Abstract
Tristetraprolin (TTP) is an RNA-binding protein that targets numerous immunomodulatory mRNA transcripts for degradation. Many TTP targets are key players in the pathogenesis of periodontal bone loss, including tumor necrosis factor–α. To better understand the extent that host immune factors play during periodontal bone loss, we assessed alveolar bone levels, inflammation and osteoclast activity in periodontal tissues, and immune response in draining cervical lymph nodes in TTP-deficient and wild-type (WT) mice in an aging study. WT and TTP-deficient (knockout [KO]) mice were used for all studies under specific pathogen-free conditions. Data were collected on mice aged 3, 6, and 9 mo. Microcomputed tomography (µCT) was performed on maxillae where 3-dimensional images were generated and bone loss was assessed. Decalcified sections of specimens were scored for inflammation and stained with tartrate-resistant acid phosphate (TRAP) to visualize osteoclasts. Immunophenotyping was performed on single-cell suspensions isolated from primary and peripheral lymphoid tissues using flow cytometry. Results presented indicate that TTP KO mice had significantly more alveolar bone loss over time compared with WT controls. Bone loss was associated with significant increases in inflammatory cell infiltration and an increased percentage of alveolar bone surfaces apposed with TRAP+ cells. Furthermore, it was found that the draining cervical lymph nodes were significantly enlarged in TTP-deficient animals and contained a distinct pathological immune profile compared with WT controls. Finally, the oral microbiome in the TTP KO mice was significantly different with age from WT cohoused mice. The severe bone loss, inflammation, and increased osteoclast activity observed in these mice support the concept that TTP plays a critical role in the maintenance of alveolar bone homeostasis in the presence of oral commensal flora. This study suggests that TTP is required to inhibit excessive inflammatory host responses that contribute to periodontal bone loss, even in the absence of specific periodontal pathogens.
Keywords: periodontitis, osteoclasts, inflammation, RNA binding proteins, microbiome, immunology
Introduction
Periodontitis is the sixth most prevalent disease in the world and affects nearly half of the American adult population (Eke et al. 2015). As a chronic inflammatory disease, constant antigen stimulation is maintained by subgingival bacterial colonization, eventually leading to epithelial and connective tissue degradation and alveolar bone loss (Graves 2008; Lorenzi et al. 2014). The balance between pro- and anti-inflammatory factors and the regulation of inflammatory mediators determines the severity and tissue damage in the periodontium (Kantarci et al. 2006; Palanisamy et al. 2012).
Tristetraprolin (TTP; also known as NUP475, GOS24, or TIS11) is an mRNA-binding protein encoded by Zfp36 that functions to inhibit the production of multiple proinflammatory cytokines (Brooks and Blackshear 2013). TTP is a key molecular target in the p38 mitogen-activated protein kinase (MAPK)/MAPK-activated protein kinase-2 (MK2) stress signaling pathways. Its primary role is binding to adenosine-uridine rich elements (AREs) in the 3′ untranslated regions by virtue of the tandem CCCH zinc finger within target mRNA transcripts, thus destabilizing and promoting mRNA degradation of targets such as tumor necrosis factor (TNF)–α, interleukin (IL)–1β, and IL-6 (Zhao et al. 2011; Palanisamy et al. 2012; Patial and Blackshear 2016). It has been proposed that TTP may bind to itself and negatively regulate its own expression (Tchen et al. 2004). However, activation of the IL-1β and toll-like receptors signal through the p38/MK2 pathway to phosphorylate could possibly inactivate TTP (Chrestensen et al. 2004; Patil and Kirkwood 2007; Herbert et al. 2016). Regardless of the phosphorylation status, once TTP dissociates from cytokine ARE mRNA, the target gene mRNA stability is enhanced, resulting in elevated immunomodulatory cytokine expression, including cytokines typically associated with periodontal disease progression and alveolar bone loss (Garlet 2010; Palanisamy et al. 2012). Because of the role of TTP in the tight regulation of proinflammatory cytokine mRNA stability, we aimed to better understand the temporal influence of TTP in alveolar bone homeostasis.
Factors mediating periodontal homeostasis and health have not been well characterized. The Zfp36-/- (TTP knockout [KO]) mice have a severe hyperinflammatory phenotype involving cachexia, arthritis, conjunctivitis, and dermatitis (Taylor et al. 1996; Qiu et al. 2012). Based on the known function of TTP, we sought to determine the role of TTP in the context of periodontal tissue homeostasis in mice postdevelopment. We report that TTP plays a critical role in periodontal homeostasis. TTP deficiency causes severe alveolar bone loss associated with increased inflammation, upregulated osteoclast cellular endpoints, aberrations in cervical lymph node (cLN) size, and adaptive cellular immune profiles. Collectively, these data support the role of TTP to play a major regulator of alveolar bone homeostasis.
Materials and Methods
Mice
Wild-type (WT; C57BL/6) and Zfp36-/- (TTP KO on a C57BL/6 background) mice (Taylor et al. 1996) were bred and cohoused from heterozygous dams and sires under specific pathogen-free (SPF) conditions. Mice were genotyped by tail snip using WT- and KO-specific primers: Fwd-5′-GGCCGAAGCTGTG CTGGGT-3′ and Rev-5′-CTGGCCAGGGAGAGCTAGGT C-3′ (Eurofins MWG Operon). Mice were aged to 3, 6, and 9 mo for experiments under SPF conditions with standard 12-h light/dark conditions and standard bedding in isolators. TTPΔARE mice were established as described recently (Patial, Curtis, et al. 2016). All work with mice was approved by the Medical University of South Carolina Animal Protocols Review Board and was performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals, and reporting fulfills criteria outlined in the ARRIVE guidelines.
Microcomputed Tomography (µCT)
Maxillae were fixed in 10% phosphate-buffered formalin for 24 h, washed with phosphate-buffered saline, and stored in 70% ethanol. Maxillae were scanned at 55 kVp, 145 µA, 16 µm voxel resolution using a Scanco Medical 40 µCT scanner (Scanco Medical). Three-dimensional images were generated and reconstructed for each specimen. These images were rotated with a standard orientation and threshold to discern mineralized and nonmineralized tissue. The region of interest (ROI) was indicated by the contour height of molars at the cementoenamel junction as the width and the molar cusp tips to root apices as the height. Depth was equal to the buccolingual size of the teeth plus 1.0 mm3. Bone volume fraction was calculated as the percentage of bone within the ROI using AnalyzePro software. Data are reported in accordance with standardized nomenclature (Bouxsein et al. 2010).
Histomorphometry
Following µCT, maxillae were decalcified as previously described (Novince et al. 2012). Specimens were paraffin embedded, and serial sagittal sections were cut through the distal maxillae for alveolar bone analyses. Sections of 7 µm were stained with hematoxylin and eosin (H&E) to assess tissue inflammation. Inflammation was scored in the maxillary periodontal tissues using the following scoring system: 0 = 0% to 5% inflammatory cell (IC) infiltration, 1 = 5% to 25% ICs, 2 = 25% to 50% ICs, and 3 = >50% ICs. Sections of 7 µm were stained with tartrate-resistant acid phosphate (TRAP) and alinine blue (counter stain) to quantify osteoclast cellular endpoints. TRAP-positive area (red color staining) and eroded bone perimeters were quantified using Visiopharm software. Data are reported in accordance with standardized nomenclature (Dempster et al. 2013).
Flow Cytometry
cLN single-cell suspensions were isolated, washed, and counted. Live cells were treated with Fc block and stained for anti-CD3-APC, anti-TCRγδ-e450, anti-CD8-PE, anti-CD4-FITC (T lymphocytes); anti-B220-FITC, anti-IgD-eF450, anti-IgM-PE, anti-CD40-APC (B lymphocytes); anti-CD11b-APC, anti-Ly6G-PacB, anti-F4/80-PE, anti-Ly6C-FITC (inflammatory monocytes); and anti-CD11c-APC, anti-MHC II-PE, anti-CD317-FITC (plasmacytoid dendritic cells). Fixed cells were treated with Fc block stained for anti-CD4-FITC, anti-CD25-PE, and anti-FoxP3-APC (T regulatory lymphocytes). Dead cells were excluded from analysis via propidium iodide viability dye (live) and e450 viability dye (fixed), and data were acquired by MACSQuant System (Miltenyi Biotec) and analyzed by FlowJo 11.0 software. Cells were gated on appreciate cell population by forward versus side scatter plots and subsequently on appropriate cell surface markers.
Oral Microbiome Analysis
Oral tissues were carefully collected by dissecting the right mandible and maxillae, preserving the buccal mucosa and gingival tissue while avoiding mouse fur and tongue. Bacterial DNA was isolated using the Qiagen QIAamp DNA Mini Kit as directed by the manufacturer. Illumina MiSeq was used for 16S bacterial sequencing analysis. The raw MiSeq reads of the 16S rRNA gene amplicons were analyzed using DADA2 package version 1.28.0 (Callahan et al. 2016). Based on the quality score profiles, the initial 12 nucleotides were trimmed from the forward and reverse sequences; these were then trimmed at 145 and 130 nucleotides, respectively. The reads were merged by concatenation and chimera identified and removed. The operational taxonomic unit (OTU) sequences were inferred by DADA2 against the mouse genome (version 1.28.0) using blast (version 2.2.25+). Taxonomy was assigned using the Greengenes database version 13.8, resulting in identification of 1,656 OTUs in the entire set of 102 samples using R package phyloseq version 1.14.0 to manage data. To control for the effects of cohousing, all analyses were stratified by the caging. Jensen-Shannon divergence beta-diversity distances have been computed and used in PERMANOVA (Anderson 2001) and within class (by cage) principal coordinates (PCoA) analysis in R package vegan version 2.4-1.
Serum Biochemical Assays
Whole blood was collected via cardiac puncture at sacrifice. Serum was isolated and stored at –80 °C for use. Mouse osteocalcin (Alfa Aesar) and CTX-1 (Immuno-diagnostics, Inc.) enzyme-linked immunosorbent assays (ELISAs) were performed following the manufacturer’s guidelines. Serum freeze/thawed once was tested for TNF-α (DuoSet, R&D Systems) by ELISA and performed per the manufacturer’s instructions.
Statistical Analysis
Unpaired t tests and 2-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test were performed using GraphPad Prism 7.0 where indicated.
Results
TTP KO Exacerbates Alveolar Bone Loss
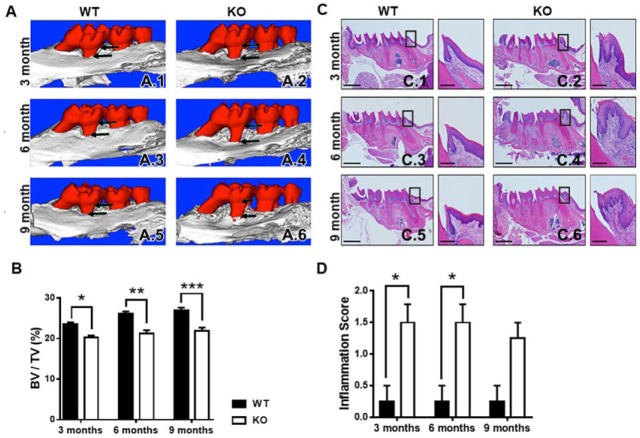
Both male and female WT and TTP-deficient mice were maintained with minimal TTP-deficient mice morbidity over the 9-mo time frame. Body weights of male and female TTP KO mice did not show any sex differences (Appendix 1A–B). Male and female mice were aged to 3, 6, and 9 mo, and maxillae were scanned by µCT to show and quantitate alveolar bone volume (Fig. 1A–B; Appendix 1C). Since bone volume fractions and body weights of TTP KO mice indicated a lack of sexual dimorphism in this mouse model, all subsequent analyses were performed in male mice. TTP KO mice have significant alveolar bone loss at 3 mo by 14.0% (0.235153 vs. 0.202162, P < 0.05; Fig. 1A.1–A.2), 6 mo by 18.7% (0.261387 vs. 0.212560, P < 0.01; Fig. 1A.3–A.4), and 9 mo by 19.0% (0.269292 vs. 0.218001, P < 0.01; Fig. 1A.5–A.6). Findings that TTP KO mice have exacerbated alveolar bone loss suggest TTP has an important role in alveolar bone maintenance.
Figure 1.
Tristetraprolin (TTP) knockout (KO) exacerbates alveolar bone loss and inflammation. (A) Representative microcomputed tomography (µCT) images showing qualitative bone loss (indicated by arrows) in TTP KO mice at 3 (A.2), 6 (A.4), and 9 (A.6) mo of age compared with wild type (WT). (B) µCT analysis of bone volume/total volume of alveolar bone; n = 4/gp. (C) Representative hematoxylin and eosin–stained maxillae from each group (scale bar = 1 mm), with insert displaying inflammation at the mesial aspect of the first molar (scale bar = 1 µm) at 3 (C.2), 6 (C.4), and 9 (C.6) mo of age. (D) Inflammation scoring of connective tissue around the first molar of maxillae with 0 = 0% to 5% inflammatory cell (IC) infiltration, 1 = 5% to 25% ICs, 2 = 25% to 50% ICs, and 3 = >50% ICs; n = 4/gp. Two-way analysis of variance with Tukey’s multiple comparisons test and unpaired t test; data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001.
TTP Modulates Inflammation and Osteoclastogenesis
Histomorphometric analysis of H&E-stained maxillae sagittal sections were performed to examine proinflammatory endpoints in TTP WT and KO mice at 3 mo (Fig. 1C.1–C.2), 6 mo (Fig. 1C.3–C.4), and 9 mo (Fig. 1C.5–C.6). Dense IC infiltration was scored in periodontal epithelial and maxillary tissues according to the following scale: 0 = minimal/normal inflammation (0 to 2 IC); 1 = light inflammation (2 to 5 IC); 2 = moderate inflammation (5 to 10 IC); or 3 = severe inflammation (>10 IC). Inflammation was mostly composed of neutrophils, with infiltration of macrophages, plasma cells, and lymphocytes in the connective tissue. TTP KO mice also showed prominent epithelium hyperplasia, migration of the junctional epithelium, and increased number of blood vessels. Inflammation scoring of the first molar on the maxillae is described as having light to moderate inflammation in the TTP KO mice compared with WT, which had an estimated 2 to 10 IC infiltration in periodontal tissues (Fig. 1D). These data suggest that TTP is essential for control of IC infiltration in maxillary soft tissue.
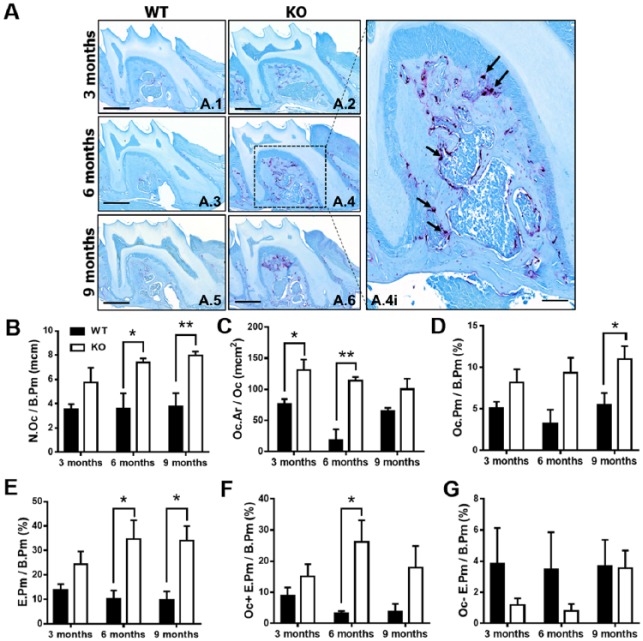
Histomorphometric analysis of osteoclastic cellular endpoints and eroded bone perimeter outcomes were performed in TRAP-stained maxillae sections to determine the role of TTP in osteoclastogenesis (Fig. 2A.1–A.6). TTP KO maxillae had elevated numbers of osteoclasts lining the alveolar bone perimeter (N. Oc/B.Pm; Fig. 2B) and increased average osteoclast cell size/area (Oc.Ar/Oc; Fig. 2C). Correlating with the increased osteoclast numbers and size, osteoclast perimeter per bone perimeter (Oc.Pm/B.Pm; Fig. 2D) was enhanced in TTP KO mice. Analysis of eroded bone perimeter was performed to evaluate the differences in osteoclast activity. TTP KO mice had an escalated eroded bone perimeter per bone perimeter (E.Pm/B.Pm) and Oc+E.Pm/B.Pm (Fig. 2F), suggesting TTP suppresses osteoclastic resorptive function.
Figure 2.
Tristetraprolin modulates inflammation and osteoclastogenesis. (A) Representative tartrate-resistant acid phosphate (TRAP) stain of maxillae (scale bar = 1 mm) at 3 (A.1, A.2), 6 (A.3, A.4), and 9 (A.5, A.6) mo of age, with insert displaying region of interest for osteoclast analysis (A.4i; scale bar = 1 µm), with arrows indicating TRAP+ osteoclasts. (B–D) Osteoclast cellular endpoints and (E–G) eroded bone analysis was performed on TRAP and alinine blue–stained wild-type and knockout maxillae sections. B. N.Oc/B. Pm = osteoclast number per bone perimeter; C. Oc.Ar/Oc = osteoclast area/size; D. Oc. Pm/B.Pm = osteoclast perimeter per bone perimeter; E. E.Pm/B.Pm = eroded perimeter per bone perimeter; F. Oc+E.Pm/B.Pm G. Oc-E.Pm/B.Pm; n = 4/gp. Two-way analysis of variance with Tukey’s multiple comparisons test and unpaired t test; data are presented as mean ± SEM, *P < 0.05, **P < 0.01.
TTP KO Mice Have Elevated Serum Bone Turnover Markers and TNF-α
Because of the elevated bone loss in TTP KO mice, bone turnover markers were assessed in serum of WT and KO to determine alterations in bone formation and resorption activities. Corroborating the increased eroded bone perimeter outcomes in TTP KO mice, serum carboxy-terminal collagen crosslinks 1 (CTX-1) was upregulated in TTP KO versus WT over time and most notably at 6 mo (Fig. 3A). There was no difference in osteocalcin (Fig. 3B), a noncollagenous protein produced by osteoblasts, suggesting TTP does not significantly regulate osteoblastic bone formation. These findings imply that TTP anticatabolic effects limit murine alveolar bone loss over time.
Figure 3.
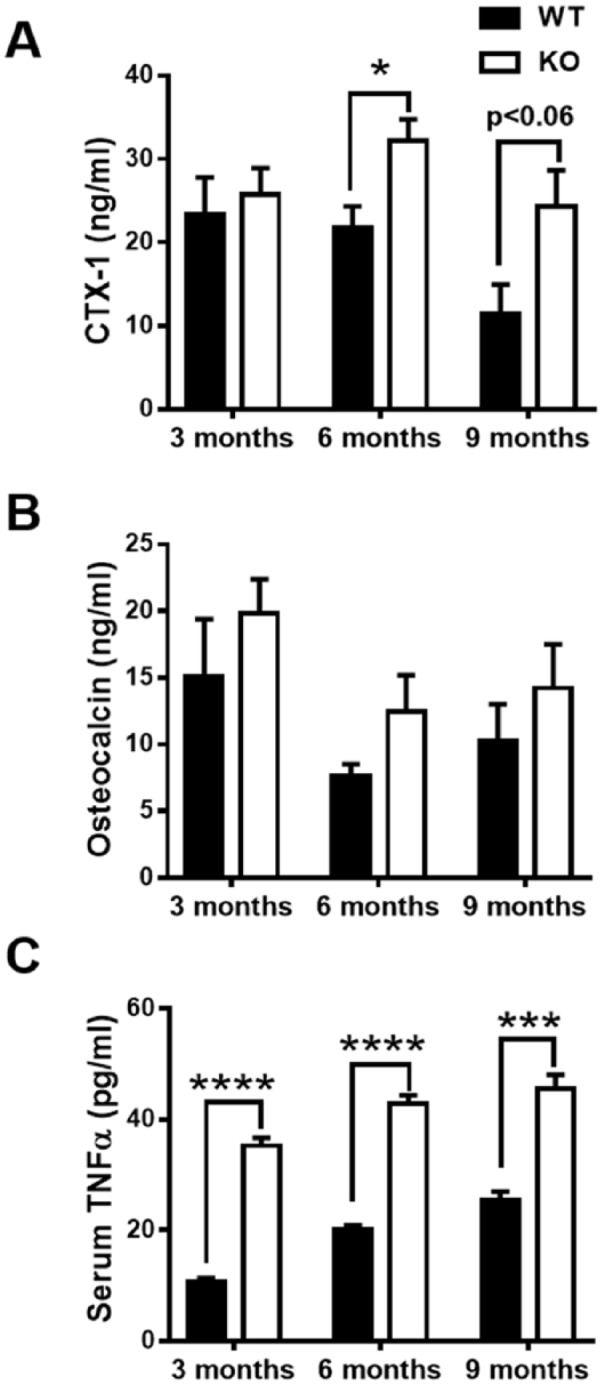
Tristetraprolin (TTP) knockout (KO) mice have elevated serum bone turnover markers and tumor necrosis factor (TNF)–α. (A, B) Serum CTX-1 (A) and osteocalcin (B) levels were tested in TTP wild-type (WT) and knockout (KO) mice aged 3, 6, and 9 mo by enzyme-linked immunosorbent assay (ELISA); n = 4/gp. (C) Serum freeze/thawed once from TTP WT and KO mice aged 3, 6, and 9 mo measured TNF-α levels by ELISA. Two-way analysis of variance with Tukey’s multiple comparisons test and unpaired t test; data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001.
In light of the increased proinflammatory/pro-osteoclastic phenotype in TTP KO mice, proinflammatory immune cytokines were assessed in serum. TNF-α, a potent cytokine that enhances osteoclastogenesis, inhibits osteoblastogenesis, and is a known TTP target (Taylor et al. 1996; Carballo et al. 1998; Boyce and Xing 2008; Redlich and Smolen 2012; Patial, Stumpo, et al. 2016), was tested in TTP WT and KO serum. Serum TNF-α levels were significantly elevated in TTP KO when compared with WT at all time points (Fig. 3C), confirming the function of TTP in chronic inflammation.
TTP Alters Innate and Adaptive Immune Cell Populations and the Oral Microbiome
Because of the altered osteoclastogenesis activity and inflammation, we further investigated the osteoimmunologic mechanisms involved in TTP WT and KO alveolar bone maintenance. When periodontal homeostasis is dysregulated, multiple immune cell types become activated within the oral cavity and further drain into the cLNs to elicit an immune response. Therefore, cLN tissues were collected, fixed, and scored. cLN weights were significantly increased in KO animals compared with WT (Fig. 4A), consistent with previous studies (Taylor et al. 1996). Histopathology of TTP KO cLNs displayed increased numbers of plasma cells in the medulla compared with WT at all ages, and the sinusoids were distended by clear space (Appendix 2). Many plasma cells present in TTP KO contained multiple eosinophilic hyaline droplet Mott cells, which suggests increased immunoglobulin (Ig) production by TTP KO animals.
Figure 4.
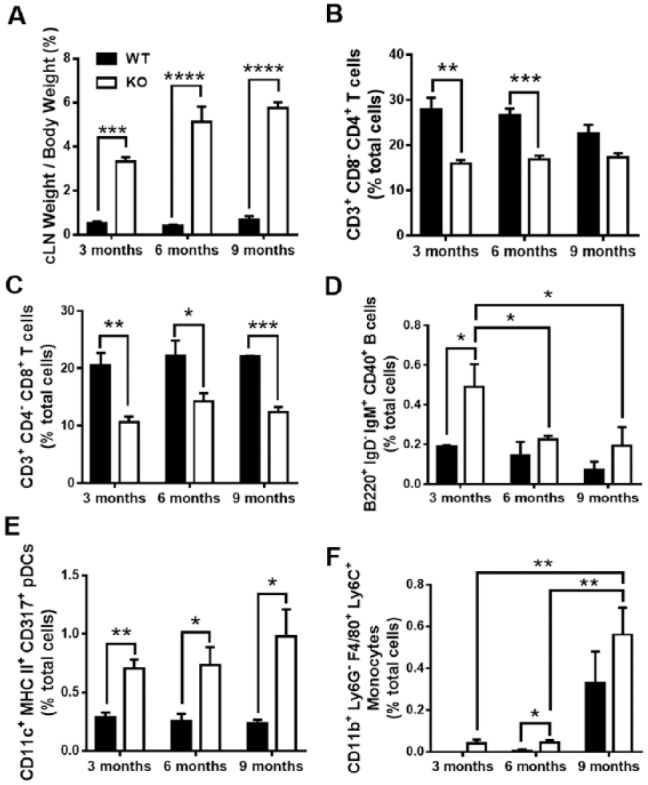
Tristetraprolin (TTP) knockout (KO) alters innate and adaptive immune cell populations. (A) Cervical lymph node (cLN) tissue weight per body weight of TTP wild-type (WT) and KO mice over time; n = 4/gp. (B–F) Flow cytometric analysis of immune cell populations in TTP WT and KO cLNs. (B) CD3+CD8-CD4+ helper T cells with percentage of total live lymphocyte population displayed. (C) CD3+CD4-CD8+ cytotoxic T cells. (D) B220+IgD-IgM+CD40+ memory B cells. (E) CD11c+MHC class II+CD317+ activated plasmacytoid dendritic cells with percentage of total live monocyte population displayed. (F) CD11b+Ly6G-F4/80+Ly6C+ inflammatory monocytes; n = 4/gp. Two-way analysis of variance with Tukey’s multiple comparisons test and unpaired t test; data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001.
Routine immune surveillance within the oral cavity involves innate naïve dendritic cells, monocytes, and macrophages taking in self-antigen. However, when an infection occurs or homeostasis is disrupted, these innate cells migrate to the lymph nodes for crosstalk and activation of the adaptive and humoral immune systems (T and B lymphocytes) to initiate an appropriate immune response. Thus, we examined the immune cell profile of TTP WT and KO mice to determine immune involvement in the draining cLNs. We collected and isolated cells from cLNs and further stained for flow cytometry for the following cell types: unactivated/activated plasmacytoid dendritic cells (pDCs), inflammatory monocytes, T-helper cells, cytotoxic T cells, T-regulatory (Treg) cells, and activated/memory B cells. CD3+CD8-CD4+ T-helper cells (Fig. 4B) and CD3+CD4-CD8+ cytotoxic T cells (Fig. 4C) showed a reduction in TTP KO compared with WT. CD4+CD25+FoxP3+ Treg cells shared a similar trend in TTP KO versus WT (Appendix 3A). Memory B-cell population was elevated in 3-mo TTP KO compared with WT, but these numbers digress in later time points (Fig. 4D). B220+IgD+IgM+CD40+ activated B cells displayed a minimal increase in TTP KO mice versus WT at all time points (data not shown). CD11c+MHC class IImidCD317- unactivated pDCs (Appendix 3B), CD11c+MHC class II+CD317+ activated pDCs (Fig. 4E), and inflammatory CD11b+Ly6G-F4/80+Ly6C+ monocytes (Fig. 4F) were gated on the monocyte population and displayed increased numbers in TTP KO, adding to the chronic inflammatory profile TTP deficiency affords.
The PERMANOVA model was used for analysis of 16S DNA of the oral microbiome in WT and TTP-deficient mice. Using this approach, the primary effect of genotype, age, and sex and all possible interactions indicated that the microbiome is different in WT and TTP KO mice (P < 0.001). A significant interaction between genotype and age (P < 0.039) indicates that the differences are amplified over time. These effects are clearly seen on PCoA plots (Appendix 4). Whereas at 3 mo, the microbial communities of both the KO and the WT mice intermix, at 6 and 9 mo of age, the differentiation of the microbial communities in the 2 groups is apparent.
TTP Overexpression Protects from Inflammation and Bone Resorption
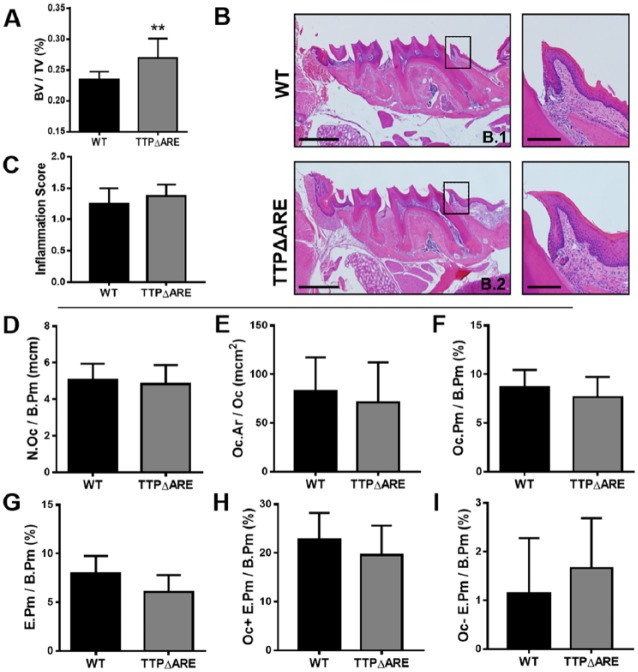
Since data collected from the TTP global KO suggest that TTP is required for alveolar homeostasis, we also investigated whether a TTP gain-of-function model may show enhanced protective effects relative to alveolar bone maintenance. Recently, mice with enhanced TTP mRNA stability and expression were generated and characterized by deleting a 130-base AU-rich region in the mouse Zfp36 locus (Patial, Curtis, et al. 2016). These TTPΔARE and WT mice were aged 8 mo, and maxillae were scanned by µCT to quantitate alveolar bone volume (Fig. 5A). Results indicate TTPΔARE animals have less bone turnover than WT mice by 13.4% (0.269779 vs. 0.233668, P < 0.01). Histomorphometric analysis of H&E-stained maxillae sections were performed to examine inflammatory components in WT (Fig. 5B.1) and TTPΔARE mice (Fig. 5B.2). Inflammation scoring of the first maxillary molar displayed normal/minimal inflammation in both groups (Fig. 5C), suggesting that overexpression of TTP may have a protective effect in alveolar inflammation infiltrates.
Figure 5.
Tristetraprolin (TTP) overexpression protects from inflammation and bone resorption. (A) Microcomputed tomography analysis of bone volume/total volume of alveolar bone in wild-type (WT) and TTPΔ adenosine-uridine rich elements (ARE) maxillae; n = 8/gp. (B.1, B.2) Representative hematoxylin and eosin–stained maxillae from each group (scale bar = 1 mm), with insert displaying inflammation at the mesial aspect of the first molar (scale bar = 1 µm). (C) Inflammation scoring of connective tissue around the first molar of WT and TTPΔARE maxillae; n = 7 to 8/gp. (D–F) Osteoclast cellular endpoints and (G–I) eroded bone analysis was performed on tartrate-resistant acid phosphate– and alinine blue–stained WT and TTPΔARE maxillae sections. (D) N.Oc/B. Pm; E. Oc.Ar/Oc; D. Oc. Pm/B.Pm; G. E.Pm/B.Pm; H. Oc+E.Pm/B.Pm I. Oc-E.Pm/B.Pm; n = 7 to 8/gp. Unpaired t test; data are presented as mean ± SEM, **P < 0.01.
TRAP-stained maxillae sections were assessed in WT and TTPΔARE mice to determine the role of TTP in osteoclastogenesis (Fig. 5D–I). Oc.Ar/Oc and N. Oc/B.Pm displayed similar numbers and size of osteoclasts between the 2 groups (Fig. 5D–E), suggesting that TTP overexpression does not alter cellular commitment to the osteoclast population. Similar findings were found when comparing Oc.Pm/B.Pm in WT and TTPΔARE animals (Fig. 5F). Eroded bone perimeter analyses showed no significant differences between groups (Fig. 5G–I). These data suggest TTP overexpression displays protective qualities from inflammation-driven bone-resorptive properties.
Discussion
Although standard periodontal therapies aim to limit the bacterial load to reduce the host response, it is quite clear that certain patients may be less responsive to customary therapies and prone to disease progression (Kassebaum et al. 2014). Thus, a keen understanding of factors that limit soft-tissue damage and bone loss is needed both to design rational therapeutic approaches to target and to understand molecular targets to avoid which are vital for integrity of the periodontium. In the present investigation, we found that TTP appears to be a key molecule required in alveolar bone maintenance and health.
Members of the TTP family of RNA-binding proteins are found in all major eukaryotic groups. In mammalian cells, these proteins promote deadenylation and decay of target transcripts. When the TTP gene is disrupted in mice, these animals develop severe syndromic arthritis, autoimmunity, cachexia, dermatitis, and myeloid hyperplasia. Conversely, recent overexpression studies have demonstrated protection against several experimental models of immune inflammatory disease, including experimental periodontitis (Patial and Blackshear 2016). Thus, this endogenous anti-inflammatory protein could serve as the basis for novel therapeutic approaches of similar human conditions.
Findings from this study highlight the significance of TTP in periodontal homeostasis, since TTP deficiency resulted in enhanced periodontal inflammatory infiltrate and hyperplasia in the connective tissues with alveolar bone loss. Furthermore, gain-of-function mutation in TTPΔARE mice further support the notion that TTP is needed for maintaining alveolar bone integrity and microarchitecture since bone volumes were increased despite that no apparent changes in inflammation or osteoclastogenesis were observed. It is important to emphasize that in all experiments, mice (both loss and gain of function) were cohoused with WT mice to ensure the commensal microflora exposure conditions were identical to gauge the effects of TTP in oral homeostasis. Despite these housing conditions, the oral microbiome was significantly altered in TTP KO compared with WT littermates with time, suggesting that the overall systemic inflammation that precedes may alter the oral microbiome. These data are consistent with recent evidence indicating that systemic inflammation, as seen in diabetes, can alter the oral microbiome, increasing its pathogenicity (Xiao et al. 2017). Future studies will need to be performed to determine if the loss of TTP creates a dysbiotic oral microbiome that has an altered pathogenicity.
Periodontal disease triggers proinflammatory reactions involving innate, humoral, and adaptive immune responses. Here, we describe that TTP regulates multiple immune cell populations during TTP deficiency. Interestingly, Treg, CD4+, and CD8+ T-cell populations are significantly downregulated in TTP KO compared with WT. Because of the chronic inflammatory environment, T-cell exhaustion or anergy may play a key role in the minimal adaptive immune responses. These findings are in line with the observed increase in pDCs with TTP KO mice. pDCs have been shown to limit T-cell growth by depleting L-tryptophan, a necessary amino acid for T-cell proliferation (Lee et al. 2003). The pDC population has also been associated with increased levels of IL-6, IL-10, CCL2, CCL5, and CXCL10, cytokines that may promote RANKL, thus leading to osteoclastogenesis induction (Chauhan et al. 2009; Sawant and Ponnazhagan 2013). With elevated proinflammatory pDCs and monocytes, and subsequently decreased T-cell populations, the humoral response was affected as well. B-cell results validate previous studies in which the change from stable to progressive periodontal bone loss was associated with increased infiltration of B cells and plasma cells (Seymour et al. 1979; Malberg et al. 1992). Memory B cells were increased at 3 mo but steadily depleted with age, correlating with less T-cell signaling to B cells and lower somatic hypermutations and Ig class switching (Blomberg and Frasca 2013). Ongoing studies are pursuing other cell populations, which may play a role in immunosuppression in alveolar bone maintenance and periodontitis.
Previously, regulators involved in periodontal health and homeostasis have not been well described. The present study highlights TTP as a candidate immunomodulatory regulator critical in alveolar bone homeostasis. Although the TTP KO phenotype has been extensively evaluated (Taylor et al. 1996; Qiu et al. 2012), our data reveal key insights into the importance of TTP in the balance between periodontal and alveolar bone health and disease, providing strong support for TTP to be a critical therapeutic target for modulation of host-inflammatory responses and subsequent alveolar bone loss.
Author Contributions
H.M. Steinkamp, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript; J.D. Hathaway-Schrader, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; M.B. Chavez, L. Zhang, T. Jensen, A. Shojaee Bakhtiari, K.L. Helke, A.V. Alekseyenko, contributed to data analysis, critically revised the manuscript; J.D. Aartun, contributed to data analysis, drafted the manuscript; D.J. Stumpo, contributed to conception and design, critically revised the manuscript; C.M. Novince, P.J. Blackshear, K.L. Kirkwood, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034518756889 for Tristetraprolin Is Required for Alveolar Bone Homeostasis by H.M. Steinkamp, J.D. Hathaway-Schrader, M.B. Chavez, J.D. Aartun, L. Zhang, T. Jensen, A. Shojaee Bakhtiari, K.L. Helke, D.J. Stumpo, A.V. Alekseyenko, C.M. Novince, P.J. Blackshear and K.L. Kirkwood in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
This work was supported by the National Institutes of Health (NIH) grants K08DE025337 (C.M.N.), R01DE021423 (K.L.K.), P30GM103331 (K.L.K.), T32DE017551 (K.L.K.) and by the Intramural Program of the National Institute of Environmental Health Sciences, NIH (P.J.B.). Supported in part by the Genomics Shared Resource, Hollings Cancer Center, Medical University of South Carolina.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26(1):32–46. [Google Scholar]
- Blomberg BB, Frasca D. 2013. Age effects on mouse and human b cells. Immunol Res. 57(1–3):354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. 2010. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 25(7):1468–1486. [DOI] [PubMed] [Google Scholar]
- Boyce BF, Xing L. 2008. Functions of rankl/rank/opg in bone modeling and remodeling. Arch Biochem Biophys. 473(2):139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SA, Blackshear PJ. 2013. Tristetraprolin (TTP): interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim Biophys Acta. 1829(6–7):666–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. 2016. Dada2: high-resolution sample inference from illumina amplicon data. Nat Methods. 13(7):581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo E, Lai WS, Blackshear PJ. 1998. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 281(5379):1001–1005. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Singh AV, Brahmandam M, Carrasco R, Bandi M, Hideshima T, Bianchi G, Podar K, Tai YT, Mitsiades C, et al. 2009. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 16(4):309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrestensen CA, Schroeder MJ, Shabanowitz J, Hunt DF, Pelo JW, Worthington MT, Sturgill TW. 2004. Mapkap kinase 2 phosphorylates tristetraprolin on in vivo sites including ser178, a site required for 14-3-3 binding. J Biol Chem. 279(11):10176–10184. [DOI] [PubMed] [Google Scholar]
- Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. 2013. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the asbmr histomorphometry nomenclature committee. J Bone Miner Res. 28(1):2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ. 2015. Update on prevalence of periodontitis in adults in the united states: Nhanes 2009 to 2012. J Periodontol. 86(5):611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlet GP. 2010. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 89(12):1349–1363. [DOI] [PubMed] [Google Scholar]
- Graves D. 2008. Cytokines that promote periodontal tissue destruction. J Periodontol. 79(8 Suppl):1585–1591. [DOI] [PubMed] [Google Scholar]
- Herbert BA, Novince CM, Kirkwood KL. 2016. Aggregatibacter actinomycetemcomitans, a potent immunoregulator of the periodontal host defense system and alveolar bone homeostasis. Mol Oral Microbiol. 31(3):207–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci A, Hasturk H, Van Dyke TE. 2006. Host-mediated resolution of inflammation in periodontal diseases. Periodontol 2000. 40:144–163. [DOI] [PubMed] [Google Scholar]
- Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. 2014. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 93(11):1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JR, Dalton RR, Messina JL, Sharma MD, Smith DM, Burgess RE, Mazzella F, Antonia SJ, Mellor AL, Munn DH. 2003. Pattern of recruitment of immunoregulatory antigen-presenting cells in malignant melanoma. Lab Invest. 83(10):1457–1466. [DOI] [PubMed] [Google Scholar]
- Lorenzi T, Nitulescu EA, Zizzi A, Lorenzi M, Paolinelli F, Aspriello SD, Banita M, Craitoiu S, Goteri G, Barbatelli G, et al. 2014. The novel role of htra1 in gingivitis, chronic and aggressive periodontitis. PLoS One. 9(6):e96978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg K, Molle A, Streuer D, Gangler P. 1992. Determination of lymphocyte populations and subpopulations extracted from chronically inflamed human periodontal tissues. J Clin Periodontol. 19(3):155–158. [DOI] [PubMed] [Google Scholar]
- Novince CM, Michalski MN, Koh AJ, Sinder BP, Entezami P, Eber MR, Pettway GJ, Rosol TJ, Wronski TJ, Kozloff KM, et al. 2012. Proteoglycan 4: a dynamic regulator of skeletogenesis and parathyroid hormone skeletal anabolism. J Bone Miner Res. 27(1):11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanisamy V, Jakymiw A, Van Tubergen EA, D’Silva NJ, Kirkwood KL. 2012. Control of cytokine mRNA expression by RNA-binding proteins and microRNAs. J Dent Res. 91(7):651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patial S, Blackshear PJ. 2016. Tristetraprolin as a therapeutic target in inflammatory disease. Trends Pharmacol Sci. 37(10):811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patial S, Curtis AD, II, Lai WS, Stumpo DJ, Hill GD, Flake GP, Mannie MD, Blackshear PJ. 2016. Enhanced stability of tristetraprolin mrna protects mice against immune-mediated inflammatory pathologies. Proc Natl Acad Sci U S A. 113(7):1865–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patial S, Stumpo DJ, Young WS, III, Ward JM, Flake GP, Blackshear PJ. 2016. Effects of combined tristetraprolin/tumor necrosis factor receptor deficiency on the splenic transcriptome. Mol Cell Biol. 36(9):1395–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil CS, Kirkwood KL. 2007. P38 mapk signaling in oral-related diseases. J Dent Res. 86(9):812–825. [DOI] [PubMed] [Google Scholar]
- Qiu LQ, Stumpo DJ, Blackshear PJ. 2012. Myeloid-specific tristetraprolin deficiency in mice results in extreme lipopolysaccharide sensitivity in an otherwise minimal phenotype. J Immunol. 188(10):5150–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich K, Smolen JS. 2012. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 11(3):234–250. [DOI] [PubMed] [Google Scholar]
- Sawant A, Ponnazhagan S. 2013. Role of plasmacytoid dendritic cells in breast cancer bone dissemination. Oncoimmunology. 2(2):e22983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour GJ, Powell RN, Davies WI. 1979. Conversion of a stable t-cell lesion to a progressive b-cell lesion in the pathogenesis of chronic inflammatory periodontal disease: an hypothesis. J Clin Periodontol. 6(5):267–277. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, et al. 1996. A pathogenetic role for tnf alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (ttp) deficiency. Immunity. 4(5):445–454. [DOI] [PubMed] [Google Scholar]
- Tchen CR, Brook M, Saklatvala J, Clark AR. 2004. The stability of tristetraprolin mrna is regulated by mitogen-activated protein kinase p38 and by tristetraprolin itself. J Biol Chem. 279(31):32393–32400. [DOI] [PubMed] [Google Scholar]
- Xiao E, Mattos M, Vieira GHA, Chen S, Correa JD, Wu Y, Albiero ML, Bittinger K, Graves DT. 2017. Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host Microbe. 22(1):120–128.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Liu M, D’Silva NJ, Kirkwood KL. 2011. Tristetraprolin regulates interleukin-6 expression through p38 mapk-dependent affinity changes with mRNA 3′ untranslated region. J Interferon Cytokine Res. 31(8):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034518756889 for Tristetraprolin Is Required for Alveolar Bone Homeostasis by H.M. Steinkamp, J.D. Hathaway-Schrader, M.B. Chavez, J.D. Aartun, L. Zhang, T. Jensen, A. Shojaee Bakhtiari, K.L. Helke, D.J. Stumpo, A.V. Alekseyenko, C.M. Novince, P.J. Blackshear and K.L. Kirkwood in Journal of Dental Research