Abstract
Considered as one of the major epidemics of the 21st century, osteoporosis affects approximately 200 million people globally, with significant worldwide impact on rates of morbidity and mortality and massive socioeconomic burdens. Mainly characterized by decreased bone mineral density (BMD) and increased risk of bone fragility/deterioration, this devastating silent epidemic typically has no symptoms until a fracture occurs. The multifactorial disease, osteoporosis is instigated by complex interactions between genetic, metabolic and environmental factors, with severe impact on the biomechanics of the musculoskeletal system. This article provides a review of the epidemiology, genetic and biomechanical aspects of primary osteoporosis. The review begins with a summary of the epidemiology and global prevalence of osteoporosis. Sections 1 and 2 discuss the genetic associations and molecular signaling pathways involved in normal and pathological osteogenesis while Section 3 explores the biomechanics of osteoporosis and its quantitative damaging effects on critical bone mechanical properties, and associated bone remodeling. Overall, this review summarizes the recent findings about osteoporosis and emphasizes the importance of an integrative holistic approach in investigating osteoporosis towards providing better informed, more effective preventive and treatment modalities. Importantly, this work also explores the limited available literature on the various aspects of osteoporosis in the United Arab Emirates (UAE), Gulf Cooperation Council (GCC), and Middle East despite its alarming prevalence in the region, and highlights the need for further research and studies taking into consideration the importance of the vitamin D receptor (VDR) gene influencing the development of osteoporosis.
Keywords: Bone, Osteoporosis
1. Introduction
Osteoporosis (porous bones) is a metabolic skeletal disorder clinically characterized by reduced bone mass density (BMD) and altered bone quality with microarchitectural and biomechanical abnormalities. This silent disease that is typically manifested by an increased risk of fracture, hence leading to significant morbidity and mortality (Marcus and Kelsey, 1996; Kanis et al., 1994; Am. J. Med., 1993; Am. J. Med., 1991), Fractures can involve any bone; however the spine, hip, wrist and proximal humerus are the most commonly affected sites (Cummings et al., 1985; Riggs and Melton, 1986; Riggs and Melton, 1992).
Traditionally, osteoporosis has been classified into primary and secondary types. Primary osteoporosis is usually associated with normal aging and reduced gonadal function, such as decreased levels of estrogen (Kanis et al., 2013; Ralston and Uitterlinden, 2010), whereas secondary osteoporosis is caused by other disease process, including Vitamin D deficiency, diabetes type 2, cardiovascular disease and certain malignancies (Miazgowski et al., 2012).
The multifactorial disease, osteoporosis is instigated by complex interactions between genetic, metabolic and environmental factors, with a severe impact on the biomechanics of the musculoskeletal system. Environmental factors include low physical activity, smoking, alcohol consumption, low sun exposure (decreased vitamin D production), and the use of certain medications, such as glucocorticoids and anticonvulsants (Lenchik and Sartoris, 1997). Ethnicity and race can also influence the incidence of osteoporosis. Understanding the risk factors for osteoporosis is critical towards the establishment of new avenues for prevention, improved clinical management, and effective healthcare.
2. The epidemiology and global prevalence of osteoporosis
Knowledge of the global prevalence of osteoporosis is relevant towards understanding its complex etiology within the associated gene pools of different races and ethnicities. It also sheds light on the serious impact of this silent killer on families and societies worldwide, and provides insights into the health planning challenges, processes and outcomes. Considered as one of the main global epidemics of the 21st century, osteoporosis affects approximately 200 million people with significant morbidity and mortality (International Osteoporosis Foundation) (Johnell and Kanis, 2006). It incurs high health care costs and imposes major socioeconomic burdens on families and societies, alike (Kanis et al., 2013). The risk of osteoporosis increases with age and is higher in women than in men, where 30% of women as compared to 12% of men suffer from osteoporosis at some point during their lifetime (one third of women, or one in every 3, and one out of eight men over the age of 50 are affected). Postmenopausal women are a high-risk, with a prevalence around 40–50% in females older than 60 years (Organization WH, 1992).
The current prevalence of osteoporosis in Europe (defined as 27 countries of the European Union) is approximately 22 million women and 5.5 million men between 50 and 84 years of age, with 3.5 million new fragility fractures. In 2015, this prevalence is expected to raise by 23% among 33.9 million individuals as compared to 27.5 million in 2010. Moreover, osteoporosis affects approximately 1.4 million Canadians, mainly postmenopausal women and the elderly. Osteoporosis and low bone mass are currently estimated to be a major public health threat for almost 44 million U.S. women and men aged 50 and older (Kanis et al., 2013; Ralston and Uitterlinden, 2010; Miazgowski et al., 2012), affecting 1 in every 4 women and >1 in 8 men over the age of 50 years, with 1 in 4 men and women presenting evidence of a vertebral fracture.
The prevalence of osteoporosis in developing countries is also increasing with a high number of osteoporotic fractures. However, the exact disease burden is difficult to estimate due to incomplete published official data. Factors that may contribute to this rise include the aging population, low bone density, high-risk ethnic groups, calcium and vitamin D deficiency, consumption of alcohol and soft drinks, smoking, soft drinks, as well as increasingly sedentary lifestyle and reduced physical activity. Despite the abundant sunlight in in the Arabian Gulf Region, there is a high prevalence of vitamin D deficiency resulting in significantly lower bone mineral density (BMD) among females as compared to Western populations (Al Taie and Rasheed, 2014).
Various studies have clearly demonstrated that race, ethnic background and genetic predisposition have a significant impact on the epidemiology and outcomes of osteoporosis. For example, Caucasians and Asians usually have lower bone density values than African Americans, Hispanics and Latin Americans (Kanis et al., 2013; García-Ibarbia et al., 2013; Liang et al., 2014).
Among the UAE female population, there is a higher rate of vitamin D deficiency and risk for osteoporotic fractures as compared to Europeans (Lenchik and Sartoris, 1997). This is likely due to a combination of conservative dressing style minimizing exposure to sunlight, spending the majority of their time indoors to avoid heat during hot weather, as well as, genetic factors. To date, accurate epidemiological prevalence figures for osteoporosis in the United Arab Emirates (UAE) are not available.
The UAE population above 50 years of age is estimated to be around 7%. It is hence not surprising that the current total number of individuals with osteoporosis is relatively small. On the other hand, the prevalence of osteoporosis in the UAE is affected by the uniquely diverse population structure as only 20% are Emirati Nationals (female/male ratio of 1/1.1) and 80% are expatriates (female/male ratio of 1 to 4). There is data documenting osteoporosis prevalence of approximately 2.5% at an average age of 42 years (based on screening of 1825 asymptomatic individuals). To date however, there is no National Hip Fracture registry in UAE. According to the records of a major hospital in the capital Abu Dhabi, there are 2.25 osteoporotic hip fractures per 100 individuals. This report recommends that the UAE health authorities should consider osteoporosis and hypovitaminosis D as major health challenges and should hence emphasize both the preventive and curative actions (Al Taie and Rasheed, 2014).
The results reported by Dubai Bone and Joint Centre and the Ministry of Health (MOH) (2007) are alarming. The data revealed that 20% of screened individuals had BMD less than −2.5 and 36% had osteopenia (low bone density). More worrying, unpublished data was observed during a superb “Hor Al Anz” screening in Dubai, which depicted that 32% of the men had low bone density, 365 postmenopausal women were osteopenic and 6% were osteoporotic. Among the younger women (<45 years old), 16% were osteopenic and 5% osteoporotic (Al Taie and Rasheed, 2014).
Overall, osteoporosis is a global health issue. It affects all populations across the world regardless of skin color, dressing style or weather conditions. Therefore, it is important to identify high-risk individuals and implement preventive and therapeutic measures to slow the disease progress and reduce fracture rates. Recognising the multifactorial nature of this silent disease, recent efforts aimed at identifying the specific genes involved in osteoporosis are in progress.
3. The molecular dilemma in osteoporosis
Despite the multiple investigations of the cellular and molecular underpinnings of osteoporosis in the last few decades, the underlying mechanisms remain largely elusive due to the complex multifactorial nature of the disease. This is not surprising considering that osteoporosis is influenced by intricate interactions among three different systems in the human body: immune, hematopoietic and musculoskeletal, hence unveiling a new field of research: osteoimmunology (Gori et al., 2015).
In order to understand the pathophysiology of the disease, it is critical to understand osteogenesis, or the process of the development of healthy bone. Bone is a highly dynamic tissue, which is continually being formed and resorbed during a person's life span. Bone morphology and function are maintained by a dynamic reconstruction process called bone remodeling.
During the remodeling cycle, the osteoblasts (OBs), or bone forming cells, orchestrate the orderly process of bone remodeling through activation signals from systemic factors including growth hormone (GH) interleukins (IL-1, IL-6) Parathyroid hormone (PTH) and withdrawal of estrogen (-E2).
Bone remodeling occurs at many focal areas throughout the skeleton. The process repairs areas of micro-cracks and helps prevent structural damage accumulation, such as that resulting from bone fatigue. Remodeling, which is also important for the maintenance of calcium homeostasis, is a bone surface phenomenon that occurs in distinct units, so-called bone remodeling units. Initially, osteoclasts (OCs), bone cells that remove the mineralized matrix, attach to the site of remodeling and drudge an erosion cavity. When this phase is complete, the OBs responsible for bone formation migrate to the newly resorbed cavity, lay down a new matrix of osteoid—composed mainly of Type I collagen—and contribute to the bone mineralization process. At steady state, the amount of new bone formed is typically more or less equal to the amount resorbed (Gori et al., 2015).
As mentioned above, bone remodeling is dependent on a precise balance between bone formation, carried out by OBs, and bone resorption caused by OCs (Gori et al., 2015; Arron and Choi, 2000). This balance is regulated by a myriad of molecular signals. Disruption in any of these molecular pathways can disturb the equilibrium of bone turnover and thereby affect bone quality. Multiple changes in cellular, microarchitectural, and humoral factors involved in bone remodeling have been identified in association with osteoporosis. Numerous studies have demonstrated that the balance between bone resorption and bone formation seems to be regulated by a variety of growth factors and immune cytokines, which play an important role in the metabolic process. The macrophage colony stimulating factor M-CSF and the receptor activator of nuclear factor kappa B ligand RANKL are the two major OBs mediated factors, which regulate the recruitment and differentiation of the OCs, or bone resorption cells. Osteoprotegerin (OPG) is synthesized by the OBs and serves as a soluble decoy receptor blocking activation of RANK. Inhibition or knockout of these signals from OBs-OCs results in reduction in bone resorption. Other cells, including activated T lymphocytes, may contribute to the marrow milieu. Not pictured below are the IGFs, which are released during bone resorption and serve as coupling factors to recruit new OBs to the surface. These peptides may also be important for osteoclast activity (Gori et al., 2015).
4. Phenotype and identification markers for osteoporosis
The recent World Health Organization (WHO) and European guidelines for the management of osteoporosis identify clinical risk factors (CRFs) and recommend the use of BMD to estimate individual probability of a fragility fracture. At present, the diagnosis of Osteoporosis depends on aereal bone mineral density (aBMD) measurement using dual energy X-ray absorptiometry (DXA). The results are reported as the difference in standard deviation (SDs) with the peak bone mass (T-score) (Kanis et al., 1994). The WHO defines osteoporosis based on a BMD T-score of −2.5 or less (Organization WH, 1992). Low BMD is usually recognised as a good predictor of osteoporatic fracture risk. The impact of certain osteoporosis risk factors differs slightly according to age and varies across sites, perhaps due in part to changing structures varying structures and compositions of cortical versus trabecular bone as well as genetic factors. The combination of bone structural parameters associated with bone mechanical properties largely determines the bone fracture risk. This is explored further in the Biomechanics section. Most of these parameters, e.g. trabecular bone density, achieve peak values at skeletal maturity (by 21 years of age) and subsequently decrease due to aging and hormonal changes associated with menopause. Microstructural features of trabecular bone, as well as those of cortical bones are known to be complex rather than Mendelian traits, determined by the cumulative effects and interactions of numerous genetic loci and environmental factors (Carmeliet et al., 2015).
On the other hand, recent evidence demonstrates that individuals with the same BMD, as measured by two-dimensional DXA scans, may have different risks for fracture. This clearly suggests that factors other than density, such as microstructural architecture and loading as well as other biomechanical factors are important determinants of skeletal health. Recently, several genome-wide association studies (GWAS), including a meta-analysis, described >50 loci associated with BMD in humans. However, many candidate genes, such as vasopressin (Avp), oxytocin (Oxt), and β-2-microglobulin (B2m) were not confirmed by the GWAS, despite their established role in bone metabolism. Failure to significantly associate these genes to bone density in GWAS suggests that there may be other bone phenotypes not yet studied, or that genetic variation segregating the populations tested does not influence the expression of these genes appreciably (Organization WH, 1992).
Importantly, almost all previous GWAS have used aBMD as the only distinctive parameter of bone phenotype. Clinically, it has been shown that aBMD and volumetric BMD (vBMD) may not accurately predict risk fracture, suggesting that site-specific changes at the microstructural level are important determinants of bone health. Using DXA, the bone is presented as a two-dimensional image that does not account for bone size or geometry, bone type (trabecular vs cortical), nor the underlying biomechanical loading and microstructure. Notably, fracture risk and bone mechanical properties, such as strength and stiffness, are closely associated with changes in the microstructure of the bone, but are not always detected by DXA and/or peripheral computed tomography (pQCT). Furthermore, there is growing evidence that cortical and trabecular bones have distinct genetic influences and should be analyzed separately (Organization WH, 1992).
Hip geometry is analyzed separately due to the high prevalence of osteoporotic fractures in that region (number one fracture risk region in postmenopausal women). Though the findings of Nissen et al. (2009) demonstrated that in healthy premenopausal Danish women, the geometric parameters of the proximal hip were not associated with any of the tested polymorphisms, other studies had argued that hip geometry contributes to fracture risk (Li et al., 2010). Furthermore, two missense polymorphisms of WNT16 were shown to be associated with hip geometry, BMD, and fractures (García-Ibarbia et al., 2013).
In addition to the above-mentioned CRFs, bone turnover markers are routinely assayed for the net amount of bone formation and resorption. Bone formation markers, which are the protein products of the OCs, include bone-specific alkaline phosphatase, procollagen-1 amino peptide, and osteocalcin, while resorption markers include C-telopeptide and N-telopeptide, which are the breakdown products of collagen type II (the main protein in bone) (Raisz, 1999).
5. Genetic basis of osteoporosis
Several genes are involved in controlling osteogenesis by acting on the target cells in a very complex manner. Investigations of the molecular signaling pathways involved in normal and pathological osteogenesis are of interest because they aim to identify the genes associated with osteoporosis and hence could be promising for the establishment of better therapeutic intervention.
5.1. Heritability
The complex etiology of osteoporosis is influenced by a variety of environmental factors including age, nutrition, and ethnicity. Considered as a multifactorial polygenic disease, genetic determinants are modulated by hormonal, environmental, and nutritional factors. Two forms of osteoporosis are typically identified: osteoporosis related to estrogen deficiency upon menopause in women; and osteoporosis related to calcium deficiency and aging of the skeleton, particularly in the elderly (Am. J. Med., 1991). As many environmental factors affect the BMD, the heritability of the BMD at the spine and hip levels has been estimated between 70 and 85% (Raisz, 1999). Research studies reveal that the susceptibility to osteoporosis has a strong genetic contribution, with genes estimated to account for about 25% of the variance in terms of susceptibility to osteoporotic fractures, 25%–54% for fractures of the wrist, and up to 48% for fractures of the hip (Raisz, 1999). Despite the moderate to high heritability, the fracture phenotype is quite challenging to incorporate in genetic studies since fracture risk is influenced by a number of diverse physiological factors, including BMD and age-related decline in bone microarchitecture and mechanical properties, muscle strength, balance, cognition, cardiovascular function, and vitamin D status. Since each of these factors is itself under at least partial genetic control, variants that influence fracture susceptibility entirely through any of these other factors should be more easily detectable in an analysis of the factor itself, rather than fracture. For example, considering the BMD, several epidemiological studies have shown that complex bone phenotypes are highly heritable, and hence a thorough understanding of osteoporosis necessitates the comprehensive genetic dissection of its component traits.
6. Methods for identifying genes associated with osteoporosis
Early scientific studies to identify specific genes related to variation in the BMD and fracture risk have focused on identifying biologically motivated candidate genes and testing specific genotyped variants for association with BMD (or fracture). However, while many positive association results were published (with few exceptions, such as the estrogen receptor 1 [ER1 gene] and low-density lipoprotein receptor-related proteins 4 and 5 [LRP4, LRP5] genes), most initially reported associations were found difficult to replicate.
With advances in genomic technology over the past ten years, numerous GWAS of BMD and related traits were published (Li et al., 2010; Levy et al., 2015). It is important to realize however the GWAS approach is designed to test the hypothesis of association with genetic variants that are common in the population, i.e., typically variants with minor allele frequencies of 5% or greater. However, the main disadvantage of GWAS is that most available marker sets are designed to identify common alleles but not rare polymorphisms (1% to 5% population frequency). Thus, many polymorphisms actually contributing to a trait, although with a small effect, might be missed, particularly with a limited sample size (Li et al., 2010).
By the end of 2014, nine GWAS and nine Meta analyses reported 107 genes and 129 Single nucleotide polymorphisms (SNPs) associated with BMD, osteoporosis or fractures with a significant threshold of 5 × 10–8. Recently Qin et al. (2016) performed a computational characterization of these SNPs and genes and reported that of 129 SNPs: 72 mapped to introns, 35 to intergenic regions, 6 to exons and 3 in 3′UTR. Osteoporosis GWAS-associated genes showed enrichment of Wnt signaling pathway, basal cell carcinoma and hedgehog signaling pathway. Highly interconnected “hub” genes, as revealed by interaction network analyses, were RUNX2, SP7, TNFRSF11B, LRP5, DKKI, ESR1 and SOST.
In addition to GWAS, microarray studies of BMD have been critical towards understanding the pathophysiology of osteoporosis and have identified a number of candidate genes. Using a network based meta-analyses, a consensus module containing 58 genes and 83 edges were detected (Qin et al., 2016). Pathway enrichment analysis of the 58 module genes revealed that these genes were enriched in several important pathways including osteoclast differentiation, B cell receptor signaling pathway, mitogen-activated protein kinase (MAPK) signaling pathway, chemokine signaling pathway, and insulin signaling pathway. Furthermore, five candidate genes ESR 1, MAP3K3, PYGM, RAC1 and SYK were identified based on gene expression meta-analysis and their associations with BMD were replicated by two BMD meta-analyses studies.
Collectively, the genes/loci identified from individual GWAS and meta analyses, to date, explain <6% of the variance in BMD deviation. Therefore, further efforts are needed to explore undiscovered genetic factors associated with BMD changes. At the DNA level, there are several possible paths towards uncovering these novel yet elusive genes. GWAS do not specifically pinpoint causal genes or provide functional context for associations. It is clear that complex bone phenotypes, such as BMD, are not exclusively determined by the cumulative effects of individual genetic influences, but instead are the result of emergent properties of biological networks. This makes it necessary for new approaches that can extend, complement, and enhance GWA by generating a systems-level view/analysis of the disease. Systems genetics is an emerging approach that can be used to investigate cell function and disease from a systems-level perspective. It focuses on determining how naturally occurring genetic variation perturbs cellular systems and ultimately trigger disease (He et al., 2016). A series of studies have nicely demonstrated how expression quantitative trait loci (eQTLs) can inform BMD GWAS. In these studies, high-density genotyping and microarray-based gene expression data were generated on primary human osteoblasts (hOBs). In the first study, the authors identified several hundred genes regulated by local eSNPs in hOBs (N095). They then cross-referenced the list of expression SNPs with the top SNPs identified in a separate BMD GWA. Two key observations were made. First, there was a significant enrichment of hOB expression SNPs among those that were also associated with BMD. A parallel analysis using lymphoblastoid cell lines (LCLs) did not reveal this enrichment. This suggested that it is advantageous to use primary bone cells (or bone tissue) for systems genetic studies of osteoporosis as compared to the more accessible cells or cells lines such as LCLs. Second, of the top 10 local eSNPs that were correlated with BMD, a variant in the serine racemase (SRR) gene was found to be associated with BMD in two independent studies, providing strong support for the hypothesis that differences in its expression lead to BMD alterations. Finding genes for fracture risk is likely to be more difficult than those for BMD due to the much smaller sample sizes generally available for studies of fracture and the complexity of the fracture phenotype. As previously noted, it is difficult enough to identify genes/SNPs associated with intermediate traits. For example, although over 60 SNPs have now been associated with BMD, for which the heritability is very high, the effect sizes of all are very small, and hence very large sample sizes were required to identify these SNPs. Of the 16 SNPs that have been associated with fracture to date, most were tested because of their initial association with BMD, and all have odds ratios for fracture of 1.11 or lower for the risk allele with the exception of one, i.e., rs13182402 in ALDH7A1 (odds ratio 2.25). This SNP was identified in a GWAS of fractures in a Chinese population (Johnell and Kanis, 2006). ALDH7A1 is a gene in the aldehyde dehydrogenase 7 family (member A1) that degrades and detoxifies acetaldehyde, which inhibits osteoblast proliferation and results in decreased bone formation (Johnell and Kanis, 2006). So far, most SNPs associated with osteoporosis and/or osteoporosis-related traits in the HuGe Navigator database and the literature are located in the noncoding intron regions of genes. Recently, Jin et al. (2015) discovered the SQRDL I264T nsSNP, which served as a significant susceptibility variant in osteoporosis among Korean postmenopausal women in a GWAS of 1180 nsSNPs. They demonstrated that overexpression of the SQRDL I264T variant in the preosteoblast MC3T3 cells results in significant changes in osteoblast differentiation markers.
Most recently, a whole-genome sequencing study (Jin et al., 2015) identified a rare nonsense novel mutation within a novel gene (LGR4) that was strongly associated with low BMD and OF (osteoporotic fracture). Interestingly, although this mutation was associated with a wide range of phenotypes across species (i.e., humans and mice), it was not present in Danish or Australian populations. The effect on other human populations needs to be further evaluated and validated.
New investigations are warranted to further clarify the underpinning mechanisms involved in the interaction between candidate genes and environmental variables leading to osteoporosis via signaling pathways in individual patients.
7. Major biological pathways involved in osteogenesis related to osteoporosis
Several pathways involved in the bone remodeling processes have been reviewed in detail elsewhere (Rosen, 2017). This review will focus on two major pathways and genes associated with the development of osteoporosis.
7.1. Wnt signaling pathway
Wnt signaling pathway is one of the most important pathways in differentiation and proliferation of bone cells. Very recently, Sharma et al. (2015) reviewed the role of polymorphism in Wnt signaling modulator, thus shedding light on the origin of various diseases, including osteoporosis, towards the identification of novel pathways for potential therapy. Within the components of WNT signaling, the gene coding for WNT16, one of the 19 WNT ligands of the human genome, was found strongly associated with specific bone traits. These included cortical bone thickness, cortical bone porosity and fracture risk, hence regulating cortical but not trabecular bone homeostasis (García-Ibarbia et al., 2013).
A new study by Luther et al. (2018) revealed that in patients with early-onset osteoporosis, the prevalence of heterozygous WNT1 mutations was remarkably high and significant. The investigators showed that spontaneous bone fractures and osteoporosis in mice resulted from the inactivation of Wnt1 in osteoblasts in mice; while conditional Wnt1 expression in osteoblasts increased bone mass (Luther et al., 2018).
More recent research had documented that sclerostin (SOST), an antagonist of bone morphogenetic protein 2 (BMP2) reportedly associated with osteoporatic fractures and BMD, is a well acclaimed gene with various SNPs associated with low and high BMD (Styrkarsdottir et al., 2010). The transforming growth factor b receptor (TGFbR) also participates in osteogenesis by modulating the biological function of BMP2. Both SOST and Dickkopf (DKK) inhibit bone formation by binding LRP5/6 receptors and blocking Wnt signaling pathway in osteoblasts. Ardawi et al. (2012) demonstrated that postmenopausal Saudi women with high circulating sclerostin have significantly increased osteoporosis fracture risk. An association of DKK-1 gene containing chromosomal region 10q 21with hip geometry, BMD, and bone turnover has also been reported (Styrkarsdottir et al., 2010; Ardawi et al., 2012), but this association was not correlated with any other studies (Styrkarsdottir et al., 2010).
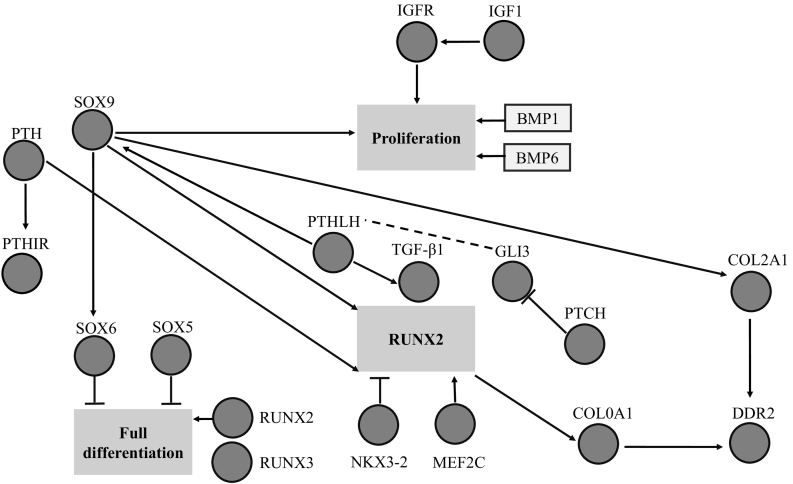
Bone tissue metabolism is modulated by estrogen through the binding estrogen receptors (ESR) in osteoblasts and osteoclasts, increasing bone formation and reducing bone resorption, respectively. Estrogen can cause a reduction of production of proinflammatory cytokines, such as interleukin-1 (IL1), IL6 and tumor necrosis factor a (TNFa) by peripheral macrophages, which is another mechanism for decreasing bone resorption. Furthermore, the effect of estrogen may be manifested through enhancing the functions of regulatory T (T-reg) cells that inhibit osteoclasts differentiation and bone resorption. CYP17 and CYP19 are also important genes involved in estrogen biosynthesis and are highly associated with BMD at various skeletal sites. The CYP17 gene encodes cytochrome P450c17a, crucial for the biosynthesis of gonadal hormones, which have positive effects on bone remodeling. Mutations in CYP17 may cause skeletal growth lag and diffuse osteoporosis (Rosen, 2017). The CYP19 gene encodes the aromatase enzyme that transforms androgen to estrogen and is essential for bone development. UDP-glucuronosyl transferase 2B17 (UGT2B17) is another important gene involved in this pathway. This enzyme catalyzes the conjugation of glucuronic acid to a variety of substrates, including steroid hormones, leading to their detoxification (Rosen, 2017) (Fig. 1).
Fig. 1.
Interaction among key genetic components of signaling pathways involved in bone formation (Rosen, 2017).
7.2. Vitamin D endocrine pathway
The vitamin D endocrine pathway is another key pathway involved in osteogenesis. In recent years, the vitamin D receptor (VDR) gene has been considered as an important candidate gene in the modification and the development of BMD and osteoporosis (Lenchik and Sartoris, 1997; Styrkarsdottir et al., 2010). Indeed, one of the first genes to be associated with the common form of osteoporosis was for the VDR (Kanis et al., 1994). Vitamin D (1,25(OH)2D3) is a steroid hormone that has a range of physiological functions in skeletal and nonskeletal tissues. Primary target of 1,25(OH)2D3 action and pathway indirectly promote calcium incorporation in bone. Severe vitamin D deficiency may thus decrease bone quality and quantity and lead to osteomalacia, whereas less severe deficiency increases the risk of osteoporosis and bone fractures. On the other hand, high vitamin D levels together with low dietary calcium intake may increase bone resorption and decrease bone mineralization in order to maintain normal serum calcium levels. Appropriate dietary calcium intake and sufficient serum vitamin D levels are critical for skeletal health (Am. J. Med., 1991). In the metabolism of bone, vitamin D increases the plasma levels of calcium and phosphorus, regulates OBs and OCs activity, and combats PTH hypersecretion, hence promoting bone formation and preventing/treating osteoporosis. This evidence is supported by most clinical studies, especially those that have included calcium and assessed the effects of vitamin D doses (≥800 IU/day) on BMD (Sadat-Ali et al., 2012). The VDR gene contains 14 exons and is located on chromosome 12q12–q14, which is a member of the nuclear receptor family of transcription factors (Miazgowski et al., 2012; Qin et al., 2016; Ardawi et al., 2012). VDR modulation influences the expression and transcription of genes involved in bone mass formation and calcium uptake, such as osteocalcin and calcium-binding proteins (Miazgowski et al., 2012; Qin et al., 2016). Since the VDR gene is polygenetic. Its SNPs could influence the expression and function of the VDR protein, which was shown to affect the risk of BMD and osteoporosis. Morrison et al. were first to postulate that genetic variants in the VDR gene could predict spinal and femoral BMD in Caucasian women (Johnell and Kanis, 2006; Gori et al., 2015) Since then, a large number of epidemiologic studies have reported the VDR genetic variants (e.g., FokI (rs10735810), BsmI (rs1544410), and ApaI (rs7975232)) are associated with BMD and osteoporosis in different ethnic groups (Lenchik and Sartoris, 1997; Styrkarsdottir et al., 2010; Haddad, 2014), however there were significant differences between the Syrian population and Asian population, including China, Japan, Thailand as well as Iran. The FokI polymorphism of the VDR gene has also shown controversial results. In an Iranian population of post-menopausal women with fokI genotype, ff exhibited a significantly lower risk for osteoporosis as compared with Ff and FF genotypes. Contrary to other investigators, Johnell and Kanis have reported that women with ff genotype experience greater bone loss as compared to Ff or FF genotypes (Johnell and Kanis, 2006).
Many essential and typical OBs genes are shown to be highly regulated by VDR, including Runt-related transcription factor 2 (RUNX2), which is considered as a key molecule in Vitamin D receptor pathway. Other transcription factors include Type I collagen (COL1A1), osteopontin, and osteocalcin COL1A is considered as the major protein building block of bone and is encoded by the COL1A1 and COL1A2 genes, respectively. Although polymorphisms of COL1A1 have been studied extensively, most researches focused on one polymorphism (rs1800012) located within intron 1, affecting the binding site for the transcription factor Sp1 (specificity protein 1) (Karasneh et al., 2013) and showing association with BMD or osteoporotic fractures (Grant et al., 1996; Judson et al., 2011; Urano et al., 2009). Despite some contradictory reports (Berg et al., 2000; Utennam et al., 2012), COL1A1 Sp1 polymorphism is common in Caucasians, although it is rare in the African subcontinent population and seems to be virtually absent from Asian populations (Ashford et al., 2001; Nakajima et al., 1999).
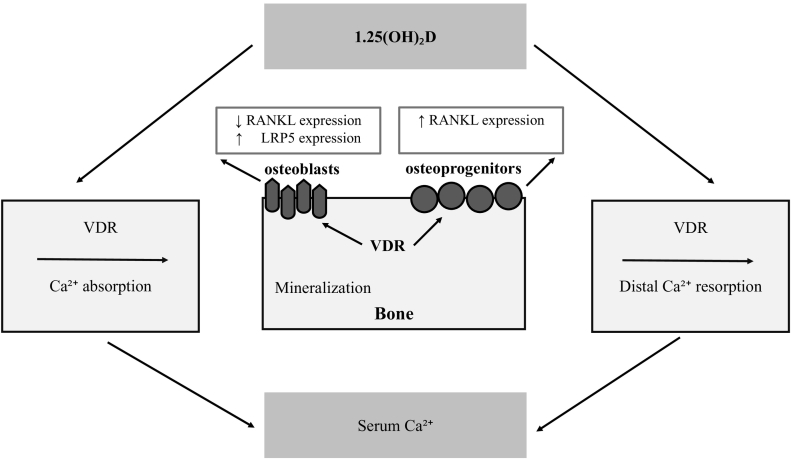
VDR stimulates RANKL/RANK -mediated osteoclastogenesis and bone resorption through up-regulation of RANKL as discussed earlier. Using microsatellite markers or short tandem repeats (STRs) situated in the vicinity (upstream and downstream) of the VDR gene, Rajesh et al. (2013) demonstrated a significant association between the allele 22 of D12S96 locus situated downstream to the VDR gene and risk of osteoporosis among Asian Indians. However, the author proposed that further studies with larger sample sizes from wider geographical areas are required for validation. Recently, Chang et al. (2015) reported the BsmI polymorphism in intestinal VDR may contribute to alterations in bone health. There is new evidence regarding the role of CD40 and CDL40 systems in the homeostasis of bone marrow cells. Panach et al. (2016) have demonstrated the association of CD40 and CDL40 genes with low BMD and osteoporosis risk. Their work also confirmed the link between SNP rs1883832, a TT homozygous mutation, and low level of OPG in bone marrow cells. The rate of gene expression by qPCR revealed that homozygous women for the C allele of SNP rs1883832 showed an increased expression of the CD40 gene in relation to other genotypes. On the other hand, they detected no expression of the CD40L gene, and hence inconclusive data. This study, however, has some limitations, including studying a population of Caucasian volunteers rather than a random population-based study (Fig. 2).
Fig. 2.
Model of normal calcium balance: normal serum 1.25(OH)2D levels promote intestinal absorption, when dietary calcium supply is low-normal to normal, and stimulate renal calcium reabsorption in the distal tubules. These pathways deliver sufficient calcium for adequate bone matrix mineralization. VDR signaling in osteoprogenitors increases RANKL expression and stimulates osteoclastogenesis, whereas VDR action in mature osteoblasts has anti-catabolic actions, by decreasing RANKL, and anabolic activity by increasing LRP5 expression (Mafi Golchin et al., 2016).
It is noteworthy here that the present review particularly addresses primary osteoporosis. The term “secondary” is applied to all patients with osteoporosis in whom the identifiable causal factors are other than menopause and aging. This includes other causal factors, such as diabetes, cardiovascular disease, autoimmune disease, etc. As established earlier, Vitamin D plays a crucial role in bone homeostasis pertaining to osteoporosis, and hence its involvement in other medical conditions such as obesity, diabetes and cardiovascular disease cannot be ignored. Vitamin D can contribute to, prevent, and/or treat osteoporosis, as well as type 2 diabetes mellitus (T2DM). Further research to investigate the genetic link between osteoporosis, diabetes and cardiovascular diseases is recommended.
8. The biomechanics of osteoporosis
8.1. Composition and structure of bone
Bone is a vital, dynamic connective tissue that plays important supportive and protective roles in the human body, by supporting its weight, protecting vital organs, as well as facilitating locomotion and providing attachment sites for muscles. A highly specialized tissue with unique structural and mechanical properties, bone is particularly designed and well equipped to enable its function. Similar to other connective tissues, bone consists of cells, as well as an organic extracellular matrix made of fibers and ground substance. Its main distinctive design feature, though, is the high content of minerals, which makes it hard and rigid as compared to soft connective tissue (Panach et al., 2016). In healthy bone, the mineral phase (inorganic portion) consists of calcium and phosphate in the form of hydroxyapatite with the chemical formula Ca10(PO4)6(OH)2 (Urano et al., 2009). Minerals account for almost 60% of weight and are responsible for hardness, rigidity, and solidarity (solid consistency) of bone (DirSci and Frankel, 2012). The Organic matrix primarily consists of collagen type I fibers, and non-collagenous protein and lipids. The collagen type 1 represents 30% of the weight, while water accounts for the remaining 10% (Panach et al., 2016).
At the cellular level, bone consists of three types of bone cells: OBs, osteocytes and OCs. OBs are cuboidal cells that are located along the bone surface representing 4–6% of the total resident bone cells. These cells are responsible for producing new bone (bone formation). Osteocytes cells comprise 90–95% of the total cells. The osteocytes are located within lacunae or cavities surrounded by mineralized bone matrix. Osteocytes act as mechanosensors and orchestrators of the bone remodeling process. Whereas, the function of OCs cells is to dispose, break down and replace old bone (DirSci and Frankel, 2012).
At the microscopic level, the fundamental structural unit of bone is the Haversian System or osteon. A typical osteon is cylindrically shaped and approximately 200 μm in diameter, with the Haversian canal that contains blood vessels and nerve fibers in the center. Each osteon consists of concentric layers of lamellae composed of mineralized matrix that surround the canal. Small cavities (lacuna), with each containing a bone cell, exist along the boundaries of each layer. Numerous small channels (canaliculi) connect the lacunae of adjacent lamellae forming a network of cell-to-cell communication. Each osteon is surrounded by a so-called cement line, where the collagen fibers do not cross, and is hence considered the weakest part of the bone's microstructure (DirSci and Frankel, 2012).
At the macroscopic level, there are two type of bone tissue: 1) Cortical or compact bone, which is the dense solid hard cortex layer of bone that forms the outer shell. Made of packed osteons, cortical bone represents most of the bone mass, accounting for 80%. 2) Cancellous bone (also referred to as trabecular or spongy bone), which forms the inner portion of bone and consists of a lattice of narrow rods and plates of calcified bone tissue called the trabeculae. The trabeculae are surrounded by bone marrow that is vascular and provides nutrients and waste disposal for the bone cells (Currey, 2013). Cortical bone always surrounds cancellous or trabecular bone but the relative quantity of each bone is different depending on the type of bone, genetics, health, age, among other factors. All bones are surrounded by a dense fibrous membrane called the periosteum which covers the entire bone expect for the joint surfaces, which are covered with articular cartilage (Currey, 2013).
8.2. Biomechanical behavior of bone
The biomechanical behavior of bone (defined here as the behavior or response to forces and moments) is mainly influenced by the bone's mechanical properties, geometric attributes, as well as loading (magnitude, rate and frequency of loading) (Panach et al., 2016).
Bone toughness, defined by the area under the stress-strain curve, is known as modulus of toughness and used as a quantitative measure of the total amount of energy absorbed to failure. Previous research has attributed bone's toughness and postyield properties of to the tough and pliable collagen fibers of the organic matrix (Marjolein and Prendergast, 2000). It has been shown that denaturing collagen in fact decreases bone toughness and strength by up to 60%. Also it has been shown that total collagen content is strongly related to failure energy and fracture toughness of bone tissue, suggesting that type 1 collagen is a primary arrestor of cracks (Marjolein and Prendergast, 2000). Bone is a highly anisotropic material (its mechanical properties depend on the direction of loading). Bone is also a viscoelastic material, since its mechanical behavior varies with the rate of loading as well (Panach et al., 2016). For example, bone is stiffer and stronger and sustains higher loads when loads are applied at a higher rate of loading. Bone also stores more energy before failure at higher rates when increases in toughness, within the physiological range.
8.3. Biomechanical characterization of bone quality
The quality of bone tissue is highly dependent on its composition and microstructure, whereas its quality as an organ depends on its macrostructure. The quality of bone can be characterized by measuring the intrinsic biomechanical properties mentioned above. These usually include the yield strength, the ultimate stress and strain, Young's modulus (stiffness) and modulus of toughness. Specialized techniques, such as nano-indentation and acoustic microscopy, allow the measurement and the high-resolution mapping of intrinsic Young's modulus of bone samples (Kini and Nandeesh, 2012). Other intrinsic tissue properties (besides Young's modulus) are difficult to measure directly and are usually inferred from whole bone empirical biomechanical tests such as low intensity ultrasound.
Osteoporosis is a condition characterized by low BMD and overall microstructural deterioration of bone tissue, leading to bone fragility and structural failure of the skeleton under low loads (Osterhoff et al., 2016). Bone fragility can be defined by the biomechanical parameters mentioned above including strength and strain measures, as well as toughness (work to failure or energy absorption) (Osterhoff et al., 2016). Often called the “silent disease”, osteoporosis typically presents no symptoms until the bone fractures. Specific biomechanical changes due to osteoporosis include an increase in bone fragility, an abnormal loss in bone volume, deterioration in the quality of the bone microarchitecture, an increased bone turnover rate, as well as a shift of BMD towards a lower mineralization density (McClung et al., 2017). The main causes postulated behind osteoporosis are multifactorial, as this review demonstrates, including genetic, physical, hormonal and nutritional factors acting alone or in concert to diminish skeletal integrity (McClung et al., 2017). Although the detailed pathophysiology of the disease remains elusive, it is agreed that an imbalance between bone resorption and formation can result in bone diseases including osteoporosis (McClung et al., 2017). The direct effect of osteoporosis is the continuous loss of bone during life, which is intensified in females after menopause and males with andropause. With aging, bone is lost from all parts of the skeleton, although not in equal amounts (Bruzzaniti and Baron, 2006). Another important factor is the decrease in bone production during maturation, resulting in an overall reduction in peak bone mass (PBM). Both cortical and cancellous bones are primarily thinned by the removal of bone at the endosteal surfaces adjacent to bone marrow. Cortical bone loss occurs mostly at the cortical endosteal surface. Age-related cancellous bone loss is mainly due to the imbalance in bone remodeling normal cycle with excessive bone resorption relative to bone formation (McClung et al., 2017). The sequence of Activation-Resorption-Formation is often uncoupled because of reducing the available trabecular rods/plates surfaces for bone formation (Currey, 2013). Another cause of increased bone resorption is calcium and vitamin D deficiency as described earlier. Age-related reduction in muscle mass and strength can also be considered as an important factor for the age-related reduction in bone apparent density and strength (McClung et al., 2017; Seeman and Delmas, 2006).
Although cortical bone plays a major role in determining the mechanical quality of bone and the risk of fracture, the age-related alterations of its geometrical features and its local porosity have long been poorly understood and underestimated (Osterhoff et al., 2016). The number of trabeculae in trabecular bone, trabecular thickness and the degree of connectivity all influence the mechanical strength of a bone. In osteoporosis, a decrease of all these characteristics is seen. Especially in bones with increased risk for osteoporotic fractures, however, the remaining trabecular tissue is largely heterogeneous, with regions of different mineralization, stiffness and strength. Both, the trabecular and the cortical component undergo different changes at different times due to the disease. Bone remodeling occurs on osseous surfaces and, thus, osteoporotic bone loss is a function of available bone surface for bone remodeling. The bone loss in early osteoporosis is mainly trabecular and with increasing age, bone loss becomes primarily endo- and intracortical (Kini and Nandeesh, 2012; McClung et al., 2017).
From a biomechanical perspective, the compressive elastic modulus, strength, and strain to failure of bone micro-beams are usually measured in order to assess the effect of osteoporosis on the mechanical properties of bone as a material at the sub-lamellar level (Osterhoff et al., 2016). Studies show a decrease in the elastic modulus of osteoporotic bone as compared to control (McClung et al., 2017; Seeman and Delmas, 2006). This decrease in the elastic modulus with osteoporosis is also associated with a relative small decrease in strength and a small increase in failure strain, as well as associated changes in material toughness (Seeman and Delmas, 2006). Compositional changes in bone material due to osteoporosis have been shown to decrease the degree of mineralization and collagen cross-linking, resulting in bone fragility (McClung et al., 2017). Reductions in the degree of mineralization have been further emphasized as detrimental to the material properties of bone (McClung et al., 2017). The stiffness versus toughness of bone is determined in part by the mineral content (McClung et al., 2017) and exhibits significant degradation in mechanical properties with relatively small mineral content changes, which increase bone fragility (Seeman and Delmas, 2006). In the case of osteoporosis, a decrease or an increase in mineralization may therefore be detrimental to the mechanical properties of bone (McClung et al., 2017). Low mineralization levels, or hypomineralization, cause reductions in stiffness and strength while high mineralization levels, or hypermineralization, reduce fracture toughness (Seeman and Delmas, 2006).
9. Concluding remarks and future perspectives
Despite the lack of data on osteoporosis in the Middle East, growing evidence indicates that the VDR gene is an important candidate gene for influencing the development of osteoporosis. VDR gene has been a focus of research in several Middle Eastern, which exhibit a high prevalence of vitamin D deficiency in the population despite the frequent sunshine. Several studies have evaluated the association between polymorphism of the VDR (Fok I, Bsm I, Taq I and Apa I) and low BMD in the contest of osteoporosis. FokI allele has been debated as it gave controversial results. Post-menopausal Iranian women with genotype ff exhibited significantly lower risk for osteoporosis, as compared to those with FF and Ff genotypes, thus demonstrating a potential protective factor. Because of the controversy in the results, it is recommended to conduct a cohort study along with a cross sectional analysis to provide a more suitable observational research design for analyzing the genetic markers as risk factors for osteoporosis.
Although it might be early to define novel biological factors as preventive or treatment targets for osteoporosis, this does not imply that the current genome-wide approaches are futile, but rather indicates that appropriate population-sensitive implementation of these studies might help to reduce potential bias confounding factors. GWAS approaches individually have specific limitations. Gene expression is a complex process that is regulated simultaneously and interactively at DNA, RNA, protein, epigenomic, and environmental levels. Therefore, a complementary genomic convergence or systems biology approach that integrates the information from studies such as GWLSs, GWASs, DNA sequencing, gene expression, proteomics (including studies of post-translational modifications), epigenomics, and gene-environment studies may help facilitate the identification of key pathways that are globally involved in the pathogenesis of osteoporosis and osteoporotic fractures. Ultimately, the functional relevance of the identified variants then needs to be confirmed by in vivo and/or in vitro molecular biology studies. Once a clear understanding of the complex nature of Osteoporosis is achieved, research can be directed using a) gene therapy approach targeted in patients at the greatest risk of osteoporosis, b) personalized medicine therapy approach by designing drugs blocking the products of susceptibility genes involved in key signaling pathways.
The key to successful prevention and treatment of osteoporosis is the identification of patients at risk for developing the disease as well as early-stage victims. Considering the multifactorial nature of osteoporosis, as presented here in this review, a holistic investigation that examines the various underlying factors of this complex disease including genetic, biological and biomechanical should be incorporated into effective risk assessment rubrics and tools. Such integrative investigations would be of great value to both clinical and research communities, alike, by shedding more light of the etiology of the disease towards more effective preventive and treatment modalities.
Transparency document
Transparency document.
Acknowledgments
We gratefully acknowledge the contribution of Ms Zahra Baalfaqih and Ms Sarah Azzam for their technical assistance in preparing this manuscript. This study was supported by research incentive funds from Zayed University granted to Dr. Fatme Al Anouti.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- Al Taie W.A., Rasheed A.M. The correlation of body mass index, age, gender with bone mineral density in osteopenia and osteoporosis: a study in the United Arab Emirates. Clin. Med. Diagn. 2014;4(3):42–54. [Google Scholar]
- Consensus development conference: prophylaxis and treatment of osteoporosisAm. J. Med. 1991;90(1):107–110. doi: 10.1016/0002-9343(91)90512-v. [DOI] [PubMed] [Google Scholar]
- Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosisAm. J. Med. 1993;94(6):646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- Ardawi M.S., Rouzi A.A., Qari M.H. Physical activity in relation to serum sclerostin, insulin-like growth factor-1, and bone turnover markers in healthy premenopausal women: a cross-sectional and a longitudinal study. J. Clin. Endocrinol. Metab. 2012;97(10):3691–3699. doi: 10.1210/jc.2011-3361. [DOI] [PubMed] [Google Scholar]
- Arron J.R., Choi Y. Bone versus immune system. Nature. 2000;408(6812):535–536. doi: 10.1038/35046196. [DOI] [PubMed] [Google Scholar]
- Ashford R.U., Luchetti M., McCloskey E.V., Gray R.L., Pande K.C., Dey A. Studies of bone density, quantitative ultrasound, and vertebral fractures in relation to collagen type I alpha 1 alleles in elderly women. Calcif. Tissue Int. 2001;68(6):348–351. doi: 10.1007/s002230010010. [DOI] [PubMed] [Google Scholar]
- Berg E.A., Johnson R.J., Leeman S.E., Boyd N., Kimerer L., Fine R.E. Isolation and characterization of substance P-containing dense core vesicles from rabbit optic nerve and termini. J. Neurosci. Res. 2000;62(6):830–839. doi: 10.1002/1097-4547(20001215)62:6<830::AID-JNR10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Bruzzaniti A., Baron R. Molecular regulation of osteoclast activity. Rev. Endocr. Metab. Disord. 2006;7(1–2):123–139. doi: 10.1007/s11154-006-9009-x. [DOI] [PubMed] [Google Scholar]
- Carmeliet G., Dermauw V., Bouillon R. Vitamin D signaling in calcium and bone homeostasis: a delicate balance. Best Pract. Res. Clin. Endocrinol. Metab. 2015;29(4):621–631. doi: 10.1016/j.beem.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Chang B., Schlussel Y., Sukumar D., Schneider S.H., Shapses S.A. Influence of vitamin D and estrogen receptor gene polymorphisms on calcium absorption: BsmI predicts a greater decrease during energy restriction. Bone. 2015;81:138–144. doi: 10.1016/j.bone.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings S.R., Kelsey J.L., O'Dowd K.J., Nevitt M.C. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol. Rev. 1985;7(1):178–208. doi: 10.1093/oxfordjournals.epirev.a036281. [DOI] [PubMed] [Google Scholar]
- Currey J.D. Princeton University Press; 2013. Bones: Structure and Mechanics. [Google Scholar]
- DirSci M.N., Frankel V.H. 4 ed. Lippincott Williams & Wilkins; New York: 2012. Basic Biomechanics of the Musculoskeletal System; p. 472. [Google Scholar]
- Dubai Bone & Joint Center Ministry of Health . 2007. Health DBJCo. 30% of women screened at ministry of health suffer from low bone mass, Your Gateway to The Middle East. (Available from: https://www.albawaba.com/news/30-women-screened-ministry-health-suffer-low-bone-mass) [Google Scholar]
- García-Ibarbia C., Pérez-Núñez M.I., Olmos J.M., Valero C., Pérez-Aguilar M.D., Hernández J.L. Missense polymorphisms of the WNT16 gene are associated with bone mass, hip geometry and fractures. Osteoporos. Int. 2013;24(9):2449–2454. doi: 10.1007/s00198-013-2302-0. [DOI] [PubMed] [Google Scholar]
- Gori F., Lerner U., Ohlsson C., Baron R. A new WNT on the bone: WNT16, cortical bone thickness, porosity and fractures. Bonekey Rep. 2015;4:669. doi: 10.1038/bonekey.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S.F.A., Reid D.M., Blake G., Herd R., Fogelman I., Ralston S.H. Reduced bone density and osteoporosis associated with a polymorphic Sp1 binding site in the collagen type I α 1 gene. Nat. Genet. 1996;14(2):203–205. doi: 10.1038/ng1096-203. [DOI] [PubMed] [Google Scholar]
- Haddad S. Vitamin-D receptor (VDR) gene polymorphisms (Taq-I & Apa-I) in Syrian healthy population. Meta Gene. 2014;2:646–650. doi: 10.1016/j.mgene.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Cao S., Niu T., Zhou Y., Zhang L., Zeng Y. Network-based meta-analyses of associations of multiple gene expression profiles with bone mineral density variations in women. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H.-S., Kim J., Park S., Park E., Kim B.-Y., Choi V.-N. Association of the I264T variant in the sulfide quinone reductase-like (SQRDL) gene with osteoporosis in Korean postmenopausal women. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0135285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnell O., Kanis J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- Judson R.S., Kavlock R.J., Setzer R.W., Cohen Hubal E.A., Martin M.T., Knudsen T.B. Estimating toxicity-related biological pathway altering doses for high-throughput chemical risk assessment. Chem. Res. Toxicol. 2011;24(4):451–462. doi: 10.1021/tx100428e. [DOI] [PubMed] [Google Scholar]
- Kanis J.A., Melton L.J., III, Christiansen C., Johnston C.C., Khaltaev N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994;9(8):1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- Kanis JA, Borgström F, Compston J, Dreinhöfer K, Nolte E, Jonsson L, et al. SCOPE: a scorecard for osteoporosis in Europe. Arch. Osteoporos.. 2013;8(1):144. [DOI] [PMC free article] [PubMed]
- Karasneh J.A., Ababneh K.T., Taha A.H., Al-Abbadi M.S., Marzouka Na-d S., Jaradat S.M. Association of vitamin D receptor gene polymorphisms with chronic and aggressive periodontitis in Jordanian patients. Eur. J. Oral Sci. 2013;121(6):551–558. doi: 10.1111/eos.12085. [DOI] [PubMed] [Google Scholar]
- Kini U., Nandeesh B.N. Physiology of bone formation, remodeling, and metabolism. In: Fogelman I., Gnanasegaran G., van der Wall H., editors. Radionuclide and Hybrid Bone Imaging. Springer Berlin Heidelberg; Berlin, Heidelberg: 2012. pp. 29–57. [Google Scholar]
- Lenchik L., Sartoris D.J. Current concepts in osteoporosis. AJR Am. J. Roentgenol. 1997;168(4):905–911. doi: 10.2214/ajr.168.4.9124138. [DOI] [PubMed] [Google Scholar]
- Levy R., Mott R.F., Iraqi F.A., Gabet Y. Collaborative cross mice in a genetic association study reveal new candidate genes for bone microarchitecture. BMC Genomics. 2015;16(1):1013. doi: 10.1186/s12864-015-2213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.-F., Hou S.-X., Yu B., Li M.-M., Férec C., Chen J.-M. Genetics of osteoporosis: accelerating pace in gene identification and validation. Hum. Genet. 2010;127(3):249–285. doi: 10.1007/s00439-009-0773-z. [DOI] [PubMed] [Google Scholar]
- Liang G., Ke T., Zhengxue Q., Zenghui Z., Dianming J. Association between seven common OPG genetic polymorphisms and osteoporosis risk: a meta-analysis. DNA Cell Biol. 2014;33(1):29–39. doi: 10.1089/dna.2013.2206. [DOI] [PubMed] [Google Scholar]
- Luther J., Yorgan T.A., Rolvien T., Ulsamer L., Koehne T., Liao N. Wnt1 is an Lrp5-independent bone-anabolic Wnt ligand. Sci. Transl. Med. 2018;10(466) doi: 10.1126/scitranslmed.aau7137. [DOI] [PubMed] [Google Scholar]
- Mafi Golchin M., Heidari L., Ghaderian S.M.H., Akhavan-Niaki H. Osteoporosis: a silent disease with complex genetic contribution. J. Genet. Genomics. 2016;43(2):49–61. doi: 10.1016/j.jgg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Marcus R.F.D., Kelsey J. Academic Press; San Diego, CA: 1996. Osteoporosis. [Google Scholar]
- Marjolein CHvdM, Prendergast P.J. Mechanics in skeletal development, adaptation and disease. Philos. Trans. Math. Phys. Eng. Sci. 2000;358(1766):565–578. [Google Scholar]
- McClung M., Baron R., Bouxsein M. An update on osteoporosis pathogenesis, diagnosis, and treatment. Bone. 2017;98:37. doi: 10.1016/j.bone.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Miazgowski T., Kleerekoper M., Felsenberg D., Stepan J.J., Szulc P. Secondary osteoporosis: endocrine and metabolic causes of bone mass deterioration. J. Osteoporos. 2012;2012 doi: 10.1155/2012/907214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T., Ota N., Shirai Y., Hata A., Yoshida H., Suzuki T. Ethnic difference in contribution of Sp1 site variation of COLIA1 gene in genetic predisposition to osteoporosis. Calcif. Tissue Int. 1999;65(5):352–353. doi: 10.1007/s002239900711. [DOI] [PubMed] [Google Scholar]
- Nissen N., Madsen J.S., Bladbjerg E.M., Beck Jensen J.E., Jørgensen N.R., Langdahl B. No association between hip geometry and four common polymorphisms associated with fracture: the Danish osteoporosis prevention study. Calcif. Tissue Int. 2009;84(4):276–285. doi: 10.1007/s00223-009-9219-9. [DOI] [PubMed] [Google Scholar]
- Organization WH . vol. 1994. 1992. Assessment of Fracture Risk and its Application to Screening for Postmenopausal Osteoporosis: Report of a WHO Study Group. meeting held in Rome from 22 to 25 June. [PubMed] [Google Scholar]
- Osterhoff G., Morgan E.F., Shefelbine S.J., Karim L., McNamara L.M., Augat P. Bone mechanical properties and changes with osteoporosis. Injury. 2016;47:S11–S20. doi: 10.1016/S0020-1383(16)47003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panach L., Pineda B., Mifsut D., Tarín J.J., Cano A., García-Pérez M.Á. The role of CD40 and CD40L in bone mineral density and in osteoporosis risk: a genetic and functional study. Bone. 2016;83:94–103. doi: 10.1016/j.bone.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Qin L., Liu Y., Wang Y., Wu G., Chen J., Ye W. Computational characterization of osteoporosis associated SNPs and genes identified by genome-wide association studies. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L.G. Physiology and pathophysiology of bone remodeling. Clin. Chem. 1999;45(8):1353–1358. [PubMed] [Google Scholar]
- Rajesh K., Rajalakshmi R., Vidyanikethan S. Formulation and evaluation of bilayer liquisolid tablets of atory astatin calcium and felodipine. Int. Res. J. Pharm. 2013;4(1):138–145. [Google Scholar]
- Ralston S.H., Uitterlinden A.G. Genetics of osteoporosis. Endocr. Rev. 2010;31(5):629–662. doi: 10.1210/er.2009-0044. [DOI] [PubMed] [Google Scholar]
- Riggs B.L., Melton L.J. Involutional osteoporosis. N. Engl. J. Med. 1986;314(26):1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- Riggs B.L., Melton L.J. The prevention and treatment of osteoporosis. N. Engl. J. Med. 1992;327(9):620–627. doi: 10.1056/NEJM199208273270908. [DOI] [PubMed] [Google Scholar]
- Rosen C.J. Endotext [Internet] MDText. com, Inc.; 2017. The epidemiology and pathogenesis of osteoporosis. [Google Scholar]
- Sadat-Ali M., Al-Habdan I.M., Al-Turki H.A., Azam M.Q. An epidemiological analysis of the incidence of osteoporosis and osteoporosis-related fractures among the Saudi Arabian population. Ann. Saudi Med. 2012;32(6):637–641. doi: 10.5144/0256-4947.2012.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E., Delmas P.D. Bone quality — the material and structural basis of bone strength and fragility. N. Engl. J. Med. 2006;354(21):2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- Sharma G., Sharma A.R., Seo E.-M., Nam J.-S. Genetic polymorphism in extracellular regulators of Wnt signaling pathway. Biomed. Res. Int. 2015;2015:9. doi: 10.1155/2015/847529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styrkarsdottir U., Halldorsson B.V., Gudbjartsson D.F., Tang N.L.S., Koh J.-M., S-m Xiao. European bone mineral density loci are also associated with BMD in East-Asian populations. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano K., Maruyama K., Ogata Y., Morishita Y., Takeda M., Sakurai N. Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J. 2009;57(6):1065–1078. doi: 10.1111/j.1365-313X.2008.03748.x. [DOI] [PubMed] [Google Scholar]
- Utennam D., Tungtrongchitr A., Phonrat B., Tungtrongchitr R., Preutthipan S. Association of T869C gene polymorphism of transforming growth factor-beta1 with low protein levels and anthropometric indices in osteopenia/osteoporosis postmenopausal Thai women. Genet. Mol. Res. 2012;11(1):1676–5680. doi: 10.4238/2012.January.13.2. (Electronic)):87-99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.