Abstract
Ocean acidification (OA) is predicted to be a major driver of ocean biodiversity change. At projected rates of change, sensitive marine taxa may not have time to adapt. Their persistence may depend on pre-existing inter-individual variability. We investigated individual male reproductive performance under present-day and OA conditions using two representative broadcast spawners, the sea urchins Lytechinus pictus and Heliocidaris erythrogramma. Under the non-competitive individual ejaculate scenario, we examined sperm functional parameters (e.g. swimming speed, motility) and their relationship with fertilization success under current and near-future OA conditions. Significant inter-individual differences in almost every parameter measured were identified. Importantly, we observed strong inverse relationships between individual fertilization success rate under current conditions and change in fertilization success under OA. Individuals with a high fertilization success under current conditions had reduced fertilization under OA, while individuals with a low fertilization success under current conditions improved. Change in fertilization success ranged from −67% to +114% across individuals. Our results demonstrate that while average population fertilization rates remain similar under OA and present-day conditions, the contribution by different males to the population significantly shifts, with implications for how selection will operate in a future ocean.
Keywords: spermatozoa, individual variability, computer-assisted sperm analysis, fertilization success, echinoderm, carbon dioxide
1. Introduction
Ocean acidification (OA) is expected to become one of the greatest drivers of global ocean biodiversity change [1]. Atmospheric CO2 levels surpassed 400 ppm in 2016 and have subsequently risen by an additional 5 ppm yr–1, above the previously projected increase (2.73 ppm yr–1, Keeling curve, Scripps Institute of Oceanography, accessed June 2018) [2]. Due to CO2 uptake, the mean pH of ocean surface water has decreased from 8.13 to 8.05 since the industrial revolution and is predicted to drop a further 0.14 to 0.40 units by 2100 (RCP2.6, 8.5) [3]. Seasonal and diurnal variability in pH is also predicted to increase, particularly in coastal areas, suggesting many species may experience greater and more variable extremes in carbonate chemistry [4]. Sensitive marine taxa may not have time to adapt to these rapid changes through new genetic mutations and so the persistence of many species may be determined by the inter-individual genetic variability or plasticity that already exists within populations [5]. Variability in sperm traits both within and among males is well recognized across animal taxa (reviews: [6,7]). Despite this, most studies investigating the impacts of OA on marine taxa have focussed on population-level responses which do not account for individual variability [8–11]. These studies indicate the general sensitivity of a population but fail to identify how inter-individual variability could influence selection and potential longer-term adaptability of a population [5]. In particular, understanding how individuals respond to environmental change may be critical for determining the adaptive potential of populations globally [12,13].
Changes in ocean chemistry have been shown to influence almost every phase of marine invertebrate life cycles, although the reported extent to which different life-history processes are affected vary [10,14–16]. Regardless, while physiological performance in later life may strongly influence the adaptability of a population, early life-history processes such as fertilization, larval development and metamorphosis, are widely understood to be the most vulnerable of life-history stages and are the critical stage for species survival [16–19]. Most marine invertebrates reproduce via broadcast spawning, releasing gametes into the water column for fertilization [20]. Organisms with this mode of reproduction may be particularly susceptible to environmental change because their gametes are exposed to anthropogenic stressors present in the environment prior to fertilization. These stressors can damage or disrupt sperm performance and fertilization processes [21]. In addition, the stress history of the male can influence sperm performance through epigenetic modifications [22,23].
Recent modifications of classic sperm theories, including both theoretical and empirical data, have identified sperm phenotypic traits that are beneficial for fertilization [24–26]. For example, fast swimming speeds have been identified to increase the likelihood of sperm reaching an oocyte first, while longevity gives sperm a greater amount of time to locate an oocyte to fertilize [24,27]. Reported changes in sperm parameters in response to CO2-induced acidified seawater are typically negative and most studies identify a reduction in sperm swimming speeds and motility in response to OA [5,10,11,28,29]. By contrast, a few studies report either a neutral or stimulatory response [15,30]. There is no clear trend on the impacts of OA on fertilization success in marine invertebrates [8,10,31], despite sperm traits including high swimming velocity being linked to fertilization efficiency [27,29]. The differences between studies might in part be explained by inter-individual male variability in the response of sperm traits to OA within a population. By looking at individual responses, we may begin to tease apart how OA influences selection across marine invertebrates and achieve a greater level of clarity than has been accomplished so far through examination of population-level responses. A positive response by a subset of individuals in a population may have implications for species genotype profiles and resilience globally.
Here, we looked at the relative responses to OA in two representative broadcast spawning invertebrates, the sea urchins Lytechinus pictus and Heliocidaris erythrogramma, that have different fertilization ecologies, where selection acting on sperm traits may therefore differ. As typical of most sea urchins, Lytechinus pictus has small sperm with a conical head (approx. 3.6 µm length, 1.3 µm diameter), small (approx. 100 µm diameter), negatively buoyant eggs and a feeding larva [32,33]. By contrast, Heliocidaris erythrogramma has large sperm with an elongated head (approx. 10.0 µm length, 2.0 µm diameter), large (approx. 400 µm diameter) positively buoyant eggs and a non-feeding larva [33,34]. Sea urchins are well-established model organisms for investigation of fertilization processes and the impacts of anthropogenic stressors [31]. We determined the level of variability of individual male reproductive performance (individual ejaculate traits and fertilization success) in response to OA conditions under non-competitive scenarios. Understanding how OA affects sperm function and fertilization success on an individual basis is required to build a better understanding of the potential that existing phenotypic variation in sperm performance traits will influence how marine species will respond globally.
2. Material and methods
(a). Gamete collection and seawater manipulation
Lytechinus pictus were provided by Dunmanus Seafoods Ltd, Ireland, and Heliocidaris erythrogramma were collected from Sydney Harbour, Australia (see electronic supplementary material for details on animal collection). Gametes were obtained from both species using standard methods of injecting 0.5 mol KCl through the peristome [35]. Sperm were collected separately from each male ‘dry' using a pipette and maintained on ice prior to use. Eggs were collected in filtered seawater FSW (L. pictus, 15°C, salinity 35; H. erythrogramma, 20°C, salinity 35). Temperatures reflected the ambient temperature at collection for each species and were maintained for all subsequent work. In total, sperm was collected from 20 male L. pictus and 16 male H. erythrogramma. Eggs were collected from 10 females from each species. All experiments were conducted directly after spawning at the maintenance temperature ± 1°C. Experimental seawater was either aerated to simulate current ocean conditions (mean ± s.e., pHNBS 8.07 ± 0.02, 506 ± 39 µatm pCO2), or received an input of CO2 pre-mixed with ambient air to achieve OA conditions (mean ± s.e., pHNBS 7.90 ± 0.02, 792 ± 20 µatm pCO2 and pHNBS 7.75 ± 0.01, 1170 ± 42 µatm pCO2). The CO2-air pre-mix was added via mass-flow controlled systems. Seawater samples were collected prior to experiments for assessment of carbonate chemistry (electronic supplementary material, table S1).
(b). Sperm functional analysis
Sperm functional characteristics were analysed by computer-assisted sperm analysis (CASA) using Microptic Sperm Class Analyser and using ImageJ software [36] with a CASA plugin [37] (electronic supplementary material). Three subsets of sperm were examined from each individual 10 min after activation in seawater. A minimum of 250 individual sperm were tracked per male for 0.5 s during analysis. Measurements of sperm concentration, proportion of motile sperm (MOT) and six CASA parameters were used for further analysis. The CASA parameters included three measures of sperm velocity (curvilinear velocity [VCL], straight-line velocity [VSL] and average-path velocity [VAP]), two measures of sperm path (linearity [LIN; VSL/VCL] and straightness [STR; VSL/VAP]), and a measure of side-to-side sperm head movement (WOB; VAP/VCL). Immotile sperm were defined as sperm swimming below threshold values of 10 µm s−1 VCL and 3.2 µm s−1 VAP [38]. The data for each CASA parameter were further split by quartiles (low = 0–25th, medium = 25–75th and high = 75–100th) and corrected for motility to determine the distribution of total sperm across these categories for each male. For every CASA parameter, the percentiles were determined for each species using the data from all males under current conditions.
(c). Fertilization success
Fertilizations were conducted using sperm from individual males and eggs pooled from 10 females. The individual male-pooled female design provided a ‘common' fertilization environment for the males thereby reducing variability caused by influential gamete incompatibility and increasing the likelihood of accurately identifying inter-individual trait variability in male responses to OA [39–40]. We note that this design does not consider the competitive fertilization scenario that can influence individual reproductive performance [24,25].
The eggs and sperm of H. erythrogramma are both considerably larger than those of L. pictus. Consequently, we used different oocyte-sperm concentrations for our fertilizations, selected to avoid either polyspermy or sperm limitation and achieve an average fertilization success rate of 75–80% under current conditions (10 000 eggs and a sperm concentration of 1 × 105 for L. pictus [28] and 350 eggs and a sperm concentration of 1 × 104 for H. erythrogramma [29]). Sperm from a single male was activated in the seawater treatment, mixed, then immediately pipetted into the centre of each well and gently mixed in a Z motion (n = 3 wells per male). Following controlled fertilizations where sperm were given 10 min to fertilize eggs, 100 eggs from each well were examined and the proportion of fertilized eggs determined. See electronic supplementary material for further details.
(d). Statistical analysis and modelling
Nested ANOVAs (analysis of variance) were used to compare fertilization success and sperm functional parameters across conditions and across males within each condition (parameter ∼ condition (male)). Where necessary, data were transformed prior to analysis to ensure homoscedasticity. Sperm functional parameters from each male were also compared to fertilization success under each condition using Pearson's correlation. For L. pictus, 20 males were included in all analyses. For H. erythrogramma, 16 males were included in fertilization success analyses but only 14 males were included in sperm parameter analyses due to problems with CASA video analysis under current conditions. For multiple independent tests, Bonferroni correction was used to maintain an overall type I error rate, α, of 0.05.
The influence of sperm parameters on fertilization success under current conditions and separately under OA were explored using linear models. Models were built using the following basic structure:
Twenty-six sperm functional parameters were included in different combinations (electronic supplementary material). Sperm parameters were added, substituted and removed to obtain the lowest Akaike information criterion (AIC) score. AIC values within 2.0 of each other were considered to be of equivalent statistical power. Beyond this, models were further optimized using the highest adjusted R2 and the highest number of degrees of freedom. Once the best-fitting model had been selected the model residuals were confirmed. Individual data points sitting outside 0.5 Cook's distance were examined and removed if deemed highly influential.
All statistical analyses were conducted in RStudio v. 1.1.423 [41] using the package ‘stats'. Figures were produced using GraphPad Prism v. 7.03 for Windows (GraphPad Software, La Jolla, CA, USA, www.graphpad.com).
3. Results
(a). Sperm functional analysis
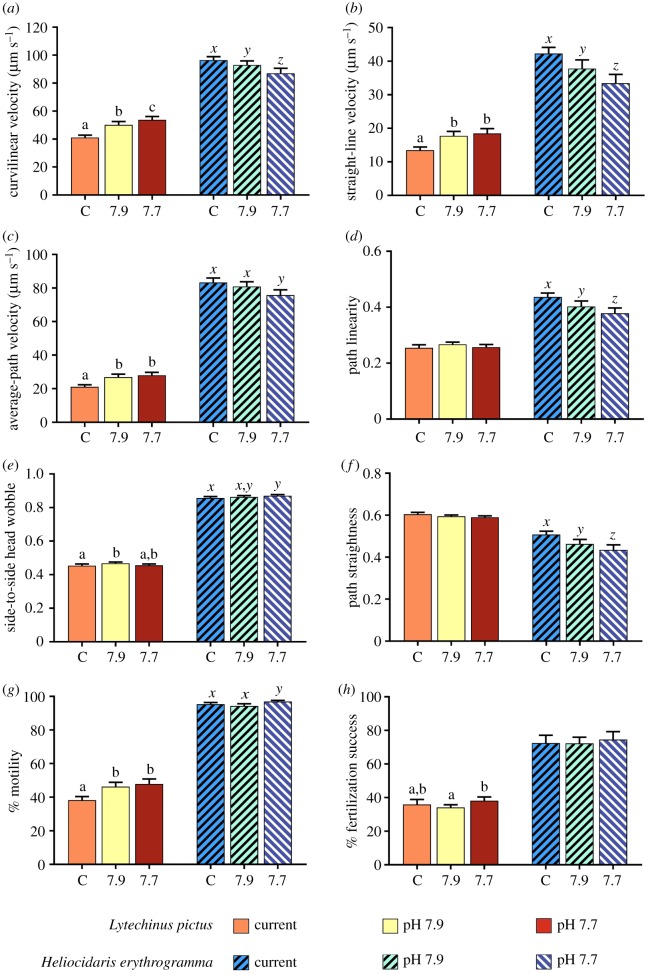
When looking at average group responses for each species, L. pictus sperm increased significantly in velocity between current and OA conditions (VCL, from 40.50 to 52.95 µm s−1; VSL, from 13.12 to 18.00 µm s−1; VAP, from 20.60 to 27.51 µm s−1) but H. erythrogramma sperm instead decreased significantly in velocity (VCL, from 95.35 to 86.46 µm s−1; VSL, from 41.79 to 33.30 µm s−1; VAP, from 82.04 to 75.22 µm s−1; table 1 and figure 1a–c). OA did not affect average sperm LIN, WOB, or STR in L. pictus. There was also no impact of OA on sperm WOB in H. erythrogramma, but there was a significant decrease in both LIN (0.44 to 0.38) and STR (0.51 to 0.43) with increasing OA (table 1 and figure 1d–f). MOT was significantly affected by OA in both species (table 1 and figure 1g); an increase in sperm MOT in response to OA conditions was observed in L. pictus (from 38.24% under current conditions to 47.88% at pH 7.70) whereas in H. erythrogramma, MOT decreased at pH 7.90 but then increased slightly again at pH 7.70 (95.39% under current conditions, 94.28% at pH 7.90, 96.95% at pH 7.70). For summary details of all sperm functional parameters, see electronic supplementary material, table S2.
Table 1.
Results of nested analysis of variance (sperm parameter ∼ condition (male)) examining sperm parameters in two species of echinoderm. The adjusted α following Bonferroni corrections was p = 0.007 for all tests. Significant p-values below the adjusted α are in italics.
| species |
Lytechinus pictus |
Heliocidaris erythrogramma |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| response variable | condition |
condition (male) |
condition |
condition (male) |
||||||||
| sperm parameter | F | d.f. | p-value | F | d.f. | p-value | F | d.f. | p-value | F | d.f. | p-value |
| curvilinear velocity | 101.56 | 2 | <0.001 | 11.15 | 57 | <0.001 | 35.60 | 2 | <0.001 | 13.63 | 39 | <0.001 |
| straight-line velocity | 67.49 | 2 | <0.001 | 14.14 | 57 | <0.001 | 62.67 | 2 | <0.001 | 17.88 | 39 | <0.001 |
| average-path velocity | 86.56 | 2 | <0.001 | 14.18 | 57 | <0.001 | 21.15 | 2 | <0.001 | 11.11 | 39 | <0.001 |
| path linearity | 2.76 | 2 | 0.067 | 3.42 | 57 | <0.001 | 32.01 | 2 | <0.001 | 11.81 | 39 | <0.001 |
| side-to-side head wobble | 4.27 | 2 | 0.016 | 4.11 | 57 | <0.001 | 3.84 | 2 | 0.025 | 4.61 | 39 | <0.001 |
| path straightness | 1.07 | 2 | 0.346 | 1.59 | 57 | 0.018 | 39.76 | 2 | <0.001 | 12.87 | 39 | <0.001 |
| motility | 41.96 | 2 | <0.001 | 7.92 | 57 | <0.001 | 7.59 | 2 | <0.001 | 5.34 | 39 | <0.001 |
Figure 1.
(a–g) Ejaculate traits and (h) fertilization success in Lytechinus pictus and Heliocidaris erythrogramma. Different letters above each trio of bars indicate a significant difference between treatments (p ≤ 0.05). (Online version in colour.)
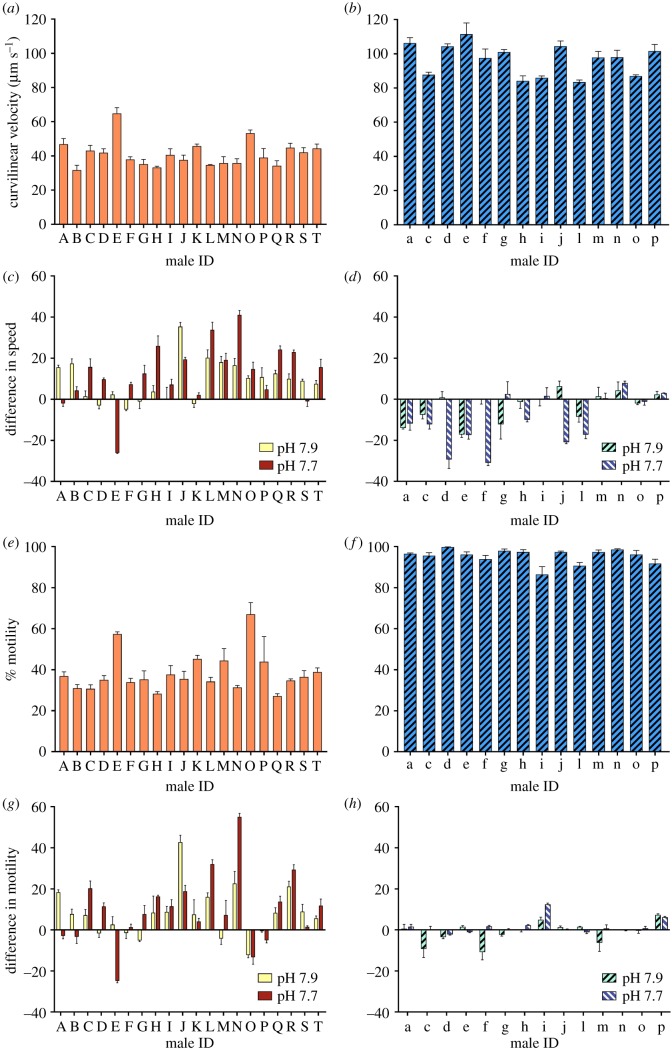
In both species, the average VCL, VSL, VAP, LIN, WOB and MOT within the ejaculate for each individual differed significantly between males within each condition. Sperm STR also differed significantly between males within each condition in H. erythrogramma but not in L. pictus (table 1). Within a condition, variability between males increased with increasing OA in VCL, VSL and VAP in both species, in MOT in L. pictus and in LIN and STR in H. erythrogramma. Variability in LIN, WOB and STR between males decreased with increasing OA in L. pictus. In H. erythrogramma variability in MOT increased between current conditions and pH 7.90, before decreasing to pH 7.70. Variability in WOB remained similar across condition in this species (electronic supplementary material, table S2). Under current conditions, average ejaculate VCL ranged from 31.8 to 64.9 µm s−1 across all individual L. pictus (figure 2a). Under OA conditions, VCL changed by between −13.20 and +93.90% at pH 7.90 (per individual relative to current conditions; average 23.80% increase) and between −40.20 and +114.70% at pH 7.70 (average 34.80) across individuals (figure 2c). In H. erythrogramma, VCL ranged from 83.00 to 107.39 µm s−1 (average 96.09 µm s−1) across all individuals under current conditions (figure 2b). Under OA conditions, VCL changed by between −14.42 and +7.28% at pH 7.90 (average −3.60%) and by between −31.23 and +8.77 at pH 7.70 (average −9.78%) across individuals (figure 2d). Under current conditions, MOT ranged from 27.1 to 67.0% (average 38.2%) in L. pictus (figure 2e) and from 87.33 to 99.86% (average 95.31%) in H. erythrogramma (figure 2f). Relative to current conditions, MOT changed by between −18.30 and +120.70% across individuals at pH 7.90 (average 24.80%) and by between −43.30 and +175.80% at pH 7.70 (average 31.80%) in L. pictus (figure 2g). In H. erythrogramma, MOT changed by between −12.28 and +8.57% across individuals at pH 7.90 (average −1.19%) and by between −2.20 and +13.23% at pH 7.70 (average 1.77%) relative to current conditions (figure 2h).
Figure 2.
Ejaculate traits of Lytechinus pictus and Heliocidaris erythrogramma. (a,b) Sperm curvilinear velocity under current conditions; (c,d) difference in curvilinear velocity between current and OA conditions; (e,f) sperm motility under current conditions; (g,h) difference in motility between current and OA conditions. Different letters below each plot indicate ejaculate from individual males. (Online version in colour.)
(b). Fertilization success
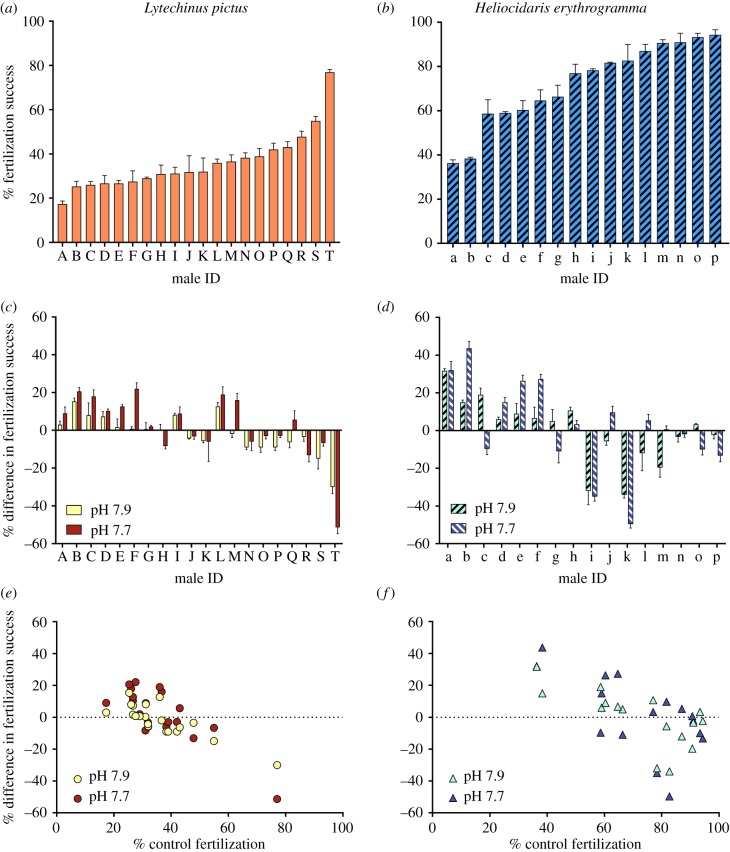
When looking at average group responses for each species, in L. pictus there was a small but significant change in fertilization success with OA (ANOVA: F = 5.73, d.f. = 2, p = 0.004). Average fertilization success decreased significantly between current conditions and pH 7.90 (from 35.97% to 34.18%), and then increased again at pH 7.70 (38.15%). Fertilization success at pH 7.70 was not significantly different to either of the other treatments (figure 1h). By contrast, OA did not affect average fertilization success in H. erythrogramma (ANOVA: F = 1.85, d.f. = 2, p = 0.164). However, for each species and within each condition, fertilization success varied significantly between males (ANOVA: L. pictus, F = 8.12, d.f. = 57, p < 0.001; H. erythrogramma, F = 15.83, d.f. = 39, p < 0.001). Under current conditions, fertilization success ranged from 17.33% to 77.00% in L. pictus (figure 3a) and from 36.33% to 94.33% in H. erythrogramma (figure 3b). Under OA, fertilization success changed by between −30.00% and +15.33% at pH 7.90 and between −51.33% and +22.05% at pH 7.70 across individuals in L. pictus (figure 3c). In H. erythrogramma, fertilization success changed by between −34.00% and +31.67% at pH 7.90 and between −49.67% and +43.67% at pH 7.70 (figure 3d). The lower-than-expected fertilization success in L. pictus was probably because this species has smaller eggs and sperm than Mesocentrotus franciscanus, on which the experimental ratios were based [28].
Figure 3.
Fertilization success under different experimental conditions. (a,b) Fertilization success under current conditions, (c,d) difference in fertilization success between current and acidified conditions and (e,f) relationship between current fertilization and difference in fertilization under acidified conditions in Lytechinus pictus and Heliocidaris erythrogramma. Different letters below plots (a–d) indicate individual males. (Online version in colour.)
In both species, males with low fertilization success under current conditions showed improved fertilization under OA, whereas the males with high fertilization success under current conditions worsened under OA (figure 3a–d). Spearman correlation revealed a strong relationship between fertilization under current conditions and the change in fertilization under OA for both L. pictus (pH 7.9: rs = −0.809, d.f. = 18, p < 0.001; pH 7.7: rs = −0.656, d.f. = 18, p < 0.001; figure 3e) and H. erythrogramma (pH 7.9: rs = −0.741, d.f. = 14, p < 0.001; pH 7.7: rs = −0.618, d.f. = 14, p = 0.006; figure 3f). The changes in fertilization with OA led to an overall shift in individual rankings with regard to fertilization success (electronic supplementary material, figure S1). For each species, the observed changes in fertilization success typically scaled with increasing pH: individuals that improved under pH 7.9 improved further under pH 7.7, and vice versa for those that worsened (figure 3c,d). In L. pictus, the change in fertilization success relative to current fertilization ranged from +60.53% to −38.96% at pH 7.9 and +81.58% to −66.67% at pH 7.7. In H. erythrogramma, the observed changes ranged from +87.16% to −41.13% at pH 7.9 and +113.91% to −60.08% at pH 7.7. See electronic supplementary material, table S2 for summary data.
(c). Sperm functional analysis and fertilization success
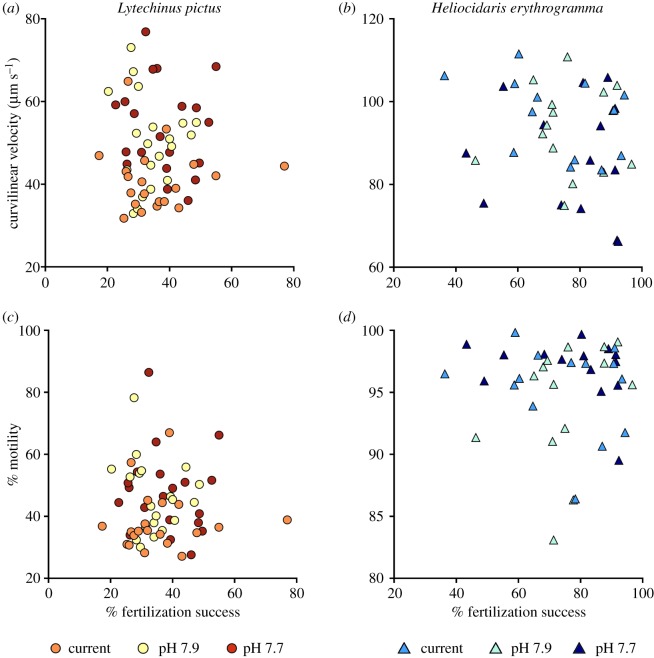
Pearson's correlation indicated no relationship between fertilization success and any measurement of sperm function (VCL, VSL, VAP, LIN, WOB, STR, MOT) for either species under current conditions, following Bonferroni adjustments (electronic supplementary material, table S3). There were also no relationships observed under OA conditions. The relationships between fertilization success and VCL, and fertilization success and MOT are displayed in figure 4.
Figure 4.
Relationship between fertilization success and key sperm parameters. (a,b) Fertilization success and curvilinear velocity, and (c,d) fertilization success and motility in Lytechinus pictus and Heliocidaris erythrogramma. (Online version in colour.)
The sperm functional parameters contributing to the ‘best-fitting' linear model explaining fertilization success under current conditions varied between the two species. For L. pictus, the ‘best-fitting' LM explained 76.97% of the observed variation in fertilization success under current conditions (F = 8.94, d.f. = 11, adjR2 = 0.7697, p < 0.001, AIC = 79.32). Based on the resulting β values, the most important sperm functional parameters for predicting fertilization success were low-to-medium swimming speed (VCL and VAP), medium path linearity and side-to-side head wobble, and low path straightness. For H. erythrogramma, the ‘best-fitting' LM explained 93.38% of the observed variation in fertilization success under current conditions (F = 31.56, d.f. = 7, adjR2 = 0.9338, p < 0.001, AIC = 45.85). Based on the resulting β values, the most important sperm functional parameters for predicting fertilization success in H. erythrogramma were fast swimming speed (VCL) and a straight path (see electronic supplementary material, table S4 for model terms, p and β values).
Under OA conditions, the sperm functional parameters contributing to the ‘best-fitting' LM explaining fertilization success shifted for both species. For L. pictus, the ‘best-fitting' LM explained 66.20% of the fertilization success under experimental conditions (F = 5.830, d.f. = 22, adjR2 = 0.662, p < 0.001, AIC = 134.94). Based on the resulting β values, the most important sperm functional parameters for predicting fertilization success in L. pictus under OA were fast swimming speed, low path straightness and medium-to-high side-to-side head wobble. For H. erythrogramma, the ‘best-fitting' LM explained 84.52% of the change in fertilization success between current and experimental conditions (F = 11.50, d.f. = 12, adjR2 = 0.845, p < 0.001, AIC = 100.89). Based on the resulting β values, the most important sperm functional parameters for predicting fertilization success under OA conditions in H. erythrogramma were medium-to-high swimming speed and low path linearity (see electronic supplementary material, table S5 for model terms, p and β values).
4. Discussion
OA is predicted to be a significant driver of global ocean biodiversity change this century, with species-specific ‘winners' and ‘losers' already being estimated based on population-level sensitivities [1,42]. Our data clearly illustrate that under non-competitive scenarios, within a species both increases and decreases in male performance can occur based on the response of sperm within a single ejaculate, with some individuals performing better under OA conditions. Importantly, we found an inverse relationship between male reproductive performance (measured as fertilization success) under current ocean conditions and change in reproductive performance under future ocean conditions. The males who performed the best under current conditions typically worsened under OA, while those who were the least successful under current conditions generally improved under OA. The same trend was evident in both L. pictus and H. erythrogramma despite the differences in their fertilization ecology. Interestingly, although to our knowledge this relationship has not previously been described, we re-examined data from the only comparable study we could find that reported data for individual males fertilized with multiple females [30] (electronic supplementary material, table S6). We identified a similar inverse relationship for males under OA in Crassostrea gigas, where following initial non-competitive fertilization success ranging from 29% to 92%, fertilization success changed by between −11% and +9% under OA (pH 7.80) conditions (electronic supplementary material, figure S2) [30].
Collectively these observed trends in fertilization success indicate the optimum pH for sperm performance varies on a species and individual male level. The differing sperm responses to pH and pCO2 may be linked to a number of biochemical and/or physiological mechanisms. For example, activation of sperm swimming involves numerous enzymes all of which have optimal pH values [43] that may differ between both individuals and species. Sperm nuclear proteins, the differing ratios of which are known to influence sperm shape and motility and hence fertility [26], may also vary between individuals. Additionally, sperm traits can be modified by epigenetic mechanisms during their development which can also influence sperm performance [22].
The relationship between average sperm swimming performance and fertilization success has been relatively well explored for broadcast spawners [24,25,35,44], but it has rarely been examined in response to OA (but see [28,29]). A change in sperm swimming performance may alter its oocyte searching efficiency, directly influencing fertilization rates. Sperm parameters including motility, velocity, length and longevity have previously been identified to partially influence fertilization success in echinoderms and other phyla [15,27,35,39,44], but we found no relationship between any single sperm functional parameter and fertilization success under the non-competitive (single male) scenarios studied here. Instead, we found that fertilization was best predicted by a range of sperm parameters that varied between both species and conditions. Under current conditions, low-to-medium speeds and low path linearity were important for predicting fertilization success in L. pictus whereas high speed and high path linearity were important in H. erythrogramma. Under OA conditions, a straighter, faster sperm path was more important for predicting fertilization success in L. pictus. Conversely, a slower, less linear path was more important in H. erythrogramma. The shifts in important parameters mirrored the observed changes in sperm functional parameters; sperm swimming speeds typically increased in L. pictus individuals in response to increasing OA but decreased in H. erythrogramma. Similar contrasting shifts in sperm parameters have been reported for two species of Helicidaris in response to OA [34], decreases in sperm velocity and path straightness were observed in H. erythrogramma, whereas in H. tuberculata, which has similar life-history mode to L. pictus (small eggs and planktotrophic larvae), sperm velocity and path linearity increased.
The observed differences in the most beneficial sperm functional traits for fertilization under current conditions between the two species studied here may be partially explained by their different reproductive ecologies. Fast swimming speeds are likely to be most beneficial for H. erythrogramma sperm which must actively swim upwards through the water column to reach the large, buoyant eggs of this species. Conversely, slower speeds may be more evolutionarily beneficial for L. pictus if they enable sperm longer periods to search for their small eggs [20,35,39]. Considering this, OA appears to reduce the influence of the most beneficial sperm functional trait for fertilization in both species (i.e. by increasing swimming speeds in L. pictus but reducing them in H. erythrogramma). Fertilization success is also influenced by other factors including sperm chemotaxis and egg penetration. For both species it is likely that some sperm were placed closer to an egg than others due to the mixing action and may have resulted in some egg–sperm contact independent of sperm swimming parameters. However, mixing is standard in fertilization studies and the methods were the same for each species.
The individual variability in sperm activity and fertilization success observed here is not surprising; inter-individual variability in sperm traits is an inherent feature of male ejaculate in broadcast spawning marine invertebrates [6,7,27,31,35]. Thus, the variability of sperm responses under OA integrates with a standing phenotypic variation and has been reported several times in response to OA [5,28–30]. Consequently, inherent variability may also help explain the conflicting data reported in previous studies examining population-level responses to OA [7–11,31]. The results of these studies are often interpreted to indicate population-level losses or gains under future ocean conditions, depending on whether the observed response is negative or positive. Our results instead highlight the importance of recognizing inter-individual variability; while the ‘average' impact of OA clearly varies species by species, individual variability within a species may drive selection, indicating a greater level of species resilience than is often interpreted. It is important to note that our experimental focus was to determine outcomes for individual males and that their performance might differ under multiple male competitive fertilization scenarios.
The variability in sperm traits identified across individuals in the present study is likely to be related to environmental heterogeneity; both L. pictus and H. erythrogramma inhabit coastal habitats where environmental variability is high and can change significantly over short spatial and temporal periods [45,46]. Individuals that are adapted to higher levels of environmental variability exhibit greater plasticity and physiological tolerances than those adapted to narrow variable regimes [47]. Environmental conditions both pre- and post-gamete release can impact sperm phenotype, with different sperm characteristics favoured under different conditions (for review see [22]). The degree to which individuals are affected may be related to genotype-by-environment interactions although further analysis is required to determine this [26,45,48,49]. Individuals and populations of the same species can also exhibit varying responses to an environmental stressor (e.g. OA, temperature, salinity), and compounding or alleviating effects of multiple stressors vary case-by-case [15,48,50]. By increasing variability in sperm traits, individuals are more likely to have some sperm that fertilize successfully across the environmental conditions they are exposed to.
Understanding individual variability within populations is clearly of great importance for predicting species resilience. A vast proportion of marine invertebrates reproduce via broadcast spawning, including many ecologically and economically important species. Interpreting how OA will impact these species is of utmost importance, and it is clear from our results that key trends in individual resilience may not be identified using a population-level approach. If the sperm functional traits that are important for fertilization success are heritable for H. erythrogramma and L. pictus, and the competitiveness of individual males also shifts with OA (as shown previously [28]), the contributions by each male to the population might shift as their fertilization capacity changes. However, the overall population numbers may not change significantly. It remains to be seen whether the same trend is evident across other species. Our results highlight the importance of examining responses to environmental change at an individual level when interpreting how future ocean conditions will affect populations of marine invertebrates; such knowledge is fundamental for predicting biodiversity change across the shifting OA landscape globally.
Supplementary Material
Acknowledgements
We thank the teams at the University of Exeter, the University of Sydney and Sydney Institute of Marine Sciences for support. In particular we thank Sergio Torres Gabarda. Heliocidaris erythrogramma were collected under permit (NSW DPI: P00/0015-6.0).
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
K.E.S., C.L. and M.B. designed the research, K.E.S., D.D., C.M.H., C.N. and A.W.-M. carried out the research, K.E.S. analysed data and all authors contributed to the final draft of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by the European Union's Horizon 2020 research and innovation programme under the Marie-Skłodowska-Curie grant agreement no. 704895 to K.E.S., travel grants from Marie Curie Alumni Association and the Company of Biologists (Journal of Experimental Biology, grant no. JEBTF-170815) to K.E.S. and the NSW Environmental Trust 2016RD0159 to M.B.
References
- 1.Doney SC, et al. 2011. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 4, 11–37. ( 10.1146/annurev-marine-041911-111611) [DOI] [PubMed] [Google Scholar]
- 2.Bopp L, et al. 2013. Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosci. 10, 6225–6245. ( 10.5194/bg-10-6225-2013) [DOI] [Google Scholar]
- 3.IPCC. 2014. Climate change 2014: synthesis report. Contribution of Working Groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change (eds Pachauri RK, Meyer LA). Geneva, Switzerland: IPCC. [Google Scholar]
- 4.Kwiatkowski L, Orr JC. 2018. Diverging seasonal extremes for ocean acidification during the twenty-first century. Nat. Clim. Change 8, 141 ( 10.1038/s41558-017-0054-0) [DOI] [Google Scholar]
- 5.Vihtakari M, Havenhand J, Renaud PE, Hendriks IE. 2016. Variable individual- and population-level responses to ocean acidification. Front. Mar. Sci. 3, 51 ( 10.3389/fmars.2016.00051) [DOI] [Google Scholar]
- 6.Pitnick S, Hosken DJ, Birkhead TR. 2009. Sperm morphological diversity. In Sperm biology: an evolutionary perspective (eds Birkhead TR, Hosken DJ, S Pitnick), pp. 69–149. London, UK: Academic Press. [Google Scholar]
- 7.Ward PI. 1998. Intraspecific variation in sperm size characters. Heredity 80, 655–659. ( 10.1046/j.1365-2540.1998.00401.x) [DOI] [PubMed] [Google Scholar]
- 8.Byrne M, Soars NA, Ho MA, Wong E, McElroy D, Selvakumaraswamy P, Dworjanyn SA, Davis AR. 2010. Fertilization in a suite of coastal marine invertebrates from SE Australia is robust to near-future ocean warming and acidification. Mar. Biol. 157, 2061–2069. ( 10.1007/s00227-010-1474-9) [DOI] [Google Scholar]
- 9.Byrne M, Soars N, Selvakumaraswamy P, Dworjanyn SA, Davis AR. 2010. Sea urchin fertilization in a warm, acidified and high pCO2 ocean across a range of sperm densities. Mar. Envviron. Res. 69, 234–239. ( 10.1016/j.marenvres.2009.10.014) [DOI] [PubMed] [Google Scholar]
- 10.Havenhand JN, Buttler FR, Thorndyke MC, Williamson JE. 2008. Near-future levels of ocean acidification reduce fertilization success in a sea urchin. Curr. Biol. 18, R651–R652. ( 10.1016/j.cub.2008.06.015) [DOI] [PubMed] [Google Scholar]
- 11.Morita M, Suwa R, Iguchi A, Nakamura M, Shimada K, Sakai K, Suzuki A. 2010. Ocean acidification reduces sperm flagellar motility in broadcast spawning reef invertebrates. Zygote 18, 103–107. ( 10.1017/S0967199409990177) [DOI] [PubMed] [Google Scholar]
- 12.Marshall DJ, Burgess SC, Connallon T. 2016. Global change, life-history complexity and the potential for evolutionary rescue. Evol. Appl. 9, 1189–1201. ( 10.1111/eva.12396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Przeslawski R, Byrne M, Mellin C. 2015. A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob. Change Biol. 21, 2122–2140. ( 10.1111/gcb.12833) [DOI] [PubMed] [Google Scholar]
- 14.Byrne M, Selvakumaraswamy P, Ho MA, Woolsey E, Nguyen HD. 2011. Sea urchin development in a global change hotspot, potential for southerly migration of thermotolerant propagules. Deep Sea Res. Part II 58, 712–719. ( 10.1016/j.dsr2.2010.06.010) [DOI] [Google Scholar]
- 15.Falkenberg LJ, Styan CA, Havenhand JN. 2019. Sperm motility of oysters from distinct populations differs in response to ocean acidification and freshening. Sci. Rep. 9, 7970 ( 10.1038/s41598-019-44321-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroeker KJ, Kordas RL, Crim RN, Singh GG. 2010. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 13, 1419–1434. ( 10.1111/j.1461-0248.2010.01518.x) [DOI] [PubMed] [Google Scholar]
- 17.Bögner D. 2016. Life under climate change scenarios: sea urchins' cellular mechanisms for reproductive success. J. Mar. Sci. Eng. 4, 28 ( 10.3390/jmse4010028) [DOI] [Google Scholar]
- 18.Kurihara H. 2008. Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar. Ecol. Prog. Ser. 373, 275–284. ( 10.3354/meps07802) [DOI] [Google Scholar]
- 19.Scanes E, Parker LM, O'Connor WA, Ross PM. 2014. Mixed effects of elevated pCO2 on fertilisation, larval and juvenile development and adult responses in the mobile subtidal scallop Mimachlamys asperrima (Lamarck, 1819). PLoS ONE 9, e93649 ( 10.1371/journal.pone.0093649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levitan DR. 1998. Sperm limitation, gamete competition, and sexual selection in external fertilizers. In Sperm competition and sexual selection (eds Birkhead TR, Møller AP), pp. 173–215. London, UK: Academic Press. [Google Scholar]
- 21.Lotterhos KE, Levitan DR. 2010. Gamete release and spawning behavior in broadcast spawning marine invertebrates. The evolution of primary sexual characters in animals (eds Leonard J, Cordoba-Aguilar A), pp. 99–120. Oxford, UK: Oxford University Press. [Google Scholar]
- 22.Marshall DJ. 2015. Environmentally induced (co) variance in sperm and offspring phenotypes as a source of epigenetic effects. J. Exp. Biol. 218, 107–113. ( 10.1242/jeb.106427) [DOI] [PubMed] [Google Scholar]
- 23.Eirín-López JM, Putnam HM. 2018. Marine environmental epigenetics. Annu. Rev. Mar. Sci. 11, 7.1–7.34. [DOI] [PubMed] [Google Scholar]
- 24.Snook RR. 2005. Sperm in competition: not playing by the numbers. Trends Ecol. Evol. 20, 46–53. ( 10.1016/j.tree.2004.10.011) [DOI] [PubMed] [Google Scholar]
- 25.Ball MA, Parker GA. 1996. Sperm competition games: external fertilization and ‘adaptive’ infertility. J. Theor. Biol. 180, 141–150. ( 10.1006/jtbi.1996.0090) [DOI] [PubMed] [Google Scholar]
- 26.Ausió J, Eirín-López JM, Frehlick LJ. 2007. Evolution of vertebrate chromosomal sperm proteins: implications for fertility and sperm competition. Soc. Reprod. Fertil. Suppl. 65, 63–79. [PubMed] [Google Scholar]
- 27.Gage MJG, Macfarlane CP, Yeates S, Ward RG, Searle JB, Parker GA. 2004. Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 14, 44–47. ( 10.1016/s0960-9822(03)00939-4) [DOI] [PubMed] [Google Scholar]
- 28.Campbell AL, Levitan DR, Hosken DJ, Lewis C. 2016. Ocean acidification changes the male fitness landscape. Sci. Rep. 6, 31250 ( 10.1038/srep31250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlegel P, Havenhand JN, Gillings MR, Williamson JE. 2012. Individual variability in reproductive success determines winners and losers under ocean acidification: a case study with sea urchins. PLoS ONE 7, e53118 ( 10.1371/journal.pone.0053118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Havenhand JN, Schlegel P. 2009. Near-future levels of ocean acidification do not affect sperm motility and fertilization kinetics in the oyster Crassostrea gigas. Biogeoscience 6, 3009–3015. ( 10.5194/bg-6-3009-2009) [DOI] [Google Scholar]
- 31.Byrne M. 2012. Global change ecotoxicology: identification of early life history bottlenecks in marine invertebrates, variable species responses and variable experimental approaches. Mar. Environ. Res. 76, 3–15. ( 10.1016/j.marenvres.2011.10.004) [DOI] [PubMed] [Google Scholar]
- 32.Raff EC, Villinski JT, Turner FR, Donoghue PC, Raff RA. 2006. Experimental taphonomy shows the feasibility of fossil embryos. Proc. Natl Acad. Sci. USA 103, 5846–5851. ( 10.1073/pnas.0601536103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raff RA, Herlands L, Morris VB, Healy J. 1990. Evolutionary modification of echinoid sperm correlates with developmental mode. Develop. Growth Differ. 32, 283–291. ( 10.1111/j.1440-169X.1990.00283.x) [DOI] [PubMed] [Google Scholar]
- 34.Foo SA, Deaker D, Byrne M. 2018. Cherchez la femme-impact of ocean acidification on the egg jelly coat and attractants for sperm. J. Exp. Biol. 221, jeb-177188 ( 10.1242/jeb.177188) [DOI] [PubMed] [Google Scholar]
- 35.Levitan DR. 2000. Sperm velocity and longevity trade off each other and influence fertilization in the sea urchin Lytechinus variegatus. Proc. R. Soc. B 267, 531–534. ( 10.1098/rspb.2000.1032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis, Nat . Methods 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson-Leedy JG, Ingermann RL. 2006. Development of a novel CASA system based on open source software for characterization of zebrafish sperm motility parameters. Theriogenology 67, 661–672. ( 10.1016/j.theriogenology.2006.10.003) [DOI] [PubMed] [Google Scholar]
- 38.Campbell AL, Mangan S, Ellis RP, Lewis C. 2014. Ocean acidification increases copper toxicity to the early life history stages of the polychaete Arenicola marina in artificial seawater. Environ. Sci. Technol. 48, 9745–9753. ( 10.1021/es502739m) [DOI] [PubMed] [Google Scholar]
- 39.Fitzpatrick JL, Simmons LW, Evans JP. 2012. Complex patterns of multivariate selection on the ejaculate of a broadcast spawning marine invertebrate. Evolution 66, 2451–2460. ( 10.1111/j.1558-5646.2012.01627.x) [DOI] [PubMed] [Google Scholar]
- 40.Kekäläinen J, Evans JP. 2018. Gamete-mediated mate choice: towards a more inclusive view of sexual selection. Proc. R. Soc. B 285, 20180836 ( 10.1098/rspb.2018.0836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 42.Fabricius KE, et al. 2011. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Change 1, 165 ( 10.1038/nclimate1122) [DOI] [Google Scholar]
- 43.Beltrán C, Galindo BE, Rodríguez-Miranda E, Sánchez D. 2007. Signal transduction mechanisms regulating ion fluxes in the sea urchin sperm. Signal Transduct. 7, 103–117. ( 10.1002/sita.200600129) [DOI] [Google Scholar]
- 44.Fitzpatrick JL, Garcia-Gonzalez F, Evans JP. 2010. Linking sperm length and velocity: the importance of intramale variation. Biol. Lett. 6, 797–799. ( 10.1098/rsbl.2010.0231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frieder CA, Nam SH, Martz TR, Levin LA. 2012. High temporal and spatial variability of dissolved oxygen and pH in a nearshore California kelp forest. Biogeoscience 9, 3917–3930. ( 10.5194/bg-9-3917-2012) [DOI] [Google Scholar]
- 46.Runcie JW, Krause C, Torres Garbarda SA, Byrne M. 2018. Technical note: continuous fluorescence-based monitoring of seawater pH in situ. Biogeoscience 15, 4291–4299. ( 10.5194/bg-15-4291-2018) [DOI] [Google Scholar]
- 47.Kapsenberg L, Okamoto DK, Dutton JM, Hofmann GE. 2017. Sensitivity of sea urchin fertilization to pH varies across a natural pH mosaic. Ecol. Evol. 7, 1737–1750. ( 10.1002/ece3.2776) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eads AR, Kennington WJ, Evans JP. 2016. Interactive effects of ocean warming and acidification on sperm motility and fertilization in the mussel Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 562, 101–111. ( 10.3354/meps11944) [DOI] [Google Scholar]
- 49.Eirin-Lopez JM, Putnam HM. 2019. Marine environmental epigenetics. Annu. Rev. Mar. Sci. 11, 335–368. ( 10.1146/annurev-marine-010318-095114) [DOI] [PubMed] [Google Scholar]
- 50.Foo SA, Qworjanyn SA, Khatkar MS, Poore AGB, Byrne M. 2014. Increased temperature, but not acidification, enhances fertilization and development in a tropical urchin: potential for adaptation to a tropicalized eastern Australia. Evol. Appl. 7, 1226–1237. ( 10.1111/eva.12218) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.