Abstract
Perinatal depression negatively impacts mother-infant health and well-being. Previous work has linked cortisol reactivity to perinatal depressive symptoms, but moderating effects including social support and neuroticism, have not been studied. Forty-nine pregnant women (9 – 30 weeks’ gestational age; GA) provided saliva samples in response to the Trier Social Stress Test (TSST) and to awakening (cortisol awakening response, CAR), and completed questionnaires on perceived social support, personality, and depressive symptoms. Two hierarchical logistic regressions, one including the TSST response and one including the CAR as predictor variables, suggest that cortisol reactivity, social support from the baby’s father, and neuroticism contribute to depressive symptoms, controlling for GA (both p < .01). Significant statistical interactions among predictors of pregnancy depressive symptoms were, however, only found in the model using the CAR. Findings highlight the importance of considering biopsychosocial interactions in studies predicting perinatal depressive symptoms.
Keywords: Trier Social Stress Test, stress reactivity, cortisol, neuroticism, social support, pregnancy
Perinatal depression, the onset of depression during pregnancy or within the first four weeks after giving birth (APA, 2013), is a global health concern affecting an estimated 11.9% of women (Woody et al., 2017) and their infants whose cognitive, emotional, and behavioral development may be impaired (Hoffman et al., 2017; Stein et al., 2014). Decades of research suggest that the etiology of perinatal depression is complex, and that perinatal depression risk is modulated by a range of biological (e.g., endocrine, inflammatory, genetic) and psychosocial (e.g., stress, social support, relationship quality) factors (e.g., Yim et al., 2015).
In terms of biological factors contributing to perinatal depression risk, the hypothalamic pituitary adrenal (HPA) axis is among the most frequently studied systems. Briefly, the activation of the HPA axis initiates a cascade of signals in which the hypothalamus releases corticotropin releasing hormone (CRH), which stimulates the anterior pituitary gland to release adrenocorticotropic hormone (ACTH), which, in turn, stimulates the adrenal cortex to release cortisol. Cortisol binds to receptors on the pituitary gland, the hypothalamus, and higher-order brain structures, establishing a negative feedback loop through which the HPA axis regulates its own activity (Smith and Vale, 2006). Hundreds of studies published over the last few decades convincingly demonstrate an association between HPA axis dysregulation and major depressive symptoms (Stetler and Miller, 2011), and it has been proposed that major depression could be reflective of a dysregulation of mineralocorticoid and glucocorticoid receptors, the major receptor types to which cortisol binds (Holsboer, 2000; Young et al., 2003).
Physiological stress-responsive systems, including the HPA axis, undergo significant changes throughout pregnancy and the postpartum period (McLean et al., 1995; Sandman et al., 2006). In pregnancy, HPA axis function changes significantly. Cortisol stimulates the production of CRH in the placenta, which leads to more ACTH and cortisol release downstream. The resulting positive feed-forward loop leads to continually increasing levels of HPA axis hormones as pregnancy progresses (Smith, 2007). While these changes are normative and necessary for a successful pregnancy, there is growing evidence suggesting that variations in pregnancy-related HPA axis activity are implicated in the pathophysiology of perinatal depression (Ehlert et al., 2001; Glynn et al., 2013; Yim et al., 2009). This makes sense because the maternal HPA axis is challenged to integrate its function with the emerging placenta, a temporary endocrine organ, and has to continually adjust not only to the increasing placental CRH production as pregnancy progresses but also to the sudden absence of the placenta after delivery. For a detailed review on the interaction between the HPA axis and the endocrine placenta in the pathophysiology of perinatal depression, interested readers are referred to Gelman et al. (2015).
The majority of studies in pregnant and postpartum women that tested the link between depressive symptoms and cortisol, the end product of the HPA axis and the most frequently studied marker of HPA axis activity, have assessed cortisol under baseline conditions and most studies yielded null findings (e.g., Orta et al., 2018; Seth et al., 2016; Yim et al., 2015). Unlike studies examining cortisol under baseline conditions, studies testing the link between cortisol responses to a stressor and depressive symptoms have yielded more promising results. This makes theoretical sense, as psychobiological stress reactivity has been hypothesized to be a possible mechanism linking effects of stress and disease (see Schlotz, Yim, Zoccola, Jansen, & Schulz, 2011). In terms of the link between stress reactivity and depressive symptoms, women who proceeded to develop postpartum depressive symptoms showed a more pronounced cortisol response to a psychosocial laboratory stressor in mid-pregnancy (Nierop et al., 2006). Similarly, Urizar et al. (in this issue) report increased cortisol responses to a laboratory stressor among pregnant women at high depression risk compared to those at low risk. One small study of 22 women found no association between cortisol responses to treadmill exercise and depressive symptoms (Jolley et al., 2007). Other studies have used the increase in cortisol levels within 30 to 45 minutes of awakening, coined the cortisol awakening response (CAR), as a measure of reactivity (Stalder et al., 2016). According to expert consensus guidelines, when looking at change in cortisol levels after awakening (i.e., reactivity) several indices are appropriate (e.g., AUCi, mean increase, baseline to peak; Stalder et al., 2016). Studies examining the CAR suggest that blunted cortisol responses are associated with concurrently assessed depressive symptoms in pregnancy (e.g., O’Connor et al., 2014) and post partum (e.g., Taylor et al., 2009). Similarly, blunted cortisol responses in pregnancy were associated with postpartum depressive symptoms (e.g., Scheyer and Urizar, 2016). Other studies, however, report null findings in pregnancy (e.g., Peer et al., 2013; Pluess et al., 2010) and post partum (e.g., Cheng and Pickler, 2009). In sum, among those studies yielding significant findings, pronounced cortisol responses to an acute lab stressor, and blunted cortisol responses to waking seem to indicate increased risk for perinatal depressive symptoms. In addition, the null findings in some studies suggest that there may be possible moderators that could play into these relationships, and which may not have been sufficiently explored.
One important moderating variable that could influence the relationship between stress reactivity and perinatal depressive symptoms is social support. A systematic review of this literature suggests that this is particularly true for perceived support and for support from the pregnant woman’s partner (Yim et al., 2015). While that review focused on postpartum depressive symptoms, perceived support and partner support are similarly important to consider, in particular because depression during pregnancy is a major risk factor for the development of postpartum depression (Norhayati et al., 2015). Across cultures, perceived social support is crucial for expectant mothers to successfully adapt to the stressors and demands of pregnancy and of caring for a newborn (e.g., Collins et al., 1993; Morling et al., 2003; Sosa et al., 1980). While the relationship between stress and perceived social support is widely studied in the general population (e.g., Cohen, 2004), studies in the perinatal literature have mostly considered stress and perceived social support separately. Findings of moderating processes obtained from the general population cannot easily be generalized to the perinatal context because the stressors and relationship challenges that occur during the perinatal transition are unique. For example, pregnancy complications and caring for a colicky baby or a baby that has trouble sleeping (e.g., Saxbe et al., 2016) are intense and novel stressors that may co-occur with other life stressors. Moreover, particularly during first pregnancies, family dynamics and individual family members’ roles change. At this time of increased stress and demand, high quality social support can provide a buffer against stressors that may accompany pregnancy, promoting healthy pregnancy outcomes (Stapleton et al., 2012).
Given that cortisol reactivity is sensitive to stress and that there is a substantial body of literature pointing to the stress buffering effects of social support (Ditzen and Heinrichs, 2014), surprisingly, there are no studies we know of that have examined how cortisol reactivity might be moderated by social support to predict depression risk (in pregnancy or otherwise). In fact, little work exists on the link between social support and cortisol reactivity in general. Frisch et al. (2015) reviewed the available work on social support and cortisol responses to a psychosocial laboratory stressor (Trier Social Stress Test) and concluded that attenuation of cortisol reactivity by social support involves important moderators like sex, attachment style, cultural background, and personality of the person receiving support. Thus, while this small literature does not directly address how these moderating effects influence perinatal depression risk, it suggests the possibility that social support might be protective, given its mitigating role in cortisol reactivity.
Aside from social support, empirical work also provides support for the idea that neuroticism might play an important role in the link between cortisol reactivity and depressive symptoms. Neuroticism is a stable personality trait that is closely tied to depression risk and can influence responsivity to stress, as it is characterized by emotional instability, negative affectivity, and high reactivity to stress (Lahey, 2009). Meta-analyses and reviews examining the Big 5 broadly (e.g., Hakulinen et al., 2015; Kotov et al., 2010) and neuroticism specifically (Lahey, 2009) consistently report an association between neuroticism and heightened risk for depression. Of the studies examining neuroticism and depressive symptoms in the perinatal period, the majority focus on the contribution of neuroticism to postpartum depressive symptoms, with most indicating a positive association between the two factors (Gelabert et al., 2012; Gelabert et al., 2011; Lee et al., 2000; Marín-Morales et al., 2014; Martin-Santos et al., 2012; Podolska et al., 2010; Saisto et al., 2001; van Bussel et al., 2009). One study examining neuroticism in late pregnancy found that non-depressed pregnant women with high neuroticism scores had four times the risk of developing depressive symptoms post partum, even after controlling for confounders (Iliadis et al., 2015).
Because neuroticism is characterized by heightened emotional reactivity to stress (Lahey, 2009), individuals high in neuroticism should display altered cortisol reactivity. A review of the literature points toward mixed findings, with studies suggesting positive, negative or no correlations between neuroticism and cortisol reactivity (see Ormel et al., 2013). One reason for the inconsistent findings could be that important modulating variables remain insufficiently explored. For example, our own work provides some evidence for a moderating role of sociocultural context (based on ethnic or cultural background) in the link between neuroticism and cortisol reactivity (Campos et al., 2014). To our knowledge, the cortisol reactivity-neuroticism link in relation to depression has not been explicitly tested in either pregnant or non-pregnant samples. However, one study suggests both depressive temperament (i.e., predisposing cognitive attitudes associated with depression) and high neuroticism are associated with a heightened cortisol response to a combined dexamethasone/CRH challenge (e.g., Zobel et al., 2004).
Finally, neuroticism and social support are also thought to affect each other, with studies in non-pregnant samples suggesting that neuroticism interferes with the effective provision and receipt of social support (Lahey, 2009). In relation to depression, there appear to be mediation effects at play. For example, one study found that the relationship between neuroticism and depression was mediated by negative social exchange (i.e., hostility, insensitivity, and interference during an interaction) and lower perceived support satisfaction (Finch and Graziano, 2001). In another study focusing on couples in which one partner was depressed, more neuroticism in the depressed partner was related to lower dyadic marital satisfaction (Cano-Prous et al., 2013).
Toward A Biopsychosocial Approach
Beyond the known individual influences of cortisol reactivity, perceived partner social support, and neuroticism on perinatal depression, it is likely that these factors work synergistically to confer greater risk or protection. The available literature indicates that cortisol reactivity, perceived partner social support, and neuroticism individually contribute to perinatal depression risk. The current study aims to make a novel contribution by integrating biological and psychosocial bodies of study to investigate how cortisol reactivity, perceived social support from the father of the baby, and neuroticism contribute to depressive symptoms during pregnancy, both additively and in terms of statistical interactions between factors. We hypothesized that high depressive symptoms would be associated with less perceived social support the father, more neuroticism, and altered cortisol reactivity (heightened for laboratory stressor, blunted for CAR), and that statistical interactions between these three variables confer an increased odds for high depressive symptoms, over and above any associations found for each individual variable.
2. Method
2.1. Participants
The data presented here are part of a larger study that followed pregnant women throughout pregnancy and into the postpartum period. Women were included in the larger study if they were 18 years or older, could speak either English or Spanish, carried a singleton pregnancy, were less than 27 weeks’ GA based on last menstrual period at the time of recruitment, were free of medical conditions that could influence their HPA axis activity (e.g. major depression, severe pregnancy complications), did not smoke, drink alcohol, use drugs or take medications that could influence HPA axis activity, or if they suffered from math, speech, or needle phobia.
Of the 104 women enrolled in the larger study, 18 women dropped out after an optional visit early in pregnancy, and 22 additional women participated in the first regular study visit but were lost to follow up 10 weeks later, reducing the available sample size to 64. Women who were lost to follow up were not different in terms of age, ethnicity, income, marital status, or gestational age (GA). For the present analyses, women were also excluded if they declined to participate in the laboratory stressor (n = 6) or did participate but had missing or insufficient cortisol data (n = 4). Finally, women were excluded if they had cortisol levels at any sampling time point that deviated by more than 3 standard deviations from the mean (n = 5). Such extreme outliers could be the result of contaminated saliva (e.g., blood traces, food), and would disproportionately influence the results, especially considering the small sample size. Thus, the final sample consisted of 49 women. These 49 women were between the ages of 18 and 39 years (M = 26.7, SD = 5.7; see Table 1 for details). Ethnicity varied, and included women of Latina (57.1%), European-American (28.6%), Asian (10.2%), and mixed ethnicity (4.1%) descent. Most women reported being from a low-income household (63.8% <$50k), having some college education (40.8%), and being married (54.3%). The average GA was 21.3 weeks at the time the laboratory stressor was conducted (range: 9–30 wks’ GA).
Table 1.
Participant Characteristics Stratified by High/Low Depressive Symptoms (mean ± SD for continuous variables or n (%) for categorical variables).
| All (n = 49) |
High Depressive Symptoms (n = 14) |
Low Depressive Symptoms (n = 35) |
Group Comparisons | |
|---|---|---|---|---|
| Age | 26.67 (5.70) | 25.46 (7.23) | 27.11 (5.07) | t(46) = 0.89 |
| Gestational Age | 21.25 (5.11) | 20.77 (5.72) | 21.44 (4.92) | t(47) = 0.41 |
| Education | ||||
| High school or less | 13 (26.5) | 6 (42.9) | 7 (20.0) | χ2(3) = 4.21 |
| Some college | 20 (40.8) | 6 (42.9) | 14 (40.0) | |
| Bachelor’s | 11 (22.4) | 1 (7.1) | 10 (28.6) | |
| Other grad | 5 (10.2) | 1 (7.1) | 4 (11.4) | |
| Income | ||||
| % extremely low | 21 (44.7) | 6 (46.2) | 15 (44.1) | χ2(4) = 4.20 |
| % very low | 9 (19.1) | 4 (30.8) | 5 (14.7) | |
| % low | 6 (12.8) | -- | 6 (17.6) | |
| % median | 2 (4.3) | 1 (7.7) | 1 (2.9) | |
| % above median | 9 (19.1) | 2 (15.4) | 7 (20.6) | |
| Marital Status | χ2(2) = 1.04 | |||
| Single | 14 (40.0) | 5 (45.5) | 9 (37.5) | |
| Married | 19 (54.3) | 6 (54.5) | 13 (54.2) | |
| Divorced | 2 (5.7) | -- | 2 (8.3) | |
| Ethnicity | χ2(2) = 3.05 | |||
| Latina | 28 (59.6) | 11 (78.6) | 17 (51.5) | |
| White | 14 (29.8) | 2 (14.3) | 12 (36.4) | |
| Asian | 5 (10.6) | 1 (7.1) | 4 (12.1) | |
| Social Support (Others) | 3.69 (0.85) | 3.51 (1.12) | 3.76 (0.71) | t(17.55) = 0.78 |
| Social Support (Father) | 3.93 (1.07) | 3.32 (1.41) | 4.17 (0.80) | t(16.45) = 2.14* |
| Neuroticism | 4.55 (1.30) | 3.93 (1.71) | 4.80 (1.03) | t(16.92) = 1.78† |
| TSST Cortisol | ||||
| AUCg (nmol/L) | 84.70 (30.22) | 92.51 (7.77) | 81.06 (5.31) | F(1, 41) = 1.48 |
| Mean increase (nmol/L) | 0.04 (0.17) | −0.01 (0.05) | 0.07 (0.03) | F(1, 43) = 1.99 |
| Max increase (nmol/L) | 0.21 (0.25) | 0.10 (0.06) | 0.25 (0.04) | F(1, 46) = 3.71† |
| CAR | ||||
| AUCg (nmol/L) | 75.32 (30.32) | 67.95 (8.81) | 79.34 (6.50) | F(1, 31) = 1.08 |
| Mean increase (nmol/L) | −0.05 (0.31) | −0.17 (0.08) | 0.02 (0.06) | F(1, 31) = 3.22† |
| Max increase (nmol/L) | 0.13 (0.25) | 0.10 (0.07) | 0.15 (0.05) | F(1, 35) = 0.37 |
| Diurnal Slope | 0.89 (0.57) | 0.79 (0.18) | 0.93 (0.12) | F(1, 33) = 0.46 |
Comparing women high and low in depressive symptoms, by χ2 for categorical variables, and by t-test or ANOVA for continuous variables.
p < .05,
p < .10 (marginal)
2.2. Overall Procedure
Women reported to the laboratory either once or twice during their pregnancy, depending on their GA at the time of recruitment. Twenty-four of the 49 women were recruited before 22 weeks’ GA and reported to the laboratory once early in pregnancy (cohort 1, first visit: Mean GA = 16.5 weeks, range: 9 – 21 weeks) and again, approximately 10 weeks later (cohort 1, second visit: Mean GA = 25.7 weeks, range: 23.6 – 28.8 weeks). Another 25 women were recruited after they had reached 22 weeks’ GA, and they completed only the mid-pregnancy visit (cohort 2: Mean GA = 25.8 weeks, range 22–30 weeks). Both cohorts were recruited concurrently into the same study, and the only difference between the two cohorts was that some measures were collected at an earlier gestational age for women in cohort 1. No evidence of cohort differences in terms of major study variables (i.e., cortisol reactivity, perceived social support, neuroticism, depressive symptoms) were observed, with the exception of the area under the TSST response curve. For the purpose of the present report, the two cohorts were therefore combined. To be conservative, GA was controlled for in all relevant analyses.
Upon arrival, participants provided written informed consent and signed a HIPAA release. Hair and blood samples as well as blood pressure, heart rate, temperature, waist and hip circumference, and skinfolds were obtained (data not reported here). After a 10-minute resting period, during which women completed questionnaires, the first saliva sample was collected (−2 min). Participants were then escorted into a separate room where they participated in the Trier Social Stress Test (TSST; Kirschbaum et al., 1993). The TSST is a frequently-used and well-validated laboratory stressor designed to induce moderate increases in HPA axis activity. It consists of an introduction period (2 min) in which the task is described, a preparation period (3 min), followed by a five minute mock job interview and a five minute mental arithmetic task. The math and speech tasks were performed in front of two neutral, non-supportive evaluators. Participants were videotaped during the TSST. After the TSST, women returned to the resting room and additional saliva samples were collected at 1, 5, 10, 20, 30, 45, 60, and 90 minutes post-TSST while they continued to fill out questionnaires. Participants took home seven saliva collection tubes at the end of each study day and were instructed to obtain saliva samples two days after the study day. Home saliva samples were obtained four times within the first hour of awakening: immediately upon waking, as well as 30, 45, and 60 minutes after waking. Three more samples were collected at 12pm, 4pm, and 8pm.
2.3. Measures
Saliva Collection and Cortisol Assay.
Saliva samples were collected with the Salivette sampling device (Sarstedt, Nümbrecht, Germany) and stored at room temperature until the end of each study day. Samples were then stored at −70 degrees C until assayed. After thawing, samples were centrifuged for 10 minutes at 2000g and 4 degrees C. Free salivary cortisol was determined in duplicate by a commercially available enzyme-linked immuno-sorbent assay (ELISA, IBL-America, Minneapolis, MN). The assay sensitivity is 0.033 nmol/L and the dynamic range is 0–82.77 nmol/L. Inter- and intraassay coefficients of variance are reported at 4.9% and 4.1%, respectively.
Depressive Symptoms.
Participants completed the 10-item Edinburgh Postnatal Depression Scale (EPDS; Cox et al., 1987) which identifies individuals at risk for perinatal depression. The items in the scale ask participants to indicate how they have felt in the past week on a scale from 0 to 3, with higher scores indicating more severe symptoms. A score below 10 indicates a low probability of perinatal depression, scores of 10 or more indicate an individual is at risk for perinatal depression, and a score of 13 or higher is considered the cut-off score for possible incidence of depression (Cox et al., 1987; Murray and Carothers, 1990). Because few women in our sample scored 13 or higher (n = 7), we here used a cutoff of ≥ 10 to distinguish between women high in depressive symptoms and women low in depressive symptoms. The EPDS demonstrates good internal consistency (α =.88; Cox et al., 1987).
Social Support.
Participants completed the 19-item Medical Outcomes Study (MOS) Social Support Survey (Sherbourne and Stewart, 1991) at the mid-pregnancy visit. Typically, the MOS Social Support Survey asks about the perceived amount of support available to the respondent and is not source specific. In this study, the survey was modified to ask about the perceived amount of social support from the baby’s father and, in a second survey, about the perceived amount of social support from all other sources of social support (e.g. family, friends). The items were rated on a Likert-type scale ranging from 1 (none of the time) to 5 (all of the time). An overall score as well as four subscales reflecting affectionate support, emotional/informational support, positive social interaction support, and tangible support were computed. The MOS Social Support Survey has demonstrated good internal consistency for all subscales (α =.92-.97; Sherbourne and Stewart, 1991).
Neuroticism.
Participants completed the Ten-Item Personality Inventory (TIPI; Gosling et al., 2003) at their first visit. The TIPI is a brief measure of the Big-Five personality dimensions extraversion, openness, agreeableness, conscientiousness, and neuroticism. Participants were asked to rate how much they agree or disagree about the extent to which a pair of traits (e.g. anxious, easily upset) applies to them on a scale from 1 (disagree strongly) to 7 (agree strongly). Based on preliminary analyses indicating that, as hypothesized, neuroticism was the only personality variable to differ between women high and low in depressive symptoms and to correlate with other variables of interest, we here only report findings for the neuroticism dimension. Neuroticism is operationalized as having a high pole of emotional stability and a low pole of neuroticism. The TIPI has adequate levels of convergent validity with longer measures of personality, discriminant validity, and test-retest reliability (Gosling et al., 2003). It has also been validated in ethnically diverse samples (e.g., Ehrhart et al., 2009).
Sociodemographics.
Relevant sociodemographic factors (e.g., age, ethnicity, income, education, marital status), pregnancy- and health-related information (e.g., GA as estimated by last menstrual period, parity, obstetric and general health), and moderators of HPA activity (e.g., smoking, drug use) were assessed by questionnaire or interview.
2.4. Statistical Methods
Descriptive statistics were reported as frequency counts and percentages for categorical variables and as mean and standard deviation for continuous variables. Summary measures were computed reflecting the maximum cortisol response to the TSST (max value – [−2 min sample]) and the CAR (max value – [+0 min sample]); the mean cortisol response to the TSST ([+1, +5, +10, +20, +30 min samples]/5 – [−2 min sample]) and the CAR ([+30, +45, +60min samples]/3 – [+0 min sample]), and overall cortisol secretion for the TSST and the CAR (the area under the curve with respect to ground; AUCg). In addition, a daily slope was computed by subtracting the evening cortisol value from the waking cortisol value (Slope = +0 min sample – 8pm sample).
Group comparisons were conducted for relevant sociodemographic (age, GA, education, income, marital status, ethnicity) and major study variables (perceived social support from baby’s father, perceived social support from everyone else, mother’s neuroticism, cortisol summary scores), using t-tests and χ2-tests, as appropriate. Point-biserial correlations were conducted between perceived social support, neuroticism and the binary depressive symptoms variable. Because cortisol was collected at different timepoints depending on cohort, partial point-biserial correlations (controlling for GA) were conducted between cortisol summary scores and the binary depressive symptoms variable.
Variables that differed significantly or were marginally significant (p < .10) between women high and low in depressive symptoms were then entered as predictor variables into a hierarchical logistical regression model, controlling for GA and with the binary EPDS score as the outcome variable1. Blocks of predictors were entered into the equation sequentially based on the following theoretical assumptions. GA was entered in Block 1 as a covariate to control for its possible influence on depressive symptoms.2 Any significant or trending cortisol summary scores and perceived social support scores were entered into the model in Block 2, because previous work indicates that cortisol responses to the TSST and perceived social support are associated with perinatal depressive symptoms (Nierop et al., 2006; Yim et al., 2015). In Block 3, mother’s neuroticism was added because a separate line of research shows that neuroticism is associated with both cortisol stress reactivity and depressive symptoms (e.g., Campos et al., 2014; Lahey, 2009). To test for possible interaction effects, two-way interaction terms were entered in Block 4. In each block, R2 change (Adjusted Nagelkerke) was evaluated to determine the proportion of variance explained by each block.
Results
3.1. Preliminary Analyses
Fourteen women (28.6%) scored high in depressive symptoms and 35 (71.4%) scored low in depressive symptoms, using an EPDS cut-off score of ≥ 10. Women high in depressive symptoms reported less perceived social support from the baby’s father, t(16.45) = 2.14, p = .05 (Table 1). Similarly, the four individual subscales of perceived father’s social support (i.e., affectionate, emotional, positive interaction, and tangible support) either differed significantly or were marginally different when comparing women high and low in depressive symptoms (all t > 1.80, all p < .10). Women high in depressive symptoms were marginally higher in neuroticism (reflected by lower scores on the TIPI), t(16.92) = 1.78, p = .09. They also had a marginally higher cortisol response to the TSST (TSST max increase), F(1, 46) = 3.71, p = .06, and a flatter cortisol awakening response (CAR mean increase), F(1, 31) = 3.22, p = .08, controlling for GA (Figure 1). No significant differences in depressive symptoms were observed for any other sociodemographic or major study variables.
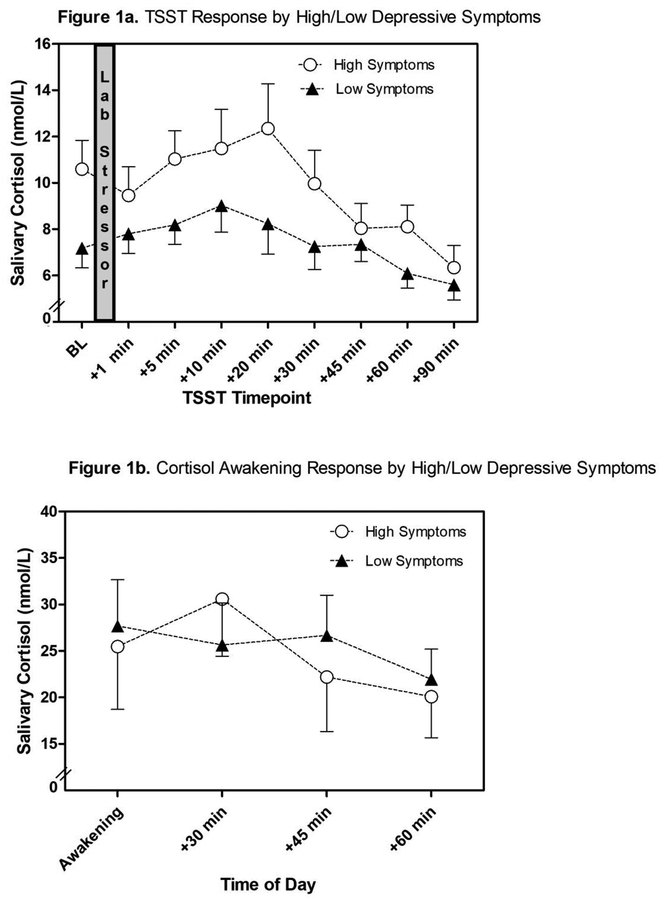
Figure 1.
Salivary cortisol responses to the Trier Social Stress Test (TSST; Figure 1a) and cortisol awakening response (Figure 1b) for women high (EPDS ≥ 10) versus low in depressive symptoms (EPDS < 10).
Note. For illustrative purposes only – the effects of neuroticism and social support are not accounted for in these graphs.
Point biserial and partial point biserial correlations were computed to examine the relation among perceived social support, neuroticism, cortisol summary measures, and the binary depressive symptoms variable (Table 2). Less perceived social support from father, rpb = −.37, p = .01, and more neuroticism, rpb = −.31, p = .03, were associated with scoring high in depressive symptoms. To test whether the overall association between perceived social support from father and depressive symptoms was driven by any of the subscales, we then correlated all subscales (i.e., affectionate, emotional, positive interaction, tangible) with the binary depressive symptoms variable. All four correlations were significant (all r < −.31, all p < .05), and therefore a decision was made to only use the overall scale for perceived father support in the regression models. Correlational analyses further suggested marginal associations between lower TSST max increase, partial rpb = −.27, p = .06 (direction opposite predicted association), and a lower CAR mean increase, partial rpb = −.33, p = .08 with high depressive symptoms, controlling for GA (see Figure 1 for an illustration of cortisol trajectories by high and low depressive symptoms). As can be seen in Figure 1a, pre-TSST cortisol concentrations were higher than cortisol 1 min after the TSST for women high in depressive symptoms, hinting at increased anticipatory stress. Notably, no discernable change in cortisol was observed for women low in depressive symptoms, which is in line with previous reports of attenuated cortisol reactivity during pregnancy (Entringer et al., 2010). Finally, more perceived social support from others was marginally associated with higher CAR AUCg, and lower neuroticism was significantly associated with blunted slope and marginally associated with lower CAR Max Increase.
Table 2.
Correlations between Major Study Variables (All Participants)
| High/Low Depressive Symptoms | Social Support (Father) | Social Support (Others) | Neuroticism | TSST AUCg | TSST Mean Increase | TSST Max Increase | CAR AUCg | CAR Mean Increase | CAR Max Increase | Diurnal Slope | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| High/Low Depressive Symptoms | – | ||||||||||
| Social Support (Father) | −.37** | – | |||||||||
| Social Support (Others) | −.14 | .11 | – | ||||||||
| Neuroticism | −.31* | .01 | −.11 | – | |||||||
| TSST AUCg | .22 | .24 | −.01 | −.14 | – | ||||||
| TSST Mean Increase | −.21 | −.07 | .26 | −.25 | −.01 | – | |||||
| TSST Max Increase | −.27† | −.09 | .18 | −.23 | −.26† | .82*** | – | ||||
| CAR AUCg | −.20 | .22 | .31† | .19 | .12 | −.04 | −.04 | – | |||
| CAR Mean Increase | −.33† | .07 | .24 | −.08 | −.04 | .40* | .36* | .40* | – | ||
| CAR Max Increase | −.11 | .05 | .07 | −.36† | .07 | 42** | .32* | −.07 | .75*** | – | |
| Diurnal Slope | −.16 | −.09 | .15 | .53** | −.33† | −.11 | −.13 | .39* | −.13 | −.13 | – |
Point-biserial correlations conducted for depressive symptoms. Partial point-biserial correlations controlling for GA conducted for all cortisol summary scores.
p < .001,
p < .01,
p < .05,
p < .10 (marginal)
In summary, simple comparisons suggested that high depressive symptoms were either associated significantly or marginally with low perceived social support from the baby’s father, high maternal neuroticism, reduced maximum cortisol increase to the TSST and reduced mean cortisol increase in response to awakening. To test the combined association of these variables with depressive symptoms, hierarchical logistic regressions were conducted. Because the TSST max increase and the CAR mean increase were significantly intercorrelated (partial r = .36, p = .04) but reflect different aspects of HPA axis function, two separate models, one including the TSST max increase and the other including the CAR mean increase as predictor variables, are reported3.
3.2. Main Results
Perceived social support from father, the TSST max increase and mother’s neuroticism predicted the binary depressive symptoms variable in a hierarchical regression model, R2 = .49, χ2 (Model) = 20.26, p < .001 (Table 3; Block 3), controlling for GA. In this model, perceived social support from father, Wald = 5.73, p = .02, the TSST max increase, Wald = 5.19, p = .02, and mother’s neuroticism, Wald = 6.49, p = .01, all emerged as significant contributors to depressive symptoms. This model correctly classified 88.6% of women as high in depressive symptoms and 57.1% of women as low in depressive symptoms, for a total accuracy rate of 79.6%. The inclusion of a two-way interaction (Block 4) did not significantly improve the model, but the emergence of a marginal interaction between perceived social support from father and mother’s neuroticism deserves mention, Wald = 3.39, p =.07. A model (Block 2) including only father’s social support and the TSST max increase (but not maternal neuroticism), yielded a weaker model fit χ2 (Block) = 11.10, p = .004, and only perceived social support from father emerged as significant, Wald = 5.47, p = .02.
Table 3.
Binary Logistic Regression Predicting the Likelihood of Being High or Low in Depressive Symptoms Based on Gestational Age, Social Support from Baby’s Father, TSST Maximum Cortisol Increase, and Neuroticism
| −2LL | R2 | X2 (Model) | X2 (Block) | B | SE (B) | Wald | OR | 95% CI OR | |
|---|---|---|---|---|---|---|---|---|---|
| Block 1 | 58.45 | 0.01 | 0.18 | 0.18 | |||||
| GA | −0.03 | 0.06 | 0.18 | 0.97 | [0.86, 1.10] | ||||
| Block 2 | 47.36 | 0.29 | 11.27* | 11.10** | |||||
| GA | −0.03 | 0.07 | 0.18 | 0.97 | [0.85, 1.11] | ||||
| Soc. Support (Father) | −0.85 | 0.37 | 5.47* | 0.43 | [0.21, 0.87] | ||||
| TSST Max Incr. | −3.74 | 1.96 | 3.64† | 0.02 | [0.001, 1.11] | ||||
| Block 3 | 38.37 | 0.49 | 20.26*** | 9.00** | |||||
| GA | −0.03 | 0.08 | 0.10 | 0.97 | [0.83, 1.14] | ||||
| Soc. Support (Father) | −1.30 | 0.54 | 5.73* | 0.27 | [0.09, 0.79] | ||||
| TSST Max Incr. | −5.82 | 2.55 | 5.19* | 0.003 | [0.00, 0.44] | ||||
| Neuroticism | −1.00 | 0.39 | 6.49* | 0.37 | [0.17, 0.79] | ||||
| Block 4 | 34.13 | 0.56 | 24.50*** | 4.24 | |||||
| GA | −0.04 | 0.09 | 0.20 | 0.96 | [0.81, 1.15] | ||||
| Soc. Support (Father) | −1.55 | 0.59 | 6.98** | 0.21 | [0.07, 0.67] | ||||
| TSST Max Incr. | −7.45 | 3.61 | 4.26* | 0.001 | [0.00, 0.69] | ||||
| Neuroticism | −1.07 | 0.43 | 6.15* | 0.34 | [0.15, 0.80] | ||||
| Soc. Support × TSST Max Incr. | 0.12 | 2.47 | 0.002 | 1.13 | [0.01, 141.66] | ||||
| Neuroticism × Soc. Support (Father) | −0.74 | 0.40 | 3.39† | 0.48 | [0.22, 1.05] | ||||
| Neuroticism × TSST Max Incr. | −1.01 | 1.85 | 0.30 | 0.36 | [0.01, 13.65] |
R2 is Adjusted Nagelkerke R2, OR = Odds ratio (Exponentiated B), C.I. = Confidence Interval; GA = gestational age, max incr. = maximum increase, Soc. = social, TSST = Trier Social Stress Test
p < .001,
p < .01,
p < .05,
p < .10 (marginal)
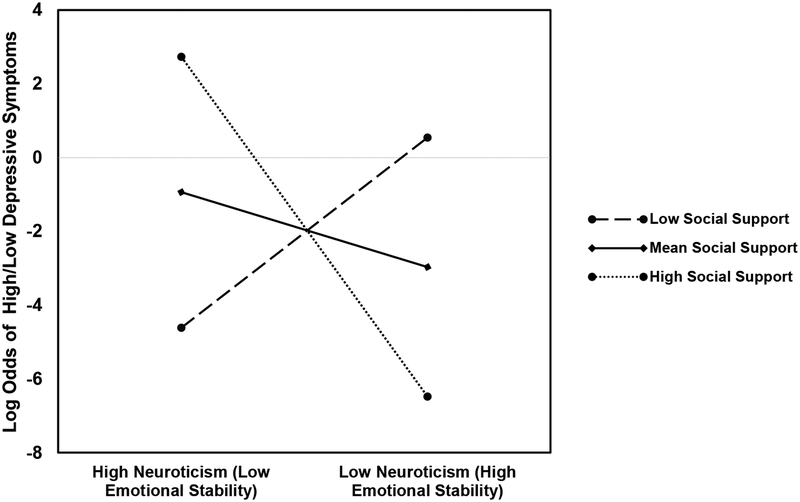
Next, the model was run again, replacing the TSST max increase with the CAR mean increase. The best-fitting model included perceived social support from father, the CAR mean increase, mother’s neuroticism, and the two-way interaction terms, R2 = 0.59, χ2(7) = 18.96, p = .01, again controlling for GA (Table 4; Block 4). In this model, the CAR mean increase, Wald = 3.77, p = .05, and the mother’s neuroticism × father’s social support interaction, Wald = 4.12, p =.04, emerged as significant. The interaction was probed using simple effects coefficients computed at high social support from father (1 SD above the mean), mean social support from father, and low social support from father (1 SD below the mean; see Figure 2 for interaction). At high levels of father support, lower levels of neuroticism (or higher levels of emotional stability) were significantly associated with a decrease in odds of depressive symptoms, b = −3.18, SE = 1.51, OR = .04, p = .04. At mean levels father support, lower levels of neuroticism were associated with a decrease in odds of depressive symptoms (b = −.70, SE = .55, OR = .50, ns.), and at low levels of support, with an increase in odds of depressive symptoms (b = 1.78, SE = 1.15, OR = 5.92, ns.), however neither of these effects were significant. This model correctly classified 95.5% of women as low in depressive symptoms and 66.7% of women as high in depressive symptoms, for a total accuracy rate of 85.3%. A reduced model not including the interaction terms (Block 3) yielded a significantly weaker fit, χ2 (Block) = 4.23, p = .04, and only marginal effects were observed for the CAR mean increase, Wald = 2.76, p = .10, and neuroticism, Wald = 3.15, p = .08. The model including only perceived social support from father and the CAR mean increase (Block 2) was not significant, R2 = .19, χ2 (3) = 5.05, p = .17.
Table 4.
Binary Logistic Regression Predicting the Likelihood of Being High or Low in Depressive Symptoms Based on Gestational Age, Social Support from Baby’s Father, CAR Mean Increase, and Neuroticism
| −2LL | R2 | X2 (Model) | X2 (Block) | B | SE (B) | Wald | OR | 95% CI OR | |
|---|---|---|---|---|---|---|---|---|---|
| Block 1 | 43.73 | 0.02 | 0.42 | 0.42 | |||||
| GA | −0.04 | 0.07 | 0.41 | 0.96 | [0.83, 1.10] | ||||
| Block 2 | 39.10 | 0.19 | 5.05 | 4.63† | |||||
| GA | −0.09 | 0.08 | 1.21 | 0.91 | [0.78, 1.07] | ||||
| Soc. Support (Father) | −0.44 | 0.38 | 1.31 | 0.65 | [0.31, 1.37] | ||||
| CAR Mean Incr. | −2.35 | 1.43 | 2.71 | 0.10 | [0.01, 1.56] | ||||
| Block 3 | 34.87 | 0.33 | 9.28* | 4.23* | |||||
| GA | −0.08 | 0.09 | 0.83 | 0.92 | [0.78, 1.10] | ||||
| Soc. Support (Father) | −0.68 | 0.43 | 2.55 | 0.51 | [0.22, 1.17] | ||||
| CAR Mean Incr. | −3.24 | 1.95 | 2.76† | 0.04 | [0.00, 1.78] | ||||
| Neuroticism | −0.67 | 0.38 | 3.15† | 0.51 | [0.25, 1.07] | ||||
| Block 4 | 25.19 | 0.59 | 18.96** | 9.68* | |||||
| GA | −0.32 | 0.19 | 2.82† | 0.73 | [0.50, 1.05] | ||||
| Social Support (Father) | 0.17 | 0.65 | 0.07 | 1.19 | [0.33, 4.27] | ||||
| CAR Mean Incr. | −6.94 | 3.57 | 3.77* | 0.001 | [0.00, 1.06] | ||||
| Neuroticism | −0.62 | 0.54 | 1.35 | 0.54 | [0.19, 1.54] | ||||
| Soc. Support (Father) × CAR Mean Incr. | −5.99 | 4.49 | 1.78 | 0.003 | [0.00, 16.67] | ||||
| Neuroticism × Soc. Support (Father) | −2.43 | 1.20 | 4.12* | 0.09 | [0.01, 0.92] | ||||
| Neuroticism × CAR Mean Incr. | −0.42 | 1.19 | 0.12 | 0.66 | [0.06, 6.81] |
R2 is Adjusted Nagelkerke R2, OR = Odds ratio (Exponentiated B), C.I. = Confidence Interval; CAR = cortisol awakening response, GA = gestational age, mean incr. = mean increase, Soc. = social
p < .01,
p < .05,
p < .10 (marginal)
Figure 2.
Log Odds of High or Low Depressive Symptoms at Low, Mean, and High Perceived Social Support from Father
Note: The graph depicts the mean, one SD above the mean and one SD below the mean for social support. At high social support, lower levels of neuroticism (or higher levels of emotional stability) were significantly associated with a decrease in odds of depressive symptoms. Full statistical findings are described in the text.
4. Discussion
The goal of the present study was to integrate biological and psychosocial bodies of work by testing whether individual associations of cortisol reactivity, perceived social support from father, and neuroticism with depressive symptoms during pregnancy would be strengthened by considering these variables additively and in terms of their statistical interactions. We ran two parallel hierarchical regression models, one conceptualizing cortisol stress reactivity as the maximum cortisol response to the TSST, the other as the mean CAR. Both models were significantly improved when multiple risk and protective factors were considered simultaneously, but the exact nature of these associations differed between the two models.
When we conceptualized cortisol reactivity as cortisol responses to the TSST, we found that low perceived support from baby’s father, a reduced cortisol maximum increase and increased maternal neuroticism were associated with scoring high in depressive symptoms. While perceived father’s support also emerged as significant in a reduced model not including maternal neuroticism, the maximum cortisol increase only emerged as significant when maternal neuroticism was included in the model. Though the direct test of the moderating effect of neuroticism on the maximum TSST cortisol response was not significant (likely due to low statistical power), these results still provide some indication that risk and protective factors do not act in isolation, but that moderating factors (e.g., neuroticism) may exist that can make the contribution of other variables (e.g., maximum TSST cortisol response) more salient. Future studies with larger sample sizes could test these moderating processes.
The direction of this association between a blunted maximum cortisol response and high depressive symptoms was unexpected and in the direction opposite to previous reports (Urizar et al., current issue; Nierop et al., 2006). Our reactivity measure was defined as the difference between the baseline value (−2 min) and each individual’s maximum cortisol level. As is evident from Figure 1a, high depressive symptom women’s baseline cortisol was elevated and, in fact, on average higher than the saliva sample obtained immediately after the TSST. Thus, the blunted response we found can be explained by increased baseline cortisol that may, at least in part, result from anticipatory stress. The increased anticipatory stress may be due to the sample characteristics of these high depressive symptom women. In our sample, the high depressive symptom group was comprised almost exclusively of Latinas, while our low depressive symptom group had a more even distribution of ethnicity. Thus, it is possible that cortisol patterns observed might be due to the compounding factors of being a low-income minority and having high depressive symptoms, though we did not have the statistical power to test group differences. Low-income U.S. born and immigrant Latinas are often overlooked in laboratory studies and in studies examining perinatal depression (Lara-Cinisomo et al., 2016; Lara-Cinisomo et al., 2015), and the setting of the TSST may be more unfamiliar or at odds with norms of culturally appropriate communication in this population. The stressors encountered by these women over the course of many years, as well as during the perinatal period, are different from those of European-American women who have been traditionally studied in the perinatal depression literature (Lara-Cinisomo et al., 2016), perhaps leading to increased anticipatory stress in novel situations, such as the TSST. To this end, more research inclusive of women from non-European backgrounds is needed.
A model conceptualizing cortisol reactivity as the CAR yielded a somewhat different pattern. Here, the best-fitting model suggests that a lower cortisol mean increase was associated with high pregnancy depressive symptoms, whereas high perceived social support from baby’s father in the context of low maternal neuroticism was protective. Of note, neither maternal neuroticism nor perceived social support from father emerged as individual significant predictors. Overall, this finding provides evidence supporting our argument that moderating processes are important to consider in studies aiming to predict depressive symptoms during the perinatal period. In terms of the direction of the association between the CAR and depressive symptoms, we found the predicted blunting with heightened depressive symptoms. However, as is illustrated in Figure 1b, this blunting is only observed in terms of the mean cortisol response (i.e., when subtracting the average of the +30 min, +45 min, and +60 min samples from baseline). The group of women high in depressive symptoms also showed a more pronounced cortisol increase from 0 to 30 minutes after waking compared to the group of women with low depressive symptoms, however, an association between this increase and depressive symptoms was not observed.
In terms of the direction of associations found for perceived social support from baby’s father and maternal neuroticism, they were in the expected direction (Gutierrez-Zotes et al., 2015; Iliadis et al., 2015). A caveat in terms of the link between neuroticism and pregnancy depressive symptoms is that a tendency toward experiencing more depressive symptoms is inherent to neuroticism. While we cannot exclude the possibility that our findings were driven mostly by the negative affect aspect of neuroticism, larger, prospective studies that accounted for negative affect facets of neuroticism scales have still found robust associations with depression (Lahey, 2009). In terms of social support, our study adds to a growing number of studies highlighting the importance of perceived social support from the baby’s father (Dennis and Letourneau, 2007; Stapleton et al., 2012)4.
The statistical interaction between perceived social support and neuroticism in this model highlights the potential influence of person-context interactions. Women low in neuroticism may be able to better capitalize on social support from baby’s father during a stressful time like pregnancy, such that this support can act as a buffer against the effects of stress on depressive symptoms. Conversely, and similar to what has been shown in non-pregnant populations, heightened neuroticism may make it more difficult for pregnant women to benefit from the social support offered by the baby’s father. This is consistent with research reporting that neuroticism likely moderates the relation between social support and depression risk (e.g., Maliszewska et al., 2016). Thus, neuroticism is one individual-level factor that may be taken into consideration when assessing perinatal depression risk. While the statistical interaction between perceived social support from baby’s father and maternal neuroticism did not emerge as significant when the TSST cortisol response was entered as a predictor variable, it should be noted that this interaction was marginally significant, and it is possible that larger studies would yield significant findings. Alternatively, it is possible that differences between the models are due to the fact that cortisol responses to the TSST result from an external, stressful stimulus whereas cortisol responses are associated with basal circadian fluctuations in cortisol tied to the sleep-wake transition (Kudielka & Wüst, 2010).
This study is not without its limitations. Most prominently, our sample size is fairly small and results should be interpreted with caution. Nonetheless, significant findings emerging in our final models are in alignment with theoretical predictions and prior empirical work. Moreover, simple correlations were strongly powered and provided a foundation to test further effects. It is also the case that by excluding women with Major Depression from our study, we truncated our symptom distribution, making our test more stringent. Future work drawing on larger cohorts should consider testing the importance of possible additional moderators, such as income and ethnicity. A second limitation lies in the combination of two cohorts of women. In Cohort 1, assessments were spread across two study visits which occurred roughly ten weeks apart whereas in Cohort 2, all assessments were obtained concurrently. While our data are limited by this design, cohort differences were not observed for major study variables included in the final models. Moreover, we controlled for GA in all relevant analyses, providing us with sufficient confidence that our findings hold despite this limitation. Third, compared to longer, more detailed measures of personality, the TIPI has low reliability (Gosling, Rentfrow, & Swann, 2003), but still has good convergent validity with longer measures of personality (e.g., Five Factor Model; Ehrhart et al., 2009). Future studies should incorporate longer measures to more accurately capture facets of personality. Finally, it is likely that perinatal depression risk is in part driven by other biological mechanisms, including for example neurological dysfunction, that have been linked to HPA axis fluctuations. However, these are beyond the scope of this study and future studies should incorporate a closer examination of the various underlying biological mechanisms that may influence perinatal depression risk.
This study is one of the first to empirically test an integrative theoretical model of predictors of depressive symptoms during pregnancy, underscoring the importance of using a biopsychosocial approach. It also contributes to a still small literature addressing these and similar questions in diverse and low SES samples. We recommend that larger studies be conducted exploring how conceptually related variables additively predict or act as modifiers to predict perinatal depression risk in diverse samples. Such studies will contribute to improving accuracy for screening women at risk for perinatal depression and stand to provide meaningful context to pregnant women’s experiences.
Highlights (Kofman et al.).
Lower cortisol reactivity, social support, and neuroticism contribute to depressive symptoms
Father’s support but not support from other sources accounted for this association
High father support in the context of low maternal neuroticism was protective
Different models emerged depending on how cortisol reactivity was conceptualized
Acknowledgments
This work was funded, in part, by the National Institute of Mental Health (R03, MH082270; Yim), a NARSAD Young Investigator Award (Yim), and a University of California Mexus – Conacyt collaborative grant (Campos, Yim). This study received further support from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR000153. The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies. Dr. Wing is now Senior Client Partner with Korn Ferry, Academic Health Center Practice.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Notes
Analyses were also conducted with the EPDS as a continuous variable using a hierarchical regression, however findings failed to replicate and were non-significant. It may be the case that a threshold approach to assessing depressive symptoms is more appropriate and clinically relevant in terms of capturing associations between depressive symptoms and other health-relevant outcomes.
All analyses were also run including the cortisol × GA interaction, which we hypothesized was likely to affect our findings. However, this variable did not emerge as significant and did not change the significance of any findings. Thus, we chose to report our findings controlling only for the direct effect of GA
A hierarchical logistic regression analysis was nonetheless conducted for a model which included both the TSST max increase and the CAR mean increase. In the best fitting model, χ2 (Model) = 15.27, p = .009, the TSST max increase emerged as significant, Wald = 3.76, p = .05, whereas the CAR mean increase did not, Wald = 1.43, p = .23, suggesting that the TSST response is the stronger of the two predictors. Because our sample size is small and the addition of another predictor variable may have resulted in a problem with statistical power, we decided to report findings for the two cortisol measures in separate regression analyses.
Due to sample size, we only included social support from the father in our overall model based on preliminary findings indicating that depressive symptom groups differed significantly in terms of father support but not support from all other sources. Nonetheless, we conducted analyses to test both models with social support from all sources (without social support from father). When testing both models with support from other sources, Model 1 (TSST Max Increase) was weaker than when using social support from father, and only Block 2 reached significance, R2 = .41, χ2 (Block) = 10.93 p = .004. The same was true for Model 2 (CAR Mean Increase) and only Block 2 reached statistical significance, R2 = .63, χ2 (Block) = 13.25 p = .001.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Campos B, Busse D, Yim IS, Dayan A, Chevez L, Schoebi D, 2014. Are the costs of neuroticism inevitable? Evidence of attenuated effects in U.S. Latinas. Cultural diversity & ethnic minority psychology 20, 430–440. [DOI] [PubMed] [Google Scholar]
- Cano-Prous A, Moya-Querejeta J, Alonso A, Martin-Lanas R, Cervera-Enguix S, 2013. Personality: a determinant in marital dissatisfaction in individuals with major depression and their couples. Actas espanolas de psiquiatria 41, 340–348. [PubMed] [Google Scholar]
- Cheng CY, Pickler RH, 2009. Effects of stress and social support on postpartum health of Chinese mothers in the United States. Research in Nursing & Health 32, 582–591. [DOI] [PubMed] [Google Scholar]
- Cohen S, 2004. Social relationships and health. Am Psychol 59, 676–684. [DOI] [PubMed] [Google Scholar]
- Collins NL, Dunkel-Schetter C, Lobel M, Scrimshaw SC, 1993. Social support in pregnancy: Psychosocial correlates of birth outcomes and postpartum depression. Journal of Personality and Social Psychology 65, 1243. [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R, 1987. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry 150, 782–786. [DOI] [PubMed] [Google Scholar]
- Dennis C-L, Letourneau N, 2007. Global and relationship-specific perceptions of support and the development of postpartum depressive symptomatology. Social Psychiatry and Psychiatric Epidemiology 42, 389–395. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Heinrichs M, 2014. Psychobiology of social support: the social dimension of stress buffering. Restorative neurology and neuroscience 32, 149–162. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M, 2001. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus–pituitary–adrenal axis. Biological Psychology 57, 141–152. [DOI] [PubMed] [Google Scholar]
- Ehrhart MG, Ehrhart KH, Roesch SC, Chung-Herrera BG, Nadler K, Bradshaw K, 2009. Testing the latent factor structure and construct validity of the Ten-Item Personality Inventory. Personality and Individual Differences 47, 900–905. [Google Scholar]
- Entringer S, Buss C, Shirtcliff EA, Cammack AL, Yim IS, Chicz-DeMet A, Sandman CA, Wadhwa PD, 2010. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress 13, 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch JF, Graziano WG, 2001. Predicting depression from temperament, personality, and patterns of social relations. Journal of personality 69, 27–55. [DOI] [PubMed] [Google Scholar]
- Frisch JU, Häusser JA, Mojzisch A, 2015. The Trier Social Stress Test as a paradigm to study how people respond to threat in social interactions. Frontiers in Psychology 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelabert E, Subirà S, García-Esteve L, Navarro P, Plaza A, Cuyàs E, Navinés R, Gratacòs M, Valdés M, Martín-Santos R, 2012. Perfectionism dimensions in major postpartum depression. Journal of Affective Disorders 136, 17–25. [DOI] [PubMed] [Google Scholar]
- Gelabert E, Subirà S, Plaza A, Torres A, Navarro P, Ímaz ML, Valdés M, García-Esteve L, Martín-Santos R, 2011. The vulnerable personality style questionnaire: psychometric properties in Spanish postpartum women. Archives of Women’s Mental Health 14, 115–124. [DOI] [PubMed] [Google Scholar]
- Gelman PL, Flores-Ramos M, López-Martínez M, Fuentes CC, Grajeda JPR, 2015. Hypothalamic-pituitary-adrenal axis function during perinatal depression. Neuroscience bulletin 31, 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, Sandman CA, 2013. New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides 47, 363–370. [DOI] [PubMed] [Google Scholar]
- Gosling SD, Rentfrow PJ, Swann WB Jr, 2003. A very brief measure of the Big-Five personality domains. Journal of Research in Personality 37, 504–528. [Google Scholar]
- Gutierrez-Zotes A, Labad J, Martin-Santos R, Garcia-Esteve L, Gelabert E, Jover M, Guillamat R, Mayoral F, Gornemann I, Canellas F, Gratacos M, Guitart M, Roca M, Costas J, Luis Ivorra J, Navines R, de Diego-Otero Y, Vilella E, Sanjuan J, 2015. Coping strategies and postpartum depressive symptoms: A structural equation modelling approach. European psychiatry: the journal of the Association of European Psychiatrists 30, 701–708. [DOI] [PubMed] [Google Scholar]
- Hakulinen C, Elovainio M, Pulkki-Raback L, Virtanen M, Kivimaki M, Jokela M, 2015. Personality and ddepressive syymptoms: IIndividual participant meta-analysis of 10 cohort studies. Depression and anxiety 32, 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman C, Dunn DM, Njoroge WF, 2017. Impact of Postpartum Mental Illness Upon Infant Development. Current Psychiatry Reports 19, 100. [DOI] [PubMed] [Google Scholar]
- Holsboer F, 2000. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23, 477–501. [DOI] [PubMed] [Google Scholar]
- Iliadis SI, Koulouris P, Gingnell M, Sylvén SM, Sundström-Poromaa I, Ekselius L, Papadopoulos FC, Skalkidou A, 2015. Personality and risk for postpartum depressive symptoms. Archives of Women’s Mental Health 18, 539–546. [DOI] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D, 2010. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychol Bull 136, 768–821. [DOI] [PubMed] [Google Scholar]
- Lahey BB, 2009. Public health significance of neuroticism. Am Psychol 64, 241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Cinisomo S, Girdler SS, Grewen K, Meltzer-Brody S, 2016. A Biopsychosocial Conceptual Framework of Postpartum Depression Risk in Immigrant and U.S.-born Latina Mothers in the United States. Women’s Health Issues 26, 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Cinisomo S, Wisner KL, Meltzer-Brody S, 2015. Advances in science and biomedical research on postpartum depression do not include meaningful numbers of Latinas. Journal of Immigrant and Minority Health 17, 1593–1596. [DOI] [PubMed] [Google Scholar]
- Lee D, Yip A, Leung T, Chung T, 2000. Identifying women at risk of postnatal depression: prospective longitudinal study. Hong Kong Med J 6, 349–354. [PubMed] [Google Scholar]
- Maliszewska K, Swiatkowska-Freund M, Bidzan M, Preis K, 2016. Relationship, social support, and personality as psychosocial determinants of the risk for postpartum blues. Ginekologia polska 87, 442–447. [DOI] [PubMed] [Google Scholar]
- Marín-Morales D, Carmona-Monge FJ, Peñacoba-Puente C, 2014. Personality, depressive symptoms during pregnancy and their influence on postnatal depression in Spanish pregnant Spanish women. [Personalidad, síntomas depresivos en el embarazo y su influencia en la depresión postparto en gestantes españolas]. Anales de Psicología 30. [Google Scholar]
- Martin-Santos R, Gelabert E, Subira S, Gutierrez-Zotes A, Langorh K, Jover M, Torrens M, Guillamat R, Mayoral F, Canellas F, Iborra JL, Gratacos M, Costas J, Gornemann I, Navines R, Guitart M, Roca M, R DEF, Vilella E, Valdes M, Esteve LG, Sanjuan J, 2012. Research letter: is neuroticism a risk factor for postpartum depression? Psychol Med 42, 1559–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R, 1995. A placental clock controlling the length of human pregnancy. Nature Medicine 1, 460. [DOI] [PubMed] [Google Scholar]
- Morling B, Kitayama S, Miyamoto Y, 2003. American and Japanese women use different coping strategies during normal pregnancy. Personality and Social Psychology Bulletin 29, 1533–1546. [DOI] [PubMed] [Google Scholar]
- Murray L, Carothers AD, 1990. The validation of the Edinburgh Post-natal Depression Scale on a community sample. The British Journal of Psychiatry 157, 288–290. [DOI] [PubMed] [Google Scholar]
- Nierop A, Bratsikas A, Zimmermann R, Ehlert U, 2006. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms? Psychosomatic Medicine 68, 931–937. [DOI] [PubMed] [Google Scholar]
- Norhayati M, Hazlina NN, Asrenee A, Emilin WW, 2015. Magnitude and risk factors for postpartum symptoms: a literature review. Journal of affective Disorders 175, 34–52. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Tang W, Gilchrist MA, Moynihan JA, Pressman EK, Blackmore ER, 2014. Diurnal cortisol patterns and psychiatric symptoms in pregnancy: short-term longitudinal study. Biological Psychology 96, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J, Bastiaansen A, Riese H, Bos EH, Servaas M, Ellenbogen M, Rosmalen JG, Aleman A, 2013. The biological and psychological basis of neuroticism: current status and future directions. Neurosci Biobehav Rev 37, 59–72. [DOI] [PubMed] [Google Scholar]
- Orta OR, Gelaye B, Bain PA, Williams MA, 2018. The association between maternal cortisol and depression during pregnancy, a systematic review. Arch Womens Ment Health 21, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer M, Soares CN, Levitan RD, Streiner DL, Steiner M, 2013. Antenatal depression in a multi-ethnic, community sample of Canadian immigrants: psychosocial correlates and hypothalamic-pituitary-adrenal axis function. The Canadian Journal of Psychiatry 58, 579–587. [DOI] [PubMed] [Google Scholar]
- Pluess M, Bolten M, Pirke KM, Hellhammer D, 2010. Maternal trait anxiety, emotional distress, and salivary cortisol in pregnancy. Biol Psychol 83, 169–175. [DOI] [PubMed] [Google Scholar]
- Podolska MZ, Bidzan M, Majkowicz M, Podolski J, Sipak-Szmigiel O, Ronin-Walknowska E, 2010. Personality traits assessed by the NEO Five-Factor Inventory (NEO-FFI) as part of the perinatal depression screening program. Medical Science Monitor 16, PH77–PH81. [PubMed] [Google Scholar]
- Saisto T, Salmela-Aro K, Nurmi JE, Halmesmäki E, 2001. Psychosocial predictors of disappointment with delivery and puerperal depression. Acta Obstetricia et Gynecologica Scandinavica 80, 39–45. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C, 2006. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides 27, 1457–1463. [DOI] [PubMed] [Google Scholar]
- Saxbe DE, Schetter CD, Guardino CM, Ramey SL, Shalowitz MU, Thorp J, Vance M, 2016. Sleep Quality Predicts Persistence of Parental Postpartum Depressive Symptoms and Transmission of Depressive Symptoms from Mothers to Fathers. Annals of behavioral medicine: a publication of the Society of Behavioral Medicine 50, 862–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer K, Urizar GG Jr., 2016. Altered stress patterns and increased risk for postpartum depression among low-income pregnant women. Arch Womens Ment Health 19, 317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth S, Lewis AJ, Galbally M, 2016. Perinatal maternal depression and cortisol function in pregnancy and the postpartum period: a systematic literature review. BMC Pregnancy Childbirth 16, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbourne CD, Stewart AL, 1991. The MOS social support survey. Social Science & Medicine 32, 705–714. [DOI] [PubMed] [Google Scholar]
- Smith R, 2007. Parturition. New England Journal of Medicine 356, 271–283. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vale WW, 2006. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in Clinical Neuroscience 8, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa R, Kennell J, Klaus M, Robertson S, Urrutia J, 1980. The effect of a supportive companion on perinatal problems, length of labor, and mother-infant interaction. New England Journal of Medicine 303, 597–600. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S, Dockray S, Smyth N, Evans P, Hellhammer DH, Miller R, Wetherell MA, Lupien SJ, Clow A, 2016. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology 63, 414–432. [DOI] [PubMed] [Google Scholar]
- Stapleton LRT, Schetter CD, Westling E, Rini C, Glynn LM, Hobel CJ, Sandman CA, 2012. Perceived partner support in pregnancy predicts lower maternal and infant distress. Journal of Family Psychology 26, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, Howard LM, Pariante CM, 2014. Effects of perinatal mental disorders on the fetus and child. The Lancet 384, 1800–1819. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE, 2011. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 73, 114–126. [DOI] [PubMed] [Google Scholar]
- Taylor A, Glover V, Marks M, Kammerer M, 2009. Diurnal pattern of cortisol output in postnatal depression. Psychoneuroendocrinology 34, 1184–1188. [DOI] [PubMed] [Google Scholar]
- van Bussel JC, Spitz B, Demyttenaere K, 2009. Depressive symptomatology in pregnant and postpartum women. An exploratory study of the role of maternal antenatal orientations. Archives of Women’s Mental Health 12, 155–166. [DOI] [PubMed] [Google Scholar]
- Woody C, Ferrari A, Siskind D, Whiteford H, Harris M, 2017. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. Journal of Affective Disorders 219, 86–92. [DOI] [PubMed] [Google Scholar]
- Yim IS, Glynn LM, Schetter CD, Hobel CJ, Chicz-DeMet A, Sandman CA, 2009. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Archives of General Psychiatry 66, 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim IS, Tanner Stapleton LR, Guardino CM, Hahn-Holbrook J, Dunkel Schetter C, 2015. Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol 11, 99–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H, 2003. Mineralocorticoid receptor function in major depression. Arch Gen Psychiatry 60, 24–28. [DOI] [PubMed] [Google Scholar]
- Zobel A, Barkow K, Schulze-Rauschenbach S, Von Widdern O, Metten M, Pfeiffer U, Schnell S, Wagner M, Maier W, 2004. High neuroticism and depressive temperament are associated with dysfunctional regulation of the hypothalamic–pituitary–adrenocortical system in healthy volunteers. Acta Psychiatrica Scandinavica 109, 392–399. [DOI] [PubMed] [Google Scholar]