Abstract
Species with wide geographical distributions are often adapted locally to the prevailing temperatures. To understand how they respond to ongoing climatic change, we must appreciate the interplay between temperature, seasonality and the organism's life cycle. The temperature experienced by many organisms results from an often-overlooked combination of climate and phenology. Summer-active (high latitude) populations are expected to adapt to local summer temperatures, but this is not expected for populations that outlive the summer in their dormant stage (low latitude, precipitation-limited). We recorded reproduction and survival in genotypes from 123 Holarctic populations of Daphnia magna during a multi-generation thermal ramp experiment. Genotypes from summer-active populations showed a positive relationship between heat tolerance and local summer temperature, whereas winter-active populations did not. These findings are consistent with the hypothesis that D. magna adapts to the local temperatures the animals experience during their planktonic phase. We conclude that predicting local temperature adaptation, in particular in the light of climate change, needs to consider the phenology of geographically wide-ranging species.
Keywords: local adaptation, temperature adaptation, life history, freshwater organisms, diversity panel
1. Introduction
As average and extreme temperatures increase [1], flora and fauna have been predicted to adapt to these new climatic conditions in their local habitats, for example, by evolving tolerance to elevated temperatures. This prediction is consistent with observations that organisms are locally adapted to their prevailing climatic conditions [2–4] and also with reports demonstrating that local populations can adapt to changing climatic conditions over short time periods [5–7]. To predict how populations cope with locally changing temperature conditions, it is necessary to understand both the selective agent (i.e. the local temperature regime as experienced by the organisms) and the ability of a population to respond to local selection (i.e. potential for local adaptation).
Latitude is often considered to be a good proxy for local temperature in studies that consider wide geographical ranges [4,8–10]: as latitude increases, temperature generally decreases. This is, however, not always the case; in fact, the correlation between latitude and experienced temperature has limits [11]. For example, some organisms start their growth period earlier in warmer climates, so the local temperature conditions that trigger the start of the active period (e.g. seed germination, hatching or eclosion from resting stages) may not differ across latitudes [12]. Latitude–temperature mismatch occurs in a more extreme way for populations that are active during different seasons of the year. At higher latitudes, for example, the growing season usually happens during the warmest part of the year, because winter temperatures prevent physiological function. Towards the equator, however, the growing season may be in the colder part of the year because temperatures are too high and/or precipitation too low during the hottest season. For example, the mosquito Culex pipiens—a vector of arboviruses, including West Nile virus—has a very wide geographical distribution [13]. In central and northern Europe, its active stage is found exclusively during the summer, while in subtropical regions, it is mainly found during the wet season—the coldest part of the year. During other seasons, it undergoes diapause. Other insect species, including butterflies, dipterans and dragonflies, exhibit similar patterns [14–17]. For these examples, temperature experienced by the population outside its dormant phase cannot be predicted by latitude alone. Therefore, the phenology of the organism needs to be taken into account.
To understand the link between phenology, diapause and local temperature adaptation, we use the planktonic crustacean Daphnia magna which lives in fresh- and brackish-water habitats of the Holarctic with a latitudinal range of about 30–70° north [18]. Because of its wide geographical distribution and the resulting wide range of local temperatures in which it is found, this species is well suited for investigating adaptation to local temperatures. Daphnia magna has been shown to respond to increased temperature with an evolutionary increase in their upper temperature tolerance [5]. Since Daphnia can only persist in the planktonic phase in temperature ranges of about 5–30°C [19], they can only be found during certain seasons in any given latitude, while they diapause in the form of resting eggs (so-called ephippia) at other times. In Europe, north of about 43°, planktonic D. magna are typically found in the warmest season (here called summer-active), while south of this latitude, the crustacean emerges from resting stages only at the beginning of the rainy season, the colder half of the year (winter-active). Thus, winter-active populations are not expected to experience and thus adapt to summer temperatures, as summer-active populations do. Previous studies of temperature adaptation in aquatic invertebrates [20–22] did not consider this variation in phenology, potentially explaining why they found poor support for the local adaptation hypothesis. Four studies testing for local temperature adaptation in Daphnia also found mixed evidence, possibly because these studies did not differentiate summer- and winter-active populations in their design [20,23–25].
The aim of this study is to test populations of D. magna for local temperature adaptation, taking the latitudinal cline in phenology into account. For this, we tested the temperature tolerance of D. magna from 123 populations across the Holarctic (using the Daphnia magna Diversity Panel) [26]. One clone per population was kept at the laboratory and tested at temperatures up to 37°C, while we recorded fecundity and survival. As predicted, we found a positive correlation between the maximum temperature tolerance and both latitude and the warmest summer temperatures only for animals from summer-active populations. By contrast, temperature tolerance of animals originating from winter-active populations could not be explained with these environmental temperature proxies. We conclude that only in summer-active populations do summer temperatures limit biological function, thus climate warming may directly influence patterns of local adaptation in these populations.
2. Material and methods
(a). Temperature loggers in natural habitats
To understand the seasonal dynamics of water presence/absence and water temperature across Europe and the Middle East, we placed TidBits dataloggers (Onset Computer Corporation, Bourne, MA, USA) close to the centre of six ponds along a transect from the White Sea (about 66° N) to Israel (about 30° N) and recorded the temperature every 30 min. The datasets cover at least 2 years in each case. Battery failure, vandalism and extreme weather conditions affected the length of time that data could be recorded.
(b). Assessment of growth season
To assess when the D. magna population is in its planktonic phase (summer- or winter-active population), we relied on information from local scientists. Furthermore, we used monthly climatic data (temperature and precipitation) from WorldClim database (www.worldclim.org) for each location and consulted published information about the water body. If more information was needed, we used diverse Internet resources such as photographs and areal pictures (current and historical) from Google Earth and Google Maps to assess the seasonality of the water body. Locations where D. magna are found year-round were recorded as summer-active, as we were interested in the upper temperature tolerance of the animals.
(c). Animals and culture conditions
We used 123 D. magna clones collected from the Holarctic climate zone (i.e. Europe, Asia, North Africa and North America; electronic supplementary material, table S1). These clones came from the Daphnia magna Diversity Panel that has been used to study geographical variation among D. magna genotypes [25–28]. Each genotype was collected from a different population and was kept clonally (iso-female lines) in standard culture conditions: 20°C, a light : dark cycle of 16 : 8, artificial Daphnia medium (ADaM), and a 1 : 1 (by cell count) mixture of Nanochloropsis limnetica and Acutodesmus obliquus algae as the only food (50 million cells per 380 ml jar three times a week).
(d). Assay of temperature tolerance
Animals were taken from the stock collection (at 20°C) and cultured in 380 ml jars filled with 350 ml Daphnia medium at 23°C. Each jar was stocked with five females and we produced five such replicate populations in 380 ml jars and kept them in one incubator. All replicate jars were assigned random numbers, to keep the identity of the clones anonymous during their handling. The medium was changed once a week, and males were removed to allow better conditions for population growth. During the acclimation phase, every two weeks, the temperature was raised (23, 25, 26, 27 and 28°C), and population survival and the presence of juveniles (as an indicator of successful reproduction) were recorded. All replicate populations that were able to reproduce were then kept at 28°C. We then took animals from these 28°C cultures and tested for reproduction and survival at nine higher temperatures (29–37°C in increments of 1°C; see electronic supplementary material, figure S1, for an overview). For each of these higher temperatures, we collected three juvenile females (1–3 days old) from each of the five replicate populations kept at 28°C and placed them in a 100 ml jar with 80 ml medium. These jars were then placed in a walk-in climate chamber at the higher temperature (29–37°C). Every second day for the following 14 days, the animals were checked for survival and signs of reproduction (eggs in brood pouch and newborns in the medium). The limited availability of climate chambers did not allow us to test more than one higher temperature at a time. If at least one animal of a replicate survived the 14-day test period, the same replicate was tested at the next higher temperature, but starting with juveniles taken from the 28°C cultures. If none of the animals survived 14 days at a certain temperature, this replicate was still tested at the next higher temperature. If all animals died again, we assumed that we had exceeded its upper temperature tolerance and discontinued with this replicate. As four replicates of one clone survived 35°C, we stopped the experiment after testing these replicates at 36 and 37°C.
(e). Data handling and statistical analysis
All data handling and analysis was done with the R software v. 3.4.1. For each replicate, we calculated the maximum survival temperature as the average between the highest temperature at which the animal was able to survive for two weeks and the lowest temperature at which it was not. Likewise, the maximum temperature for reproduction was calculated as the average of the highest temperature at which successful reproduction was observed (production of free-swimming offspring within a two-week period) and the lowest temperature at which no free-swimming offspring was observed.
Temperature data (monthly averages from years 1970 to 2000) were downloaded from the Worldclim Database for each of our sample locations ( electronic supplementary material, table S1). Locations within 1 km to the sea had to be shifted about 500 m inland to meet the requirements of the database (there are no data for marine sites). Following Yampolsky et al. [25], we calculated the mean of the highest temperature (tmax in WorldClim, in all cases either July or August) as an estimate of the average high temperature of the warmest month (AHT_warmest) for all locations.
Genetic variance components for the clones were calculated with VarCorr of R package nlme (VarCorr(lme(traits ∼ clone, data = dat, random = ∼1|clone)). We used analysis of covariance to test for the effect of latitude, average high temperature of the warmest month (AHT_warmest) and the heterogeneity of slopes for summer- and winter-active populations (lm(trait ∼ summer_active × covariable)) (with summer_active being either 0 or 1 and covariable being either latitude or the average high temperature of the warmest month (AHT_warmest)). A significant interaction term indicates a lack of homogeneity of slopes.
For the contour maps and the spatial autocorrelation analysis, we used clonal means for each trait. For the contour maps (R package fields), a thin plate spline surface was fitted to our irregularly spaced data, with the smoothing parameter determined by generalized cross-validation. These fitted surfaces were superimposed on a map of the Western Palaearctic (data points between −10° and 60°), where we had a sufficiently dense sampling grid to make this approach meaningful. We used the ‘plasma’ colour scheme of the R package viridis to better represent the results on a grey scale and to make it easier to interpret them by those with colour blindness.
For the spatial autocorrelation, the distance intervals among sampling sites were chosen to be 200 km up until 2000 km; from there, we used intervals of 2000 km up until 8000 km. Larger distances were pooled in one distance class. This method allowed each distance class to have approximately the same number of data points. For the autocorrelation analysis (calculating Moran's I), we used the function correlog in the R package ncf [29].
3. Results
(a). Seasonality and habitat suitability
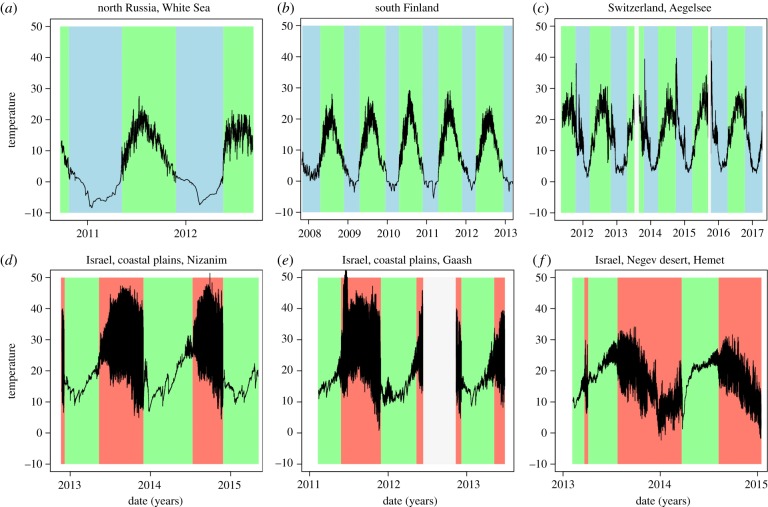
The data from all temperature loggers reveal a clear seasonality with high mid-year, summer temperatures and low winter temperatures (figure 1). The three locations in Israel show high fluctuations (black area) during the warmest part of the season, but show less daily variation during the colder period. The high daily fluctuations indicate times when these ponds were dry and the data-loggers recorded fluctuations in air temperature rather than water temperature. The coldest points of the year at these ponds (i.e. spring and winter, when the temperature is generally below 20°C) are when Daphnia typically find suitable habitats (figure 1d–f, green background). In northern populations (north Russia, southern Finland, Switzerland; figure 1a–c), Daphnia find suitable conditions, only during spring, summer and autumn when temperatures are above about 5°C.
Figure 1.
Seasonal temperature dynamics of water bodies from three summer-active northern populations (a–c) and three winter-active southern populations (d–f). The black line marks temperature recorded in 30 min intervals. Time periods with water conditions suitable for planktonic Daphnia have a green background; time periods with unsuitable conditions have a blue (less than 5°C, northern populations) or a red background (dry, southern populations). During dry periods, daily temperature fluctuations are much stronger. In northern water bodies, suitable periods are at the hottest period of the year, while in the south, suitable periods fall in the rainy winter season. Data logger failure (low battery and vandalism) is shown in white. The temperature peaks in late autumn (October/November) in the Aegelsee pond of Switzerland are caused by hot water inflow (condensation water) from a sugar factory (the pond is used as a sewage pond from October to December). The lower summer temperatures of the desert population (f) are caused by vegetation shading the data logger. (Online version in colour.)
The assessment of habitat seasonality for the populations showed a clear distinction between north and south. Summer-active populations are found mostly north of 43° N, when average high temperatures of the warmest month are below 28°C (figure 2). The spatial distribution of winter-active population corresponds very well with the distribution of the subtropical climate zone following the Köppen classification [30]. There is a wide transition zone between summer- and winter-active populations found between 35° and 55° N, because the habitat classification is not only dependent on latitude, but also on other factors, such as altitude and local climate.
Figure 2.
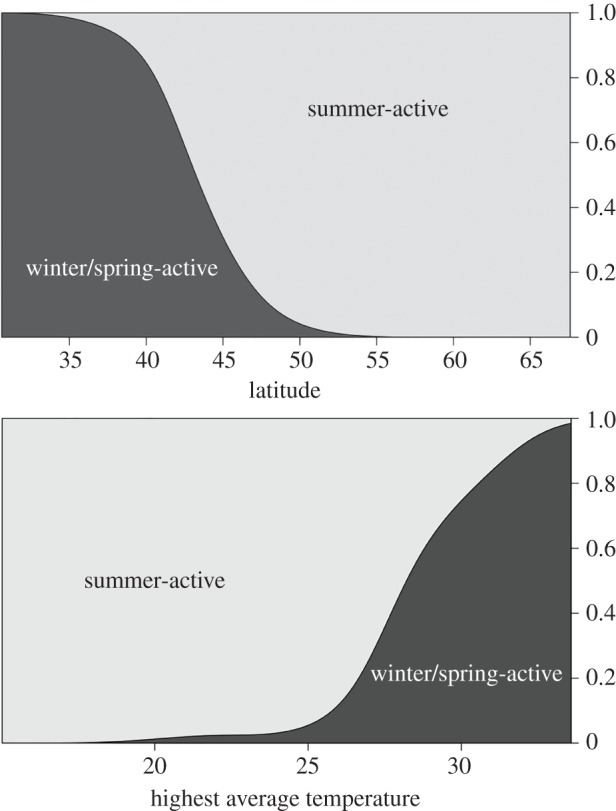
Density plots for winter-active and summer-active populations plotted against latitude and against the average temperature of the warmest month.
(b). Daphnia upper temperature tolerance
The maximal survival temperature for the tested D. magna clones ranged between 28 and 35°C, whereas the maximal reproduction temperature ranged between 26 and 32°C. Clonal means for maximal reproduction temperature and maximal survival temperature are strongly correlated (Pearson r = 0.84, p < 0.0001). This variation among clones can be largely attributed to genetic effects: clone effects explained 58.72% of the total phenotypic variation for the maximal reproduction temperature (mean2 = 5.243, F122,496 = 15.77, p < 0.0001) and 58.73% of the total variation (mean2 = 9.032, F122,496 = 22.35, p < 0.0001) for the maximal survival temperature.
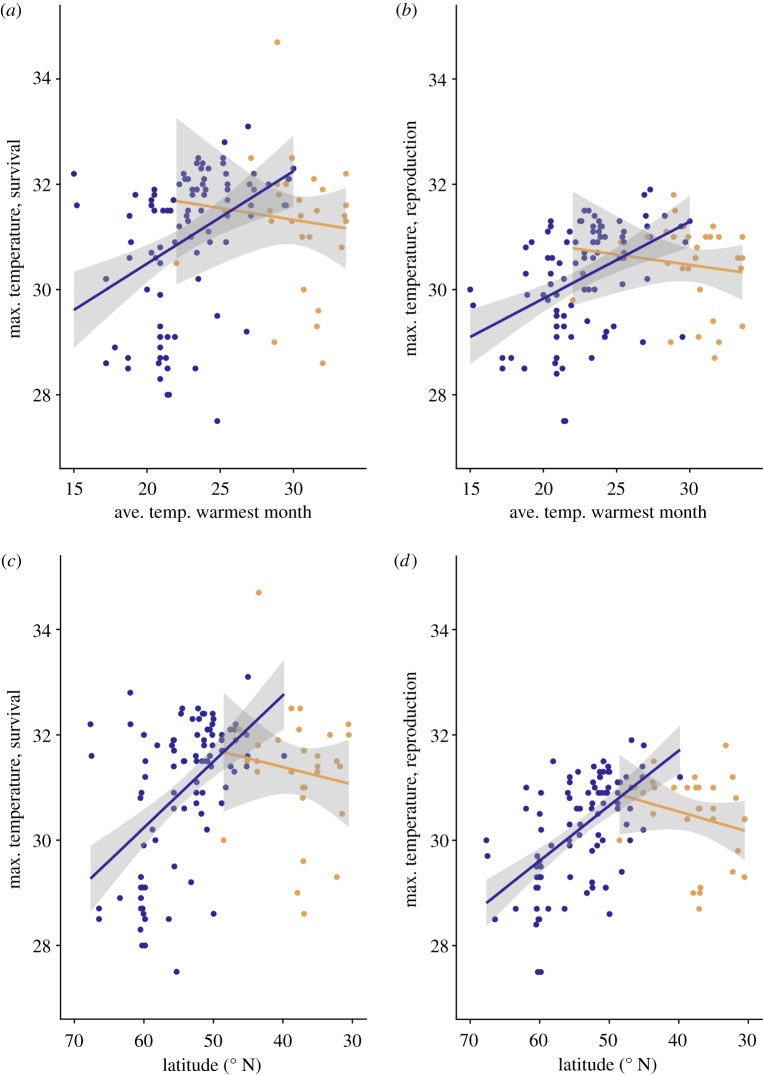
As predicted, temperature tolerance increased with increasing average high temperature of the warmest month and decreasing latitude only in the genotypes from the summer-active populations (figure 3 and table 1). Genotypes from winter-active (subtropical) locations were highly variable in their tolerance and showed no correlation with average high temperature of the warmest month and latitude (figure 3). There were no significant correlations between the winter-active population's maximal temperatures for survival and fecundity and different winter temperature estimates (average temperature in March, average temperature of the coldest quarter, average temperature of the wettest quarter) (Pearson's correlation: −0.1 < r < 0.1; p > 0.2 in all cases).
Figure 3.
Scatter plot of maximal temperature for survival (a,c) and reproduction (b,d) plotted against the average temperature of the warmest month at the sampling site (a,b) and against latitude (c,d). Solid lines are fitted models of a linear regression. Blue symbols are summer-active populations (northern) and yellow symbols are winter-active (southern) populations. See table 1 for corresponding statistics. (Online version in colour.)
Table 1.
Analysis of covariance tables with latitude or average highest temperature of the warmest month (AHT_warmest) as continuous variable and summer_active as factor (0/1). A significant interaction term indicates a lack of homogeneity of slopes for summer- and winter-active populations. In (a) and (c), statistics for the maximal survival temperature are shown; in (b) and (d), statistics for maximal reproduction temperature are shown. (a) and (b) use AHT_warmest as covariable, (c) and (d) use latitude as covariable. See figure 3 for the corresponding figure.
| d.f. | mean2 | F-value | p (>F) | |
|---|---|---|---|---|
| (a) model: maxtempsurv ∼ summer_active × AHT_warmest | ||||
| summer_active | 1 | 2.5518 | 1.5989 | 0.208528 |
| AHT_warmest | 1 | 18.9125 | 11.8501 | 0.000796*** |
| summer_active : AHT_warmest | 1 | 7.3794 | 4.6237 | 0.033556* |
| residuals | 119 | 1.5960 | ||
| (b) model: maxtempfec ∼ summer_active × AHT_warmest | ||||
| summer_active | 1 | 1.6195 | 1.7308 | 0.190835 |
| AHT_warmest | 1 | 11.1837 | 11.9523 | 0.000758*** |
| summer_active : AHT_warmest | 1 | 4.6962 | 5.0189 | 0.026928* |
| residuals | 119 | 0.9357 | ||
| (c) model: maxtempsurv ∼ summer_active × latitude | ||||
| summer_active | 1 | 2.552 | 1.8039 | 0.18180 |
| latitude | 1 | 33.925 | 23.9816 | 3.09 × 10−6*** |
| summer_active : latitude | 1 | 13.946 | 9.8583 | 0.00213** |
| residuals | 119 | 1.415 | ||
| (d) model: maxtempfec ∼ summer_active × Latitude | ||||
| summer_active | 1 | 1.6195 | 2.0762 | 0.152239 |
| latitude | 1 | 22.9662 | 29.4423 | 3.08 × 10−7*** |
| summer_active : latitude | 1 | 11.4362 | 14.6610 | 0.000207*** |
| residuals | 119 | 0.7800 | ||
Significance codes: ***p < 0.001, **p < 0.01, *p < 0.05.
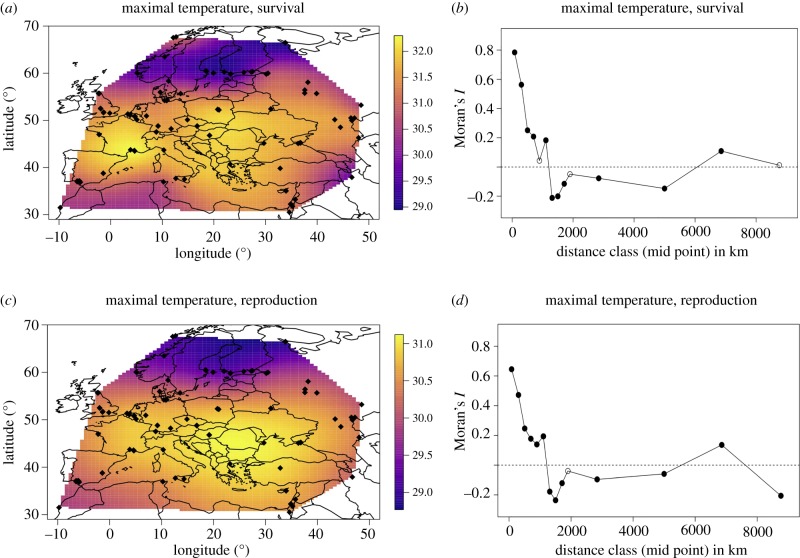
The nonlinear distribution of temperature tolerance across the Western Palaearctic is also seen when the smoothed temperature tolerance estimates are superimposed onto a map of the Western Palaearctic (between −10° and 60° E; our sampling density was too low outside this geographical region to make this approach meaningful there) (figure 4). While summer-active populations (roughly north of 43° N) show a clear north–south cline, such a cline is not recognizable for the winter-active populations.
Figure 4.
(a,c) Heat map of maximal temperature for survival (a) and reproduction (c) across the Western Palaearctic (−10° to 60° E). Small black dots mark sampling locations of D. magna genotypes. (b,d) Moran spatial autocorrelation (I) of maximal temperature for survival (b) and reproduction (d). Distance classes become larger with increasing distance. Filled symbols mark significant correlation coefficients (p < 0.05). (Online version in colour.)
An analysis of spatial autocorrelation of temperature tolerance also supported the finding that D. magna are adapted to local temperature conditions. Strong positive autocorrelations for temperature tolerance are seen on distances up to about 1000 km (figure 4), indicating that clones collected close to each other show a similar upper temperature tolerance. Since local climate changes gradually across space, such a pattern is expected for adaptation to climate variables.
4. Discussion
For species whose geographical distribution is limited by different factors across their range, latitude may be a bad proxy for expected local temperature adaptation. Freshwater organisms in small water bodies are a good test case for this: in part of their range, their habitat can be limited by the presence of water, while in other parts, high temperatures in mid-summer can limit their active phase. Adverse conditions are outlived in form of resting stages. Therefore, local adaptation to high summer temperatures is only expected in habitats with active populations during summer. We tested this prediction by investigating the upper limit of temperature tolerance for survival and reproduction of D. magna genotypes collected across large parts of the Holarctic climate zone. Unlike earlier studies, we accounted for the fact that northern populations experience the highest water temperatures in their planktonic phase in summer (July/August), while southern populations become active in winter and spring after the onset of the wet season. As predicted, we found a clear decline in temperature tolerance for summer-active populations with increasing latitude and decreasing temperature during the warmest month. For winter-active populations, this relationship was not found. For them, summer temperatures are not a limiting factor, as ponds are typically dry in summer and Daphnia undergo diapause. Thus, a high temperature tolerance is not expected for planktonic phase Daphnia from winter-active populations. Overall, our study confirms the prediction of local temperature adaptation for D. magna, and explains why earlier studies found mixed results [20,23–25].
Latitude and the average maximum temperature of the warmest month are shown here to be good predictors for upper temperature tolerance of summer-active populations. Over large geographical ranges and in the absence of strong altitudinal variation, these two variables are expected to correlate strongly. However, while the average temperature of the warmest month is a likely proximate driver of local temperature adaptation, latitude is the ultimate driver [31].
Local adaptation with regard to climatic variables is expected to change gradually across space, because sites located in close proximity are expected to have a similar climate. This is in contrast with habitat features that may vary strongly on small spatial scales (e.g. water quality and presence/absence of parasites and predators). To test this, we used a spatial autocorrelation analysis. We found very high autocorrelations for the two estimators of tolerance used here (figure 4) strongly supporting our suggestion that temperature tolerance in D. magna evolved in response to the local climate. The analysis confirms that the high variation among D. magna genotypes observed here is closely linked to geography: up to a distance of about 1000 km, the closer two populations are located to each other, the more similar their heat tolerance.
(a). Local adaptation in summer-active populations
Summer-active populations experience summer temperatures every year. Genotypes unable to tolerate the local temperatures are less likely to contribute to a population's genepool. Thus, higher temperatures are expected to select for a tolerance to higher temperatures. Experimental evolution and sediment core resurrection studies of D. magna support this interpretation [5].
Local adaptation is only found when the trait provides a specific benefit that would be disadvantageous in other locations [32], such as tolerance to high temperatures, which would be disadvantageous in colder locations. If a tolerance to high temperatures has costs, tolerance will only be selected for in climates where it is advantageous. At colder locations, selection would favour individuals that pay less costs, and these are those with a lower tolerance. Different trade-off models may apply to our findings [33]: first, by evolving a higher temperature tolerance, organisms may have costs expressed as a generally reduced fitness. Second, by evolving a higher temperature tolerance, organisms may have costs in reduced tolerance to lower temperature. This explanation was suggested for temperature tolerance in ants [3]. Both of these hypotheses can be tested with further experiments and are consistent with diverse physiological explanations, such as homeoviscous adaptation, expression of chaperonins, shifts in osmolyte systems and expression of paralogue enzymes (reviewed in [34]).
(b). Local adaptation in winter-active populations
The Daphnia genotypes collected in the species' subtropical range had large variation in heat tolerance, but that variation was not explained by summer temperatures or latitude. This does not prove that subtropical populations are not locally adapted to the ambient temperature conditions, but rather that the variables used here (local warmest summer temperature and latitude) are poorly correlated with the factor driving selection for temperature adaptation. Seasonality of precipitation in subtropical climates is likely to play a larger role because the reemergence of freshwater organisms from diapause is strongly linked to the onset of rainfall, which is usually during winter—the wettest and coldest period in the Holarctic subtropics [30]. However, because the rainfall distribution is highly variable in regions with winter-active populations, it is difficult to assess the warmest period with suitable water conditions for winter-active populations. The beginning of the rainy season varies regionally and stochastically from early autumn to late winter (figure 1). In very dry locations, entire winters may pass without sufficient rain to support a Daphnia population. Furthermore, the length of the hydroperiod may differ among locations, lasting from a few weeks to several months. Therefore, the temperature of the warmest month with suitable water conditions is much harder to estimate for winter-active populations than for summer-active populations, making it difficult to correlate estimators of temperature with temperature tolerance for winter-active populations. Thus, while our results indicate that winter-active populations are not adapted to the temperature of the warmest month of the year, we have no reason to assume that they are not adapted to their local temperature conditions, or to other habitat features. For example, Roulin et al. [27] showed local adaptation of winter-active populations of D. magna regarding resting stages, which are predominantly produced when the photoperiod is increasing, indicating the end of the winter. Other traits (e.g. emergence from diapause) may be influenced by this habitat difference as well.
(c). Spatial variation in genetic load and inbreeding
In our data, the Daphnia genotypes exhibiting the lowest temperature tolerance were mostly from northern rock pool populations (about north of 59°). These populations are part of highly dynamic metapopulations with frequent local extinction and re-colonization events [35] that often give rise to population bottlenecks, and, consequently, to a high genetic load and high levels of inbreeding [26,36]. Recent studies speculate that these recurring events cause deleterious mutations to accumulate, and, combined with inbreeding, lead to reduced fitness [26,37]. Thus, the presumably poor genetic health of these northern genotypes might explain their low tolerance to high temperatures. This alternative hypothesis could be tested in future experiments: genetic crosses among rock-pool-derived clones from locations with the same temperature profile would reduce the effect of inbreeding, while keeping the expectation for local temperature adaptation the same (for a similar argument, see [37]).
On the other hand, local adaptation at the metapopulation level has been previously reported [27] and shows evidence for directional selection [38]. Nordic rock pool populations were found to have the highest resting egg production among European populations, reflecting an adaptation to the high instability of the rock pool habitat. Thus, the poor genetic health of rock pool D. magna seems not to hinder local adaptation.
5. Conclusion
Our study demonstrates that phenological variation within a species is an essential element influencing local temperature adaptation of populations across a wide geographical range. We demonstrated this for the model system D. magna, but it also holds true for other organisms. For example, other cladoceran species (e.g. Coronatella (Alona) rectangula, Ceriodaphnia quadrangula and Simocephalus vetulus) and numerous insect species are temperature-limited at higher latitudes and precipitation-limited closer to the tropics [14–17,39–41]. More generally, our finding may apply to many species with a wide geographical range and a diapause phase that allows them to escape periods of the year with unsuitable conditions. As climate warming affects populations worldwide, populations may experience this change not only as an increase in temperature, but also with a change in phenology [12,42,43] or voltinism [17,44], which may, in some cases, even lead to counterintuitive relationships between latitude and the average temperature a population experiences during its active phase of the life cycle [11].
Supplementary Material
Supplementary Material
Acknowledgements
We thank Yan Galimov and Frida Ben-Ami for help with collecting temperature records using data loggers in the field. We thank the following people for their help in providing D. magna genotypes for this study: Adam Petrusek, Alexey Kotov, Benjamin Lange, Christoph Haag, Daniela Brunner, Dieter Ebert, Elham Sheik-Jabbari, Ellen Decaestecker, Eric von Elert, Federico Morrone, France Dufresne, Frida Ben-Ami, Iakovos Tziortzis, Ioana Enache, Ivan Gomez-Mestre, Jarkko Routtu, Jason Andras, Juergen Hottinger, Kai Lyu, Kay van Damme, Knut-Helge Jensen, Lev Yampolsky, Liron Goren, Luc DeMeester, Lukas Schaerer, Meryem Beklioglu, Nadja Brun, Peter Fields, Raquel Ortells Baneres, Samuel Pichon, Sandra Lass, Sergey Glagolev, Sigurd Einum, Souad Turki, Sue Mitchell, Theodosiou Loukas, Thomas Zumbrunn, Tom Little, Vasil Vezhnavets, Andrei Papkou, Vladimir Tchougounov and Yan Galimov. We thank Jürgen Hottinger, Michelle Krebs and Urs Stiefel for assistance in the laboratory. Maridel Fredericksen, Jonathan Stillman and the members of the Ebert research group made helpful comments on the manuscript. Suzanne Zweizig improved the language of the text.
Data accessibility
All data are available in electronic supplementary material, table S1.
Authors' contributions
L.S. and D.E. conceived and designed the experiment. L.S. performed the experiment. L.S. and D.E. conducted the analyses and wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by Swiss National Science Foundation (grant no. 310030B_166677).
References
- 1.NOAA National Centers for Environmental Information. 2017. State of the climate: global climate report for annual 2016. See https://www.ncdc.noaa.gov/sotc/global/201613.
- 2.Pereira RJ, Sasaki MC, Burton RS. 2017. Adaptation to a latitudinal thermal gradient within a widespread copepod species: the contributions of genetic divergence and phenotypic plasticity. Proc. R. Soc. B 284, 20170236 ( 10.1098/rspb.2017.0236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond SE, Chick LD. 2018. The Janus of macrophysiology: stronger effects of evolutionary history, but weaker effects of climate on upper thermal limits are reversed for lower thermal limits in ants. Curr. Zool. 64, 223–230. ( 10.1093/cz/zox072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Key FM, Abdul-Aziz MA, Mundry R, Peter BM, Sekar A, D'Amato M, Dennis MY, Schmidt JM, Andrés AM. 2018. Human local adaptation of the TRPM8 cold receptor along a latitudinal cline. PLoS Genet. 14, e1007298 ( 10.1371/journal.pgen.1007298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geerts AN, et al. 2015. Rapid evolution of thermal tolerance in the water flea Daphnia. Nat. Clim. Change 5, 665–668. ( 10.1038/nclimate2628) [DOI] [Google Scholar]
- 6.Burgarella C, Chantret N, Gay L, Prosperi JM, Bonhomme M, Tiffin P, Young ND, Ronfort J. 2016. Adaptation to climate through flowering phenology: a case study in Medicago truncatula. Mol. Ecol. 25, 3397–3415. ( 10.1111/mec.13683) [DOI] [PubMed] [Google Scholar]
- 7.Karlsson K, Puiac S, Winder M. 2018. Life-history responses to changing temperature and salinity of the Baltic Sea copepod Eurytemora affinis. Mar. Biol. 165, 30 ( 10.1007/s00227-017-3279-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly MW, Sanford E, Grosberg RK. 2012. Limited potential for adaptation to climate change in a broadly distributed marine crustacean. Proc. R. Soc. B 279, 349–356. ( 10.1098/rspb.2011.0542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauerfeind SS, Sorensen JG, Loeschcke V, Berger D, Broder ED, Geiger M, Ferrari M, Blanckenhorn WU. 2018. Geographic variation in responses of European yellow dung flies to thermal stress. J. Therm. Biol. 73, 41–49. ( 10.1016/j.jtherbio.2018.01.002) [DOI] [PubMed] [Google Scholar]
- 10.Durmaz E, Benson C, Kapun M, Schmidt P, Flatt T. 2018. An inversion supergene in Drosophila underpins latitudinal clines in survival traits. J. Evol. Biol. 31, 1354–1364. ( 10.1111/jeb.13310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmuth B, Harley CDG, Halpin PM, O'Donnell M, Hofmann GE, Blanchette CA. 2002. Climate change and latitudinal patterns of intertidal thermal stress. Science 298, 1015–1017. ( 10.1126/science.1076814) [DOI] [PubMed] [Google Scholar]
- 12.Blok SE, Olesen B, Krause-Jensen D. 2018. Life history events of eelgrass Zostera marina L. populations across gradients of latitude and temperature. Mar. Ecol. Prog. Ser. 590, 79–93. ( 10.3354/meps12479) [DOI] [Google Scholar]
- 13.Farajollahi A, Fonseca DM, Kramer LD, Kilpatrick AM. 2011. ‘Bird biting’ mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 11, 1577–1585. ( 10.1016/j.meegid.2011.08.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbet PS. 1956. Environmental factors influencing the induction and termination of diapause in the emperor dragonfly, Anax imperator Leach (Odonata, Aeshnidae). J. Exp. Biol. 33, 1–12. ( 10.2307/1781) [DOI] [Google Scholar]
- 15.Carpenter SJ, Lacasse WJ. 1974. Mosquitoes of North America (north of Mexico). Berkeley, CA: University of California Press. [Google Scholar]
- 16.Tauber MJ, Tauber CA. 1976. Insect seasonality—diapause maintenance, termination, and postdiapause development. Annu. Rev. Entomol. 21, 81–107. ( 10.1146/annurev.en.21.010176.000501) [DOI] [Google Scholar]
- 17.Denis RLH. 1993. Butterflies and climate change. Manchester, UK: Manchester University Press. [Google Scholar]
- 18.Benzie JAH. 2005. Cladocera: the genus Daphnia (including Daphniopsis). Leiden, The Netherlands: Backhuys Publisher. [Google Scholar]
- 19.Smirnov NN. 2014. The physiology of the Cladocera. Amsterdam, The Netherlands: Academic Press. [Google Scholar]
- 20.Mitchell SE, Lampert W. 2000. Temperature adaptation in a geographically widespread zooplankter, Daphnia magna. J. Evol. Biol. 13, 371–382. ( 10.1046/j.1420-9101.2000.00193.x) [DOI] [Google Scholar]
- 21.Ragland GJ, Kingsolver JG. 2008. The effect of fluctuating temperatures on ectotherm life-history traits: comparisons among geographic populations of Wyeomyia smithii. Evol. Ecol. Res. 10, 29–44. [Google Scholar]
- 22.di Lascio A, Rossi L, Costantini ML. 2011. Different temperature tolerance of northern and southern European populations of a freshwater isopod crustacean species (Asellus aquaticus L.). Fund. Appl. Limnol. 179, 193–201. ( 10.1127/1863-9135/2011/0179-0193) [DOI] [Google Scholar]
- 23.Williams PJ, Dick KB, Yampolsky LY. 2012. Heat tolerance, temperature acclimation, acute oxidative damage and canalization of haemoglobin expression in Daphnia. Evol. Ecol. 26, 591–609. ( 10.1007/s10682-011-9506-6) [DOI] [Google Scholar]
- 24.Geerts AN, De Meester L, Stoks R. 2014. Heat tolerance and its evolutionary potential along a latitudinal gradient in Daphnia magna. Evol. Ecol. Res. 16, 517–528. [Google Scholar]
- 25.Yampolsky LY, Schaer TMM, Ebert D. 2014. Adaptive phenotypic plasticity and local adaptation for temperature tolerance in freshwater zooplankton. Proc. R. Soc. B 281, 2013744 ( 10.1098/rspb.2013.3744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fields PD, Obbard DJ, McTaggart SJ, Galimov Y, Little TJ, Ebert D. 2018. Mitogenome phylogeographic analysis of a planktonic crustacean. Mol. Phylogenet. Evol. 129, 138–148. ( 10.1016/j.ympev.2018.06.028) [DOI] [PubMed] [Google Scholar]
- 27.Roulin AC, Routtu J, Hall MD, Janicke T, Colson I, Haag CR, Ebert D. 2013. Local adaptation of sex induction in a facultative sexual crustacean: insights from QTL mapping and natural populations of Daphnia magna. Mol. Ecol. 22, 3567–3579. ( 10.1111/mec.12308) [DOI] [PubMed] [Google Scholar]
- 28.Fields PD, Reisser C, Dukic M, Haag CR, Ebert D. 2015. Genes mirror geography in Daphnia magna. Mol. Ecol. 24, 4521–4536. ( 10.1111/mec.13324) [DOI] [PubMed] [Google Scholar]
- 29.Bjornstad ON. 2016. ncf: spatial nonparametric covariance functions. R package version 1.1-7.
- 30.Peel MC, Finlayson BL, Mcmahon TA. 2007. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 11, 1633–1644. ( 10.5194/hess-11-1633-2007) [DOI] [Google Scholar]
- 31.MacDougall-Shackleton SA. 2011. The levels of analysis revisited. Phil. Trans. R. Soc. B 366, 2076–2085. ( 10.1098/rstb.2010.0363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241. ( 10.1111/j.1461-0248.2004.00684.x) [DOI] [Google Scholar]
- 33.Gunderson AR, Stillman JH. 2015. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B 282, 20150401 ( 10.1098/rspb.2015.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somero GN, Lockwood BL, Tomanek L. 2017. Biochemical adaptation. Oxford, UK: Oxford University Press. [Google Scholar]
- 35.Pajunen VI, Pajunen I. 2003. Long-term dynamics in rock pool Daphnia metapopulations. Ecography 26, 731–738. ( 10.1111/j.0906-7590.2003.03542.x) [DOI] [Google Scholar]
- 36.Haag CR, Riek M, Hottinger JW, Pajunen VI, Ebert D. 2006. Founder events as determinants of within-island and among-island genetic structure of Daphnia metapopulations. Heredity 96, 150–158. ( 10.1038/sj.hdy.6800774) [DOI] [PubMed] [Google Scholar]
- 37.Lohr JN, David P, Haag CR. 2014. Reduced lifespan and increased ageing driven by genetic drift in small populations. Evolution 68, 2494–2508. ( 10.1111/evo.12464) [DOI] [PubMed] [Google Scholar]
- 38.Roulin AC, Bourgeois Y, Stiefel U, Walser JC, Ebert D. 2016. A photoreceptor contributes to the natural variation of diapause induction in Daphnia magna. Mol. Biol. Evol. 33, 3194–3204. ( 10.1093/molbev/msw200) [DOI] [PubMed] [Google Scholar]
- 39.Brooks JL. 1957. The systematics of North American Daphnia. New Haven, CT: Memoirs of the Connecticut Academy of Arts and Sciences. [Google Scholar]
- 40.Flössner D. 2000. Die Haplopoda und Cladocera (ohne Bosminidae) Mitteleuropas. Leiden, The Netherlands: Backhuys. [Google Scholar]
- 41.Vandekerkhove J, Declerck S, Brendonck L, Conde-Porcuna JM, Jeppesen E, De Meester L. 2005. Hatching of cladoceran resting eggs: temperature and photoperiod. Freshw. Biol. 50, 96–104. ( 10.1111/j.1365-2427.2004.01312.x) [DOI] [Google Scholar]
- 42.Adrian R, Wilhelm S, Gerten D. 2006. Life-history traits of lake plankton species may govern their phenological response to climate warming. Glob. Change Biol. 12, 652–661. ( 10.1111/j.1365-2486.2006.01125.x) [DOI] [Google Scholar]
- 43.Carter JL, Schindler DE. 2012. Responses of zooplankton populations to four decades of climate warming in lakes of southwestern Alaska. Ecosystems 15, 1010–1026. ( 10.1007/s10021-012-9560-0) [DOI] [Google Scholar]
- 44.Altermatt F. 2010. Climatic warming increases voltinism in European butterflies and moths. Proc. R. Soc. B 277, 1281–1287. ( 10.1098/rspb.2009.1910) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in electronic supplementary material, table S1.