Abstract
Parental care has evolved repeatedly and independently across animals. While the ecological and evolutionary significance of parental behaviour is well recognized, underlying mechanisms remain poorly understood. We took advantage of behavioural diversity across closely related species of South American poison frogs (Family Dendrobatidae) to identify neural correlates of parental behaviour shared across sexes and species. We characterized differences in neural induction, gene expression in active neurons and activity of specific neuronal types in three species with distinct care patterns: male uniparental, female uniparental and biparental. We identified the medial pallium and preoptic area as core brain regions associated with parental care, independent of sex and species. The identification of neurons active during parental care confirms a role for neuropeptides associated with care in other vertebrates as well as identifying novel candidates. Our work is the first to explore neural and molecular mechanisms of parental care in amphibians and highlights the potential for mechanistic studies in closely related but behaviourally variable species to help build a more complete understanding of how shared principles and species-specific diversity govern parental care and other social behaviour.
Keywords: parental care, poison frog, phosphoTRAP, preoptic area, hippocampus, galanin
1. Background
Parental care is an important adaptation that allows exploitation of novel habitats, influences fitness and survival of parents and offspring, and serves as an evolutionary precursor to other affiliative behaviour [1,2]. Specialized parental care strategies have evolved repeatedly and independently across animals, yet the mechanisms underlying parental behaviour and its evolution remain poorly understood. The neural mechanisms promoting parental care in females are best understood in mammals [3]. However, female uniparental care evolved at the base of the mammalian lineage and therefore provides limited clues to the evolutionary origins of parenting. Moreover, studies of male parental care come mostly from biparental systems [4,5] in which parental behaviour cannot easily be dissociated from pair bonding. What is needed to further understand the mechanisms underlying parental behaviour and its evolution are studies across closely related species that vary in care strategies.
Parental care can be conceptualized as a complex set of inter-related behaviours controlled by brain regions involved in the integration of sensory, social, motivational and cognitive aspects of care [6]. Across vertebrates, many of these functions are performed by the social decision-making network (SDMN) [7], a highly interconnected group of evolutionarily ancient and functionally conserved brain regions. Although studies on the neural mechanisms of parental behaviour are sparse outside mammals—and particularly lacking in amphibians and reptiles—the SDMN provides an ideal starting point for this work as network nodes and connectivity are well understood and highly conserved, and behaviourally important ligand/receptor complexes have been characterized [7,8].
Dendrobatid poison frogs show remarkable diversity in parental care across closely related species, including male uniparental, female uniparental and biparental care. Parental care in poison frogs generally involves egg attendance during embryo development, followed by transportation of tadpoles ‘piggyback’ to pools of water upon hatching [9–11]. In some species, mothers nourish growing tadpoles with unfertilized, trophic eggs until metamorphosis [10–12]. Importantly, both male and female care occurs with and without pair bonding in this clade [13], allowing the dissociation of pair bonding from parental care. The diversity of behavioural care strategies among poison frogs affords a unique opportunity to identify physiological, neural and molecular contributions to parental care across sexes of closely related species as well as across the convergent evolution of parental behaviour in all major vertebrate lineages.
In the current study, we take advantage of three focal species with distinct care strategies: Dendrobates tinctorius (male uniparental), Ranitomeya imitator (biparental and monogamous) and Oophaga sylvatica (female uniparental). By comparing neural activity in parental frogs and their non-caregiving reproductive partners, we identify core brain regions active during tadpole transport independent of sex and species. To identify neuronal types mediating tadpole transport, we characterize gene expression and activity patterns specifically in behaviourally relevant neurons within core brain regions. Our experiments are the first to explore neural and molecular mechanisms of parental care in amphibians and demonstrate the utility of mechanistic studies in closely related but behaviourally distinct species to identify core neural correlates of parental behaviour.
2. Methods
(a). Laboratory sample collection
Dendrobates tinctorius and R. imitator frogs were housed in breeding pairs in the laboratory, allowing us to identify both parental individuals and their non-caregiving reproductive partners. To control for effects of experience, all pairs successfully reared at least one clutch from egg-laying through tadpole transport prior to the experiment. For the non-parental group, we collected frog pairs between parental bouts when they were not caring for eggs or tadpoles, collecting individuals of both the caregiving sex (non-transport; n = 10 D. tinctorius, n = 7 R. imitator) and their opposite sex reproductive partners (non-transport partner; n = 9 D. tinctorius, n = 8 R. imitator). For the tadpole transport group, when we found transporting frogs, we collected both the transporting individual (tadpole transporter; n = 13 D. tinctorius, n = 7 R. imitator) and its opposite-sex, non-caregiving partner (transport partner; n = 11 D. tinctorius, n = 6 R. imitator). All brain tissue was collected in an identical manner: frogs were captured, anaesthetized with benzocaine gel, weighed and measured, and euthanized by rapid decapitation. This entire process took less than 5 min.
(b). Field sample collection
Oophaga sylvatica (Puerto Quito-Santo Domingo population) were collected in field enclosures in Ecuador in April and May 2016. We collected non-parental control females (n = 8) from enclosures containing only mature females to ensure that frogs were not currently caring for eggs or tadpoles. We collected tadpole transporting females (n = 5) from enclosures containing multiple males and females and therefore could not identify their non-caregiving male reproductive partners. Tissue was collected as described above.
(c). Immunohistochemistry
Whole brains were placed into 4% paraformaldehyde at 4°C overnight and then transferred to 30% sucrose for cryoprotection. Once dehydrated, brains were embedded in Tissue-Tek OCT Compound (Electron Microscopy Sciences, Hatfield, PA, USA), rapidly frozen and stored at −80°C until cryosectioning. We sectioned brains into four coronal series at 14 µm, allowed slides to dry completely and stored slides at −80°C.
To assess the level of neural activity across brain regions, we used an antibody for phosphorylated ribosomes (pS6; phospho-S6 Ser235/236; Cell Signaling, Danvers, MA, USA) and followed standard immunohistochemical procedures for 3′,3′-diaminobenzadine (DAB) antibody staining (as in [14]). To ask whether neural activity was higher specifically in galanin neurons, we combined the pS6 antibody with a custom-made galanin antibody (peptide sequence: CGWTLNSAGYLLGPHAVDNHRSFNDKHGLA; Pocono Rabbit Farm & Laboratory, Inc., Canadensis, PA, USA) and followed standard immunohistochemical procedures for fluorescent double labelling (as in [4]). Additional methodological details are in the electronic supplementary material.
(d). Microscopy and cell counts
Stained brain sections were photographed on a Leica DMRE connected to a QImaging Retiga 2000R camera at 20× magnification. We quantified labelled cells from photographs using FIJI image analysis software [15]. Brain regions were identified using a custom dendrobatid frog brain atlas (electronic supplementary material). We measured the area of candidate SDMN brain regions and counted all labelled cells in a single hemisphere for each brain region across multiple sections. We quantified cell number in the nucleus accumbens, the basolateral nucleus of the stria terminalis, the habenula, the lateral septum, the magnocellular preoptic area (POA), the medial pallium (Mp) (homologue of the mammalian hippocampus), the anterior POA, the suprachiasmatic nucleus, the striatum, the posterior tuberculum (homologue of the mammalian midbrain dopamine cells representing the ventral tegmental area and substantia nigra), the ventral hypothalamus and the ventral pallium.
Fluorescently stained brain sections were photographed at 20× magnification on a Leica DM4B compound microscope attached to a fluorescent light source. Each section was visualized at three wavelengths (594, 488, 358 nm) and images were pseudo-coloured to reflect these spectra. We used DAPI nuclear staining to identify brain regions as above and quantified the number of galanin-positive cells, pS6-positive cells and co-labelled cells from photographs of the POA using FIJI [15]. We combined counts for all POA subregions due to the low overall number of galanin-positive neurons and because this more closely reflected the neuroanatomical resolution of tissue punches used in PhosphoTRAP (see below).
(e). Statistical analyses of cell counts
We analysed the relationship between parental behaviour and pS6 neural activity to identify brain regions whose activity differed during tadpole transport independent of sex and species (i.e. core parental care brain regions). We used generalized linear mixed models with a negative binomial distribution appropriate for count data with unequal variances to test for differences in pS6-positive cell number. For laboratory animals, behavioural group (tadpole transport versus non-parental), sex, brain region and their interactions were included as main effects predicting the number of pS6-positive cells. For field-sampled O. sylvatica, sex was omitted from the model as we could not identify non-caregiving reproductive partners and collected only females. Rather than averaging cell counts across brain regions, we included frog identity as a repeated measure to control for both random and systematic variation across the large number of tissue sections quantified for each individual. Brain region area was included as an offset variable to control for body size differences between frogs, size differences between brain regions, and rostral to caudal size/shape variation within brain regions. We explored main effects of group, sex and regional differences in further detail using post hoc comparisons Tukey adjusted for multiple hypothesis testing.
We tested for differences in the number and activity of galanin neurons using generalized linear mixed models. To compare the number of galanin neurons, we included behavioural group (tadpole transport versus non-parental), sex and their interactions as main effects predicting the median number of galanin-positive cells using a negative binomial distribution appropriate for count data with unequal variances. To analyse activity differences in POA galanin neurons, we included behavioural group, sex and their interactions as main effects predicting the proportion of pS6-positive galanin (i.e. co-labelled) cells using a binomial distribution. All analyses were performed separately for each species using SAS statistical software (SAS v. 9.4; SAS Institute for Advanced Analytics). Raw cell counts and representative SAS code are provided in the electronic supplementary material.
(f). PhosphoTRAP library construction and sequencing
We collected D. tinctorius males found transporting tadpoles and males that currently had tadpoles present in the leaf litter but had not yet transported them. Males were euthanized as described above (n = 9 per group). Brains were removed, embedded in Tissue-Tek OCT Compound, frozen on dry ice and stored at −80°C for no more than one month. Once all animals had been collected, brains were sectioned at 100 µm on a cryostat and thaw mounted onto SuperFrost Plus slides. A 0.96 mm tissue micro punch tool was used to isolate the Mp and rostral hypothalamus (anterior, medial, and magnocellular POA and suprachiasmatic nucleus). To provide enough starting material for PhosphoTRAP, tissue punches from three individuals were combined into a single sample, for a total of three biological replicates per group. PhosphoTRAP libraries for total (TOT) and immunoprecipitated (IP) RNA from each sample were constructed following [16] (details in electronic supplementary material). Libraries were pooled in equimolar amounts and sequenced on an Illumina HiSeq 2500.
(g). PhosphoTRAP analysis
To analyse PhosphoTRAP data, we first quantified gene expression by mapping sequenced reads back to a brain tissue-specific D. tinctorius transcriptome (E.K.F. & L.A.O. 2019, unpublished data) and estimated their abundance using Kallisto [17]. As gene expression is known to differ across brain regions [18], we performed all subsequent analysis steps separately for the Mp and POA. Analysis methods are described in detail in the electronic supplementary material. Briefly, we normalized read counts using DESeq2 [19] and quantified transcript enrichment/depletion in active neurons as a log-fold difference between transcript counts from immunoprecipitated (IP) and total (TOT) mRNA for each sample. We then calculated differential fold enrichment between parental and non-parental individuals by dividing the mean log-fold expression values from the two behavioural groups. We refer to this final metric as the log-fold difference ratio between tadpole transport and non-transport behavioural groups.
Our primary objective was to use PhosphoTRAP data to identify cell types whose activity differed between tadpole transport and non-parental individuals. To this end, we restricted further analysis to a subset of 158 transcripts representing cell types with known roles in parental care (electronic supplementary material, table S1). We identified transcripts as significantly enriched/depleted based on a combination of log-fold enrichment thresholds (greater than 4) and permutation testing (electronic supplementary material). Permutation testing and visualization were done using R statistical software (v. 3.5.0; the R Foundation for Statistical Computing).
3. Results
(a). Neural induction during tadpole transport
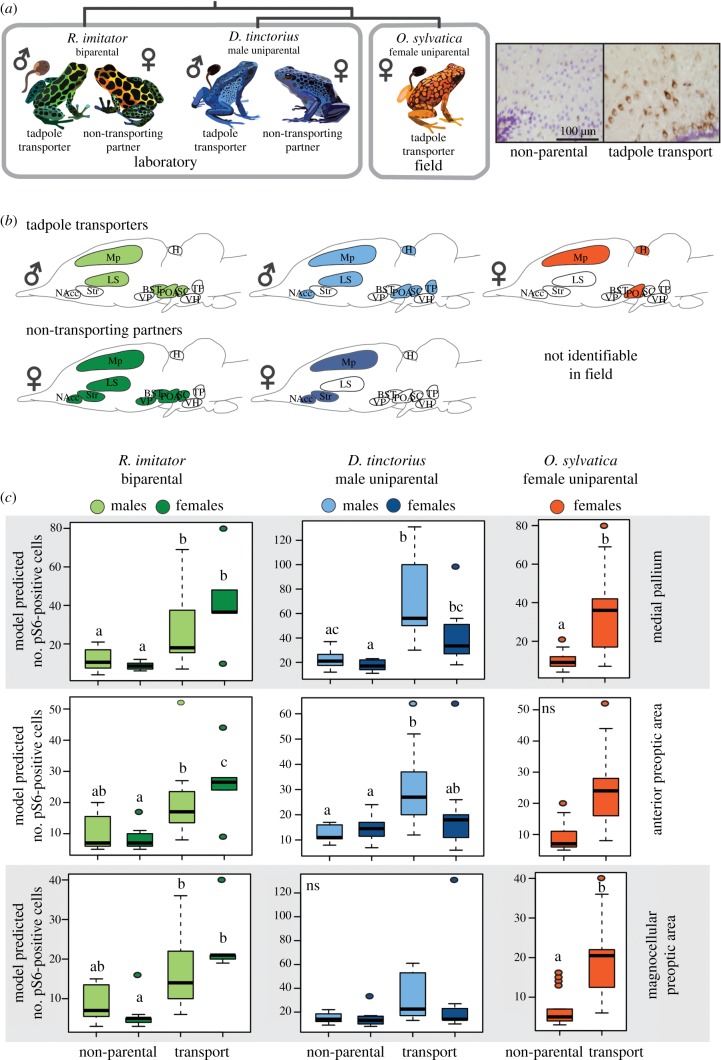
We compared neural activity patterns in tadpole transporters and their non-transporting reproductive partners across three closely related poison frog species with distinct parental care strategies (figure 1a). Differences in neural activity depended on behavioural group, sex and brain region (figure 1b and table 1), and associations between behavioural group and neural induction were brain region-specific (table 1; group × region: D. tinctorius: F1,2515 = 5.00, p < 0.0001; R. imitator: F12,557 = 6.85, p < 0.0001; O. sylvatica: F12,557 = 5.53, p < 0.0001). We found overall differences between the transporting and non-transporting sex in male uniparental D. tinctorius (sex × group × region: F1,2515 = 3.89, p < 0.0001) but not biparental R. imitator (table 1 and figure 1b). Indeed, post hoc analyses of region-specific differences revealed greater similarity between sexes in biparental, monogamous R. imitator than male uniparental D. tinctorius (figure 1b,c; electronic supplementary material, table S2).
Figure 1.
Patterns of neural induction associated with parental care. (a) Overview of experimental design, which allowed us to identify brain regions important in parental care independent of sex and species. (b) Overview of brain regions showing differences in neural activity between parental and non-parental individuals (shaded) for tadpole transporting sex and their non-transporting partners. Symbols indicate the sex of transporting and non-transporting partner individuals. Comparing across species, we identified Mp and POA as active during tadpole transport regardless of sex and species (i.e. as core parental care brain regions). (c) Detailed results for core brain regions. Letters above the box plots indicate statistical differences: comparing groups pairwise, shared letters indicate no significant differences and non-shared letters indicate significant differences (p < 0.05). Representative micrographs of pS6 staining (brown) with cresyl violet nuclear stain (purple) from the mPOA are shown at top right. BST, basolateral nucleus of the stria terminalis; H, habenula; Ls, lateral septum; Mp, medial pallium (homologue of the mammalian hippocampus); NAcc, nucleus accumbens; aPOA, anterior preoptic area; mPOA, magnocellular preoptic area; SC, the suprachiasmatic nucleus; Str, striatum; TP, posterior tuberculum; VH, ventral hypothalamus; VP, ventral pallium. (Online version in colour.)
Table 1.
Summary of main statistical effects for neural induction differences.
| dfN, dfD | F-value | p-value | ||
|---|---|---|---|---|
| R. imitator | group | 1, 1731 | 13.83 | 0.0002 |
| sex | 1, 1731 | 0.64 | 0.4242 | |
| region | 12, 1731 | 87.48 | <0.0001 | |
| sex × group | 1, 1731 | 1.25 | 0.2630 | |
| group × region | 12, 1731 | 6.85 | <0.0001 | |
| sex × region | 12, 1731 | 2.69 | 0.0013 | |
| sex × group × region | 12, 1731 | 1.45 | 0.1344 | |
| D. tinctorius | group | 1, 2519 | 9.73 | 0.0018 |
| sex | 1, 2519 | 1.36 | 0.2443 | |
| region | 12, 2519 | 80.15 | <0.0001 | |
| sex × group | 1, 2519 | 1.76 | 0.1844 | |
| group × region | 12, 2519 | 5.00 | <0.0001 | |
| sex × region | 12, 2519 | 3.39 | <0.0001 | |
| sex × group × region | 12, 2519 | 3.89 | <0.0001 | |
| O. sylvatica | group | 1, 557 | 1.02 | 0.3126 |
| region | 12, 557 | 9.40 | <0.0001 | |
| group × region | 12, 557 | 5.53 | <0.0001 |
Comparing neural activity patterns associated with parental care across species allowed us to identify brain regions important in parental care independent of sex and species (i.e. core parental care brain regions). We observed parallel increases in neural activity in tadpole transporting individuals in two core brain regions across all species: the POA and the Mp (homologue of the mammalian hippocampus). In the POA, patterns differed by subdivision, with female-specific effects in the magnocellular POA and male-specific effects in the anterior POA (figure 1c). We also observed increased neural activity in the Mp of non-caregiving female reproductive partners in D. tinctorius and R. imitator (figure 1c).
(b). Gene expression in behaviourally relevant neurons
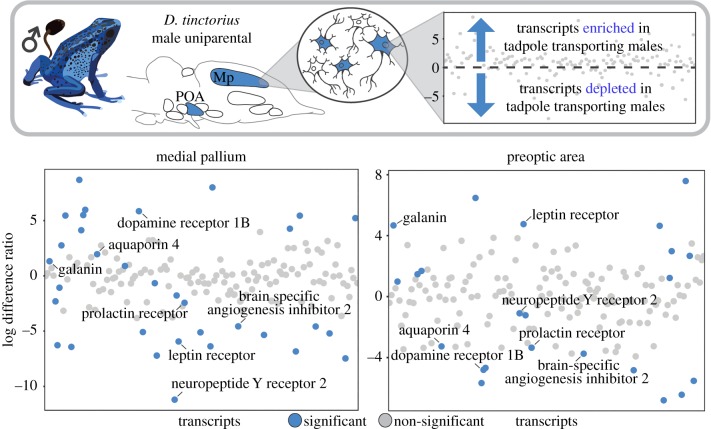
Following identification of core brain regions active during tadpole transport, we sought to identify behaviourally relevant neuronal types within these regions. We found 25 transcripts with significant expression enrichment/depletion in the POA and 32 transcripts with significant enrichment/depletion in the Mp. Seven transcripts were overlapping between brain regions (figure 2 and table 2). Of the overlapping transcripts, four had log-fold expression differences in the same direction (galanin, prolactin receptor, neuropeptide Y receptor 2, brain-specific angiogenesis inhibitor-associated protein 2) and three had log-fold expression differences in opposite directions (aquaporin 4, dopamine receptor 1B, leptin receptor) between brain regions (figure 2).
Figure 2.
Gene expression in behaviourally relevant neurons. We identified significant expression differences in neurons active during parental care in the POA and Mp of tadpole transporting versus non-transporting D. tinctorius males. Those transcripts with significant expression enrichment or expression depletion in tadpole transporting males when compared with control males are highlighted in blue, and the seven transcripts overlapping between brain regions are labelled. The same candidate transcripts are plotted in the same order along the x-axis for both brain regions. We found some unique and some shared transcripts differentially expressed across brain regions (e.g. distribution of blue dots between plots). (Online version in colour.)
Table 2.
Gene expression in behaviourally relevant neurons. Summary of transcripts significantly enriched (log different ratio greater than 0) or depleted (log difference ratio less than 0) in tadpole transporting when compared with non-transporting male D. tinctorius in the POA and Mp.
| preoptic area |
medial pallium |
||
|---|---|---|---|
| gene | log difference ratio | gene | log difference ratio |
| 5-hydroxytryptamine receptor 5A | 6.48 | 5-hydroxytryptamine receptor 1A | 8.69 |
| aquaporin 4 | −3.27 | 5-hydroxytryptamine receptor 1B | −4.58 |
| bombesin | 2.68 | 5-hydroxytryptamine receptor 1D | 4.27 |
| brain-specific angiogenesis inhibitor 1-associated protein 2 | −3.75 | 5-hydroxytryptamine receptor 3A | −7.21 |
| centromere-associated protein | −5.06 | androgen receptor | 5.44 |
| cocaine- and amphetamine-regulated transcript protein | 7.77 | angiotensin-converting enzyme | −5.34 |
| corticotropin-releasing factor binding protein | −6.44 | anoctamin 3 | −5.21 |
| dopamine D1 receptor | −4.80 | aquaporin 4 | 1.97 |
| oestrogen receptor β | −3.64 | brain-specific angiogenesis inhibitor 1-associated protein 2 | −4.57 |
| ETS translocation variant 1 | −6.81 | chondroitin sulfate proteoglycan | 5.50 |
| galanin | 4.67 | cocaine- and amphetamine-regulated transcript protein | 5.98 |
| galanin receptor type 2 | −5.67 | corticotropin-releasing factor receptor 2 | 8.01 |
| γ-aminobutyric acid receptor subunit α1 | −4.83 | dopamine β-hydroxylase | −6.38 |
| gonadotropin-releasing hormone II receptor | 4.64 | dopamine D1 receptor | 5.86 |
| gonadotropin-releasing hormone II receptor | −4.67 | dopamine D4 receptor | −1.07 |
| leptin receptor | 4.76 | Fez family zinc finger protein 1 | 0.90 |
| myelin basic protein | 0.99 | galanin | 1.32 |
| netrin G1 | 2.99 | leptin receptor | −5.94 |
| neuropeptide Y receptor type 2 | −1.09 | myosin-11 | −5.10 |
| neurotensin/neuromedin N | 1.68 | neuroligin 3 | −6.28 |
| nitric oxide synthase | −5.53 | neuropeptide Y receptor type 2 | −11.20 |
| prolactin receptor | −3.35 | perilipin 3 | 4.13 |
| synaptotagmin 2 | 1.22 | pro-neuropeptide Y | −7.46 |
| urocortin 3 | 7.59 | pro-opiomelanocortin | 5.23 |
| vasopressin V1b receptor | 3.39 | pro-thyrotropin-releasing hormone | −6.43 |
| proenkephalin A | 5.46 | ||
| progesterone receptor | −5.08 | ||
| prolactin receptor | −2.43 | ||
| secretogranin 2 | −2.30 | ||
| secretogranin 2 | −0.66 | ||
| thyrotropin-releasing hormone receptor | −6.84 | ||
| vasotocin | 2.77 | ||
(c). Galanin neuron number and activity
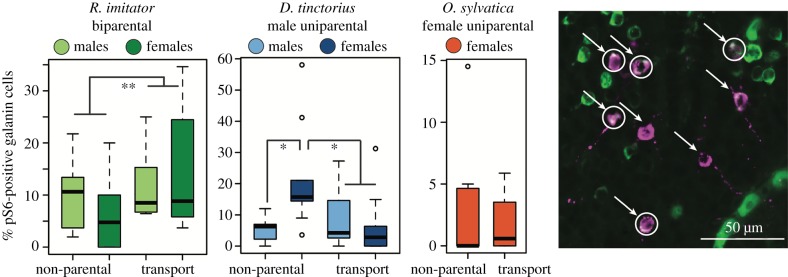
Recent demonstrations of the importance of POA galanin neurons in mediating parental care in mice [20,21] and our own findings of galanin transcript enrichment in neurons active during tadpole transport led us to ask whether activity differences specifically in POA galanin neurons were associated with parental care. Parental R. imitator had significantly more galanin neurons than did non-parental R. imitator, independent of sex (behavioural group: F1,404 = 4.58, p = 0.0329). There were no differences in galanin neuron number in D. tinctorius or O. sylvatica (electronic supplementary material, figure S2). Both D. tinctorius and R. imitator showed differences in galanin neuron activity associated with parental care, but not in the same manner: in D. tinctorius, the proportion of active galanin neurons was greater in the female partners of non-transporting males than any other group (sex × behavioural group: F1,40 = 12.73, p = 0.0010; figure 3). By contrast, in R. imitator, the proportion of active galanin neurons was greater during tadpole transport in both males and females (behavioural group: F1,26 = 8.15, p = 0.0083; figure 3). We observed no differences in the proportion of active galanin neurons between transporting and non-transporting O. sylvatica females.
Figure 3.
POA galanin neuron activity. Parental R. imitator had a greater proportion of active galanin neurons, as did the female partners of non-parental D. tinctorius. Representative micrograph: magenta, galanin-positive neurons (arrows); green, pS6-positive neurons; white, co-localization indicating active galanin neurons (circles). *p < 0.05; **p < 0.01. (Online version in colour.)
4. Discussion
Parental care requires the coordination of hormonal, neural and molecular changes, many of which remain poorly understood. We took advantage of shared parental behaviour across three poison frog species with distinct parental care strategies, combining laboratory and field data to disentangle sex- and species-specific mechanisms from core neural mechanisms. We identified the Mp and POA as core brain regions associated with parental care and demonstrated expression changes in genes associated with parental care in other vertebrates. Mechanistic studies in closely related, behaviourally variable poison frogs offer an opportunity to distinguish shared principles from neural diversity in the mechanisms mediating parental care.
(a). Core brain regions for parental care
By comparing patterns of neural activity across closely related species with distinct care strategies, we were able to identify core brain regions in which increased neural induction during parental care was sex- and species-independent. We observed increased neural induction in the Mp and one or more subdivisions of the POA during parental care in all focal species. The POA's widespread connections with other brain regions and high density of neuromodulators make it ideally positioned to modulate complex social behaviour, including parental care. Although data outside mammals are sparse, POA activity is associated with parental behaviour across vertebrates, including mammals [3], birds [3,22], fish [23] and now frogs. In brief, the POA appears to be a core node in parental care circuitry across vertebrates. Importantly, parental care has evolved independently across these clades, indicating convergence across behavioural and neural levels.
Although the precise function of the hippocampus and its non-mammalian homologues remains an area of active research, this brain region is classically implicated in memory, and specifically spatial memory [24,25]. Poison frogs inhabit complex rainforest environments in which tadpole deposition sites are a limited resource of variable quality. Behavioural studies in poison frogs document the use of cognitive spatial maps [26], and demonstrate the importance of spatial memory for navigating back to high-quality tadpole deposition pools [27] and for relocating offspring in egg provisioning species [28]. Increased neural induction in the Mp during tadpole transport is therefore in line with the unique ecological and evolutionary pressures associated with parental care in poison frogs. Indeed, spatial cognition is an important, but rarely examined, component of parental care [29,30], and motherhood is associated with changes in hippocampal plasticity in rodents (reviewed in [31]). Comparisons of hippocampal involvement in parental care across species may yield particularly interesting results given the functional—but not anatomical—conservation of this structure across vertebrates [32].
(b). Shared parental care circuitry across sexes
The strength of our design is highlighted by identification of interspecific variation in neural activity patterns between sexes that provide exciting, mechanistic hypotheses to be tested by future studies. Patterns of neural activity during tadpole transport differed between males and females in uniparental D. tinctorius, but not biparental, pair bonding R. imitator. Females are not directly involved in tadpole transport in either species; however, biparental R. imitator females provide parental care in the form of tadpole provisioning [13,33,34]. Thus, similar patterns of neural activity in male and female R. imitator could arise either because both sexes are in a ‘parental state’ that modulates long-term circuit activity or because even indirect involvement in tadpole transport activates parental circuitry (i.e. female frogs must know where their tadpoles are transported in order to return to feed them). In either case, similarities in neural activity patterns associated with parental care in males and females suggest that parental care circuitry is conserved across sexes.
In addition to broad similarities in R. imitator, we also observed increased neural activity in the Mp of non-caregiving D. tinctorius females. While they are not the typically caregiving sex and do not appear to pair bond with a single male partner, females of D. tinctorius and related species will occasionally perform tadpole transport [34,35]. This behavioural flexibility demonstrates that parental circuits are present and can be activated under certain circumstances in females. We suggest that an increase in Mp neural activity is related to females' monitoring of their partners’ behaviour (even in the absence of increased or preferential behavioural affiliation) and ability to perform tadpole transport in the absence of their male partners. The diversity of behavioural care strategies between species combined with behavioural flexibility within species in poison frogs affords a unique opportunity to further disentangle the evolution of sex-specific parental care circuits in future.
(c). Expression variation in behaviourally relevant neurons
Using D. tinctorius males, we characterized gene expression differences specifically in neurons active within the POA and Mp during tadpole transport, focusing our analyses on genes previously identified as markers of neuronal types involved in parental care [36]. Of particular interest in the POA were increased expression of the vasopressin 1b receptor, a gonadotropin-releasing hormone receptor and a number of stress response-related genes (Urocortin-3, CART, CRF binding protein) (table 2). Links between vasopressin and parental care have been demonstrated in rodents [37,38] and vasopressin and gonadotropin-releasing hormone may additionally influence parental care via regulation of other molecules with known roles in parental behaviour (e.g. oxytocin, prolactin) [3]. Stress hormones are known to increase in response to the behavioural and metabolic demands of parental care [39,40] providing a link between parental behaviour and the observed upregulation of stress-related signalling pathways.
Notable in the Mp were increased expression of vasopressin and androgen receptor transcripts. As described above, vasopressin signalling is widely implicated in parental care, and has been specifically linked to space use and behavioural and life-history trade-offs in prairie voles [29,30]. Space use and navigational abilities differ between males and females in many species, and it has been proposed that greater navigational abilities in males are a side effect of increased androgen signalling [41]. Increased androgen signalling during parental care in D. tinctorius could facilitate the heightened spatial cognition important during tadpole transport. Furthermore, increasing signalling via region-specific receptor expression could overcome the lower testosterone levels typically observed in parental males [42].
In addition to changes specific to either the POA or Mp, we observed a number of transcripts with significant expression differences in both regions. Among them were dopamine and prolactin receptors, and a number of molecules and receptors most commonly implicated in feeding behaviour (galanin, leptin receptor, NPY receptor). Dopamine and prolactin play known roles in parental care [42–44], while other shared transcripts (and some of those unique to a single brain region) are traditionally associated with feeding behaviour. There is growing recognition that molecules traditionally classified as feeding-related play important roles in mediating social behaviour, providing exciting opportunities to explore the repeated targeting of feeding-related mechanisms in the convergent evolution of parental care [45].
(d). Galanin and parental care
Initially described in relation to feeding behaviour, recent work uncovered a role for POA galanin neurons in driving parental care in both male and female mice [20,21]. We found a positive association between parental care and galanin neuron number and activity in biparental R. imitator, but not in male uniparental D. tinctorius, nor female uniparental O. sylvatica. Indeed, the only significant difference outside R. imitator was a relative increase in galanin neuron activity in the female partners of non-transporting male D. tinctorius, and we note that the per cent of active galanin neurons was overall low in all species.
While recent work demonstrates a sex-independent, behaviour-specific link between galanin neuron activity and parental care [20,21], the earliest work on POA galanin in rodents showed that microinjection of galanin into the POA of male rats facilitated copulatory behaviour [46], and work in fish similarly suggests an association between male courtship behaviour and galanin signalling [47,48]. Thus, species in which the role of galanin in social behaviour has been explored vary in parental care strategy: rats are female uniparental, only some male mice exhibit parental care, and fish include both male uniparental and female uniparental species. Together with our findings in poison frogs, these observations suggest that the role of galanin signalling in parental care may be mediated—both acutely and evolutionarily—by life-history differences related to parental care, interactions among partners and male courtship strategy. In brief, galanin appears to have been repeatedly evolutionarily co-opted to modulate social behaviour, but the type(s) of social behaviour influenced by galanin signalling are complex, mediated by behavioural variation and evolutionary history, and provide fertile ground for future comparative research.
5. Conclusion
Our findings lay the foundation for exciting work using poison frogs as a model to explore neural and molecular mechanisms of parental care, sex-specific behavioural patterns, and the integration of social and environmental cues to coordinate complex social behaviour. We identified core brain regions associated with tadpole transport across dendrobatid poison frogs with distinct care strategies. Moreover, we confirmed a role in amphibians for hormones and neuropeptides associated with parental care in other vertebrates. While increased POA activity was associated with parental care across species, activity specifically of galanin neurons differed between species, suggesting that shared brain regions may nonetheless rely on unique neuronal types to mediate similar behaviour. Studies in closely related but behaviourally distinct species across the animal kingdom provide opportunities to build a more holistic understanding of how shared principles and species-specific diversity govern parental behaviour.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the O'Connell Lab frog caretakers for help with animal care, Lola Guarderas (Wikiri) and Manuel Morales-Mite (Centro Jambatu) for fieldwork support, and Julie Butler, Hans Hofmann and the members of the O'Connell Lab for comments on previous versions of the manuscript. We thank Allen Moore and one anonymous reviewer for feedback that improved the manuscript during the review process.
Ethics
All laboratory procedures were approved by the Harvard University Animal Care and Use Committee (protocol no. 12-10-1). Field sample procedures were approved by the Harvard University Animal Care and Use Committee (protocol no. 15-03-239). All samples were collected and imported in accordance with Ecuadorian and US Law (collection permits: 005-15-IC-FAU-DNB/MA and 007-2016-IC-FAU-DNB/MA; CITES export permit 16EC000007/VS issued by the Ministerio de Ambiente de Ecuador).
Data accessibility
Cell counts, read counts from PhosphoTRAP, R code for PhosphoTRAP analysis and the D. tinctorius brain atlas are available as electronic supplementary material associated with the manuscript. Raw sequencing reads are available through the NCBI SRA repository (SUB5832932).
Authors' contributions
L.A.O. conceived of the study with input from K.S. and L.A.C.; L.A.O., E.K.F., A.B.R., N.A.M. and E.E.T. collected samples; E.K.F. and L.A.O. performed molecular work and data analysis; E.K.F. and L.A.O. wrote the manuscript with input from all authors. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
We gratefully acknowledge support from a Harvard University Bauer Fellowship, the International Society for Neuroethology Konishi Research Award and the Graduate Women in Science Adele Lewis Grant Fellowship to L.A.O., and a postdoctoral fellowship (NSF-1608997) to E.K.F. L.A.C. and E.E.T. were supported by Wikiri and the Saint Louis Zoo to Centro Jambatu.
References
- 1.Clutton-Brock TH. 1991. The evolution of parental care. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Royle NJ, Smiseth PT, Kölliker M. 2012. The evolution of parental care. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Numan M, Insel TR. 2006. The neurobiology of parental behavior. Berlin, Germany: Springer. [Google Scholar]
- 4.O'Connell LA, Matthews BJ, Hofmann HA. 2012. Isotocin regulates paternal care in a monogamous cichlid fish. Horm. Behav. 61, 725–733. ( 10.1016/j.yhbeh.2012.03.009) [DOI] [PubMed] [Google Scholar]
- 5.Kirkpatrick B, Kim JW, Insel TR. 1994. Limbic system fos expression associated with paternal behavior. Brain Res. 658, 112–118. ( 10.1016/S0006-8993(09)90016-6) [DOI] [PubMed] [Google Scholar]
- 6.Pereira M, Ferreira A. 2016. Neuroanatomical and neurochemical basis of parenting: dynamic coordination of motivational, affective and cognitive processes. Horm. Behav. 77, 72–85. ( 10.1016/j.yhbeh.2015.08.005) [DOI] [PubMed] [Google Scholar]
- 7.O'Connell LA, Hofmann HA. 2012. Evolution of a vertebrate social decision-making network. Science 336, 1154–1157. ( 10.1126/science.1218889) [DOI] [PubMed] [Google Scholar]
- 8.Goodson JL, Kingsbury MA. 2013. What's in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks. Horm. Behav. 64, 103–112. ( 10.1016/j.yhbeh.2013.05.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown JL, Morales V, Summers K. 2010. A key ecological trait drove the evolution of biparental care and monogamy in an amphibian. Am. Nat. 175, 436–446. ( 10.1086/650727) [DOI] [PubMed] [Google Scholar]
- 10.Pröhl H, Hödl W. 1999. Parental investment, potential reproductive rates, and mating system in the strawberry dart-poison frog, Dendrobates pumilio. Behav. Ecol. Sociobiol. 46, 215–220. ( 10.1007/s002650050612) [DOI] [Google Scholar]
- 11.Weygoldt P. 2009. Evolution of parental care in dart poison frogs (Amphibia: Anura: Dendrobatidae). J. Zool. Syst. Evol. Res. 25, 51–67. ( 10.1111/j.1439-0469.1987.tb00913.x) [DOI] [Google Scholar]
- 12.Summers K, Earn DJD. 1999. The cost of polygyny and the evolution of female care in poison frogs. Biol. J. Linn. Soc. 66, 515–538. ( 10.1111/j.1095-8312.1999.tb01924.x) [DOI] [Google Scholar]
- 13.Summers K, Tumulty J. 2014. Parental care, sexual selection, and mating systems in neotropical poison frogs. In Sexual selection: perspectives and models from the neotropics (eds Macedo RH, Machado G), pp. 289–320. New York, NY: Academic Press. [Google Scholar]
- 14.Fischer EK, Westrick SE, Hartsough L, Hoke KL. 2018. Differences in neural activity, but not behavior, across social contexts in guppies, Poecilia reticulata. Behav. Ecol. Sociobiol. 72, 131 ( 10.1007/s00265-018-2548-9) [DOI] [Google Scholar]
- 15.Schindelin J, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. ( 10.1038/nmeth.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight ZA, Tan K, Birsoy K, Schmidt S, Garrison JL, Wysocki RW, Emiliano A, Ekstrand MI, Friedman JM. 2012. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell 151, 1126–1137. ( 10.1016/j.cell.2012.10.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bray NL, Pimentel H, Melsted P, Pachter L. 2016. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527. ( 10.1038/nbt.3519) [DOI] [PubMed] [Google Scholar]
- 18.Lein ES, et al. 2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176. ( 10.1038/nature05453) [DOI] [PubMed] [Google Scholar]
- 19.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 ( 10.1186/s13059-014-0550-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. 2014. Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330. ( 10.1038/nature13307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohl J, et al. 2018. Functional circuit architecture underlying parental behaviour. Nature 556, 326–331. ( 10.1038/s41586-018-0027-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruscio MG, Adkins-Regan E. 2004. Immediate early gene expression associated with induction of brooding behavior in Japanese quail. Horm. Behav. 46, 19–29. ( 10.1016/j.yhbeh.2004.02.002) [DOI] [PubMed] [Google Scholar]
- 23.Demski LS, Knigge KM. 1971. The telencephalon and hypothalamus of the bluegill (Lepomis macrochirus): evoked feeding, aggressive and reproductive behavior with representative frontal sections. J. Comp. Neurol. 143, 1–16. ( 10.1002/cne.901430102) [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez F, López JC, Vargas JP, Gómez Y, Broglio C, Salas C. 2002. Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. J. Neurosci. 22, 2894–2903. ( 10.1523/JNEUROSCI.22-07-02894.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson RF. 1986. The neurobiology of learning and memory. Science 233, 941–947. ( 10.1126/science.3738519) [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Day LB, Summer K, Burmeister SS. 2019. A cognitive map in a poison frog. J. Exp. Biol. 222, jeb197467. [DOI] [PubMed] [Google Scholar]
- 27.Pašukonis A, Ringler M, Brandl HB, Mangione R, Ringler E, Hödl W. 2013. The homing frog: high homing performance in a territorial dendrobatid frog (Dendrobatidae). Ethology 119, 762–768. ( 10.1111/eth.12116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stynoski JL. 2009. Discrimination of offspring by indirect recognition in an egg-feeding dendrobatid frog, Oophaga pumilio. Anim. Behav. 78, 1351–1356. ( 10.1016/j.anbehav.2009.09.002) [DOI] [Google Scholar]
- 29.Okhovat M, Berrio A, Wallace G, Ophir AG, Phelps SM. 2015. Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science 350, 1371–1374. ( 10.1126/science.aac5791) [DOI] [PubMed] [Google Scholar]
- 30.Ophir AG, Wolff JO, Phelps SM. 2008. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proc. Natl Acad. Sci. USA 105, 1249–1254. ( 10.1073/pnas.0709116105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leuner B, Glasper ER, Gould E. 2010. Parenting and plasticity. Trends Neurosci. 33, 465–473. ( 10.1016/j.tins.2010.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler AB. 2017. Of horse-caterpillars and homologies: evolution of the hippocampus and its name. Brain Behav. Evol. 90, 7–14. ( 10.1159/000475981) [DOI] [PubMed] [Google Scholar]
- 33.Brown JL, Morales V, Summers K. 2008. Divergence in parental care, habitat selection and larval life history between two species of Peruvian poison frogs: an experimental analysis. J. Evol. Biol. 21, 1534–1543. ( 10.1111/j.1420-9101.2008.01609.x) [DOI] [PubMed] [Google Scholar]
- 34.Tumulty J, Morales V, Summers K. 2014. The biparental care hypothesis for the evolution of monogamy: experimental evidence in an amphibian. Behav. Ecol. 25, 262–270. ( 10.1093/beheco/art116) [DOI] [Google Scholar]
- 35.Ringler E, Pasukonis A, Fitch WT, Huber L, Hödl W, Ringler M. 2015. Flexible compensation of uniparental care: female poison frogs take over when males disappear. Behav. Ecol. 26, 1219–1225. ( 10.1093/beheco/arv069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moffitt JR, et al. 2018. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 362, eaau5324 ( 10.1126/science.aau5324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bester-Meredith JK, Marler CA. 2003. Vasopressin and the transmission of paternal behavior across generations in mated, cross-fostered Peromyscus mice. Behav. Neurosci. 117, 455–463. ( 10.1037/0735-7044.117.3.455) [DOI] [PubMed] [Google Scholar]
- 38.Rilling JK, Mascaro JS. 2017. The neurobiology of fatherhood. Curr. Opin. Psychol. 15, 26–32. ( 10.1016/j.copsyc.2017.02.013) [DOI] [PubMed] [Google Scholar]
- 39.Jeffrey JD, Cooke SJ, Gilmour KM. 2014. Regulation of hypothalamic-pituitary-interrenal axis function in male smallmouth bass (Micropterus dolomieu) during parental care. Gen. Comp. Endocrinol. 204, 195–202. ( 10.1016/j.ygcen.2014.05.023) [DOI] [PubMed] [Google Scholar]
- 40.O'Connor CM, Gilmour KM, Arlinghaus R, Van Der Kraak G, Cooke SJ. 2009. Stress and parental care in a wild teleost fish: insights from exogenous supraphysiological cortisol implants. Phyisiol. Biochem. Zool. 82, 709–719. ( 10.1086/605914) [DOI] [PubMed] [Google Scholar]
- 41.Clint EK, Sober E, Garland T Jr, Rhodes JS. 2012. Male superiority in spatial navigation: adaptation or side effect? Q. Rev. Biol. 87, 289–313. ( 10.1086/668168) [DOI] [PubMed] [Google Scholar]
- 42.Adkins-Regan E. 2013. Hormones and animal social behavior. Princeton, NJ: Princeton University Press. [Google Scholar]
- 43.Angelier F, Wingfield JC, Tartu S, Chastel O. 2016. Does prolactin mediate parental and life-history decisions in response to environmental conditions in birds? A review. Horm. Behav. 77, 18–29. ( 10.1016/j.yhbeh.2015.07.014) [DOI] [PubMed] [Google Scholar]
- 44.Schradin C, Anzenberger G. 1999. Prolactin, the hormone of paternity. News Physiol. Sci. 14, 223–231. ( 10.1152/physiologyonline.1999.14.6.223) [DOI] [PubMed] [Google Scholar]
- 45.Fischer EK, O'Connell LA. 2017. Modification of feeding circuits in the evolution of social behavior. J. Exp. Biol. 220, 92–102. ( 10.1242/jeb.143859) [DOI] [PubMed] [Google Scholar]
- 46.Bloch GJ, Butler PC, Kohlert JG, Bloch DA. 1993. Microinjection of galanin into the medial preoptic nucleus facilitates copulatory behavior in the male rat. Physiol. Behav. 54, 615–624. ( 10.1016/0031-9384(93)90068-Q) [DOI] [PubMed] [Google Scholar]
- 47.Partridge CG, MacManes MD, Knapp R, Neff BD. 2016. Brain transcriptional profiles of male alternative reproductive tactics and females in bluegill sunfish. PLoS ONE 11, e0167509 ( 10.1371/journal.pone.0167509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tripp JA, Feng NY, Bass AH. 2018. Behavioural tactic predicts preoptic-hypothalamic gene expression more strongly than developmental morph in fish with alternative reproductive tactics. Proc. R. Soc. B 285, 20172742 ( 10.1098/rspb.2017.2742) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Cell counts, read counts from PhosphoTRAP, R code for PhosphoTRAP analysis and the D. tinctorius brain atlas are available as electronic supplementary material associated with the manuscript. Raw sequencing reads are available through the NCBI SRA repository (SUB5832932).