Supplemental Digital Content is available in the text.
Keywords: cerebral infarction, heparin, reperfusion, stroke, thrombectomy
Abstract
Background and Purpose—
Intravenous administration of heparin during endovascular treatment for ischemic stroke may improve outcomes. However, risks and benefits of this adjunctive therapy remain uncertain. We aimed to evaluate periprocedural intravenous heparin use in Dutch stroke intervention centers and to assess its efficacy and safety.
Methods—
Patients registered between March 2014 and June 2016 in the MR CLEAN Registry (Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke), including all patients treated with endovascular treatment in the Netherlands, were analyzed. The primary outcome was functional outcome (modified Rankin Scale) at 90 days. Secondary outcomes were successful recanalization (extended Thrombolysis in Cerebral Infarction ≥2B), symptomatic intracranial hemorrhage, and mortality at 90 days. We used multilevel regression analysis to evaluate the association of periprocedural intravenous heparin on outcomes, adjusted for center effects and prognostic factors. To account for possible unobserved confounding by indication, we analyzed the effect of center preference to administer intravenous heparin, defined as percentage of patients treated with intravenous heparin in a center, on functional outcome.
Results—
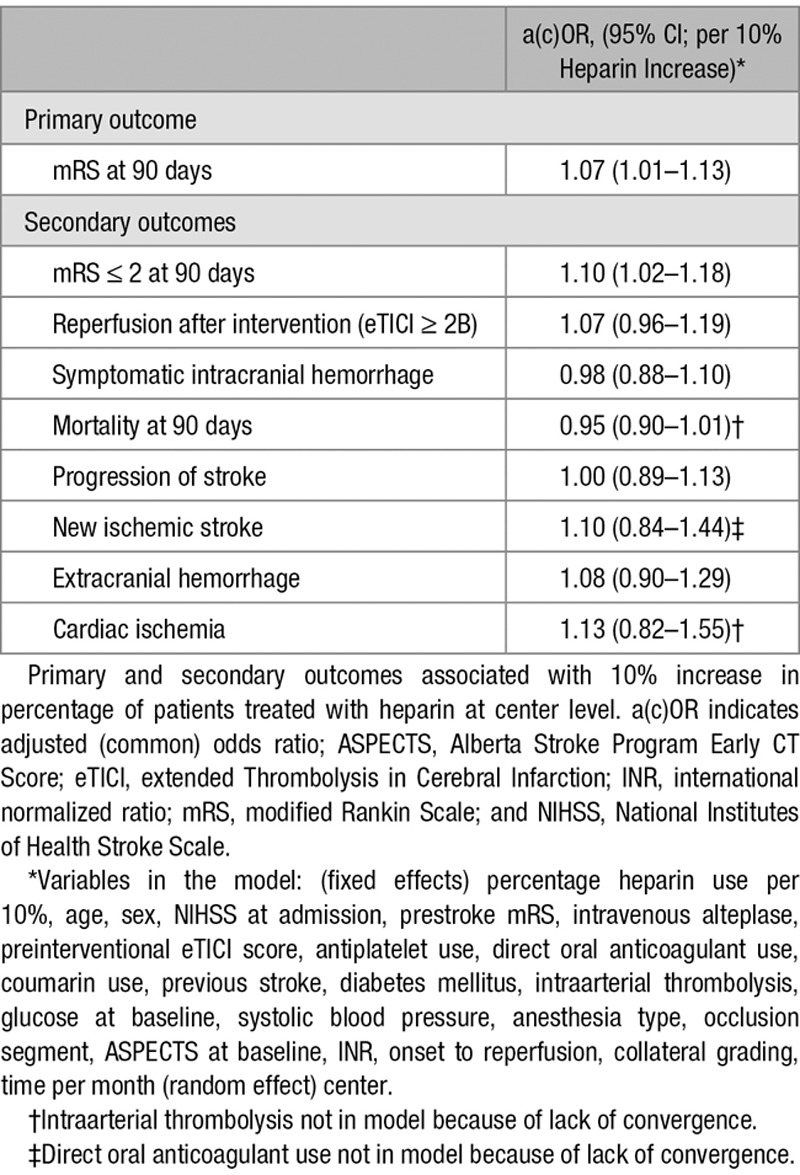
One thousand four hundred eighty-eight patients from 16 centers were analyzed, of whom 398 (27%) received intravenous heparin (median dose 5000 international units). There was substantial between-center variability in the proportion of patients treated with intravenous heparin (range, 0%–94%). There was no significant difference in functional outcome between patients treated with intravenous heparin and those without (adjusted common odds ratio, 1.17; 95% CI, 0.87–1.56), successful recanalization (adjusted odds ratio, 1.24; 95% CI, 0.89–1.71), symptomatic intracranial hemorrhage (adjusted odds ratio, 1.13; 95% CI, 0.65–1.99), or mortality (adjusted odds ratio, 0.95; 95% CI, 0.66–1.38). Analysis at center level showed that functional outcomes were better in centers with higher percentages of heparin administration (adjusted common odds ratio, 1.07 per 10% more heparin, 95% CI, 1.01–1.13).
Conclusions—
Substantial between-center variability exists in periprocedural intravenous heparin use during endovascular treatment, but the treatment is safe. Centers using heparin more often had better outcomes. A randomized trial is needed to further study these effects.
About one-third of the patients with ischemic stroke caused by an intracranial large vessel occlusion do not recover to functional independence, despite early and complete recanalization by endovascular treatment (EVT).1 Although EVT is successful in reopening large intracranial arteries, it does not always restore microvascular perfusion. This incomplete microvascular reperfusion, also described as the no-reflow phenomenon, has first been reported in animal studies.2–4 One of the causes of microvascular obstruction is the formation of neutrophil extracellular traps, which are known to be present in all thrombi of ischemic stroke patients irrespective of stroke cause.5 neutrophil extracellular traps are resistant to r-tPA (recombinant tissue-type plasminogen activator), but experimental studies show that unfractionated heparin is able to dissolve neutrophil extracellular traps at the microvascular level.6–9 The effect of unfractionated heparin on neutrophil extracellular traps in humans has not been evaluated. In the pre-EVT era, no benefit of heparin use on outcome in ischemic stroke patients was seen, with a concomitant 1.2% increase in occurrence of symptomatic intracranial hemorrhage (sICH).10 However, as the rate of successful recanalization is high in patients treated with EVT, heparin is now more capable of penetrating the downstream microvessels and targeting the no-reflow areas. That heparin may contribute to the treatment effect of EVT is not a new concept but originates from cardiology practices: periprocedural heparin has been used since the first percutaneous coronary intervention performed in 1977 and is standard practice since then.11 By contrast, heparin is not the standard anticoagulant in EVT for ischemic stroke, which might be related to the perceived risk of sICH. In a systematic literature review, we found that heparin use during EVT indeed seems to be associated with an increased risk of sICH, but this increase seems to be outweighed by a higher overall chance of a good functional outcome.12 The risk-benefit ratio of periprocedural intravenous heparin in patients with ischemic stroke undergoing EVT is still unclear. The uncertainty regarding this risk-benefit ratio is also reflected in the wide variation in the use of heparin in randomized trials that investigated the effect of EVT.13 We aimed to evaluate the use of intravenous heparin during EVT in Dutch stroke intervention centers and to assess its efficacy and safety.
Methods
Study Design
We used data from the MR CLEAN Registry (Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke), which is an ongoing, nationwide, multicenter, prospective, observational study, including all consecutive patients treated with EVT for ischemic stroke in the Netherlands. The complete methods and description of variables of the MR CLEAN Registry have been described elsewhere.14 For the present study, we selected patients who were registered between March 2014 and June 2016 and adhered to the following criteria: age of ≥18 years; treatment in a center that participated in the MR CLEAN trial; presence of a proximal intracranial occlusion in the anterior circulation confirmed on noninvasive vascular imaging (intracranial carotid artery [internal carotid artery (terminus)], middle cerebral artery [M1/M2], anterior cerebral artery [A1/A2]); and groin puncture within 6.5 hours after symptom onset. The current observational study was guided by the STROBE statement (Strengthening the Reporting of Observational Studies in Epidemiology).15 Data cannot be made available, as no patient approval has been obtained for sharing coded data. However, syntax files and output of statistical analyses in R will be made available on request.
Unfractionated Heparin Administration
Heparin administration was defined as any intravenous dose of unfractionated heparin administered during EVT. We explored the variability in doses of heparin used and percentages of patients treated with heparin within and between centers and over time. When information on heparin administration was missing, we assumed no heparin was administered to the patient. We performed 2 sensitivity analyses on this matter. First, we compared baseline characteristics of the group of patients whom we assumed not to have been treated with heparin to the patients explicitly registered as not treated with heparin. Second, we performed a complete case analysis of the primary and secondary outcomes in patients explicitly registered as treated with heparin versus no heparin.
Outcome Measures
The primary outcome was functional outcome at 90 days (range 14 days either way), assessed with the modified Rankin Scale (mRS), which is a 7-point ordinal scale ranging from 0 no symptoms to 6 dead.16 Secondary outcomes were good functional outcome (mRS ≤2) at 90 days, successful recanalization rate (extended Thrombolysis in Cerebral Infarction grade ≥ 2B) assessed by an independent imaging core laboratory, occurrence of sICH, defined as patient neurological deterioration (decline of 4 points or more on the National Institutes of Health Stroke Scale) and a compatible hemorrhage seen on imaging assessed by an independent imaging core laboratory (according to the Heidelberg criteria), mortality at 90 days, progression of ischemic stroke (resulting in a decline of at least 4 points on the National Institutes of Health Stroke Scale), new ischemic stroke (imaging of new brain infarction with corresponding clinical neurological deficit), extracranial hemorrhage, and cardiac ischemia (myocardial ischemia confirmed by ECG and release of appropriate biomarkers).
Statistical Methods
Differences in baseline characteristics were analyzed for both categorical and dichotomous variables using χ2 statistics. Continuous data were assessed for normality both visually and by means of Kolmogorov-Smirnov testing. One-way ANOVA was used for parametric and Kruskal-Wallis for nonparametric testing. A P value of <0.05 was considered significant in all applied tests. All baseline data and outcomes that are reported are crude and not imputed. Any mRS score assessed within 30 days of symptom onset was considered invalid and treated as missing. For the purpose of unbiased estimation of associations of outcome with baseline characteristics, we replaced missing outcome values when missing in <10% of the patients (eg, mRS) by values derived from multiple imputation.17 Multiple imputed data were used in the adjusted outcome analyses. We used multilevel logistic and ordinal regression analyses to compare outcomes of patients treated with and without periprocedural intravenous heparin, with center as random effect and relevant factors as fixed effects (ie, heparin use, age, sex, National Institutes of Health Stroke Scale at admission, prestroke mRS, antiplatelet use, direct oral anticoagulant use, coumarin use, previous stroke, diabetes mellitus, glucose level at baseline, international normalized ratio, baseline systolic blood pressure, occlusion segment, Alberta Stroke Program Early CT Score at baseline, collateral grading, treatment with intravenous alteplase, anesthesia type, preinterventional extended Thrombolysis in Cerebral Infarction (eTICI) score, intraarterial thrombolysis, and onset-to-reperfusion time). Effects are presented as (adjusted common) odds ratios (OR) with 95% CI. To account for possible confounding by indication, we also analyzed the effect of center preference to administer heparin, defined as percentage of patients treated with heparin in a center, on outcome. All statistical analyses were performed with R version 3.5.0 (R foundation for Statistical Computing, Vienna, Austria) with the packages: tableone, mice, Hmisc, ggplot, and ordinal.
Results
Patient Population
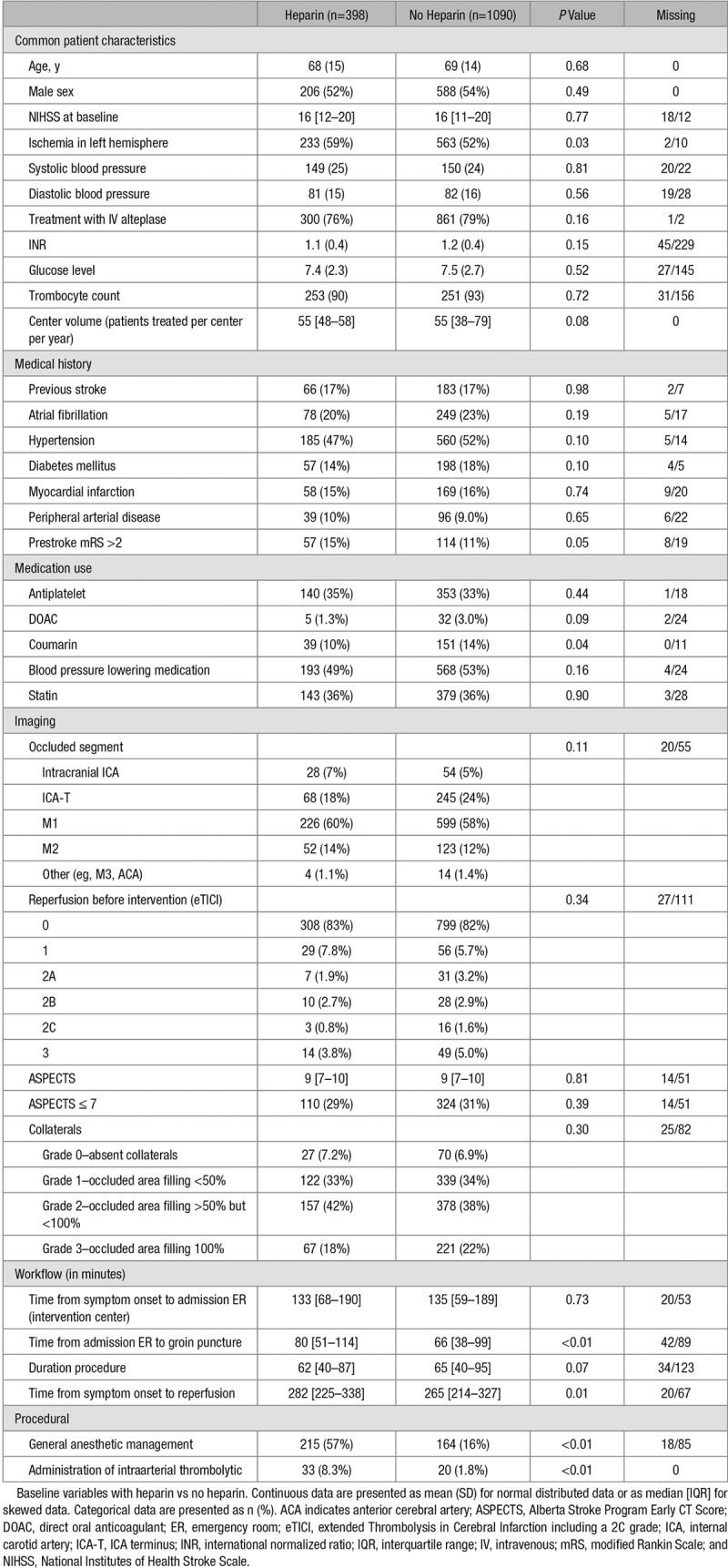
From the total cohort of 1627 patients, 1488 patients from 16 centers were included and analyzed, of whom 398 (27%) received intravenous heparin (Figure 1). Among patients who received intravenous heparin, the median dose was 5000 international units (IU), ranging from 1250 to 10 000 IU (Figure I in the online-only Data Supplement). The percentage of patients within a center treated with intravenous heparin ranged from 0% to 94% (Figure 2). Over the investigated time period, both the total proportion of patients receiving heparin and the proportion of patients receiving heparin per center remained stable (Figure II in the online-only Data Supplement). Patients receiving heparin presented more often with a stroke in the left hemisphere (233/398 [59%] versus 563/1090 [52%], P=0.03) and used coumarins less often (39/398 [10%] versus 151/1090 [14%], P=0.04; Table 1). Median time from emergency room admission at the intervention center to groin puncture (80 [51, 114] versus 66 [38, 99] minutes, P<0.01) and time from symptom onset to reperfusion (282 [225, 338] versus 265 [214, 327] minutes, P=0.01) were both longer in the heparin group. In the heparin group, patients received more often general anesthesia (215/398 [57%] versus 164/1090 [16%], P<0.01) and intraarterial thrombolytics (33/398 [8.3%] versus 20/1090 [1.8%], P<0.01) during EVT. The sensitivity analysis showed no substantial baseline differences between patients in whom no heparin use was explicitly registered and those with missing heparin administration in whom we assumed no heparin was administered (Table I in the online-only Data Supplement).
Figure 1.
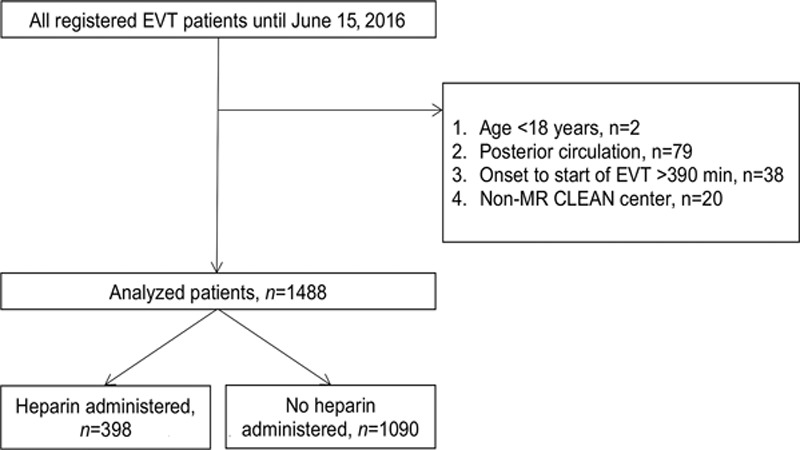
Flowchart. EVT indicates endovascular treatment; and MR CLEAN, Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke.
Figure 2.
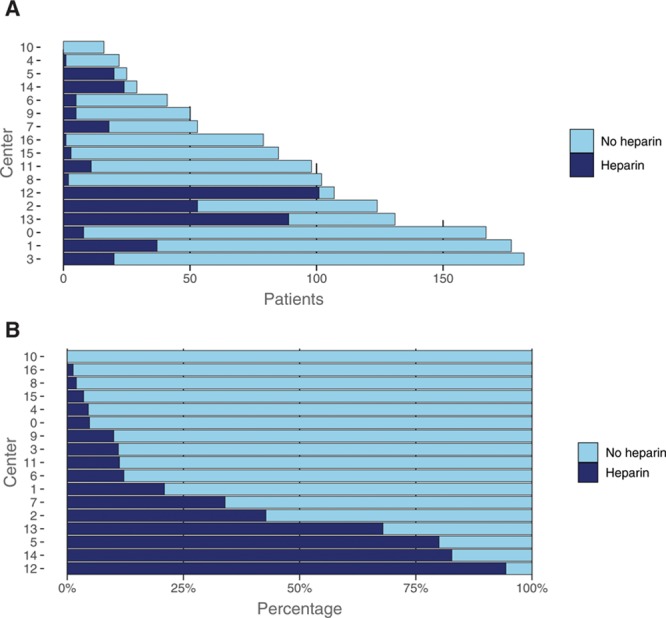
Heparin use during the total time period among Dutch stroke intervention centers in frequencies (A), and in percentages (B).
Table 1.
Baseline Demographics
Outcome Measures
No statistically significant difference in median mRS was observed between patients who received heparin and those who did not (3 [2, 6] versus 3 [2, 6]; adjusted common OR 1.17; 95% CI, 0.87–1.56; Figure 3). No statistically significant associations were found between heparin use and good functional outcome (adjusted odds ratio [aOR], 1.29; 95% CI, 0.88–1.88; Table 2), successful recanalization (aOR, 1.24; 95% CI, 0.89–1.79), sICH (aOR, 1.13; 95% CI, 0.65–1.99), and mortality (aOR, 0.95; 95% CI, 0.66–1.38). There were also no statistically significant differences between both groups in any of the other secondary outcomes. Multiple imputation was performed for 125/1488 (<10%) of the main outcome. The complete case analysis showed similar results (Table II in the online-only Data Supplement). The analyses of center preference to administer heparin showed that functional outcomes were better in centers with higher percentages of heparin administration (adjusted common OR, 1.07 per 10% increase in heparin use; 95% CI, 1.01–1.13 and for good functional outcome aOR 1.10 per 10% increase in heparin; 95% CI, 1.02–1.18; Table 3). In the center preference analyses, there was no association between an increase in heparin use and successful recanalization (aOR, 1.07; 95% CI, 0.96–1.19), sICH (aOR, 0.98; 95 % CI, 0.88–1.10), mortality (aOR, 0.95; 95% CI, 0.90–1.01), and other secondary outcomes.
Figure 3.
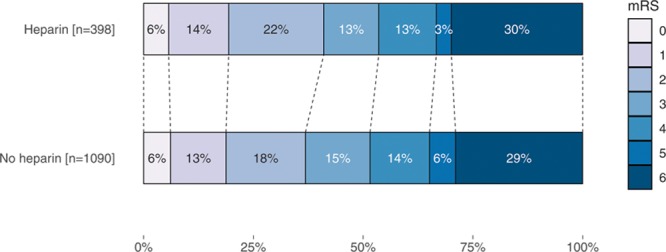
Primary outcome on the modified Rankin Scale (mRS) at patient level.
Table 2.
Secondary Outcomes in Patients Treated With Heparin vs No Heparin
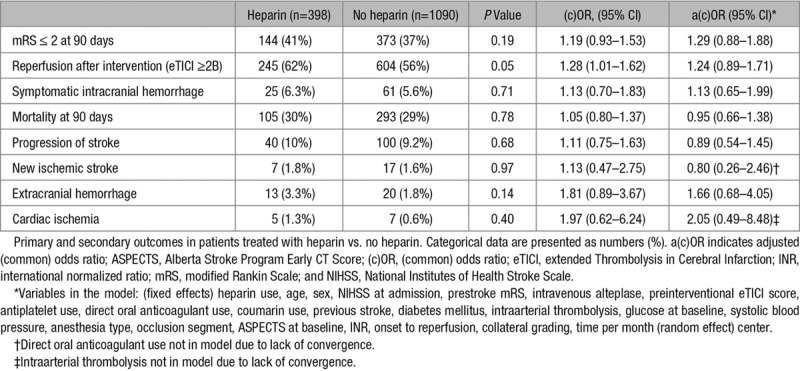
Table 3.
Primary and Secondary Outcomes Associated With Percentage Heparin Use Per Center (Per 10% Heparin Increase)
Discussion
In the present observational study, substantial between-center variability was found in the percentage of patients treated with periprocedural intravenous heparin. We did not find a significant effect of intravenous heparin use on functional outcome at the level of the individual patient. After mitigating potential unmeasured confounding by indication through analysis at the center level, we found a modest beneficial effect of heparin on functional outcome. Patients in centers that treat more patients with intravenous heparin had better functional outcomes, without increased sICH risk.
One of the first studies that introduced periprocedural use of intravenous heparin during EVT (by means of intraarterial prourokinase) was the PROACT II trial (Prolyse in Acute Cerebral Thromboembolism II), in which a nonsignificant increase in the risk of sICH was observed in the EVT arm compared with the control arm, with an improvement in functional outcome (significant after stratification for stroke severity).18 Patients in both arms received a total dose of 4000 IU of heparin. Afterward several EVT trials implemented this as part of their protocol with doses ranging from 2000 to 5000 IU, whereas other trials did not.12,13 The uncertainty regarding the risk-benefit ratio and absence of recommendations in the guidelines explains the variability in periprocedural intravenous heparin use in Dutch stroke intervention centers.19 In prior studies on periprocedural heparin use, the doses used are comparable to the median dose of 5000 IU of heparin in this study.13,20–22 Furthermore, we found that patients receiving heparin were less often on coumarins, which suggests that interventionists are more cautious to administer heparin in anticoagulated patients because of an allegedly higher sICH risk or the indication to administer heparin has already been treated by the coumarin. By contrast, we found that patients who received heparin were more likely to receive intraarterial thrombolytics, which could probably be related to center policy. This might also be the case for general anesthesia, which was also more often used in the heparin group. The longer emergency room to groin puncture time in the heparin group may be explained by the fact that heparin was less often used in the 3 largest centers, in which the workflow may be more optimized in comparison to the workflow of the other centers. The median duration of the procedure was, however, comparable between groups.
Two smaller post hoc analyses of randomized controlled trials (Multi MERCI [Multi Mechanical Embolus Removal in Cerebral Ischemia] and TREVO-II [Thrombectomy Revascularization of Large Vessel Occlusions in Acute Ischemic Stroke II]) investigating the effects of EVT also addressed the question of whether periprocedural heparin is beneficial.21,22 In both studies, periprocedural intravenous heparin use was associated with higher rates of good functional outcomes. The beneficial effect might be explained by the ability of intravenous heparin to restore incomplete microvascular reperfusion. The use of periprocedural heparin seems safe. In all our analyses, there was no statistically significant association between heparin use and sICH or mortality. This is also in line with the findings of the 2 aforementioned post hoc analyses of trials, which, however, did not adjust for risk factors for sICH. Finally, it is important to realize that periprocedural use of heparin is not novel in EVT practices as heparin has been used ever since the introduction of percutaneous coronary intervention in cardiology.23 The rationale for heparin use during percutaneous coronary intervention is that the intervention is associated with factors that predispose to thrombosis (eg, stasis within the coronary artery, stasis within the catheters, and exposure of blood coagulation factors to injured endothelium, catheters, and guidewires) and is, therefore, used as part of protocol care.11 One reason why neuro-interventionists have not fully adopted heparin use in current practice might be the fear of sICH, which, based on our results, seems to be unjustified.
Given the variability in heparin administration among Dutch stroke intervention centers and the promising results regarding outcome, a randomized controlled trial is warranted to prospectively evaluate adjunctive therapy and assess whether this is beneficial. In the ongoing trial MR CLEAN-MED (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands; the Effect of Periprocedural Medication: Heparin, Antiplatelet Agents, Both or Neither, ISRCTN76741621), patients are randomized to intravenous heparin and/or acetylsalicylic acid to investigate whether this will affect microvascular reperfusion and improve functional outcome. Our observational study showed a nonsignificant absolute difference of 4% in good functional outcome (mRS 0–2) in favor of heparin. This supports the sample size calculation of MR CLEAN-MED, which is powered to detect an absolute difference in good functional outcome of 5%.
Limitations
Because of the observational design of our study, confounding by indication could have influenced the results. For example, patient-related factors that are associated with the outcome could have influenced the treating physician’s decision whether or not to administer heparin. For this reason, we adjusted for relevant prognostic factors that were likely to be associated with the administration of heparin. Furthermore, we performed an additional analysis in which we incorporated center preference to administer heparin to reduce the risk of possible unmeasured confounding by indication. In the latter analysis, confounding by indication at the interventionist level diminishes as the analysis at center level is less likely to suffer from this type of confounding (not decision or indication dependent). Also, this analysis takes into account specific center-related factors not included in the model—residual confounding—which could have influenced the physician’s choice to administer heparin. However, even in this center preference analysis, some residual confounding might be present. Possible examples of residual confounding are that centers using heparin more frequently could have been better equipped or that interventionists administering heparin have more experience. Unfortunately, we could not adjust for this. Furthermore, as the distribution of heparin use among centers varied widely, we considered it interesting to explore if center preference is actually the preference of the specific center or rather the preference of the specific interventionist within the center. However, because some interventionists work at different sites and as part of an intervention team with changing staff, it was not feasible to perform this more in-depth exploration. Another limitation regarding this study is that activated clotting times were not measured, leaving the question unanswered if activated clotting times were adequately influenced by the treatment.
Conclusions
Substantial between-center variability exists in intravenous heparin use during EVT procedures in patients with ischemic stroke, but treatment is safe. Patients treated in centers that treat more patients with intravenous heparin have better functional outcomes. A randomized trial is warranted to further study the effects of this treatment.
*Appendix
Coinvestigators MR CLEAN Registry
Executive Committee
Diederik Dippel, Department of Neurology, Erasmus MC University Medical Center; Aad van der Lugt, Department of Radiology, Erasmus MC University Medical Center; Charles Majoie, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Yvo Roos, Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam; Robert van Oostenbrugge, Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Wim van Zwam, Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Jelis Boiten, Department of Neurology, Haaglanden MC, the Hague; Jan Albert Vos, Department of Radiology, Sint Antonius Hospital, Nieuwegein
Study Coordinators
Ivo Jansen, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Maxim Mulder, Department of Neurology, Erasmus MC University Medical Center and Department of Radiology, Erasmus MC University Medical Center; Robert-Jan Goldhoorn, Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM), Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Kars Compagne, Department of Radiology, Erasmus MC University Medical Center; Manon Kappelhof, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam.
Local Principal Investigators
Wouter Schonewille, Department of Neurology, Sint Antonius Hospital, Nieuwegein; Jan Albert Vos, Department of Radiology, Sint Antonius Hospital, Nieuwegein; Charles Majoie, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Jonathan Coutinho, Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam; Marieke Wermer, Department of Neurology, Leiden University Medical Center; Marianne van Walderveen, Department of Radiology, Leiden University Medical Center; Julie Staals, Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Wim van Zwam, Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Jeannette Hofmeijer, Department of Neurology, Rijnstate Hospital, Arnhem; Jasper M. Martens, Department of Radiology, Rijnstate Hospital, Arnhem; Geert Lycklama à Nijeholt, Department of Radiology, Haaglanden MC, the Hague; Jelis Boiten, Department of Neurology, Haaglanden MC, the Hague; Bob Roozenbeek, Department of Neurology, Erasmus MC University Medical Center; Bart Emmer, Department of Radiology, Erasmus MC University Medical Center; Sebastiaan de Bruijn, Department of Neurology, HAGA Hospital, the Hague; Lukas van Dijk, Department of Radiology, HAGA Hospital, the Hague; H. Bart van der Worp, Department of Neurology, University Medical Center Utrecht; Rob Lo, Department of Radiology, University Medical Center Utrecht; Ewoud van Dijk, Department of Neurology, Radboud University Medical Center, Nijmegen; Hieronymus Boogaarts, Department of Neurosurgery, Radboud University Medical Center, Nijmegen; Paul de Kort, Department of Neurology, Sint Elisabeth Hospital, Tilburg; Jo Peluso, Department of Radiology, Sint Elisabeth Hospital, Tilburg; Jan van den Berg, Department of Neurology, Isala Klinieken, Zwolle; Boudewijn van Hasselt, Department of Radiology, Isala Klinieken, Zwolle; Leo Aerden, Department of Neurology, Reinier de Graaf Gasthuis, Delft; René Dallinga, Department of Radiology, Reinier de Graaf Gasthuis, Delft; Maarten Uyttenboogaart, Department of Neurology, University Medical Center Groningen; Omid Eshghi, Department of Radiology, University Medical Center Groningen; Tobien Schreuder, Department of Neurology, Atrium Medical Center, Heerlen; Roel Heijboer, Department of Radiology, Atrium Medical Center, Heerlen; Koos Keizer, Department of Neurology, Catharina Hospital, Eindhoven; Lonneke Yo, Department of Radiology, Catharina Hospital, Eindhoven; Heleen den Hertog, Department of Neurology, Isala Klinieken, Zwolle; Emiel Sturm, Department of Radiology, Medical Spectrum Twente, Enschede
Imaging Assessment Committee
Charles Majoie, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam (chair); Wim van Zwam, Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Aad van der Lugt, Department of Radiology, Erasmus MC University Medical Center; Geert Lycklama à Nijeholt, Department of Radiology, Haaglanden MC, the Hague; Marianne van Walderveen, Department of Radiology, Leiden University Medical Center; Marieke Sprengers, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Sjoerd Jenniskens, Department of Radiology, Radboud University Medical Center, Nijmegen; René van den Berg, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Albert Yoo, Department of Radiology, Texas Stroke Institute, Texas, United States of America; Ludo Beenen, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Alida Postma, Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Stefan Roosendaal, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Bas van der Kallen, Department of Radiology, Haaglanden MC, the Hague; Ido van den Wijngaard, Department of Radiology, Haaglanden MC, the Hague; Adriaan van Es, Department of Radiology, Erasmus MC University Medical Center; Bart Emmer, Department of Radiology, Erasmus MC University Medical Center, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Jasper Martens, Department of Radiology, Rijnstate Hospital, Arnhem; Lonneke Yo, Department of Radiology, Catharina Hospital, Eindhoven; Jan Albert Vos, Department of Radiology, Sint Antonius Hospital, Nieuwegein; Joost Bot, Department of Radiology, Amsterdam UMC, Vrije Universiteit van Amsterdam, Amsterdam; Pieter-Jan van Doormaal, Department of Radiology, Erasmus MC University Medical Center.
Writing Committee
Diederik Dippel, Department of Neurology, Erasmus MC University Medical Center (chair); Aad van der Lugt, Department of Radiology, Erasmus MC University Medical Center; Charles Majoie, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Yvo Roos, Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam; Robert van Oostenbrugge, Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Wim van Zwam, Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Geert Lycklama à Nijeholt, Department of Radiology, Haaglanden MC, the Hague; Jelis Boiten, Department of Neurology, Haaglanden MC, the Hague; Jan Albert Vos, Department of Radiology, Sint Antonius Hospital, Nieuwegein; Wouter Schonewille, Department of Neurology, Sint Antonius Hospital, Nieuwegein; Jeannette Hofmeijer, Department of Neurology, Rijnstate Hospital, Arnhem; Jasper Martens, Department of Radiology, Rijnstate Hospital, Arnhem; Bart van der Worp, Department of Neurology, University Medical Center Utrecht; Rob Lo, Department of Radiology, University Medical Center Utrecht.
Adverse Event Committee
Robert van Oostenbrugge, Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM) (chair); Jeannette Hofmeijer, Department of Neurology, Rijnstate Hospital, Arnhem; Zwenneke Flach, Department of Radiology, Isala Klinieken, Zwolle
Trial Methodologist;
Hester Lingsma, Department of Public Health, Erasmus MC University Medical Center.
Research Nurses / Local Trial Coordinators
Naziha el Ghannouti, Department of Neurology, Erasmus MC University Medical Center; Martin Sterrenberg, Department of Neurology, Erasmus MC University Medical Center; Corina Puppels and Wilma Pellikaan, Department of Neurology, Sint Antonius Hospital, Nieuwegein; Rita Sprengers, Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam; Marjan Elfrink, Department of Neurology, Rijnstate Hospital, Arnhem; Joke de Meris, Department of Neurology, Haaglanden MC, the Hague; Tamara Vermeulen, Department of Neurology, Haaglanden MC, the Hague; Annet Geerlings, Department of Neurology, Radboud University Medical Center, Nijmegen; Gina van Vemde, Department of Neurology, Isala Klinieken, Zwolle; Tiny Simons, Department of Neurology, Atrium Medical Center, Heerlen; Cathelijn van Rijswijk, Department of Neurology, Sint Elisabeth Hospital, Tilburg; Gert Messchendorp, Department of Neurology, University Medical Center Groningen; Hester Bongenaar, Department of Neurology, Catharina Hospital, Eindhoven; Karin Bodde, Department of Neurology, Reinier de Graaf Gasthuis, Delft; Sandra Kleijn, Department of Neurology, Medical Spectrum Twente, Enschede; Jasmijn Lodico, Department of Neurology, Medical Spectrum Twente, Enschede; Hanneke Droste, Department of Neurology, Medical Spectrum Twente, Enschede; M. Wollaert, Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); D. Jeurrissen, Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Ernas Bos, Department of Neurology, Leiden University Medical Center; Yvonne Drabbe, Department of Neurology, HAGA Hospital, the Hague; Nicoline Aaldering, Department of Neurology, Rijnstate Hospital, Arnhem; Berber Zweedijk, Department of Neurology, University Medical Center Utrecht; Mostafa Khalilzada, Department of Neurology, HAGA Hospital, the Hague
PhD / Medical Students
Esmee Venema, Department of Public Health, Erasmus MC University Medical Center; Vicky Chalos, Department of Neurology, Erasmus MC University Medical Center and Department of Public Health, Erasmus MC University Medical Center; Ralph Geuskens, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Tim van Straaten, Department of Neurology, Radboud University Medical Center, Nijmegen; Saliha Ergezen, Roger Harmsma, Daan Muijres, and Anouk de Jong, Department of Neurology, Erasmus MC University Medical Center. Wouter Hinseveld, Department of Neurology, Sint Antonius Hospital, Nieuwegein; Olvert Berkhemer, Department of Neurology, Erasmus MC University Medical Center, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, and Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Anna Boers, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam and Department of Biomedical Engineering and Physics, Amsterdam UMC, University of Amsterdam, Amsterdam; J. Huguet, P. Groot, Marieke Mens, Katinka van Kranendonk, Kilian Treurniet, Manon Tolhuijsen, and Heitor Alves, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam.
Acknowledgments
We thank the MR CLEAN Registry Investigators. A list of all investigators is given in Appendix.
Sources of Funding
The authors received no funding for this study. The MR CLEAN Registry is partially funded by unrestricted grants from Toegepast Wetenschappelijk Instituut voor Neuromodulatie, Twente University (TWIN), Erasmus MC University Medical Center, Maastricht University Medical Center, and Amsterdam UMC.
Disclosures
All authors are directly or indirectly involved as investigators for the MR CLEAN-MED (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands; the Effect of Periprocedural Medication: Heparin, Antiplatelet Agents, Both or Neither; ISRCTN76741621). Dr Emmer is the recipient of compensation fees for review work from DEKRA and speaker fees from Novartis. Dr van der Worp has received speaker’s fees Boehringer Ingelheim and has served as a consultant to Boehringer Ingelheim. In addition, Dr van der Worp is the recipient of unrestricted grants from Dutch Heart Foundation and the European Union for the conduct of trials on acute treatment for stroke. Erasmus MC received compensation from Stryker, Medtronic, and Bracco Imaging Ltd for activities of Drs van der Lugt and Dippel as consultants. In addition, of Drs van der Lugt and Dippel are the recipients of unrestricted grants from Dutch Heart Foundation, Dutch Brain Foundation, The Netherlands Organisation for Health Research and Development, Health Holland Top Sector Life Science, AngioCare BV, Covidien/EV3, MEDAC Gmbh/LAMEPRO, Top Medical/Concentric, Thrombolytic Science LLC, Stryker, Medtronic, and Penumbra, Inc for the conduct of trials on acute treatment for stroke. The other authors report no conflicts.
Supplementary Material
Footnotes
A list of all MR CLEAN Registry Investigators is given in the Appendix
Guest Editor for this article was Harold P. Adams, MD.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.119.025329.
References
- 1.Fransen PS, Berkhemer OA, Lingsma HF, Beumer D, van den Berg LA, Yoo AJ, et al. Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands Investigators. Time to reperfusion and treatment effect for acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2016;73:190–196. doi: 10.1001/jamaneurol.2015.3886. doi: 10.1001/jamaneurol.2015.3886. [DOI] [PubMed] [Google Scholar]
- 2.del Zoppo GJ, Schmid-Schönbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991;22:1276–1283. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]
- 3.Mori E, del Zoppo GJ, Chambers JD, Copeland BR, Arfors KE. Inhibition of polymorphonuclear leukocyte adherence suppresses no-reflow after focal cerebral ischemia in baboons. Stroke. 1992;23:712–718. doi: 10.1161/01.str.23.5.712. [DOI] [PubMed] [Google Scholar]
- 4.Okada Y, Copeland BR, Fitridge R, Koziol JA, del Zoppo GJ. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke. 1994;25:1847–53. doi: 10.1161/01.str.25.9.1847. discussion 1853. [DOI] [PubMed] [Google Scholar]
- 5.Ducroux C, Di Meglio L, Loyau S, Delbosc S, Boisseau W, Deschildre C, et al. Thrombus neutrophil extracellular traps content impair tpa-induced thrombolysis in acute ischemic stroke. Stroke. 2018;49:754–757. doi: 10.1161/STROKEAHA.117.019896. doi: 10.1161/STROKEAHA.117.019896. [DOI] [PubMed] [Google Scholar]
- 6.Dalkara T, Arsava EM. Can restoring incomplete microcirculatory reperfusion improve stroke outcome after thrombolysis? J Cereb Blood Flow Metab. 2012;32:2091–2099. doi: 10.1038/jcbfm.2012.139. doi: 10.1038/jcbfm.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Zoppo GJ, Copeland BR, Harker LA, Waltz TA, Zyroff J, Hanson SR, et al. Experimental acute thrombotic stroke in baboons. Stroke. 1986;17:1254–1265. doi: 10.1161/01.str.17.6.1254. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laridan E, Denorme F, Desender L, François O, Andersson T, Deckmyn H, et al. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;82:223–232. doi: 10.1002/ana.24993. doi: 10.1002/ana.24993. [DOI] [PubMed] [Google Scholar]
- 10.The international stroke trial (IST) A randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International stroke trial collaborative group. Lancet. 1997;349:1569–1581. [PubMed] [Google Scholar]
- 11.Neumann FJ, Sousa-Uva M. ‘Ten Commandments’ for the 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2018;39:3759. doi: 10.1093/eurheartj/ehy658. doi: 10.1093/eurheartj/ehy658. [DOI] [PubMed] [Google Scholar]
- 12.van de Graaf RA, Chalos V, del Zoppo GJ, van der Lugt A, Dippel DWJ, Roozenbeek B. Periprocedural antithrombotic treatment during acute mechanical thrombectomy for ischemic stroke: a systematic review. Front Neurol. 2018;9:238. doi: 10.3389/fneur.2018.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreitzer N, Khatri P, Adeoye O, Abruzzo T, Grossman A, Ringer A, et al. Heparin use across major endovascular trials. Stroke. 2016;47 ATP2. [Google Scholar]
- 14.Jansen IGH, Mulder MJHL, Goldhoorn RB MR CLEAN Registry Investigators. Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (MR CLEAN Registry). BMJ. 2018;360:k949. doi: 10.1136/bmj.k949. doi: 10.1136/bmj.k949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 16.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 17.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 19.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. American Heart Association Stroke Council. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 20.del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in Acute Cerebral Thromboembolism. Stroke. 1998;29:4–11. doi: 10.1161/01.str.29.1.4. [DOI] [PubMed] [Google Scholar]
- 21.Nahab F, Walker GA, Dion JE, Smith WS. Safety of periprocedural heparin in acute ischemic stroke endovascular therapy: the multi MERCI trial. J Stroke Cerebrovasc Dis. 2012;21:790–793. doi: 10.1016/j.jstrokecerebrovasdis.2011.04.009. doi: 10.1016/j.jstrokecerebrovasdis.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Winningham MJ, Haussen DC, Nogueira RG, Liebeskind DS, Smith WS, Lutsep HL, et al. Periprocedural heparin use in acute ischemic stroke endovascular therapy: the TREVO 2 trial. J Neurointerv Surg. 2018;10:611–614. doi: 10.1136/neurintsurg-2017-013441. doi: 10.1136/neurintsurg-2017-013441. [DOI] [PubMed] [Google Scholar]
- 23.Grüntzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary-artery stenosis: percutaneous transluminal coronary angioplasty. N Engl J Med. 1979;301:61–68. doi: 10.1056/NEJM197907123010201. doi: 10.1056/NEJM197907123010201. [DOI] [PubMed] [Google Scholar]


