
- [4].Lee AYY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickies FR, Julian JA, Haley S, Kovacs MJ, Gent M, Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators, Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer., N. Engl. J. Med. 349 (2003) 146–53. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- [5].Khorana AA, Yannicelli D, McCrae KR, Milentijevic D, Crivera C, Nelson WW, Schein JR, Evaluation of US prescription patterns: Are treatment guidelines for cancer-associated venous thromboembolism being followed?, Thromb. Res 145 (2016) 51–53. doi: 10.1016/j.thromres.2016.07.013. [DOI] [PubMed] [Google Scholar]
- [6].Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri MF, Meyer G, Segers A, Shi M, Wang T-F, Yeo E, Zhang G, Zwicker JI, Weitz JI, Büller HR, Hokusai VTE Cancer Investigators, Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism., N. Engl. J. Med. 378 (2018) 615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
- [7].Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, Hale D, Dunn JA, Lyman GH, Hutchinson C, MacCallum P, Kakkar A, Richard Hobbs FD, Petrou S, Dale J, Poole CJ, Maraveyas A, Levine M, Hobbs FDR, Petrou S, Dale J, Poole CJ, Maraveyas A, Levine M, Comparison of an oral factor xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D), J. Clin. Oncol 36 (2018) 2017–2023. doi: 10.1200/JCO.2018.78.8034. [DOI] [PubMed] [Google Scholar]
- [8].Li A, Garcia DA, Lyman GH, Carrier M, Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta-analysis., Thromb. Res. 173 (2019) 158–163. doi: 10.1016/j.thromres.2018.02.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Discount Window, Fed. Reserv (2018). https://www.frbdiscountwindow.org. [Google Scholar]
- [10].DerSimonian R, Laird N, Meta-analysis in clinical trials., Control. Clin. Trials. 7 (1986) 177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- [11].Chee CE, Ashrani AA, Marks RS, Petterson TM, Bailey KR, Melton III LJ, Heit JA, Melton LJ, Heit JA, Predictors of venous thromboembolism recurrence and bleeding among active cancer patients: a population-based cohort study, Blood. 123 (2014) 3972–3978. doi: 10.1182/blood-2014-01-549733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Book Red, Heal Truven. Anal. Aver. Wholes. Price. (2018). http://truvenhealth.com/Products/Micromedex/Product-Suites/Clinical-Knowledge/RED-BOOK. [Google Scholar]
- [13].Preblick R, Kwong WJ, White RH, Goldhaber SZ, Cost-effectiveness of edoxaban for the treatment of venous thromboembolism based on the Hokusai-VTE study., Hosp. Pract. (1995). 43 (2015) 249–57. doi: 10.1080/21548331.2015.1099412. [DOI] [PubMed] [Google Scholar]
- [14].Consumer Price Index, All Urban Consumers, Medical care – Not Seasonally Adjusted, U.S. Dep. Labor Bur. Labor Stat (2018). http://data.bls.gov/cgi-bin/surveymost?cu. [Google Scholar]
- [15].Locadia M, Bossuyt PMM, Stalmeier PFM, Sprangers MAG, van Dongen CJJ, Middeldorp S, Bank I, van der Meer J, Hamulyák K, Prins MH, Treatment of venous thromboembolism with vitamin K antagonists: patients’ health state valuations and treatment preferences., Thromb. Haemost. 92 (2004) 1336–41. doi: 10.1160/TH04-02-0075. [DOI] [PubMed] [Google Scholar]
- [16].Hogg K, Kimpton M, Carrier M, Coyle D, Forgie M, Wells P, Estimating quality of life in acute venous thrombosis., JAMA Intern. Med. 173 (2013) 1067–72. doi: 10.1001/jamainternmed.2013.563. [DOI] [PubMed] [Google Scholar]
- [17].Macbeth F, Noble S, Evans J, Ahmed S, Cohen D, Hood K, Knoyle D, Linnane S, Longo M, Moore B, Woll PJPJ, Appel W, Dickson J, Ferry D, Brammer C, Griffiths G, Randomized Phase III Trial of Standard Therapy Plus Low Molecular Weight Heparin in Patients With Lung Cancer: FRAGMATIC Trial., J Clin Oncol. 34 (2016) 488–94. doi: 10.1200/JCO.2015.64.0268. [DOI] [PubMed] [Google Scholar]
- [18].Marchetti M, Pistorio A, Barone M, Serafini S, Barosi G, Low-molecular-weight heparin versus warfarin for secondary prophylaxis of venous thromboembolism: a cost-effectiveness analysis., Am. J. Med. 111 (2001) 130–9. doi: 10.1016/S0002-9343(01)00793-8. [DOI] [PubMed] [Google Scholar]
- [19].Kraaijpoel N, Di Nisio M, Mulder FI, van Es N, Beyer-Westendorf J, Carrier M, Garcia D, Grosso M, Kakkar AK, Mercuri MF, Middeldorp S, Hernandez CR, Santamaria A, Schwocho L, Segers A, Verhamme P, Wang T-F, Weitz JI, Zhang G, Zwicker JI, Büller HR, Raskob GE, Clinical Impact of Bleeding in Cancer-Associated Venous Thromboembolism: Results from the Hokusai VTE Cancer Study., Thromb. Haemost. 118 (2018) 1439–1449. doi: 10.1055/s-0038-1667001. [DOI] [PubMed] [Google Scholar]
- [20].Mahé I, Chidiac J, Heifer H, Noble S, Factors influencing adherence to clinical guidelines in the management of cancer-associated thrombosis, J. Thromb. Haemost. 14 (2016)2107–2113. doi: 10.1111/jth.13483. [DOI] [PubMed] [Google Scholar]
- [21].Elting LS, Escalante CP, Cooksley C, Avritscher EBC, Kurtin D, Hamblin L, Khosla SG, Rivera E, Outcomes and cost of deep venous thrombosis among patients with cancer., Arch. Intern. Med. 164 (2010) 1653–61. doi: 10.1001/archinte.164.15.1653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.