Inflammatory bowel disease (IBD) is associated with immune dysregulation triggered by environmental factors, microbial dysbiosis, and genetical susceptibility. Regulatory T cells (Tregs) are critical in controlling intestinal immune homeostasis and Treg deficiencies trigger intestinal inflammation.1, 2 Interleukin (IL)-2 is a key cytokine controlling differentiation, survival, and function of Tregs.3 In contrast to conventional T cells (Tcon), Tregs exhibit higher sensitivity to IL-2 due to constitutive expression of CD25, the high-affinity subunit of the IL-2 receptor.3 Low-dose (LD) IL-2 has been reported to selectively expand Tregs and used as a therapeutic strategy in chronic graft-versus-host disease, hepatitis C virus–induced vasculitis, systemic lupus erythematosus, and Wiskott-Aldrich syndrome.4, 5, 6, 7 Thus, we sought to investigate LD IL-2 as an IBD therapeutic using humanized mice.
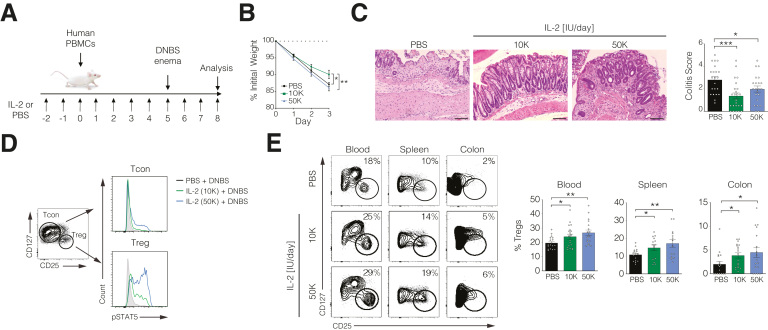
Similar to other disease settings,4, 5, 6, 7 LD IL-2 specifically activated peripheral blood and colonic lamina propria Tregs from patients with IBD in vitro (Supplementary Figure 1). To study the efficacy of LD IL-2 in a preclinical setting, we treated NSG mice reconstituted with healthy donor peripheral blood mononuclear cells with phosphate-buffered saline (PBS) or LD IL-2 (1.0 × 104 IU/day [10K]; 5.0 × 104 IU/day [50K]) (Figure 1A). On day 5 following immune reconstitution, colitis was induced by rectal application of 2,4-dinitrobenzene sulfonic acid. Mice receiving 10K IL-2 had reduced weight loss and reduced histology scores compared with mice treated with PBS or 50K IL-2 (Figure 1B and C). Phospho-flow analysis of STAT5 confirmed that 10K IL-2 specifically activated Tregs, whereas STAT5 phosphorylation was also detected in Tcons from mice receiving 50K IL-2 (Figure 1D). Expansion of Tregs was observed in blood, spleen, and colon of mice treated with either dose of IL-2 (Figure 1E). However, reduced body weight loss upon treatment with 10K IL-2 associated with Treg expansion in the absence of significant Tcon activation suggest that a therapeutic range of LD IL-2 is critical (Figure 1C–E).
Figure 1.
Low-dose (LD) interleukin (IL)-2 selectively expands human regulatory T cells (Tregs) in vivo and alleviates experimental colitis. (A) Schematic of low-dose interleukin-2 treatment of 2,4-dinitrobenzene sulfonic acid (DNBS)–induced colitis in NSG mice reconstituted with human peripheral blood mononuclear cells (PBMCs). (B) Percent initial body weight and (C) histological analysis of distal colons following 3 days post-DNBS enema. With 20× magnification. Scale bars = 100 μm. (D) Phospho-flow analysis of pSTAT5 in splenic conventional T cells (Tcons) and Tregs. (E) Representative FACS showing Tregs in blood, spleen, and colon. Graphs are pooled data from 5 experiments. 10K, 1.0 × 104 IU/day; 50K, 5.0 × 104 IU/day; PBS = phosphate-buffered saline. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001.
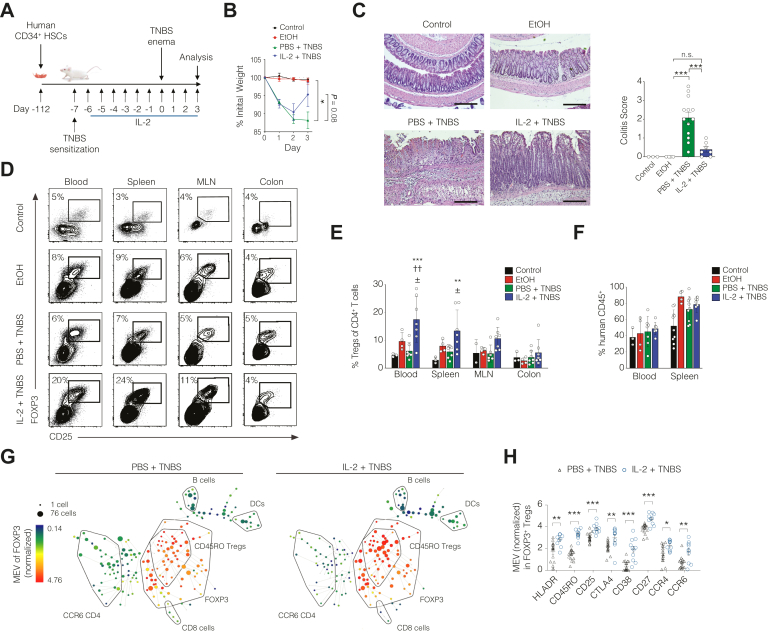
To evaluate the efficacy of LD IL-2 in a fully reconstituted humanized murine system, we developed NSG mice that lack murine MHCII but express human HLA-DQ8 (NSGIIDQ8 mice). Sixteen-week-old mice reconstituted with human healthy donor CD34+ hematopoietic stem cells at birth were sensitized with 2,4,6-trinitrobenzenesulfonic acid (TNBS) and treated with 10K IL-2 daily followed by induction of colitis with TNBS rectal challenge (Figure 2A). In contrast to mice treated with PBS, mice receiving LD IL-2 exhibited significant improvement in histological disease activity with a trend in reduced weight loss (Figure 2B and C). LD IL-2 was associated with significant expansion of human Tregs in the blood and spleen but not in the mesenteric lymph nodes or colon (Figure 2D and E). Correspondingly, no difference in the frequency of FOXP3-expressing T cells was detected using RNAscope analysis of paraffin-embedded colonic lamina propria sections (Supplementary Figure 2). The expansion of peripheral Tregs and improvement in disease activity in LD IL-2-treated mice was not attributed to alterations in the human immune reconstitution between groups (Figure 2F) or frequency of effector T or natural killer cells (Supplementary Figure 3).
Figure 2.
Low-dose (LD) interleukin (IL)-2 expands human CD45RO+regulatory T cells (Tregs) and ameliorates colitis in humanized mice. (A) Schematic depicting 2,4,6-trinitrobenzenesulfonic acid (TNBS) in NSGIIDQ8 mice reconstituted with CD34+ hematopoietic stem cells (HSCs). (B) Percent initial body weight and (C) representative hematoxylin and eosin–stained distal colon sections with histological colitis scores from 2 independent experiments. With 10× magnification. Scale bars = 200 μm. (D and E) Representative flow cytometry plots and statistical analysis of human Tregs in blood, spleen, mesenteric lymph node (MLN), and colon. ∗∗P < .01 or ∗∗∗P < .001 compared to control, ††P < .01 compared to EtOH, ± P < .001 compared to TNBS. (F) Frequency of human CD45+ cells in blood and spleen. (G) SPADE analysis from 37 analyte CyTOF of splenic CD25+ cells from phosphate-buffered saline (PBS)– and LD IL-2–treated mice. Each circle represents cells with a similar phenotype. Circle size is proportional to the number of cells. Heat color is the median expression value (MEV) of FOXP3 in arcsinh15 scale. (H) MEV quantified for various activation or functional Treg markers in splenic CD25+ cells based on the top 10 nodes of the SPADE analysis within the FOXP3+ cells. Data are pooled from 3 independent experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001.
Tregs have been reported to suppress pathogenic effector T cell function and autoimmunity through both contact-dependent and contact-independent mechanisms.8 CyTOF analysis of splenic CD25+ cells showed an increased frequency of FOXP3+ T cells in mice receiving LD IL-2 with majority of expanded cells falling within the CD45RO+FOXP3+ Treg cluster (Figure 2G). The majority of FOXP3+ Tregs that expanded after LD IL-2-treatment exhibited increased expression of molecules associated with Treg activation or function (HLA-DR, CD45RO, and CTLA4) or chemokines important for trafficking and migration to sites of inflammation (CCR4 and CCR6) (Figure 2H). Our data suggest that expansion and activation of memory Tregs might be critical clinical determinants.
Taken together, our study demonstrates that LD IL-2 expands Tregs and ameliorates experimental colitis in humanized mice. While these data support a rationale for LD IL-2 in IBD therapy, the safety of long-term drug administration needs further investigation to ensure continued selective activation of Tregs over Tcons. Based on these promising results, we have initiated a phase 1b/2a clinical trial investigating the safety and therapeutic efficacy of LD IL-2 in patients with moderate to severe ulcerative colitis (NCT02200445).
Acknowledgments
The authors thank the Harvard Digestive Disease Center for their core support services.
Footnotes
Conflicts of interest These authors declare the following: Aisling O’Hara Hall, Joshua R. Friedman, Jennifer E. Towne, and Scott E. Plevy are employed by Janssen Research and Development. Scott B. Snapper consults for Amgen and Hoffman La Roche and has been on scientific advisory boards of Janssen, Pfizer, Celgene, IFM Therapeutics, and Pandion Inc. Jeremy A. Goettel has been on the scientific advisory board of Allergan, and has grant support from Pandion Inc. The remaining authors declare no conflicts.
Funding The work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) DK106311 (to Jeremy A. Goettel), Crohn's and Colitis FoundationCDA 352644 (to Jeremy A. Goettel) and 422348 (to Liza Konnikova), NIDDK P30 DK034854, the Helmsley Charitable Trust, Boston Children’s Hospital Translational Investigator Service Award, and the Wolpow Family Chair in IBD Treatment and Research (to Scott B. Snapper), the DAAD network “Research for Rare Diseases and Personalised Medicine” (David Illig), Reinhard-Frank Stiftung, the International Pediatric Research Foundation, and the Daimler und Benz Stiftung (Daniel Kotlarz). The authors declare that financial support for some of the mouse studies was provided by Janssen Research and Development.
Contributor Information
Jeremy A. Goettel, Email: jeremy.goettel@vumc.org.
Scott B. Snapper, Email: scott.snapper@childrens.harvard.edu.
Supplementary Material
References
- 1.Brunkow M.E. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 2.Bennett C.L. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 3.Klatzmann D. Nat Rev Immunol. 2015;15:283–294. doi: 10.1038/nri3823. [DOI] [PubMed] [Google Scholar]
- 4.He J. Nat Med. 2016;22:991–993. doi: 10.1038/nm.4148. [DOI] [PubMed] [Google Scholar]
- 5.Koreth J. N Engl J Med. 2011;365:2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saadoun D. N Engl J Med. 2011;365:2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 7.Jyonouchi S. Clin Immunol. 2017;179:47–53. doi: 10.1016/j.clim.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.