Abstract
Nowadays, there is an increasing tendency toward using probiotics in different food systems. In this work, probiotic survival, texture features and sensory properties of synbiotic chewing gum containing encapsulated probiotic organisms (Lactobacillus reuteri) were studied. Probiotics were encapsulated using alginate, inulin (0–1%) and lecithin (0–1%). Storage trials showed that, unlike control, the viability of the probiotic in encapsulated samples was retained after 21 days. Probiotic survival was increased by increasing of inulin and lecithin in cell walls. Samples containing encapsulated organisms had different texture parameters compared to the control. Sensory panelists liked the chewing gum with encapsulated lactobacilli. Thus, chewing gum has been shown to be an excellent food for delivery of probiotic lactobacilli. Principal component analysis (PCA) allowed discriminating among probiotics survival and chewing gum specialties. Partial least squares regression (PLSR) models were applied to find out the relationships between sensory and instrumental data.
Keywords: Food science, Food engineering, Food technology, Food quality, Food processing, Food analysis, Chewing gum, Synbiotic, Inulin, Lecithin, Encapsulation
1. Introduction
Chewing gum as a junk food could be an appropriate delivery system for active agents such as probiotics. Chewing gum is increasingly being viewed for improving memory, increasing focus, alertness and concentration, weight manage, improving oral health and stress reduction (Dodds, 2012; Onyper et al., 2011; Palabiyik et al., 2018).
Recently, probiotics have been used in different types of food products because of their beneficial effects on human health. Probiotic bacteria as live microorganisms play an important role in host health (Sanders, 2000). The use of probiotics for the prevention or treatment of dental caries and gingival diseases has been investigated in several in vitro and in vivo studies. Lactobacillus reuteri has anti-microbial and anti-inflammatory properties (Kaur et al., 2018). Acceptable dose levels of probiotics are 10−6–10−7 CFU/g of product per day according to the considered strain (Krasaekoopt et al., 2006). It is one of the most important factors in producing probiotic food (Palabiyik et al., 2018). Protection of probiotics can be realized by several methods including encapsulation with alginates to enhance their survival in foods. Encapsulation is a mechanical or physicochemical process, in which particles containing active components are coated with other materials. Control of the oxidative reaction, hiding the taste, color and aroma, improving the maintenance and control of release, increasing the shelf life, and protecting the cells from harmful environments are some advantages of probiotics encapsulation. There are various types of encapsulation; among which extrusion is an easy and inexpensive method that does not damage probiotics and increases their bioavailability. This technology does not impose harmful solutions and can be carried out under aerobic and anaerobic conditions (Burgain et al., 2011).
Chewing gum has through the years gained increasing acceptance as a delivery system for active ingredients. Several ingredients are now incorporated in chewing gum. Due to the best of our knowledge, there is no work that investigates the encapsulation of probiotics for addition in chewing gum. Thus, the aims of the present study were as follows: (a) encapsulation of L. reuteri (b) production of gum containing the produced capsules evaluating the effect of inulin and lecithin as wall compounds of the capsules on sensory and textural characteristics of the gum (c) evaluating the survival of L. reuteri in produced chewing gum (d) to obtain the relationship between chewing gum parameters using multivariate mathematical-statistical methods such as partial least squares regression analysis (PLSR) and principal components analyses (PCA) as ways to propose simple procedures to quantify texture, sensory and survival aspects.
2. Material and methods
2.1. Preparation of probiotic microorganism
The standard strain of L. reuteri (PTCC 1655, ATCC 32272-DSM 200016) was provided from Iran collection of industrial and pathogenic bacteria and fungi. MRS broth medium was prepared and autoclaved. Lyophilized ampoules were broken under a laminar air flow chamber and the bacteria were inoculated under sterile culture medium and incubated at 37 °C for 24 h. Then, the bacterial suspension was isolated by centrifugation (1560 RCF, 10 min) and washed twice with 0.9% w/v saline solution, and used with certain percentages of inulin and lecithin for producing capsules. The turbidity test was performed by spectrophotometer at 600 nm wavelength (Bengtsson-Riveros et al., 2012).
2.2. Encapsulation of probiotics
Encapsulation of probiotics was carried out using extrusion under sterile conditions (Krasaekoopt et al., 2003; Zhou et al., 1998). Bacterial suspension was used in 5 mL solution of inulin and lecithin according to Table 1 (at 121 °C for 15 min) that were mixed with 20 mL of 2% sterile sodium alginate solution (w/v) (at 121 °C for 15 min). The cell suspension was injected into a container containing 0.05 M solution of sterile calcium chloride by a sterile 1 mL syringe (30G × 8mm) and the capsules were kept in the solution for 30min. Then they were washed and kept in 0.1% sterile peptone water at 4 °C for not more than 1 h and used on the same day (Krasaekoopt and Watcharapoka, 2014). The samples were then filtered using a sterile filter paper and excess water was removed using a sterile blotting paper before using in the experiments.
Table 1.
Treatments used in the production of encapsulated samples.
| Sample | Inulin (%) | Lecithin (%) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 0.5 | 0 |
| 3 | 1 | 0 |
| 4 | 0 | 0.5 |
| 5 | 0.5 | 0.5 |
| 6 | 1 | 0.5 |
| 7 | 0 | 1 |
| 8 | 0.5 | 1 |
| 9 | 1 | 1 |
| 10 | Non-capsulated treatment | Non-capsulated treatment |
2.3. Chewing gum preparation
The base of gum was purchased from Iran Zak Company (Qazvin, Iran). Sucrose, lecithin, glycerin, glucose, sorbitol, inulin, and edible mint essence were provided by Below Company (Tehran, Iran). To prepare the chewing gum, the gum base was first transferred to the laboratory mixer and kneaded at 45–50 °C to provide soft dough. Then encapsulated probiotics, dry and liquid material was gradually added to the base of the chewing gum and finally edible mint essence was added (Table 2) (Miao et al., 2009; Palabiyik et al., 2018; Sh, 2005). It should be mentioned that the formulation of chewing gum was chosen by optimizing on the preliminary screening tests.
Table 2.
Formulation of chewing gum.
| Ingredients | Weight % |
|---|---|
| Gum base | 25 |
| Sweeteners | 68 |
| Lecithin | 0.5 |
| Glycerin | 0.5 |
| Essence | 1 |
| Inulin | 5 |
2.4. Counting the number of trapped bacteria in capsules
In order to break down the capsules and release of the contents, the first dilution of the samples was made in a 2% sodium citrate solution. Then, 10 g of homogenized sample was weighed in sterile zipper bags containing 90 mL of 2% sterile sodium citrate and homogenized by vortex for 5 min until the capsules were completely opened. The next dilution series was prepared with an increase of 1 mL of each dilution to 9 mL of 0.1% sterile peptone water. Lactobacillus counting was performed in an agar MRS medium under anaerobic conditions in anaerobic lava at 37 °C for 72 h (Dave and Shah, 1997; Krasaekoopt et al., 2003). Direct counting was done by an automatic colony counter (Biotec, Germany).
2.5. Encapsulation efficiency
Encapsulation efficiency was determined from the actual number of bacteria loaded in the capsules and the theoretical bacterial loading. The encapsulation efficiency of the formulation for the probiotic bacteria was determined according to:
| (1) |
2.6. Morphology and particle size analysis of capsules
A sample of 10 g of the encapsulated particles were scanned by flatbed HP Scanjet G4010 Photo Scanner (Hewlett-Packard, Palo-Alto, CA, USA) supporting Desk Scan II software (Hewlett Packard, USA). JPEG image file format was analyzed with ImageJ 1.4g (National Institute of Health, USA). To obtain particle size, color images were converted to 8-bits 256 gray level images. The thresholding process of the gray level digital images was used for image segmentation according to Pourfarzad and Habibi-Najafi (2012). In order to investigate the shape and morphology of particles, the solidity (area to convex area) and circularity (4 × pi × area/perimeter2) were also obtained.
2.7. Texture analysis
Studied texture parameters were hardness, adhesiveness, cohesiveness, springiness and chewiness. For this purpose, a Brookfield texture analyzer (CT3, Middleboro, MA, United States) with a cylindrical probe (TA25/1000) and velocity of 2 mm/s was used. The samples had 10 mm long, 10 mm wide and 10 mm height (Potineni and Peterson, 2008).
2.8. Sensory analysis
Sensory analysis was carried out using a 5-point scale scoring 1 (lowest) to 5 (highest) by 10 trained panelists. The overall quality of chewing gum was evaluated by considering the sensory characteristics including overall acceptance, chewing ability, adhesion to the wrapper, adhesion to the teeth, texture, taste and aroma. Sensory analysis was performed by 10 trained panelists.
2.9. Statistical analyses
Data analysis and evaluating the effect of inulin and lecithin on the survival of bacteria and physical and sensory properties of produced gum samples were carried out using a factorial completely randomized design. After analysis of variance, Duncan's multiple range test was used to investigate the significant differences between the means of the data with 95% confidence. Results were reported as the average of six replications. PCA and PLSR were performed on mean survival, texture and sensory data sets. Statistical analysis of the data was done using Minitab software (Minitab 15, Minitab Inc., State College, PA, USA).
3. Results and discussion
3.1. Encapsulation efficiency and survival of Lactobacillus reuteri
This study showed a decrease in the number of probiotic bacteria in the encapsulated beads in comparison with the bacteria added to the feed suspension (Table 3). It might be due to the removal of the free cells from the surface during the washing of beads. Also, the ion equilibrium of bacterial cytoplasmic membranes could be destabilized because of Ca+2 ions used during the bead formation (Reid et al., 2005). Also, there wasn't ant significant difference between encapsulation efficiency of all the treatments (p > 0.05).
Table 3.
Effect of capsulation, lecithin and inulin on the encapsulation efficiency and survival of Lactobacillus reuteri in chewing gum samples during storage.
| Treatment | Encapsulation efficiency (%) |
Lactobacillus reuteri (Log (CFU/ml)) |
||||
|---|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 14 | Day 21 | |||
| Non-capsulated | – | 4.01b | 0b | 0b | 0b | |
| Capsulated | 93.76 | 7.52a | 7.09a | 6.17a | 4.19a | |
| SEM (±) | 0.612 | 0.004 | 0.022 | 0.005 | 0.029 | |
| Lecithin (%) | 0 | 94.15a | 7.53a | 7.10a | 6.11c | 4.16c |
| 0.5 | 93.57a | 7.48a | 7.08a | 6.42b | 4.55b | |
| 1 | 93.55a | 7.49a | 7.17a | 6.67a | 5.17a | |
| SEM (±) | 0.532 | 0.001 | 0.076 | 0.062 | 0.016 | |
| Inulin (%) | 0 | 93.90a | 7.51a | 7.03a | 6.05c | 3.83c |
| 0.5 | 93.83a | 7.48a | 7.12a | 6.35b | 4.26b | |
| 1 | 93.54a | 7.51a | 7.19a | 6.80a | 5.79a | |
| SEM (±) | 0.623 | 0.001 | 0.002 | 0.001 | 0.001 | |
Each observation is a mean±SD of three replicate experiments (n=3).
Values in columns with different letters are significantly different (p ≤ 0.05).
SEM, standard error of the mean.
As shown in Table 3, at the end of the maintenance period, the number of L. reuteri in the encapsulated probiotic chewing gum was higher than the free probiotic. Microbial counting of probiotics showed that probiotics were alive in capsules and the encapsulation process didn't reduce their number. This behavior showed the protective effect of capsules on probiotics against environmental conditions. Reducing the number of bacteria in the capsulated forms could be attributed to containment of produced metabolites by in-capsule bacterial activity, which had a deterrent effect on the growth of bacteria and resulted in a reduction in their number during storage (Edgar and Geddes, 1990). Also, it was reported that the encapsulation of probiotics with calcium alginate didn't reduce the number of bacteria (Sultana et al., 2000). The evaluation of bacterial survival in capsules over a 21-day period showed that increasing the concentration of inulin and lecithin in the wall of encapsulated seeds increased the bacterial survival of the samples. This behavior could be attributed to the ability of inulin and lecithin to improve trapping efficiency and bacterial accumulation capability during the encapsulation process (Donthidi et al., 2010).
3.2. Morphology and particle size analysis
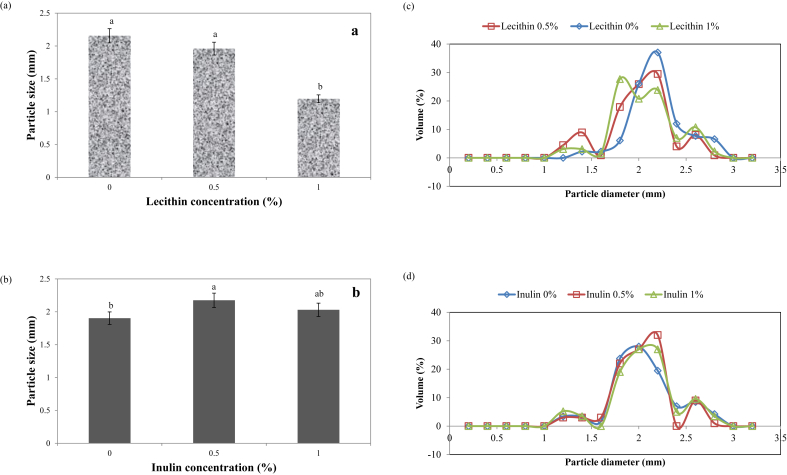
As it could be seen in Fig. 1, particle size of the capsules was significantly decreased and increased with addition of lecithin and inulin in walls, respectively. Furthermore, addition of lecithin and inulin extend the size distribution of capsules. It gives a smooth texture when the capsules are incorporated into chewing gum. Also, circularity and solidity as components of the shape and morphology of capsules were investigated (Table 4). As it could be seen, there wasn't any significant difference between shape and morphology of treatments. However, capsules produced this way is exceptionally good raw material for adding in chewing gum because of its relatively homogeneous particle size distribution and accepted, spherical shape. Also, the developed technology is a manufacturing process which facilitates forming of a chewing gum containing valuable probiotic spheres having unique characteristics.
Fig. 1.
The effect of lecithin (a and c) and inulin (b and d) on average particle size (a and b) and particle size distribution (c and d) of microcapsules.
Table 4.
Effect of lecithin and inulin on morphology of capsules.
| Treatment | Circularity | Solidity | |
|---|---|---|---|
| Lecithin (%) | 0 | 0.877a | 0.902a |
| 0.5 | 0.874a | 0.905a | |
| 1 | 0.872a | 0.905a | |
| SEM(±) | 0.0208 | 0.0096 | |
| Inulin (%) | 0 | 0.869a | 0.898a |
| 0.5 | 0.881a | 0.908a | |
| 1 | 0.873a | 0.906a | |
| SEM (±) | 0.0220 | 0.0097 |
Each observation is a mean±SD of three replicate experiments (n=3).
Values in columns with different letters are significantly different (p ≤ 0.05).
SEM, standard error of the mean.
3.3. Texture analysis
The factors derived from the texture analysis of the chewing gum samples are presented in Table 5. Addition of capsules to gum caused a significant decrease in the hardness of the samples compared to the control sample. The main volume of the samples was occupied with microcapsules, which had low density. This probably led to a reduction in their hardness (Santos et al., 2014). Besides, increasing the moisture content of gum due to the presence of these seeds can also make the texture of the chewing gum softer (Habibi Najafi et al., 2011). Addition of microcapsules into chewing gum matrix resulted in the less dense crystalline networks and reduced particle–particle interactions, with more open structures and void spaces between the crystals. This could be related to the higher moisture content in the matrix, which tends to wet the matrix thereby opening up the moisture phase, as moisture filled the voids within the crystal network. The increase of lecithin and inulin in the capsule wall resulted in a decrease and increase in hardness of chewing gum samples, respectively. Beckett (1999) attributed this to the free-moving lubricating plastic flow, more connected with forces between solid particles. Moisture relatively fills spaces between solid particles in chewing gum and reduces resistance to chewing, with greatest effect at lower particle sizes.
Table 5.
Effect of capsulation, lecithin and inulin on texture analysis of chewing gum samples.
| Treatment | Hardness (N) | Adhesiveness (N.S) | Cohesiveness | Springiness | Chewiness (N) | |
|---|---|---|---|---|---|---|
| Non-capsulated | 46.06a | 2.3b | 0.43a | 1.12b | 22.18a | |
| Capsulated | 5.81b | 3.46a | 0.06b | 3.81a | 1.33b | |
| SEM (±) | 0.111 | 0.051 | 0.003 | 0.128 | 0.083 | |
| Lecithin (%) | 0 | 6.23a | 2.53c | 0.36b | 3.39b | 7.60c |
| 0.5 | 6.25a | 3.32b | 0.38b | 4.81a | 11.42a | |
| 1 | 4.95b | 4.53a | 0.54a | 3.21b | 8.58b | |
| SEM(±) | 0.508 | 0.504 | 0.049 | 0.442 | 0.446 | |
| Inulin (%) | 0 | 5.79b | 3.98a | 0.47a | 3.53b | 9.61b |
| 0.5 | 5.32c | 3.13b | 0.39b | 4.18a | 8.67c | |
| 1 | 6.33a | 3.27b | 0.42b | 3.72b | 9.89a | |
| SEM (±) | 0.092 | 0.332 | 0.018 | 0.163 | 0.106 |
Each observation is a mean±SD of three replicate experiments (n=3).
Values in columns with different letters are significantly different (p ≤ 0.05).
SEM, standard error of the mean.
The adhesion of the specimens to the teeth or the amount of force needed to separate the food particles from the teeth surface is defined as adhesion (Munksgaard et al., 1995). Adding the microcapsules to the chewing gum formulation increased the adhesiveness of the samples compared to the control sample. This behavior can be attributed to increased moisture content of the samples due to the presence of the microcapsules (Habibi Najafi et al., 2011). Besides, an increase in the concentration of lecithin in particles walls resulted in higher adhesiveness of chewing gum samples. But additions of inulin lead to decrease of adhesiveness. It could be explained by change trend of particle size. Lower microcapsules had higher surface to volume ratio and subsequently higher surface moisture and adhesiveness (Martynenko and Janaszek, 2014).
Cohesiveness is the internal force between components and is defined as the amount of force needed to modify the sample before breaking. In other words, it shows the quantity of sample initial deformation before rupture when the sample is pressed with teeth (Meullenet et al., 1998). By addition of the microcapsules, the cohesiveness of chewing gum was significantly decreased. It should be noted that cohesiveness was increased and decreased as a consequence of lecithin and inulin addition to microcapsules walls. This could be explained with particle size trend of microcapsules which affect the density of chewing gum matrix.
Springiness is the degree or severity that sample returns to its initial form after the partial pressure between the tongue and the mouth, or it is the intensity of a deformed specimen after the removal of force to its original state (Fox et al., 2017). Springiness also shows the elasticity of the sample. The higher springiness in sample results in the higher elasticity which increases acceptability (Habibi Najafi et al., 2011). Addition of microcapsules into chewing gum matrix lead to increase of springiness and the most springiness was observed for samples containing 0.5% lecithin and 0.5% inulin.
Chewiness shows the number of masticates required for certain amount of sample in order to decrease the consistency satisfactory for swallowing. It is the product of hardness × cohesiveness × springiness (Fox et al., 2017). The chewing gums with microcapsules had lower chewiness properties than samples without microcapsule. It was due to the important influence of hardness on calculation of chewiness. Similar to trend of hardness changes, they were affected by the reverse relationship between moisture and chewiness. Thus, by increasing the moisture content, the chewiness was reduced (Habibi Najafi et al., 2011). The samples containing capsules with 1% inulin and 0.5% lecithin showed the highest chewiness.
3.4. Sensory analysis
No significant difference was observed in taste and aroma between the chewing gums treated with and without capsules (p > 0.05) (Table 6). The score of texture, chewing ability and overall acceptance parameters in samples containing capsules was significantly higher than that of control samples, indicating the acceptance of texture by evaluators. While in non-capsulated samples, the scores of adhesion to the teeth and wrapper were higher than the control group. In terms of aroma, taste, smell, there was no significant difference between different levels of lecithin and inulin. The highest score texture was observed in the specimen containing 1% lecithin and 0.5% inulin, which can be due to the hardness of the seeds wall. The adhesion to wrapper score was increased by increasing lecithin, but the highest amount was reported at 0.5% inulin level. The highest chewing ability was observed in the non-lecithin sample containing 0.5% inulin. By addition of lecithin and inulin, adhesion to teeth and overall acceptance of the specimens were significantly increased. It was expected that due to the moisture content around the seeds, the encapsulated samples stick to teeth during chewing. By increasing the amount of lecithin and inulin in the seeds wall, low difference was observed between the control and treatment samples in term of the adhesion of the chewing gum to the teeth. The more adhesion to the teeth shows the undesirable characteristics of sensory analysis. In general, comparison of capsulated and non-capsulated treatments indicated that the moisture from the seeds in the capsulated treatment caused the adhesion of the gum to the wrapper. These results are in agreement with those reported by other researchers (Devereux et al., 2003; Frank-Frippiat, 1995; Santos et al., 2014).
Table 6.
Effect of capsulation, lecithin and inulin on the sensory properties of chewing gum samples during storage.
| Treatment | Aroma | Taste | Texture | Adhesion to the teeth | Adhesion to the wrapper | Chewing ability | Overall acceptance | |
|---|---|---|---|---|---|---|---|---|
| Non-capsulated | 3.9a | 4a | 3.7b | 4.3a | 4.3a | 3.4b | 3.95b | |
| Capsulated | 4.03a | 4a | 3.76a | 4.26b | 4.26b | 3.65a | 4.2a | |
| SEM (±) | 0.325 | 0.357 | 0.0003 | 0.0003 | 0.0003 | 0.0003 | 0.0003 | |
| Lecithin (%) | 0 | 3.93a | 4.10a | 3.71b | 4.16c | 4.20b | 3.73a | 3.90b |
| 0.5 | 3.96a | 3.86a | 3.73b | 4.26b | 4.33a | 3.63b | 4.00a | |
| 1 | 4.15a | 4.05a | 3.85a | 4.36a | 4.25ab | 3.50c | 4.01a | |
| SEM (±) | 0.538 | 0.480 | 0.079 | 0.105 | 0.174 | 0.212 | 0.139 | |
| Inulin (%) | 0 | 4.16a | 3.96a | 3.76b | 4.23b | 4.26a | 3.43c | 1.90b |
| 0.5 | 4.05ab | 4.11a | 3.83a | 4.31a | 4.35a | 3.80a | 2.17a | |
| 1 | 3.83b | 3.93a | 3.70c | 4.25a | 4.16b | 3.63b | 2.03ab | |
| SEM (±) | 0.524 | 0.479 | 0.053 | 0.098 | 0.157 | 0.141 | 0.131 |
Each observation is a mean±SD of three replicate experiments (n=3).
Values in columns with different letters are significantly different (p ≤ 0.05).
SEM, standard error of the mean.
3.5. Principal component analysis (PCA)
Data on quality, texture and sensory aspects of chewing gum and probiotic survival were subjected to PCA (Fig. 2). The multivariate treatment of the data obtained for the samples permitted the reduction of the variables to two principal components, which together explained 77.1% of the total variability. The first axis reported for 58.1% and the second axis for 19%. According to the PCA biplot, hardness, springiness, chewiness, survival at first day, chewing ability and particle size were negatively correlated to PC1 axis whereas other chewing gum characteristics were positively correlated to PC1 axis. Hardness, adhesiveness, cohesiveness, chewiness and survival during storage were negatively correlated to PC2 axis while other chewing gum characteristics were positively correlated to PC2 axis. As clearly revealed in the PCA plot (Fig. 2), encapsules containing 1% inulin and 1% lecithin had higher survival during storage. There were positive and high correlations between springiness, chewing ability and particle size. The chewing gum samples containing encapsule with 0 or 0.5% lecithin and 0.5% inulin had higher springiness, chewing ability and particle size. The results represented by PCA seemed to be in agreement with the results discussed above.
Fig. 2.
Principal component analysis biplot on properties of chewing gums containing microcapsules.
3.6. Correlation of texture and sensory properties of chewing gum using PLSR method
Multivariate techniques can be applied to establish relationships between texture and sensory data. Statistical techniques commonly used can be either asymmetric or symmetric (Dijksterhuis, 1994). The PLSR as a asymmetric method is particularly used to predict one data set from the other while PCA as a symmetric method only depicts the relationship between data sets (Martens and Martens, 2001). Several researchers have attempted to correlate various characteristics of different products (Najafi, Pourfarzad, Zahedi, Ahmadian-Koochaksaraei and Khodaparast, 2016; Hejri-Zarifi et al., 2013; Pourfarzad et al., 2012; Sai Manohar and Haridas Rao, 2002). PLSR was applied to examine the relationship between textural X and sensory Y data. The prediction of sensory aspects using texture analysis as an instrumental measurement had a principal importance in the food industry because it help us to gather much information about the acceptability and quality of the final product. Thus, four instrumental properties, which had the most significant interrelationships with other properties, were used as predictors: hardness, adhesiveness, cohesiveness and springiness. The regression equations output to predict important parameters are given in Table 7. The coefficients of obtained equations were higher than 90%, which show the applicability of the regression model between the ranges of variables comprised.
Table 7.
Partial least squares regression (PLSR) models for sensory features of chewing gums containing encapsulated probiotics.
| Sensory attributes | Mathematical models | R2 |
|---|---|---|
| Texture | 4.449 − 0.122 Hardness +0.006 Adhesiveness − 0.155 Cohesiveness +0.014 Springiness | 99.60 |
| Adhesion to teeth | 3.057 − 0.079 Hardness − 0.379 Adhesiveness +4.869 Cohesiveness +0.209 Springiness | 99.71 |
| Adhesion to wrapper | 5.774 − 0.099 Hardness +0.377 Adhesiveness − 4.551 Cohesiveness − 0.048 Springiness | 99.37 |
| Chewing ability | 3.128 − 0.125 Hardness − 0.790 Adhesiveness +6.952 Cohesiveness +0.275 Springiness | 99.20 |
| Aroma | 8.472 − 0.206 Hardness +1.134 Adhesiveness − 13.423 Cohesiveness − 0.424 Springiness | 90.35 |
| Taste | 6.179 − 0.176 Hardness − 0.049 Adhesiveness – 1.019 Cohesiveness – 0.121 Springiness | 93.82 |
| Overall acceptance | −72.939 + 15.285 Hardness +3.162 Adhesiveness +160.114 Cohesiveness − 0.781 Springiness | 99.28 |
4. Conclusion
Generally, the results of the present research showed that encapsulation with L. reuteri can improve its survival in chewing gum so that after 21 days, the survival of encapsulated bacteria was in the standard level of probiotics. Besides, this study indicated that a product such as encapsulated chewing gum could be a good choice for consumption of probiotics. Also, inulin and lecithin, as compositions of capsule wall, had no negative effect on its sensory and texture characteristic and was accepted by evaluators. It was proved that PCA is able to extract relevant information and offer an easy and talented method for the explanation of properties of encapsulated and chewing gum samples. As the combination of inulin and lecithin improved the stability of the encapsulated bacteria, this technology could be utilized to develop chewing gum systems encapsulated with probiotics.
Declarations
Author contribution statement
Sahar Dehqan Ghaziyani, Amir Pourfarzad, Siamak Gheibi, Leila Roozbeh Nasiraie: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was partially supported by the Guilan Science and Technology Park as a creativity and flourishing project.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The Department of Food Science and Technology at University of Guilan is warmly acknowledged for providing necessary research facilities. The authors wish to thank Dr. S. Gheibi for his invaluable guidance and assistance in food microbiology laboratory. We also thank Eng. Safaie (Iran Zak Company, Qazvin, Iran) for providing gum base.
References
- Bengtsson-Riveros, Annmarie, De Reu, Johannes, Wood, Robert Dustan, Darbyshire, John, Knauf, Hermann, & Cavadini, Christoph. (2012). Consumable Product Containing Probiotics: Google Patents.
- Beckett S.T. 3rd edn. Blackwell Science; Oxford: 1999. Industrial Chocolate Manufacture and Use. [Google Scholar]
- Burgain Jennifer, Gaiani Claire, Linder Michel, Scher Joel. Encapsulation of probiotic living cells: from laboratory scale to industrial applications. J. Food Eng. 2011;104(4):467–483. [Google Scholar]
- Dave Rajiv I., Shah Nagendra P. Viability of yoghurt and probiotic bacteria in yoghurts made from commercial starter cultures. Int. Dairy J. 1997;7(1):31–41. [Google Scholar]
- Devereux H.M., Jones G.P., McCormack L., Hunter W.C. Consumer acceptability of low fat foods containing inulin and oligofructose. J. Food Sci. 2003;68(5):1850–1854. [Google Scholar]
- Dijksterhuis G. Procrustes analysis in studying sensory-instrumental relations. Food Qual. Prefer. 1994;5(1):115–120. [Google Scholar]
- Dodds Michael WJ. The oral health benefits of chewing gum. J. Ir. Dent. Assoc. 2012;58(5):253–261. [PubMed] [Google Scholar]
- Donthidi A.R., Tester R.F., Aidoo K.E. Effect of lecithin and starch on alginate-encapsulated probiotic bacteria. J. Microencapsul. 2010;27(1):67–77. doi: 10.3109/02652040902982183. [DOI] [PubMed] [Google Scholar]
- Edgar W.M., Geddes D.A. Chewing gum and dental health--a review. Br. Dent. J. 1990;168(4):173. doi: 10.1038/sj.bdj.4807129. [DOI] [PubMed] [Google Scholar]
- Fox Patrick F., Guinee Timothy P., Cogan Timothy M., McSweeney Paul LH. Springer; 2017. Fundamentals of Cheese Science. [Google Scholar]
- Frank-Frippiat A. Paper Presented at the 1st Orafti Research Conference Proceedings. Brussels, Belgium. 1995. Innovative food products with inulin and oligofructose. [Google Scholar]
- Habibi Najafi M.B., Vahedi N., Yaghanehzad S., Hoseini Z. Effects of milk permeate addition on physicochemical and textural properties of toffee. J. Food Res. 2011;21(2):229–246. [Google Scholar]
- Hejri-Zarifi Sudiyeh, Ahmadian-Kouchaksaraei Zahra, Pourfarzad Amir, Khodaparast Mohammad Hossein Haddad. Dough performance, quality and shelf life of flat bread supplemented with fractions of germinated date seed. J. Food Sci. Technol. 2013:1–9. doi: 10.1007/s13197-013-0929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur Kanwardeep, Nekkanti Sridhar, Madiyal Mridula, Choudhary Prashant. Effect of chewing gums containing probiotics and xylitol on oral health in children: a randomized controlled trial. [Original Research] J. Int. Oral Health. 2018;10(5):237–243. [Google Scholar]
- Krasaekoopt Wunwisa, Watcharapoka S. Effect of addition of inulin and galactooligosaccharide on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT-Food Sci. Technol. 2014;57(2):761–766. [Google Scholar]
- Krasaekoopt Wunwisa, Bhandari Bhesh, Deeth Hilton. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 2003;13(1):3–13. [Google Scholar]
- Krasaekoopt Wunwisa, Bhandari Bhesh, Deeth Hilton C. Survival of probiotics encapsulated in chitosan-coated alginate beads in yoghurt from uht-and conventionally treated milk during storage. LWT-Food Sci. Technol. 2006;39(2):177–183. [Google Scholar]
- Martens H., Martens M. J. Wiley & Sons Ltd; London, UK: 2001. Analysis of Two Data Tables X and Y: Partial Least Squares Regression (Plsr) Multivariate Analysis of Quality: an Introduction; pp. 111–125. [Google Scholar]
- Martynenko Alex, Janaszek Monika A. Texture changes during drying of apple slices. Dry. Technol. 2014;32(5):567–577. [Google Scholar]
- Meullenet J.F., Lyon B.G., Carpenter John A., Lyon C.E. Relationship between sensory and instrumental texture profile attributes. J. Sens. Stud. 1998;13(1):77–93. [Google Scholar]
- Miao Dagang, Blom Dan, Zhao Hongmei, Luan Xuefei, Chen Tongzhi, Wu Xiaohui. The antibacterial effect of cmcts-containing chewing gum. J. Nanjing Med. Univ. 2009;23(1):69–72. [Google Scholar]
- Munksgaard E.C., Nolte J., Kristensen K. Adherence of chewing gum to dental restorative materials. Am. J. Dent. 1995;8(3):137–139. [PubMed] [Google Scholar]
- Najafi Mohammad B Habibi, Pourfarzad Amir, Zahedi Hoda, Ahmadian-Kouchaksaraie Zahra, Khodaparast Mohammad H Haddad. Development of sourdough fermented date seed for improving the quality and shelf life of flat bread: Study with univariate and multivariate analyses. J. Food Sci. Technol. 2016;53(1):209–220. doi: 10.1007/s13197-015-1956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyper Serge V., Carr Timothy L., Farrar John S., Floyd Brittney R. Cognitive advantages of chewing gum. Now you see them, now you don’t. Appetite. 2011;57(2):321–328. doi: 10.1016/j.appet.2011.05.313. [DOI] [PubMed] [Google Scholar]
- Palabiyik Ibrahim, Pirouzian Haniyeh Rasouli, Konar Nevzat, Toker Omer Said. Springer; 2018. A Novel Delivering Agent for Bioactive Compounds: Chewing Gum Bioactive Molecules in Food; pp. 1–39. [Google Scholar]
- Potineni Rajesh V., Peterson Devin G. Influence of flavor solvent on flavor release and perception in sugar-free chewing gum. J. Agric. Food Chem. 2008;56(9):3254–3259. doi: 10.1021/jf072783e. [DOI] [PubMed] [Google Scholar]
- Pourfarzad Amir, Habibi-Najafi Mohammad Bagher. Optimization of a liquid improver for barbari bread: staling kinetics and relationship of texture with dough rheology and image characteristics. J. Texture Stud. 2012;43(6):484–493. [Google Scholar]
- Pourfarzad Amir, Mohebbi Mohebbat, Mazaheri-Tehrani Mostafa. Interrelationship between image, dough and barbari bread characteristics; use of image analysis to predict rheology, quality and shelf life. Int. J. Food Sci. Technol. 2012;47(7):1354–1360. [Google Scholar]
- Reid A Ainsley, Vuillemard J.C., Britten M., Arcand Y., Farnworth E., Champagne C.P. Microentrapment of probiotic bacteria in a ca2+-induced whey protein gel and effects on their viability in a dynamic gastro-intestinal model. J. Microencapsul. 2005;22(6):603–619. doi: 10.1080/02652040500162840. [DOI] [PubMed] [Google Scholar]
- Sai Manohar R., Haridas Rao P. Interrelationship between rheological characteristics of dough and quality of biscuits; use of elastic recovery of dough to predict biscuit quality. Food Res. Int. 2002;35(9):807–813. [Google Scholar]
- Sanders Mary Ellen. Considerations for use of probiotic bacteria to modulate human health. J. Nutr. 2000;130(2):384S–390S. doi: 10.1093/jn/130.2.384S. [DOI] [PubMed] [Google Scholar]
- Santos Milla G., Carpinteiro Débora A., Thomazini Marcelo, Rocha-Selmi Glaucia A., da Cruz Adriano G., Rodrigues Christiane EC. Coencapsulation of xylitol and menthol by double emulsion followed by complex coacervation and microcapsule application in chewing gum. Food Res. Int. 2014;66:454–462. [Google Scholar]
- Sh Porkar. Islamic Azad University; Tehran. Iran: 2005. Evaluation of Manna Taranjebin Effect on Reduction of Jaundice in Infants. Pharm. D. Thesis under the guidance of Dr. Sharif. [Google Scholar]
- Sultana Khalida, Godward Georgia, Reynolds N., Arumugaswamy R., Peiris Pt, Kailasapathy Kaila. Encapsulation of probiotic bacteria with alginate–starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int. J. Food Microbiol. 2000;62(1-2):47–55. doi: 10.1016/s0168-1605(00)00380-9. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Martins E., Groboillot A., Champagne C.P., Neufeld R.J. Spectrophotometric quantification of lactic bacteria in alginate and control of cell release with chitosan coating. J. Appl. Microbiol. 1998;84(3):342–348. [Google Scholar]