Abstract
Background: Studies of morphometric variation make it possible to delimit species and geographic intraspecific variation, mainly in species with wide distribution ranges. In the Neotropical otter, Lontra longicaudis, variation in the shape of the rhinarium of three potential subspecies has been described but it is not known whether there is a pattern to the morphometric variation in the skull throughout the distribution of this species. We analyzed morphological variation in the cranium (ventral view) and the mandible (lateral view) of the Neotropical otter, comparing male and female specimens and evaluating the differences between specified geographic units utilizing methods from geometric morphometrics. Specimens from the entire distribution of the species were analyzed. Between sexes, variability in the shape was determined by calculating the Procrustes distances and using Goodall’s F test. Geographic variationwas analyzed using a discriminant analysis, an analysis of variance (ANOVA) on a matrix of partial warp scores, and a cluster analysis with Mahalanobis distances, allowing for similarities in shape to be identified between different geographic units. Variation in the size of the two structures was calculated based on the values for centroid size using a one-way ANOVA with a Bonferroni correction and a 95 % confidence interval.
Results: There was sexual dimorphism in shape for both views, with males the largest. In general, there was geographic variation in the shape and size of both the cranium and the mandible in the Neotropical otter, exhibiting a pattern that resembled Bergmann’s rule. Variation in shape between geographic units could result from the presence of geographic barriers, the spatial configuration of hydrological regions, and/or the large distances between populations throughout this species’ distribution.
Conclusions: The Neotropical otter exhibits dimorphism in the size, but not in the shape of the skull. There is geographic variation between geographic units, and our results suggest that L.longicaudis could bea group of species. An integrative study using molecular and morphological data could elucidate its taxonomy.
Keywords: Geographic variation, Geometric morphometrics, Lontra longicaudis, Sexual dimorphism
BACKGROUND
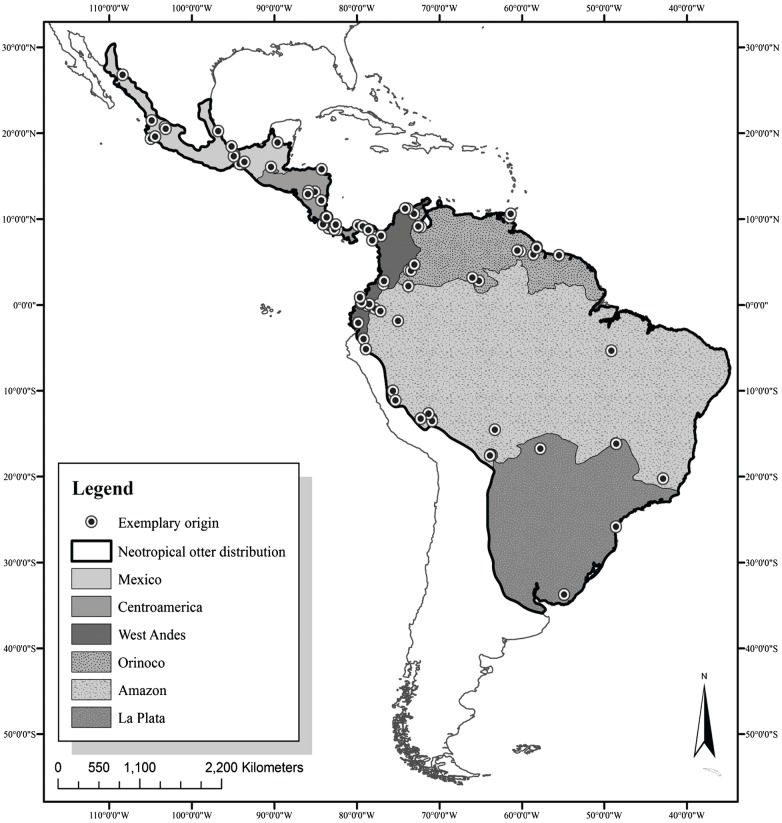
The Neotropical otter (Lontra longicaudis) is an aquatic species with a wide distribution that is limited to the terrestrial-aquatic ecotone that corresponds to lagoon and riparian systems (Gallo-Reynoso et al. 2008). This species is Neotropical in origin and has a continuous distribution from Argentina in South America to the Trans-Mexican Volcanic Belt (Fig. 1). The latter is a mountain range that divides the country in two, follows the outline of both the Atlantic and Pacific slopes, and ultimately reaches its northern extreme in the state of Sonora on the Pacific slope with its other extreme in Tamaulipas on the Gulf of Mexico slope (Gallo-Reynoso 1997). Sexual dimorphism has been reported for L. longi- caudis, with males larger than females (Kruuk 2006). Along its distribution, this otter occupies habitats such as coastal lagoons, rivers, and lakes that are located in distinct geo- graphic and climatic regions, from arid zones to wetlands and from tropical to temperate forests (Larivière 1999). The composition of its diet is also variable between regions and seasons, though different studies have shown its diet is largely made up of fish and crustaceans and to a lesser extent amphibians, mammals, birds, and turtles (Gallo- Reynoso et al.2008; Carvalho-Junior et al. 2010;Chemes et al.2010; Platt and Rainwater 2011). Owing to these factors and the variation in its habitat in terms of vegetation, cli- mate, and food availability throughout its extensive distri- bution, potential changes in the cranial morphology of L. longicaudis may be interpreted as local adaptations to en- vironmental variables such as different types of prey.
Fig. 1.
Fig. 1 Geographic distribution of Lontra longicaudis, indicating the origin of the specimens used, and the six geographic units: Mexico (MEX), Central America (CEAM), Western Andes (AND), Orinoco (ORI), Amazon (AMA), and La Plata (PLA)
The wide geographic distribution of L. longicaudis and the variation in its aquatic habitats give rise to two hy- potheses of morphological variation. The first hypothesis is that there should be a differentiation in the shape and size of the cranium and mandible between the sexes, and the second is that these variables differ between populations of the different geographic regions throughout its distribution.
In order to test these hypotheses, we used tech- niques based on geometric morphometrics (Kendall 1984; Bookstein 1991; Lawing and Polly 2010) which describe and interpret changes in the shape of different anatomical structures of organisms, based on comparisons of homologous structures (Adams et al. 2004). This ana- lysis of anatomical shape allows for a better under- standing of morphological evolution and adaptive effects (Zelditch et al. 2004) since variation in shape is influenced by environmental factors (Rohlf and Marcus 1993).
Morphometric analysis of the cranium of several species has indicated that patterns of morphological variation in species with large distribution areas might be adapta- tions to the range of environmental conditions occurring throughout their distribution (Zelditch et al. 2004; Cardini et al. 2007). This pattern has been observed in mammals such as the puma (Puma concolor), the tiger (Panthera tigris), and the brown bear (Ursus arctos), for which variation in the size and configuration of the cranium are correlated with the different geographic regions through- out which their populations are distributed (Gay and Best 1996; Baryshnikov and Puzachenko 2011; Mazák 2011). These patterns of morphological variation are associated with habitat variables such as temperature, precipitation, and latitude (Platz et al. 2011). Patterns of change in the mandible associated with contrasting habitats have been observed in several species of rodents (Duarte et al. 2000). This also occurs for species like the titus monkey, genus Leontopithecus (Freitas et al. 1999), and for bats of the genus Monophyllus (Mancina et al. 2010). And for the green monkey, Cercopithecus aethiops, there is a close re- lationship between rainfall pattern and the length of its face (clinal variation) (Cardini et al. 2007).
Patterns of morphological change associated with geo- graphic regions are even more notable in species with a high level of habitat specialization, such as aquatic spe- cies (Langerhans et al. 2003; Jonsson and Jonsson 2001). For the cichlid Biotodoma wavrini and the characid Bryco- nops caudomaculatus, distinct morphological adaptations have been found for their two contrasting habitats, riverchannels and lagoons, where morphological variation increases with increasing geographic distance between populations and with variation in other environmental factors (Langerhans et al. 2003).
Morphological variation in the Eurasian otter (Lutra lutra) has only been assessed using linear morphometric analysis. For example, Ruiz-Olmo et al. (1998) reported that the age of L. lutra may be determined on the basis of its cranial morphology. And variation in cranium size has also been found over the range of this species, with more variability in animals in the tropics (Baryshnikov and Puzachenko 2012). This technique demonstrated thatthere is a significant difference in cranial morphology between healthy populations of otters and populations considered at risk due to decreased population size and habitat quality (Pertoldi et al. 2000). In addition, these studies have made it possible to explore any effects of contaminants on the morphology and ontogenesis of the cranium of L. lutra (Pertoldi et al. 1998) and other species such as Ursusmaritimus (Pertoldi et al.2012).
Several taxonomic studies of L. longicaudis have pos- tulated that it is potentially composed of a number of species based primarily on the differentiation of its rhi- narium (Pohle 1920; Cabrera 1957; Harris 1968). The most recent studies have suggested that populations with distinct rhinarium morphology may be conspecific and that these populations may be subdivided geographically into three subspecies: (I) L. longicaudis annectens, found from Mexico to Colombia and Ecuador; (II) L. longicaudis enudris, distributed in French Guiana, Suriname, Peru, and Trinidad and Tobago; and (III) L. longicaudis longicaudis, distributed throughout most of South America from Brazil to Uruguay and Argentina (Van Zyll 1972; Larivière 1999). However, this classification has also been questioned recently by Trinca et al. (2012) as a result of new insights into the phylogenetic structure of the South American populations based on mitochondrial sequence analysis. These authors mentioned that an intra- specific subdivision continues to be controversial due to genetic differentiation within the different geographic re- gions, and indicated that an integrative study would be ne- cessary to examine morphological variation in the species, including an analysis of cranial morphometrics.
The present study has the objectives of evaluating changes in the morphology of the cranium and mandible of L. longicaudis using geometric morphometric tech- niques and examining the variation in the configuration (shape and size) of the cranium and the mandible between sexes and among the different geographic regions of its distribution. Morphological variation associated with distinct geographic regions throughout which the otter is distributed would support previously reported gen- etic differences.
METHODS
Sampling
The crania of 151 specimens of L. longicaudis from local- ities throughout its distribution were examined (Fig. 1). The specimens were housed in the National Collection of Mammals of the National Autonomous University of Mexico, the Mammal Collection of the Autonomous University of Campeche, the Vertebrate Collection of the Biological Station of the National Autonomous University of Mexico in Los Tuxtlas, the Mammal Collection of the University of Kansas, the American Museum of Natural History, the Mammal Collection of the Field Museum, the National Museum of Natural History of the Smithsonian Institute, the Carnegie Museum, and the Michigan Uni- versityMuseum (see list of specimens in Additional file 1). Since the Neotropical otter is a threatened species and protected in the majority of the countries where it is found, the analysis was limited to cranium samples from the aforementioned collections.
Each specimen was assigned to one of the three age categories using the criteria of Van Zyll (1972): juvenile (presence of deciduous teeth), subadult (a full set of fully erupted teeth, presence of nasal sutures, and occipital- parietal sutures), and adult (absence of maxillary-nasal and occipital-parietal sutures). For the morphometric analysis, only adult samples were used to avoid the er- rors associated with the modifications that the cranium undergoes when the animal is growing (Caumul and Polly 2005).
The samples were grouped a priori into six geographic units (GUs): Mexico, Central America, Western Andes, Orinoco, Amazon, and La Plata (Fig. 1) based on the main hydrological delimitations throughout this otter’s distribution and on patterns of morphological and gen- etic structure observed among the different populations of other aquatic species in South America (Banguera- Hinestroza et al. 2002; Hubert and Renno 2006; Arza- mendia and Giraudo 2009), including the Neotropical otter (Trinca et al. 2012).
Digitization of images, recording landmarks, and semi- landmarks
The analysis of variation in cranial morphology was based on digital images of the cranium in ventral view (n = 64) and the mandible in lateral view (n = 87). All photographs were taken by the same person to avoid any variation associated with possible different points of view of observers. Specimen placement was stan- dardized, and the crania were photographed on the right side of the face, from a distance of 40 cm from the camera lens and oriented at an angle of 90°. All images were captured using a Sony Alpha 200 reflex camera with a resolution of 3872 × 2592 pixels. A ruler was included in each photograph as a reference scale.
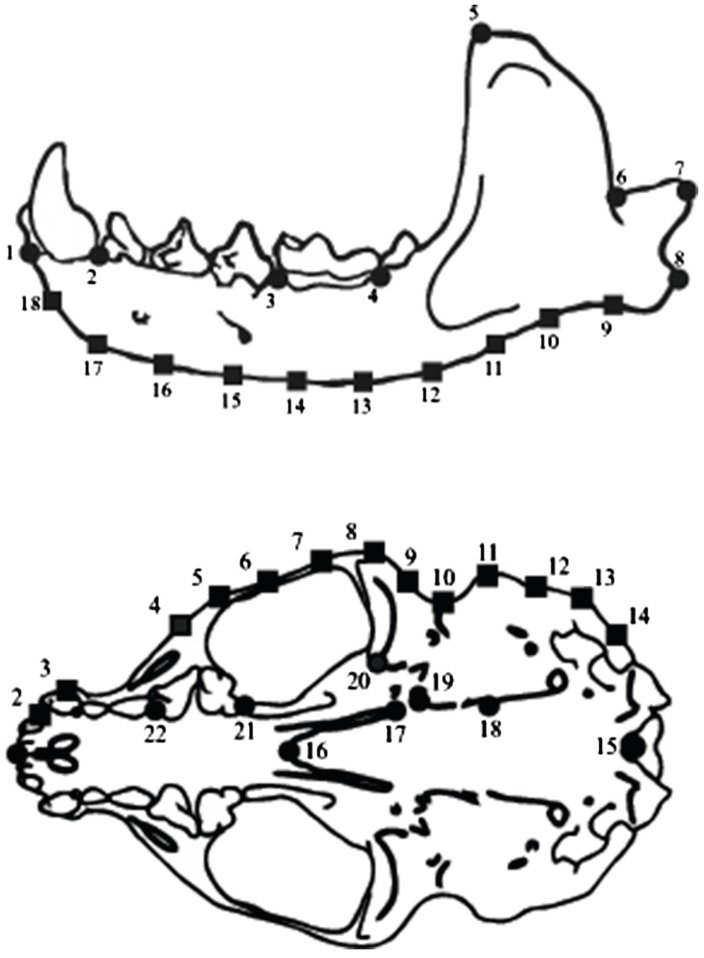
The use of only the right side of the cranium and the mandible to locate the landmarks assumes bilateral symmetry in these structures. The configuration of the cranium and the mandible was recorded using land- marks (LMs), which are natural points located on homologous sites and that can be easily located on any specimen (Bookstein 1997), and semi-landmarks (SLMs), which are located along the homologous out- lines of the structures and are based on templates drawn between the LM points (Bookstein 1991; Gunz and Mitteroecker 2013). Outlines were drawn using MakeFan6 software (Sheets 2003) to facilitate the loca- tion of the SLMs and were based on two LMs (num- bers 1 and 8 for the mandible and 1 and 16 for the cranium, Fig. 2). LMs and SLMs were digitized for each specimen using the TpsDig 2.15 software (Rohlf 2001), and configuration matrices obtained for each view. For these matrices, a generalized Procrustes superposition adjustment (Rohlf and Slice 1990) was applied using the software CoorGen6f (Sheets 2005a), where variation associated with the effects of positioning, orientation, and scale is minimized by calculating the sum of the squared differences between corresponding landmarks (Bookstein 1991; Zelditch et al. 2004). The placement of the outlines for the positioning of the SLMs results in a shape distor- tion during the Procrustes adjustment (Bookstein 1996; Gunz and Mitteroecker 2013), so a second adjustment was made to eliminate variation by sliding the SLMs based on the alignment distance criteria of the Semiland soft- ware (Sheets 2002).
Fig. 2.
Fig. 2 Landmarks used to capture the configuration of the mandible (LM = 8 and SLM = 10) and the ventral view of the cranium (LM = 9 and SLM = 14) of Lontra longicaudis. Circles are LM and squares SLM
For each configuration of LMs and SLMs (2 LMs and 21 SLMs on the ventral cranium view; 2 LMs and 16 SMLs on the lateral mandible view), an eigen analysis was done following the methodology of Bookstein (1991) and Rohlf (1996). This analysis allows a tangent space to be converted to a Euclidian space, making it possible to use conventional multivariate statistical ana- lyses (Rohlf 1996). The average general shape was used as a reference, and each sample was described in terms of its deviation from the average form for all specimens. The resulting matrices describe change in shape (partial warp scores, PWS) and size as measured from the cen- troid size (CS). These variables were used in subsequent analyses to describe the change in shape and size of the skull and mandible.
Statistical analysis
A two-way analysis of variance (ANOVA) for centroid size and a two-way multivariate analysis of variance (MANOVA) for shape were performed to test a priori the differences between sexes and among GUs. Speci- mens from La Plata were excluded from both analyses of the ventral view because only females were available for this GU. These analyses were run using Statistica 8.0 software (StatSoft 2007).
Sexual dimorphism in shape and size
Because sexual dimorphism has been reported for sev- eral species of otter (Kruuk 2006), we analyzed shape and size differences between sexes, based on 70 man- dible images (36 male and 34 female) and 56 cranium images (29 male and 27 female). To this end, a princi- pal component analysis (PCA) and two-way MANOVA were run to test for sexual dimorphism in the shape of the mandible and the cranium. Subsequently, a permutation test was performed using Goodall’s F test, designed to compare Procrustes coordinates (Goodall 1991, Zelditch et al. 2004), as implemented in the soft- ware TwoGroup6 (IMP series; Sheets and Zeldich 2001). The significance of the differences was tested with 2500 bootstrap permutations. The variation in size between the sexes was analyzed using an ANOVA with Bonferroni correction and a 95 % confidence interval and run in Statistica 8.0 (StatSoft 2007). These analyses were run to determine whether the data for males and females could be pooled for subsequent tests.
Geographic variation in shape and size
Due to the low number of specimens used for the ana- lysis of geographic variation and the fact that no signifi- cant sexual dimorphism was found for either structures (see “Results”), the data for both sexes were combined to analyze for geographic variation.
Canonical variables were extracted in a multidimen- sional space in order to explain the majority of the vari- ation between groups for which the partial warp scores matrix was used. To assess shape variation between GUs in both views, a canonical variate analysis (CVA) was run in CVAGen6 software (Sheets 2005b). We obtained thin-plate spline plots (deformation grids describing shape changes) by regressing the PWS onto the first and second canonical variables. Subsequently, a discriminant function analysis (DFA) using Mahalanobis distances was calculated to assess the validity of the a priori classification of the specimens into six GUs.
Applying a MANOVA, a significant difference in shape was detected between the distinct GUs. To describe morphological similarity between GUs, a cluster analysis with the Unweighted Pair Group Method with Arith-metic Mean (UPGMA) was used, using the Mahalanobis distance between GUs. The DFA and UPGMA were run in Statistica 8.0 (StatSoft 2007).
Variation in cranium and mandible size between the six GUs of L. longicaudis was analyzed using a one-way ANOVA, with log-natural-transformed values of centroid size. Post hoc comparisons among GUs were run using a Bonferroni correction and a 95 % confidence interval in Statistica 8.0 (StatSoft 2007).
RESULTS
Sexual dimorphism in shape and size
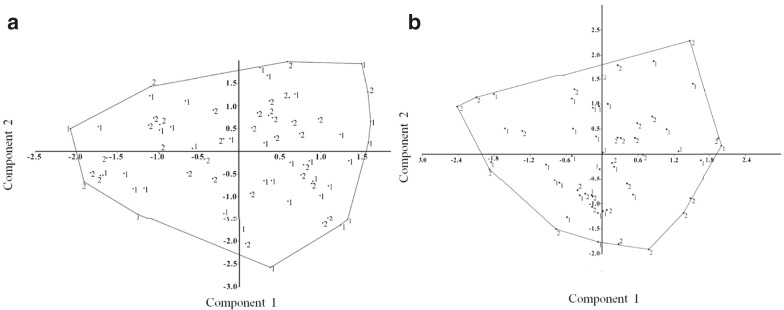
The PCA of the skull and the mandible indicate a small proportion of the variance was explained by the first two principal components, 63.6 and 42.9 %, respectively. Plots of these two components for each structure showed continuous variation between male and females, indi- cating the absence of sexual dimorphism (Fig. 3). This was confirmed by Goodall’s F tests which showed that there were no significant differences in the shape of either structure between sexes (mandible: Goodall’s F(32) = 0.75, p= 0.84224; skull: Goodall’s F(42) =1.62, p= 0.1271). Hence, we pooled female and male samples in subsequent analyses. The results of the ANOVA for centroid size indicated that males are significantly different from females in both skull size (F(1,54) = 6.2030, p = 0.0158) and mandible size (F(1,68) = 9.8040, p = 0.0025), with males larger (me- dian CS mandible = 103.76, median CS skull = 169.76) than females (median CS mandible = 95.76, median CS skull = 161.53) for both views.
Fig. 3.
Fig. 3 Principal component analysis (PCA) of the variation in shape between sexes, where 1 = males and 2 = females. a PCA of the mandible with 49.9 % of the variation explained by the first axis and 12.7 % by the second. b PCA of the ventral view of the cranium with 30.3 % of the variation explained by the first axis and 12.6 % by the second
Geographic variation in the shape of the mandible and the cranium
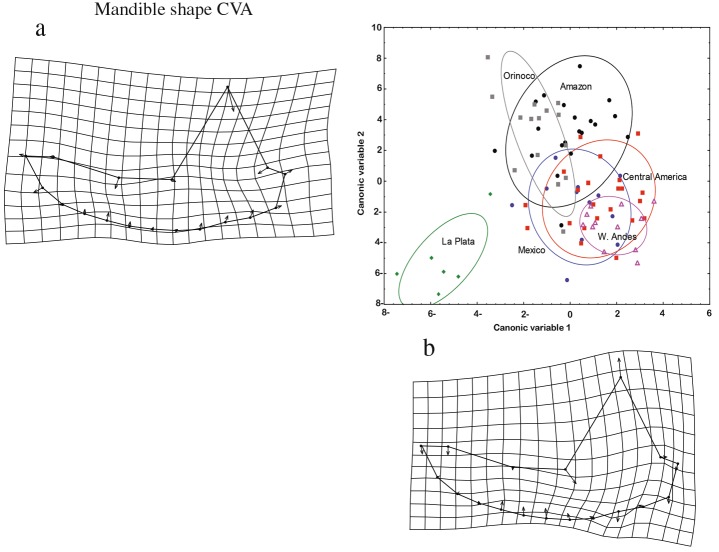
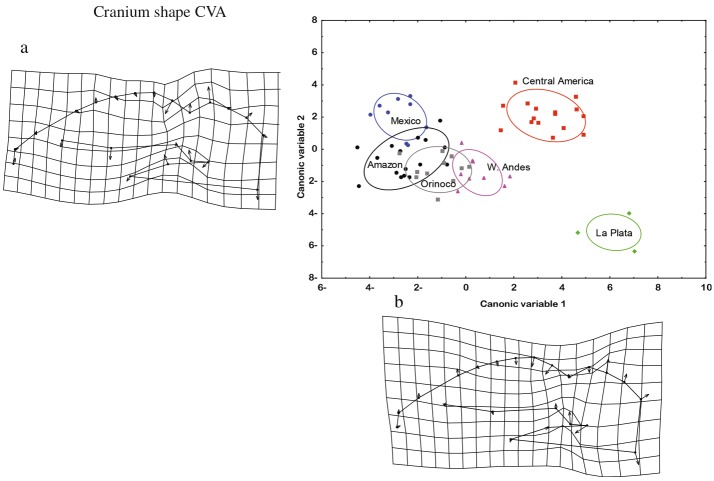
The MANOVA detected significant differences in skull (Wilks’λ=0.014, F= 1.362, p< 0.05) and mandible (Wilks’ λ = 0.013, F = 1.606, p < 0.001) shapes between GUs. The CVA plots depict the patterns of change between the GUs defined in this study for both structures (Fig. 4). For both views, the samples from La Plata differed the most from the samples from the other GUs. The shape of the cra- nium had the most marked differences between GUs. For the change in the deformation axes of the mandible be- tween GUs, the VC1 values indicate a change in the height of the coronoid process, which includes the emergence of the first and second molars, and in the width of the set of teeth. For the second canonical correlation (VC2), a change in shape was found between landmarks 1 and 8, which corresponds to the length of the mandible.
Fig. 4.
Fig. 4 a, b Analysis of the canonical variables and planes of deformation for the shape of the mandible in lateral view between geographic units. The arrows indicate the direction and magnitude of change
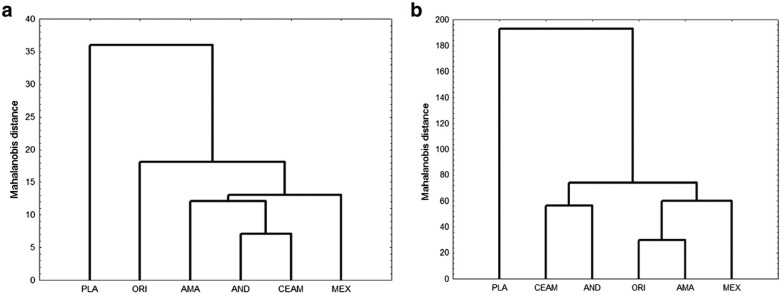
The deformation grid of the cranium between the GUs along VC1 (Fig. 5) shows a change in the shape of the posterior neurocranium (LMs 12–14) and the foramen magnum (LM 15), in addition to a decrease in the widthof the zygomatic arch. For VC2, there is an increase in the length of the mastoid bone (LM 11), along with an increase in the distance between the pterygoid muscles (LM 17) and a decrease in the distance between the rows of molars and premolars (LMs 21 and 22). The DFA of the mandible demonstrated that the a priori grouping of the specimens in six GUs is appropriate, with the majority of mandible samples correctly assigned (89.6 %) (Fig. 6). The analysis of the mandible for specimens from the six GUs using the Mahalanobis dis- tances resulted in three groups. The first group is formed by the Amazon region, the Western Andes, Central Amer- ica, and Mexico, Mexico being the region with the most differences within this grouping. The second group has the specimens from the Orinoco, and the third group is formed by the specimens from La Plata, the region with the lowest level of similarity with respect to the other GUs (Fig. 6).
Fig. 5.
Fig. 5 a, b Analysis of the canonical variables and planes of deformation for the shape of the cranium in ventral view between geographic units. The arrows indicate the direction and magnitude of change
Fig. 6.
Fig. 6 UPGMA dendrogram of geographic samples based on the Mahalanobis distances matrix for the a mandible and b cranium. MEX Mexico, CEAM Central America, PLA La Plata, AND Western Andes, AMA Amazon, and ORI Orinoco
The deformation grid of the cranium between theGUs along VC1 (Fig. 5) shows a change in the shape of the posterior neurocranium (LMs 12–14) and the foramen magnum (LM 15), in addition to a decrease in thewidth of the zygomatic arch. For VC2, there is an increase in the length of the mastoid bone (LM 11), along with an increase in the distance between the pterygoid muscles (LM 17) and a decrease in the distance between the rows of molars and premolars (LMs 21 and 22).
The DFA of the cranium samples indicated that 98.43 % were correctly assigned to the GUs by the discriminant analysis (Fig. 6). The analysis of the grouping of the six GUs using the Mahalanobis distances shows that the vari- ation in shape of the mandible of L. longicaudis allowed the specimens to be classified into three groups. The first group includes specimens from the Amazon, Orinoco, and Mexico, Mexico being the unit with the most differ- ences in shape within this larger grouping. The second most different group is that of the Western Andes and Central America. The third group has the samples from La Plata, the region with the lowest similarity with respect to the other GUs (Fig. 6).
Geographic variation in size
Differences werefound (F(5,81) = 3.9603, p= 0.0029) in the size of the mandible between the specimens from distinct GUs. The multiple comparison test with a Bonferroni cor- rection classified the GUs into three groups. The first group included Western Andes, the Amazon, and Central America (average centroid size 107.27); the second Ori- noco and La Plata (average centroid size 112.28), and the third Mexico (average centroid size 122.59). There were marginally significant differences in size of the cranium (F(5,58) = 2.3123, p = 0.0553) between the GUs.
DISCUSSION
Sexual dimorphism in shape and size
This is the first study to use geometric morphometric techniques to describe the sexual and geographic variations in the cranium and mandible of L. longicaudis throughout its distribution. The results indicate that there is no sexual dimorphism in the shape of the mandible or the skull of the Neotropical otter, though both structures do differ in size. This difference corresponds to a pattern of sexual dimorphism common in the Mustelidae family where males are generally larger than females (Moors 1980). The Eurasian otter L. lutra (Lynch and O’Sullivan 1993; Lynch et al. 1996; Baryshnikov and Puzachenko 2012) and the river otter Lontra canadensis (Pertoldi et al. 2000; Kruuk 2006) also have this pattern. Given that the difference in the size of the cranium and the mandible of the otters is correlated with body size (Pertoldi et al. 1998), the difference in size between sexes of L.longicaudis could be associated with its characteristically solitary habit and territorial behavior. The males of L. lutra must defend their territory, and a larger body ensures a higher position in the hierarchy and potentially better territory (Pertoldi et al. 1997; Kruuk 2006). For L. canadensis, social behavior has been reported and there are even cases of overlapping territories between males (Blundell et al. 2002; Melquist et al. 2003), though the territories of the males are larger insize than those of the females possibly due to the larger energetic requirement of having a larger body (Sandell 1996; Thom et al. 2004). On the other hand, females do not engage in territorial disputes and are smaller, with lower energy requirements for daily maintenance. It is thought that the excess of energy is allocated to raising the litters (Moors 1980; Pertoldi et al. 1997; Gorman et al. 2006).
Geographic variation in the size and shape of the skull and mandible
The size of the mandible differs between GUs: animals from Mexico and La Plata have larger mandibles relative to those from the other GUs. These two GUs are the most northern (Mexico) and southern (La Plata) distri- butions of L. longicaudis. This variation is congruent with the size patterns reported for other species, like the American puma, P. concolor, which in addition to having a large distribution, like the Neotropical otter, also has a larger cranium size in temperate regions and with increas- ing latitudinal distance from the Equator (Iriarte et al. 1990; Gay and Best 1996). These patterns of change correspond to Bergmann’s rule, which dictates that populations located furthest from the Equator have a relatively larger body. It is assumed that this pattern is the result of an adaptation that allows the animal to maintain its body temperature and confers greater endurance during aestivation (Blackburn and Hawkins 2004).
Cranium size does not follow the pattern observed for the mandible: the specimens from Mexico had a larger centroid size, while for the specimens from La Plata, it was smaller. In this study, only four samples were used from the La Plata region, so it is necessary to increase the sample size to determine whether this pattern would still be observed.
Our results indicated that significant variation in the shape of the cranium and the mandible of the Neotrop- ical otter is associated with geography. In other species of Mustelidae such as weasels, European minks, and European badgers (Lee and Mill 2004), it is assumed that this type of variation responds to the variation in envir- onmental conditions throughout the distribution of the species (Kruuk 2006). The morphology of a species re- sponds to select factors in the environment, in addition to limitations in the response of the phenotype to such factors. This has led to the proposal that the ecological and geographic distribution of populations is a reflection of the ecological range of a phenotype (Ricklefs and Miles 1994). In contrast, it has been reported that geographic differences do not always lead to a homologous effect on the shape and size of an anatomical structure, in this case the cranium, due to the high levels of plasti- city and adaptation in size that have been observed in other mammals (Cardini et al. 2007). Our data indicates that for L. longicaudis, there are changes in shape as well as size between the GUs studied, suggesting the existence of different selective pressures to which the shape and size of the cranium and the mandible re- spond and different means of adaptation.
In general, variation in shape corresponded to an increase in distance between the GUs. For example, the shortest Mahalanobis distances were between the Orinoco and the Amazon for the cranium and between Central America and the Western Andes for the mandible. In both cases, the GUs are adjacent hydrological regions and the proxim- ity of the regions could allow for regional migration. The similarity between neighboring hydrological regions is exemplified by the genetic distance between the giant otter populations of Pteronura brasiliensis, located in the Amazon Basin, the Orinoco, and Guyana, suggesting that these populations originated from an ancestral popu- lation that inhabited the central region of the Amazon. Several populations of giant otter subsequently diverged and were partially isolated, although similarity may have been maintained between them by gene flow (Pickles et al. 2011). Likewise, for those GUs that are geographically most distant, such as Mexico and La Plata, greater Maha- lanobis distances were also found for both structures. To elaborate, it was expected that these two regions would have the greatest differences in shape for the two views: not only due to geographic distance but also because these GUs are located at the limits of the Neotropical otter’s dis- tribution. Organisms that live at the limits of their dis- tribution generally exhibit the greatest morphological change, as they are generally subject to suboptimal condi- tions that may challenge their limits of tolerance (Gay and Best 1996).
Morphological variation is not always associated with geographic distance, as in some cases it may be ex- plained by the presence of different evolutionary units that are delimited by geographic barriers that succes- sively give origin to vicariance events (Patton and Smith 1994; De Queiroz and Good 1997; Smith et al. 1997). This pattern is observed in the GUs of the Western Andes, for which there was less variation in shape com- pared with Central America than with the adjacent re- gions of the Orinoco and the Amazon. The Andes mountain range is a potential geographic barrier that may give rise to vicariance events.
The hypothesis of vicariance events caused by the Andes supports the genetic distances reported by Trinca et al. (2012), who found large genetic distances between the phylogenetic groups of South America and those of the Western Andes, where genetic distances are greater be- tween populations of L. longicaudis than with its sister spe- cies, Lontra provocax. This separation also corresponds to a subspecies division: L. longicaudis annectens is distributed throughout the Western Andes and L. longicaudis enudris is distributed throughout the Eastern Andes (Larivière 1999). The effect of population separation by the Andes mountain range has also been reported for other vertebrate species including some South American fish (Hubert et al. 2007) and bats belonging to the genus Artibeus (Larsen et al. 2007), where the elevation of the Andes prevents gene flow and has resulted in different species on each side of the mountain range. The results of the present study offer morphological information that concurs with the molecular data from Trinca et al. (2012) on the separation of popula- tions of the Eastern and Western Andes. This indicates that sufficient time has passed for both the morpho- logical and the genetic divergence of the populations of L. longicaudis on both sides of the mountain range.
Another important pattern that reinforces the possibility of divergence events for the Neotropical otter was the notable differentiation in the shape of the mandible and cranium of the animals from La Plata with respect to those from the other five GUs. This reinforces the possibility of divergence events for the Neotropical otter. This dissimilarity for La Plata corresponds to the geo- graphic group labeled Eastern South America and re- ported by Trinca et al. (2012), which was formed by a clade that does not share a single haplotype with the other clades that were reported on by the authors. This pattern of differentiation corresponds to the genetic variation between the populations of Pantanal, which belongs to the hydrological region of La Plata, and the other populations of the giant otter P. brasiliensis (Pickles et al. 2011).
In addition, this pattern of differentiation in shape maybe influenced by the two biogeographically distinct regions in the La Plata Basin—tropical in the north and subtropical-temperate in the south—where climatic zoning has promoted changes in distribution and diversification patterns in both aquatic and terrestrial (Arzamendia and Giraudo 2009) species, and has possibly had the same effect on the Neotropical otter. Added to this effect is the presence of prairies that may be functioning as a bar- rier between the Amazon and La Plata during the dry season when bodies of water become scarce, preventing the Neotropical otter from migrating from one hydro- logical region to another.
Finally, it is important to address the pattern found for the Mahalanobis distance values and for the UPGMA, and the variation in the shape of the mandible and the cranium for specimens found in Mexico. These speci- mens showed no association with any of the other GUs, thereby indicating that the otters of this region could be under selective forces that are allowing for this differen- tiation. A similar pattern occurred in the morphology of the cranium of Panthera onca (Larson 1997), con- firmed by molecular analysis (Eizirik et al. 2001), for which there is also a pattern of geographic division for populations in Mexico and Central America in compari- son to the populations of South America. This pattern has also been found for the ocelot, Leopardus pardalis, and the margay, Leopardus wiedii, using molecular data (Eizirik et al. 1998), species which both have distribu- tions similar to that of the Neotropical otter.
This supports the idea that the otters in Mexico and Central America have been separated from the more southern populations and are subject to a distinct set of selective pressures, resulting in important morphological variations that are reflected in the shape and size of the cranium and the mandible. On the other hand, since the Pacific and Gulf of Mexico slopes where L. longicaudis is distributed are separated by the Central Mexican Plat- eau, it is possible that the shape and size of the cranium also vary between the two coastlines. We recommend that a morphometric study be undertaken to compare populations from the two regions separated by the Trans-Mexican Volcanic Belt that bifurcates and creates the Pacific and the Atlantic ridge. This would allow for a better description of any vicariance event that has hap- pened between these hydrological regions of Mexico.
The hypothesis that L. longicaudis is a species complex is supported by the geographic distances, the presence of geographic barriers, and the morphological adaptations to distinct environmental conditions that exist throughout its distribution, as well as by the congruence between the morphological differences we report here and the genetic information reported in Trinca et al. (2012). This hypothesis should be further tested using an integrative approach that includes phylogeographic information and geometric morphometrics with an appropriate number of sequences and representative sampling for the entire dis- tribution of the Neotropical otter, especially in Mexico and the La Plata region. This would elucidate the current status of L. longicaudis as a possible group of species, as proposed by other authors based on the form of the rhinarium (Pohle 1920; Cabrera 1957; Harris 1968). The results of such a study would have important conse- quences for the knowledge and conservation of this species given that L. longicaudis is on the Red List of Threatened Species of the International Union for Conservation of Nature (IUCN) and is currently catego- rized as data deficient, due to uncertainty about the rate decline of its populations and debate over its taxonomic status (IUCN 2015).
CONCLUSIONS
The present results add new information about morpho- metricskull variation in L.longicaudis. There is significant variation in the size of the cranium between male and fe- male Neotropical otters, with the males’craniathe biggest. In contrast, the variation in shape is not significant be- tween sexes.
The cranial and mandible shape analysis indicated the morphometricdistance was greatest between the La Plata and the other GUs, whereas the smallest distance was found between the Western Andes and Central America for the cranium and Amazonia and Orinoco for the mandible.
For size variation, both views indicated that specimens from Mexico are the biggest relative to the other GUs, in concurrence with Bergmann’s rule. The presence of geographic barriers and the large distances between the GUs could have given rise to the morphological variation observed in the Neotropical otter. The results of thisstudy and previous research on genetic variability suggest that L. longicaudis could actually be a group of species and therefore requires an integrative study to clarify its taxonomy.
Additional file
Additionalmaterial: origin of specimens. Examined specimens of Lontra longicaudis for the mandible. National Mammal Collection of the Biological Institute of the Autonomous National University of Mexico (NMC-UNAM), Vertebrate Collection of the Autonomous University of Campeche(VC-AUC), Mammal Collection of the Biological Research Station of Los Tuxtlas, Autonomous National University of Mexico (MCT-UNAM), American Natural History Museum (ANHM), Carnegie Museum of Natural History (CM), Field Museum of Chicago (FIELD), National Museum of Natural History-Smithsonian (SM), Museum of Michigan State University (MSU), Mammal Collection of Kansas University (KU), and private collections (PC).
Acknowledgements
This study was started with a Seed Fund Grant awarded by the State University of New York (SUNY) and funding from the Instituto de Ecología A.C.(INECOL). PCHR received a doctoral studies scholarship from CONACyT. Carla Gutierrez and Ella Vázquez contributed to this article with ideas and comments. We thank all the curators for their kindness and permission to use their Lontra longicaudis samples and for access to the following collections: National Collection of Mammals of the National Autonomous University of Mexico, the Mammal Collection of the Autonomous University of Campeche, the Vertebrate Collection of the Biological Station of the National Autonomous University of Mexico in Los Tuxtlas, the Mammal Collection of the University of Kansas, the American Museum of Natural History, the Mammal Collection of the Field Museum, the National Museum of Natural History of the Smithsonian Institute, the Carnegie Museum, and the Michigan University Museum. Bianca Delfosse revised the English.
Footnotes
Authors’ contributions: PCHR conceived this study and acquired the data. PCHR and JAG coordinated this study and performed data analysis. PCHR, JAG, and CV interpreted the data and drafted and revised the manuscript. All authors read and approved the final manuscript.
Competing interests: The authors declare that they have no competing interests.
References
- Adams D C, Rohlf F J, Slice D E. Geometric morphometrics: ten years of progress following the "revolution. Italian J Zool. 71:5–16. [Google Scholar]
- Arzamendia V, Giraudo A R. Influence of large South American rivers of the Plata Basin on distributional patterns of tropical snakes: a panbiogeographical analysis. J Biogeogr. 36:1739–1749. [Google Scholar]
- Banguera-Hinestroza E, Cárdenas H, Ruiz-García M, Marmontel M, Gaitán E, Vázquez R, García-Vallejo F. Molecular identification of evolutionarily significant units in the Amazon River dolphin Inia sp. (Cetacea: Iniidae) Journal of Heredity. 93:312–322. doi: 10.1093/jhered/93.5.312. [DOI] [PubMed] [Google Scholar]
- Baryshnikov G F, Puzachenko A Y. Craniometrical variability in the cave bears (Carnivora, Ursidae): multivariate comparative analysis. Quat Int. 245:350–368. [Google Scholar]
- Baryshnikov G F, Puzachenko A Y. Craniometrical variability of the Eurasian otter (Lutra lutra: Carnivora: Mustelidae) from the Northern Eurasia. Zoological Institute Russian Academy of Sciences. 316:203–222. [Google Scholar]
- Blackburn T M, Hawkins B A. Bergmann's rule and the mammal fauna of northern North America. Ecography. 27:715–724. [Google Scholar]
- Blundell G M, Ben-David M, Bowyer R T. Sociality in river otters: cooperative foraging or reproductive strategies? Behav Ecol. 13:134–141. [Google Scholar]
- Bookstein E L. Morphometric tools for landmark data: geometry and biology. London): Cambridge University Press; 1991. [Google Scholar]
- Bookstein E L. Biometrics, biomathematics and the morphometric synthesis. Bull Math Biol. 58:313–365. doi: 10.1007/BF02458311. [DOI] [PubMed] [Google Scholar]
- Bookstein E L. Landmark methods for forms without landmarks: morphometrics of group differences in outline shape. Med Image Anal. 1:225–243. doi: 10.1016/s1361-8415(97)85012-8. [DOI] [PubMed] [Google Scholar]
- Cabrera A. Catálogo de los mamíferos de América del Sur. I (MetatheriaUnguiculata-Carnivora) Revista del Museo Argentino de Ciencias Naturales "Bernardino Rivadavia" e Instituto Nacional de Investigación de las Ciencias Naturales. 4:1–307. [Google Scholar]
- Cardini A, Jansson A U. A geometric morphometric approach to the study of ecogeographical and clinal variation in vervet monkeys. J Biogeogr. 34:1663–1678. [Google Scholar]
- Carvalho-Junior O, Macedo-Soares L, Birolo A B. Annual and interannual food habits variability of a Neotropical otter (Lontra longicaudis) population in Conceicao Lagoon, south of Brazil. IUCN Otter Special Group Bulletin. 27:1–15. [Google Scholar]
- Caumul R, Polly P D. Phylogenetic and environmental components of morphological variation: skull, mandible, and molar shape in marmots (Marmota, Rodentia) Evolution. 59:2460–2472. [PubMed] [Google Scholar]
- Chemes B S, Graudo R A, Gil G. Dieta de Lontra longicaudis (Carnivora: Mustelidae) en el Parque Nacional El Rey (Salta, Argentina) y su comparación con otras poblaciones de la Cuenca del Paraná. Mastozoología Neotropical. 17:19–29. [Google Scholar]
- De Queiroz K, Good D A. Phenetic clustering in biology: a critique. Q Rev Biol. 72:3–30. [Google Scholar]
- Duarte L C, Rabello M L, Zuben Von, Furtado F J. Variation in mandible shape in Thrichomys apereoides (Mammalia: Rodentia): geometric analysis of a complex morphological structure. Syst Biol. 49:563–578. doi: 10.1080/10635159950127394. [DOI] [PubMed] [Google Scholar]
- Eizirik E, Bonato S L, Johnson P, Vié J C, Brousset D M. Phylogeographic patterns and evolution of the mitochondrial DNA control region in two Neotropical cats (Mammalia, Felidae) J Mol Evol. 47:613–624. doi: 10.1007/pl00006418. [DOI] [PubMed] [Google Scholar]
- Eizirik E, Kim J, Menotti-Raymond M, 'brien O, We Johnson. Phylogeography, population history and conservation genetics of jaguars (Panthera onca, Mammalia, Felidae) Mol Ecol. 10:67–79. doi: 10.1046/j.1365-294x.2001.01144.x. [DOI] [PubMed] [Google Scholar]
- Freitas Bch, -D M, Pissinatti L. Cranial and mandibular morphometry in Leontopithecus Lesson, 1840 (Callitrichidae, Primates) Am J Primatol. 48:185–196. doi: 10.1002/(SICI)1098-2345(1999)48:3<185::AID-AJP2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gallo-Reynoso J P. Situación y distribución de las nutrias en México, en énfasis en Lontra longicaudis annectens MAJOR, 1897. Revista Mexicana de Mastozoologia. 2:10–32. [Google Scholar]
- Gallo-Reynoso J P, Ramos-Rosas N, Rangel-Aguilar O. Depredación de aves acuáticas por la nutria neotropical (Lontra longicaudis annectens), en el Río Yaqui. Revista Mexicana de la Biodiversidad. 79:275–279. [Google Scholar]
- Gay S W, Best T L. Relationships between abiotic variables and geographic variation in skulls of pumas (Puma concolor: Mammalia, Felidae) in North and South America. Zool J Linn Soc. 117:259–282. [Google Scholar]
- Goodall C R. Procrustes methods in the statistical analysis of shape (with discussion and rejoinder) J R Stat Soc, Ser B. 53:285–339. [Google Scholar]
- Gorman T A, Erb J D, Mcmillan B R, Martin D J. Space use and sociality of river otters (Lontra canadensis) in Minnesota. J Mammal. 87:740–747. [Google Scholar]
- Gunz P, Mitteroecker P. Semilandmarks: a method for quantitative curves and surfaces. Hystrix. 24:103–109. [Google Scholar]
- Harris C J. Otters: a study of the recent Lutrinae. London): Weinfield and Nicholson; 1968. [Google Scholar]
- Hubert N, Renno J F. Historical biogeography of South American freshwater fishes. J Biogeogr. 33:1414–1436. [Google Scholar]
- Hubert J M, Duponchelle F, Nuñez J, Garcia-Davila C, Paugy D, Renno J F. Phylogeography of the piranha genera Serrasalmus and Pygocentrus: implications for the diversification of the Neotropical ichthyofauna. Mol Ecol. 16:2115–2136. doi: 10.1111/j.1365-294X.2007.03267.x. [DOI] [PubMed] [Google Scholar]
- Iriarte J A, Franklin W L, Johnson W E, Redford K H. Biogeographic variation of food habits and body size of the America puma. Oecologia. 85:185–190. doi: 10.1007/BF00319400. [DOI] [PubMed] [Google Scholar]
- The IUCN Red List of Threatened Species. Version 2015.1. <www.iucnredlist.org>. IUCN. 2015.
- Jonsson B, Jonsson N. Polymorphism and speciation in Arctic charr. J Fish Biol. 58:605–638. [Google Scholar]
- Kendall D G. Shape-manifolds, procrustean metrics, and complex projective spaces. Bull Lond Math Soc. 16:81–121. [Google Scholar]
- Kruuk H. Habitat-associated morphological divergence in two Neotropical fish species. Oxford University Press. 80:689–698. [Google Scholar]
- Larivière S. Lontra longicaudis, Olfers 1818. Mamm Species. 609:1–5. [Google Scholar]
- Larsen P A, Hoofer S R, Bozeman M C, Pedersen S C, Genoways H H, Phillips C J, Pumo D E, Bajer R J. Phylogenetics and Phylogeography of the Artibeus jamaicensis complex based on Cytochrome-b DNA sequences. J Mammal. 88:712–727. [Google Scholar]
- Larson S E. Taxonomic re-evaluation of the jaguar. Zoo Biol. 16:107–120. [Google Scholar]
- Lawing A M, Polly P D. Geometric morphometrics: recent applications to the study of evolution and development. J Zool (Lond) 280:1–7. [Google Scholar]
- Lee S, Mill P J. Cranial variation in British mustelids. J Morphol. 260:57–64. doi: 10.1002/jmor.10212. [DOI] [PubMed] [Google Scholar]
- Lynch J M, Sullivan O. Cranial form and sexual dimorphism in the Irish otter Lutra lutra. Biology and Environment: Proceedings of the Royal Irish Academy. 93:97–105. [Google Scholar]
- Lynch J M, Conroy Jwh, Kitchener A C, Jefferies D J, Hayden T J. Variation in cranial form and sexual dimorphism among five European populations of the otter Lutra lutra. Zoology. 238:81–96. [Google Scholar]
- Mancina C A, Balsero F. Variación en la forma de la mandíbula en Monophyllus redmani (Chiroptera: Phyllostomidae): análisis geométrico de la variación sexual y geográfica. Mastozoología Neotropical. 17:87–95. [Google Scholar]
- Mazák J H. Craniometric variation in the tiger (Panthera tigris): implications for patterns of diversity, taxonomy and conservation. Mamm biol. 75:45–68. [Google Scholar]
- Melquist W E, Toweill D. River otter. Johns Hopkins University Press. pp. 708–734.
- Moors P J. Sexual dimorphism in the body size of Mustelids (Carnivora): the roles of food habits and breeding systems. Oikos. 34:147–158. [Google Scholar]
- Patton J L, Smith M F. Paraphyly, polyphyly, and the nature of species boundaries in pocket gophers (genus Thomomys) Syst Biol. 43:11–26. [Google Scholar]
- Pertoldi C, Loeschcke V, Madsen Bo. Developmental stability in the Eurasian otter (Lutra lutra) in Denmark. Ann Zool Fenn. 34:187–196. [Google Scholar]
- Pertoldi C, Madsen Bo, Braun Randi E, Loeschcke A. Variation of skull morphometry of Eurasian otters (Lutra lutra) in Denmark and Germany. Ann Zool Fenn. 35:87–94. [Google Scholar]
- Pertoldi C, Loeschcke V, Braun A, Madsen Bo. Craniometrical variability and developmental stability. Two useful tools for assessing the population viability of Eurasian otter (Lutra lutra) population in Europe. Biol J Linn Soc. 70:309–323. [Google Scholar]
- Pertoldi C, Sonne C, Wiig Ø, Baagøe H J, Loeschcke V, Bechshøft T Ø. East Greenland and Barents Sea polar bears (Ursus maritimus): adaptive variation between two populations using skull morphometrics as an indicator of environmental and genetic differences. Hereditas. 149:99–107. doi: 10.1111/j.1601-5223.2012.02259.x. [DOI] [PubMed] [Google Scholar]
- Pickles Rsa, Groombridge J J, Zambrana-Rojas V D, Van Damme P, Gotelli D, Kundu S. Evolutionary history and identification of conservation units in the giant otter Pteronura brasiliensis. Mol Phylogenet Evol. 61:616–627. doi: 10.1016/j.ympev.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Platt G S, Rainwater R T. Predation by Neotropical otter (Lontra longicaudis) on turtles in Belize. IUCN Otter Specialist Group Bulletin. 28:4–10. [Google Scholar]
- Platz S, Hertwig S T, Jetschke G, Krüger M, Fischer M. Comparative morphometric study of the Slovakian wildcat population (Felis silvestris silvestris): evidence for a low rate of introgression? Mamm Biol. 76:222–233. [Google Scholar]
- Pohle H. Die Unterfamilie der Lutrinae. Eine systematisch tiergeographische studie an dem Material der. Berliner Messen Archiv für Naturgeschichte. 85:1–247. [Google Scholar]
- Ricklefs R E, Miles D B. Ecological and evolutionary inferences from morphology: An ecological perspective. USA): University of Chicago Press; 1994. [Google Scholar]
- Rohlf F J. Morphometric spaces, shape components, and the effects of linear transformations. Plenum Publishing Corporation. pp. 117–129.
- Rohlf F J. TPSdig, version 1.23. State University at Stony Brook. New York, software): 2001. [Google Scholar]
- Rohlf F J, Marcus L F. A revolution in morphometrics. Trends Ecol Evol. 8:129–132. doi: 10.1016/0169-5347(93)90024-J. [DOI] [PubMed] [Google Scholar]
- Rohlf F J, Slice D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst Zool. 39:40–59. [Google Scholar]
- Ruiz-Olmo J, Delibes M, Zapata S C. External morphometry, demography and mortality of the Otter Lutra lutra (Linneo, 1758) in the Iberian Peninsula. Galemys. 10:239–251. [Google Scholar]
- Sandell M. The mating tactics and spacing patterns of solitary carnivores. In: Gittleman JL (ed) Carnivore behavior, ecology, and evolution. Cornell University Press. pp. 164–182.
- Sheets H D. Semiland6. A tool for processing semi-landmarks. Physics Department; 2002. [Google Scholar]
- Sheets H D. MakeFan6 [Online. 2003.
- Sheets H. CoordGen6, Coordinate Generation program for calculating shape coordinates. 2005.
- Sheets H. CVAGen6, Canonical Variates Analysis program for the analysis of shape. 2005.
- Sheets H D, Zeldich Ml ;, Chiszar D, Montanucci R R. Tmorphgen6traditional morphometrics variables generation utility part of IMPIntegrated Morphometrics Package. Physics Department, Canisius College. 28:13–16. [Google Scholar]
- Statsoft ;, Tulsa Oklahoma, Thom M D, Harrington L A, Macdonald D W. Why are American mink sexually dimorphic? A role for niche separation. Oikos. 105:522–535. [Google Scholar]
- Trinca C S, Thoisy B, Rosas F, Waldemarin H F, Koepfli K P, Vianna J. Phylogeography and demographic history of the Neotropical otter (Lontra longicaudis) J Hered. 103:479–492. doi: 10.1093/jhered/ess001. [DOI] [PubMed] [Google Scholar]
- Van Zyll C G. A systematic review of the Nearctic and Neotropical river otters (Genus Lutra, Mustelidae, Carnivora) Royal Ontario Museum Life Sciences Contributions; 1972. 80 [Google Scholar]
- Zelditch M L, Swidwerski D L, Sheets H D. Geometric morphometrics for biologists: a primer. Academic Press of Elsevier; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additionalmaterial: origin of specimens. Examined specimens of Lontra longicaudis for the mandible. National Mammal Collection of the Biological Institute of the Autonomous National University of Mexico (NMC-UNAM), Vertebrate Collection of the Autonomous University of Campeche(VC-AUC), Mammal Collection of the Biological Research Station of Los Tuxtlas, Autonomous National University of Mexico (MCT-UNAM), American Natural History Museum (ANHM), Carnegie Museum of Natural History (CM), Field Museum of Chicago (FIELD), National Museum of Natural History-Smithsonian (SM), Museum of Michigan State University (MSU), Mammal Collection of Kansas University (KU), and private collections (PC).