Highlights
-
•
Survival time of lung cancer with BM observed was very lower than other foundings.
-
•
The only two homogeneous factors were male gender and metastasis to the liver.
-
•
A number of heterogeneous risk factors have been found like age.
Keywords: Risk factors, Survival, Occurrence, Prognosis, Clinical guidelines
Abstract
Purpose
To analyse the homogeneous and heterogeneous risk factors for occurrence and prognosis in lung cancer patients diagnosed with bone metastasis (BM) by using the Surveillance, Epidemiology, and End Results (SEER) database.
Patients and methods
The medical records of lung cancer patients with or without bone metastasis were identified in the SEER database between 2010 and 2015. A multivariate logistic regression analysis was performed to identify risk factors, and a multivariate Cox regression was used to determine the prognostic effects of every variable on survival.
Results
In total, 34,585 eligible patients from the SEER database were included in the analysis. Male gender and metastasis to the liver were factors that were both positively associated with a risk for the development and prognosis of bone metastasis in patients with lung cancer. Younger age, poor tumour differentiation grade, higher N stage (N3), adenocarcinoma and metastasis to the brain were all positively correlated with a risk of occurrence of BM, but these factors were not correlated with an unfavourable prognosis. Age, race, marital status, tumour size and pathologic type were independent risk factors for the prognosis of bone metastasis.
Conclusion
The morbidity of bone metastasis in lung cancer patients is dismal, with a rate of 25.9%. The findings of this study estimate the homogeneous and heterogeneous risk factors for the occurrence and prognosis of bone metastasis in lung cancer patients, which may provide clinical guidelines for physicians.
Graphical abstract
1. Introduction
Lung cancer is the most common cancer in men and the leading cancer-related death in women in most developed countries, which lead to an enormous burden on family and social [1]. Even if the technology of lung cancer screening have been growing for several years, the reduction in lung cancer mortality was not extremely lower than before [2]. Although the number of smokers has decreased significantly in recent years, lung cancer still remained high mortality accounting for nearly 27.4% of all cancer deaths and 5-year survival rates of lung cancer ranged from 4 to 17% for regional differences [3], [4]. Lung cancer incidence trends was significantly different by gender, race, sex and histology in different countries [5], [6], [7], [8], [9], [10].
Bone metastasis (BM) was one of the most common distant metastases in patients with lung cancer, which brought about poor prognosis as an incurable disease. Nearly 20% of lung cancer patients aged ≥65 years were diagnosed with BM [11]. BM in non-small cell lung cancer was predominant in different metastatic patterns, with a rate of 37.1% [12]. Although survival time for lung cancer patients has been increased through improving medical care, the risk of BM which resulted in poor prognosis remained increasing [13], [14], [15]. It was a great pity that there was not a standard and palliative management strategy to reduce the odds of BM and an assessment method for prognosis.
The purpose of this study is to use the Surveillance, Epidemiology, and End Results (SEER) to analyze the risk factors of BM and estimate prognosis for BM in patients diagnosed with lung cancer, as well as further analyze homogeneous and heterogeneous factors. Factors include age, race, histological type, marital status, T stage, N stage, grade classification, the tumor size, brain metastasis, and liver metastasis.
2. Methods
2.1. Study population
Lung and bronchus cancer cases were obtained from the National Cancer Institute's SEER program (https://seer.cancer.gov), which consisted of 18 population-based cancer registries. The SEER database collected and published cancer data covering nearly 28% of the total population in the United States. We send the data agreement to the SEER administration and accepted the agreement from the administrator. We had the right to obtain the information of patients with personal account. In the SEER database, all available data were retrospective, so Institutional Review Board approval was not required in our study.
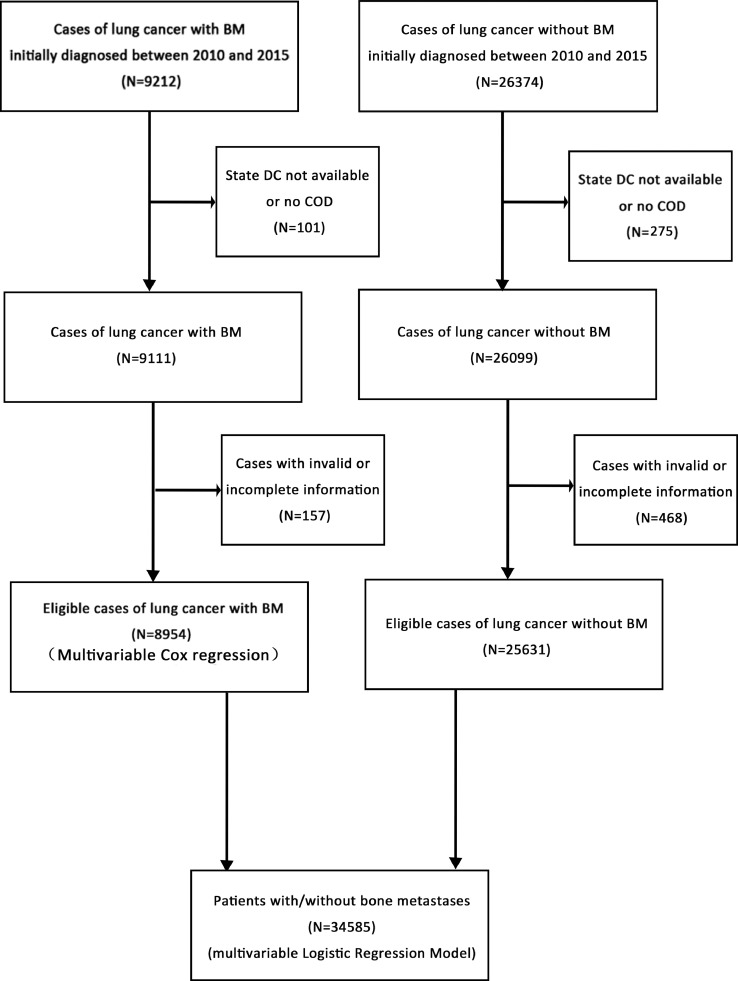
Because this database began collecting the information on bone metastases at the time of diagnosis in 2010 and related information was updated until 2015, we extracted data on lung cancer patients with the presence or absence of BM at the time of diagnosis from 2010 to 2015. The data was selected and listed in flow-chart (Fig. 1). In total, 9212 patients who were diagnosed as lung cancer with BM and 26,374 patients who were diagnosed as lung cancer without BM from 2010 to 2015 were selected. Subsequently, we removed patients with invalid information, leaving 8954 patients eligible for survival analysis and 25,631 patients eligible for multivariable logistic regression model.
Fig. 1.
The flow-chart of the data selection for analyzing the risk factors of the morbidity and prognosis of bone metastasis from lung cancer patients
Abbreviations: BM, bone metastasis.
2.2. Statistical analysis
Demographic data, including sex(male and female), age (≤40, 41–60, 61–80, and ≥81years), race [white, black, American Indian/Alaska Native (AI) and Asian or Pacific Islander (API)], marital status (married and unmarried), tumor sites (< 2;≥2,< 4; ≥4,< 6;>6,≤8;≥8,< 10;≥10,< 12;≥12,< 14;≥14), grade(I,II,III,IV), T stage(T0,T1, T2, T3, and T4), N stage(N0,N1, N2 and N3), metastasis at brain(yes and no) and metastasis at liver(yes and no). In addition, the histology codes were grouped into five categories based largely on the International Agency for Research on Cancer (IARC) classifications [16]: adenocarcinoma (histologic codes 8140,8230,8244,8255,8260,8310,8323,8480,8481,8490,8507,8550,8570,8574,8576),squamous cell carcinoma (histologic code 8050,8070-8074,8123), large cell carcinoma(histologic codes 8012-8014,8020–8022,8030,8031), small cell carcinoma (8041-8045,8246) and others(8000-8004,8010,8032-8034,8046,8082,8200,8240,8241,8247,8249-8254,8342,8430,8560,8575,8980).
Multivariable logistic regression was used to show the odds ratios (ORs) with 95% confidence intervals (CIs) and distinguish the risk factors for developing BM at diagnosis. Kaplan-Meier method was used to analyze survival duration; Log-rank test were tested to distinguish the differences between the curves. Multivariable Cox proportional hazards regression was performed to show the hazard ratios (HRs) with 95% CIs and determine prognostic effects of every variable on survival.
All statistical analyses were performed using SPSS 22.0 (Chicago, IL, USA). Two-sided P-values < 0.05 were considered significant statistically.
3. Results
3.1. Morbidity analysis
For the 34,585 eligible lung cancer patients who were diagnosed with BM or without BM between 2010 and 2015 in the study, Table 1 shows the number of the two cohorts according to different variables in the SEER. Of them, 8954 (25.9%) were diagnosed with BM at the initial diagnosis and we had their complete information. Thus, they were included in the survival analysis, and their demographic and clinical characteristics were shown in Table 2. Within this cohort, the median survival time was only 2 ± 0.12 months and the mean was just 6.61±0.26 months, while the time was 4 ± 0.16 months and 13.18±0.27 month for the patients without BM respectively.
Table 1.
Multivariable logistic regression for analyzing the demographic information and risk factors for occurrence of bone metastasis in patients diagnosed lung cancer (diagnosed 2010–2015).
| Subject characteristics | No. of patients with LC |
OR (95%CI) | P value | |
|---|---|---|---|---|
| With bone metastasis | Without bone metastasis | |||
| Sex | <0.001 | |||
| Male | 5163 | 13,253 | 1 (reference) | 1.000 |
| Female | 3791 | 12,378 | 0.804 (0.763–0.847) | <0.001 |
| Age, in years | <0.001 | |||
| ≤40 | 94 | 188 | 1 (reference) | 1.000 |
| 41–60 | 2254 | 5000 | 0.876 (0.670–1.145) | 0.331 |
| 61–80 | 5221 | 14,766 | 0.739 (0.567–0.963) | 0.025 |
| ≥81 | 1385 | 5677 | 0.536 (0.409–0.702) | <0.001 |
| Race | 0.004 | |||
| Black | 1002 | 3002 | 1 (reference) | 1.000 |
| White | 7265 | 20,851 | 1.080 (0.995–1.171) | 0.066 |
| AI | 46 | 145 | 0.915 (0.637–1.314) | 0.631 |
| API | 631 | 1556 | 1.153 (1.016–1.307) | 0.027 |
| Unknown | 10 | 77 | NA | NA |
| Marital status | 0.012 | |||
| Married | 4343 | 11,573 | 1 (reference) | 1.000 |
| Unmarried | 4168 | 12,554 | 0.953 (0.903–1.007) | 0.084 |
| Unknown | 443 | 1504 | NA | NA |
| Hist | ||||
| AD | 4144 | 10,235 | 1 (reference) | 1.000 |
| SQCC | 833 | 3319 | 0.629 (0.574–0.688) | <0.001 |
| LCLC | 166 | 424 | 0.786 (0.646–0.956) | 0.016 |
| SCLC | 1768 | 5220 | 0.544 (0.504–0.586) | <0.001 |
| Other | 2043 | 6433 | NA | NA |
| Gleason grade | <0.001 | |||
| I | 90 | 656 | 1 (reference) | 1.000 |
| II | 348 | 1528 | 1.456 (1.124–1.887) | 0.004 |
| III | 1209 | 3760 | 1.778 (1.400–2.258) | <0.001 |
| IV | 230 | 708 | 2.044 (1.542–2.710) | <0.001 |
| Unknown | 7077 | 18,979 | NA | NA |
| tumor size(cm) | 0.313 | |||
| ≤2 | 795 | 2805 | 1 (reference) | 1.000 |
| >2, ≤4 | 874 | 2959 | 1.140 (0.989–1.315) | 0.071 |
| >4, ≤6 | 723 | 2142 | 1.177 (1.010–1.373) | 0.037 |
| >6, ≤8 | 449 | 1364 | 1.099 (0.929–1.300) | 0.270 |
| >8, ≤10 | 245 | 746 | 1.080 (0.887–1.314) | 0.444 |
| >10, ≤12 | 122 | 339 | 1.168 (0.909–1.501) | 0.225 |
| >12, ≤14 | 31 | 144 | 0.772 (0.506–1.177) | 0.229 |
| >14 | 38 | 130 | 0.935 (0.628–1.393) | 0.743 |
| Unknown | 5677 | 15,002 | NA | NA |
| T Stage | <0.001 | |||
| T0 | 400 | 1238 | 1 (reference) | 1.000 |
| T1 | 200 | 1129 | 0.685 (0.553–0.848) | 0.001 |
| T2 | 867 | 3204 | 0.836 (0.692–1.010) | 0.064 |
| T3 | 1736 | 4128 | 1.195 (0.999–1.429) | 0.051 |
| T4 | 2468 | 7158 | 1.021 (0.858–1.215) | 0.811 |
| TX | 3283 | 8774 | NA | NA |
| N Stage | <0.001 | |||
| N0 | 1695 | 7452 | 1 (reference) | 1.000 |
| N1 | 610 | 1504 | 1.544 (1.377–1.731) | <0.001 |
| N2 | 3338 | 9010 | 1.372 (1.279–1.472) | <0.001 |
| N3 | 1718 | 3843 | 1.605 (1.476–1.744) | <0.001 |
| NX | 1593 | 3822 | NA | NA |
| Brain Met | <0.001 | |||
| Yes | 1733 | 3352 | 1 (reference) | 1.000 |
| None | 6678 | 22,101 | 0.708 (0.661–0.758) | <0.001 |
| Unknown | 543 | 178 | NA | NA |
| Liver Met | <0.001 | |||
| Yes | 2985 | 3617 | 1 (reference) | 1.000 |
| None | 5473 | 21,807 | 0.304 (0.286–0.323) | <0.001 |
| Unknown | 496 | 207 | NA | NA |
Abbreviations: LC, lung cancer; AI, American Indian/Alaska Native; API, Asian or Pacific Islander; Met, metastasis; AD, adenocarcinoma; SQCC, squamouscell carcinoma; LCLC, large cell carcinoma; SCLC, small cell carcinoma; NA, not avail.
Table 2.
Multivariable Cox regression for analyzing prognostic factors among lung cancer patients diagnosed bone metastasis (diagnosed 2010–2015).
| Subject characteristics | No. of patients with LC with bone metastasis | Survival, Median (IQR), mo | Cox HR (95% CI) | P value |
|---|---|---|---|---|
| Sex | <0.001 | |||
| Male | 5163 | 2 (1.848–2.152) | 1 (reference) | 1 |
| Female | 3791 | 3 (2.823–3.177) | 0.883 (0.843–0.924) | <0.001 |
| Age, in years | <0.001 | |||
| ≤40 | 94 | 9 (5.101–12.899) | 1 (reference) | 1 |
| 41–60 | 2254 | 4 (3.667–4.333) | 1.651 (1.297–2.100) | <0.001 |
| 61–80 | 5221 | 2 (1.852–2.148) | 2.091 (1.647–2.655) | <0.001 |
| ≥81 | 1385 | 1 (0.846–1.154) | 2.701 (2.116–3.448) | <0.001 |
| Race | <0.001 | |||
| Black | 1002 | 2 (1.672–2.328) | 1 (reference) | 1 |
| White | 7265 | 2 (1.871–2.129) | 0.978 (0.912–1.050) | 0.544 |
| AI | 46 | 2 (0.792–3.208) | 0.785 (0.567–1.087) | 0.145 |
| API | 631 | 3 (2.164–3.836) | 0.733 (0.656–0.819) | <0.001 |
| Unknown | 10 | NA | NA | NA |
| Marital status | <0.001 | |||
| Married | 4343 | 3 (2.807–3.193) | 1 (reference) | 1 |
| Unmarried | 4168 | 2 (1.839–2.161) | 1.196 (1.142–1.254) | <0.001 |
| Unknown | 443 | NA | NA | NA |
| Hist | <0.001 | |||
| AD | 4144 | 3 (2.814–3.186) | 1 (reference) | <0.001 |
| SQCC | 833 | 2 (1.678–2.322 | 1.170 (1.081–1.266) | 0.011 |
| LCLC | 166 | 2 (1.394–2.606) | 1.232 (1.050–1.466) | 0.024 |
| SCLC | 1768 | 4 (3.560–4.440) | 0.929 (0.871–0.990) | 0.001 |
| Other | 2043 | NA | NA | NA |
| Gleason grade | 0.106 | |||
| I | 90 | 4 (2.495–5.505) | 1 (reference) | 1 |
| II | 348 | 3 (2.236–3.764) | 1.257 (0.978–1.616) | 0.074 |
| III | 1209 | 3 (2.713–3.287) | 1.342 (1.064–1.693) | 0.013 |
| IV | 230 | 3 (2.286–3.714) | 1.402 (1.076–1.827) | 0.012 |
| Unknown | 7077 | NA | NA | NA |
| Tumor size (cm) | 0.015 | |||
| ≤2 | 795 | 4 (3.449–4.551) | 1 (reference) | 1 |
| >2, ≤4 | 874 | 3 (2.628–3.372) | 1.144 (1.007–1.301) | 0.039 |
| >4, ≤6 | 723 | 3 (2.622–3.378) | 1.187 (1.036–1.360) | 0.013 |
| >6, ≤8 | 449 | 2 (1.478–2.522) | 1.237 (1.066–1.436) | 0.005 |
| >8, ≤10 | 245 | 3 (2.071–3.929) | 1.111 (0.932–1.324) | 0.239 |
| >10, ≤12 | 122 | 1 (0.414–1.586) | 1.382 (1.114–1.716) | 0.003 |
| >12, ≤14 | 31 | 2 (0.971–3.029) | 1.564 (1.053–2.322) | 0.027 |
| >14 | 38 | 3 (0.448–5.552) | 1.318 (0.928–1.873) | 0.123 |
| Unknown | 5677 | NA | NA | NA |
| T Stage | 0.465 | |||
| T0 | 400 | 3 (2.247–3.753) | 1 (reference) | 1 |
| T1 | 200 | 4 (3.079–4.921) | 0.829 (0.678–1.013) | 0.067 |
| T2 | 867 | 3 (2.602–3.398) | 0.989 (0.835–1.171) | 0.899 |
| T3 | 1736 | 2 (1.738–2.262) | 0.965 (0.823–1.131) | 0.657 |
| T4 | 2468 | 3 (2.791–3.209) | 0.975 (0.833–1.141) | 0.749 |
| TX | 3283 | NA | NA | NA |
| N Stage | 0.113 | |||
| N0 | 1695 | 3 (2.734–3.266) | 1 (reference) | 1 |
| N1 | 610 | 2 (1.608–2.392) | 1.077 (0.976–1.188) | 0.142 |
| N2 | 3338 | 2 (1.799–2.201) | 1.084 (1.018–1.155) | 0.012 |
| N3 | 1718 | 3 (2.706–3.294) | 1.029 (0.957–1.107) | 0.443 |
| NX | 1593 | NA | NA | NA |
| Brain Met | 0.071 | |||
| Yes | 1733 | 2 (1.770–2.230) | 1 (reference) | 1 |
| None | 6678 | 3 (2.863–3.137) | 0.936 (0.884–0.992) | 0.025 |
| Unknown | 543 | NA | NA | NA |
| Liver Met | <0.001 | |||
| Yes | 2985 | 2 (1.835–2.165) | 1 (reference) | 1 |
| None | 5473 | 3 (2.836–3.164) | 0.760 (0.723–0.798) | <0.001 |
| Unknown | 496 | NA | NA | NA |
Abbreviations: LC, lung cancer; AI, American Indian/Alaska Native; API, Asian or Pacific Islander; Met, metastasis; AD, adenocarcinoma; SQCC, squamouscell carcinoma; LCLC, large cell carcinoma; SCLC, small cell carcinoma; NA, not available.
3.2. Risk factors for developing bone metastasis
As shown in Table 1, the possibility of BM at diagnosis were significantly associated with male, younger age, adenocarcinoma, poor tumor differentiation grade, higher N stage(N3), brain metastasis and liver metastasis, while the tumor size was demonstrated not to be an independent risk factor. In addition, there was no difference between T0 and higher T stage, but patients with T1 showed less chance of BM.
3.3. Survival and prognostic factors for BM
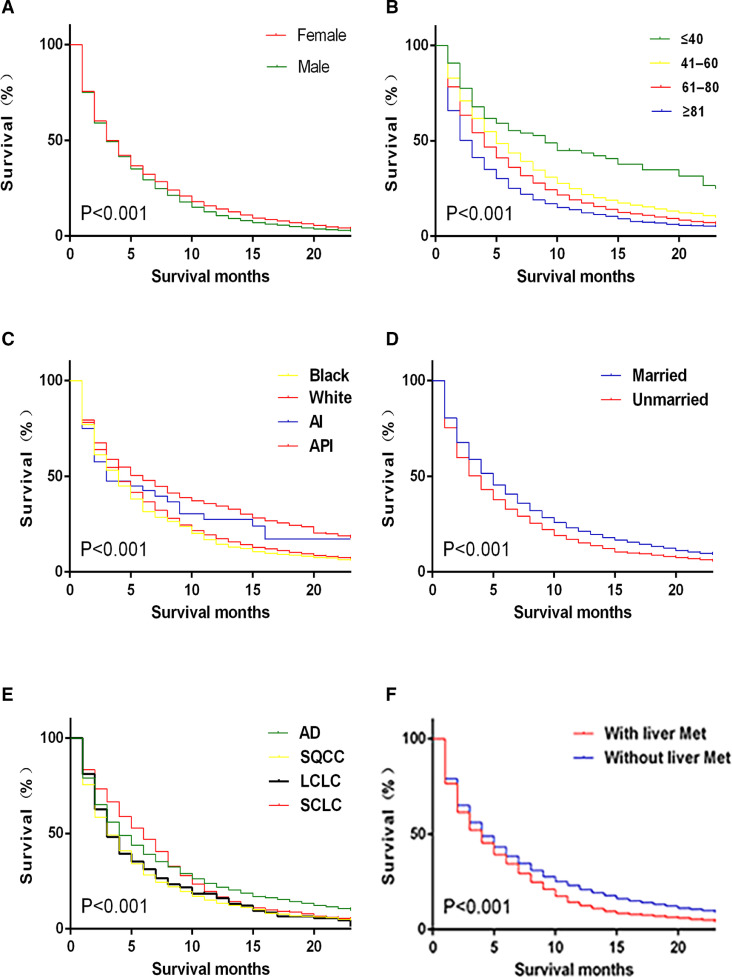
Vital prognostic factors selected by sex (Fig. 2A), age (Fig. 2B), race (Fig. 2C), marital status (Fig. 2D), pathologic type (Fig. 2E) and liver metastasis (Fig. 2F) were graphically displayed.
Fig. 2.
Kaplan–Meier analysis of overall survival among lung cancer patients with bone metastasis were displayed by sex (A), age (B), race (C), marital status (D), pathologic type (E) and liver metastasis (F).
Abbreviations: AI, American Indian/Alaska Native; API, Asian or Pacific Islander; Met, metastasis; AD, adenocarcinoma; SQCC, squamouscell carcinoma; LCLC, large cell carcinoma; SCLC, small cell carcinoma.
AS shown in Table 2, the prognostic factors for BM in lung cancer patients were observed clearly. In the multivariate Cox regression model, patients of male, older age, unmarried status, large cell carcinoma, squamouscell carcinoma, metastasis at liver were correlated with higher risk of poor prognosis. Grade, T stage, N stage and brain metastasis were not significantly correlated with survival. Although the tumor size was also an independent prognostic factor, the larger tumor size resulting in the worse the outcome was not observed. Patients with BM in Asian or Pacific Islander (PAI) displayed the better outcome, while there was no difference among other races on survival.
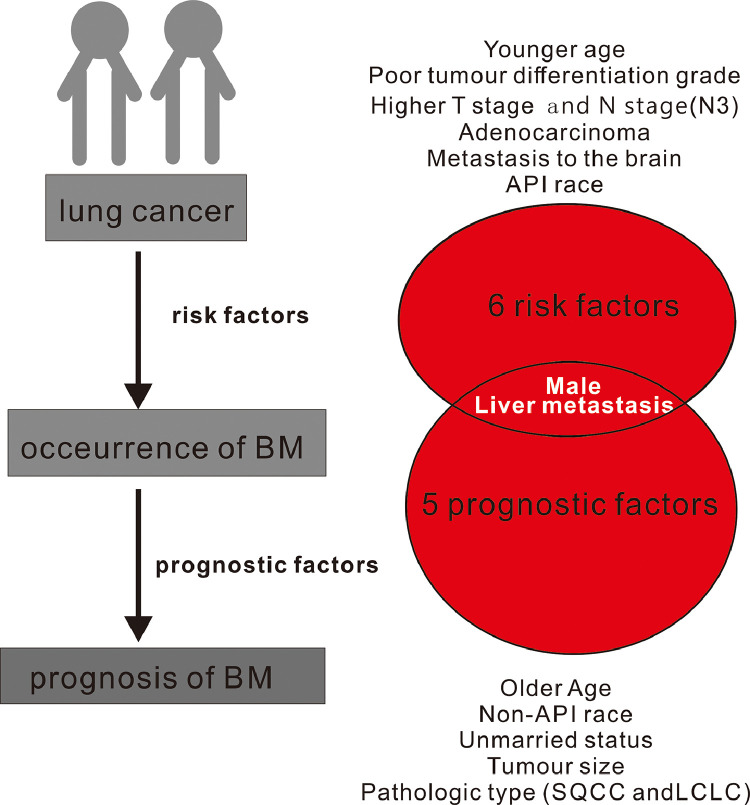
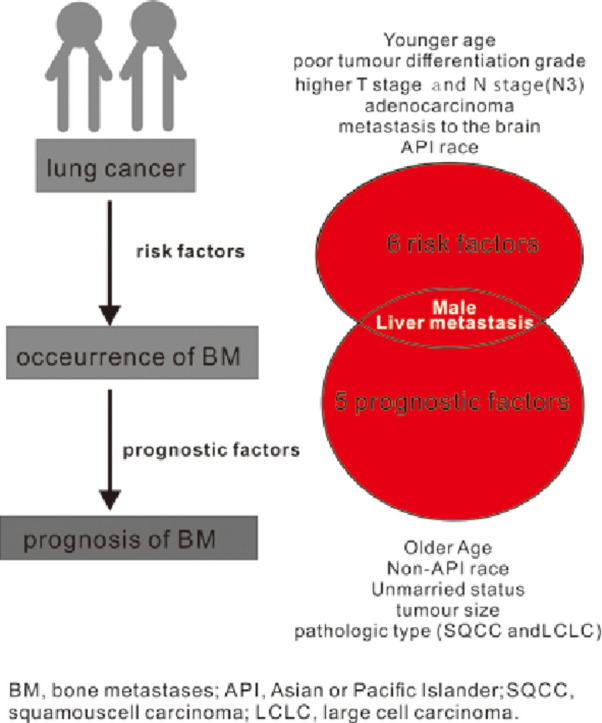
In our study, the homogeneous risk factors for the odds and prognosis of BM in lung cancer patients were male and metastasis at liver (Fig. 3). However, younger age, married status, poor tumor differentiation grade, higher T stage, higher N stage, metastasis at brain were all positively correlated with the development of BM but they were not the prognostic factors of BM. Older age and unmarried status both cause the worse survival of patients with BM but not influenced the occurrence of BM. Meanwhile, adenocarcinoma was prone to be the most risk factors for the occurrence of BM in different pathologic types, but did not cause the worst prognosis.
Fig. 3.
The homogeneous and heterogeneous risk factors and prognosis factor of BM in patients with lung cancer.
Abbreviation: BM, bone metastases; API, Asian or Pacific Islander; SQCC, squamous cell carcinoma; LCLC, large cell carcinoma.
4. Discussion
Based on the SEER database, we found that the amount of BM in lung cancer patients was staggering, accounting for 25.89%. In addition, BM morbidity still might be underestimated for ineligible cases. Therefore, it is vital to estimate the risk factors for patients in lung cancer and the prognosis for patients diagnosed with BM. Previously, only some small sample size has been used to estimate the prognosis of BM [17], [18]. Li Zhang et al. and Sugiura et al. only retrospectively observed 168 and 118 patients with BM and discussed the prognostic factors, respectively. In the large population-based cohort study, larger cases were carried out to analyze and predict the prognosis. In the study, we observed a number of lung cancer patients whose survival time is less than two months, and the dismal median survival of lung cancer with BM observed was lower than previous reports [17], [19]. This was not difficult to understand that lots of patients with lung cancer were not willing to accept treatment in hospital and chose natural death because of the poor physical state, so the part of cases might be missed and not be included in the previous literatures. However, based on the reliable and large data from SEER program, the survival time obtained from this study could reflect the real length of life of cancer patients.
A number of risk factors of BM development in lung cancer patients were found, including male, younger age, adenocarcinoma, poor tumor differentiation grade, higher N stage, metastasis at brain and metastasis at liver. Thus, doctors should pay more attention to these risk factors for their lung cancer patients. Furthermore, a skeletal scanning should be advised timely for their patients with these risk factors. In the future, the risk factors might be considered to be predictive factors of BM for lung cancer patients.
A number of prognostic factors of BM in lung cancer patients were correlated with male, older age, large cell carcinoma, squamouscell carcinoma, unmarried status, metastasis at liver and the tumor size. It was surprising that grade, T and N stage were able to confirm the prognosis of BM. Based on the above prognostic factors in the study, doctors might be capable of making a prognostic estimation and clinical guidelines for the lung cancer patients with BM effectively.
In our study, male carried a higher risk for the development of BM and poorer prognosis in lung cancer patients with BM. A previous study have shown that the median survival time did make the difference between two genders in lung cancer with bone metastasis; for male patients was 7.9 months, versus 13 months in female [17]. Male has been shown to portend poor survival in that study, and the difference was demonstrated to be statistically significant in our larger cohort study. We suspect that this phenomenon may be related to smoking and social stress in men, but we can't capture some basic information in the database.
Adenocarcinoma was considered to be the highest risk factors of BM development as a kind of pathologic type. To our knowledge, this is the first report to identify the risk factor at the time of diagnosis of BM. However, the prognosis of BM with large cell carcinoma or squamouscell carcinoma becomes worse than adenocarcinoma. The previous study by Sugiura et al. has reported that the patients of BM with nonadenocarcinoma had a worse prognosis, while those with adenocarcinoma alone had the favorable prognosis, which was consistent with our finding [17].
In addition, black race compared with whites were not found to have a higher risk of development and prognosis of BM, while PAI had the best outcome. As we all known, race diversity in cancer patients have been still existing and black men have higher cancer rates than other races. Probably, blacks were diagnosed with more advanced cancers than other races in the US [20]. In a latest report, the five-year survival of blacks was lower than whites for most cancers, but the disparity has been narrowed in men which can explain our founding to some extent [21]. Anyway, in our study, race diversity is not only a risk factor for the occurrence of BM, but also a risk factor for the prognosis of patients with BM. Therefore, racial disparities must be focused efforts on reducing.
Lung cancer with liver metastasis or brain metastasis continues to have a poor prognosis despite recent advances in medical level [22], [23], [24], [25], [26], [27]. Interestingly, BM patients with liver metastasis had a worse prognosis, but the presence or absence of brain metastasis did not affect survival. Because brain metastasis was not, like liver metastasis, significantly associated with the survival of lung cancer with BM, oncologists could try to conversely evaluate the risks of BM and/or liver metastasis on lung cancer with brain metastasis. Alternatively, they could evaluate the risks of brain metastasis and/or BM on lung cancer with liver metastasis. By doing these, they may hopefully identify factors which prolong patients' survival significantly longer than those identified by us in our manuscript. Similarly, younger people with lung cancer is more likely to have BM, but older patients with BM have a worse prognosis. The poorer prognosis related to age in non-small cell lung cancer with bone metastasis have been observed by Bae et al. [28]. Since unmarried status has a serious impact on the prognosis of patients with BM, we advocated maintaining a good marriage which could extend the life span of patients.
These astonishing discoveries deserved further analysis and exploration by medical researchers for the unclear causes. As mentioned above, it would be important that physicians must pay more attention to the heterogeneous risk factors for occurrence and prognostic of BM in patients with lung cancer. Based on our research, further studies looking into the potential explanations on the heterogeneous and heterogeneous risk factors were needed.
Except for the intention of estimating the risk factors, the other significance of this research related to the factors was the finding of cancer care and management. Even if, most of the factors, except age, associated with improved prognosis of lung cancer patients with BM only averagely prolong patients' survival for one month. But significant, it seemed that one month survival clinically also impacted and benefited patients too much on account of the poor prognosis. Furthermore, based on several meaningful risk factors observed in our study, patients' survival might be more different in the process of clinical evaluation or intervention. At the same time, we acknowledged that the difference of the possibility of BM or the survival time would be more significant through interfering with these factors, if we collected the cancer patient whose survival time was more than one year. However, oncologists were not able to recognize the accurate life span, so we considered that all lung cancer patients with adequate information initially diagnosed should be included.
Of course, several limitations existed in the present study. Firstly, a certain number of patients with incomplete and invalid information were excluded. Secondly, we can't research some basic information in the SEER database which could had an impact on patients, such as smoking, Body Mass Index, family history. Thirdly, several selection bias might exist in the retrospective trial.
5. Conclusion
In this study, we estimated the risk factors for occurrence and prognosis of lung cancer patients with BM in an effort to provide some information for making a potential treatment plan and clinical evaluation. In addition, there were two homogeneous risk factors and a number of heterogeneous factors, which deserved our attention.
CRediT authorship contribution statement
Wang Ben: . Chen Lijie: Writing - review & editing. Huang Chongan: Writing - review & editing. Lin Jialiang: Writing - review & editing. Pan Xiangxiang: Writing - review & editing. Shao Zhenxuan: Software. Hu Sunli: Software. Zhang Xiaolei: . Wang Xiangyang: .
Acknowledgments
Acknowledgments
This work is supported by grants from the National Nature Foundation of China (Grant nos. 81871806), the Zhejiang Public service technology research program/social development (LGF18H060008).
Disclosure
The author reports no conflict of interest in this work.
Contributor Information
Xiaolei Zhang, Email: zhangxiaolei@wmu.edu.cn.
Xiangyang Wang, Email: xiangyangwang@wmu.edu.cn.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Ten Haaf K., van Rosmalen J., de Koning H.J. Lung cancer detectability by test, histology, stage, and gender: estimates from the NLST and the PLCO trials. Cancer Epidemiol. Biomarkers Prev. 2015;24(1):154–161. doi: 10.1158/1055-9965.EPI-14-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch F.R., Scagliotti G.V., Mulshine J.L., Kwon R., Curran W.J., Jr., Wu Y.L., Paz-Ares L. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 5.Devesa S.S., Bray F., Vizcaino A.P., Parkin D.M. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int. J. Cancer. 2005;117(2):294–299. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 6.Kinoshita F.L., Ito Y., Morishima T., Miyashiro I., Nakayama T. Sex differences in lung cancer survival: long-term trends using population-based cancer registry data in Osaka, Japan. Jpn. J. Clin. Oncol. 2017;47(9):863–869. doi: 10.1093/jjco/hyx094. [DOI] [PubMed] [Google Scholar]
- 7.Lewis D.R., Check D.P., Caporaso N.E., Travis W.D., Devesa S.S. US lung cancer trends by histologic type. Cancer. 2014;120(18):2883–2892. doi: 10.1002/cncr.28749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meza R., Meernik C., Jeon J., Cote M.L. Lung cancer incidence trends by gender, race and histology in the United States, 1973–2010. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel M.I., McKinley M., Cheng I., Haile R., Wakelee H., Gomez S.L. Lung cancer incidence trends in California by race/ethnicity, histology, sex, and neighborhood socioeconomic status: an analysis spanning 28 years. Lung Cancer. 2017;108:140–149. doi: 10.1016/j.lungcan.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Tse L.A., Mang O.W., Yu I.T., Wu F., Au J.S., Law S.C. Cigarette smoking and changing trends of lung cancer incidence by histological subtype among Chinese male population. Lung Cancer. 2009;66(1):22–27. doi: 10.1016/j.lungcan.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 11.Sathiakumar N., Delzell E., Morrisey M.A., Falkson C., Yong M., Chia V., Blackburn J., Arora T., Kilgore M.L. Mortality following bone metastasis and skeletal-related events among patients 65 years and above with lung cancer: a population-based analysis of U.S. Medicare beneficiaries, 1999-2006. Lung India. 2013;30(1):20–26. doi: 10.4103/0970-2113.106127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J., Zhang Y., Sun X., Gusdon A.M., Song N., Chen L., Jiang G., Huang Y. The prognostic value of multiorgan metastases in patients with non-small cell lung cancer and its variants: a SEER-based study. J. Cancer Res. Clin. Oncol. 2018 doi: 10.1007/s00432-018-2702-9. [DOI] [PubMed] [Google Scholar]
- 13.Cook R.J., Major P. Multistate analysis of skeletal events in patients with bone metastases. Clin. Cancer Res. 2006;12(20 Pt 2):6264s–6269s. doi: 10.1158/1078-0432.CCR-06-0654. [DOI] [PubMed] [Google Scholar]
- 14.Luksanapruksa P., Buchowski J.M., Hotchkiss W., Tongsai S., Wilartratsami S., Chotivichit A. Prognostic factors in patients with spinal metastasis: a systematic review and meta-analysis. Spine J. 2017;17(5):689–708. doi: 10.1016/j.spinee.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Zhang R., Wang Z.Y., Li Y.H., Lu Y.H., Wang S., Yu W.X., Zhao H. Dynamic contrast-enhanced MRI to predict response to vinorelbine-cisplatin alone or with rh-endostatin in patients with non-small cell lung cancer and bone metastases: a randomised, double-blind, placebo-controlled trial. Lancet 388 Suppl. 2016;1:S95. doi: 10.1016/S0140-6736(16)32022-0. [DOI] [PubMed] [Google Scholar]
- 16.Cancer Incidence in Five Continents, IX, International Association of Cancer Registries, 2008, pp. 1–837.
- 17.Sugiura H., Yamada K., Sugiura T., Hida T., Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin. Orthop. Relat. Res. 2008;466(3):729–736. doi: 10.1007/s11999-007-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L., Gong Z. Clinical characteristics and prognostic factors in bone metastases from lung cancer. Med. Sci. Monit. 2017;23:4087–4094. doi: 10.12659/MSM.902971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decroisette C., Galerneau L.M., Hominal S., Chouaid C. [Epidemiology, management and cost of bone metastases from lung cancer] Rev. Mal. Respir. 2013;30(4):309–315. doi: 10.1016/j.rmr.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Jemal A., Simard E.P., Dorell C., Noone A.M., Markowitz L.E., Kohler B., Eheman C., Saraiya M., Bandi P., Saslow D., Cronin K.A., Watson M., Schiffman M., Henley S.J., Schymura M.J., Anderson R.N., Yankey D., Edwards B.K. Annual report to the nation on the status of Cancer, 1975-2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J. Natl. Cancer Inst. 2013;105(3):175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.C.E. DeSantis, R.L. Siegel, A.G. Sauer, K.D. Miller, S.A. Fedewa, K.I. Alcaraz, A. Jemal, Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities, CA Cancer J. Clin.66(4) (2016) 290–308. [DOI] [PubMed]
- 22.Bates J.E., Milano M.T. Prognostic significance of sites of extrathoracic metastasis in patients with non-small cell lung cancer. J. Thorac. Dis. 2017;9(7):1903–1910. doi: 10.21037/jtd.2017.06.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nussbaum E.S., Djalilian H.R., Cho K.H., Hall W.A. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer Cancer. 1996;78(8):1781–1788. [PubMed] [Google Scholar]
- 24.Ren Y., Dai C., Zheng H., Zhou F., She Y., Jiang G., Fei K., Yang P., Xie D., Chen C. Prognostic effect of liver metastasis in lung cancer patients with distant metastasis. Oncotarget. 2016;7(33):53245–53253. doi: 10.18632/oncotarget.10644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riihimaki M., Hemminki A., Fallah M., Thomsen H., Sundquist K., Sundquist J., Hemminki K. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86(1):78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Takayama K., Atagi S., Imamura F., Tanaka H., Minato K., Harada T., Katakami N., Yokoyama T., Yoshimori K., Takiguchi Y., Hataji O., Takeda Y., Aoe K., Kim Y.H., Yokota S., Tabeta H., Tomii K., Ohashi Y., Eguchi K., Watanabe K. Quality of life and survival survey of cancer cachexia in advanced non-small cell lung cancer patients-Japan nutrition and QOL survey in patients with advanced non-small cell lung cancer study. Support. Care Cancer. 2016;24(8):3473–3480. doi: 10.1007/s00520-016-3156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu K.L., Tsai M.J., Yang C.J., Chang W.A., Hung J.Y., Yen C.J., Shen C.H., Kuo T.Y., Lee J.Y., Chou S.H., Liu T.C., Chong I.W., Huang M.S. Liver metastasis predicts poorer prognosis in stage IV lung adenocarcinoma patients receiving first-line gefitinib. Lung Cancer. 2015;88(2):187–194. doi: 10.1016/j.lungcan.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Bae H.M., Lee S.H., Kim T.M., Kim D.W., Yang S.C., Wu H.G., Kim Y.W., Heo D.S. Prognostic factors for non-small cell lung cancer with bone metastasis at the time of diagnosis. Lung Cancer. 2012;77(3):572–577. doi: 10.1016/j.lungcan.2012.05.094. [DOI] [PubMed] [Google Scholar]