Abstract
Objectives:
Global increases in Caesarean deliveries are exposing more infants to perinatal environments that are evolutionarily novel and potentially increasing their risks for inflammatory conditions. Yet, the pathways linking Caesareans to later health outcomes are not well understood, particularly in dual burden contexts. We test two of the hypothesized pathways, altered immune function and gut microbiota, that may link delivery mode to later health outcomes and test whether these associations persist when controlling for postnatal nutritional and pathogenic exposures.
Methods:
Data come from infants, aged 0–2 (n=41), and young children, aged 2–11 (n=135), from the Galápagos, Ecuador. Differences in morbidity, C-reactive protein (CRP), and gut microbiota by delivery type were tested using linear and logistic regression models adjusted for nutritional and pathogenic exposures and infant age.
Results:
Over half of infants and over 40% of children were delivered by Caesarean. Morbidity and CRP did not differ between infants or children born by Caesarean or vaginally. Microbial taxa abundance differed by delivery mode. Infants born by Caesarean had a higher abundance of Firmicutes and a lower relative abundance of Bacteroidales. Children born by Caesarean had a higher abundance of Proteobacteria and Enterobacteriales. These differences remained after adjustment for environmental exposure.
Conclusions:
Caesarean delivery is associated with differences in gut microbiota across childhood even in this dual burden context. Our results highlight the importance of examining Caesarean delivery across diverse contexts to better understand the impact of perinatal interventions on short- and longer- term health outcomes.
Keywords: Caesarean delivery, microbiome, morbidity, dual burden, inflammation
The rate of Caesarean section is increasing globally, particularly in low and middle income countries (LMICs) where rates had traditionally been low (Betrán et al. 2016). This increasing prevalence may be exposing more infants to perinatal experiences and environments that are evolutionarily novel with detrimental consequences for short and long-term growth and development (Rosenberg and Trevathan 2018). Caesarean deliveries, particularly elective Caesarean deliveries occurring prior to labor and rupture of the membranes, have been associated with poorer neonatal outcomes including greater risk of respiratory distress and necrotizing enterocolitis (NEC) (Collado et al. 2012). Longer-term, Caesarean delivery may increase risk of allergy and asthma (Kristensen and Henriksen 2016), juvenile-onset diabetes (Cardwell et al. 2008) and inflammatory bowel disease (Bager et al. 2012) in childhood and adolescence. The risk of asthma, for example, is 20% higher in children and adults born by Caesarean in meta-analyses controlling for known confounders, including maternal smoking, low birth weight and duration of breastfeeding (Thavagnanam et al. 2008). Analyses of national health registries have documented that Caesarean delivery is associated with greater risk of allergy, asthma, and autoimmune conditions, even when adjusting for maternal history of disease, socioeconomic status, and maternal smoking (Sevelested et al. 2015). The variety of conditions associated with Caesarean delivery and the persistence of these effects into adulthood support the importance of perinatal exposures for healthy growth and development.
Evidence suggests that several interrelated pathways may underlie these associations between mode of delivery and later health outcomes, including altered immune system development and perturbed gut microbiota colonization. First, labor appears to be important for the development and proper activation of the immune response. Contractions of the uterus and hypoxia during passage through the birth canal elicit a stress response in the fetus that leads to the production of catecholamines and cortisol. In turn, these stress hormones redistribute and activate immune cells (Almanzar et al. 2015). Neonates born vaginally have higher leukocyte counts in their cord blood, with higher proportions of neutrophils, monocytes, natural killer cells, T-cells, B-cells, and granulocytes compared to infants born after elective Caesarean delivery (Almanzar et al. 2015; Thysen et al. 2015). These immune cells also function differently; the leukocytes of neonates born by Caesarean produce lower levels of cytokines, including IL-I, TNF-a, IL-6 and IL-12 (Thysen et al 2015), that regulate the innate immune response and inflammation. Likely due to these differences in cytokine production, levels of C-reactive protein (CRP), a measure of systemic inflammation stimulated by cytokines, are also lower in these neonates (Kaapa and Koistinen 1993, Logan et al 2016). Differences in the ability to mount an inflammatory response are important since, at an acute scale, inflammation helps fight infection and promotes the repair of damaged tissues. Together the greater numbers of immune cells and elevated inflammatory biomarkers seen with vaginal delivery likely serve as an adaptation, enhancing the defense mechanisms of the immune system and protecting neonates against immediate infection in the external environment (Gollwixter and Marsland 2015; Weineberger et al. 2007). While the persistence of these immune alterations through infancy and childhood remain unknown, the experience of labor may be “stored” in the immune system through memory T-cells and/or epigenetic modifications (Romero and Korzeniewski 2013). Some, but not all studies, have found that infants born by pre-labor Caesarean have higher global DNA methylation in umbilical cord leukocytes than infants born vaginally (Cho and Norman 2013; Schlinzig et al 2009; Virani et al. 2012), indicating that vaginal delivery may up-regulate gene expression in immune cells.
Alongside these alterations in immune cell development and function, Caesarean delivery also influences neonatal bacterial exposures. Unlike vaginally born infants, Caesarean delivered infants are not exposed to their mothers’ vaginal and fecal bacteria during delivery and, instead, have bacterial profiles more like their mother’s skin and the hospital environment (Dominguez-Bello et al. 2010). Vaginally-delivered infants are exposed to and colonized by Bacteroides and Bifidobacterium species thought to be important for infant health, and they show differences in the proportion of these bacteria in their stool as early as the third day of life (Biasucci et al. 2010). While the gut microbiota of vaginally and Caesarean born infants become more similar over the first year of life, the gut microbiome of infants delivered by Caesarean remain less diverse with a delayed colonization by Bacteroides (Arrieta et al. 2014). Caesarean delivery may compromise the intergenerational transmission of these and other beneficial maternal bacteria, an exposure important for the development of healthy gut function and immune regulation in early life (Huda et al. 2014).
How these delivery-related differences in immune function and bacterial exposure are further modified by other postnatal exposures, such as pathogens and infant diet, has received less attention. The majority of studies examining the association between Caesarean delivery and immune function and gut microbiome development have been conducted in the United States and other industrialized countries. In these settings, Caesarean deliveries are highly correlated with maternal factors such as health conditions, obesity and advanced age (Herstad et al. 2016; Li, Zhou and Liu 2013) and are a strong risk factor for a lack of breastfeeding or delayed onset (Prior et al. 2012). Since maternal obesity during pregnancy (McCloskey et al. 2018) and lack of breastfeeding (McDade et al. 2010) are associated with inflammation in infants and young adults, these exposures, rather than the Caesarean section procedure, may alter the development of the immune system in ways that make infants and children more prone to inflammatory conditions, like asthma or autoimmune conditions. Ignoring these confounders may over-emphasize the importance of Caesarean delivery in disease pathways in Western populations. Conversely, postnatal pathogenic exposures in Western environments may be more limited (Dowd, Zajacova and Aiello 2010), leading to less acute inflammation and potentially masking important differences in immune response or gut microbial colonization in response to pathogens based on delivery experience. Thus, examining the association between Caesarean delivery and immune and gut development in diverse developmental contexts is important for understanding and potentially isolating the effects of Caesareans from these other confounding factors (Veile and Kramer 2017; Carrillo-Larco et al 2015). Of particular interest are LMICs, like Ecuador, where rates of Caesareans are increasing and patterns of immune activation, inflammation and gut microbiota may differ due to the higher rates of breastfeeding and persistent pathogenic environmental exposures.
Thus, we examine the association between Caesarean delivery and immune function and the gut microbiota in infants and children from Galápagos, Ecuador. Ecuador, and the Galápagos, in particular, serve as an important case study for addressing these questions. The Galápagos suffer from a dual burden of prevalent infectious disease and high levels of overweight and obesity in children and adults due to poor water infrastructure and the reliance on processed foods (Houck 2017; Page, Bentley, and Waldrop 2013). In this environment, infants are simultaneously exposed to factors that may increase risk (e.g., pathogen exposure, formula feeding, stunting and obesity) or buffer against (e.g., breastfeeding) inflammation and/or perturbation of the gut microbiota. Rates of Caesarean delivery are also high in Ecuador. Nationally, the prevalence of Caesareans has almost doubled in the past 15 years, from 22% in 1989 to 41% in 2012. Caesarean rates show significant geographic, socioeconomic, and ethnic differences with higher rates seen in coastal areas, among women with higher income, and among non-indigenous women (Jahnke et al, under review). As a relatively high-income province with mainly mestiza inhabitants, the Galápagos have some of the highest Caesarean rates in the country with 36–45% of infants being delivered by Caesarean (Freire et al. 2015). Yet, previous work indicates that rates of breastfeeding are also high, with the vast majority of mothers breastfeeding their infants. In the Galápagos, nearly 100% of mothers initiate breastfeeding and almost 44% of all infants are exclusively breastfed until 6 months of age (Freire et al. 2015). At the same time, because of the limited water and sanitation infrastructure, rates of diarrheal disease and pathogenic exposures remain high among infants and children on the islands (Houck 2017; Page, Bentley, and Waldrop 2013).
Given these potential pro- and anti-inflammatory postnatal exposures, we describe the birth practices and postnatal feeding and pathogenic environments for infants and children in the Galápagos, and test two of the hypothesized pathways, altered immune function and gut microbiota, that may link birth practices to later health outcomes. We ask: 1) does immune activation (measured by illness symptoms and C-reactive protein) differ by delivery type? 2) do gut microbiota diversity and predominant taxa differ by delivery type? And, lastly, 3) do differences in immune activation and microbiota persist after controlling for relevant pathogenic (household size and bath water source) and nutritional (formula feeding, stunting, and obesity) exposures?
Sample and Methods
Setting:
Participants come from San Cristóbal, the island that serves as the provincial capital of the Galápagos. Because the majority of the island is national park land, most of the island’s nearly 7500 residents (82%) live in the concentrated urban area of Puerto Baquerizo Moreno and its surrounding neighborhoods, which account for only 3% of the island’s land (CGREG and INEC 2010). As of the 2010 census, the island has a low unemployment rate (approximately 2%) with residents working in public administration (21%), tourism (21%), and agriculture or fishing (11%). Although poverty is present on the island, household incomes are higher than on the mainland, and no households are considered to live in extreme poverty (Granda Leon, Gonzalez Cambra, and Calvopina Carvajal 2013). Over 99% of households have electricity, approximately 75% have indoor bathrooms connected to the municipal sewage system, and another 23% have septic tanks (CGREG and INEC 2010). Most residents self-identify as mestizo (a mix of Indigenous Ecuadorian and Spanish descent) at 81%, with smaller proportions identifying as indigenous ethnicities from the mainland (9%), European (6%), and Afro-Ecuadorian (4%; CGREG and INEC 2010). As in the rest of Ecuador, Spanish is the most commonly spoken language followed by indigenous languages. Health services are provided by a free, public hospital, which provides both emergency services and primary care, and a smaller public health clinic, which provides basic services, in the highlands. Nearly all births (98.1%) take place in a hospital or clinic either on the island or on the continent, where some women travel to give birth in private hospitals or to stay closer to their families (Freire et al. 2015).
San Cristóbal, like the rest of the archipelago, suffers from a dual burden of undernutrition and infectious disease alongside obesity and cardiometabolic disease. Migration to the island has contributed to dramatic popuation growth of >300% in the past decade and tourism brings an additional 225,000 tourists to the island yearly, putting strain on the island’s already limited water and food resources (Walsh et al 2010). Residents rely on municpal water supplied by a water treatment plant, but have concerns about its safety and tend to purchase bottled water or treat tap water before drinking (Houck 2017). Water quality testing has documented high levels of E. coli in household tap water (Gerhard et al. 2017), and morbidity from gastrointestinal, respiratory, and skin infections is common (Walsh et al. 2010). In addition to pathogen exposure through water, children also come into frequent contact with animals as many households have cats, dogs and chickens. Residents primarily rely on food shipped from the mainlaind, which tends to be energy-dense processed foods. Relatively little fresh produce is avaialble and prices tend to be high (Page, Waldrop and Bentley, 2010). Levels of stunting remain relatively high (11% in children <5), and micronutrient deficiencies of iron (16% in children <5) and zinc (57% of reproductive age women) are common (Freire et al. 2015). At the same time, the prevalence of overweight and cardiometabolic disease are the highest in Ecuador, with over 40% of children aged 5–11 and 75% of adults aged 19–59 overweight or obese and over 20% of adults aged 19–59 having impaired glucose tolerance or diabetes (Freire et al. 2015).
Sample:
Data come from two different studies conducted between 2014 and 2016 in San Cristóbal, Galápagos, Ecuador. The first study, conducted from 2014–2015, included 159 children, aged 2–11, participating in the Child Gut Health Study, who were measured in their homes to assess the association between diet, water quality, gut health, and growth. A subsample of 135 of these children with information on birth practices and gut microbiota measures are included in the current analysis. The second sample comes from a pilot study, the Galápagos Birth Practices Study, conducted in June-July 2016. This study collected in-home surveys assessing birth practices, blood spots, and stool samples from 40 mothers and 41 infants and young children, aged 1 month-2 years. Both studies received approval for human subjects research from the University of North Carolina Institutional Review Board and the local review board of the Universidad de San Francisco Quito.
Household Survey:
In each study, mothers were surveyed about their birth histories, child morbidity, household hygiene, feeding practices and sociodemographic characteristics. Mothers reported whether the index child’s birth had been vaginal or by Caesarean. Additionally, in the infant study, mothers giving birth by Caesarean were asked whether the procedure had been planned and reasons for it. Along with the birth characteristics, we used the survey data to define our nutritional and pathogenic exposures from measures available across both studies. In the infant study, mothers were asked whether they had ever breastfed their infants, whether they were currently breastfeeding, and whether they had used infant formula. Similarly, mothers in the child study were asked to recall whether they had breastfed their child, the age at which they stopped breastfeeding, and whether their child had ever received formula. Since there was little variability in whether mothers had breastfed, we used formula use as a marker of early nutritional exposures. Our prior work indicates that formula may influence the development of the gut microbiota independently of breastfeeding (Thompson et al 2015). While formula is unlikely to influence inflammation directly, it may introduce infants to contaminated water or displace breastmilk, which is associated with lower inflammation (Kendall-Tackett 2007).
Mothers in both studies were asked about their hygiene practices, including the source of water used for tasks like bathing their infants, and location of bathing. These data were used to create an “improved” bath water variable which indicates that infants were bathed in boiled, filtered or bottled water vs. tap water and that children were bathed indoors (vs. outdoors). Outdoor bathing was done with non-improved water sources. Additionally, household size, defined as the number of individuals currently residing in the home, was used as a marker of pathogenicity since larger household size has been associated with greater morbidity across a number of contexts (e.g., Dowd et al. 2011; Pan et al 2010).
Anthropometry:
For both infants and children, length/height and weight were collected following standardized methods (WHO 1995). Length was measured to the nearest 0.1cm in infants and children under age 2 using a portable length board. Height was measured to the nearest 0.1cm in children over the age of 2 using a portable stadiometer. Weight was measured using a digital scale to the nearest 10gm. Body mass index (BMI) was calculated from mass(kg)/height(m)2. Sex- and age- specific BMI and length-for-age/height-for-age z-scores were calculated using the WHO reference data and cut-points for comparison of overweight and stunting, respectively, across sex and age groups (de Onis et al. 2006). Overweight was defined as >1 BMIz and stunting as <−2 LAZ/HAZ.
Immune activation:
Immune activation was assessed through two measures, illness symptoms and C-reactive protein levels (CRP). Mothers of infants and children were asked whether their children had experienced any of a list of symptoms, including fever, diarrhea, cough/cold, vomiting and rash, over the past 2 weeks. Children were considered to have gastrointestinal (GI) infections if they experienced diarrhea or vomiting with fever. CRP was obtained from a minimally invasive 50μL blood sample collected by pricking the finger or heel (in younger infants) with a sterile, disposable microlancet. Blood spots were collected on protein saver cards (Whatman 903) (McDade, Williams, and Snodgrass 2007). Eluted blood spots were analyzed using Quantikine’s Human High Sensitivity C-Reactive Protein ELISA (R&D Systems, Inc. Minneapolis, MN). Following previous work (Wander, Shell-Duncan, and McDade 2009, McDade et al. 2008), CRP above 2 mg/L was considered elevated.
Gut microbiota:
Mothers were asked to collect a small sample of feces and store it in provided containers inside their freezers until the interviewers retrieved the samples (normally within 24 hours). Collected samples were stored in −25C freezers at the Galápagos Science Center until transported frozen to UNC for analysis at the Microbiome Core Laboratory at the completion of fieldwork. DNA was isolated using the Qiagen BioRobot Universal (Qiagen, Valencia, CA) with the Qiagen Blood and Tissue and QIAmp DNA Stool protocols modified as previously described to ensure isolation of DNA from Gram-positive and Gram-negative bacteria (Thompson et al 2015). Initial amplification of the V1-V2 region of the bacterial 16S gene was performed on samples collected from both studies (Devine et al. 2013). The 16S rDNA amplicons were sequenced on a Roche GS FLX Titanium instrument (Microbiome Core Facility, Chapel Hill NC). Analysis of sequencing data was carried out using the QIIME pipeline (Caporaso et al. 2010). Raw sequencing data were filtered for quality control and denoised (Denoiser (Reeder and Knight 2010). Sequences were grouped into Operational Taxonomic Units (OTUs) at a 97% level using Uclust (Edgar 2010). Alpha diversity measures were calculated using QIIME to quantify microbiome community structure. We measured the observed species per sample and phylogenetic diversity (PD) to provide a measure of the diversity of taxa present. Taxa abundance was assessed at the phyla, order and family levels for taxa having >1% abundance in each group of samples.
Analytic Methods:
Descriptive statistics and bivariate tests (chi-square tests for categorical variables and t-tests for continuous variables) were used to assess whether maternal, child or household characteristics differed by mode of delivery. Logistic regression was used to test whether morbidity or elevated CRP differed by birth type, first in minimally adjusted models controlling for only for age, and then in more fully adjusted models, additionally controlling for pathogenic and nutritional exposures that were significantly associated with delivery mode. We tested for differences in the alpha diversity and taxa abundance of the gut microbiota by delivery mode using t-tests. For taxa showing evidence of difference in bivariate testing (p<0.20), we ran multivariate regression models to assess whether the abundance of taxa differed by delivery mode when controlling for our pathogenic and nutritional exposures. All models were run separately for infants and children to account for differences in study measures and timing of data collection. Models for children were also adjusted for siblings clustered in households. Stata version 14 was used for all analyses.
Results
The majority of infants (57.5%) and nearly half of children (47.4%) were born by Caesarean. Mothers of infants reported that 72% of these Caesarean deliveries were planned due to previous Caesareans, presentation of the baby, fetal size, maternal pelvic size or other complications. No infant and few maternal characteristics differed by delivery type, except for maternal ethnicity (Table 1). Indigenous mothers had a lower prevalence of Caesarean delivery than mestiza mothers in both samples. The duration of breastfeeding did not differ between vaginally and Caesarean delivered infants in either sample. The duration of breastfeeding in the child sample was at least 15 months for both Caesarean and vaginally delivered children. The majority of infants in the infant study (65%) were still breastfeeding at the time of the interview. Among those who had ceased breastfeeding, the mean duration was 5 months (SD 7 months) and did not differ by delivery mode. Over one-third of infants and children were overweight or obese, but the prevalence did not differ by delivery mode in either sample. Only one child in the sample was underweight. Vaginally delivered infants and children were shorter than those delivered by Caesarean, although this difference was only significant among children. Morbidity from common childhood illnesses was high for both infants and children. Illness symptoms were present in 85% of infants and 70% of children, with cold and flu symptoms being most common followed by diarrhea or GI infection. The prevalence of illness did not differ by delivery type in bivariate testing.
Table 1:
Sample Characteristics
| Infant Study | Child Study | |||
|---|---|---|---|---|
| Vaginal (N=17) | C-section (N=23) | Vaginal (N=71) | C-section (N=64) | |
| Maternal characteristics | ||||
| Maternal age, years | 28.3 (6.5) | 27.4 (6.4) | 30.1 (7.0) | 30.8 (7.5) |
| Maternal ethnicity1, %mestizo | 87.5 (14) | 91.3 (21)* | 67.6 (71) | 96.9 (62)* |
| % indigenous | 12.5 (2) | 4.4 (1)* | 30.0 (21) | 1.6 (1)* |
| Marital status, %unmarried | 50.0 (16) | 50.0 (16) | 64.8 (46) | 67.2 (43) |
| Child characteristics | ||||
| Sex, %male (n) | 47.4 (9) | 43.5 (10) | 46.5 (33) | 57.8 (37) |
| Age at survey2, mo or yr | 6.3 (4.8) | 8.8 (5.8) | 6.2 (2.5) | 5.7 (2.6) |
| Gestational age (wks) | 39.5 (2.7) | 38.5 (2.3) | -- | -- |
| Birthweight, gm | 3252 (457) | 3404 (539) | 3288 (511) | 3223 (480) |
| First born, %yes | 47.1 (8) | 30.4 (7) | -- | -- |
| Breastfeeding, mo | 5.7 (4.9) | 6.9 (6.4) | 15.2 (9.3) | 15.0 (11.9) |
| BMIz3, current | 0.79 (1.2) | 0.79 (1.1) | .89 (1.2) | .85 (1.2) |
| BMI category4 | ||||
| Underweight, % (n) | 0 (0) | 0 (0) | 1.4 (1) | 0 (0) |
| Overweight, % (n) | 13.3 (2) | 35.0 (7) | 25.4 (18) | 17.2 (11) |
| Obese, % (n) | 20.0 (3) | 10.0 (2) | 15.5 (11) | 18.8 (12) |
| Length/height-for- age, z-score3 | −0.62 (2.1) | −0.25 (1.39) | −.78 (1.1) | −.33 (.9)* |
| Any illness symptoms, % yes | 93.8 (15) | 77.3 (17) | 67.6 (71) | 71.9 (46) |
| Respiratory Symptoms5 | 54.6 (6) | 52.3 (12) | 49.3 (35) | 48.4 (31) |
| Diarrhea5 | 45.5 (5) | 16.7 (2) | 16.9 (12) | 20.3 (13) |
p<0.05 in chi square tests (categorical variables) or t-test (continuous variables)
Maternal self-report of race/ethnicity
Shown in months for infants and year for children
Calculated using WHO growth standards (de Onis)
Defined as: underweight BMIz<−2, overweight BMIz> 1 and ≤2, and obese BMIz>2
Represents portion of infants or children with any illness symptoms
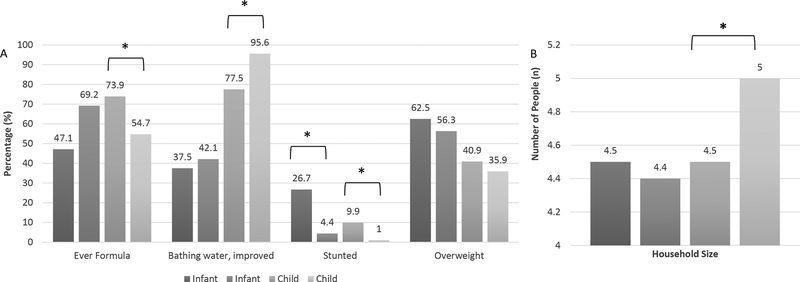
We next tested whether the postnatal environments of infants and children differed for those born vaginally vs. those born by Caesarean (Figure 1). We examined three markers of diet and nutritional status, formula feeding, stunting and overweight, and two markers of potential pathogen exposure, source of bath water and household size, measured in both studies. Fewer children born by Caesarean received formula than those born vaginally (p=0.02). This contrasts with the pattern seen in infants where more of those born by Caesarean had received formula (69% compared to 47%) though these differences were not statistically significant (p=0.18). Patterns of stunting were similar in both infants and children, with a higher prevalence of stunting seen in those born vaginally (p<0.05 for both infants and children). No differences were seen in overweight by delivery mode for either infants or children. Both pathogenic markers differed by delivery mode for children, albeit in the contrary directions. Children born by Caesarean were more commonly bathed in improved water sources, which could lower exposure to fecally contaminated household water, but they also tended to live in larger households, which could be associated with greater pathogen exposure.
Figure 1: Pathogenic and nutritional exposures by delivery mode in infants and children from Galápagos, Ecuador.
*p<0.05 in bivariate testing (chi square for categorical and t-test for continuous variables)
To examine the first pathway, whether immune activation differed by delivery mode, we tested whether morbidity from all symptoms, gastrointestinal symptoms, and respiratory symptoms differed for infants and children born vaginally vs. by Caesarean (Table 2). No significant differences were seen in any morbidity measures in infants. In children, Caesarean delivery was associated with a marginally increased odds of GI infection (p=0.08) in fully adjusted models controlling for postnatal nutritional and pathogenic exposures. Delivery mode wasn’t significantly associated with the odds of having elevated CRP in infants and children in either bivariate analysis or in the fully adjusted models.
Table 2:
Adjusted Odds of Infant and Child Immune Activation by Delivery Modea
| Infants | Children | |||||||
|---|---|---|---|---|---|---|---|---|
| Any symptomb | Diarrhea | Respiratoryc Symptoms | Elevated CRP (>2mg/L) | Any symptom | GI infectiond | Respiratory Symptoms | Elevated CRP (>2mg/L) | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Caesarean delivery | 0.11 (0.06, 2.29) | 0.38 (0.04, 3.85) | 0.48 (0.05, 4.52) | 0.25 (0.01, 4.06) | 1.44 (0.60, 3.47) | 2.33 (.90,6.02)* | 0.96 (0.39, 2.39) | 1.58 (0.63, 3.95) |
| Age | 1.10 (0.88, 1.01) | 0.93 (0.74, 1.15) | 1.05 (0.86, 1.29) | 0.99 (0.80, 1.21) | 0.91 (0.78, 1.05) | 1.01 (0.85, 1.19) | 0.85 (0.73, 0.98) | 0.98 (0.81, 1.18) |
| Any formula | 2.72 (.25, 29.0) | 1.65 (0.16, 16.57) | 2.65 (0.30, 23.29) | 0.73 (0.06, 9.81) | 1.57 (0.71, 3.44) | 2.55 (1.06, 6.11)* | 1.64 (0.75, 3.57) | 2.01 (0.79, 5.09) |
| Stuntede | -- | -- | -- | -- | 0.53 (0.12, 2.40) | 0.38 (0.05, 2.72) | 1.95 (0.41, 9.16) | 0.41 (0.05, 3.50) |
| Clean Bathwaterf | 0.51 (0.04, 6..44) | 0.59 (0.07, 4.96) | 0.87 (0.12, 6.26) | 0.36 (0.02, 6.35) | 0.57 (0.17, 1.88) | 0.54 (0.19, 1.53) | 3.24 (0.87, 12.11)* | 0.37 (0.13,1.04)* |
| Household size | 0.67 (0.30, 1.51) | 1.09 (0.72, 1.67) | 0.93 (0.61, 1.41) | 1.44 (0.68, 3.06) | 0.91 (0.73, 1.13) | 0.84 (0.63, 1.11) | 0.87 (0.71, 1.08) | 1.15 (0.91, 1.45) |
p<0.05
Results for logistic regression models for each morbidity outcome separately; models in children additionally account for clustering by household.
Includes maternal report of symptoms including fever, diarrhea, cough/cold, flu, vomiting and rash, over the past 2 weeks
Includes cough, cold and flu symptoms
Includes diarrhea, vomiting and fever
<−2 SD length-for-age for infants or height-for-age in children
Defined as bathing in boiled, filtered or bottled water (vs. tap water) for infants and indoor bathing (vs. outdoors) for children
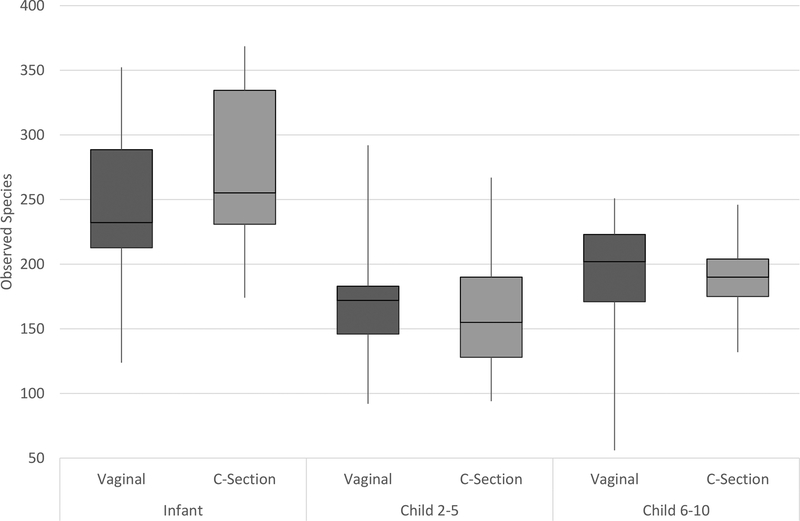
To examine the second pathway, whether the gut microbiome differs in infants and children born by Caesarean, we tested for differences in overall microbiota diversity and the abundance of specific taxa by delivery mode. We first examined two measures of alpha diversity, observed OTUs and phylogenetic diversity (shown for observed OTUs in Figure 2), in infants, children aged 2–5, and children aged 6–11. Similar patterns were seen for both measures. In general, diversity was higher in infants than in children, and we found contrasting results between the infants and children. In infants, vaginal delivery was associated with a lower number of observed species, and in children this relationship was reversed with Caesarean born children showing lower levels of diversity, though these differences did not reach statistical significance. No significant differences were seen in phylogenetic diversity for infants or children by delivery type.
Figure 2: Diversity in observed species by delivery mode in infants and children.
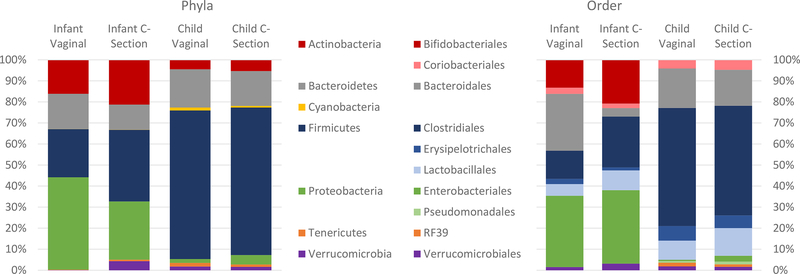
In contrast, we found a number of significant differences in taxa abundance by delivery mode in infants and children (Figure 3). At the phyla level, infants born vaginally had a lower abundance of Firmicutes and a higher abundance of Proteobacteria, though these results did not reach statistical significance (p=0.14 and p=0.13, respectively), and children born vaginally had a significantly lower abundance of Proteobacteria (p=0.01). In models controlling for nutritional and pathogenic exposures, Caesarean delivery was significantly associated with a higher abundance of Firmicutes in infants and a higher abundance of Proteobacteria in children (Table 3). Interestingly, stunting and formula were independently associated with phyla abundance in infants and children, respectively.
Figure 3: Relative abundance of phyla and order by delivery mode in infants and children.
Table 3.
Differences in Microbiota Taxa in Infants and Childrena
| Infants | Children | |||||
|---|---|---|---|---|---|---|
| Phyla | Order | Phyla | Order | |||
| Firmicutes (β) | Bacteroidales (β) | Proteobacteria (β) | Enterobacteriales (β) | Pseudomonadales (β) | Clostridiales (β) | |
| Caesarean delivery | 0.184* | −0.260* | 0.030* | 0.022* | 0.006 | −0.058 |
| Age | −0.014 | 0.013 | −0.003 | −0.003 | −0.001 | 0.023 |
| Any Formula | 0.043 | −0.016 | 0.022* | 0.012 | 0.009* | −0.073* |
| Stuntedb | 0.418* | −0.127 | −0.003 | −0.003 | 0.001 | 0.002 |
| Clean Bathwaterc | −0.013 | 0.013 | −0.007 | −0.002 | 0.008 | 0.071 |
| Household Size | 0.039 | −0.016 | −0.001 | 0.000 | −0.001 | −0.017* |
Indicates p-value < 0.05
Results for regression models for each taxa separately; models in children additionally account for clustering by household.
<−2 SD length-for-age for infants or height-for-age in children
Defined as bathing in boiled, filtered or bottled water (vs. tap water) for infants and indoor bathing (vs. outdoors) for children
At the order level, the relative abundance of Bacteroidales was significantly higher in infants born vaginally (p=0.002) and the proportion of Clostridiales was marginally higher in infants born vaginally (p=0.09) compared to those born by Caesarean (Figure 3). Children born vaginally also had higher relative abundance of Clostridiales, though this was not statistically different (p=0.15). Caesarean born children had a significantly higher abundance of Enterobacteriales (p=0.01), marginally higher Pseudomonadales (p=0.10) and Lactobacillales (p=0.12). In fully adjusted models, Bacteroidales remained significantly lower in Caesarean delivered infants while the differences in Clostridiales were attenuated (Table 3). Among children, Caesarean delivery was associated with a significantly higher abundance of Enterobacteriales (p=0.03) and marginally higher abundances of Pseudomonadales (p=0.07) and Lactobacillales (p=0.11) and marginally lower abundances of Clostridiales (p=0.06). Similar results were seen at the family level (data not shown). For infants, Bacteroidaceae were more abundant in infants born vaginally (p<0.002), and these differences remained significant in adjusted models. For children, vaginal births had higher abundance of Ruminococcaceae (p=0.12), and lower abundance of Streptococcaceae (p=0.15), Enterobacteriaceae (p=0.01) and Moraxellaceae (p=0.07). In adjusted models, Enterobacteriaceae were more abundant in children born by Caesarean (p=0.03) while Streptococcaceae and Moraxellaceae were marginally more abundant in children born by Caesarean.
Discussion
In this dual burden setting, rates of Caesarean are high. Over half of participating infants and over 40% of participating children were born by Caesarean delivery. Unlike previous research in the US and other industrialized countries, however, breastfeeding is nearly universal in our sample. Rates of morbidity from common childhood illness, such as cold, flu and GI infection, are also high and do not differ by delivery mode. While pathogenic and nutritional exposures --formula use, stunting, bath water source, and household size-- do vary by delivery mode across infancy and childhood, our results do not show a clear pattern of more or less pro-inflammatory environments for infants and children born vaginally or by Caesarean. Perhaps because of these multiple, contrasting exposures, we saw few differences in immune activation between infants and children by delivery mode, the first proposed pathway linking Caesarean delivery to later health outcomes. We did, however, find support for the second proposed pathway. Caesarean delivery was associated with differences in the abundance of microbial taxa from infancy through childhood in our sample. Importantly, we found differences in bacterial groups associated with immune development, healthy gut function and weight gain, and these differences mostly persisted when controlling for nutritional and pathogenic environmental exposures. These results suggest that, even in this dual burden context, birth practices may be associated with immune function and health in early life, highlighting the need to understand the pathways linking delivery type to long-term health in diverse contexts.
Contrary to previous results in neonates (Logan et al. 2016; Thysen et al. 2015) and our prior analysis of immune cell types and inflammation among a nationally-representative sample of nearly 900 Ecuadorian infants, including 45 infants from Galápagos, participating in the Encuesta Nacional de Salud y Nutricion 2012 study (Freire et al. 2015; Thompson 2017), neither morbidity nor CRP differed across infancy or childhood by delivery type in the current study. Previous work has shown that CRP is lower in neonates born by elective Caesarean compared to those born by emergency Caesarean or vaginally and that the duration of labor is a particularly important predictor of CRP levels in cord blood (Logan et al. 2016). In these German neonates, CRP did not seem to be associated with underlying maternal infection since infants with levels indicative of infection were excluded as were those who were transferred to the neonatal intensive care unit. Thus, the authors suggest that the process of labor may be important for initiating an acute phase response in neonates. In our previous secondary data analysis of the association between delivery mode, CRP and morbidity in Ecuadorian infants, we found that the association between delivery and inflammation persisted into the second year of life (Thompson 2017). In that analysis, Caesarean delivery was associated with higher morbidity from respiratory and diarrheal symptoms but a lower likelihood of inflammation than infants born vaginally, suggesting that Caesarean delivery may alter the immune response to infection. Few studies compare morbidity from common childhood symptoms by delivery mode, but Caesarean delivery has been linked to more severe complications such as respiratory distress and NEC in early life (Collado et al. 2012). While the lack of association between delivery mode and inflammation in the current study may be due to the older age of the children studied, Caesarean delivery has been linked to numerous inflammatory conditions in childhood and adolescence, including asthma (Leung et al 2015) and inflammatory bowel disease (Bager et al. 2012). Alternatively, the lack of differences in infant and child immune function by delivery mode in the current study may be due to the varying pro- and anti-inflammatory environmental factors seen in the dual burden environment of the Galápagos. Infants and children were exposed to both pro-inflammatory risk factors, including unclean water, larger household size and obesogenic diets, and anti-inflammatory factors, such as breastfeeding. While the patterning of some of these exposures, such as exposure to clean bath water or household size, differed between Caesarean and vaginally delivered children, others, such as breastfeeding duration and obesity did not. Thus, in this context, infant and childhood exposures may be more important in shaping the development of the immune response.
Yet, delivery mode remained an important predictor of some aspects of the gut microbiota in infants and children in this sample. Similar to some (Lee et al. 2016), but not other studies (Jakobsson et al 2013; Azad et al. 2013), measures of alpha diversity did not differ significantly between vaginally or Caesarean-born infants or children in our study. The overall observed diversity was high for infants compared to other samples (e.g., Avershina et al. 2014; Thompson et al. 2015), likely due the age span (1 month to 2 years) included in our pilot sample and/or to more diverse postnatal dietary and environmental exposures experienced by the infants in our study compared to American and Western European samples, a pattern seen in comparative studies (Yatsunenko et al. 2012). Diversity tends to increase over the first years of life as infants are exposed to new solid foods (Backhed et al 2016; Thompson et al 2015) and new environmental conditions, such as siblings, household pets, daycare and illnesses, and tends to stabilize through middle childhood (Collado et al. 2012), a developmental trajectory that may explain the higher diversity we found in infants than in children.
Unlike our diversity results, we do find significant differences in the relative abundance of specific taxa based on delivery mode for both infants and children. At the phyla level, we found a higher relative abundance of Firmicutes in infants born by Caesarean and a higher abundance of Proteobacteria in children born by Caesarean. At the order level, we found lower relative abundances of Bacteroidales in infants born by Caesarean and higher abundances of Enterobacteriales and Pseudomonadales, and lower abundances of Clostridiales in children born by Caesarean. The higher abundance of Proteobacteria in Caesarean-delivered children in our study is similar to findings from a study of 6-week old American infants, where Caesarean delivery was associated with a higher abundance of Proteobacteria, particularly Escherichia spp and other Enterobacteria, and concomitant enrichment in genes coding for putative membrane transport proteins (Brumbaugh et al., 2016). These metabolic pathways are involved in the transport of metals essential for microbial growth, suggesting that a higher Proteobacteria abundance may be associated with increased risk of infectious morbidity. Further, infant and childhood colonization of Enterobacteriales, in particular, seem to play an important role in the immune dysregulation associated with atopic disorders and inflammation. In a study of Swedish and Estonian children, 2-year-olds with allergies had increased neonatal colonization of Enterobacteriaceae (Bjorksten et al. 1999). The abundance of Proteobacteria serves as a key marker of gut dysbiosis associated with chronic intestinal inflammation (Litvak et al. 2017; Shin et al. 2015). Thus, these high levels seen with Caesarean delivery may have long term health impacts.
Our study also revealed a negative association between Caesarean birth and Clostridiales abundance, similarly to a study from Finland which found that seven-year-old children born vaginally had higher levels of Clostridia (Salminen et al. 2004). Among Western European infants, Caesarean delivery has been associated with higher levels of Clostridium and lower early colonization by E. coli, Bacteroides and Bifidobacterium (Adlerberth et al. 2007). Research from the KOALA Birth Cohort Study in the Netherlands has demonstrated a similar pattern of higher levels of Clostridium and lower levels of Bifidobacteria and Bacteroides among Caesarean-born infants while adjusting for breastfeeding practices and the presence of siblings (Penders et al. 2006). Since Clostridium difficile and E. coli have been associated with a higher risk of atopic disease (Penders et al. 2007), more research is needed to investigate the developmental windows (infancy vs. childhood, for example) during which Clostridia colonization may shape immune function and what other factors, such as feeding practices (Alderbath et al. 1991), may interact to influence this development. Such research is particularly important for Ecuador and other South American countries, where rates of allergy and asthma are already comparable to those reported in Western Europe and are increasing at a steeper rate (Pitrez et al. 2008). In the Galápagos, 15% of children participating in the Child Gut Health Study had been diagnosed with allergies or asthma (Houck 2017) further supporting the need for more research in this and other LMIC settings.
Importantly, our results support past research finding delayed colonization and potential dysbiosis in Bacteroides species amongst infants and children born by Caesarean (Salminen et al. 2004). The higher abundance of Firmicutes and reduced abundance of Bacteroidales seen in the infants in our study suggest that the Caesarean-delivered infants have reduced Bacteriodetes colonization, a risk factor for both immune development and overweight (Magne et al. 2017). Bacteroidetes play an important role in gut health, regulating the gut immune system, maintaining gut barrier function and stimulating an inflammatory response (He et al. 2007; Magne et al. 2017). A delayed or disturbed colonization with Bacteroidetes may also contribute to the development of obesity since, in children and adults, a higher ratio of Firmicutes to Bacteroidetes accompanies obesity (Ley et al. 2005). Given the high rate of overweight and obesity in children and adults on the Galápagos, 13% and 76%, respectively, (Freire et al 2015), preventing such dysbiosis and promoting colonization by Bacteroidetes may be particularly important in this setting.
Among infants and children in our sample, several postnatal exposures were independently associated with taxa abundance, highlighting the complex interactions between delivery mode, feeding practices and early life environmental exposures in the establishment of the gut microbiota. In our study, stunting was associated with significantly lower abundance of Firmicutes in infants, while formula use during infancy was associated with significantly greater Pseudomonadales, and formula use and larger households with lower Clostridiales in children. Each of these factors has previously been linked to microbiota diversity in infants and children (Adlerberth et al. 2007; Dinh et al. 2016; Thompson et al. 2015). A study of infants from three Western European countries, for example, found that being the first born (or having no older siblings) was associated with high Clostridium colonization (Adlerberth et al. 2007). The KOALA Birth Cohort study found that having older siblings was associated with higher levels of Bifidobacteria; yet there were no significant effects of exposure to farm animals or pets on bacteria species (Penders et al. 2006). In the present study, controlling for pathogenic and nutritional exposures did not attenuate the differences we saw in microbiota by delivery mode. Thus, while the early feeding and the postnatal pathogenic/microbial environments may modulate microbial colonization and its impacts on immune function and dysfunction, birth practices may nonetheless have persistent effects on the gut microbiota throughout infancy and childhood.
Our study is one of the few examining the association between Caesarean delivery and immune function and the gut microbiota in a LMIC context. However, our results are limited by several important factors and should be considered preliminary. While our sample size of children is relatively large (n=135), our sample size of infants is considerably smaller (n=41). This small sample size may have limited our power to find statistically significant differences in pathogenic and nutritional environmental characteristics and the relative abundance of microbial taxa. Nevertheless, the infants included in the study represent nearly ¼ of births on the islands over two years, since the annual number of births is around 80 (Dr. Byron Tobar, personal communication), and likely represents the range of exposures infants face. We also were not able to include information on several factors that may influence microbiota colonization and immune development, including whether Caesarean deliveries were emergency or planned and the use of antibiotics during delivery and/or to treat illness postnatally. While we were not able to test these impacts directly, our data suggests that these variables may have a limited impact on our results. The majority of Caesarean deliveries in infants were planned (72%) and occurred prior to the onset of contractions (65%). Similarly, antibiotic use was relatively rare in infants and children. The local hospital provides prophylactic antibiotics (tetracycline) to mothers prior to Caesarean delivery, but antibiotics are only given to infants who show evidence of infection or sepsis (Dr. Juan Ochoa, personnel communication). Postnatally, only 3% of the children studied received antibiotics in the week prior to fecal sample collection. Our measure of infant feeding, whether infants and children “ever received formula,” is a crude measure that does not capture differences in the magnitude of formula feeding. While the amount of formula infants receive may be important for both their immune development and gut microbiota composition, our previous work in American infants found that the gut microbiota of exclusively breastfed infants differed significantly from infants who had received breastmilk and any amount of formula (Thompson et al. 2015). Thus, even with this crude measure we are likely identifying important differences in exposure. Perhaps even more importantly, the infants and children included in this analysis come from two different studies and include individuals measured at two different times and selected for different aims. Thus, we had a limited number of comparable variables measured across studies to describe pathogenic and nutritional exposures. Our results nevertheless suggest that additional research with more comprehensive measures of postnatal environments is warranted.
Despite these weaknesses, our study contributes an important case study to the literature on the health impacts of Caesarean delivery. We are able to characterize morbidity and gut microbiota across a wide age range, one month to 11 years of age, in a unique context where high rates of Caesarean deliveries coexist alongside prevalent breastfeeding, persistent pathogenic exposures, and increasingly obesogenic diets and lifestyles. In this context, delivery mode was not associated with measures of immune activation, but did remain a significant predictor of gut microbiota colonization across infancy and childhood. These findings suggest that delivery mode has persistent impacts on the gut microbiota that can be seen across infancy and childhood even in a dual burden context. Further work is needed to understand whether exposure to these postnatal environmental factors may have independent and, perhaps, mitigating impacts on the development of the immune system and its function. Nonetheless, our results highlight the importance of examining Caesarean delivery across diverse contexts to better understand the impact of perinatal interventions on short- and longer- term growth, metabolism, and health.
Acknowledgments
We would like to thank the participating families and Dr. Jaime Ocampo, Dr. Peggy Bentley, Berenice Norris, Noemi Arias, Stefani Esteves and Gyssell Zapata. We are particularly thankful for the support and assistance of Dr. Tobar and the staff at the Hospital Oskar Jandl. This research received support from the National Science Foundation (BCS-1341156), the Wenner-Gren Foundation, the Triangle Center for Evolutionary Medicine, and, at the University of North Carolina at Chapel Hill: University Research Council, Center for Galápagos Studies, Institute for Global Health and Infectious Disease, Royster Society of Fellows, and the Population Research infrastructure Program awarded to the Carolina Population Center (P2C HD050924) at the University of North Carolina at Chapel Hill by the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adlerberth IMDP, Strachan DPMD, Matricardi PMMD, Ahrné SP, Orfei LM, Åberg NMDP, Perkin MRMD, Tripodi SMD, Hesselmar BMDP, Saalman RMDP et al. . 2007. Gut microbiota and development of atopic eczema in 3 European birth cohorts. Journal of Allergy and Clinical Immunology 120(2):343–350. [DOI] [PubMed] [Google Scholar]
- Almanzar G, Schönlaub J, Hammerer-Lercher A, Koppelstaetter C, Bernhard D, & Prelog M (2015). Influence of the delivery modus on subpopulations and replication of lymphocytes in mothers and newborns. Early human development, 91(12), 663–670. [DOI] [PubMed] [Google Scholar]
- Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, & Finlay B (2014). The intestinal microbiome in early life: health and disease. Front Immunol, 5, 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avershina E, Storro O, Oien T, Johnsen R, Pope P, & Rudi K (2014). Major faecal microbiota shifts in composition and diversity with age in a geographically restricted cohort of mothers and their children. FEMS Microbiol Ecol, 87(1), 280–290. [DOI] [PubMed] [Google Scholar]
- Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, … Kozyrskyj AL (2013). Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. Canadian Medical Association Journal, 185(5), 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, … Wang J (2015). Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe, 17(6), 852. [DOI] [PubMed] [Google Scholar]
- Bager P, Simonsen J, Nielsen NM, Frisch M. (2012) Cesarean section and offspring’s risk of inflammatory bowel disease: a national cohort study. Inflamm Bowel Dis. 18: 857–862. [DOI] [PubMed] [Google Scholar]
- Betrán AP, Ye J, Moller A-B, Zhang J, Gülmezoglu AM, & Torloni MR (2016). The increasing trend in Caesarean section rates: global, regional and national estimates: 1990–2014. PLOS ONE, 11(2), e0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, & Retetangos C (2010). Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev, 86 Suppl 1, 13–15. [DOI] [PubMed] [Google Scholar]
- Brumbaugh DE, Arruda J, Robbins K, Ir D, Santorico SA, Robertson CE, & Frank DN (2016). Mode of delivery determines neonatal pharyngeal bacterial composition and early intestinal colonization. Journal of pediatric gastroenterology and nutrition, 63(3), 320–328. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, and Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5): 335–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardwell CR, Stene LC, Joner G, Cinek O, Svensson J, Goldacre MJ, … & Urbonaitė B (2008). Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia; 51: 726–35. [DOI] [PubMed] [Google Scholar]
- Carrillo-Larco RM, Miranda JJ, & Bernabé-Ortiz A (2015). Delivery by caesarean section and risk of childhood obesity: analysis of a Peruvian prospective cohort. PeerJ, 3, e1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CGREG, and INEC. 2010. Encuesta de condiciones de vida Galápagos 2009–2010. edited by Consejo de Gobierno del Regimen Especial de Galápagos and Instituto Nacional de Estadistica y Censos. Quito, Ecuador. [Google Scholar]
- Cho CE, and Norman M. (2013) Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol. 208:249–54. [DOI] [PubMed] [Google Scholar]
- Collado MC, Cernada M, Baüerl C, Vento M, & Pérez-Martínez G (2012). Microbial ecology and host-microbiota interactions during early life stages. Gut microbes, 3(4), 352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Onis M (2006). WHO Child Growth Standards based on length/height, weight and age. Acta paediatrica, 95(S450), 76–85. [DOI] [PubMed] [Google Scholar]
- Devine AA, Gonzalez A, Speck KE, Knight R, Helmrath MA, Lund PK, and Azcarate Peril MA. (2013) Impact of ileocecal resection and concomitant antibiotics on the microbiome of the murine jejunum and colon. PLoS One 8(8): e73140. doi: 10.1371/journal.pone.0073140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, & Knight R (2010). Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA, 107: 11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh DM, Ramadass B, Kattula D, Sarkar R, Braunstein P, Tai A, et al. (2016) Longitudinal Analysis of the Intestinal Microbiota in Persistently Stunted Young Children in South India. PLoS ONE 11(5): e0155405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Zajacova A, & Aiello AE (2010). Predictors of inflammation in US children aged 3–16 years. American journal of preventive medicine, 39(4), 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire WB, Belmont P, Rivas-Mariño G, Larrea A, Ramírez-Luzuriaga MJ, Silva-Jaramillo KM, Valdivia C (2015). Tomo II Encuesta Nacional de Salud y Nutrición. Salud Sexual y Reproductiva. ENSANUT-ECU 2012. Ministerio de Salud Pública/Instituto Nacional de Estadística y Censos. Quito-Ecuador. [Google Scholar]
- Edgar RC. (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26(19): 2460–1. [DOI] [PubMed] [Google Scholar]
- Gerhard WA, Choi WS, Houck KM, & Stewart JR (2017). Water quality at points-of-use in the Galápagos Islands. International Journal of Hygiene and Environmental Health, 220, 485–93. [DOI] [PubMed] [Google Scholar]
- Granda Leon, Marianita Sandra, Gonzalez Cambra, and Vilma Calvopina Carvajal. 2013. Measuring poverty in Galápagos. In Galápagos Report 2011–2012. Puerto Ayora, Galápagos: Galápagos National Park Service, Governing Council of Galápagos, Charles Darwin Foundation, Galápagos Conservancy. [Google Scholar]
- Gollwitzer ES, & Marsland BJ (2015). Impact of early-life exposures on immune maturation and susceptibility to disease. Trends in immunology, 36(11), 684–696. [DOI] [PubMed] [Google Scholar]
- He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, et al. (2007) Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 26:812–26. [DOI] [PubMed] [Google Scholar]
- Herstad L, Klungsøyr K, Skæjrven R et al. (2016) Elective cesarean section or not? Maternal age and risk of adverse outcomes at term: a population-based registry study of low-risk primiparous women. BMC Pregnancy Childbirth; 16:230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck KM . 2017. Early life effects of a dual burden environment: Childhood intestinal health and immune function in Galápagos, Ecuador. Ph.D., Anthropology, University of North Carolina; (10681674). [Google Scholar]
- Huda MN, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, … Stephensen CB (2014). Stool microbiota and vaccine responses of infants. Pediatrics, 134(2), e362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. (2013) Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced responses in infants delivered by caesarean section. Gut. 63:559–66. [DOI] [PubMed] [Google Scholar]
- Kaäaäpaä P and Koistinen E (1993) Maternal and neonatal C-reactive protein after interventions during delivery, Acta Obstetricia et Gynecologica Scandinavica, 72: 543–546 [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett K (2007). A new paradigm for depression in new mothers: the central role of inflammation and how breastfeeding and anti-inflammatory treatments protect maternal mental health. International Breastfeeding Journal, 2(1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen K, and Henriksen L (2016). Cesarean section and disease associated with immune function. Journal of Allergy and Clinical Immunology, 137(2), 587–590. [DOI] [PubMed] [Google Scholar]
- Lee E, Kim B-J, Kang M-J, Choi KY, Cho H-J, Kim Y, et al. (2016) Dynamics of gut microbiota according to the delivery mode in healthy Korean infants. Allergy Asthma Immunol Res 8:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JYY, Li AM, Leung GM, Schooling CM. (2015) Mode of delivery and childhood hospitalizations for asthma and other wheezing disorders. Clin Exp Allergy. 45: 1109–1117. [DOI] [PubMed] [Google Scholar]
- Ley RE, Backhed F, Turnbaugh P, et al. (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci. 102:11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HT, Zhou YB, Liu JM. (2013) The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int J Obes. 37:893–9. [DOI] [PubMed] [Google Scholar]
- Litvak Y, Byndloss MX, Tsolis RM, and Bäumler AJ. 2017. Dysbiotic Proteobacteria expansion: a microbial signature of epithelial dysfunction. Current Opinion in Microbiology 39:1–6. [DOI] [PubMed] [Google Scholar]
- Logan CA, Thiel L, Bornemann R, Koenig W, Reister F, Brenner H, et al. (2016) Delivery Mode, Duration of Labor, and Cord Blood Adiponectin, Leptin, and C-Reactive Protein: Results of the Population-Based Ulm Birth Cohort Studies. PLoS ONE 11(2): e0149918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magne F, Puchi Silva A, Carvajal B and Gotteland M (2017) The Elevated Rate of Cesarean Section and Its Contribution to Non-Communicable Chronic Diseases in Latin America: The Growing Involvement of the Microbiota. Front. Pediatr 5:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey K, Ponsonby AL, Collier F, Allen K, Tang MLK, Carlin JB, Saffery R, Skilton MR, Cheung M, Ranganathan S and Dwyer T (2018) The association between higher maternal pre-pregnancy body mass index and increased birth weight, adiposity and inflammation in the newborn. Pediatric obesity, 13(1): 46–53. [DOI] [PubMed] [Google Scholar]
- McDade TW, Reyes García V, Tanner S, Huanca T, and Leonard WR. (2008). Maintenance versus growth: investigating the costs of immune activation among children in lowland Bolivia. American Journal of Physical Anthropology 136 (4):478–484. [DOI] [PubMed] [Google Scholar]
- McDade TW, Metzger MW, Chyu L, Duncan GJ, Garfield C, & Adam EK (2014). Long-term effects of birth weight and breastfeeding duration on inflammation in early adulthood. Proc. R. Soc. B, 281(1784), 20133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WKY, Erlien C, & Bilsborrow RE (2010). Morbidity and mortality disparities among colonist and indigenous populations in the Ecuadorian Amazon. Social science & medicine, 70(3), 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page R, Bentley ME, and Waldrop J. 2013. “People Live Here: Maternal and Child Health on Isla Isabela, Galápagos.” In Science and Conservation in the Galápagos Islands, edited by Walsh SJ and Mena CF, 141–153. Springer. [Google Scholar]
- Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, and Stobberingh EE. 2007. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut 56(5):661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, and Stobberingh EE. 2006. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics 118(2):511–521. [DOI] [PubMed] [Google Scholar]
- Pitrez PM, Stein RT. Asthma in Latin America: the dawn of a new epidemic. Curr Opin Allergy Clin Immunol (2008) 8:378–83. [DOI] [PubMed] [Google Scholar]
- Prior E, Santhakumaran S, Gale C, Philipps LH, Modi N, Hyde MJ. 2012. Breastfeeding after cesarean delivery: a systematic review and meta- analysis of world literature. Am J Clin Nutr 95:1113–35. [DOI] [PubMed] [Google Scholar]
- Reeder J and Knight R (2010) Rapidly denoising pyrosequencing amplicon reads by exploiting rank-abundance distributions. Nature methods 7(9): 668–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, & Korzeniewski SJ (2013). Are infants born by elective cesarean delivery without labor at risk for developing immune disorders later in life?. American Journal of Obstetrics & Gynecology, 208(4), 243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg KR, & Trevathan WR (2018). Evolutionary perspectives on cesarean section. Evolution, Medicine, and Public Health, 2018(1), 67–81. [Google Scholar]
- Salminen S, Gibson G, McCartney A, & Isolauri E (2004). Influence of mode of delivery on gut microbiota composition in seven year old children. Gut, 53(9), 1388–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinzig T, Johansson S, Gunnar A, Ekstrom TJ, Norman M. (2009) Epigenetic modulation at birth: altered DNA-methylation in white blood cells after caesarean section. Acta Paediatr. 98:1096–9.] [DOI] [PubMed] [Google Scholar]
- Sevelsted A, Stokholm J, Bønnelykke K, & Bisgaard H (2014). Cesarean section and chronic immune disorders. Pediatrics, 135: e92. [DOI] [PubMed] [Google Scholar]
- Shin N-R, Whon TW, and Bae J-W. 2015. Proteobacteria : microbial signature of dysbiosis in gut microbiota. Trends in Biotechnology 33(9):496–503. [DOI] [PubMed] [Google Scholar]
- Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. (2008) A meta-analysis of the association between cesarean section and childhood asthma. Clin Exp Allergy. 38: 629–33. [DOI] [PubMed] [Google Scholar]
- Thompson AL, Monteagudo-Mera A, Cadenas MB, Lampl ML, & Azcarate-Peril MA (2015). Milk- and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front Cell Infect Microbiol, 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AL, Jahnke HR, and Houck KM. 2017. Birth practices and infant immune development in Ecuador. American Journal of Human Biology 29(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thysen AH, Larsen JM, Rasmussen MA, Stokholm J, Bønnelykke K, Bisgaard H, & Brix S (2015). Prelabor cesarean section bypasses natural immune cell maturation. Journal of Allergy and Clinical Immunology, 136: 1123–1125. [DOI] [PubMed] [Google Scholar]
- Veile A, & Kramer KL (2017). Childhood body mass is positively associated with cesarean birth in Yucatec Maya subsistence farmers. American Journal of Human Biology, 29(2), e22920. [DOI] [PubMed] [Google Scholar]
- Virani S, Dolinoy DC, Halubai S, et al. (2012) Delivery type not associated with global methylation at birth. Clin Epigenetics. 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SJ, McCleary AL, Heumann BW, Brewington L, & Raczkowski EJ (2010). Community expansion and infrastructure development: Implications for human health and environmental quality in the Galápagos Islands of Ecuador. Journal of Latin American Geography, 9(3), 137–159 [Google Scholar]
- Wander K, Shell-Duncan B, and McDade TW. (2009). Evaluation of Iron Deficiency As a Nutritional Adaptation to Infectious Disease: An Evolutionary Medicine Perspective. American Journal of Human Biology 21 (2):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger B, Vetrano AM, Syed K, Murthy S, Hanna N, Laskin JD, & Laskin DL (2007). Influence of labor on neonatal neutrophil apoptosis, and inflammatory activity. Pediatric research, 61: 572. [DOI] [PubMed] [Google Scholar]
- WHO. 1995. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. In World Health Organization Technical Report Series. Geneva. [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, … & Heath AC (2012). Human gut microbiome viewed across age and geography. nature, 486(7402), 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Gaskins AJ, Blaine AI, Zhang C, Gillman MW, Missmer SA, Field AE & Chavarro JE (2016). Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA pediatrics, 170(11), e162385–e162385. [DOI] [PMC free article] [PubMed] [Google Scholar]