Abstract
Background:
Women with vulvodynia, a chronic pain condition, experience vulvar pain and dyspareunia. Few studies examine the range and combination of treatment strategies which women are actually using to reduce vulvodynia.
Aim:
To describe pain experiences and pain relief strategies of women with vulvodynia.
Methods:
Convenience sample, 60 women with vulvodynia (median age 32.5 (ICR 8.5) years; 50 White, 10 racial/ethnic minorities) completed PAINReportIt and reported use of drugs and alcohol and responded to open-ended questions. Univariate descriptive statistics and bivariate inferential tests were used to describe average pain intensity (API) scores, alcohol use, smoking, number of pain relief strategies, and their associations. Women’s open-ended responses about their pain experiences and drug and non-drug pain relief strategies were analyzed for patterns.
Outcomes:
Our mixed methods analysis connected data from pain measures, prescribed treatments and self-reported behaviors with women’s free responses. This enabled nuanced insights into women’s vulvodynia pain experiences.
Results:
Women’s descriptions of their pain and suffering aligned with their reported severe pain and attempts to control their pain, with a median pain intensity of 6.7(ICR 2.0) despite use of adjuvant drugs (median2.0 [ICR=2.0]) and opioids (median 1.0[ICR=2.0]). Thirty-six women (60%) used alcohol to lessen their pain. Twenty-six women (43%) listed combining analgesics and alcohol to relieve their pain. Thirty women (50%) smoked cigarettes. Fifty-four women (90%) used at least one non-drug pain relief strategy (NDPRS). The mean number of NDPRS used was 2.1±1.3 (range 0 to 6). The 5 most common NDPRS from women’s comments were herbal medicine (40%), acupuncture (27%), massage (22%), hypnosis (15%), and mental healthcare (13%).
Clinical Implications:
Severe pain in women with vulvodynia may be a clinical indicator of those at higher risk of combining prescription pain medications with alcohol which are all central nervous system depressants (CNS), and may potentiate overdose.
Strengths and Limitations:
This pilot demonstrated that the mixed methods approach to help understand the complexity of vulvodynia was feasible. We identified data showing a reliance on a high-risk mix of prescriptions and alcohol to reduce vulvodynia pain and a high prevalence of cigarette smoking. However, as a pilot study, these results are considered preliminary; the sample may not be representative. Perhaps only women at the extreme end of the pain continuum participated or women took the survey twice since identifiers were not collected.
Conclusion:
Despite attempts to reduce pain using multiple therapies, including alcohol, women’s vulvodynia pain is severe and not controlled.
Keywords: vulvodynia, vestibulodynia, pain, chronic pain, alcohol, opioids, cigarette smoking
Introduction
Vulvodynia is a chronic pain condition in which women experience vulvar pain and dyspareunia, leaving them desperate for relief and rendering sexual intercourse almost impossible. [1, 2] Global rates of vulvodynia are unknown, however, it is estimated to affect up to 7 million American women. [3–5] Women are prescribed a myriad of treatments to reduce their pain, but none are consistently effective. [1, 6, 7] Poor pain control is associated with the self-management of pain through the use of alcohol and marijuana.[8, 9] The self-management of vulvodynia pain using alcohol, a central nervous system (CNS) depressant, puts sufferers at risk for acute liver failure from mixing acetaminophen and alcohol, gastric bleeding from mixing aspirin and alcohol, and overdose and death due to the combined analgesic and sedative effects of mixing opioids and alcohol.[8] Few studies [1, 6, 7] have examined the strategies that women use to manage pain from this life-altering and debilitating condition.
To fill this gap, we piloted a nationwide online survey and performed exploratory analyses in which we used a mixed methods approach to examine women’s vulvodynia pain experience. The aim of this pilot study was to document the pain experiences of women with vulvodynia and the drug and non-drug pain relief strategies (NDPRS) used.
Material and Methods
Design
Between November 2017 and January 2018, we piloted an anonymous, cross-sectional online survey to better understand women’s pain experiences with vulvodynia. The University of Illinois at Chicago Institutional Review Board approved this study.
Sample
Women were eligible to participate if they had a previous diagnosis of vulvodynia, were between 18 – 45 years of age, able to read English, not pregnant or in menopause. They were recruited through a link to the study page posted on the National Vulvodynia Association (NVA) website.
Procedure and Measurement
Participants accessed the online study link where the study purpose and procedures were explained, eligibility questions were answered, and online consent was obtained. The survey included two modules from PAINReportIt (Nursing Consultant LLC, Seattle, Washington), [10] a computerized version of the McGill Pain Questionnaire (MPQ). [11] PAINReportIt [11] has equivalence to the paper MPQ. [12] The MPQ and PAINReportIt have been validated in women with vulvodynia and other pain conditions. [13, 14] The demographics module included questions about participants’ age, marital status, race, educational level, and income.
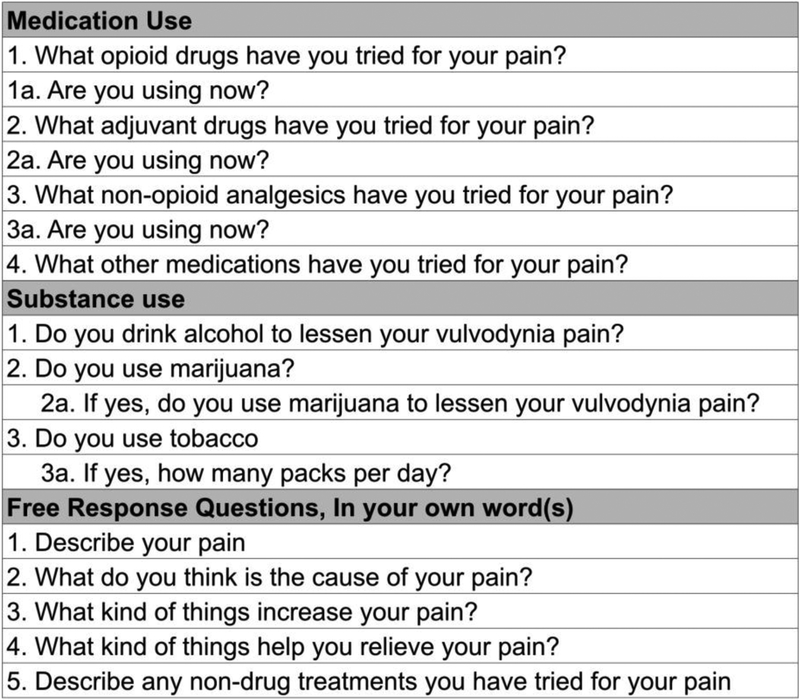
The pain assessment module captured women’s sensory pain experience including pain intensity. Participants selected a number on a scale of 0 (no pain) to 10 (pain as bad as it could be) on the pain intensity numbers scale (PINS) to record their current, worst, and least pain in the last 24 hours.[15] PINS has been shown to have construct and concurrent validity as well as sensitivity and reliability.[16–19] The following questions were asked: 1) On a scale of 0 to 10, with 0 being no pain and 10 being extreme pain, select a number to represent the pain you’re feeling right now; 2) On a scale of 0 to 10, what was the least amount of pain you’ve experienced in the last 24 hours?; 3) On a scale of 0 to 10, what was the worst amount of pain you’ve experienced in the last 24 hours? Additional questions are in Figure 1.
Figure 1.
Survey Questions Completed by Participants*
For the medication module, women reported if they ever or currently used opioid analgesics, adjuvants, non-opioid analgesics, and other medications used to reduce their pain. Data were collected on alcohol and marijuana use and cigarette smoking. Women also provided free responses to open-ended questions asking them to describe their pain, what causes their pain, what increases their pain, and the pain relief strategies (prescription drugs and NDPRS) they use to reduce their pain (Figure 2).
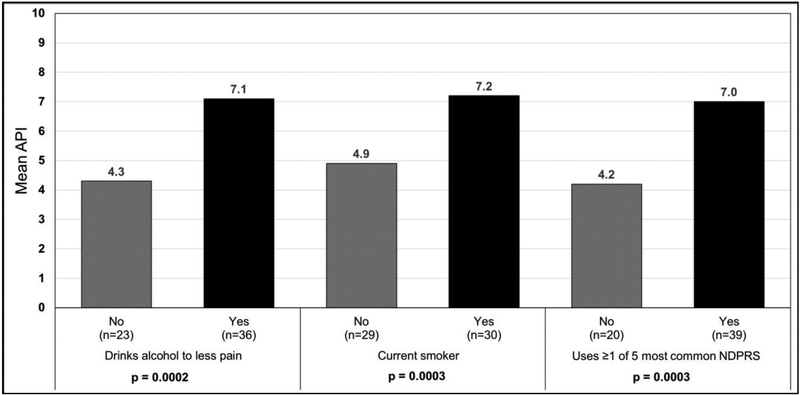
Figure 2.
Mean Average Pain Intensity by Alcohol Use to Lessen Vulvodynia Pain, Current Smoking Status, and Non-Drug Pain Relief Strategies (NDPRS)
n=59, 1 participant is missing API data
For the medication module, women reported if they ever or currently used opioid analgesics, adjuvants, non-opioid analgesics, and other medications used to reduce their pain. Data were collected on alcohol and marijuana use and cigarette smoking. Women provided free responses to open-ended questions asking them to describe their pain, what causes their pain, what increases their pain, and the pain relief strategies (prescription drugs and NDPRS) they use to reduce their pain (Figure 1) Analysis.
Quantitative and qualitative data were transferred from PAINReportIt into Microsoft Excel and then imported into SAS 9.4 and MAXQDA 2018 for analyses. We used univariate analysis to calculate frequencies and percentages. An average pain intensity (API) score was calculated by averaging the current, worst, and least pain scores from the PINS. We tabulated types and total number of medications that each woman reported and calculated the mean and median number of medications used from each drug category. Bivariate analyses, using independent t-tests, were performed to assess API differences among those who reported alcohol use, smoking, number of pain relief strategies (adjuvants, opioids, alcohol), and NDPRS with statistical significance set at an α level <0.05.
For the qualitative analysis, inductive thematic content analysis was undertaken with the goal of describing participants’ comments about their pain experiences and how they experience pain and link it to situations and context. [20] This approach allowed for the unexpected and included more socially-located responses, such as beliefs or linkages to important events in their lives to be included. To move to themes, our analysis process involved interpretation to explain the findings, contribute to the larger the research question of characterizing pain by expanding it to include respondents’ insights. We began by reading each woman’s free responses to the open-ended questions multiple times to gain a global perspective about their experiences. Through open coding of each questions, we descriptively coded women’s comments and collapsed the number of codes to reflect patterns across participants’ experiences. After coding the first few transcripts, three team members met to compare and agree on the set of codes to apply to all subsequent comments. We also recategorized women’s NDPRS to reflect the designations used by the 2012 U.S. Department of Health and Human Services, National Health Interview Survey. [21] Coding and NDPRS category assignment discrepancies were discussed by team members until consensus was reached. We identified qualitative patterns in conjunction with results from self-reported pain measures and data on alcohol use, marijuana use, and cigarette smoking provided by women to provide insight into their vulvodynia pain experiences.
Results
We screened 121 women; 87 (72%) were eligible for participation, of those nine did not consent (10%). Sixty of those eligible completed the survey (50%), 58 (48%) in its entirety. Of the 60 participants only 47 (78%) answered all five open-ended questions. Thirty-four women (28%) were ineligible: 11 did not complete the screening questions (9%), 18 had at least one exclusionary criteria (14%), 1 had at least one exclusionary criteria and did not pass the bogus screening question (1%), and 5 did not pass a bogus screening question (4%).Participants’ mean age was 32.7 ± 5.5 years and they self-identified as White (n=50), Black/ (n=2), Hispanic/Latino (n=4), or Native American/Alaska Native (n=4). Demographic characteristics are summarized in Table 1.
Table 1.
Vulvodynia Participant Characteristics (n=60)
| Variable | Category | n | Mean (SD), Median (IQR), or Percent |
|---|---|---|---|
| Age | 32.7(5.5), 32 5 (IQR= 8.5) |
||
| Marital status | Single | 7 | 12 |
| Married/Partnered | 53 | 88 | |
| Race | White | 50 | 83 |
| Black/African-American | 2 | 3 | |
| Native American/Alaska Native | 4 | 7 | |
| Hispanic | 4 | 7 | |
| Income | <40k | 16 | 27 |
| 40–50k | 20 | 33 | |
| 50k+ | 24 | 40 | |
| Education | < HS, HS, Vocational School | 18 | 30 |
| Some college or Associates degree | 19 | 32 | |
| Bachelor’s degree or higher | 23 | 39 |
Pain Intensity
Previous analyses showed that 41women in this sample (69%) reported severe pain (API ≥ 6 and ≤ 10/10).[22] The average current pain score was 7.4 ± 3.2, the median was 6.7 (IQR=2.0); the average worst pain in the last 24 hours was 7.3 ± 2.8, the median was 8.0 (IQR=3.0); the average least pain score in the last 24 hours was 3.4 ± 2.3, the median was 4.0 (IQR=3.0); and the API (average of current pain, least and worst pain in the last 24 hours) was 6.1 ± 2.4, the median was 6.7 (IQR=2.0). [22] Sixty-Nine percent of the sample had an API indicating severe pain In this current analysis, along with these high API scores, women’s descriptions of their pain highlighted the intensity of their suffering. Some women could not find words to describe their vulvodynia pain. For example, one 33-year-old woman wrote, “the pain I experience cannot be described with words.” Another woman wrote her pain is “all words horrible and bad [these words] are not able to describe the pain that I experienced.” Other women were able to describe their suffering. Their pain description comments were assigned to one of three major categories described in more detail below: physical pain, pain linked to its emotional burden, and personified suffering representing pain that was described as though it embodied a human form.
Physical pain descriptors written by women were the same or similar to the MPQ pain quality descriptors and included burning, tingling, electrical sensations, ripping, tearing, cutting, tightness, or heaviness. [22] A 28-year-old woman wrote that she feels “heaviness in the abdomen/pelvis, gnawing pain/discomfort like a tight muscle…tearing/ripping of skin at [the] vaginal opening…[and] tingling/prickling shooting pain when pressing upper vaginal opening towards urethral opening or clitoris.” Reflecting her frustration with her level of pain, a 38-year-old woman, wrote, “I want to throw away [my] vagina.” Some women used analogies to make their pain relatable to other pain experiences. For example, the pain is “worse than childbirth” and is “invincible, it feels [like] a hundred toothaches”. A 40-year-old woman wrote that it feels “like someone put a cigarette out on my outer pubic area.” Another described the pain as feeling “like peroxide poured on an open wound.”
In addition to physical descriptors, 22 women included statements linking vulvodynia pain to its emotional burden. A 38-year-old woman wrote that vulvodynia pain has had a “very negative impact on [the] quality of my life, because [my] physical health and mental health were badly affected…” A few women explicitly stated that the condition “causes me emotional distress.” Eleven women wrote statements that linked vulvodynia to their value as a person and the toll that living with vulvodynia has taken on their mental well-being. A 35-year-old woman wrote that she “regretted being born.” One 38- year-old woman said “I became worthless because of pain that I experienced.” Another simply stated that this disease “has made my life like hell.” Others described the emotional impact of this condition as frustrating and unfair and one said that her “…future looks horrible.” A well-known consequence of vulvodynia is the inability to have intercourse. Surprisingly, only one 39-year-old woman mentioned vulvodynia’s impact on her sex life when she wrote, “it has taken away my enjoyment of intercourse.”
Several women expressed their pain experience by describing pain as an embodied a human form or active agent with intention to cause suffering. Pain described this way turned pain into a person that that caused their suffering. For example, a 35-year-old woman assigned pain to being a male when she wrote: “…he [is] beating all the desire in the life of a woman, he really has had no pity. He was more than ruthless killer.”
Another woman linked pain to some who inflicts pain on someone as a punishment when she wrote, “vulvodynia pain is a really great torturer.” A 35-year-old woman wrote that the pain is “like a curse, my life is now crumbling, filled with anguish and mental pressure.” Eight other women used phrases like “unbelievably cruel” or “killing”, “he [is] killing me slowly.” Instead of describing her pain, a 39-year-old woman asked “[why] did God create [vulvodynia] pain, while I am not a sinner and why should I suffer?” Similarly, another woman stated that “…this pain [in] my life has no meaning…[it] destroys [me] mentally and physically slowly.” Women’s high API scores and words capturing the emotional toll and distress illustrate how burdensome vulvodynia pain is in these women’s lives.
Pain Relief Strategies
Prescription Medications
On the survey, women reported non-opioid, adjuvant and opioid analgesics prescription drugs they had ever used to reduce their pain (Table 2). We previously reported the mean number of adjuvant drugs ever used per participant, 1.8 ± 1.2, the median was 2.00 (IQR=2.0); and the mean number of opioid drugs ever used per participant, 1.1 ± 1.1, the median was 1.0 (IQR=2.0). [22] The vast majority of participants reported using adjuvant medication (82%) and opioids (62%) (Table 3).
Table 2.
Reported Use of Medications Used to Control Vulvodynia Pain, by Drug Category*
| Opioid Analgesic | n | % ever used |
|---|---|---|
| Methadone (dolophine) | 15 | 25.9 |
| Fentanyl (duragesic, oralet, sublimaze) | 14 | 24.1 |
| Codeine | 8 | 13.8 |
| Meperidine (demerol, pethadol) | 7 | 12.1 |
| Codeine with acetaminophen (tylenol with codeine) | 7 | 12.1 |
| Propoxyphene HCL (darvon,dolene) | 3 | 5.2 |
| Hydrocodone (lorcet, lortab, vicodin | 3 | 5.2 |
| Tramadol (ultram) | 2 | 3.4 |
| n=1 for each of the following drugs: Propoxyphene Napsylate (darvocet n, darvon n); Hydromorphone (dilaudid); Levorphanol (levodromoran); Oxycodone-controlled release (oxycontin); Codeine phosphate hemihydrate (paralgin forte); Oxycodone-immediate release (percocet, percodan, roxicodone) | ||
| Adjuvant Analgesic | n | % ever used |
| Carbamazepine (epitol, tegretol) | 27 | 46.6 |
| Gabapentin (neurontin) | 19 | 32.8 |
| Amitriptyline (elavil) | 19 | 32.8 |
| Paroxetine (paxil) | 12 | 20.7 |
| Phenytoin (dilantin) | 7 | 12.1 |
| Methylphenidate (ritalin) | 7 | 12.1 |
| Hydroxyzine (atarax) | 3 | 5.2 |
| Imipramine (tofranax) | 2 | 3.4 |
| n=1 for each of the following drugs: Duloxatine (cymbalta); Trazodone (desyrel); Dextroamphetamine (dexedrine); Gamma-Aminobutyric acid (GABA); Escitalopram Oxalate (lexapro); Nefazodone (Serzone) | ||
| Adjuvant Analgesic | n | % ever used |
| Lidocaine | 48 | 82.7 |
| Non-opioid Analgesic | n | % ever used |
| Acetylsalicylic acid (aspirin) | 36 | 62.1 |
| Acetaminophen (tempra, tylenol) | 31 | 53.4 |
| Ibuprophen (advil, motrin) | 27 | 46.6 |
| Ketorolac (toradol) | 12 | 20.7 |
| Etodolac (Iodine) | 10 | 17.2 |
| Nabumetone (relafen) | 8 | 13.8 |
| Ketoprofen (orudis) | 5 | 8.6 |
| Piroxicam (feldene) | 4 | 6.9 |
| Choline Magnesium Trisalicylate (trilisate) | 4 | 6.9 |
| Diflunisal (dolobid) | 3 | 5.2 |
| n=1 for each of the following drugs: Capsaicin 0.025%; Sulindac (clinoril); Indomethacin (indocin) | ||
| Other Drugs | n | h% ever usedh |
| Topical Estrogen Cream | 4 | 8.3 |
| Ganglion impar block; Internal botox injections; Pudendal nerve block; Valium suppository | ||
n = 58, two participants are missing drug data
Table 3.
Reported Prescription and Substance Use*
| Reported Use | ||
|---|---|---|
| Prescription Medication Use | n* | % |
| Adjuvant | 49 | 82 |
| Opioid | 37 | 62 |
| Adjuvant + Opioid | 36 | 62 |
| Substance Use | ||
| Do you use alcohol? | 50 | 83 |
| Do you smoke cigarettes? | 30 | 50 |
| Reducing Pain | ||
| Do you use alcohol to lessen your pain? | 36 | 60 |
| Do you use marijuana to lessen your pain? | 4 | 7 |
| Adjuvant + Opioid + Alcohol | 27 | 47 |
n=58, two participants are missing medication and alcohol data
Substance Use
In addition to their prescription medications, women were asked to report their alcohol, marijuana, and tobacco use. Fifty women (83%) reported drinking alcohol; the majority (36 women, 60%) reported using alcohol specifically to lessen their pain. Four women (7%) reported using marijuana to self-medicate (Table 3). When asked to describe what relieves their pain, 32 women (53%) included alcohol in their response with many of them emphasizing getting drunk. One woman said, “I sleep in a drunken state.” A 35- year-old woman wrote, “drink a lot of alcohol to fall asleep” while a 41-year-old woman wrote, “drink alcohol to unconsciousness.” Another 39-year-old woman wrote, “pain killers, sleep in a drunken state” and another woman wrote, “taking drugs that make [me] fall asleep, drinking alcohol.” Most women listed the three or four items together. For example, “drinking, smoking and painkillers,” “painkillers, sedatives, drunk,” or “painkillers, opioids, alcohol drunk.” Out of the 58 participants who responded to this question, 26 women (45%) explicitly listed combining painkillers and alcohol to relieve their pain.
Using bivariate analysis, we compared mean API among subgroups who did and did not use alcohol and tobacco and found higher mean API among women using alcohol to lessen their pain and also for women using tobacco (Figure 2). Half of the women in this sample smoked, with an average of 2.5 packs per day.
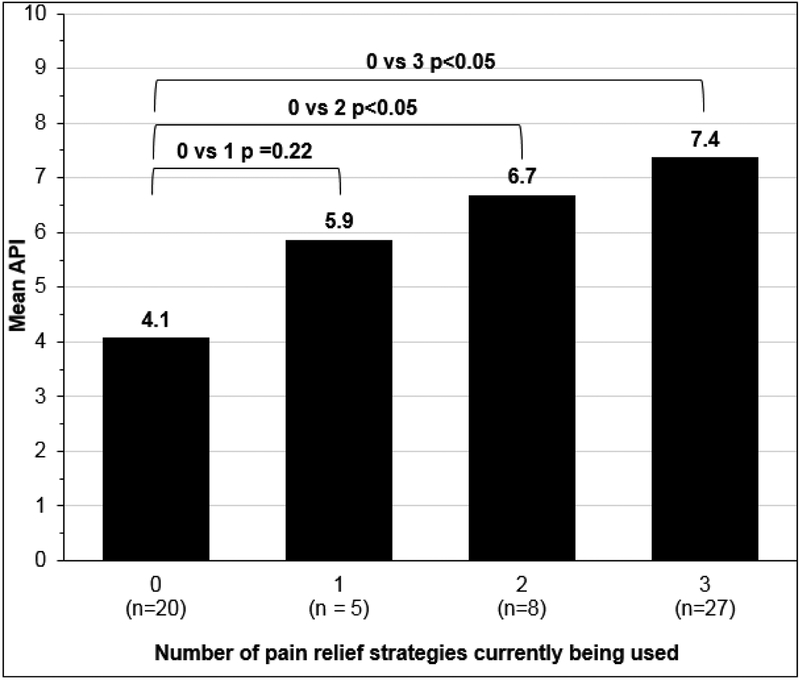
In addition, there were significant differences in API in women who did not use adjuvant, opioid, and alcohol strategies compared to women who reported using 2 or 3 of these pain relief strategies (Figure 3). The higher the API, the more pain relief strategies women reported using.
Figure 3.
Mean API by Number of Pain Relief Strategies (opioids, adjuvants, and alcohol to reduce pain)*
*n=57, One participant is missing API data, 2 are missing drug data Pain Relief strategies= current opioid use, current adjuvant use, drinking alcohol to lessen pain
Non-Drug Pain Relief Strategies (NDPRS)
Fifty-eight women (97%) completed responses to the open-ended question “Describe any non-drug treatments you have tried for your pain.” Fifty-four women (90%) reported using at least one NDPRS. The mean number of NDPRS used was 2.1 ± 1.3, the median was 2.0 (IQR= 2.0) and the range was 0 6. Only 6 women (10%) reported they did not use any NDPRS but did use at least one drug treatment. Thirty-nine women (65%) reported using 1 or more of the 5 most commonly reported NDPRS. A 28-year- old woman listed multiple strategies when she wrote, “acupuncture, biofeedback therapy, using vaginal dilators, Kegel exercises, pelvic stretches.” Similarly, a 27-year- old woman listed using eight NDPRS including “physiotherapy, chiropractic, Neuro Trac pelvitone exerciser, stretching with a dilator, psychotherapy, healing, acupuncture, [and a] mind/body toolbox (audiotapes) by Abigail Stedley.” Table 4 presents all of the NDPRS reported by women in this sample. Using a NDPRS was also associated with pain. Women who used at least one of the five most commonly reported NDPRS had significantly higher mean API scores compared to women who did not use of one these five strategies (Figure 2).
Table 4.
Type and Frequency of Use of Non-Drug Pain Relief Strategies
| Non-drug Pain Relief Strategy | n | % |
|---|---|---|
| Herbal medicine | 24 | 40 |
| Acupuncture | 16 | 27 |
| Massage | 13 | 22 |
| Hypnosis | 9 | 15 |
| Mental health care | 8 | 13 |
| Dilator | 7 | 12 |
| Physical therapy | 5 | 8 |
| Psychic/paranormal | 5 | 8 |
| Yoga/stretching/pilatesa | 3 | 5 |
| Pressure avoidanceb | 3 | 5 |
| Ice/Cold to vulva | 3 | 5 |
| Homeopathic | 3 | 5 |
| Prayer rituals | 3 | 5 |
| Diet | 2 | 3 |
| Meditation | 2 | 3 |
| Relaxation | 2 | 3 |
| Sleep/rest | 2 | 3 |
| Sex therapy | 2 | 3 |
| n=1 for each of the following: | ||
| Biofeedback, chiropractic, coconut oil, “creams”, digital pelvic floor stimulator, drink water, epsom salts, kegels, mild soaps, positive affirmation, finding a respectful sexual partner, self-help workbooks, stress reduction, washing with water only no soap | ||
combined yoga, stretching, pilates
combined cotton underwear, vulvar hygiene, and pressure avoidance
Use of the 5 most common NDPRS was related to opioid use, drinking alcohol, and smoking (all p<.001). Of the 39 women who used at least one of the top 5 NDPRS, 85% had used opioids, 79% drank alcohol to lessen their pain, and 67% were current smokers. Of the 21 women who had not used one of the 5 most common NDPRS, 19% had used opioids, 24% drank alcohol to lessen their pain, and 19% were current smokers. Twenty-nine (48%) of women in our sample used all 3 substances -opioids, alcohol, and smoking, and 26 (90%) of them had also tried one of the 5 most common NDPRS.
Discussion
This is the first study to examine the association between pain and the use of opioids, adjuvants, alcohol, and NDPRS in women with vulvodynia. This is also the first report of the high prevalence of cigarette smoking in women with vulvodynia. Despite using multiple pain relief strategies, the women in this pilot sample had pain that was severe (API ≥ 6 and ≤ 10/10) [22] and not controlled. Higher pain intensity was associated with the use of more prescription drugs, alcohol, and NDPRS. The majority of women who reported using the 5 most common NDPRS also reported experiencing severe pain, had used opioids and alcohol to lessen their pain, and smoked cigarettes. These findings suggest that women use non-drug therapies to reduce the pain of vulvodynia as adjunctive treatment to prescription medications that are not controlling their pain.
Only 4 women (7%) reported using marijuana to reduce their pain, which appears low for this sample of women with chronic pain. This survey was completed between November 2016 and January 2017. Trends in the legalization of marijuana and public awareness of using marijuana to reduce chronic pain continue to evolve.[23] As of November 2016, medical marijuana was legal in 28 states, the District of Columbia, Guam, and Puerto Rico;[24] 4 states and the District of Columbia had legalized marijuana for recreational use.[25] As of March 2019, medical marijuana was legal in 34 states, the District of Columbia, Guam, and Puerto Rico;[26] 10 states and the District of Columbia had legalized marijuana for recreational use.[27] Participants were asked if they smoked marijuana to reduce their pain, but of those who did, information was not collected as to whether they received medical marijuana for the treatment of vulvodynia. It is possible women did not reside in states where marijuana was legal and did not use it, or they did not admit to using it because it is not legal in their state. Research on marijuana use for the treatment of vulvodynia is needed. The smoking rate (50%) in this small sample was nearly 4 times that of the current U.S. prevalence of women smokers (13.5%).[28] It is known that there is a higher prevalence of cigarette smoking in patients living with chronic pain, including fibromyalgia which is a comorbid syndrome associated with vulvodynia.[29, 30] Also, there is a causal relationship between smoking and lower back pain [31, 32] as well as one type of migraine.[33] Because there was a high prevalence of heavy smokers (mean 2.5 packs per day) in our sample, smoking should be investigated as a potential cause of as well as an aggravating factor associated with vulvodynia Smokers are more likely to use opioids and there is an association between smoking and opioid dose.[30, 34, 35] Future vulvodynia studies may include an analysis of the amount of cigarettes smoked and current opioid dose. Also, because there is a well-known association between smoking and depression, [36] future studies should include a depression measure in the assessment of women with vulvodynia. It should be determined if smoking may be a proxy for depression in women with vulvodynia Cigarette smoking is a coping strategy used by individuals with chronic pain and is significantly and positively associated with fear of pain, interruption of pain, and pain intensity. [29] Women in this sample smoked heavily and may have used cigarette smoking as a coping strategy for their chronic pain.
Because vulvodynia pain is not controlled, women’s desperate search for relief leaves them vulnerable and at high-risk for serious health problems. Given the danger of combining multiple CNS depressants, drug overdose is an important concern. Collectively, these qualitative and quantitative pilot data suggest a public health problem. More effective methods of controlling vulvodynia pain and reducing negative health behaviors, such as tobacco and alcohol use, are needed. This is especially important given that the women in this study reported mixing opioids and adjuvants with alcohol.
This study has several important limitations that may influence the reproducibility of our findings. As a pilot, the sample size, albeit appropriate for a pilot study, was small so is likely not representative of the U.S. vulvodynia population. Generalizability is limited by the paucity of Hispanic/Latino, Black, and other minority women who participated. We are not able to analyze these data by vulvodynia subtype. Even though women were recruited through the NVA website, diagnosis of vulvodynia was through self-report; it would have increased study rigor for the diagnosis of vulvodynia to be made by the investigators after performance of a pelvic exam.
We also acknowledge the possibility of volunteer bias arising from differences in who chose to participate in the study compared to those who did not. [37] Our survey population may over-represent those with uncontrolled pain. Further, without zip code data, it is possible that participants could have responded multiple times to the survey with the use of additional e-mail addresses. Because of the small sample size we did not analyze prescription drug, alcohol, and marijuana use, or cigarette smoking by vulvodynia subtype. This must be done in a future study with a larger sample size. Also, wWe did not compare study respondents with vulvodynia to a healthy control group of women to enable baseline pain and behavior results to be compared to a non- pain population. The comments format for qualitative investigation enabled collection of only rudimentary data. Unlike focus group or individual interviews, further discussion and exploration based on individual’s response was impossible.
Last, the source of the opioids used by women in this sample is unknown. It is important to obtain these data as they may reflect prescribing patterns by providers in primary care, specialty care, or emergency departments, and/or they may represent desperate women seeking “street” medications in order to obtain symptom relief.
Conclusion
This pilot study provides insights into the characterization of the complexity of vulvodynia pain. Women in this sample used the opportunity to provide comments to succinctly express elements of their suffering and that they rely on a high-risk mix of prescription drugs and alcohol to reduce vulvodynia pain and have a high prevalence of heavy cigarette smoking. Even with a high percentage of women using both prescription medications and NDPRS, dangerous polypharmacy practices persist. This suggests that the treatment regimens available to women in this sample and possibly to the American population of women with vulvodynia may not be sufficiently effective and desperate women are putting their health at risk by using a combination of CNS depressants (opioids, adjuvants, and alcohol).
Additional research is needed to replicate this study in a larger representative national survey of U.S. women with vulvodynia and assess whether volunteer bias meant that we captured only those on the extreme end of the pain continuum. Additional qualitative studies might further characterize the complexity of vulvodynia pain by comparing the pain experiences of women whose pain is well-controlled and to those whose pain is not well-controlled. This study shows that additional research exploring the pain experience, its burden and impact on quality of life, and how it relates to motivations for seeking non-prescription treatments is needed. Future research aimed at developing non-opioid and non-addictive pain therapies [38, 39] is critical to reducing the risk of overdose or death from simultaneous use of multiple CNS depressants among women with uncontrolled vulvodynia pain.
Funding:
This research was supported by a grant from the University of Illinois at Chicago, Office of the Vice-Chancellor for Research, Campus Research Board Award. This publication was made possible by Grant Number R01HD091210 from the National Institutes of Health, National Institute of Child Health and Human Development (NICHD). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NICHD. The final peer-reviewed manuscript is subject to the National Institutes of Health Public Access Policy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No author has any conflicts of interest to report.
References
- [1].Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Low Genit Tract Dis. 2005;9: 40–51. [DOI] [PubMed] [Google Scholar]
- [2].Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. The journal of sexual medicine. 2016;13: 607–12. [DOI] [PubMed] [Google Scholar]
- [3].Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58: 82–8. [PubMed] [Google Scholar]
- [4].Reed BD, Crawford S, Couper M, Cave C, Haefner HK. Pain at the vulvar vestibule: a web-based survey. J Low Genit Tract Dis. 2004;8: 48–57. [DOI] [PubMed] [Google Scholar]
- [5].Harlow BL, Kunitz CG, Nguyen RH, Rydell SA, Turner RM, MacLehose RF. Prevalence of symptoms consistent with a diagnosis of vulvodynia: population-based estimates from 2 geographic regions. American Journal of Obstetrics & Gynecology. 2014;210: 40 e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stockdale CK, Lawson HW. 2013 Vulvodynia Guideline update. J Low Genit Tract Dis. 2014;18: 93–100. [DOI] [PubMed] [Google Scholar]
- [7].Goldstein AT, Pukall CF, Brown C, Bergeron S, Stein A, Kellogg-Spadt S. Vulvodynia: assessment and treatment. The journal of sexual medicine. 2016;13: 572–90. [DOI] [PubMed] [Google Scholar]
- [8].Using Alcohol to Relieve Your Pain: What are the Risks? National Institute on Alcohol Abuse and Alcoholism Website2013.
- [9].Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA. 2003;289: 2370–8. [DOI] [PubMed] [Google Scholar]
- [10].Wilkie DJ, Judge MKM, Berry DL, Dell J, Zong S, Gilespie R. Usability of a computerized PAINReportIt in the general public with pain and people with cancer pain. J Pain Symptom Manage. 2003;25: 213–24. [DOI] [PubMed] [Google Scholar]
- [11].Melzack R The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1: 277–99. [DOI] [PubMed] [Google Scholar]
- [12].Huang H-Y, Wilkie DJ, Zong S-PS, et al. Developing a computerized data collection and decision support system for cancer pain management. Comput Inform Nurs. 2003;21:206–17. [DOI] [PubMed] [Google Scholar]
- [13].Dargie E, Holden RR, Pukall CF. The Vulvar Pain Assessment Questionnaire inventory. Pain. 2016;157: 2672–86. [DOI] [PubMed] [Google Scholar]
- [14].Wilkie DJ, Molokie R, Boyd-Seal D, et al. Patient-reported outcomes: descriptors of nociceptive and neuropathic pain and barriers to effective pain management in adult outpatients with sickle cell disease. J Natl Med Assoc. 2010;102: 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wilkie DJ, Savedra MC, Holzemer WL, Tesler MD, Paul SM. Use of the McGill Pain Questionnaire to measure pain: a meta-analysis. Nurs Res. 1990;39: 36–41. [PubMed] [Google Scholar]
- [16].Wilkie D, Lovejoy N, Dodd M, Tesler M. Cancer pain intensity measurement: concurrent validity of three tools--finger dynamometer, pain intensity number scale, visual analogue scale. Hosp J.1990;6: 1–13. [DOI] [PubMed] [Google Scholar]
- [17].Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27: 117–26. [DOI] [PubMed] [Google Scholar]
- [18].Coward DD, Wilkie DJ. Metastatic bone pain: Meanings associated with self-report and self- management decision making. Cancer Nurs. 2000;23: 101–8. [DOI] [PubMed] [Google Scholar]
- [19].Du Pen SL, Du Pen AR, Polissar N, et al. Implementing guidelines for cancer pain management: results of a randomized controlled clinical trial. Journal of Clinical Oncology. 1999;17: 361. [DOI] [PubMed] [Google Scholar]
- [20].Creswell JW, Poth CN. Qualitative inquiry and research design: Choosing among five approaches: Sage publications; 2017. [Google Scholar]
- [21].Health UDo, Services H National Center for Health Statistics 2012 National Health Interview Survey Description. 2012.
- [22].Schlaeger JM, Patil CL, Steffen AD, et al. Sensory pain characteristics of vulvodynia and their association with nociceptive and neuropathic pain: an online survey pilot study. PAIN Reports. 9000; Latest Articles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Azofeifa A, Mattson ME, Grant A. Monitoring Marijuana Use in the United States: Challenges in an Evolving EnvironmentMonitoring Marijuana Use in the United StatesMonitoring Marijuana Use in the United States. JAMA. 2016;316: 1765–6. [DOI] [PubMed] [Google Scholar]
- [24].Project MP. State-by-State Medical Marijuana Laws 2015 with a December 2016 Supplement. https://www.scribd.com/document/264980279/State-by-State-Laws-Report-2015.
- [25].Azofeifa A, Mattson ME, Grant A. Monitoring marijuana use in the United States: challenges in an evolving environment. JAMA. 2016;316:1765–6. [DOI] [PubMed] [Google Scholar]
- [26].Legislatures NCoS. State Medical Marijuana Laws. http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Last accessed March 6, 2019/2019.
- [27].Legislatures NCoS. Marijuana Overview. http://www.ncsl.org/research/civil-and-criminal-justice/marijuana-overview.aspx.
- [28].Jamal A, Phillips E, Gentzke AS, et al. Current cigarette smoking among adults—United States, 2016. Morbidity and Mortality Weekly Report. 2018;67: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Patterson AL, Gritzner S, Resnick MP, Dobscha SK, Turk DC, Morasco BJ. Smoking cigarettes as a coping strategy for chronic pain is associated with greater pain intensity and poorer pain-related function. The Journal of Pain. 2012;13: 285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Orhurhu VJ, Pittelkow TP, Hooten WM. Prevalence of smoking in adults with chronic pain. Tobacco induced diseases. 2015;13: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between smoking and low back pain: a meta-analysis. Am J Med. 2010; 123:87 e7-. e35. [DOI] [PubMed] [Google Scholar]
- [32].Leboeuf–Yde C and Smoking and low back pain: a systematic literature review of 41 journal articles reporting 47 epidemiologic studies. Spine. 1999;24: 1463. [DOI] [PubMed] [Google Scholar]
- [33].Rozen TD. A history of cigarette smoking is associated with the development of cranial autonomic symptoms with migraine headaches. Headache: The Journal of Head and Face Pain. 2011;51: 85–91. [DOI] [PubMed] [Google Scholar]
- [34].Hooten WM, Shi Y, Gazelka HM, Warner DO. The effects of depression and smoking on pain severity and opioid use in patients with chronic pain. PAIN®. 2011;152: 223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hooten WM, Townsend CO, Bruce BK, Shi Y, Warner DO. Sex differences in characteristics of smokers with chronic pain undergoing multidisciplinary pain rehabilitation. Pain Med. 2009;10: 1416–25. [DOI] [PubMed] [Google Scholar]
- [36].Vogt MT, Hanscom B, Lauerman WC, Kang JD. Influence of smoking on the health status of spinal patients: the National Spine Network database. Spine. 2002;27:313–9. [DOI] [PubMed] [Google Scholar]
- [37].Karos K, Alleva JM, Peters ML. Pain, please: An investigation of sampling bias in pain research. The Journal of Pain. 2018;19: 787–96. [DOI] [PubMed] [Google Scholar]
- [38].Schlaeger JM, Xu N, Mejta CL, Park CG, Wilkie DJ. Acupuncture for the treatment of vulvodynia: a randomized wait-list controlled pilot study. The Journal of Sexual Medicine. 2015;12: 1019–27. [DOI] [PubMed] [Google Scholar]
- [39].Morin M, Dumoulin C, Bergeron S, et al. Randomized clinical trial of multimodal physiotherapy treatment compared to overnight lidocaine ointment in women with provoked vestibulodynia: design and methods. Contemp Clin Trials. 2016;46:52–9. [DOI] [PubMed] [Google Scholar]