Abstract
Humans create social closeness with one another through a variety of shared social activities in which they align their emotions or mental states towards an external stimulus such as dancing to music together, playing board games together or even engaging in minimal shared experiences such as watching a movie together. Although these specific behaviours would seem to be uniquely human, it is unclear whether the underlying psychology is unique to the species, or if other species might possess some form of this psychological mechanism as well. Here we show that great apes who have visually attended to a video together with a human (study 1) and a conspecific (study 2) subsequently approach that individual faster (study 1) or spend more time in their proximity (study 2) than when they had attended to something different. Our results suggest that one of the most basic mechanisms of human social bonding—feeling closer to those with whom we act or attend together—is present in both humans and great apes, and thus has deeper evolutionary roots than previously suspected.
Keywords: great ape social behaviour, shared cognition, social closeness, joint attention
1. Introduction
Humans create and maintain social relationships in ways that are seemingly unique in the animal kingdom. Specifically, humans are able to create social closeness through all kinds of shared activities and experiences that do not require direct physical interaction but instead seem to satisfy a fundamental need to share the experience with other individuals [1]. Although the precise psychological mechanisms through which such activities result in social closeness remain unclear, humans have been shown to connect with one another by doing such things as making music together [2], acting together in synchrony [3], dancing together [4,5], playing team sports together [6] or by sharing experiences through gossip [7] or attitudes [8], or disclosing personal information [9]. In a recent study, Wolf et al. [10] demonstrated that even after a minimal shared interaction in which participants were attending to the same thing without otherwise communicating, they reported feeling closer to that participant [11].
Throughout the animal kingdom, the individuals of many species act in coordination with conspecifics. For example, dolphins often behave in synchrony [12], many bird species coordinate their song and dance in a mating context [13,14], and great apes travel together [15] and sometimes hunt monkeys together [16]. But do behavioural interactions in which individuals focus on an external stimulus together create stronger social relationships or bonds between participants? To our knowledge, there are no studies examining such a relationship in any non-human species, and indeed some theorists have suggested that this method of social bonding might be uniquely human [5,10].
As always in comparison with humans, great apes are a special case because of their close phylogenetic connection. Operational definitions of social closeness (bonding) in great ape research usually rely on interactions involving physical closeness (e.g. grooming and physical play [17–19] and/or spatial proximity [20]. However, given that apes do engage in a variety of coordinated (and even to some degree cooperative activities) such as building and fighting in coalitions and alliances [21], as well as travelling and hunting in groups [22], the question is whether, like humans, great apes have evolved a psychological mechanism that leads them to create social closeness with others through shared experiences. On the other hand, it might be that connecting with others through shared experiences is a uniquely human phenomenon.
To answer this question, we adapted Wolf et al.’s [10] paradigm for apes and conducted two studies in which participants shared the experience of attending to a video together with a human experimenter (study 1) or a conspecific (study 2). In the control condition, a human experimenter (study 1) or conspecific (study 2) sat in the same place but was not watching the video. We then compared the apes' subsequent behaviour towards their partner—approaching and/or remaining in physical proximity—between the two conditions.
2. Study 1
(a). Methods
(i). Participants and design
Nineteen chimpanzees (Pan troglodytes) and seven bonobos (Pan paniscus) at the Wolfgang Kohler Primate Research Center (WKPRC) in the Leipzig Zoo participated in a two-condition (watching together (WT) versus control) within-subject experiment. All of them had previously engaged in social and cognitive experiments with humans. For two chimpanzees, one of the trials yielded uninterpretable behaviour1 and was therefore excluded from the analysis. The final sample for which we collected behavioural data thus consisted of 17 chimpanzees (mean age = 26.4 years, 7 males) and 7 bonobos (mean age = 18.6, 3 males). Additionally, we also collected eye-tracking data for 15 chimpanzees (for 2 chimpanzees, eye-tracking data were not collected due to their physiology2 interfering with the eye-tracking set-up).
(ii). Ethics statement
Study 1 was approved by the Wolfgang Köhler Primate Center Animal Research Committee and was done in accordance with all of the governing laws and regulations concerning research with animals in Germany. In addition, the WKPRC has additional animal health and safety standards.
(iii). Set-up
Participants were sitting in a booth, looking through a Plexiglas screen to a pc monitor placed in front of them. Underneath the monitor, there was an eye-tracking camera. Outside the room were two laptops, one of which was used for displaying stimuli on the monitor that the participants were looking at, while the other one was controlling the eye tracker. All trials were videotaped using three cameras. Participants sat down in front of a Plexiglas screen through which a mouthpiece of a juice tube stuck out (figure 1).
Figure 1.
Visual experience of the ape in the watching together condition (a) and the control condition (b). Note the eye tracker on the table in front of the television monitor. (Online version in colour.)
(iv). Procedure
The experimental coordinator (EC) set up the room for the appropriate condition and turned on the cameras. Next, the EC filled the juice tube with diluted grape juice, waited for the subject to come in,3 made sure the subject was drinking from the juice tube and left the room. The EC then started the eye tracker and signalled to experimenter 1 (E1) that they could go in.
Participants engaged in two trials of the procedure. There were two female research assistants that assisted in the procedure, each of which the participants had never seen or interacted with before.4 Each experimenter only engaged with each participant once and all participants thus interacted only one time with each experimenter. The experimenters were counterbalanced across conditions to prevent potentially confounding effects of a systematic preference of the participants for one of the two experimenters. At the same time, the order of the conditions was also counterbalanced to prevent potential learning effects from biasing the results.
When E1 got into the room, they sat down next to the screen. In the WT condition, the screen was turned towards the experimenter so that both the participant and the experimenter could see the screen (figure 1a). In the control condition, the screen was turned away from the experimenter so that only the participant could see the screen (figure 1b).
After E1 had sat down, EC started one of several 1 min videos (see electronic supplementary material, video S1 for an example). These videos were excerpts from a longer video of a playing juvenile chimpanzee. This video was chosen based on a recommendation of researchers conducting studies with chimpanzees and bonobos using video stimuli. The key consideration was that the videos should be (1) interesting enough for participants to sit down and attend to the video, and (2) not so arousing that they would elicit stress and/or behaviour that would cause them to disengage from the video. We were therefore advised to show participants a video of great apes that were not in their own group, which would certainly capture their interest. However, showing them a video of adult males and/or females might contain cues of dominance or mating, which would make the video too arousing. We therefore decided that a video of a playing juvenile chimpanzee would serve the purposes of the current study best.
In the WT condition, experimenter 2 watched the video together with the participant, whereas in the control condition, the experimenter looked down and read her own clipboard, not looking at the pc monitor at all. To prevent auditory joint attention, the video had no sound. In order to make sure that the participants (1) had attended to the video and (2) had looked at the experimenter sitting next to the screen, we used eye-tracking cameras to collect eye movement data from the participants, starting at the moment the experimenter sat down (see electronic supplementary material, video S2 for an example of the eye tracking). After the video had ended, the experimenter got up, walked to the other side of the enclosure, and sat down. In the meantime, experimenter 1 entered, took out the juice tube and left the room again. We then measured the approach latency of the participant towards experimenter 2.
(v). Eye-tracking apparatus and usage
To measure eye movement, we used a 60 Hz, ×120 Tobii infrared eye tracker (Tobii Technology AB, Stockholm, Sweden), commonly used at the WKPRC. This eye-tracking apparatus records eye movement in a non-invasive manner, without head restraint. Previous research in this facility has shown that the acrylic safety panels do not interfere with the eye-tracking measurements (e.g. [23]). Participants were kept relatively stationary by supplying them with diluted grape juice through a custom-made juice tube device, created from a medical drip that was fitted with a mouthpiece at the end of the tube, which fitted through a hole in the Plexiglas screen. The provision of juice was not contingent on the participants’ eye gaze.
The eye tracker was calibrated on the real-world visual space of the participant. To do so, a separate camera was fixed on the glass above the participant. Furthermore, a black screen was placed behind the scene on which the eye tracker needed to be calibrated. This screen contained two marks which indicated the corners of the visual field of the camera. Each participant was calibrated by presenting them with physical objects (e.g. toy fruit) in those two corners. All calibrations were checked manually and replaced if necessary. For analysis, we created two areas of interest. One area of interest encompassed the area of the scene in which the screen was placed during the manipulation. The other area encompassed the visual field of the participant in which the experimenter was sitting during the manipulation.
(vi). Approach latency
To get a behavioural measure of the attitude of the participants towards the experimenter, we used approach latency towards that experimenter. Approach latency was operationalized as the participant crossing the area four tiles behind the Plexiglas screen behind which experimenter 2 was now sitting (see electronic supplementary material, video S3 for an example). A ceiling camera was used to determine if the participant stepped over that line. If the participant did not approach after 30 s, the experimenter started rattling the mesh to prompt the participant to come over. If they still had not approached after 60 s, the experimenter, in addition to rattling the mesh, also called out their name. All participants approached within 90 s. The moment the participant entered the approach area, the trial was finished.
(b). Results
(i). Eye-tracking manipulation check
The eye-tracking data showed that all participants in all trials had attended the video during the manipulation (mean looking time = 26.03, s.d. = 12.13) and that all of them had looked at the experimenter at some point after the experimenter had sat down (mean looking time = 5.68, s.d. = 7.03). As the data were not normally distributed, we conducted a within-subjects Wilcoxon signed-rank test (two-sided) on the 30 trials (two times 15 subjects). We found no differences in the time the participants spent watching (1) the experimenter or (2) the screen between the WT condition and the control condition (all p > 0.21).
(ii). Approach latency
Approach latency was coded using the ‘interact’ software package. A second coder double coded 50% of the trials (24 out of 48). The angle of the videos that were coded was such that it was impossible to see what condition that subject was in. Coder reliability was high (Interclass correlation = 0.999). As the data were not normally distributed and data transformations did not resolve this issue, non-parametric statistics were warranted. To test if the manipulation had an effect, as well as whether this effect was different for the different species, we conducted a two (condition: watching together versus watching alone) by two (species: chimpanzee versus bonobo) non-parametric mixed model with approach latency as the dependent variable [24]. Results showed no main effect of species on approach latency (modified ATS5 (d.f. = 1, 10.90) = 3.98, p = 0.07), nor an interaction effect between condition and species (ATS (d.f. = 1) = 0.01, p = 0.92). Crucially, we did find an effect of condition on approach latency (ATS (d.f. = 1) = 5.61, p = 0.017; figure 2). Participants from both species approached the experimenter with whom they had watched the video together faster (M = 14.86, s.d. = 13.18) than the experimenter that had been reading her own clipboard (M = 26.78, s.d. = 22.93).6
Figure 2.
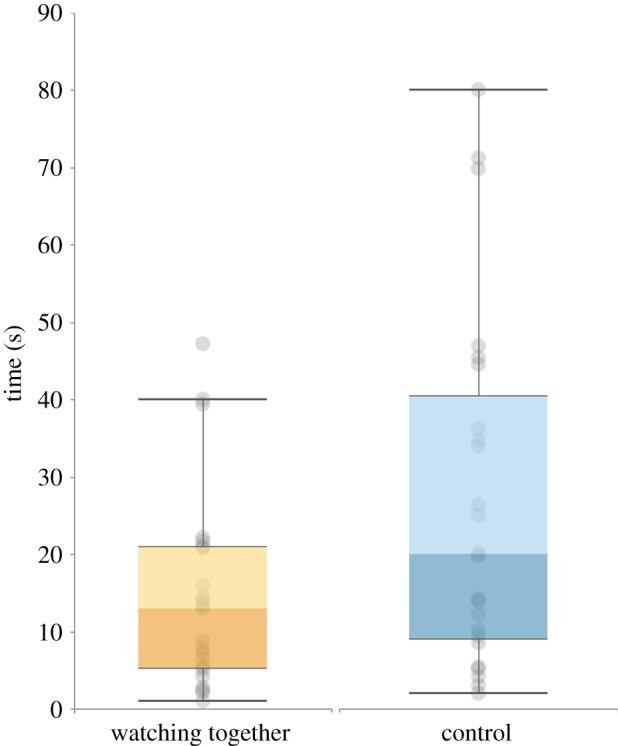
Approach latency per condition (study 1). (Online version in colour.)
(c). Discussion
These results suggest that visually attending to a stimulus together creates some kind of social connection between a great ape and a human, such that the ape experiences an increased motivation to approach the human experimenter. What is still unknown, however, is whether these results indicate a general psychological mechanism in great apes, or rather a particular way that apes in zoos relate to humans based on their extensive experience with them. Previous research has found that great apes are, for example, more likely to understand and produce an imperative pointing gesture while interacting with humans than when interacting with conspecifics [25–28]. Similarly, the elaborate interactions with humans of the sample in study 1, including frequent testing, as well as their dependence on humans for food, might make them specifically attuned to what humans are looking at, and this might not generalize to interactions with conspecifics. In study 2, therefore, we aimed at replicating these findings, but between two chimpanzees, and with a different group of chimpanzees, that is, from the Ngamba Island Chimpanzee Sanctuary (NICS).
3. Study 2
(a). Methods
(i). Participants and design
Twenty-one chimpanzees (mean age = 19.57, s.d. = 3.88, 10 males) at the NICS in Uganda participated in a study which had a design similar to study 1. All of them were part of a single group and had previously engaged in social and cognitive experiments with humans before. In contrast to study 1, the procedure of study 2 required the participation of pairs of chimpanzees. The creation of the pairs was based on the judgement of the NICS keepers. We asked them to help us create a list with same-sex pairs of individuals who were likely to be tolerant of one another while being alone in the same room. In addition, we asked them to exclude pairs in which one of the individuals was likely to be scared or intimidated by the other individual and would try to hide or avoid their partner while being in the same room. Finally, we also decided that individuals should not go through the procedure more than four times (i.e. eight trials). Based on these constraints, we ended up with 36 pairs which we tested over 72 trials7 (one WT trial and one control trial per pair). Electronic supplementary material, data table S3 shows how many times each individual was tested.
(ii). Ethics statement
Study 2 was approved by the Ugandan Wildlife Authority (UWA) in accordance with governing laws and regulations concerning research with animals in Uganda.
(iii). Set-up
Participants were led into two adjacent rooms with a closed door between them. One room was smaller than the other, allowing us to seat participants in a 90° angle from each other (figure 3; electronic supplementary material, figure S1). The participants sat down behind two Plexiglas screens, each of which had a juice tube mouthpiece sticking out from which they could drink diluted mango juice. In the WT condition, one computer monitor was placed in front of the participants so that both participants could see the same screen (figure 3a). In the control condition, two monitors were placed with a plastic barrier in between the screens, so that participants could not see each other's screen (figure 3b). In both conditions, monitors were placed so that the participants could still see their partner in his or her entirety. The monitors were connected to a laptop controlling stimulus presentation. Furthermore, the room in which the monitors were placed contained three cameras, which recorded the chimpanzees' behaviour during and after the manipulation.
Figure 3.
Set-up of study 2 for the watching together condition (a) and control condition (b). (Online version in colour.)
(iv). Procedure: individual exposure trial
Before we started any of the experimental trials, we wanted to expose the participants to the juice tubes. Furthermore, we wanted them to gain experience with the two-monitor set-up, because we wanted to make sure participants were aware that from the perspective of the different Plexiglas screens, it was impossible to see what was happening on the other screen (an inference which previous research has shown great apes are able to make [28]). To do so, participants entered the rooms8 by themselves while the door between the rooms was open. To reduce the likelihood of carry-over effects from the exposure trials into the experimental trials, the stimulus for the exposure trials was an unrelated video of (non-chimpanzee) animal behaviour used in a previous study with human children. All chimpanzees almost instantly understood the mechanics of the juice tube and drank from them during the exposure trials.
The first phase of the exposure trial consisted of participants freely roaming around the two rooms for 3 min. In the second phase, we wanted to expose the participants to the juice tube and make sure they had experienced the perspective from both sides of the set-up (as some individuals merely sat down at the first Plexiglas screen they came across and did not roam around any further). In the second phase, we therefore offered them juice through the juice tube at the Plexiglas screen of the room they were not in at that moment. This way, all the apes walked around to the other room and thus saw the set-up from both perspectives at least once.
(v). Procedure: experimental trial
After we had completed all the exposure trials, we started engaging pairs of participants in the experimental trials. All pairs engaged in a WT trial and a control trial, at least a day apart, with the order of the conditions counterbalanced across pairs. In both conditions, two keepers of the NICS let the two individuals into their individual rooms to engage in the manipulation. During the manipulation, the door between the rooms was closed. The moment the participants came in the experimenter turned on the juice tube. In all cases, this motivated the participants to come to the Plexiglas screen and drink from the juice tube. The experimenter then started the same videos used in study 1 (i.e. the videos of the playing juvenile chimpanzee) and left the room. When the video was finished, the experimenter came back into the room and took out the juice tubes. He then left the room, while at the same time, one of the keepers opened the door between the rooms the participants were sitting in. Next, the experimenter and both of the keepers moved to a location where the participants would not be able to see them anymore.9 The participants were then left to do what they wanted for 3 min, during which their behaviour was recorded.
(vi). Measures
As the manipulation in study 2 was no longer an interaction between an ape participant and an experimenter who stayed in one place, but instead a spontaneous interaction between two apes, we could no longer use approach latency as a proxy for social closeness. Instead, we measured participants’ subsequent physical proximity as our main dependent variable, consistent with previous research on social networks in great apes [20]. As a secondary dependent measure, we also looked at interactive behaviours after the manipulation. In order to obtain sufficient behaviour to analyse in the absence of experimental prompts (as in the first study), we decided that a longer time window during which behaviour was recorded was necessary. However, at the same time, we also had to consider the possibility that the effect of the manipulation would wear off over time, potentially adding noise to the data. Additionally, the staff and researchers that had worked with these subjects before cautioned us that for some subjects, keeping them in their enclosure with nothing to do while part of their group members had already left for the forest would make them uncomfortable. Based on these methodological and animal welfare considerations, we decided to set the time window during which their behaviour was measured to 3 min. After 3 min, data collection stopped, as the keepers and experimenter returned to the enclosure to let the subjects out.
(vii). Proximity
Proximity was coded on three different levels. First, we coded the time the participants spent in the same room. However, as the surface of the big room in the enclosure was twice as large as the surface of the small room (electronic supplementary material, figure S2), the base rate probability of a participant spending time in the big room was twice as high as the probability of them spending time in the small room. Furthermore, the rooms were several metres high, with hammocks hanging from the ceiling, where some of the apes spend at least part of the 3 min following the manipulation. As such, a proximity measure merely constrained by which room the apes were in did not account for their actual physical proximity in three-dimensional space. Also, it is important to note that the enclosures were surrounded by bars (and not opaque walls). This means that subjects could still see each other while being in different rooms, meaning that they could not simply hide away from their conspecific's attention by going into a different room. As such, compared with actual physical proximity, being in a separate room, at least in this context, did not seem to be relevant for measuring social closeness.
To address these issues and reduce the amount of noise in the data, the main variable of interest was how much time participants spent in the same part of the room. That is, during coding, the big room was split up into two equal parts, causing the total surface area of the big and small room together to now be divided into three more or less equal spaces (electronic supplementary material, figure S3). Furthermore, we only coded time spent in the same part of the room if participants were on the same level of the enclosure. This means that either both of them had to be on the floor, or both had to be hanging from the ceiling or sitting in a hammock attached to the ceiling in order for that time to qualify as time spent in the same part of the room.
Finally, we also coded the amount of time the participants spent at arm's length. In these cases, participants were sitting so close to one another that they were either touching or would have been able to touch each other if one of the individuals would have stretched their arms. We also coded this for instances where the two participants were not technically in the same part of the room.
In addition to measures of physical proximity, we also measured the frequency with which two types of interactive behaviours occurred between participants. We coded the time participants spent grooming (i.e. sifting through the hair of another individual [12]), a common indicator of social affiliation in great apes [17,19]. Additionally, we coded the time participants spent fighting (i.e. aggressively chasing or using physical aggression) with each other during the 3 min after the manipulation as a counter-indicative measure of social closeness. The amount of time individuals spend fighting within a dyad was subsequently subtracted from all the time individuals spend together in the proximity measures, as this was not the type of proximity that can be used as a proxy measure of social closeness.
(b). Results
All behaviour was coded with the BORIS software package for behavioural coding. In total, 25% of the trials (i.e. 18 out of 72) were double coded. Coder discrepancies were discussed and resolved, resulting in high coder agreement (Interclass correlation r = 0.995). Next, we compared the participants' WT trials with their control trials in terms of the time spent in each other's proximity and the time engaged in interactive behaviours (table 1). As none of the data were normally distributed, we conducted a within-subjects Wilcoxon signed-rank test for each variable (two-sided) on the 72 trials (two times 36 pairs).
Table 1.
Descriptive statistics of coded behaviours in study 2.
| watching together |
control |
|||||
|---|---|---|---|---|---|---|
| mean | s.d. | Na | mean | s.d. | Na | |
| in same part of the room | 31.5 | 38.61 | 25 | 19.25 | 36.27 | 22 |
| in the same room | 72.47 | 61.07 | 28 | 65.22 | 58.57 | 30 |
| arm's length | 4.61 | 10.12 | 11 | 2.00 | 4.52 | 7 |
| grooming | 1.13 | 6.75 | 1 | 0 | 0 | 0 |
| fighting | 0 | 0 | 0 | 1.03 | 4.04 | 3 |
aThe number of trials in which this behaviour occurred at least once.
We found an effect of condition on the amount of time participants spent in the same part of the room (p = 0.033 r = 0.251; figure 4). Participants spent more time in the same part of the room in the WT condition (M = 31.50, s.d. = 6.44) than in the control condition (M = 19.25, s.d. = 6.04).
Figure 4.
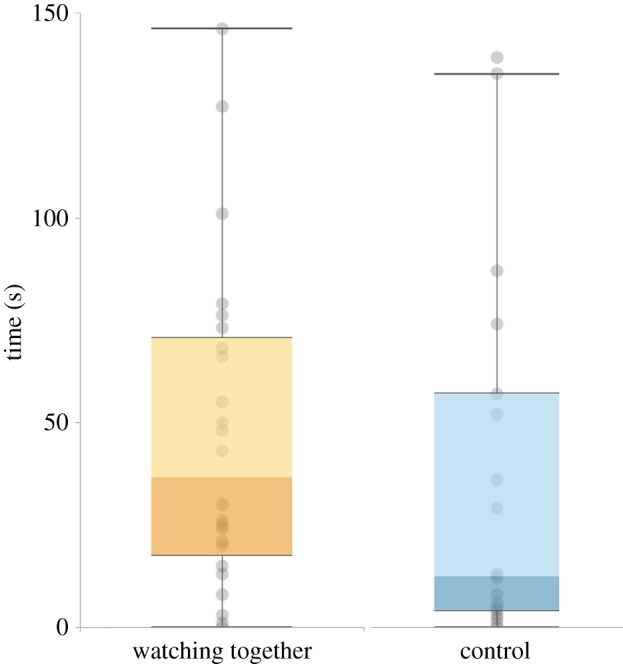
Time spent in the same part of the room (study 2). (Online version in colour.)
We found no effect of any of the other variables (p's greater than 0.05). As expected, the more general ‘in the same room’ measure seemed not sensitive enough to pick up a difference between conditions. Furthermore, proximity at arm's length (n = 18), as well as fighting (n = 3) and grooming (n = 1) did not occur frequently enough to do statistical analyses on. With regard to grooming, this is not surprising. As research has shown that chimpanzees only spend 6.8% of their daytime grooming (i.e. around 45 min a day [20]), a 3 min window to measure grooming might simply have been too short for such behaviour to occur frequently enough to make statistical inferences. However, as table 1 shows, the time spent (1) in the same room, (2) at arm's length, (3) grooming and (4) fighting showed a converging trend. Grooming only occurred after WT trials while fighting only occurred during control trials. Furthermore, the condition means of the time participants spent in the same room and the time participants spent at arm's length, despite lacking sensitivity or occurring too infrequent to analyse, show a trend similar to the effect found in the time spent in the same part of the room.
(c). Discussion
Extending the results of the first experiment, these results suggest that visually attending to a stimulus together with another individual elicits social closeness in great apes not only with human experimenters but also with conspecifics. Furthermore, the results were obtained from a different population of apes, living in a larger group, in an African sanctuary instead of a European zoo, suggesting that the effect of watching something together on great ape social closeness is not limited to apes living in a zoo environment.
4. General discussion
The current results show that great apes behave more socially after an interaction in which they align their attention to an external stimulus. Study 1 showed that both chimpanzees and bonobos approach a human experimenter faster after having watched a video with them, suggesting that this effect can be found in the entire genus Pan. Study 2 replicated these findings in a different sample and extended them by showing that this effect is not limited to great ape's interactions with humans, but also seems to occur in interactions between great apes. As such, the current findings shed new light on great ape social cognition and social behaviour, as well as the evolutionary origin of connecting through shared experiences in humans.
Becoming socially closer to others through shared experiences such as dancing to music together or communicating about shared experiences has only been described in humans. It has therefore been suggested that this bonding mechanism is uniquely human, explaining (at least in part) why humans have larger social networks with more complex social relationships than other species [29,30]. The current results imply, however, that some of the basic elements of this social bonding mechanism—eliciting social closeness by visually attending to something together with another individual—are present in humans through shared descent with other apes.
This is surprising because many researchers have argued that the capacity to experience reality as shared is uniquely human [1,31], which implies that other species, including great apes, lack the psychological mechanisms to interpret an experience as shared and therefore do not engage in social activities solely for the purpose of generating social closeness. However, one can imagine that great ape activities such as fighting together in a coalition or traveling together in a small group—based at least partly on visually attending to things together—already elicit social closeness among ape individuals. This suggests that such activities, aside from their instrumental purpose (e.g. travelling safely, acquiring or maintaining dominance in a group), might also function as a way to generate social closeness between the individuals partaking in the activity. As such, this psychological mechanism might be a previously unnoted facilitator of great ape social relationships.
One must, however, be cautious when extrapolating a human shared experience-based bonding mechanism to great apes on the basis of the current studies. The current control condition for both studies was designed to keep all parts of the experience, aside from whether it was shared or not, as constant as possible, similar to studies on shared experiences in humans [10,11]. Based on the current results, it is therefore not possible to know whether the results of the current studies generalize to any other stimulus than the video stimulus we used, or to social activities that do not include watching a video. Additionally, the results of the current study do not give insight into how the effect of sharing an experience compares to other factors influencing social closeness, such as, for example, eye contact, which has to been shown to play an important role in chimpanzee interactions [32].
Furthermore, it is hard to tell if the apes' psychological experience of watching a video together with a partner in the current study is cognitively similar to the psychological effects that occur when humans share an experience. Also, we do not know if the short-term psychological effects found in the current paradigm are sufficient to influence great ape social relationships in the long term. Additionally, although the approach and proximity measures tell us something about a general social interest in and/or motivation to interact with a social partner, the question remains how this compares with the social closeness that humans feel after sharing an experience. Nevertheless, the current results demonstrate that on a basic level, socially relating to others via shared experiences seems not to be uniquely human but instead deeply rooted in our evolutionary history.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the staff at the Wolfgang Kohler Primate Research Center and the Ngamba Island Chimpanzee Sanctuary for help with all aspects of the study.
Footnotes
The videos showed that in these trials, the participant did not enter the area in front of the experimenter, but merely walked by. However, the experimenter had interpreted that behaviour as an approach and had therefore stopped the trial, meaning that no definitive approach latency could be coded for these trials.
One chimpanzee (Jeudi) had a condition in one of her eyes which caused the eye tracker not to be able to track that eye. The other chimpanzee (Riet) had a sizable swelling in the right part of her upper lip which caused her to tilt her head to the side while drinking from the juice tube. This caused the eye tracker not to be able to track her right eye.
All participants were used to being moved around by keepers. Sometimes, they were motivated by placing some food in the area where they had to wait for the study to begin. Furthermore, all subjects in study 1 had extensive experience with juice tubes and therefore required no training in using them.
The chimpanzees and bonobos participating in the study have extensive research experience (i.e. testing occurs daily, throughout the year, for over 20 years), and are therefore used to interacting with humans they have not encountered before.
The between-subjects factor in this model is evaluated with a modified ANOVA-type statistic (ATS) [24].
See electronic supplementary material, figure S1 for an overview of the individual response times per condition, grouped by species.
Due to technical problems, the videos of two trials were lost, and these trials were therefore replaced with a new trial. Excluding these pairs from the analysis does not meaningfully change the results of study 2.
As in study 1, subjects in this group were used to being moved around by keepers, who sometimes used food to direct them towards the right location where they waited to be let into the testing enclosure. This is very similar to the strategy the keepers use every evening when trying to get chimpanzees in specific sleeping enclosures (to minimize conflict at night) by throwing small food pallets in specific areas. As such, the movement of subjects in study 2 was in line with the chimpanzees’ daily routine.
One of the keepers hid behind the enclosure, where he was able to look around the corner in case he heard aggressive vocalizations. This allowed him to intervene if, in his judgement, the fighting put one of the animals at risk for serious physical harm. In none of the three instances of fighting that occurred did the keeper feel the fighting was severe enough to intervene, and no injuries were sustained by any of the participants.
Ethics
Study 1 was approved by the Wolfgang Köhler Primate Center Animal Research Committee and was done in accordance with all of the governing laws and regulations concerning research with animals in Germany. In addition, the WKPRC has additional animal health and safety standards. Study 2 was approved by the Ugandan Wildlife Authority (UWA) in accordance with governing laws and regulations concerning research with animals in Uganda.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
W.W. and M.T. designed the experiments together. M.T. facilitated data collection. W.W. collected the data. W.W. analysed the data, under supervision of M.T. W.W. and M.T. wrote the manuscript together.
Competing Interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Echterhoff G, Higgins ET, Levine JM. 2009. Shared reality: experiencing commonality with others’ inner states about the world. Perspect. Psychol. Sci. 4, 496–521. ( 10.1111/j.1745-6924.2009.01161.x) [DOI] [PubMed] [Google Scholar]
- 2.Pearce E, Launay J, Dunbar RIM. 2015. The ice-breaker effect: singing mediates fast social bonding. R. Soc. open sci. 2, 150221 ( 10.1098/rsos.150221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hove MJ, Risen JL. 2009. It's all in the timing: interpersonal synchrony increases affiliation. Soc. Cogn. 27, 949–960. ( 10.1521/soco.2009.27.6.949) [DOI] [Google Scholar]
- 4.Tarr B, Launay J, Cohen E, Dunbar RIM. 2015. Synchrony and exertion during dance independently raise pain threshold and encourage social bonding. Biol. Lett. 11, 20150767 ( 10.1098/rsbl.2015.0767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarr B, Launay J, Dunbar RIM. 2016. Silent disco: dancing in synchrony leads to elevated pain thresholds and social closeness. Evol. Hum. Behav. 37, 343–349. ( 10.1016/j.evolhumbehav.2016.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artinger L, Clapham L, Hunt C, Meigs M, Milord N, Sampson B, Forrester SA. 2006. The social benefits of intramural sports. J. Stud. Aff. Res. Pract. 43, 69–86. ( 10.2202/1949-6605.1572) [DOI] [Google Scholar]
- 7.Dunbar RIM. 2004. Gossip in evolutionary perspective. Rev. Gen. Psychol. 8, 100–110. ( 10.1037/1089-2680.8.2.100) [DOI] [Google Scholar]
- 8.Bosson JK, Johnson AB, Niederhoffer K, Swann WB. 2006. Interpersonal chemistry through negativity: bonding by sharing negative attitudes about others. Pers. Relat. 13, 135–150. ( 10.1111/j.1475-6811.2006.00109.x) [DOI] [Google Scholar]
- 9.Aron A, Melinat E, Aron EN, Vallone RD, Bator RJ. 1997. The experimental generation of interpersonal closeness: a procedure and some preliminary findings. Pers. Soc. Psychol. 23, 363–377. ( 10.1177/0146167297234003) [DOI] [Google Scholar]
- 10.Wolf W, Launay J, Dunbar RIM. 2015. Joint attention, shared goals, and social bonding. Br. J. Psychol. 107, 322–337. ( 10.1111/bjop.12144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rennung M, Göritz AS. 2015. Facing sorrow as a group unites. Facing sorrow in a group divides. PLoS ONE 10, 1–22. ( 10.1371/journal.pone.0136750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fellner W, Bauer GB, Harley HE. 2006. Cognitive implications of synchrony in dolphins: a review. Aquat. Mamm. 32, 511–516. ( 10.1578/AM.32.4.2006.511) [DOI] [Google Scholar]
- 13.Williams H. 2001. Choreography of song, dance and beak movements in the zebra finch (Taeniopygia guttata). J. Exp. Biol. 10, 3497–3506. [DOI] [PubMed] [Google Scholar]
- 14.Ullrich R, Norton P, Scharff C. 2016. Waltzing Taeniopygia: integration of courtship song and dance in the domesticated Australian zebra finch. Anim. Behav. 112, 285–300. ( 10.1016/j.anbehav.2015.11.012) [DOI] [Google Scholar]
- 15.Symington MM. 1990. Fission–fusion social organization in Ateles and Pan. Int. J. Primatol. 11, 47–61. ( 10.1007/BF02193695) [DOI] [Google Scholar]
- 16.Boesch C. 1994. Cooperative hunting in wild chimpanzees.pdf. Anim. Behav. 48, 653–667. ( 10.1006/anbe.1994.1285) [DOI] [Google Scholar]
- 17.Hare B, Schroepfer-Walker K, Wobber V. 2015. Experimental evidence that grooming and play are social currency in bonobos and chimpanzees. Behaviour 152, 545–562. ( 10.1163/1568539X-00003263) [DOI] [Google Scholar]
- 18.Behncke I. 2015. Play in the Peter Pan ape. Curr. Biol. 25, 24–27. ( 10.1016/j.cub.2014.11.020) [DOI] [PubMed] [Google Scholar]
- 19.Lehmann J, Korstjens AH, Dunbar RIM. 2007. Group size, grooming and social cohesion in primates. Anim. Behav. 74, 1617–1629. ( 10.1016/j.anbehav.2006.10.025) [DOI] [Google Scholar]
- 20.Kasper C, Voelkl B. 2009. A social network analysis of primate groups. Primates 50, 343–356. ( 10.1007/s10329-009-0153-2) [DOI] [PubMed] [Google Scholar]
- 21.Harcourt AH, De Waal FBM. 1992. Coalitions and alliances in humans and other animals. Oxford, UK: Oxford University Press. [Google Scholar]
- 22.Boinski S, Garber PA. 2000. On the move: how and why animals travel in groups. Chicago, IL: University of Chicago Press. [Google Scholar]
- 23.Krupenye C, Kano F, Hirata S, Call J, Tomasello M. 2016. Great apes anticipate that other individuals will act according to false beliefs. Science 354, 107–110. ( 10.1126/science.aaf8110) [DOI] [PubMed] [Google Scholar]
- 24.Noguchi K, Gel YR, Brunner E, Konietschke F. 2012. nparLD: an R software package for the nonparametric analysis of longitudinal data in factorial experiments. J. Stat. Softw. 50 ( 10.18637/jss.v050.i12) [DOI] [Google Scholar]
- 25.Leavens DA, Hopkins WD, College B. 1999. The whole-hand point: the structure and function of pointing from a comparative perspective. J. Comp. Psychol. 113, 417–425. ( 10.1037/0735-7036.113.4.417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tempelmann S, Kaminski J, Liebal K. 2013. When apes point the finger: three great ape species fail to use a conspecific's imperative pointing gesture. Interact. Stud. 14, 7–23. ( 10.1075/is.14.1.02tem) [DOI] [Google Scholar]
- 27.Lyn H, Russell JL, Hopkins WD. 2010. The impact of environment on the comprehension of declarative communication in apes. Psychol. Sci. 21, 360–365. ( 10.1177/0956797610362218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hare B, Call J, Agnetta B, Tomasello M. 2000. Chimpanzees know what conspecifics do and do not see. Anim. Behav. 59, 771–785. ( 10.1006/anbe.1999.1377) [DOI] [PubMed] [Google Scholar]
- 29.Dunbar RIM. 2008. Mind the gap or why humans are not just great apes. In Proceedings of the British academy (ed. Johnston R.), pp. 402–423. London, UK: British Academy; See http://www.britishacademypublications.com/view/10.5871/bacad/9780197264355.001.0001/upso-9780197264355-chapter-15. [Google Scholar]
- 30.Tomasello M, Carpenter M, Call J, Behne T, Moll H. 2005. Understanding and sharing intentions: the origins of cultural cognition. Behav. Brain Sci. 28, 675–691. discussion 691–735 ( 10.1017/S0140525X05000129) [DOI] [PubMed] [Google Scholar]
- 31.Tomonaga M, Tanaka M, Matsuzawa T, Myowa-Yamakoshi M, Kosugi D, Mizuno Y, Okamoto S, Yamaguchi MK, Bard KA. 2004. Development of social cognition in infant chimpanzees (Pan troglodytes): face recognition, smiling, gaze, and the lack of triadic interactions. Jpn. Psychol. Res. 46, 227–235. ( 10.1111/j.1468-5584.2004.00254.x) [DOI] [Google Scholar]
- 32.Tomonaga M. 2006. Development of chimpanzee social cognition in the first 2 years of life. In Cognitive development in chimpanzees, vol. 46 (eds T Matsuzawa, M Tomonaga), pp. 182–197. Berlin, Germany: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.




