Abstract
Background
Study coordinators play an essential role on study teams; however, there remains a paucity of research on the supports and services they need to effectively recruit and retain study participants.
Methods
A cross-sectional survey was conducted with 147 study coordinators from a large academic medical center. Survey items assessed barriers and facilitators to recruitment and retention, anxiety about reaching enrollment numbers, confidence for talking to potential study participants about research involvement, awareness and use of CTSA resources, and PI involvement with recruitment planning.
Results
Significant associations were found between anxiety about reaching target enrollment numbers and whether the study coordinator was the primary person responsible for developing a recruitment strategy. Three years or more serving as a study coordinator and levels of anxiety for reaching enrollment numbers was also significant.
Conclusion
More institutional level supports and formal training opportunities are needed to enhance study coordinators’ effectiveness to recruit participants.
Keywords: Research personnel, Patient selection, Clinical trials as topic, National center for advancing translational sciences (U.S.), Cross-sectional studies, Academic medical centers
1. Introduction
Prior research has demonstrated that including a study coordinator on a research team significantly improves recruitment numbers, improves participant retention, and increases study efficacy [[1], [2], [3], [4]]. However, there remains a paucity of research specifically on study coordinators as a workforce population. A study coordinator's responsibilities can vary across trials, but generally include recruitment and retention of participants, completion of study visits and procedures for each participant, maintaining study documents, and data management [[1], [2], [3],5]. Given that no systematic process exists for onboarding study coordinators, there is ongoing skill development that needs to occur [6]. Work-related anxiety and burnout may arise if adequate training and supports are not received [1].
In 2006, the NIH National Center for Advancing Translational Sciences created the Clinical and Translational Science Awards program (CTSA) to advance biomedical research, with a focus on training and cultivating the translational science workforce [3]. The Research Coordinator Taskforce was later implemented to increase efforts to better provide support and training to study coordinators across the CTSA network [3]. In 2008, the taskforce conducted two surveys among study coordinators to enhance understanding of job responsibilities and the use and availability, of training opportunities [3]. Completed by 1597 coordinators in 22 CTSA academic centers, 45% of respondents that completed this survey reported receiving adequate training for all required job tasks [3]. However, those CTSA academic centers who reported providing some form of training opportunity, also reported training that varied greatly in terms of length, frequency, and areas of focus. In an effort to streamline the training resources available across CTSA academic centers, the Joint Task Force (JTF) was created in 2013 [6]. The JTF created a framework is comprised of 8 core domains and 48 core competency statements that have laid the foundation of professional competencies required for clinical research workforce development [6].
This paper presents the results from a study coordinator survey conducted as part of the Novel Approaches to Recruitment Planning parent project. The primary objectives of this survey were to assess: (1) how study coordinators approach recruitment, (2) what strategies were most helpful for recruitment and retention, (3) interest in future trainings on recruitment and retention, and (4) the supports needed by study coordinators to successfully improve recruitment and retention of research participants in the future. For purposes of this paper, we use the broad term study coordinator although other related job titles include: data manager, research nurse, research coordinator, research assistant, clinical research coordinator, and clinical research associate.
2. Methods
2.1. Participants
Participants were recruited through NYU Langone Health's Association of Clinical Coordination and Research Management (ACCRM). ACCRM is comprised of more than 600 members and provides a variety of resources to help support clinical research staff. ACCRM manages a database of clinical research staff who proactively joined the group or those who attended a 2-day training for new clinical research staff. Study coordinators received an introductory email about the study along with an invitation to participate. This email provided a basic overview of the survey, information regarding compensation for survey completion, as well as a direct online link to the survey via Open RedCap. RedCap is a secure, HIPAA-compliant, web-based database application designed to support data capture, management, and descriptive statistics [7]. The survey items were original questions developed by our study team, although many of the concepts were informed by the literature when available [2,3,8], and focus groups conducted with survey coordinators at our institution in 2015. Before the survey was implemented, it was reviewed and taken online by 5 study coordinators and/or clinical trial professionals for understanding and ease of use. Adjustments were made based on their feedback. All study procedures were approved by NYU School of Medicine's Institutional Review Board.
Participants were eligible to complete the online survey if they were: (1) 18 years of age or older, (2) self-identified as a study coordinator, 3) currently employed at NYU Langone Health, and 4) agreed to the terms of the study. Since this was an online survey, a PDF version of the informed consent was available for participants to download via Open RedCap. Data collection took place from September 2017 through November 2017. Participants who provided informed consent online (n = 190) were invited to complete the survey. Out of the 190 interested respondents, n = 147 (77%) completed the survey. Participants who completed the survey (n = 147) received a $15 Visa gift card as reimbursement for time and effort.
3. Measures
3.1. Sociodemographic factors
Age, race/ethnicity, gender, and education were captured. Race/ethnicity was collapsed into two categories: White and non-White. Gender was identified as male and female.
3.2. Barriers and facilitators to recruitment and retention
Respondents were asked to identify potential barriers to successful recruitment (e.g. patient refusal, not enough money in the budget for study related needs, insufficient time). A list of recruitment facilitators was also presented, with response options including, but not limited to, monetary incentives for participants, physician mention or recommendation, and more time for study procedures.
3.3. Most helpful resources for strengthening the study Coordinator's recruitment skills
Respondents were prompted to identify internal (i.e., within the institution) and external resources (i.e. outside the institution) that were the most helpful in strengthening recruitment skills. Example responses included live webinars, self-paced online trainings, and guidance from a colleague.
3.4. Perspectives about the importance of participant-study coordinator concordance
Respondents were asked to assess the importance of participant-study coordinator concordance regarding age, gender, race/ethnicity, and language spoken on a Likert scale of 1–5, with 1 being not at all important and 5 being very important.
3.5. Anxiety about recruitment numbers
Respondents were asked to measure their level of anxiety about meeting their target enrollment numbers for research trials. Response options were scaled from 1 to 5, with 1 being not all anxious to 5 being very anxious.
3.6. Overall PI involvement and awareness
Regarding the level of the PI's involvement in developing a recruitment plan for studies and the PI's awareness of the study coordinator's challenges with recruitment, respondents measured these items via a Likert scale from 1 to 5, with 1 being not at all aware/involved to 5 being very aware/involved.
3.7. Study coordinator job satisfaction
Respondents were prompted to report their level of satisfaction in their current role as a study coordinator and the degree to which study team members got along (excluding the PI) on a Likert scale of 1–5, with 1 being not at all satisfied and 5 being very satisfied. Additionally, respondents were asked if they planned to stay in their current position in the next 1–2 years (yes or no response), and then asked to provide a brief text response as to why they selected this option.
3.8. CTSA awareness and usage
We aimed to capture respondent's awareness and usage of CTSA resources (referred to as the Clinical and Translational Science Institute or CTSI at NYULH). Participants were asked, “Have you ever heard of the CTSI,” and “Have you ever used any of the CTSI tools and services specifically related to recruitment.” Response options were yes and no. If respondents indicated that they had used CTSI resources, they were asked to identify which resources they used (e.g. participant recruitment e-book, consultation with the Recruitment and Retention Unit). If CTSI resources were not used, participants were asked they reasons why they did not use existing resources. Example response options included: didn't have time, the services were too expensive, and not aware of CTSA services.
The data was cleaned and analyzed using R version 3.4.3. Categorical variables were analyzed using chi square test or Fisher's exact test, dependent upon if conditions were met. Student t-tests and ANOVAs were used to compare continuous data, and nonparametric equivalent tests were used when appropriate.
4. Results
4.1. Sociodemographic factors, prior training, and CCRC credential
Of the 147 respondents, 122 (83%) identified as female and 72 (49%) identified as white. There was almost an even spread with regard to highest education level with 61 (41.5%) study coordinators reported having an associate degree and 65 (44.2%) reported having a bachelor's degree (Table 1). The median age of the respondents was 29, with a minimum of 21 years and a maximum of 70 years. The median years as a study coordinator were 3 years.
Table 1.
| Demographics | Frequency (%) |
|---|---|
| Gender | |
| Male | 24 (16.3) |
| Female | 122 (83.0) |
| Transgender | 1 (0.7) |
| Missing | 0 (0.0) |
| Ethnicity | |
| Hispanic | 34 (23.1) |
| Not Hispanic | 112 (76.2) |
| Missing | 1 (0.7) |
| Race | |
| Black | 19 (12.2) |
| White | 72 (49.0) |
| Asian | 21 (14.3) |
| American Indian or Alaska Native | 1 (0.7) |
| Native Hawaiian or Other Pacific Islander | 0 (0.0) |
| Other | 20 (13.6) |
| N/A | 11 (7.5) |
| Missing | 3 (2.04) |
| Education Level | |
| H.S Grad, Diploma or Equivalent | 4 (2.7) |
| Some College, No Degree | 2 (1.4) |
| Trade/Tech Vocational Training | 1 (0.7) |
| Associates Degree | 61 (41.5) |
| Bachelor's Degree | 65 (44.2) |
| Master's Degree | 12 (8.2) |
| Doctorate Degree | 1 (0.7) |
| Missing | 1 (0.7) |
| Clinical Training | |
| Yes | 32 (21.8) |
| No | 115 (78.2) |
| Missing | 0 (0.0) |
| CCRC or CCRA | |
| Yes | 40 (27.2) |
| No | 107 (72.8) |
| Missing | 0 (0.00) |
| Licensure or certification required | |
| Yes | 28 (19.0) |
| No | 117 (79.6) |
| Missing | 2 (1.4) |
Regarding prior training, the majority of respondents, 115 (78.2%), reported not ever receiving prior training on recruitment strategies. Only 40 (21.8%) respondents received their Certified Clinical Research Coordinator (CCRC) certification.
4.2. Barriers and facilitators to recruitment and retention
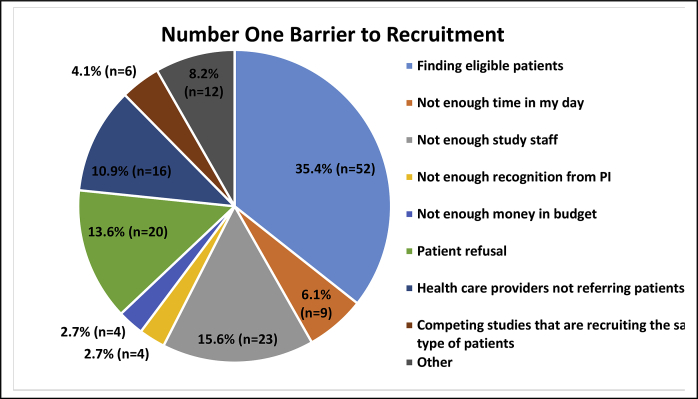
Fig. 1 illustrates that the largest barrier to recruitment reported by study coordinators were finding eligible research participants (n = 52, 35.4%), followed by lack of study staff (n = 23, 15.6%), and patients’ refusal (n = 20, 13.6%).
Fig. 1.
Number one barrier to recruitment.
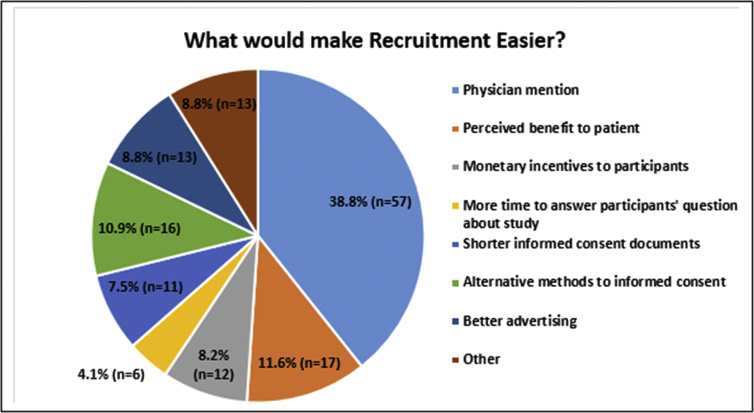
As shown in Fig. 2, study coordinators reported that recruitment would be easier if physicians referred eligible patients into ongoing studies (physician mention; n = 57, 38.8%). Other key facilitators included the perceived benefit to the patient (n = 17, 11.6%) and finding alternative methods for informed consent (n = 16, 10.9%).
Fig. 2.
What would make Recruitment Easier?.
Some of the biggest challenges with participant retention perceived by respondents included that there was too much time involved in the study for the participant (n = 26, 17.7%) and lack of a perceived benefit of staying in the study (n = 17, 11.6%). Additional challenges in retention can be viewed in Table 2.
Table 2.
| Biggest challenge retaining participants | Frequency (%) |
|---|---|
| Too much time involved | 26 (17.7) |
| N/A | 18 (12.2) |
| Participants don't see the benefit of staying in the study | 17 (11.6) |
| Travel burden to study site | 16 (10.9) |
| Lost to follow up | 13 (8.8) |
| Other life challenges that impact study participation | 12 (8.2) |
| Lost interest | 11 (7.5) |
| Other health challenges that impact study participation | 6 (4.1) |
| Moved out of the area | 4 (2.7) |
| No or insufficient incentives | 4 (2.7) |
| I'm not responsible for retention | 3 (2.0) |
| Other | 3 (2.0) |
| Missing | 2 (1.4) |
| Death | 1 (0.7) |
| Poorly designed or non-existent retention plan | 1 (0.7) |
4.3. Most helpful resources for strengthening the study Coordinator's recruitment skills
When asked what the most helpful resources was for strengthening recruitment skills, 57 (38.5%) study coordinators reported that they received training on participant recruitment strategies at the current institution. Respondents reported that other study coordinators were the most helpful resource when they first started (n = 58, 39.5%) and for strengthening ongoing recruitment skills (n = 69, 46.9%).
4.4. Perspectives about the importance of participant-study coordinator concordance
As shown in Table 3, the majority of participants listed that language was the single most important factor with regard to participant-study coordinator concordance (n = 87, 58%), compared to age, gender, and race/ethnicity were not as highly ranked.
Table 3.
| Participant/Study Coordinator Concordance | 1 (Not at all important) | 2 | 3 | 4 | 5 (Very important) |
|---|---|---|---|---|---|
| Age | 35 (23.8%) | 30 (20.4%) | 41 (27.9%) | 26 (17.7%) | 14 (9.5%) |
| Gender | 54 (36.7%) | 33 (22.4%) | 41 (27.9%) | 15 (10.2%) | 2 (1.4%) |
| Race/ethnicity | 37 (25.2%) | 23 (15.6%) | 44 (30%) | 28 (19.0%) | 14 (9.5%) |
| Language | 2 (1.4%) | 5 (3.4%) | 17 (11.6%) | 35 (23.8%) | 87 (58.2%) |
4.5. Anxiety about recruitment numbers
Respondents were asked to measure their level of anxiety regarding meeting enrollment numbers. Of those that responded, 36.7% reported none or very little anxiety, while 62.6% answered a 3 or above.
The level of anxiety regarding achieving target enrollment numbers and median years as study coordinator (3 years) was compared. Table 4 illustrates that there was a significant difference between the distribution of anxiety scores and years as a study coordinator (p-value = 0.011). Those who have been a study coordinator for 3 years or less, tended to report experiencing either none or very little anxiety about reaching their recruitment numbers when compared with those having more years of experience as a study coordinator (47.4% compared to 25.3%).
Table 4.
| Level of Anxiety and Years as a Study Coordinators | Anxiety about meeting your enrollment numbers |
||||
|---|---|---|---|---|---|
| 1 (None) | 2 | 3 | 4 | 5 (A lot) | |
| Years as a study coordinator ≤ 3 years | 14 17.9% |
23 29.5% |
17 21.8% |
21 26.9% |
3 3.8% |
| Years as a study coordinator > 3 years | 10 14.9% |
7 10.4% |
24 35.8% |
17 25.4% |
9 13.4% |
Additionally, there was a significant difference (p-value = 0.034) between anxiety about achieving target enrollment numbers and the study coordinator having primary responsibility for developing the recruitment strategy (Table 5). When the study coordinator was not primarily responsible for recruitment strategy development, the majority of the responses (77.3%) were a 1, 2, or 3, on a scale of 1–5, indicating small to moderate levels of anxiety. However, there was a greater proportion of study coordinators that selected scores 4 or 5 that had the primary responsibility of developing a recruitment strategy compared to those that did not have this responsibility.
Table 5.
| Level of Anxiety and Primary Responsibility for Recruitment Strategy Development | Anxiety about meeting your enrollment numbers |
||||
|---|---|---|---|---|---|
| 1 (None) | 2 | 3 | 4 | 5 (A lot) | |
| Study coordinator is NOT primarily responsible for recruitment strategy development | 13 17.3% |
17 22.7% |
28 37.3% |
13 17.3% |
4 5.3% |
| Study coordinator is primarily responsible for recruitment strategy development | 11 15.5% |
13 18.3% |
14 19.7% |
25 35.2% |
8 11.3% |
There was no association between anxiety levels and prior trainings (p-value > 0.05), indicating that prior training does not seem to influence the amount of anxiety felt by study coordinators for reaching enrollment numbers. Moreover, there were not associations between anxiety levels for meeting enrollment numbers and CCRC certification, awareness of CTSI resources, and use of CTSI resources.
4.6. Study coordinator job satisfaction
Overall, 68.0% of participants were satisfied or very satisfied in their role as a study coordinator and 83.7% reported that they got along ‘well’ or ‘very well’ with their study team members (not including PI). Additionally, 73.5% of study coordinators planned to stay in their current position for the next year or two.
4.7. CTSI awareness and usage
While there was high CTSI awareness (n = 117, 82.3%) among study coordinators, only 14.5% (n = 17) had used its services and resources. For those that had used the CTSI before, the most used resource was a consultation with the CTSI Recruitment and Retention Unit.
5. Discussion
The purpose of this study was to identify barriers and facilitators of the recruitment and retention of research participants as experienced by study coordinators. We also aimed to assess interest in future trainings on recruitment and retention, and what services or supports are needed by study coordinators to improve recruitment and retention in the future. As highlighted by the study results, finding eligible participants remains a major challenge in clinical research worldwide. This was an interesting finding considering that our institution has various options for estimating the pool of potentially eligible patients. These options include i2b2 and more recently Epic's SlicerDicer tool for de-identified queries based on problem lists or ICD-10 codes which are identified through electronic health records. Study teams are also able to utilize the CTSI's DataCore service, which can create identified reports on patients in our electronic health record system once IRB approval is obtained. For studies that accept people from the community or nationally, we also have access to ResearchMatch and various community partnerships. Nevertheless, this lack of awareness about ways to find eligible patients signals that more advertising about internal and external resources warrants attention. It also signals that partnerships with marketing and communication departments may help to publicize our clinical trials and health research studies.
Our results are consistent with other prior research that have demonstrated that under recruitment, or lack of finding and enrolling eligible patients, continues to be the most challenging aspect of research [9,10]. Some of the open text responses may shed light on the need to distinguish between how target enrollment numbers are established. As stated by one respondent, “PIs equate statistical power with realistic recruitment goals.” Another respondent wrote, “Not enough participants to even screen or find that may be eligible.” Lack, or the inability to find eligible patients, contributes to early study termination due to failure in enrollment. Greater emphasis needs to be placed on “real world” contexts in deciding target enrollment numbers.
In regard to specific training opportunities and supports for study coordinators, most respondents reported that they did not receive prior recruitment training (78.2%). “Other study coordinators” were the most important resource for both initial (within first 6 months) and ongoing recruitment skill development; however, more formal training opportunities were still desired. A sentiment that was expressed by several coordinators who completed the survey was the need for formal training. In principle, CTSI/As across the U.S. are tasked with providing research infrastructure and related supports to study teams. While there was high awareness of our institution's CTSI, there was low usage of its services. Respondents may not perceive its value, relevance, or possible receptiveness to their needs.
Services and tools that have been developed to aid study coordinators at NYU Langone Health include: (1) an in-person mandatory two-day Clinical Research Coordinator Foundational Program which includes a recruitment strategy and resource segment, (2) a recruitment eBook outlining various resources at the institution including those offered by the CSTI, approved translation and interpreter service vendors, and online tools (e.g., stock photos, health literacy calculator), (3) several online training modules that highlight pre and post award research tasks, and (4) a Clinical Research Coordinator Mentorship program. While the two-day training is widely used across the institution since it is a requirement, the other services are not nearly as utilized but are additional helpful resources that are available.
In an effort to identify additional training programs at other institutions, the CTSA recruitment and retention network was contacted. While responses were limited, we were able to identify a few with the assistance of additional clinical research professionals that are members of the CTSA. The University of Rochester's Study Coordinators Organization for Research & Education (SCORE) program is a coordinator-run forum to create opportunities for sharing, learning and connecting with peer research coordinators. Furthermore, their Office for Human Subject Protection recorded a module on recruitment and retention that is available through their internal continuing education portal called My Path. Additionally, they offer a 3-credit online course on recruitment and retention. Another institution is exploring training study coordinators in basic social marketing principles in regard to recruitments skills. In addition, responses from other institutions in the network revealed that they would like more “formal” study coordinator training opportunities.
National organizations also support training of study coordinators. These include, but are not limited to, the Collaborative Institutional Training Initiative program (CITI) and The Association of Clinical Research Professionals (ACRP) [6]. Both organizations provide online training modules/courses that are tailored to address what is needed to efficiently conduct clinical research, specifically geared for study coordinators. In addition, ACRP provides a variety of certification opportunities related to the various roles in clinical research.
Despite the availability of potentially helpful resources, there appears to be a gap in making these resources used by study coordinators. Understandably so, there are generally great efforts to make PIs aware and use recruitment and retention resources at various institutions. While indeed important, our findings suggest that increased attention needs to be paid to the critical, yet sometimes underappreciated role that study coordinators play regarding recruitment and retention. For example, one reason why study coordinators may not be using the CTSI is that they are unaware of the services it has to offer. A suggestion was made to send out an email blast to all coordinators that contains all available resources since many of them are unknown.” Moving forward, targeted communications and ongoing updates about relevant resources may improve the capacity of study coordinators to engage patients in research.
An additional major finding of this study was that higher levels of anxiety were reported among study coordinators that had the primary responsibility for developing recruitment plans, as well as study coordinators who were in their current role for 3 or more years. While lack of training was not found to be significantly associated with higher levels of anxiety in reaching enrollment number (p-value > 0.05), future studies with a broader pool of study coordinators are needed to better understand this relationship.
5.1. Strengths, limitations, and future directions
Strengths of this study include identifying barriers and facilitators of recruitment and retention among study coordinators from a variety of departments in the medical, nursing, and dental school. Few research studies have exclusively focused on the role and needs of study coordinators; thus, our findings add a unique perspective on how to enhance the skill sets of this group. We were able to examine anxiety levels that study coordinators face and key associations such as median years in their role. Despite its strengths, some limitations should be noted. First, this survey was conducted at a single institution; therefore, it may not be generalizable to wider populations. Second, we may have missed study coordinators who were not active members of ACCRM, or who performed study coordinator functions but did not have the title of study coordinator, or those who were unhappy in their role and therefore less willing to take our survey. Third, although we strived for a representative sample of study coordinators, study coordinators from all departments/divisions were not captured. Forth, some questions were broad in nature. Despite piloting the questions with a small group of study coordinators, we do not know if the questions were interpreted in the way we originally intended. Lastly, in order to obtain their gift card, study coordinators had to provide their name and contact information. It is possible that study coordinators would have answered differently if the survey was completely anonymous.
Future research should explore the general experiences of study coordinators regarding recruitment and retention issues in a larger sample. This could mean distributing a similar, but more generalizable survey through the existing CTSA study coordinator network or via a national organization like the Association of Clinical Research Professionals or Society of Clinical Research Associates. More research is needed to explore these trends by the type of study (e.g., behavioral or clinical drug study), phase of study (e.g., phase 1 vs. phase 3), and the health condition or behavior (e.g., Parkinson's disease, clinical depression, smoking, cancer screening). It is likely that the barriers, facilitators to recruitment are different by clinical trial characteristics, which may thereby affect study coordinators' anxiety levels. Finally, more research is needed on the types of trainings desired by study coordinators and efficacy of various training strategies with regard to meeting recruitment targets.
6. Conclusions
Study coordinators play an important role in the recruitment and retention of study participants; however, they are often unaware of resources available to them for this purpose and report that they desire more training in these areas. Study coordinators find their peers to be their most helpful resource; thus, more strategies are needed to enhance the professional network of study coordinators. Ongoing skills development is needed for study coordinators, especially those who have been in their role for more than 3 years and those who are responsible for the development of recruitment strategies. CTSAs are well suited to enhance study coordinators’ ability to effectively recruit and retain study participants; however, better advertisement of services is necessary.
Funding source
This research was supported in part by the NYU CTSA grant UL1 TR001445 from the National Center for Advancing Translational Sciences, National Institutes of Health and a diversity supplement from NCATS to support Dr. Langford's “Novel Approaches to Recruitment Planning” study (5 UL1 TR001445-03S2). The sponsor did not participate in any aspect of the program design, data collection, data analysis, or manuscript development.
Acknowledgements
The authors wish to thank the New York University (NYU) – Health + Hospitals Corporation (H + H) Clinical and Translational Science Institute leadership for their ongoing support: Bruce Cronstein, MD (Director); Judith Hochman, MD (Co-Director), and Deborah Chavis-Keeling, MS (Executive Director). We would also like to thank Judith Goldberg, PhD, Program Director for the CTSI Biostatistics Epidemiology and Research Design for early consultations on the project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2019.100424.
Contributor Information
Ebony Scott, Email: Escott19@gmail.com.
Bryan McComb, Email: Bryan.McComb@nyulangone.org.
Howard Trachtman, Email: Howard.Trachtman@nyulangone.org.
Lois Mannon, Email: Lois.Mannon@nyulangone.org.
Peri Rosenfeld, Email: Peri.Rosenfeld@nyulangone.org.
Rachel Thornton, Email: Rachel.Thornton@nyulangone.org.
Nassira Bougrab, Email: Nassira.Bougrab@nyulangone.org.
Scott Sherman, Email: Scott.Sherman@nyulangone.org.
Aisha Langford, Email: Aisha.Langford@nyulangone.org.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Fisher J.A., Kalbaugh C.A. Altruism in clinical research: coordinators' orientation to their professional roles. Nurs. Outlook. May-Jun 2012;60(3):143–148. doi: 10.1016/j.outlook.2011.10.002. 148.e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis A.M., Hull S.C., Grady C., Wilfond B.S., Henderson G.E. The invisible hand in clinical research: the study coordinator's critical role in human subjects protection. J. Law Med. Ethics. 2002;30(3):411–419. doi: 10.1111/j.1748-720x.2002.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speicher L.A., Fromell G., Avery S. The critical need for academic health centers to assess the training, support, and career development requirements of clinical research coordinators: recommendations from the clinical and translational science award research coordinator taskforce. Clin. Transl. Sci. 2012;5(6):470–475. doi: 10.1111/j.1752-8062.2012.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher J.A. Co‐ordinating ‘ethical’clinical trials: the role of research coordinators in the contract research industry. Sociol. Health Illn. 2006;28(6):678–694. doi: 10.1111/j.1467-9566.2006.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rico-Villademoros F., Hernando T., Sanz J.-L. The role of the clinical research coordinator–data manager–in oncology clinical trials. BMC Med. Res. Methodol. 2004;4(1) doi: 10.1186/1471-2288-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonstein S.A., Jones C.T. Joint task Force for clinical trial competency and clinical research professional workforce development. Front. Pharmacol. 2018;9(1148) doi: 10.3389/fphar.2018.01148. 2018-October-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith W., Salenius S., Cobb C. A survey of clinical research coordinators in the cooperative group setting of the American College of Radiology Imaging Network (ACRIN) Acad. Radiol. 2010;17(11):1449–1454. doi: 10.1016/j.acra.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan-Bolyai S., Bova C., Deatrick J.A. Barriers and strategies for recruiting study participants in clinical settings. West. J. Nurs. Res. 2007;29(4):486–500. doi: 10.1177/0193945907299658. [DOI] [PubMed] [Google Scholar]
- 10.Stein M.A., Shaffer M., Echo‐Hawk A., Smith J., Stapleton A., Melvin A. Research START: a multimethod study of barriers and accelerators of recruiting research participants. Clin. Transl. Sci. 2015;8(6):647–654. doi: 10.1111/cts.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.