Abstract
Purpose of Review:
Research over the past decade has shown that immunologic and metabolic pathways are intricately linked. This burgeoning field of immunometabolism, includes intrinsic and extrinsic pathways, and is known to be associated with obesity-accelerated metabolic disease. Intrinsic immunometabolism includes the study of fuel utilization and bioenergetic pathways that influence immune cell function. Extrinsic immunometabolism includes the study of immune cells and products that influence systemic metabolism.
Recent Findings:
Th2 immunity, macrophage iron handling, adaptive immune memory, and epigenetic regulation of immunity, which are all require intrinsic metabolic changes, play a role in systemic metabolism and metabolic function, linking the two arms of immunometabolism. Together, this suggests that targeting intrinsic immunometabolism can directly affect immune function and ultimately systemic metabolism.
Summary:
We highlight important questions for future basic research that will help improve translational research provide therapeutic targets to help establish new treatments for obesity and associated metabolic disorders.
Keywords: Immunometabolism, obesity, adipose tissue, immunology, Th2 immunity, adaptive immunity, iron handling, epigenetics, therapeutic development
Introduction
Obesity affects approximately 38% of adults in the United States (US)[1] and increases the risk for nearly every chronic disease, including heart disease, stroke, diabetes, some forms of cancer, and dementia [2]. Roughly $190 billion, or approximately 21% of total healthcare costs, are spent on obesity-related diseases in the US annually [2], and the American Medical Association formally recognized obesity as a disease in 2013. However, there are still very few effective treatments for obesity, as less than 20% of individuals who lose weight are able to maintain ≥10% weight loss for more than a year [3]. Bariatric surgery has a much higher success rate than lifestyle interventions [4], but it is not a feasible solution for every individual. Therefore, additional research is necessary to improve patient health and obesity-related outcomes.
Obesity is a multifaceted disease, associated with environmental, genetic, endocrine, neural, and microbiota-associated risk factors, [reviewed in [5]], as well as functional changes within the brain, adipose tissue (AT), liver, pancreas, skeletal muscle, and intestine. There are many drugs in development to target neurotransmitters, which are involved in appetite regulation and satiety, and GLP-1 and cAMP, which are involved in blood glucose control and insulin action [6]. Additionally, research over the past 15 years suggests that the immune system contributes to the pathogenesis of obesity, and therefore is an emerging target to alleviate health consequences of obesity.
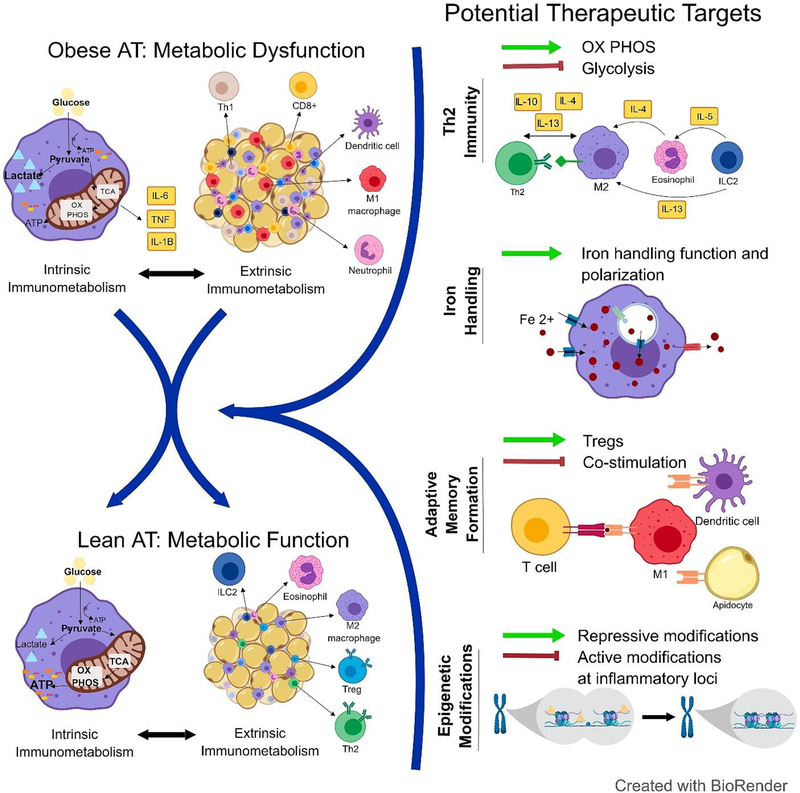
Immune cell presence and function within metabolic tissues is closely linked to systemic glucose and lipid homeostasis, a field of research we refer to as “extrinsic immunometabolism” in this article. Macrophage accumulation in AT of obese mice was first shown in seminal papers published in 2003 [7,8], and by 2013, our field had substantial evidence that obesity effects immune cell populations not only in AT, but also the liver, bone marrow and hypothalamus [as reviewed in [9]]. These immune populations contribute to tissue homeostasis during health; however, shifts in number and phenotype can promote an inflammatory milieu in obesity and can contribute to insulin resistance and glucose intolerance, which together are referred to metabolic dysfunction. Simultaneously, immunologists began to uncover the mechanisms by which changes in intracellular bioenergetic pathways can alter immune cell function [10], a field of research that we refer to as “intrinsic immunometabolism” that has also been specifically linked to obesity-associated inflammation [11**]. Emerging evidence suggests that these to concepts in immunometabolism are interconnected (Figure 1). In this review, we will highlight extrinsic and intrinsic studies of immunometabolism in obesity. Further, we propose future areas of research that can harness both sides of immunometabolism to help identify therapeutic targets for translational use in obesity and other associated metabolic disorders.
Figure 1.
Intrinsic and extrinsic immunometabolic pathways are intricately linked in AT homeostasis and metabolic dysfunction associated with obesity. We propose four distinct areas of research that may provide potential therapeutic targets for the treatment of obesity and associated metabolic disease: Th2 immunity, macrophage iron handling, adaptive memory formation, and epigenetic regulation. Importantly, these targets will likely influence both intrinsic and extrinsic immunometabolism, strengthening the potential likelihood for success as therapeutic targets.
Extrinsic Immunometabolism
There has been a substantial body of work published in the area of extrinsic immunometabolism. While this review will focus on AT, it should be noted that immune cells are also present in other metabolic organs such as muscle, liver, brain and pancreas, where they likely play an important role in systemic metabolic homeostasis. Additionally, we want to emphasize that resident immune cells in the AT can contribute to both AT homeostasis and metabolic disease, which have been nicely summarized in many reviews [12–14]. In healthy AT, there is an abundance of cells with regulatory functions associated with Th2 immunity, including M2 macrophages, invariant natural killer T cell (iNKT)s, innate lymphoid cell (ILC2)s, T regulatory (Treg) cells, T helper (Th)2s cells, and eosinophils. These cell types are traditionally associated with protection against parasites, but are elevated in lean AT and are considered protective against metabolic dysfunction [14]. Following weight gain with high fat diet (HFD)-feeding and in genetically obese models, the Th2-associated cells are reduced and there is an influx of Th1-associated cells. M1 macrophages, Th1 cells, CD8+ T cells, dendritic cells (DCs), and neutrophils infiltrate and expand, contributing to metabolic dysfunction. Interestingly, myeloid derived suppressor cells (MDSCs), which are typically associated with anti-inflammatory effects, increase with weight gain to limit metabolic dysfunction associated with obesity; however, this feedback is not enough to prevent worsened glucose tolerance and insulin resistance (IR) [15]. AT macrophages (ATM)s have received the most attention in the literature [16] and have recently been characterized to have a “metabolically activated” (MMe) phenotype in obesity [17], which is essentially inflammatory. However, both pro- and anti-inflammatory signaling pathways are activated in these cells, which may explain the chronic non-resolving inflammation in obesity. Additionally, recent work has begun to classify the expression pattern of additional populations, such AT DCs, which also accumulate with obesity and contribute to IR [18].
Much of the data above has been discovered by modulating immune populations in knockout mice or transgenic mice. Drug therapies which target chemokines, cytokines, and their receptors have also been used to target specific immune populations of interest. One of the most commonly studied pathways is chemokine receptor 2 (CCR2). Disrupting monocyte chemoattractant protein 1 (MCP-1, also called CCL2)-mediated macrophage infiltration into the AT is associated with improved IR after HFD-feeding [19–22]. However, ATM numbers in CCR2-deficient HFD-fed mice do not return to lean values and less promising results have been shown with other chemokine receptor knockouts [23,24], suggesting that multiple pathways may need to be simultaneously targeted for optimal responses. Additionally, a study on the administration of IL-33 recently elucidated protective effects on metabolic function in obesity [25], however administration also drives allergy and asthma. Importantly, recent adoptive transfer experiments of helminth-induced M2 macrophages[26] and B1-B cells [27] and older experiments with Treg cells [28] suggest that immunotherapy can improve metabolic function in obese mice and should be considered as a potential future therapeutic strategy. This suggests that promoting protective Th2-immunity while limiting detrimental Th1-associated inflammation may improve AT function and more generally, systemic metabolism.
The role of immune cells in AT homeostasis and metabolic dysfunction following weight gain is well understood, however the best therapeutic target to reverse HFD-induced changes in inflammatory populations is not known. Moreover, immune cell populations change in response to weight loss, and may provide further insight into metabolic function with weight gain. Following HFD-induced obesity, weight loss initially accompanies a lipolysis-mediated increase in ATMs and then a decrease with reduced AT mass and a normalization in glucose tolerance [29,30]. However CD11c+ ATMs were still elevated up to 6 months post weight loss and insulin sensitivity was not completely normalized [31]. More work is needed to address the mechanism by which weight loss changes immune cells abundance and how these changes contribute to metabolic function. Potential strategies to modulate immune cell metabolism and relatedly, immune function, are discussed below.
Intrinsic Immunometabolism
It is well known that macronutrient catabolism is required for energy production; however, the importance of intrinsic immunometabolism for immune function and systemic metabolism has only recently been investigated (reviewed in [32]). While immune cell metabolites continuously flux through both glycolysis and oxidative phosphorylation (OX PHOS), the relative contribution of each pathway to ATP versus metabolite production influences immune cell differentiation, polarization, and effector function [33,34]. Fatty acid oxidation (FAO) and OX PHOS are primarily used by regulatory and anti-inflammatory cells, including Treg cells, M2 macrophages, and MDSC, while glycolysis is primarily used by inflammatory effector cells, such as Th1 and Th17 cells, M1-like macrophages and DCs [35–38]. OX PHOS is more efficient than glycolysis, producing 32 versus 2 ATPs per glucose molecule; however, glycolysis is often utilized because it can increase ATP availability rapidly, operate under low oxygen tension, and provide pentose phosphate pathway and tricarboxylic acid (TCA) cycle intermediates for the synthesis of nucleotides, amino acids, and lipids [33,35]. Additionally, the metabolites generated can contribute to oxidation-reduction reactions, pro- and anti-oxidant balance, and protein modifications such as acetylation, methylation, prenylation, and palmitoylation. Metabolic enzymes, nutrient sensors, and nutrient transporters are amenable to pharmacological intervention and offer promising opportunities for selective regulation of the immune response. Moreover, because intrinsic metabolism is tightly linked to immune function, targeting intrinsic immunometabolism may offer potential opportunities to improve systemic metabolism.
Healthy, lean AT has an abundance of Treg cells, which primarily utilize FAO and OX PHOS. Lean AT also has an abundance of Th2 cells, M2 macrophages, ILC2s, and eosinophils which utilize glycolysis at a rate similar to Th1 cells and neutrophils; however, they too have elevated glucose oxidation, FAO, and OX PHOS [39*, 40–41]. On the contrary, Th1 cells, M1-like macrophages, and effector CD8+ T cells, which are associated with obesity, primarily utilize glycolysis [42–44]. This suggests that increasing OX PHOS could support healthy AT-associated cells and blunting OX PHOS could support obese AT-associated cells, but very little research has been done to modulate energy metabolism strictly in immune cells in the context of obesity. A few studies have shown that systemic suppression of glycolysis can improve obesity-related metabolic dysfunction in mice. For example, administration of oxamate, which blocks the conversion of pyruvate to lactate and reduces glycolytic flux, resulted in reduced inflammatory cytokine secretion, improved insulin sensitivity, and improved glycemic control in leptin receptor deficient obese mice [45,46]. This effect was observed with oxamate alone as well as when combined with metformin [47]. The mechanism for these results was attributed to reduced plasma lactate, a metabolite known to impair insulin sensitivity; however, it is plausible that immune effects also contributed to improved metabolic function, as both oxamate and metformin can suppress immune cell glycolysis and inflammatory cytokine production [48]. In a second example, HFD fed pyruvate dehydrogenase (PDK2) deficient mice demonstrated improved blood glucose and insulin and reduced weight gain compared with control mice [49]. PDK2 increases the flux of pyruvate through the TCA cycle and indirectly suppresses glycolysis, again suggesting that glycolytic suppression, however these studies could not clarify a role for immune specific metabolic changes. In contrast to studies targeting glycolysis, a recent study of macrophage-specific deficiency in Raptor, part of the mammalian target of rapamycin complex 1 (mTORC1) that promotes OX PHOS, improved glycemic control and blunted inflammatory parameters in the liver and AT of HFD-fed mice. This data supports older studies showing that impingement on FAO and OX PHOS in macrophages generally impairs metabolic health following HFD feeding [50–53]. Together, these studies suggest that blunting glycolysis may be protective against obesity-induced metabolic dysfunction, while blunting FAO and OX PHOS may be detrimental.
There is still a lot to understand about modulating metabolic pathways for therapeutic targeting. A recent study with a global knock out of the glycolytic enzyme pyruvate kinase (PKM2) reported PKM1 compensation [54], suggesting the importance of considering feedback loops when modulating metabolic targets. Furthermore PKM2, as well as the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH), have been shown to translocate to the nucleus and alter gene transcription [55,56], suggesting that non-glycolytic functions for target proteins should be better explored to understand the overall impact of these systems. In a myeloid-specific glucose transporter (GLUT)1 deficiency model, GLUT1 deficient BMDMs increased oleate and glutamine metabolism [57*]. These results suggest that other metabolic pathways can compensate when glucose is limited and should be considered when inhibiting any metabolic pathway. Additionally, deficiency of the fatty acid transporter carnitine palmitoyltransferase (Cpt)1b shows opposite effects on weight gain and metabolic dysfunction when mice are fed HFD for 8 weeks versus 7 months [58,59], suggesting that acute versus long term needs may be different for each cell type and that targeting these mediators may induce adaptation or feedback regulation over time. Moreover, it’s recently been reported that the drug etomoxir, which is commonly used to inhibit FAO, has non-specific effects at the concentrations used in many previous immunometabolism studies [60*]. Previous studies suggest that etomoxir treatment inhibits M2 polarization, however this effect happens in the absence of the target enzymes Cpt1 and Cpt2, as shown in a double knockout model. Instead, this recent study showed that the depletion of intracellular free coenzyme A is responsible for the M2 polarizing effects of etomoxir, suggesting that M2 macrophages may be less reliant on FAO than we previously believed.
New discoveries in intrinsic immunometabolism offer the opportunity to selectively target specific immune cell subsets by modifying the metabolic pathways essential for their function. Recent studies support the need for better mouse models to test deficiency of immune cell-specific pathways. Additionally, these studies support the need to develop more specific pharmacological drugs with fewer off-target effects. Mechanisms to target specific cells would be optimal, as these pathways are utilized in all cell types, and any modulation should be reversible in order to reverse immunosuppression in order to effectively fight bacterial and viral infections as well as cancer. Furthermore, future studies should examine the full impact of immune cell subset metabolic regulation on metabolic function and how modulating these pathways change over time with feedback regulation.
Current Research and Potential Avenues for Therapeutic Development
An enhanced understanding of immune cell networks and metabolic pathways may help us to target immune responses associated with obesity and metabolic dysfunction more effectively. In light of this, there are many ongoing studies that aim to understand and modulate both intrinsic and extrinsic immunometabolism to improve metabolic health. In this section, we will review current and ongoing areas of research that simultaneously impact both extrinsic and intrinsic immunometabolism that we believe are promising areas for therapeutic development.
Th2 immunity and intrinsic immunometabolism
There is substantial evidence for the role of Th2 immunity in AT and metabolic health, yet targets for modulating Th2 immunity are still unclear. While eosinophils are associated with healthy AT and positively correlated with metabolic health in IL-5 transgenic and IL-5 deficient mice [61,62], increasing AT eosinophils via IL-5 injections or via CCR2 deficiency had no effect on metabolic function [20,63], suggesting that eosinophil infiltration alone does not contribute functionally to metabolic health. It’s plausible that eosinophil activation or polarization is required or that AT-associated tissue resident cells are distinct from circulating cells of the same type. Additionally, it’s plausible that that additional cell types, such as ILC2, Th2 cells, or M2 macrophages, are required for improved metabolic function [64]. Targeting Th2 intrinsic immunometabolism may be a therapeutic strategy to influence systemic immunometabolism. Immunometabolism-targeted treatment strategies in autoimmunity and cancer research should be considered for use in obesity-driven metabolic disease to promote Th2 immunity and polarization. In a model of allograft rejection, simultaneously blocking glycolysis and glutamine metabolism prevented the induction of CD4+ and CD8+ effector T cells while promoting the generation of allospecific Treg cells [65], suggesting that inhibiting more than one pathway can be an effective strategy to encourage anti-inflammatory immunometabolism. Additionally, anti-cancer agents and metabolic inhibitors have been conjugated to glucose to selectively deliver the highest concentration of drug to cancer cells that have much higher rates of glucose uptake than surrounding cell populations [66], suggesting that a similar strategy could exert selectivity on immune subsets by affecting the populations with the greatest demand for a particular substrate. Moreover, nanoparticles, glucan-shells, and liposomes can be engineered to express ligands or antibodies to target specific cell types, and carry siRNA or different drugs, as further reviewed here in the context of ATMs [67]. These therapeutics can even induce tissue specificity, as intraperitoneal administration of siRNA encapsulated by glucan shells in obese mice selectively silences genes in epididymal ATMs, whereas macrophages within lung, spleen, kidney, heart, skeletal muscle, subcutaneous adipose, and liver were not targeted [68]. Additional research is needed to determine a full view of metabolic pathways utilization by AT immune cells during obesity. Technologies such as RNA sequencing, proteomics, and phage display [69] can help us to further identify peptides targets for drug delivery.
Macrophage Iron Handling
Targeting macrophage iron handling may also be a therapeutic strategy to influence both intrinsic and extrinsic immunometabolism. Our lab has recently discovered a population of ATMs with high iron content in lean AT [70]. Coined “MFehi” cells, these iron handling cells can take in, store, and release iron in response to environmental demands, similar to iron handling Kupffer cells in the liver [71,72]. Additionally, MFehi cells have similar gene expression to M2 macrophages and are reduced with HFD-feeding, which suggests they may play a role in metabolic function [70–72]. In general, iron metabolism in macrophages reduces the expression of genes associated with antigen presentation while increasing gene expression for antioxidant and glucose metabolism proteins [73], and iron is well known for its role within iron-sulfur clusters, re-dox reactions, and enzyme function in the mitochondria [74]. Based on these studies, it is possible that iron handling in macrophages may impact intrinsic immunometabolism and therefore polarization and effector function. Moreover, MFehi macrophages may prevent iron overload and oxidative stress in adipocytes [75], which in turn improves systemic metabolic function. Iron chelation and intravenous iron treatments have been shown to play a direct role on tumor-associated macrophage polarization and indirectly impacting cancer survival [76], suggesting that targeting macrophage iron handling and polarization may also be an effective target for extrinsic metabolism. Future studies should determine how iron handling in macrophages is regulated, the effects on intrinsic metabolism, and how therapeutic drugs could target these pathways to improve metabolic function.
Adaptive Immune Memory
Macrophages are considered a major player in extrinsic immunometabolism; however, T cell populations are also altered in obesity and weight cycling (WC), which is defined by repeated cycles of weight gain and loss that impairs metabolic function beyond that of obesity alone. Our lab has recently shown that CD8+ and CD8+ memory T cells are elevated in the AT after HFD-feeding in mice, and that CD4+, CD8+, and CD8+ memory T cells are even further increased with WC [30]. The role of CD4+ T cells in WC has also recently supported by another group [77**]. This data suggests that the immune system recognizes weight gain in a manner similar to its recognition of pathogens and develops an adaptive memory response that may affect subsequent bouts of weight gain. Additionally, we recently found that T cells isolated from HFD-fed mice have reduced TCR diversity and increased clonality, with TCR-CDR3 regions enriched for positively charged and less polar amino acids [78**], further supporting the role for antigenic memory. These regions can recognize isolevuglandins, gamma reactive ketoaldehyde and isoprostenoid derivatives, suggesting these may be relevant neoantigens in AT. T cells have been well characterized in light of intrinsic immunometabolism, and bioenergetic pathways contribute to polarization, memory development, and proliferation, suggesting that future therapeutics may target both intrinsic and extrinsic immunometabolism.
There are many promising mechanisms by which T cell memory development in the AT could be bunted to reduce AT inflammation and metabolic dysfunction. First, blocking antigen presentation may ameliorate initial memory formation. Dendritic cells, macrophages, and adipocytes reside in AT and upregulate MHCII in obesity [79,80], and a recent study showed that adipocyte-specific MHCII deficiency improves insulin sensitivity in HFD-fed mice [81]. This suggests that targeting MHCII interactions in the AT may reduce memory formation. Second, modulating checkpoint co-inhibitory interactions have been successful in animal models of obesity-associated inflammation and metabolic dysfunction as well as in humans for lupus, Crone’s disease, and transplant rejection. However, long-term treatment targeting general co-inhibitory molecules can leave patients susceptible to bacterial and viral infections, as well as cancer [82]. A recent study showed that the interaction between CD40-TNF receptor-associated factor (TRAF) 2/3/5 is protective against metabolic dysfunction and inflammation associated with obesity; while, the CD40-TRAF6 pathway contributes to the detrimental consequences of obesity [83]. Administration of an inhibitor of the CD40-TRAF6 interaction improved IR in mice, suggesting that specific co-inhibitory interactions and/or blocking co-stimulatory interactions in the AT may be plausible targets to modulate T cell activation and memory formation.
Finally, Treg immunotherapy has potential use in transplant patients, graft versus host disease, and autoimmunity, and could be expanded to metabolic diseases. Ex vivo expanded Tregs have been shown to polarize M2 macrophages more effectively than freshly isolated Treg cells [84*], and progenitor populations can be differentiated and expanded ex vivo using anti-CD3/CD28 beads, IL-2 and rapamycin [85]. The ThRIL clinical trial at King’s College London is currently studying Treg immunotherapy to prevent organ transplant rejection while minimizing long-term immunosuppression, and if successful, may have potential use in obesity- and WC- related metabolic dysfunction. Interestingly, AT Tregs have been shown to have distinct TCR repertoires from both the lymph cells and conventional AT T cells and express high levels of PPARy [86,87]. Using this technique in AT would require selective isolation and expansion of the AT Tregs or their direct progenitors. Future research should elucidate additional AT antigens and determine the best mechanism to target adaptive memory formation to improve metabolic function in obesity.
Epigenetic regulation of immunity and microRNAs (mIRs)
Another emerging field in immunometabolism is the epigenetic control of immunity [88]. Epigenetics changes such as DNA methylation, can have long-lasting effects on hematopoietic lineage commitment and cell differentiation. In contrast, acute changes to covalent histone modifications such as methylation, acetylation, phosphorylation, sumolyation, ubiquitination, and monoaminylation, can impact cell polarization and function. These modifications influence the phenotype, polarization, and activation stage of the cells as well as their metabolic phenotypes. For example, CD4+ and Th2 cells are marked by H3K27me3, a repressive histone modification at the interferon gamma locus, whereas Th1 cells show increased levels of histone H3K4me2, a histone mark associated with actively transcribed chromatin, at the same locus [89]. Monocyte to macrophage differentiation and the development of tolerance or trained immunity are associated with the acquisition of distinct epigenetic signatures (H3K4me1, H3K4me3, and H3K27ac) at promoter and enhancer regions of inflammatory and glycolytic genes [90]. In addition, H3K4me3 is required for M1 macrophage cytokine production and H3K27 demethylation is required for M2 polarization [91]. Furthermore, metabolic pathway utilization can drive epigenetic changes. Glycolysis and β-oxidation increase acetyl-coA availability which can be used by histone acetyltranferase enzymes to promote an open chromatin state and increase transcription. Interestingly, histone deacetylase (HDAC)11 deficient mice are resistant to HFD-induced obesity and have greater glucose tolerance and insulin sensitivity when compared to wild type controls [92], while sirtuin (SIRT)2 deficiency and macrophage-specific SIRT1 deficiency increase weight gain and impair metabolic parameters associated with HFD-feeding [93,94]. This data suggests that specific modulation of epigenetic changes is important, as both HDAC and sirtuin proteins function as deacetylase enzymes. Additional research is needed to determine how obesity affects immune cell histone modifications and what therapeutic targets may be useful to improve metabolic function.
Another element of epigenetics that has being recently explored in obesity is the role of miRs [88]. MiRs are non-coding RNA molecules that bind mRNA and prevent translation. Like epigenetic changes, miRs can simultaneously influence metabolic and inflammatory function. miRs-155 is well known to augment receptor signaling in immune cells and has been shown to increase glycolysis in cancer cells [95–97]. Deletion of miR-155 in female mice protects against HFD-induced obesity[98]; however, there were immune-independent effects on AT beiging and male mice did not demonstrate the same protection [99]. Additionally, miRs can be secreted. MiR-34a has been shown to be secreted from adipocytes, suppress M2 polarization, and worsen metabolic dysfunction [100], suggesting that targeting exosome release could modulate miR delivery and function. Future studies should further examine the contribution of specific miRs to inflammation and metabolic function in obesity. Additionally, researchers can utilize nanoparticle, β-glucan coated, or liposome delivery methods to deliver miR mimics or antagomiRs, antisense oligonucleotides that prevent the interaction of miRNAs with their target mRNAs, to cells of interest in order to target immune cell function and moreover, metabolic function.
Conclusions
Intrinsic immunometabolism is the study of how bioenergetics pathway utilization is linked to inflammatory function, and is closely linked to extrinsic immunometabolism, which is the study of how immune cell distribution and function broadly influences systemic metabolism. Both intrinsic and extrinsic immunometabolism are linked to obesity and metabolic dysfunction. Targeting different areas of intrinsic immune cell metabolism may therefore influence immune function and more largely, metabolic function in obesity. While many studies have been designed to target macrophage metabolism and function in obesity, targeting Th2 immunity, iron-handling, adaptive memory, or epigenetic regulation in immune cells may also be worthwhile targets to better understand immunometabolism and to develop better pharmacological treatments (Figure 1). Future basic science research will help us to better understand which metabolic pathways and controls to target to improve obesity-related inflammatory diseases without dampening host immunity against pathogens like bacteria and viruses.
Acknowledgements
We would like to acknowledge Ellen T. Yu for her essential help in creating the figure for this manuscript, created in ©BioRender - biorender.com. HL Caslin is supported by the Molecular Endocrinology Training Grant (DK07563). AH Hasty is supported by a Merit Award from the Veterans Affairs (5I01BX002195) and an Innovative Basic Science award from the American Diabetes Association (1-17-IBS-140).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
Heather L. Caslin and Alyssa H. Hasty declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hruby A, Hu FB. The Epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33:673–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S–225S. [DOI] [PubMed] [Google Scholar]
- 4.Courcoulas AP, Yanovski SZ, Bonds D, Eggerman TL, Horlick M, Staten MA, et al. Long-term outcomes of bariatric surgery: a National Institutes of Health Symposium. JAMA Surg. 2014;149:1323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trandafir LM, Temneanu OR. Pre and post-natal risk and determination of factors for child obesity. J Med Life. 2016;9:386–91. [PMC free article] [PubMed] [Google Scholar]
- 6.Kim GW, Lin JE, Blomain ES, Waldman SA. Antiobesity pharmacotherapy: new drugs and emerging targets. Clin Pharmacol Ther. 2013;95:53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrante AW. The immune cells in adipose tissue. Diabetes Obes Metab. 2013;15:34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathis D, Shoelson SE. Immunometabolism: an emerging frontier. Nat Rev Immunol. 2011;11:81–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.** Boutens L, Hooiveld GJ, Dhingra S, Cramer RA, Netea MG, Stienstra R. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia. 2018;61:942–53.This is the first study to metabolically phenotype ATM. ATM from HFD-fed mice had enhanced glycolytic metabolism and OX PHOS supporting the research which shows that they are different from M1 (glycolytic) or M2 (oxidative) cells. It is interesting that both metabolic pathways are enhanced in one cell type. The authors showed that cytokine production was directly tied to elevated glycolytic metabolism (and not dependent on HIF-1), and hypothesize that OX PHOS contributes to lysosomal biogenesis or phagocytosis.
- 12.Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155:407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotamisligil GS. Foundations of immunometabolism and implications for metabolic health and Disease. Immunity. 2017;47:406–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolus WR, Hasty AH. Contributions of innate type 2 inflammation to adipose function. J Lipid Res. 2018;jlr.R085993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem. 2011;286:23591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill AA, Reid Bolus W, Hasty AH. A decade of progress in adipose tissue macrophage biology. Immunol Rev. 2014;262:134–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, et al. Metabolic dysfunction drives a mechanistically distinct pro-inflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB, et al. Adipose tissue dendritic cells are independent contributors to obesity-induced inflammation and insulin resistance. J Immunol. 2016;197:3650–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutierrez DA, Kennedy A, Orr JS, Anderson EK, Webb CD, Gerrald WK, et al. Aberrant accumulation of undifferentiated myeloid cells in the adipose tissue of CCR2-deficient mice delays improvements in insulin sensitivity. Diabetes. 2011;60:2820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolus WR, Gutierrez DA, Kennedy AJ, Anderson-Baucum EK, Hasty AH. CCR2 deficiency leads to increased eosinophils, alternative macrophage activation, and type 2 cytokine expression in adipose tissue. J Leukoc Biol. 2015;98:467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Chung K, Choi C, Beloor J, Ullah I, Kim N, et al. Silencing CCR2 in macrophages alleviates adipose tissue inflammation and the associated metabolic syndrome in dietary obese mice. Mol Ther Nucleic Acids. 2016;5:e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orr JS, Kennedy AJ, Hill AA, Anderson-Baucum EK, Hubler MJ, Hasty AH. CC-chemokine receptor 7 (CCR7) deficiency alters adipose tissue leukocyte populations in mice. Physiol Rep. 2016;4:e12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy A, Webb CD, Hill AA, Gruen ML, Jackson LG, Hasty AH. Loss of CCR5 results in glucose intolerance in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2013;305:E897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller AM, Asquith DL, Hueber AJ, Anderson LA, Holmes WM, McKenzie AN, et al. IL-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res. 2010;107:650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. [DOI] [PubMed] [Google Scholar]
- 27.Harmon DB, Srikakulapu P, Kaplan JL, Oldham SN, McSkimming C, Garmey JC, et al. Protective role for B-1b B cells and IgM in obesity-associated inflammation, glucose intolerance, and insulin resistance significance. Arterioscler Thromb Vasc Biol. 2016;36:682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilan Y, Maron R, Tukpah A-M, Maioli TU, Murugaiyan G, Yang K, et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. PNAS. 2010;107:9765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson EK, Gutierrez DA, Kennedy A, Hasty AH. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes. 2013;62:3180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamarron BF, Mergian TA, Cho KW, Martinez-Santibanez G, Luan D, Singer K, et al. Macrophage proliferation sustains adipose tissue inflammation in formerly obese mice. Diabetes. 2016;db160500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neill LAJ, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loftus RM, Finlay DK. Immunometabolism: cellular metabolism turns immune regulator. J Biol Chem. 2016;291:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norata GD, Caligiuri G, Chavakis T, Matarese G, Netea MG, Nicoletti A, et al. The cellular and molecular basis of translational immunometabolism. Immunity. 2015;43:421–34. [DOI] [PubMed] [Google Scholar]
- 35.Kelly B, O’neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bantug GR, Galluzzi L, Kroemer G, Hess C. The spectrum of T cell metabolism in health and disease. Nat Rev Immunol. 2017;18:19–34. [DOI] [PubMed] [Google Scholar]
- 38.Wahl DR, Byersdorfer CA, Ferrara JLM, Opipari AW, Glick GD. Distinct metabolic programs in activated T cells: opportunities for selective immunomodulation. Immunol Rev. 2012;249:104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.* Porter L, Toepfner N, Bashant KR, Guck J, Ashcroft M, Farahi N, et al. Metabolic profiling of human eosinophils. Front Immunol. 2018;9.This study shows that human eosinophils have high glycolytic metabolism similar to neutrophils, however eosinophils also have elevated OX PHOS. Like the study by Boutens, et al above, it is interesting that eosinophils use both energy generating pathways at a substantial rate.
- 40.Yang J-Q, Kalim KW, Li Y, Zhang S, Hinge A, Filippi M-D, et al. RhoA orchestrates glycolysis for TH2 cell differentiation and allergic airway inflammation. J Allerg Clin Immunol. 2016;137:231–245.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelgrom LR, Everts B. Metabolic control of type 2 immunity. Eur J Immunol. 2017;47:1266–75. [DOI] [PubMed] [Google Scholar]
- 42.Wang T, Liu H, Lian G, Zhang S-Y, Wang X, Jiang C. HIF1α-induced glycolysis metabolism Is essential to the Aativation of inflammatory macrophages. Mediators Inflamm. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng M, Yin N, Chhangawala S, Xu K, Leslie CS, Li MO. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science. 2016;354:481–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phan AT, Doedens AL, Palazon A, Tyrakis PA, Cheung KP, Johnson RS, et al. Constitutive glycolytic metabolism supports CD8+ T cell effector memory differentiation during viral infection. Immunity. 2016;45:1024–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu M, Ye W, Zheng Y, Zhang S. Oxamate enhances the anti-inflammatory and insulin-sensitizing effects of metformin in diabetic mice. Pharmacology. 2017;100:218–28. [DOI] [PubMed] [Google Scholar]
- 46.Ye W, Zheng Y, Zhang S, Yan L, Cheng H, Wu M. Oxamate improves glycemic control and insulin sensitivity via inhibition of tissue lactate production in db/db mice. PLoS One. 2016;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woo S-L, Xu H, Li H, Zhao Y, Hu X, Zhao J, et al. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS One. 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caslin HL, Taruselli MT, Haque T, Pondicherry N, Baldwin EA, Barnstein BO, et al. Inhibiting glycolysis and ATP production attenuates IL-33-mediated mast cell function and peritonitis. Front Immunol. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Go Y, Jeong JY, Jeoung NH, Jeon J-H, Park B-Y, Kang H-J, et al. Inhibition of pyruvate dehydrogenase kinase 2 protects against hepatic steatosis through modulation of tricarboxylic acid cycle anaplerosis and ketogenesis. Diabetes. 2016;65:2876–87. [DOI] [PubMed] [Google Scholar]
- 50.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawano Y, Nakae J, Watanabe N, Fujisaka S, Iskandar K, Sekioka R, et al. Loss of Pdk1-Foxo1 signaling in myeloid cells predisposes to adipose tissue inflammation and insulin resistance. Diabetes. 2012;61:1935–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, et al. Adipocyte-derived Th2 cytokines and myeloid PPAR delta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson AR, Qin Y, Cozzo AJ, Freemerman AJ, Huang MJ, Zhao L, et al. Metabolic reprogramming through fatty acid transport protein 1 (FATP1) regulates macrophage inflammatory potential and adipose inflammation. Mol Metab. 2016;5:506–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dayton TL, Gocheva V, Miller KM, Israelsen WJ, Bhutkar A, Clish CB, et al. Germline loss of PKM2 promotes metabolic distress and hepatocellular carcinoma. Genes Dev. 2016;30:1020–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kong Q, Li N, Cheng H, Zhang X, Cao X, Qi T, et al. HSPA12A Is a novel player in nonalcoholic steatohepatitis via promoting nuclear PKM2-mediated M1 macrophage polarization. Diabetes. 2019;68:361–76. [DOI] [PubMed] [Google Scholar]
- 56.Sheng W-Y, Wang T-CV. Proteomic analysis of the differential protein expression reveals nuclear GAPDH in activated T lymphocytes. PLoS ONE. 2009;4:e6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.* Freemerman AJ, Zhao L, Pingili AK, Teng B, Cozzo AJ, Fuller AM, et al. Myeloid Slc2a1 -deficient murine model revealed macrophage activation and metabolic phenotype are fueled by GLUT1. J Immunol. 2019;ji1800002.This study shows that BMDM from myeloid-specific glucose transporter GLUT1 (Slc2a1) deficient mice had reduced glycolysis (as expected), and reduced maximal respiratory capacity, despite compensatory oleate and glutamine metabolism. Myeloid Slc2a1M deficiency was not protective for obesity-induced metabolic dysregulation and induced unstable atheroma formation in an Ldlr−/− atherosclerosis model, which suggests that this deficiency affects phagocytic capacity more than cytokine production. This was the first study to show that blunting glycolysis in macrophages does not affect systemic metabolism and reiterates the importance of examining many functional parameters when modulating metabolism.
- 58.Kim T, Moore JF, Sharer JD, Yang K, Wood PA, Yang Q. Carnitine palmitoyltransferase 1b deficient mice develop severe insulin resistance after prolonged high fat diet feeding. J Diabetes Metab. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim T, He L, Johnson M, Li Y, Zeng L, Ding Y, et al. Carnitine palmitoyltransferase 1b deficiency protects mice from diet-induced insulin resistance. J Diab Metab. 2014;05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Divakaruni AS, Hsieh WY, Minarrieta L, Duong TN, Kim KKO, Desousa BR, et al. Etomoxir inhibits macrophage polarization by disrupting CoA homeostasis. Cell Metab. 2018;28:490–503.e7.Etomoxir has long been used to inhibit carnitine palmitoyltransferase-1 and make inferences about FAO. However, many studies have used concentrations of etomoxir that far exceed what is required to inhibit enzyme activity (EC90 < 3 μM), and many published results are therefore due to off target effects. This study showed that etomoxir blocks M2 polarization even in the absence of Cpt1a and Cpt2 expression, and that OX PHOS is dispensable for M(IL-4). Interestingly, the reduced polarization was traced to depletion of intracellular free coenzyme A (CoA). This study is the first to reveal the off target effects of etomoxir and dispute prior studies which link M2 polarization to FAO.
- 61.Molofsky AB, Nussbaum JC, Liang H-E, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu D, Molofsky AB, Liang H-E, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bolus WR, Peterson KR, Hubler MJ, Kennedy AJ, Gruen ML, Hasty AH. Elevating adipose eosinophils in obese mice to physiologically normal levels does not rescue metabolic impairments. Mol Metab. 2018;8:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bolus WR. Diversity of adipose tissue immune cells: are all eosinophils created equal? BioEssays. 2018;40:1800150. [DOI] [PubMed] [Google Scholar]
- 65.Lee C-F, Lo Y-C, Cheng C-H, Furtmüller GJ, Oh B, Andrade-Oliveira V, et al. Preventing allograft rejection by targeting immune metabolism. Cell Rep. 2015;13:760–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calvaresi EC, Granchi C, Tuccinardi T, Di Bussolo V, Huigens RW, Lee HY, et al. Dual targeting of the Warburg Effect with glucose-conjugated lactate dehydrogenase inhibitor. Chembiochem. 2013;14:2263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peterson KR, Cottam MA, Kennedy AJ, Hasty AH. Macrophage-targeted therapeutics for metabolic disease. Trends Pharmacol Sci. 2018;39:536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aouadi M, Tencerova M, Vangala P, Yawe JC, Nicoloro SM, Amano SU, et al. Gene silencing in adipose tissue macrophages regulates whole-body metabolism in obese mice. PNAS. 2013;110:8278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miettinen HM, Gripentrog JM, Lord CI, Nagy JO. CD177-mediated nanoparticle targeting of human and mouse neutrophils. PLoS ONE. 2018;13:e0200444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orr JS, Kennedy A, Anderson-Baucum EK, Webb CD, Fordahl SC, Erikson KM, et al. Obesity alters adipose tissue macrophage iron content and tissue iron distribution. Diabetes. 2014;63:421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clementi AH, Gaudy AM, van Rooijen N, Pierce RH, Mooney RA. Loss of Kupffer cells in diet-induced obesity is associated with increased hepatic steatosis, STAT3 signaling, and further decreases in insulin signaling. Biochim Biophys Acta. 2009;1792:1062–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mayoral Monibas R, Johnson AMF, Osborn O, Traves PG, Mahata SK. Distinct hepatic macrophage populations in lean and obese mice. Front Endocrinol. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaempfer T, Duerst E, Gehrig P, Roschitzki B, Rutishauser D, Grossmann J, et al. Extracellular hemoglobin polarizes the macrophage proteome toward Hb-clearance, enhanced antioxidant capacity and suppressed HLA class 2 expression. J Proteome Res. 2011;10:2397–408. [DOI] [PubMed] [Google Scholar]
- 74.Paul BT, Manz DH, Torti FM, Torti SV. Mitochondria and iron: current questions. Expert Rev Hematol. 2017;10:65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hubler MJ, Erikson KM, Kennedy AJ, Hasty AH. MFe hi adipose tissue macrophages compensate for tissue iron perturbations in mice. Am J Physiol Cell Physiol. 2018;315:C319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong H, Bullock TNJ. Metabolic influences that regulate dendritic cell function in tumors. Front Immunol. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.** Zou J, Lai B, Zheng M, Chen Q, Jiang S, Song A, et al. CD4+ T cells memorize obesity and promote weight regain. Cell Mol Immunol. 2018;15:630–9.This study showed that previously obese mice regain weight much faster than mice that have never been obese, a phenotype that lasts at least 2 months. This memory was attenuated by dexamethasone treatment and in immunodeficient Rag1−/− and H2A−/− mice. Memory was restored after introducing CD4+ T cells from previously obese mice in the Rag1−/−, and depletion of CD4+ T cells led to obesity memory ablation. This is the first study to show that CD4+ T cells modulate weight cycling- associated metabolic parameters.
- 78.** McDonnell WJ, Koethe JR, Mallal SA, Pilkinton MA, Kirabo A, Ameka MK, et al. High CD8 T-cell receptor clonality and altered CDR3 properties are associated with elevated isolevuglandins in adipose tissue during diet-induced obesity. Diabetes. 2018;67:2361–76.This study showed that HFD feeding reduced TCR diversity and increased TCR CDR3 regions with positively-charged and less polar amino acids in AT CD8+ T cells. Moreover, negatively-charged and nonpolar isolevuglandin-adducted protein species were higher in AT macrophages of HFD-fed mice. This is the first study to examine the presence and clonal expansion of specific TCR sequences in obesity and suggest a plausible antigen.
- 79.Xiao L, Yang X, Lin Y, Li S, Jiang J, Qian S, et al. Large adipocytes function as antigen-presenting cells to activate CD4+ T cells via upregulating MHCII in obesity. Int J Obes. 2016;40:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morris DL, Cho KW, Delproposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, et al. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013;62:2762–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deng T, Liu J, Deng Y, Minze L, Xiao X, Wright V, et al. Adipocyte adaptive immunity mediates diet-induced adipose inflammation and insulin resistance by decreasing adipose Treg cells. Nature Comm. 2017;8:15725. [Google Scholar]
- 82.Seijkens T, Kusters P, Chatzigeorgiou A, Chavakis T, Lutgens E. Immune cell crosstalk in obesity: a key role for costimulation? Diabetes. 2014;63:3982–91. [DOI] [PubMed] [Google Scholar]
- 83.Chatzigeorgiou A, Seijkens T, Zarzycka B, Engel D, Poggi M, Berg S van den, et al. Blocking CD40-TRAF6 signaling is a therapeutic target in obesity-associated insulin resistance. PNAS. 2014;111:2686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.* Romano M, Fanelli G, Tan N, Nova-Lamperti E, McGregor R, Lechler RI, et al. Expanded regulatory T cells induce alternatively activated monocytes with a reduced capacity to expand T helper-17 cells. Front Immunol. 2018;9:1625.This study showed that expanded Tregs promote M2 polarization more efficiently than freshly isolated Tregs. Expanded Tregs suppressed monocyte NF-κB activation, IL-6 and TNF production, co-stimulatory and MHC-class II expression, and Th17 expansion. Expanded Tregs also increased CD206 and heme oxygenase-1 expression, and IL-10 production. This was the first study to identify the mechanism by which expanded Tregs suppress autoimmunity and transplant rejection.
- 85.Safinia N, Vaikunthanathan T, Fraser H, Thirkell S, Lowe K, Blackmore L, et al. Successful expansion of functional and stable regulatory T cells for immunotherapy in liver transplantation. Oncotarget. 2016;7:7563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Fat Treg cells: a liaison between the immune and metabolic systems. Nat Med. 2009;15:930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raghuraman S, Donkin I, Versteyhe S, Barrès R, Simar D. The emerging role of epigenetics in inflammation and immunometabolism. Trends Endocrinol Metab. 2016;27:782–95. [DOI] [PubMed] [Google Scholar]
- 89.Schoenborn JR, Dorschner MO, Sekimata M, Santer DM, Shnyreva M, Fitzpatrick DR, et al. Comprehensive epigenetic profiling identifies multiple distal regulatory elements directing transcription of the gene encoding interferon-gamma. Nat Immunol. 2007;8:732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saeed S, Quintin J, Kerstens HHD, Rao NA, Aghajanirefah A, Matarese F, et al. Epigenetic programming during monocyte to macrophage differentiation and trained innate immunity. Science. 2014;345:1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takeuch O, Akira S. Epigenetic control of macrophage polarization. Eur J Immunol. 2011;41:2490–3. [DOI] [PubMed] [Google Scholar]
- 92.Sun L, Marin De Evsikova C, Bian K, Achille A, Telles E, Seto E. HDAC11 deficiency prevents high-fat diet-induced obesity and metabolic syndrome. bioRxiv. 2018. [Google Scholar]
- 93.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. PNAS. 2008;105:9793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, et al. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol. 2010;30:4712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang S, Zhang L-F, Zhang H-W, Hu S, Lu M-H, Liang S, et al. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. EMBO J. 2012;31:1985–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim S, Lee E, Jung J, Lee JW, Kim HJ, Kim J, et al. microRNA-155 positively regulates glucose metabolism via PIK3R1-FOXO3a-cMYC axis in breast cancer. Oncogene. 2018;37:2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huffaker TB, O’Connell RM. miR-155-SOCS1 as a functional axis: satisfying the burden of proof. Immunity. 2015;43:3–4. [DOI] [PubMed] [Google Scholar]
- 98.Gaudet AD, Fonken LK, Gushchina LV, Aubrecht TG, Maurya SK, Periasamy M, et al. miR-155 deletion in female mice prevents diet-induced obesity. Sci Rep. 2016;6:22862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Velázquez KT, Enos RT, Carson MS, Cranford TL, Bader JE, Sougiannis AT, et al. miR155 deficiency aggravates high-fat diet-induced adipose tissue fibrosis in male mice. Physiol Rep. 2017;5:e13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pan Y, Hui X, Hoo RLC, Ye D, Chan CYC, Feng T, et al. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]