Abstract
Background & Aims:
Therapeutic drug monitoring (TDM) is widely available for biologic therapies in patients with inflammatory bowel disease (IBD). We reviewed current data and provided expert opinion regarding the clinical utility of TDM for biologic therapies in IBD.
Methods:
We used a modified Delphi method to establish consensus. A comprehensive literature review was performed regarding the use of TDM of biologic therapy in IBD and presented to international IBD specialists. Subsequently, 28 statements on the application of TDM in clinical practice were rated on a scale of 1 to 10 (1=strongly disagree and 10=strongly agree) by each of the panellists. Statements were accepted if 80% or more of the participants agreed with a score ≥7. The remaining statements were discussed and revised based on the available evidence followed by a second round of voting.
Results:
The panel agreed on 24 (86%) statements. For anti-tumor necrosis factor (anti-TNF) therapies, proactive TDM was found to be appropriate after induction and at least once during maintenance therapy, but this was not the case for the other biologics. Reactive TDM was appropriate for all agents both for primary non-response and secondary loss of response. The panellists also agreed on several statements regarding TDM and appropriate drug and anti-drug antibody (ADA) concentration thresholds for biologics in specific clinical scenarios.
Conclusion:
Consensus was achieved towards the utility of TDM of biologics in IBD, particularly anti-TNF therapies. More data are needed especially on non-anti-TNF biologics to further define optimal drug concentration and ADA thresholds as these can vary depending on the therapeutic outcomes assessed.
Keywords: consensus statement, Crohn’s disease, ulcerative colitis, immunogenicity, anti-TNF, vedolizumab, ustekinumab
INTRODUCTION
Biologic therapies, including the anti-tumor necrosis factor (anti-TNF) agents (infliximab, adalimumab, certolizumab pegol and golimumab), the adhesion molecule inhibitors (vedolizumab and natalizumab), and the p-40 interleukin-12/23 inhibitor ustekinumab, are effective treatments for patients with moderate to severe inflammatory bowel disease (IBD).1, 2 Nevertheless, up to 1/3 of patients with Crohn’s disease (CD) and ulcerative colitis (UC) show primary non-response (PNR) to biologic therapies and up to 50% of patients after an initial clinical response stop therapy either for secondary loss of response (SLR) or a serious adverse event.3, 4 Both PNR and SLR are due to either pharmacokinetic (PK) or pharmacodynamic (PD) problems. PK issues are associated with inadequate drug exposure, often due to the development of anti-drug antibodies (ADA), whereas PD issues are typically related to inflammatory process unrelated to the targeted immunoinflammatory pathway.5, 6
Numerous studies have demonstrated a positive correlation between serum biologic drug concentrations and favorable therapeutic outcomes, while low or undetectable drug concentrations can lead to immunogenicity and treatment failure (Tables 1–3 and supplementary table 1).7–95 Therapeutic drug monitoring (TDM), defined as the assessment of drug concentrations and ADA, is an important tool for optimizing biologic therapy. Reactive TDM has rationalized the management of PNR and SLR and has proven more cost-effective when compared to empiric dose escalation.96–102 Preliminary data suggest that proactive TDM, with drug titration to a target trough concentration, performed in patients with clinical response/remission can also improve the efficacy of anti-TNFs.38, 39, 103, 104 Moreover, proactive TDM may also improve the cost-effectiveness and safety of biologic therapy via the implementation of a de-escalation strategy in patients with supra-therapeutic drug concentrations by reducing the dose, increasing the time interval and/or stopping the immunomodulator in patients on combination therapy (optimized monotherapy).39, 82, 105–107
Table 1.
Serum adalimumab concentration thresholds associated with therapeutic outcomes in inflammatory bowel disease.
| IBD type | Threshold (μg/ml) | Therapeutic outcome | TDM assay | Assay type | Ref. |
|---|---|---|---|---|---|
| Induction (week 2) | |||||
| CD | >6.7 | Clinical remission (w14) | ELISA | AHLC | 23 |
| Post-induction (week 4) | |||||
| CD | >5 | Drug retention | HMSA | Prometheus | 29 |
| CD | >12 | Normal CRP (≤5mg/L) | ELISA | LFA/ELISA (R-Biopharm AG) | 31 |
| UC | ≥7.5 | Mucosal healing (w10–14) | ELISA | Leuven assay | 30 |
| UC | >4.6 | Clinical response (w12) | ELISA | Leuven assay | 26 |
| UC | >7 | Clinical response (w52) | ELISA | Leuven assay | 26 |
| Maintenance | |||||
| CD | >5.9 | Normal CRP (≤5mg/L) | ELISA | AHLC | 15 |
| CD | >5.9 | Normal CRP (≤3mg/L) | ELISA | Sumitomo Bakelite Co Ltd | 16 |
| CD | >8.1 | Mucosal healing | HMSA | Prometheus | 18 |
| CD | >5.6 | Normal CRP (≤3mg/L) | ELISA | In-house | 19 |
| CD | >7.9 | Mucosal healing | ELISA | In-house | 19 |
| CD | >10.3 | Mucosal healing | ELISA | In-house | 20 |
| CD | >5 (w26) | Clinical remission (w52) | ELISA | Sanquin Diagnostics | 21 |
| CD | ≥12 | Endoscopic remission | HMSA | Prometheus | 22 |
| CD | ≥12.2 | Histologic remission | HMSA | Prometheus | 22 |
| CD | ≥3.7 (w14) | CRP normalization (w14) | ELISA | AHLC | 23 |
| CD/UC | >6.6 | Normal CRP (≤5mg/L) | ELISA | AHLC | 13 |
| CD/UC | ≥6.9 | No SLR | RIA | Biomonitor A/S | 14 |
| CD/UC | >7.1 | Mucosal healing | ELISA | AHLC | 13 |
| CD/UC | >4.9 | Mucosal healing | ELISA | Theradiag | 9 |
| CD/UC | >7.8 | Histologic remission | HMSA | Prometheus | 12 |
| CD/UC | >7.5 | Mucosal healing | HMSA | Prometheus | 12 |
| CD/UC | >12.2 | Successful dose reduction | ELISA | Promonitor Grifols | 11 |
| CD/UC | >9 | Clinical response | ELISA | Promonitor Grifols | 11 |
| CD/UC | >6.6 | Normal CRP (≤5mg/L) | ELISA | Promonitor Grifols | 11 |
| CD/UC | >4.5 | When SLR, better long-term outcome when change to a biological with a different mechanism of action compare to anti-TNF dosage increase or a switch within class | ELISA | AHLC | 10 |
| CD/UC | ≥3 | No active inflammationa | ELISA | AHLC | 10 |
| CD/UC | >4.9 | When SLR, high risk of failure who subsequently after changing to infliximab | ELISA | Theradiag | 8 |
| CD/UC | >7.3 | Clinical remission | ELISA | New Zealand assay | 7 |
defined as increased CRP level and/or endoscopic/imaging documentation of inflammation.
ELISA: enzyme-linked immunosorbent assay; HMSA: homogeneous mobility shift assay; CRP: C-reactive protein, TDM: therapeutic drug monitoring; RIA: Radioimmunoassay; SLR: secondary loss of response; TNF: tumor necrosis factor; CD: Crohn’s disease; UC: ulcerative colitis; LFA: lateral flow-based assay; Ref.: references, AHLC: antihuman lambda chain.
Table 3.
Association of anti-drug antibodies with therapeutic outcomes in inflammatory bowel disease.
| Drug | IBD type | ADA | Therapeutic outcome | TDM assay | Assay type | Ref. | |
|---|---|---|---|---|---|---|---|
| IFX | CD | ≥282 ng/mL-eq | Lower success rate of treatment optimization | ELISA | Leuven drug-tolerant assay | 75 | |
| IFX | CD | >8 μg/mL-eq | Shorter clinical response | ELISA | Prometheus | 28 | |
| IFX | CD | Detectable | Lack of mucosal healing | ELISA | MP Biomedicals | 17 | |
| IFX | CD | Detectable | Elevated CRP (>5 mg/L) | HMSA | Prometheus | 56 | |
| IFX | CD | Detectable | Elevated CPP (>5 mg/L) | HMSA | Prometheus | 60 | |
| IFX | CD | Detectable | Lack of fistula healing | HMSA | Prometheus | 12 | |
| IFX | CD | Detectable | SLR | ELISA | Prometheus | 88 | |
| IFX | CD | Detectable | SLR | RIA | Biomonitor A/S | 87 | |
| IFX | UC | Detectable | Lack of endoscopic response | HMSA | Prometheus | 33 | |
| IFX | UC | Detectable | Lack of mucosal healing | ELISA | Leuven drug-tolerant assay | 67 | |
| IFX | CD/UC | ≥8.8 U/ml | Drug discontinuation | HMSA | Prometheus | 86 | |
| IFX | CD/UC | Detectable | PNR | ELISA | AHLC | 73 | |
| IFX | CD/UC | Detectable | Drug discontinuation | HMSA | Prometheus | 63 | |
| IFX | CD/UC | >9.1 U/ml | Failure of dose intensification after SLR | HMSA | Prometheus | 63 | |
| IFX | CD/UC | >12 U/mL | Surgery | HMSA | Prometheus | 85 | |
| IFX | CD/UC | Undetectable | Mucosal healing | ELISA | AHLC | 13 | |
| IFX | CD/UC | Undetectable | Short-term clinical response | HMSA | Prometheus | 27 | |
| IFX | CD/UC | Detectable | SLR | ELISA | AHLC | 32 | |
| IFX | CD/UC | Detectable | SLR | ELISA | AHLC | 84 | |
| IFX | CD/UC | >9 μg/mL-eq | When SLR, longer duration of response when anti-TNF agents are switched than when dosage is increased | ELISA | AHLC | 10 | |
| IFX | CD/UC | ≥3.3 U/mL | Lack of post-adjustment endoscopic remission | HMSA | Prometheus | 37 | |
| IFX | CD/UC | Detectable | Treatment related adverse events | ELISA | Promonitor Menarini / ImmunDiagnostik | 83 | |
| IFX | CD/UC | Detectablea | PNR (w14) | ELISA | AHLC | 73 | |
| IFX | CD/UC | >4.3 μg/mL-eqb | PNR (w14) | ELISA | AHLC | 73 | |
| IFX | CD/UC | >9.1 U/mL | IFX discontinuation | HMSA | Prometheus | 82 | |
| IFX | CD/UC | >9.1 U/mL | Infusion reactions | HMSA | Prometheus | 82 | |
| IFX | CD/UC | >200 ng/mL-eq | No response to treatment optimization | ELISA | Theradiag | 81 | |
| ADM | CD | Detectable | PNR | ELISA | AHLC | 23 | |
| ADM | CD | Detectable | Drug discontinuation | HMSA | Prometheus | 29 | |
| ADM | CD | Detectable | Drug discontinuation | ELISA | In-house | 57 | |
| ADM | CD | >12 U/mL | Lack of clinical response | RIA | Biomonitor A/S | 58 | |
| ADM | CD | Detectable | Active disease | ELISA | AHLC | 15 | |
| ADM | CD | Detectable | Higher CRP and ESR | ELISA | Sumitomo Bakelite Co., Ltd | 16 | |
| ADM | CD | Detectabled | No clinical remission (w52) | RIA | Sanquin | 21 | |
| ADM | CD | Detectable (w12) | Higher needs for dose escalation less frequently sustained clinical benefit due to PNR or SLR | ELISA | R-Biopharm AG | 31 | |
| ADM | CD/UC | Detectable | Drug discontinuation | RIA | Biomonitor A/S | 80 | |
| ADM | CD/UC | >4 μg/mL-eq | When SLR, longer duration of response when anti-TNF agents are switched than when dosage is increased | ELISA | AHLC | 10 | |
| ADM | CD/UC | Detectable | SLR | RIA | Biomonitor A/S | 14 | |
either week 2 or 6;
week 2;
Université François-Rabelais, Immuno-Pharmaco-Genetics of Therapeutic Antibodies, Tours, France;
week 26.
ADA: anti-drug antibody; IFX: infliximab; ADM: adalimumab; ELISA: enzyme-linked immunosorbent assay; CD: Crohn’s disease; UC: ulcerative colitis; CRP: C-reactive protein; RIA: Radio-immunoassay; eq: equivalent; SLR: secondary loss of response; U: units; HMSA: homogeneous mobility shift assay; ESR: erythrocyte sedimentation rate; AHLC: antihuman lambda chain antibody; TDM: therapeutic drug monitoring; TNF: tumor necrosis factor; w: week; PNR: primary non-response; Ref.: references.
However, there are still some limitations when applying TDM into clinical practice, such as when to utilize TDM, proper interpretation and application of the results, and the identification of the optimal window/thresholds to target. These therapeutic windows or thresholds appear to vary based on the outcome of interest and the IBD phenotype (Tables 1 and 2 and supplementary table 1). Moreover, most of the data on implementation of TDM refer to anti-TNF therapies and the maintenance phase of treatment.
Table 2.
Association of serum certolizumab pegol, golimumab, vedolizumab and ustekimumab concentration thresholds with therapeutic outcomes in inflammatory bowel disease.
| IBD type | Time point | Threshold (μg/ml) | Therapeutic outcome | TDM assay | Assay type | Ref. | |
|---|---|---|---|---|---|---|---|
| A. Certolizumab pegol | |||||||
| CD | Post-induction (w6) | >31.8 | Clinical response/remission (w6) | ELISA | UCB Pharma | 94 | |
| CD | Post-induction (w6) | >31.9 | Normal CRP (≤5mg/L) (w6) | ELISA | UCB Pharma | 94 | |
| CD | Post-induction (w6) | >32.7 | Normal FC (<250mg/g) (w6) | ELISA | UCB Pharma | 94 | |
| CD | Post-induction (w6) | >34.5 | Normal FC (<250mg/g) and CDAI (≤150) (w6) | ELISA | UCB Pharma | 94 | |
| CD | Post-induction (w6) | >36.1 | Normal FC (<250mg/g) and CDAI (≤150) (w26) | ELISA | UCB Pharma | 94 | |
| CD | Post-induction (w8) | >23.3 | Endoscopic remission (w10) | ELISA | UCB Pharma | 95 | |
| CD | Maintenance (w12) | >13.8 | Normal FC (<250mg/g) (w26) | ELISA | UCB Pharma | 94 | |
| CD | Maintenance (w12) | >14.8 | Normal FC (<250mg/g) and CDAI (≤150) (w26) | ELISA | UCB Pharma | 94 | |
| B. Golimumab | |||||||
| UC | Induction (w2) | >8.9 | Clinical response (w6) | ECLIA | Janssen Biotech Inc | 48 | |
| UC | Post-induction (w4) | >7.4 | Clinical response (w6) | ECLIA | Janssen Biotech Inc | 48 | |
| UC | Post-induction (w6) | >2.5 | Clinical response (w6) | ECLIA | Janssen Biotech Inc | 48 | |
| UC | Post-induction (w6) | >2.6 | Partial clinical response (w14) | ELISA | In house Leuven | 93 | |
| UC | Maintenance (w28) | >0.9 | Clinical remission (w30 and 54) | ECLIA | Janssen Biotech Inc | 48 | |
| UC | Maintenance (w44) | >1.4 | Clinical remission (w30 and 54) | ECLIA | Janssen Biotech Inc | 48 | |
| C. Vedolizumab | |||||||
| CD | Induction (w2) | >35.2 | Biological remission (w6) | ELISA | Leuven assay | 90 | |
| UC | Induction (w2) | >28.9 | Clinical response (w14) | ELISA | Leuven assay | 90 | |
| UC | Induction (w2) | >23.7 | Mucosal healing (w14) | ELISA | Leuven assay | 90 | |
| CD/UC | Induction (w2) | ≥24.5 | No drug optimization (within w24) | ELISA | Theradiag | 92 | |
| UC | Induction (w6) | >20.8 | Clinical response (w14) | ELISA | Leuven assay | 90 | |
| CD/UC | Induction (w6) | ≥18.5 | No need for extended therapy | ELISA | Theradiag | 92 | |
| CD/UC | Induction (w6) | >27.5 | Sustained clinical response | ELISA | Theradiag | 92 | |
| CD/UC | Induction (w6) | >18 | Mucosal healing (within w54) | ELISA | Theradiag | 91 | |
| UC | Post-induction (w14) | >12.6 | Clinical response (w14) | ELISA | Leuven assay | 90 | |
| UC | Post-induction (w14) | >17 | Mucosal healing (w14) | ELISA | Leuven assay | 90 | |
| CD | Maintenance (w22) | >13.6 | Mucosal healing (w22) | ELISA | Leuven assay | 90 | |
| CD | Maintenance (w22) | >12 | Biological remission (w22) | ELISA | Leuven assay | 90 | |
| D. Ustekinumab | |||||||
| CD | Post-induction (w8) | >3.3 | Clinical remission (w8) | ECLIA | Janssen Biotech Inc | 49 | |
| CD | Maintenance | >4.5 | Endoscopic response | HMSA | Prometheus | 89 | |
| CD | Maintenance (w24)a | >0.8 | Clinical remission (w24) | ECLIA | Janssen Biotech Inc | 49 | |
| CD | Maintenance (w40)b | >1.4 | Clinical remission (w44) | ECLIA | Janssen Biotech Inc | 49 | |
Combined q8w and q12w;
q8w only.
ELISA: enzyme-linked immunosorbent assay; HMSA: homogeneous mobility shift assay; CRP: C-reactive protein, FC: fecal calprotectin; ECLIA: electrochemiluminescence immunoassay; w: week; TDM: therapeutic drug monitoring; CD: Crohn’s disease; UC: ulcerative colitis; CDAI: Crohn’s disease activity index; Ref.: reference.
We aimed to reach a consensus on when and how to utilize TDM of biologic therapies during different phases of the treatment (i.e. induction, post-induction, and maintenance therapy) and sought to identify clinically relevant drug concentrations and ADA thresholds to help physicians apply TDM in clinical practice.
METHODS
We applied a modified Delphi method to establish consensus similar to that described in the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) program.108 A comprehensive literature review was performed regarding the use of TDM of biologic therapies in IBD using PubMed and Medline databases. We utilized the search terms: ‘inflammatory bowel disease’; ‘Crohn’s disease’; ‘ulcerative colitis’; ‘anti-drug antibodies’; ‘therapeutic drug monitoring’ AND ‘infliximab’ OR ‘adalimumab’ OR ‘certolizumab pegol’ OR ‘golimumab’ OR ‘vedolizumab’ OR ‘ustekinumab’. The literature was then presented to a panel of 13 international IBD specialists. Subsequently, based on this review, 28 statements were formulated (K.P., A.S.C, C.A.S.) describing when and how to apply TDM in clinical practice. An Expert Consensus Development Meeting consisting of members of the BRIDGe group (www.BRIDGeIBD.com) and TDM specialists was held in New Orleans, on December 9, 2017, to refine and vote anonymously on the statements. Each statement was rated on a scale of 1 to 10 (1=strongly disagree, 10=strongly agree). Statements were accepted if 80% or more of the participants agreed with a score ≥7. If less than 80% of the panelists agreed with a score ≥7, statements were discussed and revised based on the available evidence followed by a second round of voting. The word ‘appropriate’ was used for each statement to suggest that application of TDM for treatment optimization in a particular clinical scenario is a good option. However, these are not recommendations applicable to every patient.
RESULTS
The panel reached consensus on 24 out of 28 (86%) statements (Tables 4 and 5).
Table 4.
Scenarios of applying therapeutic drug monitoring of biological therapy in patients with inflammatory bowel disease.
| Statement | Vote agreement, % |
|---|---|
| A. Anti-TNFs | |
| 1. It is appropriate to order drug/antibody concentration testing, in responders at the end of induction for all anti-TNFs. | 92 (12/13) |
| 2. It is appropriate to order drug/antibody concentration testing at least once during maintenance for patients on all anti-TNFs. | 100 (13/13) |
| 3. It is appropriate to order drug/antibody concentration testing of anti-TNFs at the end of induction in primary non-responders. | 100 (13/13) |
| 4. It is appropriate to order drug/antibody concentration testing for all anti-TNFs, in patients with confirmed secondary loss of response. | 100 (13/13) |
| B. Vedolizumab | |
| 5. It is appropriate to order drug/antibody concentration testing for vedolizumab, in responders at the end of induction. | 15 (2/13)a |
| 6. It is appropriate to order drug/antibody concentration testing at least once during maintenance for patients on vedolizumab. | 46 (6/13)a |
| 7. It is appropriate to order drug/antibody concentration testing for vedolizumab in non-responders at the end of induction. | 92 (12/13) |
| 8. It is appropriate to order drug/antibody concentration testing for vedolizumab, in patients with confirmed secondary loss of response. | 83 (10/12)a |
| C. Ustekinumab | |
| 9. It is appropriate to order drug/antibody concentration testing for ustekinumab, in responders at the end of induction. | 39 (5/13)a |
| 10. It is appropriate to order drug/antibody concentration testing at least once during maintenance for patients on ustekinumab. | 31 (4/13)a |
| 11. It is appropriate to order drug/antibody concentration testing for ustekinumab in non-responders at the end of induction (at 8 weeks). | 92 (12/13) |
| 12. It is appropriate to order drug/antibody concentration testing for ustekinumab, in patients with confirmed secondary loss of response. | 83 (10/12)a |
After a second round of voting.
TNF: tumor necrosis factor
Table 5.
Biological drug concentrations and anti-drug antibodies when applying therapeutic drug monitoring in inflammatory bowel disease.
| Statement | Vote agreement, % |
|---|---|
| A. General | |
| 13. There is no difference in indication for ordering drug/antibody concentrations or interpretation of results for biosimilars or the originator drug. | 100 (13/13) |
| 14. The threshold drug concentration may vary depending on disease phenotype and desired therapeutic outcome. | 100 (13/13) |
| 15. In the presence of adequate trough drug concentrations, anti-drug antibodies are unlikely to be clinically relevant. | 100 (12/12) |
| 16. Other than for anti-infliximab antibodies, there are not enough data to recommend a threshold for high anti-drug antibody titers for the biologic drugs. | 100 (12/12) |
| B. Infliximab | |
| 17. The current evidence suggests that the variability of infliximab concentrations between the different assays is unlikely to be clinically significant. | 100 (13/13)a |
| 18. There is insufficient evidence that inter-assay drug concentration results are comparable for biologic drugs other than for infliximab. | 100 (13/13) |
| 19. The minimal trough concentration for infliximab post-induction at week 14 should be greater than 3 μg/ml, and concentrations greater than 7 μg/ml are associated with an increased likelihood of mucosal healing. | 100 (13/13) |
| 20. During maintenance the minimal trough concentration for infliximab for patients in remission should be greater than 3 μg/ml. For patients with active disease infliximab should generally not be abandoned unless drug concentrations are greater than 10 μg/ml. | 92 (12/13) |
| 21. In the absence of detectable infliximab, high titer anti-infliximab antibodies require a change of therapy. Low level antibodies can sometimes be overcome. For the ANSER assay, a high titer anti-infliximab antibody at trough is defined as 10 U/ml, for RIDAscreen the cut-off is 200 ng/ml, for InformTx/Lisa Tracker the cut-off is 200 ng/ml. For other assays, there is insufficient data to define an adequate cut-off for a high titer anti-infliximab antibody. | 100 (13/13) |
| C. Adalimumab | |
| 22. The minimum drug concentration at week 4 for adalimumab should at least be 5 μg/ml. Drug concentrations greater than 7 μg/ml are associated with an increased likelihood of mucosal healing. | 83 (10/12)a |
| 23. During maintenance the minimum trough concentration for adalimumab for patients in remission should be greater than 5 μg/ml. For patients with active disease adalimumab should generally not be abandoned unless drug concentrations are greater than 10 μg/ml. | 100 (12/12) |
| D. Certolizumab pegol | |
| 24. The minimum concentrations for certolizumab pegol at week 6 should be greater than 32 μg/ml. | 100 (12/12) |
| 25. During maintenance the minimum trough concentration for certolizumab pegol for patients in remission should be 15 μg/ml. | 92 (11/12) |
| E. Golimumab | |
| 26. The minimum drug concentration at week 6 for golimumab should at least be 2.5 μg/ml. | 92 (11/12) |
| 27. During maintenance the minimum trough concentration for golimumab for patients in remission should be greater than 1 μg/ml. | 92 (11/12) |
| F. Vedolizumab / Ustekinumab | |
| 28. Although there are emerging data that may show an association between drug concentrations and outcomes, they are not sufficient to guide specific induction and maintenance drug concentrations for vedolizumab and ustekinumab other than confirming that there is detectable drug. | 100 (12/12) |
After a second round of voting.
HMSA: homogeneous mobility shift assay; TNF: tumor necrosis factor.
Scenarios when TDM of biologic therapies should be performed
Anti-TNF therapy
Based on the literature review, consensus was reached on all 4 statements regarding anti-TNFs (Table 4A).
-
1
It is appropriate to order drug/antibody concentration testing in responders at the end of induction for all anti-TNFs.
-
2
It is appropriate to order drug/antibody concentration testing at least once during maintenance for patients on all anti-TNFs.
-
3
It is appropriate to order drug/antibody concentration testing of anti-TNFs at the end of induction in primary non-responders.
-
4
It is appropriate to order drug/antibody concentration testing for all anti-TNFs in patients with confirmed secondary loss of response.
Numerous studies have demonstrated a positive correlation between anti-TNF drug concentrations and favorable therapeutic outcomes (Table 1, Table 2A and 2B, supplementary table 1). However, the great majority of TDM studies refer to infliximab. A large retrospective study showed that at least one TDM, either proactive and/or reactive of infliximab compared to lack of any TDM was associated with less treatment failure.109 Several studies have shown that reactive TDM can better identify the cause and consequently manage SLR to anti-TNF therapy, although the data for PNR are more scarce.4, 8, 10, 110, 111 Reactive TDM to guide infliximab dose adjustment compared to clinical decision making alone is associated with higher post-adjustment clinical response and endoscopic remission and fewer hospitalizations.37 Moreover, reactive TDM of infliximab was found more cost-effective than utilizing clinical symptoms alone to guide therapeutic decisions.99, 101, 102, 112
Proactive TDM of infliximab compared to empiric dose escalation and/or reactive TDM was found to be associated with increased drug retention.39 The landmark randomized controlled trial (RCT), Trough Concentration Adapted Infliximab Treatment (TAXIT), despite failing to meet its primary endpoint, showed that proactive TDM of infliximab compared to clinically-based dosing was associated with lower frequency of undetectable drug concentrations and lower risk of relapse.104 Additionally, in patients with CD and subtherapeutic drug concentrations a one-time dose optimization improved clinical remission rates and C-reactive protein.104 Furthermore, proactive compared to reactive TDM of infliximab was associated with greater drug durability, less need for IBD-related surgery or hospitalization, and lower risk of antibodies to infliximab or serious infusion reactions.38 Recently, proactive following reactive TDM of infliximab was found to be associated with greater drug persistence and fewer IBD-related hospitalizations than reactive TDM alone.103 Proactive TDM can also efficiently guide immunomodulator withdrawal in patients on combination therapy. This concept of ‘optimized monotherapy’ was introduced in a retrospective study showing that patients with infliximab concentrations ≥5 μg/mL had similar drug persistence when treated with infliximab monotherapy or combination therapy with an immunomdulator5 and is further supported by a recent post-hoc analysis of the RCT Study of Biologic and Immunomodulator Naïve Patients in Crohn’s Disease (SONIC) which demonstrated that patients stratified by infliximab trough quartiles had comparable outcomes regardless of concomitant azathioprine.113
Vedolizumab
Consensus was reached on only 2 out of 4 statements regarding vedolizumab (Table 4B).
-
5
It is appropriate to order drug/antibody concentration testing for vedolizumab in non-responders at the end of induction.
-
6
It is appropriate to order drug/antibody concentration testing for vedolizumab in patients with confirmed secondary loss of response.
The current evidence supporting the role of TDM regarding vedolizumab derives only from exposure-response relationship studies showing that higher vedolizumab concentrations are associated with better therapeutic outcomes (Table 2C).90–92, 114 In particular, a large single-center retrospective cohort study of 179 patients (66 with UC and 113 with CD) showed that higher vedolizumab trough concentrations at week 2 and 6 were associated with a higher probability of attaining endoscopic healing, clinical response and biologic response or remission assessed at week 14 for UC and week 22 for CD.90 A multi-center prospective observational study identified a vedolizumab trough concentration cut-off of 18 μg/mL at week 6 as the only independent variable associated with mucosal healing within the first year of treatment.91 Currently, there are no studies comparing either proactive or reactive TDM with symptom-based vedolizumab optimization.
Ustekinumab
Consensus was reached on only 2 out of 4 statements regarding ustekinumab (Table 4C).
-
7
It is appropriate to order drug/antibody concentration testing for ustekinumab in non-responders at the end of induction (at 8 weeks).
-
8
It is appropriate to order drug/antibody concentration testing for ustekinumab in patients with confirmed secondary loss of response.
The current evidence supporting the role of TDM regarding ustekinumab is based on two exposure-response relationship studies showing that higher ustekinumab concentrations correlate to better therapeutic outcomes (Table 2D).49, 89 At this time, there are still no studies comparing either proactive or reactive TDM with empiric ustekinumab optimization.
Assays, drug concentrations and anti-drug antibodies
General
Consensus was reached on all 4 statements regarding the use of biologic drug concentrations and anti-drug antibodies (Table 5A).
-
9
There is no difference in indication for ordering drug/antibody concentrations or interpretation of results for biosimilars or originator drug.
Current data suggest that infliximab enzyme-linked immunosorbent assay (ELISA)s for evaluating either drug concentrations or ATI are suitable for monitoring the infliximab biosimilars SB2 and CT-P13.115–118
-
10
The threshold drug concentration may vary depending on disease phenotype and desired therapeutic outcome.
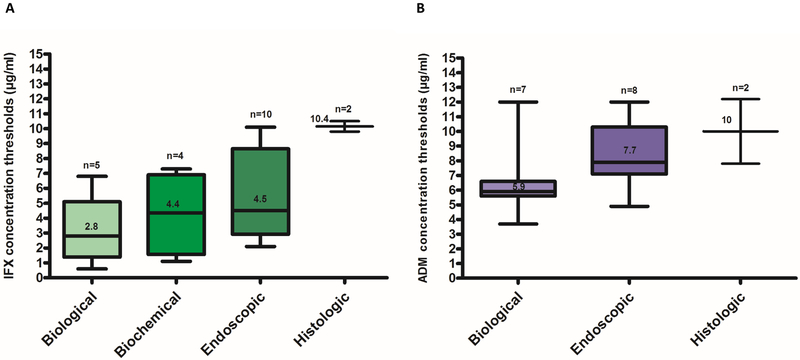
Numerous studies have shown an association between higher induction or maintenance biologic drug concentrations and favorable therapeutic outcomes in IBD (Tables 1 and 2, supplementary table 1). Current exposure-response relationship studies suggest that biologic drug concentration thresholds and ranges appear to differ depending on treatment goals and/or disease phenotypes. In general, higher drug concentrations tend to be associated with more stringent outcomes and higher drug concentrations appear to be needed for phenotypes with a higher inflammatory burden, such as fistulising CD (Tables 1 and 2, supplementary table 1, Figure 1).
Figure legend 1.
Infliximab (A)13, 17, 20, 40–43, 45, 46, 53, 55, 59–61, 64, 67 and adalimumab (B)9, 11–13, 15, 16, 18–23, 30, 31 concentration thresholds associated with biological (based on CRP), biochemical (based on FC), endoscopic or histologic remission in inflammatory bowel disease. Box whisker plots show the median (solid line within box), interquartile range (upper and lower box boundaries) and 5–95% lower and upper extreme (whiskers).
IFX: infliximab; ADM: adalimumab; CRP: C - reactive protein; FC: fecal calprotectin.
-
11
In the presence of adequate trough drug concentrations, anti-drug antibodies are unlikely to be clinically relevant.
A study from Steenholdt et. al. showed that most antibodies to infliximab (ATI) detected via the drug tolerant homogeneous mobility-shift assay (HMSA) lack neutralising potential when tested via a functional cell-based reporter-gene assay, suggesting that they may not be clinically significant.119 A post-hoc analysis of the TAXIT study, which investigated the additional benefit of a drug-tolerant assay, concluded that although it allowed closer follow-up of ATI concentrations and identification of true transient versus persistent antibodies, it offered no clinical benefit over a drug-sensitive assay.120 Nevertheless, other studies have suggested that ‘double positive’ patients (with positive ATI and drug on board) may be prone to SLR or lack of mucosal healing.60, 67, 121
-
12
Other than for anti-infliximab antibodies, there are not enough data to recommend a threshold for high anti-drug antibody titers for the biologic drugs.
Numerous studies have shown that ADA are associated with sub-therapeutic drug trough concentrations, loss of response and lack of recapture of response following dose escalation (Table 3).10, 12–17, 21, 23, 27–29, 31–33, 37, 56–58, 60, 63, 67, 73, 75, 80–88 However, the great majority of them and specifically the ones suggesting a threshold of high-titer ADA refer to ATI (Table 3).
Infliximab
Consensus was reached on all statements regarding infliximab concentrations and ATI (Table 5B).
-
13
The current evidence suggests that the variability of infliximab concentrations between the different assays is unlikely to be clinically significant.
-
14
There is insufficient evidence that inter-assay drug concentration results are comparable for biologic drugs other than for infliximab.
Current evidence suggests that although absolute drug concentrations can differ between different assays, including the commonly used ELISA, radio-immunoassay, HMSA and the recently developed electrochemiluminescence immunoassay, they correlate well and generally lead to the same therapeutic decision.83, 119, 122–124 However, these data refer mostly to infliximab, while there are only scarce data for adalimumab and none for non-anti-TNF agents.
-
15
The minimal trough concentration for infliximab post-induction at week 14 should be greater than 3 μg/ml, and concentrations greater than 7 μg/ml are associated with an increased likelihood of mucosal healing.
-
16
During maintenance the minimal trough concentration for infliximab for patients in remission should be greater than 3 μg/ml. For patients with active disease infliximab should generally not be abandoned unless drug concentrations are greater than 10 μg/ml.
These drug concentration thresholds were mainly based on infliximab exposure-response relationship studies depicted in supplementary table 1.
-
17
In the absence of detectable infliximab, high titer anti-infliximab antibodies require a change of therapy. Low level antibodies can sometimes be overcome. For the ANSER assay, a high titer anti-infliximab antibody at trough is defined as 10 U/ml, for RIDAscreen the cut-off is 200 ng/ml, for InformTx/Lisa Tracker the cut-off is 200 ng/ml. For other assays, there is insufficient data to define an adequate cut-off for a high titer anti-infliximab antibody.
Differences in assay methodology result in varying sensitivity to detect ADA and discrepancies when reporting ADA titers.123 Therefore, clinically relevant ADA cut-offs are assay specific, referring mostly to ELISAs and the HMSA (Table 3). Vande Casteele et al, showed that ATI >9.1 U/ml (measured with the HMSA) at time of loss of response resulted in a likelihood ratio of 3.6 for an unsuccessful intervention, suggesting these ATI are sustained and probably very hard to overcome.63 Moreover, Yanai et al. showed ATI >9 μg/mL-eq can identify patients who do not respond to an increased drug dosage with 90% specificity.10Additionally, a small retrospective study of IBD patients in whom infliximab was optimized, either proactively or reactively, to overcome immunogenicity showed that an ATI titer < 8.8 U/mL (measured with the HMSA) was associated with drug retention, suggesting that lower titer ATI can often be overcome with dose intensification.86 A post-hoc analysis of the TAXIT trial showed that ATI> 222 ng/mL eq (measured with an in-house developed drug tolerant ELISA) was not possible to be overcome following infliximab optimization.120
Adalimumab
Consensus was reached on all 2 statements regarding adalimumab concentrations and antibodies to adalimumab (Table 5C).
-
18
The minimum drug concentration at week 4 for adalimumab should at least be 5 μg/ml. Drug concentrations greater than 7 μg/ml are associated with an increased likelihood of mucosal healing.
-
19
During maintenance the minimum trough concentration for adalimumab for patients in remission should be greater than 5 μg/ml. For patients with active disease adalimumab should generally not be abandoned unless drug concentrations are greater than 10 μg/ml.
These drug concentration thresholds were based mainly on adalimumab exposure-response relationship studies depicted in Table 1.
Certolizumab pegol
Consensus was reached on all 2 statements regarding certolizumab pegol concentrations and antibodies to certolizumab pegol (Table 5D).
-
20
The minimum concentrations for certolizumab pegol at week 6 should be greater than 32 μg/ml.
-
21
During maintenance the minimum trough concentration for certolizumab pegol for patients in remission should be 15 μg/ml.
These drug concentration thresholds were based on certolizumab pegol exposure-response relationship studies depicted in Table 2A.
Golimumab
Consensus was reached on all 2 statements regarding golimumab concentrations and antibodies to golimumab (Table 5E).
-
22
The minimum drug concentration at week 6 for golimumab should at least be 2.5 μg/ml.
-
23
During maintenance the minimum trough concentration for golimumab for patients in remission should be greater than 1 μg/ml.
These drug concentration thresholds were based on exposure-response relationship studies depicted in Table 2B.
Vedolizumab and ustekinumab
Consensus was reached on the statement regarding vedolizumab and ustekinumab concentrations and antibodies to vedolizumab or ustekinumab (Table 5F).
-
24
Although there are emerging data that may show an association between drug concentrations and outcomes, they are not sufficient to guide specific induction and maintenance drug concentrations for vedolizumab and ustekinumab other than confirming that there is detectable drug.
At the time of the consensus meeting there were only limited data available from exposure-response relationship studies to suggest a clinically relevant vedolizumab (Table 2C) or ustekinumab (Table 2D) threshold or range associated with favorable therapeutic outcomes.
DISCUSSION
Unlike for rheumatoid arthritis and psoriasis, there are only a limited number of biologic agents approved for the treatment of IBD. Additionally, current data demonstrate that patients who fail anti-TNF therapies do no respond as well to subsequent agents.125, 126 Thus, optimizing the use of biologic therapies is of the utmost importance. TDM is one strategy to optimize biologics and maximise their effectiveness. Reactive TDM can better explain and manage SLR, and there is emerging evidence that proactive TDM further improves outcomes and is being used more frequently.127, 128
In the recent American Gastroenterological Association guidelines, no recommendation was made regarding proactive TDM of anti-TNFs for patients who have quiescent disease due to a ‘knowledge gap’.96 However, the IBD Sydney Organisation and the Australian Inflammatory Bowel Diseases Consensus Working Group recommended that in patients in clinical remission following anti-TNF therapy induction, TDM should be considered to guide management and additionally TDM should be considered periodically in patients in clinical remission if the results are likely to impact management.97 Although well designed large prospective studies are lacking there are preliminary data mainly from retrospective studies which demonstrate that proactive TDM is associated with better therapeutic outcomes compared to empiric dose optimization and/or reactive TDM.38, 39, 103, 104, 129 Furthermore, numerous retrospective studies23, 24, 26, 29, 31–33, 67, 73, 74, 77–79, 130, 131 and some post-hoc analyses of RCT47–49, 71, 76, 94, 132, 133 have shown that higher biologic drug concentrations are associated with favourable short- and long-term therapeutic outcomes in IBD (supplementary Table 1, Table 2 and Table 3). There do appear to be certain clinical scenarios that proactive TDM of anti-TNF therapy can efficiently guide therapeutic decisions, such as treatment de-escalation,134 the application of ‘optimized monotherapy’ instead of combo therapy with IMM,82 re-starting therapy after a long ‘drug holiday’135 and treatment cessation upon deep remission.50, 51
Nevertheless, before TDM can be widely applied in clinical practice there are several obstacles to their regular use including when to utilize TDM, how to accurately interpret and apply the results of such testing, and in defining the optimal drug concentration thresholds and ranges to target.136 We feel these consensus statements help address these issues and hope they will aid physicians in better understanding and utilizing TDM.
Major limitations of the evidence and consequently these consensus statements relate to the lack of large prospective studies and RCT on TDM of biologic therapy applied on different IBD phenotypes, and sparse data on induction therapy and on biologic agents other than infliximab and adalimumab. Moreover, it is unclear if trough concentrations are the best predictor of initial response to biologics, compared to peak drug concentrations or total drug exposure. However, in the absence of RCT, consensus guidelines synthesizing the literature and extrapolating from available data serve to support clinicians in clinical decision making.
Further RCT to establish the utility of proactive TDM, particularly during the induction phase, should be performed. Additional future directions should include the development of accurate, easily accessible and affordable rapid assays and dashboards to allow fast dosing adaptation and incorporation of predictive PK models based on patient and disease characteristics.137, 138
In conclusion, there is a growing body of evidence that demonstrates the clinical utility of TDM of biologic therapy in IBD. This is a big step towards personalised medicine and optimizing the care of patients with IBD. Although more prospective data are needed especially for proactive TDM, induction therapy, and non-anti-TNF biologics, these consensus statements provide a practical guide to apply TDM for optimizing biologic therapy in patients with IBD.
Supplementary Material
DISCLOSURES:
G.Y.M has received research funding from Pfizer, Prometheus, and Shire; and is a consultant for Abbvie, Given Imaging, Luitpold Pharmaceuticals, Janssen, UCB, Celgene, Takeda, Genentech, and Pfizer. P.M.I is on the Advisory Board and Speaker’s Bureau for Abbvie, MSD, and Takeda. L.E.R. has served on the Advisory Board for Ferring Pharmaceuticals with all honoraria paid to Mayo Clinic and is a consultant for Alivio Therapeutics, L.B. has served as a consultant for Pfizer, Janssen, Shire, and Takeda; and served as speaker for Janssen, Shire, and Takeda. J.J. has served as a speaker for Jansen, Merck, Schering-Plough, Abbot, and Abbvie; and has participated in advisory boards for Janssen, Abbott, and Takeda. G.G.K. has served as a speaker for Pfizer, Janssen, Merck, Schering-Plough, and Abbvie; has participated in advisory board meetings for Jansen and Abbvie; and has received research support from GlaxoSmithKline, Merck, Abbvie. M.P.S. has received educational grants and research support from Ferring Pharmaceuticals and Orphan Pharmaceuticals; speaker’s fees from Janssen, Abbvie, Ferring, Takeda, Pfizer and Shire, and is on the Advisory Boards of Janssen, Takeda, Pfizer, Celgene, Abbvie, and MSD. B.B. is on the Advisory Board of Abbvie, Janssen, Takeda, Shire, Genentech, Ferring, and Warner Chillcott; the Speaker’s Bureau of Abbvie, Janssen, Takeda, and Forrest Laboratory; is a consultant for Celltrion and Pendopharm; and has received research support from Abbvie, Amgen, BMS, Genentech, Janssen, BI, and GlaxoSmithKline. A.S.C. has served on advisory boards for Abbvie, Janssen Takeda, Pfizer, Arena, Samsung and Bacainn and has received research support from Miraca. S.M.D. has served on Speaker’s Bureau and as a consultant for Takeda, Janssen, and Abbvie. N.V.C. has received consultancy fees from Pfizer, Progenity, Takeda; and has received research support from Takeda. C.A.S. has received research funding from Abbvie, Janssen, Takeda, and UCB; delivered CME lectures for Abbvie, Janssen, Merck, and Takeda; and served as an advisor/consultant for Abbvie, Amgen, Janssen, Lilly, Pfizer, Takeda, and Theradiag. The remaining authors disclose no conflicts.
GRANT SUPPORT:This project was supported by unrestricted educational grants from Takeda, Pfizer and AbbVie. Funders had no role in the study design, analysis or interpretation of data, review of the manuscript, or decision to publish. Funders were not present at the moderated panel discussions. K.P. is supported by Ruth L. Kirschstein NRSA Institutional Research Training Grant 5T32DK007760–18. The content of this project is solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH).
ABBREVIATIONS:
- ADA
anti-drug antibodies
- ATI
antibodies to infliximab
- CD
Crohn’s disease
- CI
confidence interval
- ELISA
enzyme-linked immunosorbent assay
- HMSA
homogeneous mobility shift assay
- IBD
inflammatory bowel disease
- IMM
immunomodulator
- TDM
therapeutic drug monitoring
- TNF
tumor necrosis factor
- UC
ulcerative colitis
- PNR
primary non response
- SLR
secondary loss of response
- PK
pharmacokinetic
- PD
pharmacodynamic
- RCT
randomized controlled trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miligkos M, Papamichael K, Vande Casteele N, et al. efficacy and safety profile of anti-tumor necrosis factor-α versus anti-integrin agents for the treatment of Crohn’s disease: a network meta-analysis of indirect comparisons. Clin Ther 2016;38:1342–1358. [DOI] [PubMed] [Google Scholar]
- 2.Katsanos KH, Papamichael K, Feuerstein JD, et al. Biological therapies in inflammatory bowel disease: Beyond anti-TNF therapies. Clin Immunol. 2018. March 12 pii: S1521–6616(17)30901–4. doi: 10.1016/j.clim.2018.03.004. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Horin S, Chowers Y. Loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther 2011;33:987–95. [DOI] [PubMed] [Google Scholar]
- 4.Papamichael K, Vande Casteele N, Ferrante M, et al. Therapeutic drug monitoring during induction of anti-tumor necrosis factor therapy in inflammatory bowel disease: defining a therapeutic drug window. Inflamm Bowel Dis 2017;23:1510–5. [DOI] [PubMed] [Google Scholar]
- 5.Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis 2015;21:182–97. [DOI] [PubMed] [Google Scholar]
- 6.Papamichael K, Cheifetz AS. Use of anti-TNF drug levels to optimise patient management. Frontline Gastroenterol 2016;7:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barclay ML, Karim S, Helms ETJ, et al. Infliximab and adalimumab concentrations and anti-drug antibodies in inflammatory bowel disease control using New Zealand assays. Intern Med J. 2018. August 8. doi: 10.1111/imj.14064. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Roblin X, Rinaudo M, Tedesco E, et al. Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am J Gastroenterol 2014;109:1250–1256. [DOI] [PubMed] [Google Scholar]
- 9.Roblin X, Marotte H, Rinaudo M, et al. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014;12:80–4. [DOI] [PubMed] [Google Scholar]
- 10.Yanai H, Lichtenstein L, Assa A, et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol 2015;13:522–30. [DOI] [PubMed] [Google Scholar]
- 11.Aguas Peris M, Bosó V, Navarro B, et al. , Serum adalimumab levels predict successful remission and safe deintensification in inflammatory bowel disease patients in clinical practice. Inflamm Bowel Dis 2017;23:1454–1460. [DOI] [PubMed] [Google Scholar]
- 12.Yarur AJ, Jain A, Hauenstein SI, et al. Higher adalimumab levels are associated with histologic and endoscopic remission in patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 2016;22:409–15. [DOI] [PubMed] [Google Scholar]
- 13.Ungar B, Levy I, Yavne Y, et al. Optimizing Anti-TNF-α Therapy: Serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2016;14:550–7. [DOI] [PubMed] [Google Scholar]
- 14.Frederiksen MT, Ainsworth MA, Brynskov J, et al. Antibodies against infliximab are associated with de novo development of antibodies to adalimumab and therapeutic failure in infliximab-to-adalimumab switchers with IBD. Inflamm Bowel Dis 2014;20:1714–21. [DOI] [PubMed] [Google Scholar]
- 15.Mazor Y, Almog R, Kopylov U, et al. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn’s disease. Aliment Pharmacol Ther 2014;40:620–8. [DOI] [PubMed] [Google Scholar]
- 16.Imaeda H, Takahashi K, Fujimoto T, et al. Clinical utility of newly developed immunoassays for serum concentrations of adalimumab and anti-adalimumab antibodies in patients with Crohn’s disease. J Gastroenterol 2014;49:100–9. [DOI] [PubMed] [Google Scholar]
- 17.Imaeda H, Bamba S, Takahashi K, et al. Relationship between serum infliximab trough levels and endoscopic activities in patients with Crohn’s disease under scheduled maintenance treatment. J Gastroenterol 2014;49:674–82. [DOI] [PubMed] [Google Scholar]
- 18.Zittan E, Kabakchiev B, Milgrom R, et al. Higher adalimumab drug levels are associated with mucosal healing in patients with Crohn’s disease. J Crohns Colitis 2016;10:510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morita Y, Imaeda H, Nishida A, et al. Association between serum adalimumab concentrations and endoscopic disease activity in patients with Crohn’s disease. J Gastroenterol Hepatol 2016;31:1831–1836. [DOI] [PubMed] [Google Scholar]
- 20.Morita Y, Bamba S, Takahashi K, et al. Prediction of clinical and endoscopic responses to anti-tumor necrosis factor-α antibodies in ulcerative colitis. Scand J Gastroenterol 2016;51:934–41. [DOI] [PubMed] [Google Scholar]
- 21.Nakase H, Motoya S, Matsumoto T, et al. Significance of measurement of serum trough level and anti-drug antibody of adalimumab as personalised pharmacokinetics in patients with Crohn’s disease: a subanalysis of the DIAMOND trial. Aliment Pharmacol Ther 2017;46:873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juncadella A, Papamichael K, Vaughn B, Cheifetz AS. Maintenance adalimumab concentrations are associated with biochemical, endoscopic and histologic remission in inflammatory bowel disease. Dig Dis Sci. 2018. July 13. doi: 10.1007/s10620-018-5202-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ungar B, Engel T, Yablecovitch D, et al. Prospective observational evaluation of time-dependency of adalimumab immunogenicity and drug concentrations: The Poetic Study. Am J Gastroenterol. 2018;113:890–8. [DOI] [PubMed] [Google Scholar]
- 24.Ungar B, Glidai Y, Yavzori M, et al. Association between infliximab drug and antibody levels and therapy outcome in pediatric inflammatory bowel diseases. J Pediatr Gastroenterol Nutr 2018;67:507–12. [DOI] [PubMed] [Google Scholar]
- 25.Van Hoeve K, Dreesen E, Hoffman I, et al. Higher infliximab trough levels are associated with better outcome in paediatric patients with inflammatory bowel disease. J Crohns Colitis. 2018;12:1316–25. [DOI] [PubMed] [Google Scholar]
- 26.Baert F, Vande Casteele N, Tops S, et al. Prior response to infliximab and early serum drug concentrations predict effects of adalimumab in ulcerative colitis. Aliment Pharmacol Ther 2014;40:1324–32. [DOI] [PubMed] [Google Scholar]
- 27.Baert F, Drobne D, Gils A, et al. Early trough levels and antibodies to infliximab predict safety and success of re-initiation of infliximab therapy. Clin Gastroenterol Hepatol 2014;12:1474–81. [DOI] [PubMed] [Google Scholar]
- 28.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003;348:601–8. [DOI] [PubMed] [Google Scholar]
- 29.Baert F, Kondragunta V, Lockton S, et al. Antibodies to adalimumab are associated with future inflammation in Crohn’s patients receiving maintenance adalimumab therapy: a post hoc analysis of the Karmiris trial. Gut 2016;65:1126–31. [DOI] [PubMed] [Google Scholar]
- 30.Papamichael K, Baert F, Tops S, et al. Post-Induction Adalimumab concentration is associated with short-term mucosal healing in patients with ulcerative colitis. J Crohns Colitis 2017;11:53–9. [DOI] [PubMed] [Google Scholar]
- 31.Verstockt B, Moors G, Bian S, et al. Influence of early adalimumab serum levels on immunogenicity and long-term outcome of anti-TNF naive Crohn’s disease patients: the usefulness of rapid testing. Aliment Pharmacol Ther 2018;48:731–9. [DOI] [PubMed] [Google Scholar]
- 32.Ungar B, Chowers Y, Yavzori M, et al. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut 2014;63:1258–64. 33. [DOI] [PubMed] [Google Scholar]
- 33.Brandse JF, Mathot RA, van der Kleij D, et al. Pharmacokinetic features and presence of anti-drug antibodies associate with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol 2016;14:251–8. [DOI] [PubMed] [Google Scholar]
- 34.Brandse JF, Mould D, Smeekes O, et al. A real-life population pharmacokinetic study reveals factors associated with clearance and immunogenicity of infliximab in inflammatory bowel disease. Inflamm Bowel Dis 2017;23:650–660. [DOI] [PubMed] [Google Scholar]
- 35.Drobne D, Bossuyt P, Breynaert C, et al. Withdrawal of immunomodulators after co-treatment does not reduce trough level of infliximab in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2015;13:514–21. [DOI] [PubMed] [Google Scholar]
- 36.El-Matary W, Walters TD, Huynh HQ, et al. Higher postinduction infliximab serum trough levels are associated with healing of fistulizing perianal Crohn’s disease in children. Inflamm Bowel Dis 2019;25:150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly OB, Donnell SO, Stempak JM, et al. Therapeutic drug monitoring to guide infliximab dose adjustment is associated with better endoscopic outcomes than clinical decision making alone in active inflammatory bowel disease. Inflamm Bowel Dis 2017;23:1202–1209. [DOI] [PubMed] [Google Scholar]
- 38.Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017;15:1580–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaughn BP, Martinez-Vazquez M, Patwardhan VR, et al. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis 2014;20:1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roblin X, Boschetti G, Duru G, Williet N, et al. Distinct thresholds of infliximab trough level are associated with different therapeutic outcomes in patients with inflammatory bowel disease: A prospective observational study. Inflamm Bowel Dis 2017;23:2048–53 [DOI] [PubMed] [Google Scholar]
- 41.Marits P, Landucci L, Sundin U, et al. Trough s-infliximab and antibodies towards infliximab in a cohort of 79 IBD patients with maintenance infliximab treatment. J Crohns Colitis 2014;8:881–9. [DOI] [PubMed] [Google Scholar]
- 42.Yarur A, Kubiliun M, Czul F, et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol 2015;13:1118–1124. [DOI] [PubMed] [Google Scholar]
- 43.Huang VW, Prosser C, Kroeker KI, et al. Knowledge of fecal calprotectin and infliximab trough levels alters clinical decision-making for IBD outpatients on maintenance infliximab therapy. Inflamm Bowel Dis 2015;21:1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steenholdt C, Bendtzen K, Brynskov J, et al. Cut-off levels and diagnostic accuracy of infliximab trough levels and anti-infliximab antibodies in Crohn’s disease. Scand J Gastroenterol 2011;46:310–8. [DOI] [PubMed] [Google Scholar]
- 45.Papamichael K, Rakowsky S, Rivera C, et al. Infliximab trough concentrations during maintenance therapy are associated with endoscopic and histologic healing in ulcerative colitis. Aliment Pharmacol Ther 2018;47:478–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magro F, Afonso J, Lopes S, et al. Clinical performance of an infliximab rapid quantification assay. Therap Adv Gastroenterol 2017;10:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014;147:1296–307. [DOI] [PubMed] [Google Scholar]
- 48.Adedokun OJ, Xu Z, Marano CW, et al. Pharmacokinetics and exposure-response relationship of golimumab in patients with moderately-to-severely active ulcerative colitis: results from phase 2/3 PURSUIT induction and maintenance studies. J Crohns Colitis 2017;11:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adedokun OJ, Xu Z, Gasink C, et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn’s disease. Gastroenterology 2018;154:1660–71. [DOI] [PubMed] [Google Scholar]
- 50.Louis E, Mary JY, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology 2012;142:63–70. [DOI] [PubMed] [Google Scholar]
- 51.Papamichael K, Vande Casteele N, Gils A, et al. Long-term outcome of patients with Crohn’s disease who discontinued infliximab therapy upon clinical remission. Clin Gastroenterol Hepatol. 2015;13:1103–10. [DOI] [PubMed] [Google Scholar]
- 52.Kang B, Choi SY, Choi YO, et al. Subtherapeutic infliximab trough levels and complete mucosal healing are associated with sustained clinical remission after infliximab cessation in paediatric-onset Crohn’s disease patients treated with combined immunosuppressive therapy. J Crohns Colitis 2018;12:644–52. [DOI] [PubMed] [Google Scholar]
- 53.Yarur AJ, Kanagala V, Stein DJ, et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn’s disease. Aliment Pharmacol Ther 2017;45:933–40. [DOI] [PubMed] [Google Scholar]
- 54.Fay S, Ungar B, Paul S, et al. The association between drug levels and endoscopic recurrence in postoperative patients with Crohn’s disease treated with tumor necrosis factor inhibitors. Inflamm Bowel Dis 2017;23:1924–9. [DOI] [PubMed] [Google Scholar]
- 55.Ward MG, Warner B, Unsworth N, et al. Infliximab and adalimumab drug levels in Crohn’s disease: contrasting associations with disease activity and influencing factors. Aliment Pharmacol Ther 2017;46:150–161. [DOI] [PubMed] [Google Scholar]
- 56.Levesque BG, Greenberg GR, Zou G, et al. A prospective cohort study to determine the relationship between serum infliximab concentration and efficacy in patients with luminal Crohn’s disease. Aliment Pharmacol Ther 2014;39:1126–35. [DOI] [PubMed] [Google Scholar]
- 57.Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn’s disease. Gastroenterology 2009;137:1628–40. [DOI] [PubMed] [Google Scholar]
- 58.West RL, Zelinkova Z, Wolbink GJ, et al. Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn’s disease. Aliment Pharmacol Ther 2008;28:1122–6. [DOI] [PubMed] [Google Scholar]
- 59.Papamichael K, Rakowsky S, Rivera C, Cheifetz AS, Osterman MT. Association between serum infliximab trough concentrations during maintenance therapy and biochemical, endoscopic and histologic remission in Crohn’s disease. Inflamm Bowel Dis 2018; 24: 2266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut 2015;64:1539–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reinisch W, Colombel JF, Sandborn WJ, et al. Factors associated with short- and long-term outcomes of therapy for Crohn’s disease. Clin Gastroenterol Hepatol 2015;13:539–47. [DOI] [PubMed] [Google Scholar]
- 62.Bodini G, Giannini EG, Savarino V, et al. Infliximab trough levels and persistent vs transient antibodiesvmeasured early after induction predict long-term clinical remission inpatients with inflammatory bowel disease. Dig Liver Dis 2018. May;50:452–6. [DOI] [PubMed] [Google Scholar]
- 63.Vande Casteele N, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol 2013;108:962–71. [DOI] [PubMed] [Google Scholar]
- 64.Singh N, Rosenthal CJ, Melmed GY, et al. Early infliximab trough levels are associated with persistent remission in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2014;20:1708–13 [DOI] [PubMed] [Google Scholar]
- 65.Farkas K, Rutka M, Golovics PA, et al. efficacy of infliximab biosimilar CT-P13 induction therapy on mucosal healing in ulcerative colitis. J Crohns Colitis 2016;10:1273–8. [DOI] [PubMed] [Google Scholar]
- 66.Van Stappen T, Bollen L, Vande Casteele N, et al. Rapid test for infliximab drug concentration allows immediate dose adaptation. Clin Transl Gastroenterol 2016;7:e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2016;14:543–9. [DOI] [PubMed] [Google Scholar]
- 68.Arias MT, Vande Casteele N, Vermeire S, et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2015;13:531–8. [DOI] [PubMed] [Google Scholar]
- 69.Bortlik M, Duricova D, Malickova K, et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn’s disease. J Crohns Colitis 2013;7:736–43. [DOI] [PubMed] [Google Scholar]
- 70.Hibi T, Sakuraba A, Watanabe M, et al. C-reactive protein is an indicator of serum infliximab level in predicting loss of response in patients with Crohn’s disease. J Gastroenterol 2014;49:254–62. [DOI] [PubMed] [Google Scholar]
- 71.Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut 2014;63:1721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stein R, Lee D, Leonard MB, et al. Serum infliximab, antidrug antibodies, and tumor necrosis factor predict sustained response in pediatric Crohn’s disease. Inflamm Bowel Dis. 2016;22:1370–7. [DOI] [PubMed] [Google Scholar]
- 73.Bar-Yoseph H, Levhar N, Selinger L, et al. Early drug and anti-infliximab antibody levels for prediction of primary nonresponse to infliximab therapy. Aliment Pharmacol Ther 2018;47:212–8 [DOI] [PubMed] [Google Scholar]
- 74.Davidov Y, Ungar B, Bar-Yoseph H, et al. Association of induction infliximab levels with clinical response in perianal Crohn’s disease. J Crohns Colitis 2017;11:549–55 [DOI] [PubMed] [Google Scholar]
- 75.Dreesen E, Van Stappen T, Ballet V, et al. Anti-infliximab antibody concentrations can guide treatment intensification in patients with Crohn’s disease who lose clinical response Aliment Pharmacol Ther 2018;47:346–55. [DOI] [PubMed] [Google Scholar]
- 76.Dreesen E, D’Haens G, Baert F, et al. Infliximab exposure predicts superior endoscopic outcomes in patients with active Crohn’s disease: pharmacokinetic–pharmacodynamic analysis of TAILORIX. J Crohns Colitis 2018; 12(suppl 1): S063–S064. [Google Scholar]
- 77.Gonczi L, Vegh Z, Golovics PA, et al. Prediction of short- and medium-term efficacy of biosimilar infliximab therapy. Do trough levels and antidrug antibody levels or clinical and biochemical markers play the more important role? J Crohns Colitis 2017;11:697–705 [DOI] [PubMed] [Google Scholar]
- 78.Kobayashi T, Suzuki Y, Motoya S, et al. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis-results from a multicenter prospective randomized controlled trial and its post hoc analysis. J Gastroenterol 2016;51:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Papamichael K, Rivals-Lerebours O, Billiet T, et al. Long-term outcome of patients with ulcerative colitis and primary non-response to infliximab. J Crohns Colitis. 2016;10:1015–23. [DOI] [PubMed] [Google Scholar]
- 80.Steenholdt C, Frederiksen MT, Bendtzen K, et al. Time course and clinical implications of development of antibodies against adalimumab in patients with inflammatory bowel disease. J Clin Gastroenterol 2016;50:483–9. [DOI] [PubMed] [Google Scholar]
- 81.Paul S, Del Tedesco E, Marotte H, et al. therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: A Prospective Study. Inflamm Bowel Dis 2013;19:2568–2576. [DOI] [PubMed] [Google Scholar]
- 82.Lega S, Phan BL, Rosenthal CJ, et al. Proactively optimized infliximab monotherapy is as effective as combination therapy in IBD. Inflamm Bowel Dis 2019;25:134–41. [DOI] [PubMed] [Google Scholar]
- 83.Guiotto C, Daperno M, Frigerio F, et al. Clinical relevance and inter-test reliability of anti-infliximab antibodies and infliximab trough levels in patients with inflammatory bowel disease. Dig Liver Dis 2016;48:138–43. [DOI] [PubMed] [Google Scholar]
- 84.Ben-Horin S, Yavzori M, Katz L, et al. The immunogenic part of infliximab is the F(ab’)2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut 2011; 60:41–8. [DOI] [PubMed] [Google Scholar]
- 85.Buurman DJ, Maurer JM, Keizer RJ, et al. Population pharmacokinetics of infliximab in patients with inflammatory bowel disease: potential implications for dosing in clinical practice. Aliment Pharmacol Ther 2015;42:529–39. [DOI] [PubMed] [Google Scholar]
- 86.Papamichael K, Vajravelu RK, Osterman MT, Cheifetz AS. Long-term outcome of infliximab optimization for overcoming immunogenicity in patients with inflammatory bowel disease. Dig Dis Sci 2018;63:761–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ainsworth MA, Bendtzen K, Brynskov J. Tumor necrosis factor alpha binding capacity and anti-infliximab antibodies measured by fluid-phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn’s disease. Am J Gastroenterol 2008;103:944–8. [DOI] [PubMed] [Google Scholar]
- 88.Farrell RJ, Alsahli M, Jeen YT et al. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn’s disease: a randomized controlled trial. Gastroenterology 2003;124:917–24. [DOI] [PubMed] [Google Scholar]
- 89.Battat R, Kopylov U, Bessissow T, et al. Association between ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2017;15:1427–1434. [DOI] [PubMed] [Google Scholar]
- 90.Dreesen E, Verstockt B, Bian S, et al. evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:1937–46. [DOI] [PubMed] [Google Scholar]
- 91.Yacoub W, Williet N, Pouillon L, et al. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther 2018;47:906–12. [DOI] [PubMed] [Google Scholar]
- 92.Williet N, Boschetti G, Fovet M, et al. Association between low trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin Gastroenterol Hepatol 2017;15:1750–7. [DOI] [PubMed] [Google Scholar]
- 93.Detrez I, Dreesen E, Van Stappen T, et al. Variability in golimumab exposure: a ‘real-life’ observational study in active ulcerative colitis. J Crohns Colitis. 2016;10:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vande Casteele N, Feagan BG, Vermeire S, et al. Exposure–response relationship of certolizumab pegol induction and maintenance therapy in patients with Crohn’s disease. Aliment Pharmacol Ther 2018;47:229–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Colombel JF, Sandborn WJ, Allez M, et al. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:423–31. [DOI] [PubMed] [Google Scholar]
- 96.Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017;153:827–34. [DOI] [PubMed] [Google Scholar]
- 97.Mitrev N, Vande Casteele N, Seow CH, et al. Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2017;46(11–12):1037–53. [DOI] [PubMed] [Google Scholar]
- 98.Restellini S, Chao CY, Lakatos PL, et al. Therapeutic drug monitoring guides the management of crohn’s patients with secondary loss of response to adalimumab. Inflamm Bowel Dis 2018;24:1531–8. [DOI] [PubMed] [Google Scholar]
- 99.Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27. [DOI] [PubMed] [Google Scholar]
- 100.Steenholdt C, Brynskov J, Thomsen OO, et al. Individualized therapy is a long-term cost-effective method compared to dose intensification in Crohn’s disease patients failing infliximab. Dig Dis Sci 2015;60:2762–70. [DOI] [PubMed] [Google Scholar]
- 101.Velayos FS, Kahn JG, Sandborn WJ, et al. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn’s disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol 2013;11:654–66. [DOI] [PubMed] [Google Scholar]
- 102.Guidi L, Pugliese D, Panici Tonucci T, et al. Therapeutic drug monitoring is more cost-effective than a clinically-based approach in the management of loss of response to infliximab in inflammatory bowel disease: an observational multi-centre study. J Crohns Colitis. 2018. May 31. doi: 10.1093/ecco-jcc/jjy076. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 103.Papamichael K, Vajravelu RK, Vaughn BP, et al. Proactive infliximab monitoring following reactive testing is associated with better clinical outcomes than reactive testing alone in patients with inflammatory bowel disease. J Crohns Colitis 2018;12:804–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9. [DOI] [PubMed] [Google Scholar]
- 105.Amiot A, Hulin A, Belhassan M, et al. Therapeutic drug monitoring is predictive of loss of response after de-escalation of infliximab therapy in patients with inflammatory bowel disease in clinical remission. Clin Res Hepatol Gastroenterol 2016;40:90–8. [DOI] [PubMed] [Google Scholar]
- 106.Selinger CP, Lenti MV, Clark T, et al. Infliximab therapeutic drug monitoring changes clinical decisions in a virtual biologics clinic for inflammatory bowel disease. Inflamm Bowel Dis 2017;23:2083–8. [DOI] [PubMed] [Google Scholar]
- 107.Attar A, Duru G, Roblin X, et al. Cost savings using a test-based de-escalation strategy for patients with Crohn’s disease in remission on optimized infliximab: A discrete event model study. Dig Liver Dis. 2018. September 5 pii: S1590–8658(18)30917–4. doi: 10.1016/j.dld.2018.08.029. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 108.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- 109.Billiet T, Cleynen I, Ballet V, et al. Prognostic factors for long-term infliximab treatment in Crohn’s disease patients: a 20-year single centre experience. Aliment Pharmacol Ther 2016;44:673–83. [DOI] [PubMed] [Google Scholar]
- 110.Afif W, Loftus EV Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:1133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Papamichael K, Gils A, Rutgeerts P, Levesque BG, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis 2015;21:182–97. [DOI] [PubMed] [Google Scholar]
- 112.Steenholdt C, Bendtzen K, Brynskov J, et al. Changes in serum trough levels of infliximab during treatment intensification but not in anti-infliximab antibody detection are associated with clinical outcomes after therapeutic failure in Crohn’s disease. J Crohns Colitis 2015;9:238–245. [DOI] [PubMed] [Google Scholar]
- 113.Colombel JF, Adedokun OJ, Gasink C, et al. Combination therapy with infliximab and azathioprine improves infliximab pharmacokinetic features and efficacy-a post-hoc analysis. Clin Gastroenterol Hepatol. 2018. September 26 pii: S1542–3565(18)31024–3. doi: 10.1016/j.cgh.2018.09.033. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 114.Al-Bawardy B, Ramos GP, Willrich MAV, et al. Vedolizumab drug level correlation with clinical remission, biomarker normalization, and mucosal healing in inflammatory bowel disease. Inflamm Bowel Dis 2018. August 24. doi: 10.1093/ibd/izy272. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 115.Jentzer A, Emmanuelle Berger A, Labetoulle R, et al. Short Communication: Evaluation of infliximab and anti-infliximab LISA-TRACKER immunoassays for the therapeutic drug monitoring of SB2 infliximab biosimilar. Ther Drug Monit. 2018. September 21. doi: 10.1097/FTD.0000000000000565. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 116.Afonso J, de Sousa HT, Rosa I, et al. Therapeutic drug monitoring of CT-P13: a comparison of four different immunoassays. Therap Adv Gastroenterol 2017;10:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schulze K, Koppka N, Lutter F, et al. CT-P13 (Inflectra™, Remsima™) monitoring in patients with inflammatory bowel disease. Biologicals. 2016;44:463–6. [DOI] [PubMed] [Google Scholar]
- 118.Gils A, Van Stappen T, Dreesen E, harmonization of infliximab and anti-infliximab assays facilitates the comparison between originators and biosimilars in clinical samples. Inflamm Bowel Dis 2016;22:969–75. [DOI] [PubMed] [Google Scholar]
- 119.Steenholdt C, Bendtzen K, Brynskov J, et al. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in Crohn’s disease: post hoc analysis of a randomized controlled trial. Am J Gastroenterol 2014;109:1055–1064. [DOI] [PubMed] [Google Scholar]
- 120.Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut 2018;67:818–26. [DOI] [PubMed] [Google Scholar]
- 121.Kopylov U, Mazor Y, Yavzori M, et al. Clinical utility of antihuman lambda chain-based enzyme-linked immunosorbent assay (ELISA) versus double antigen ELISA for the detection of anti-infliximab antibodies. Inflamm Bowel Dis 2012;18:1628–33. [DOI] [PubMed] [Google Scholar]
- 122.Vande Casteele N, Buurman DJ, Sturkenboom MG, et al. Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays. Aliment Pharmacol Ther 2012;36:765–71. [DOI] [PubMed] [Google Scholar]
- 123.Vande Casteele N Assays for measurement of TNF antagonists in practice. Frontline Gastroenterol 2017;8:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marini JC, Sendecki J, Cornillie F, et al. comparisons of serum infliximab and antibodies-to-infliximab tests used in inflammatory bowel disease clinical trials of Remicade®. AAPS J 2017;19:161–71. [DOI] [PubMed] [Google Scholar]
- 125.Singh S, George J, Boland BS, et al. Primary non-response to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with inflammatory bowel diseases: a systematic review and meta-analysis. J Crohns Colitis 2018;12:635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014;147:618–27. [DOI] [PubMed] [Google Scholar]
- 127.Papamichael K, Cheifetz AS. Therapeutic drug monitoring in IBD: The new standard-of-care for anti-TNF Therapy. Am J Gastroenterol 2017;112:673–6. [DOI] [PubMed] [Google Scholar]
- 128.Papamichael K, Osterman MT, Siegel CA, et al. Using proactive therapeutic drug monitoring of anti-tumor necrosis factor therapy in inflammatory bowel disease: from an old concept to a future standard of care? Gastroenterology 2018;154:1201–2. [DOI] [PubMed] [Google Scholar]
- 129.Papamichael K, Juncadella A, Wong D, et al. Proactive therapeutic drug monitoring of adalimumab is associated with better long-term outcomes compared to standard of care in patients with inflammatory bowel disease. J Crohns Colitis. 2019. January 21. doi: 10.1093/eccojcc/jjz018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Verstockt B, Dreesen E, Noman M, et al. Ustekinumab exposure-outcome analysis in Cohn’s disease only in part explains limited endoscopic remission rates. J Crohns Colitis. 2019. January 30. doi: 10.1093/ecco-jcc/jjz008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 131.Pouillon L, Rousseau H, Busby-Venner et al. Vedolizumab trough levels and histological healing during maintenance therapy in ulcerative colitis. J Crohns Colitis 2019. January 29. doi: 10.1093/ecco-jcc/jjz029. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 132.Osterman MT, Rosario M, Lasch K, et al. Vedolizumab exposure levels and clinical outcomes in ulcerative colitis: determining the potential for dose optimisation. Aliment Pharmacol Ther 2019;49:408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vande Casteele N, Jeyarajah J, Jairath V, et al. infliximab exposure-response relationship and thresholds associated with endoscopic healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2018. October 26 pii: S1542–3565(18)31200-X. doi: 10.1016/j.cgh.2018.10.036. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 134.Lucidarme C, Petitcollin A, Brochard C, et al. Predictors of relapse following infliximab de-escalation in patients with inflammatory bowel disease: the value of a strategy based on therapeutic drug monitoring. Aliment Pharmacol Ther 2019;49:147–54. [DOI] [PubMed] [Google Scholar]
- 135.Baert F, Drobne D, Gils A, et al. Early trough levels and antibodies to infliximab predict safety and success of reinitiation of infliximab therapy. Clin Gastroenterol Hepatol 2014;12:1474–81. [DOI] [PubMed] [Google Scholar]
- 136.Grossberg LB, Papamichael K, Feuerstein JD, et al. A survey study of gastroenterologists’ attitudes and barriers toward therapeutic drug monitoring of anti-TNF therapy in inflammatory bowel disease. Inflamm Bowel Dis 2017;24:191–7. [DOI] [PubMed] [Google Scholar]
- 137.Mould DR, Upton RN, Wojciechowski J, et al. Dashboards for Therapeutic Monoclonal Antibodies: Learning and Confirming. AAPS J 2018;20:76. [DOI] [PubMed] [Google Scholar]
- 138.Van Stappen T, Bollen L, Vande Casteele N, et al. rapid test for infliximab drug concentration allows immediate dose adaptation. Clin Transl Gastroenterol 2016;7:e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.