Abstract
Background:
Siglec-7 is an inhibitory receptor (IR) expressed on human blood eosinophils. Whereas activation of other IRs, including Siglec-8 and CD300a, has been shown to downregulate eosinophil function, little is known about the role of Siglec-7 on human eosinophils.
Objective.
To examine Siglec-7 expression and function in eosinophils from normal (ND) and eosinophilic (EO) donors.
Methods:
Eosinophil expression of Siglec-7 was quantified by flow cytometry and quantitative PCR. Soluble Siglec-7 (sSiglec-7) levels were measured by ELISA in serum. The effect of Siglec-7 on eosinophil viability and degranulation was assessed in vitro by AnnexinV-FITC/7-AAD staining and by measuring GM-CSF-induced mediator release in culture supernatants. Signal transduction was studied by Western Blot.
Results:
Siglec-7 was expressed ex vivo on blood eosinophils from all eosinophilic and normal individuals studied. Siglec-7 surface, but not SIGLEC-7 mRNA expression, was correlated with absolute eosinophil count (AEC). Siglec-7 was upregulated on purified eosinophils after in vitro stimulation with GM-CSF or IL-5. Serum sSiglec-7 was detectable in 133/144 subjects tested and correlated with AEC. Siglec-7 crosslinking inhibited GM-CSF-induced release of eosinophil peroxidase, TNFα and IL-8 (n=7–8) but did not promote eosinophil apoptosis (n=5). Finally, Siglec-7 crosslinking on GM-CSF-activated eosinophils induced phosphorylation of SHP-1 and de-phosphorylation of ERK½ and p38.
Conclusions:
Siglec-7 is constitutively expressed on human eosinophils and downmodulates eosinophil activation. Targeting of Siglec-7 on eosinophils might enhance treatment efficacy in eosinophil-driven disorders. Conversely, therapeutic interventions that inhibit Siglec-7 could have unanticipated consequences and promote eosinophilic inflammation.
Keywords: apoptosis, eosinophilia, eosinophils, hypereosinophilic syndrome, ITIM, Siglec-7, Siglec-8, signaling
Capsule Summary
Siglec-7 is an inhibitory receptor expressed on human blood eosinophils. Siglec-7 activation downregulates eosinophil activation but does not affect survival. Targeting of Siglec-7 on eosinophils could be useful in the treatment of eosinophilic inflammation.
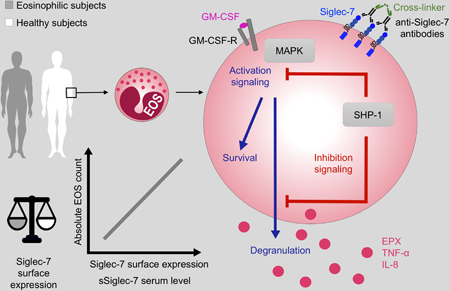
Introduction
Eosinophils are main effector cells in allergic inflammation and have been implicated in the late and chronic phases of the allergic response. Their recruitment and activation are regulated by a complex network of activating and inhibitory signals that represent key therapeutic targets1,2. Initial attention focused primarily on the IL-5 axis in eosinophil-associated disorders, leading to the recent approval of mepolizumab, reslizumab and benralizumab for the treatment of eosinophilic asthma3,4 and mepolizumab for the treatment of eosinophilic granulomatosis with polyangiitis5. The role of inhibitory receptors (IRs), many of which are shared by eosinophils, basophils and mast cells, has been less well-explored.
IRs, including members of the Sialic acid binding Ig-like lectin (Siglec) family6 and the Ig-like superfamily member CD300a7, are characterized by immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their cytoplasmic tail, which, when phosphorylated, decrease cellular activation and/or induce apoptosis. The precise effects of an individual IR may vary depending on the cell type. For example, crosslinking of Siglec-8, an IR expressed on eosinophils8, mast cells9 and basophils10, leads to apoptosis of eosinophils11,12, but inhibits degranulation of mast cells without promoting cell death9. In contrast, CD300a activation causes decreased function and apoptosis of both mast cells and eosinophils in the presence of their respective growth factors13,14.
Originally detected on Natural Killer cells (NK cells) and monocytes 15,16, Siglec-7 (p75/AIRM) is an additional inhibitory member of the Siglec family that is expressed on a wide variety of cells, including human eosinophils13, mast cells and basophils17. Siglec-7 displays two signaling motifs: a membrane-proximal ITIM and a membrane-distal motif (ITIM-like)15. On NK cells, the Siglec-7 cytoplasmic domain associates with the protein tyrosine phosphatases Src homology-2 (SH2) domain-containing protein-tyrosine phosphatase-1 (SHP-1), which mediates the Siglec-dependent inhibitory signal18.
In contrast to Siglec-8 which binds sialylated ligands on human airways cells19, Siglec-7 binds preferentially to α2,8-disialyl and branched α2,6-sialyl carbohydrate structures20, which are expressed on various tumor cells and normal colonic epithelial cells21. The function of Siglec-7 has been best studied in the context of tumor biology, where expression of Siglec-7 ligands on tumor cells suppress NK cell function leading to increased metastatic potential22. These findings have stimulated the development of novel strategies to inhibit Siglec-7 function, including the development of low molecular weight, high affinity Siglec-7 ligands that block the inhibitory effects of tumor cells on NK cells in vitro23. Such strategies may have unanticipated consequences on cells other than NK cells that express Siglec-7, including the cells involved in allergic inflammation. Although activation of Siglec-7 on mast cells can inhibit IgE-mediated mast cell and basophil activation17, the function of Siglec-7 on eosinophils is unknown. The aim of the present study was to characterize the expression of Siglec-7 on eosinophils from normal donors (ND) and subjects with eosinophilia (EO) and to explore the functional consequences of Siglec-7 engagement on eosinophils.
Materials and Methods
Study subjects
Eosinophilic subjects (EO) underwent detailed clinical evaluation at the NIH Clinical Center as part of an NIAID Institutional Review Board (IRB)-approved protocol to study eosinophilic disorders (NCT00001406). For the purposes of this study, hypereosinophilic syndrome (HES) is defined as hypereosinophilia (AEC ≥1500/μL) with evidence of eosinophil-associated clinical manifestations. EO included subjects with idiopathic HES (n=32), myeloid HES (n=19), lymphocytic HES (n=15), overlap HES (n=30), hypereosinophilia of unknown significance (n=8), familial eosinophilia (n=3), episodic angioedema and eosinophilia (n=3), and hypereosinophilia associated with neoplasia (n=5), immunodeficiency (n=3) and atopy (n=1). Forty-seven subjects were untreated at the time of analysis (geometric mean (GM) AEC 2505/μL, range 470–68220/μL) and 72 were receiving treatment at the time of the study (GM AEC 871/μL, range 30–26420/μL). See Supplementary Table 1 for details. Normal donors (ND) were recruited under NIAID IRB (NCT00090662) and Hadassah-Hebrew University Human Experimentation Helsinki Committee approved protocols for in vitro research. All participants gave written informed consent.
Eosinophil purification
Eosinophils were purified by sedimentation on Ficoll-Hypaque and magnetic bead purification, as previously described 13,24. Eosinophil purity was >98% in all experiments with a viability of >98%, as assessed by trypan blue staining.
Real-time quantitative PCR
Total RNA was extracted from 107 purified eosinophils using TriZol (Invitrogen) prior to cDNA synthesis using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, Calif), according to the manufacturer’s protocol. SIGLEC-7 and SIGLEC-8 mRNA, and 18S rRNA were amplified in a 96-well plate using the following commercially available TaqMan primers (Applied Biosystems) in a final volume of 10 μL: Hs01100854_m1, Hs00274289_m1, and X03205.1, respectively. Each sample was run in triplicate, and cycle threshold levels were normalized using the 18S cycle threshold values from corresponding samples. mRNA levels are expressed in arbitrary units (x 10−5) related to 18S rRNA.
Assessment of surface receptor expression by flow cytometry on whole blood
Surface expression of Siglec-7 was quantified by multiparameter flow cytometry ex vivo on eosinophils (CD45+CD16- granulocytes) in whole blood as previously described24 and in vitro using purified blood eosinophils (2 × 106/mL) incubated with or without 10 ng/mL of rhIL-5 (R&D, Minneapolis, MN, USA) or rhGM-CSF (PeproTech, Rocky Hill, NJ, USA). The antibodies used are provided in the online Supplementary Materials and Methods.
Modulation of Siglec-7 expression on purified eosinophils in vitro
Purified eosinophils (2×106/mL/wells) were incubated overnight with or without 10ng/mL of recombinant human cytokines (rhGM-CSF, rhIL-5, rhIL-33, rhIFN-α, rhIFN-γ), SEB (5 μg/mL) or E. coli extracts (4 μg/mL). Surface expression of Siglec-7 was determined by flow cytometry on viable eosinophils (7-AADneg), and RNA was extracted from TriZol and mRNA levels assessed after overnight incubation with and without GM-CSF (10 ng/mL).
Measurement of soluble Siglec-7 receptor levels in serum.
Serum levels of soluble Siglec-7 (sSiglec-7) were quantified using a commercially available sandwich ELISA (Human Siglec-7/CD328 DuoSet ELISA, R&D) according to the manufacturer’s instructions. All assays were performed in singlicate using undiluted serum, and values were calculated on the basis of a recombinant standard curve. The minimal level of detection of sSiglec-7 in serum was 125 pg/mL.
In vitro eosinophil stimulation and inhibition assays
Freshly isolated eosinophils (1.5 × 105/150 μL in culture medium) were blocked in 5% goat serum in PBS prior to incubation for 30 minutes at 4°C in 96-well U shape plates (Nunc, Roskilde, Denmark) in the presence of anti-Siglec-7 (QA79, eBiosciences), anti-Siglec-8 (7C9, Biolegend), or matched control antibodies (mouse IgG1k isotype control, eBiosciences) (0.62–5 μg/mL). After washing, crosslinker (F(ab′)2fragment goat anti-mouse IgG (H+L) (10 μg/mL), Jackson Laboratories, West Grove, PA, US) and rhGM-CSF (50 ng/mL) were added simultaneously and the cells were incubated for 40 minutes (eosinophil peroxidase, EPX release) or overnight (CD69 expression and cytokine release) at 37°C, 5% CO2 in phenol-free RPMI 5% FCS. Supernatants were collected and stored at −20°C. Anti-CD300a (1 μg/mL, from hybridoma #12, provided by Prof. O. Mandelboim) was used as a positive control for inhibition in all experiments.
Measurement of EPX and cytokines in supernatants
EPX release was measured by colorimetric assay using freshly prepared peroxidase substrate solution containing o-phenylenediamine (OPD) as described previously25. A standard curve was constructed using purified human EPX (provided by Dr. Gerald J. Gleich, University of Utah Health Sciences Center, Salt Lake City, UT, USA). The reaction was stopped by the addition of 100 μl of 4 mM sulfuric acid (BDH), and absorbance was determined at 492 nm in a spectrophotometer (PowerWave XS; Bio-Tek Instruments). IL-8 and TNFα levels were detected in supernatants by ELISA (Peprotech, minimum detectable level 16 pg/mL). Data are expressed as % of control, calculated by dividing the concentration in the antibody-treated well by that in the corresponding uninhibited control well (GM-CSF only) and multiplying by 100.
Assessment of eosinophil apoptosis.
Eosinophil cell death was quantified by flow cytometry using AnnexinV-FITC/7-AAD staining (BD, San Jose, CA, USA). Briefly, after overnight incubation with the cytokines (GM-CSF or IL-5 at 10 ng/mL), anti-Siglec-7, anti-Siglec-8 or their respective isotype controls were added for another overnight incubation at 37°C, 5% CO2. The cells were then washed in PBS 1X, stained with AnnexinV-FITC and 7-AAD probes (5 μL/tube) in a binding buffer (BD) during 15 minutes room temperature. The samples were acquired on a LSRII flow cytometer (BD).
Western Blot
Crosslinking with anti-Siglec-7, anti-CD300a or matched control antibody was performed on freshly isolated peripheral blood eosinophils (2 × 106/ 200 μL) in culture medium for 30 minutes at 4°C as described above. Cells were activated for the indicated times in Eppendorf tubes in a 37°C water bath in the presence of rhGM-CSF (50 ng/mL) and sheep anti-mouse F(ab’)2 crosslinker antibodies (CL), (10 μg/mL). Activation was stopped by adding ice-cold PBS to the pellet followed by centrifugation. Cell pellets were lysed using a cell lysis buffer (#7018 Cell Signaling Technology, Danvers, MA) supplemented with protease inhibitor cocktail (1:100, Sigma-Aldrich, Rehovot, Israel), and cell debris was removed from the lysates by centrifugation (14 000g, 1 minute 4°C). Western blot analysis was performed using standard techniques (see Supplement for details).
Statistical analysis
Group means were compared using the Mann-Whitney U test, ANOVA (for multiple group comparisons) and Wilcoxon signed rank test (for paired analyses). Spearman rank was used for determination of correlation and a custom rank trend test for Western blot analysis (see Supplemental Methods). P values less than .05 were considered significant for all analyses.
Results
Eosinophil expression of Siglec-7
Siglec-7 expression was detected on eosinophils from all 23 ND and 31 EO subjects tested. Geometric mean (GM) Siglec-7 expression showed a tendency towards an increase in EO subjects compared to ND with GM ΔMFI of 183 vs 105, respectively, (P=0.065; Figure 1A) and was significantly correlated with absolute eosinophil count (AEC) (n=52, P<0.05, Figure 1B). Siglec-7 was expressed on blood eosinophils at lower levels than the inhibitory receptors, CD300a and Siglec-8 (GM ΔMFI 271 vs 667 and 980, respectively, n=20 EO; GM ΔMFI 307 vs 852 vs 837, n=11 ND; Wilcoxon test, P<0.05 to P<0.0001; Figure 1C) and was positively correlated with Siglec-8 expression (r=+0.52, P<0.0001, n=47; Figure 1D) and CD300a (r=+0.7, P<0.0001, n=27, Supplementary Figure 1).
Figure 1. Siglec-7 expression on peripheral blood eosinophils.
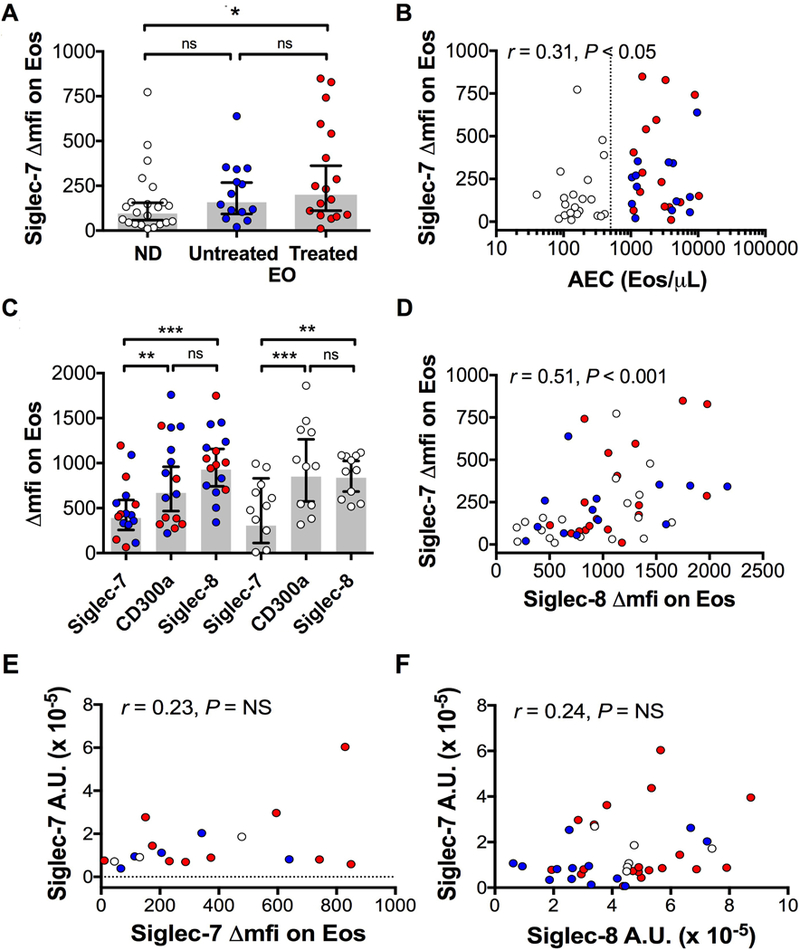
A, Siglec-7 expression (expressed as ΔMFI) on eosinophils in normal donors (ND) and eosinophilic subjects (EO), untreated and treated. B, Correlation between Siglec-7 expression and AEC. C, Comparison with eosinophil expression of Siglec-8 and CD300a. D-F, Correlation between Siglec-7 and Siglec-8 surface expression (D), SIGLEC-7 mRNA levels and cell surface expression (E), and SIGLEC-7 and SIGLEC-8 mRNA levels (F). Symbols represent individual subjects (white = ND, blue = untreated EOS, red = EOS on treatment). EOS subjects were comprised of subjects with MHES (n=6), LHES (n=6), Overlap HES (n=5), IHES (n=9) and other eosinophilic disorders (n=3). The grey bars indicate geometric mean. Error bars represent 95% CI. The vertical dotted line in panel B is drawn at the laboratory cutoff for eosinophilia (500/μL). *P < .05; **P < .001, ***P< .0001.
SIGLEC-7 mRNA was quantified in purified peripheral blood eosinophils from 45 subjects (13 ND and 32 EO) and levels were detectable in all subjects tested (data not shown). SIGLEC-7 mRNA levels were not correlated with the surface Siglec-7 levels measured on blood eosinophils by flow cytometry (Figure 1E). Moreover, contrary to what was seen at the cell surface levels by flow cytometry (Figure 1 D), levels of mRNA for SIGLEC-7 and SIGLEC-8 were not correlated (Figure 1F).
Measurement of soluble Siglec-7 receptor levels in serum
sSiglec-7 was measured by ELISA in the serum of normal and eosinophilic donors (n=16 and 126) and was detectable in 133 of 142 subjects tested. Compared to ND (GM 270 pg/mL; n=16) and treated EOS patients (GM 366 pg/mL; n=81), serum sSiglec-7 levels were increased in untreated EOS subjects (GM 501 pg/mL; n=45) (Figure 2A). Untreated EO subjects also had higher GM AEC than ND or treated patients (GM 2281 vs 250 vs 907 Eos/μL, Figure 2B). Serum levels of sSiglec-7 were weakly but significantly correlated with AEC (n=135, r = +0.23, P<0.001, Figure 2C). However, there was no significant correlation between sSiglec-7 levels in serum and surface expression of Siglec-7 on blood eosinophils for the 32 subjects tested including 11ND and 21EOS (r=−0.14, P=0.42; Figure 2D). Levels of serum sSiglec-7 were comparable in untreated subjects of different HES subtypes, with the exception of MHES subjects who had significantly higher sSiglec-7 levels than ND (942 vs 270 pg/mL respectively, Figure 2E). Of note, GM AEC was the highest in this HES subgroup (Figure 2F), and sSiglec-7 levels and AEC were correlated (r= +0.6, P<0.05, n=15, data not shown).
Figure 2. Serum levels of soluble Siglec-7.
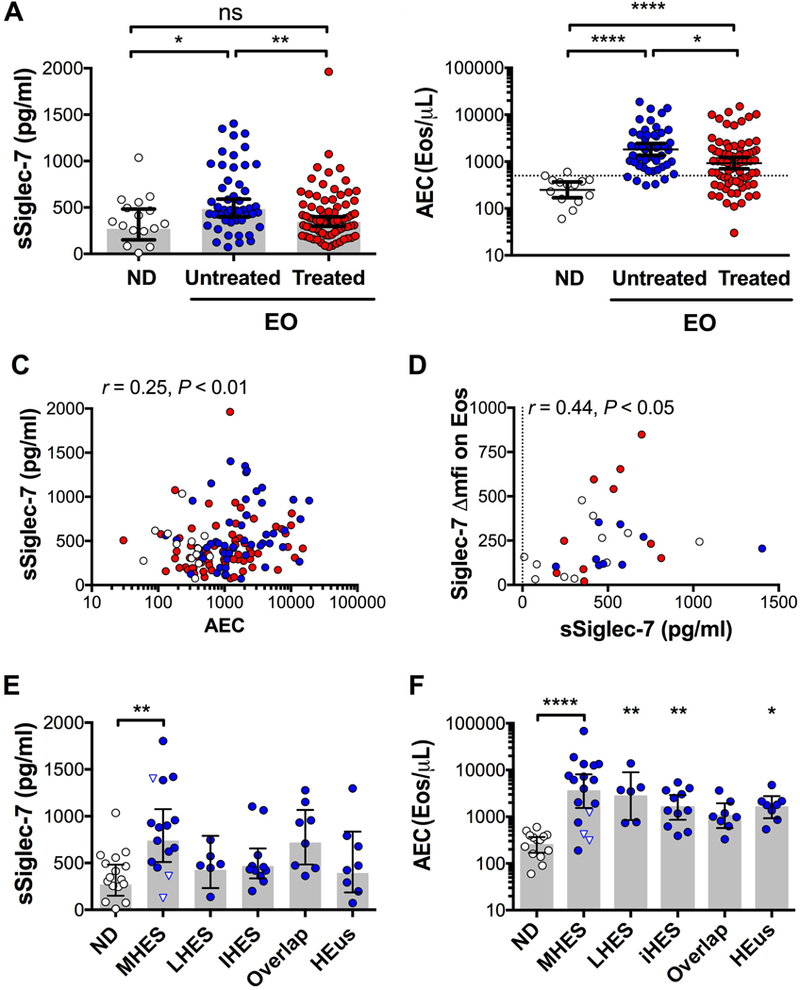
A and B, sSiglec-7 levels in serum (left) and AEC (right) from ND and EO subjects. C and D, Comparison of sSiglec-7 levels (left) and AEC (right) between EO clinical subgroups in untreated patients (blue), inverted triangles indicate FIP1L1-PDGFRAneg MHES. ND are shown as a control. E, Correlation of sSiglec-7 levels with AEC. F, Correlation with surface expression of Siglec-7 quantified on blood eosinophils by flow cytometry. Symbols represent individual subjects (white = ND, blue = untreated EO, red = EO on treatment). The grey shading indicates GM. Error bars represent 95% CI. *P < .05; **P < .001; ***P< .0001, ****P< .00001
Modulation of Siglec-7 expression in vitro
Geometric mean surface expression of Siglec-7 on purified blood eosinophils increased from an ΔMFI of 67.3 to 101.1 and 100.8 after an overnight incubation with GM-CSF or IL-5 (10 ng/mL), respectively (P <0.05, n=7; Figure 3A). Other stimuli, including IL-33, SEB (Figure 3A), E. Coli extracts, rhIFN-α and rhIFN-γ, had no detectable effect on Siglec-7 surface expression (n=6, Supplementary data Figure 2). Similarly, SIGLEC-7 mRNA levels significantly increased from 7.0 to 14.5 A.U. x10−5 in purified eosinophils incubated overnight with GM-CSF (P<0.05; n=7; Figure 3B).
Figure 3. Siglec-7 is upregulated by stimulation with GM-CSF or IL-5.
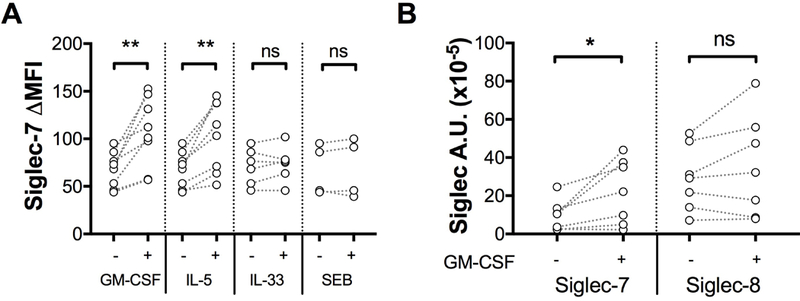
A, Surface expression of Siglec-7 after overnight incubation in media (−) or media supplemented with GM-CSF, IL-5, IL-33, or SEB at 10ng/mL (+). B, SIGLEC-7 and SIGLEC-8 mRNA levels in eosinophils after overnight incubation in media (−) or media supplemented with GM-CSF at 10ng/mL (+). *P < .05 (n=7). Symbols represent individual subjects (white = ND). **P<0.01
Effect of Siglec-7 engagement on eosinophil viability and function
In contrast to anti-Siglec-8 antibodies, which induced eosinophil apoptosis, as assessed by total Annexin-V staining, after overnight priming with GM-CSF or IL-5 (10ng/mL), anti-Siglec-7 antibodies had no effect on eosinophil apoptosis under the same conditions (n=5; Figure 4). The % of 7-AAD+ eosinophils was also unchanged in response to anti-Siglec-7 (data not shown).
Figure 4. Siglec-7 does not induce eosinophil cell death.
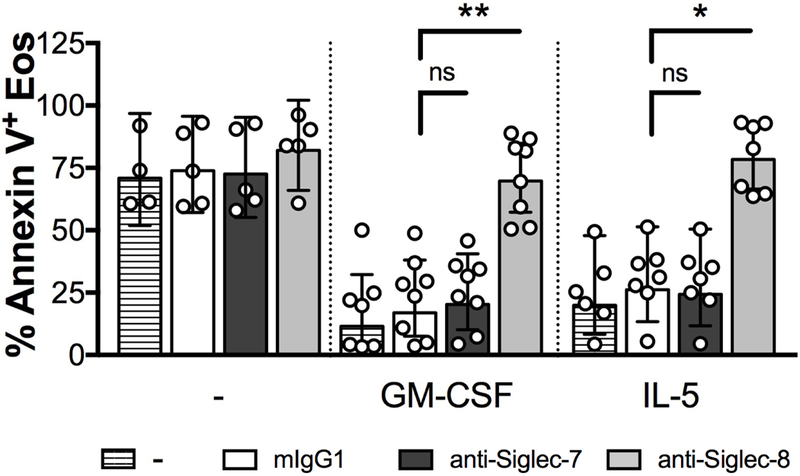
A, Eosinophil cell death after overnight incubation with GM-CSF or IL-5, eosinophils were incubated with anti-Siglec antibodies (10μg/mL) for 18hrs. Cell death was evaluated by Annexin-V/7-AAD staining and FACS analysis. Data are expressed as % geomean with 95% CI (n = 8). Symbols represent individual subjects (white = ND). *P<0.05, **P<0.01
Crosslinking of Siglec-7 in the presence of GM-CSF reduced eosinophil activation in vitro, as assessed by surface expression of CD69 (Figure 5A, n=6, P<0.05). Crosslinking of Siglec-7, but not Siglec-8, on eosinophils also significantly inhibited the release of EPX, TNFα, and IL-8 induced by GM-CSF (50 ng/mL) compared to the isotype control (mIgG1) (n=7–8; Wilcoxon test, Figure 5 B, C, D).
Figure 5. Crosslinking of Siglec-7 inhibits GM-CSF-induced eosinophil activation.
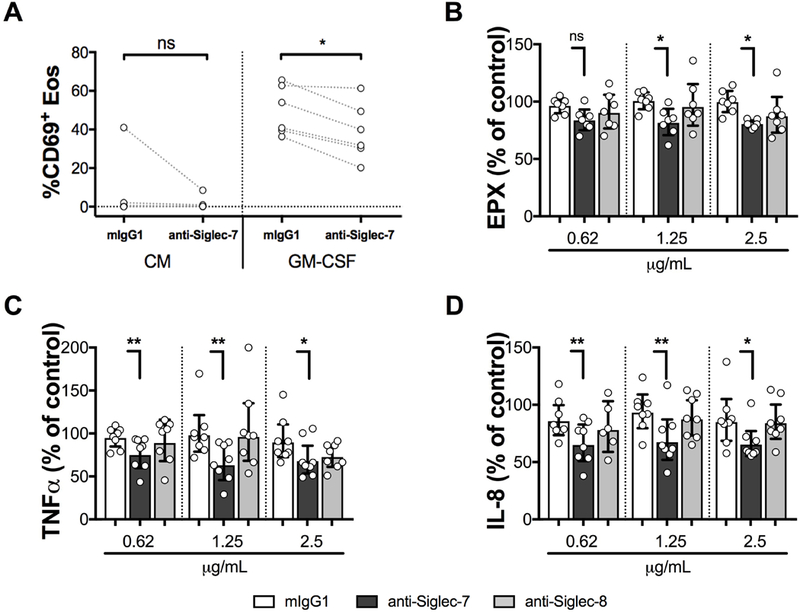
A, Eosinophil surface expression of CD69 after overnight incubation in culture medium (CM) +/− GM-CSF (50ng/mL). The antibodies were crosslinked with F(ab’)2 goat anti-mouse (10 μg/mL). Measurement of EPX (B), TNFα (C) and IL-8 (D) in culture supernatants following crosslinking of isotype control (white bars), anti-Siglec-7 (dark gray bars), or anti-Siglec-8 (light gray bars) at antibody concentrations ranging from 0.62–2.5 μg/mL on eosinophils for 40 minute (EPX), or overnight (TNFα and IL-8) in the presence of GM-CSF (n=7–8). Mediator release is expressed as the % of the level measured in the control well containing 50 ng/mL GM-CSF alone. Data are expressed as % GM with 95% CI. *P < .05; **P < .001; Wilcoxon matched-pairs signed rank test. Symbols represent individual subjects (white = ND).
Activation of Siglec-7 on eosinophils induces SHP-1 phosphorylation and decreases MAPK phosphorylation in a time-dependent fashion
Siglec-7 crosslinking of GM-CSF-activated eosinophils showed a trend towards induction of a time-dependent phosphorylation of SHP-1 (Figure 6A and 6B; p=0.054, rank trend test). Conversely, GM-CSF induction of phosphorylation of ERK½ and p38 (Figure 6C, 6D and 6E) were reduced by Siglec-7 crosslinking in a time-dependent manner, although this was not statistically significant. Notably, eosinophils incubated with anti-Siglec-7 alone displayed a basal phosphorylated state. Activation of CD300a (positive control) produced a pattern similar to that seen with Siglec-7.
Figure 6. Activation of Siglec-7 on eosinophils induces SHP-1 phosphorylation and reduces MAPK phosphorylation in a time-dependent fashion.
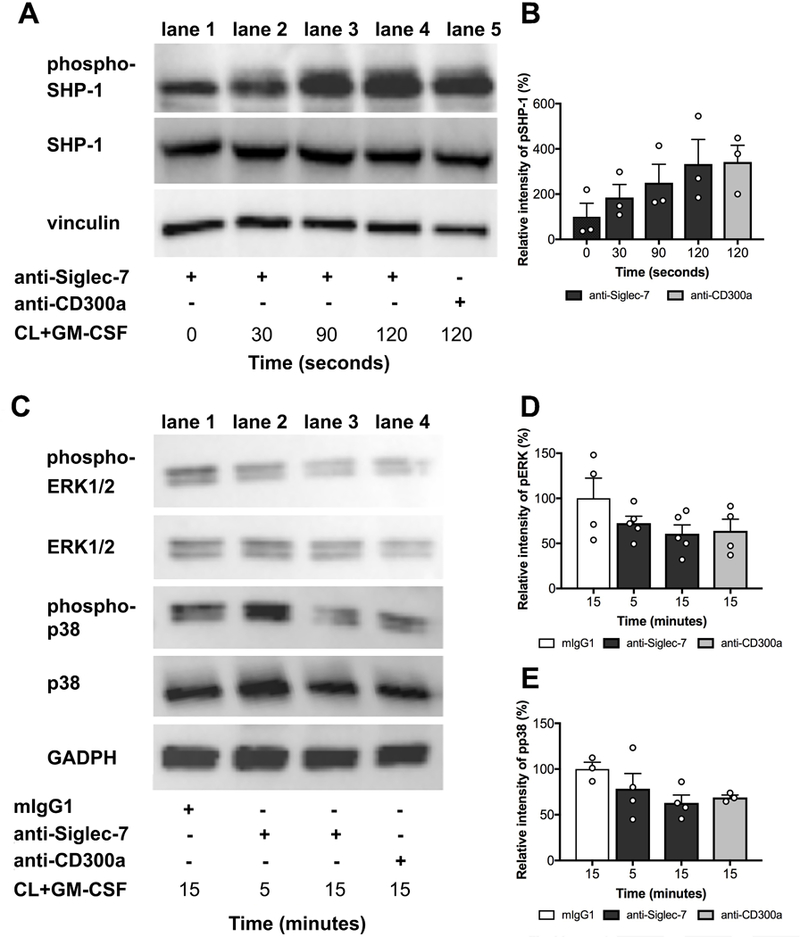
Western blot detecting phosphorylation of SHP-1 (A and B), ERK1/2 and p38 (C, D and E). Eosinophils were incubated with IgG1, anti-Siglec-7 or CD300a antibodies (10μg/mL) followed by crosslinking (CL) in the presence of GM-CSF (n=4). Phosphorylation of SHP-1 and MAPK (ERK1/2 and p38) was determined in the time interval of 0–120 sec and 5–15 min respectively. Vinculin and GAPDH were used as loading controls. Western blots shown are representative of 4 independent experiments performed on normal donors; pooled data are shown in the densitometry graphs. Relative intensity of phospho-SHP-1 (B), phospho-ERK1/2 (D) and phospho-p38 (E) are shown. Symbols represent individual subjects (white = ND).
Discussion
Inhibitory receptors (IRs) play important roles in modulating the function of innate immune cells, including eosinophils and mast cells, and have been proposed as therapeutic targets in allergic inflammation26. Siglec-7 is an IR expressed on a wide variety of human hematopoietic cells including NK cells, monocytes27, mast cells, basophils and eosinophils13,17. Decreased expression of Siglec-7 on selected cell populations has been reported in obesity28 and diabetes29 and has been associated with impaired NK cell function during chronic viral infections30,31. Although Siglec-7 expression was previously demonstrated on eosinophils13, the regulation of eosinophil expression of Siglec-7 in eosinophilic disorders and the functional consequences of Siglec-7 engagement on eosinophils has been largely unexplored.
In the present study, Siglec-7 expression was examined in normal donors and a large, heterogeneous cohort of eosinophilic subjects. Unlike Siglec-8, surface expression of Siglec-7 on eosinophils was positively correlated with absolute eosinophil count and was upregulated in vitro by IL-5 and GM-CSF, cytokines involved in eosinophilopoiesis and survival, but not by any of the other stimuli examined. These data, together with the finding that serum levels of sSiglec-7 are increased in eosinophilic donors, suggest that expression of Siglec-7 is actively regulated on the surface of eosinophils and may play a role in down-modulating eosinophil activation in the setting of allergic inflammation.
In support of this hypothesis, antibody-induced Siglec-7 crosslinking inhibited GM-CSF-mediated release of EPX, IL-8 and TNFα. This suppression of mediator release is similar to what has been described in response to crosslinking of Siglec-7 on anti-IgE-activated human cord blood derived mast cells17 and may be important in the context of the “allergic effector unit” where both mast cells and eosinophils interact via physical and soluble mechanisms32. A similar inhibition of mediator release has been reported with activation of the inhibitory receptor, CD300a, on eosinophils 13 and may be explained by the ability of these two IRs to phosphorylate common pathways with a critical role in signal transduction, such as SHP-1 . In this context, the failure of Siglec-7 to induce eosinophil apoptosis (unlike CD300a) may be due to relatively weaker SHP-1 recruitment by Siglec-718. In contrast to Siglec-7 and CD300a, Siglec-8, which signals through a pathway involving Akt, p38, and c-Jun N-terminal kinase 1, appears to function predominantly as an activating receptor on IL-5 primed eosinophils leading to increased release of ROS and eosinophil apoptosis33.
The major limitation of the present study was the technical challenges of working with human eosinophils, which are present in relatively low numbers in peripheral blood of normal individuals and do not survive in culture without the addition of exogenous cytokines. Moreover, eosinophils purified from different individuals exhibit differences in the basal activation state. Unfortunately, Siglec-E, the murine ortholog of Siglec-7 and Siglec-9, is not expressed on murine eosinophils34 and humanized mouse models expressing Siglec-7 on eosinophils are unavailable at the present time.
In summary, Siglec-7 was constitutively expressed on eosinophils from all normal and eosinophilic donors tested, and expression was increased in the context of eosinophilia and by stimulation with cytokines that lead to eosinophilia and eosinophil activation.
Although the effect of Siglec-7 on eosinophil function was relatively modest, it appeared to be increased by eosinophil activation and was similar to the effect previously described on mast cell activation17. Whether targeting Siglec-7 (alone or in combination with other IRs) would be useful as an additional means of reducing allergic inflammation remains unknown. Conversely, the effects of agents currently in development designed to block Siglec-7 function in the setting of tumors may have unexpected effects on eosinophil and mast cell activation.
Supplementary Material
Key Messages.
Siglec-7 is expressed at comparable levels on blood eosinophils from normal and eosinophilic subjects and expression correlates with AEC or soluble Siglec-7 levels in serum.
Siglec-7 inhibits eosinophil activation but does not affect survival.
Acknowledgments
The authors would like to thank Michael Fay, Biostatistics Research Branch, NIAID, NIH for his assistance with the statistical analyses.
Funding
This study was funded in part by the Division of Intramural Research, NIAID, NIH. (AK) and in part by the Aimwell Charitable Trust Gutterman Funds and by the Israel Basic Science Foundation (FLS).
Abbreviations
- AEC
Absolute eosinophil count
- CL
Crosslinker
- EO
Eosinophilic donors
- Eos
Eosinophils
- EPX
Eosinophil peroxidase
- E. Coli
Escherichia coli
- GM
Geometric mean
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- HES
Hypereosinophilic syndromes
- IL
Interleukin
- IR
Inhibitory receptor
- ITAM
Immunoreceptor tyrosine-based activating motifs
- ITIM
Immunoreceptor tyrosine-based inhibitory motif
- mAbs
Monoclonal Antibodies
- NK
Natural killer cells
- ND
Normal donors
- Rh
Recombinant human
- RT
Room temperature
- SEB
Staphylococcus aureus Enterotoxin B
- SHP-1
Src homology-2 (SH2) domain-containing protein-tyrosine phosphatase-1
- Siglec
Sialic acid binding Ig-like lectin
- TNFα
Tumor necrosis factor alpha
Footnotes
Competing interests
None of the authors report any conflicts of interest related to this work.
Bibliography
- 1.Gangwar RS, Landolina N, Arpinati L, Levi-Schaffer F. Mast cell and eosinophil surface receptors as targets for anti-allergic therapy. Pharmacol Ther 2017;170:37–63. [DOI] [PubMed] [Google Scholar]
- 2.Legrand F, Klion AD. Biologic therapies targeting eosinophils: current status and future prospects. J Allergy Clin Immunol Pract 2015;3:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landolina N, Levi-Schaffer F. Monoclonal antibodies: the new magic bullets for allergy: IUPHAR Review 17. Br J Pharmacol 2016;173:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev 2017;9:CD010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N Engl J Med 2017;376:1921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochner BS, Zimmermann N. Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. J Allergy Clin Immunol 2015;135:598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zenarruzabeitia O, Vitallé J, Eguizabal C, Simhadri VR, Borrego F. The biology and disease relevance of cd300a, an inhibitory receptor for phosphatidylserine and phosphatidylethanolamine. J Immunol 2015;194:5053–60. [DOI] [PubMed] [Google Scholar]
- 8.Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol Ther 2012;135:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokoi H, Choi OH, Hubbard W, Lee H-S, Canning BJ, Lee HH, et al. Inhibition of FcepsilonRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J Allergy Clin Immunol 2008;121:499–505.e1. [DOI] [PubMed] [Google Scholar]
- 10.Kikly KK, Bochner BS, Freeman SD, Tan KB, Gallagher KT, D’alessio KJ, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J Allergy Clin Immunol 2000;105:1093–100. [DOI] [PubMed] [Google Scholar]
- 11.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood 2003;101:5014–20. [DOI] [PubMed] [Google Scholar]
- 12.Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun 2005;336:918–24. [DOI] [PubMed] [Google Scholar]
- 13.Munitz A, Bachelet I, Eliashar R, Moretta A, Moretta L, Levi-Schaffer F. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood 2006;107:1996–2003. [DOI] [PubMed] [Google Scholar]
- 14.Bachelet I, Munitz A, Moretta A, Moretta L, Levi-Schaffer F. The inhibitory receptor IRp60 (CD300a) is expressed and functional on human mast cells. J Immunol 2005;175:7989–95. [DOI] [PubMed] [Google Scholar]
- 15.Nicoll G, Ni J, Liu D, Klenerman P, Munday J, Dubock S, et al. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J Biol Chem 1999;274:34089–95. [DOI] [PubMed] [Google Scholar]
- 16.Falco M, Biassoni R, Bottino C, Vitale M, Sivori S, Augugliaro R, et al. Identification and molecular cloning of p75/AIRM1, a novel member of the sialoadhesin family that functions as an inhibitory receptor in human natural killer cells. J Exp Med 1999;190:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizrahi S, Gibbs BF, Karra L, Ben-Zimra M, Levi-Schaffer F. Siglec-7 is an inhibitory receptor on human mast cells and basophils. J Allergy Clin Immunol 2014;134:230–3. [DOI] [PubMed] [Google Scholar]
- 18.Yamaji T, Mitsuki M, Teranishi T, Hashimoto Y. Characterization of inhibitory signaling motifs of the natural killer cell receptor Siglec-7: attenuated recruitment of phosphatases by the receptor is attributed to two amino acids in the motifs. Glycobiology 2005;15:667–76. [DOI] [PubMed] [Google Scholar]
- 19.Jia Y, Yu H, Fernandes SM, Wei Y, Gonzalez-Gil A, Motari MG, et al. Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J Allergy Clin Immunol 2015;135:799–810.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaji T, Teranishi T, Alphey MS, Crocker PR, Hashimoto Y. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to alpha 2,8-disialyl and branched alpha 2,6-sialyl residues. A comparison with Siglec-9. J Biol Chem 2002;277:6324–32. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki K, Sakuma K, Kawamura YI, Izawa M, Ohmori K, Mitsuki M, et al. Colonic epithelial cells express specific ligands for mucosal macrophage immunosuppressive receptors siglec-7 and −9. J Immunol 2012;188:4690–700. [DOI] [PubMed] [Google Scholar]
- 22.Jandus C, Boligan KF, Chijioke O, Liu H, Dahlhaus M, Démoulins T, et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J Clin Invest 2014;124:1810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prescher H, Frank M, Gütgemann S, Kuhfeldt E, Schweizer A, Nitschke L, et al. Design, Synthesis, and Biological Evaluation of Small, High-Affinity Siglec-7 Ligands: Toward Novel Inhibitors of Cancer Immune Evasion. J Med Chem 2017;60:941–56. [DOI] [PubMed] [Google Scholar]
- 24.Legrand F, Tomasevic N, Simakova O, Lee C-CR, Wang Z, Raffeld M, et al. The eosinophil surface receptor epidermal growth factor-like module containing mucin-like hormone receptor 1 (EMR1): a novel therapeutic target for eosinophilic disorders. J Allergy Clin Immunol 2014;133:1439–47, 1447.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adamko DJ, Wu Y, Ajamian F, Ilarraza R, Moqbel R, Gleich GJ. The effect of cationic charge on release of eosinophil mediators. J Allergy Clin Immunol 2008;122:383–90, 390.e1. [DOI] [PubMed] [Google Scholar]
- 26.Munitz A, Levi-Schaffer F. Inhibitory receptors on eosinophils: a direct hit to a possible Achilles heel? J Allergy Clin Immunol 2007;119:1382–7. [DOI] [PubMed] [Google Scholar]
- 27.Varchetta S, Brunetta E, Roberto A, Mikulak J, Hudspeth KL, Mondelli MU, et al. Engagement of Siglec-7 receptor induces a pro-inflammatory response selectively in monocytes. PLoS ONE 2012;7:e45821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenstock P, Horstkorte R, Gnanapragassam VS, Harth J, Kielstein H. Siglec-7 expression is reduced on a natural killer (NK) cell subset of obese humans. Immunol Res 2017;65:1017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dharmadhikari G, Stolz K, Hauke M, Morgan NG, Varki A, de Koning E, et al. Siglec-7 restores β-cell function and survival and reduces inflammation in pancreatic islets from patients with diabetes. Sci Rep 2017;7:45319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varchetta S, Mele D, Lombardi A, Oliviero B, Mantovani S, Tinelli C, et al. Lack of Siglec-7 expression identifies a dysfunctional natural killer cell subset associated with liver inflammation and fibrosis in chronic HCV infection. Gut 2016;65:1998–2006. [DOI] [PubMed] [Google Scholar]
- 31.Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, et al. The decreased expression of Siglec-7 represents an early marker of dysfunctional natural killer-cell subsets associated with high levels of HIV-1 viremia. Blood 2009;114:3822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landolina N, Gangwar RS, Levi-Schaffer F. Mast cells’ integrated actions with eosinophils and fibroblasts in allergic inflammation: implications for therapy. Adv Immunol 2015;125:41–85. [DOI] [PubMed] [Google Scholar]
- 33.Carroll DJ, O’Sullivan JA, Nix DB, Cao Y, Tiemeyer M, Bochner BS. Sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8) is an activating receptor mediating β2-integrin-dependent function in human eosinophils. J Allergy Clin Immunol 2017;8;141:2196–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol 2004;34:1175–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


