Abstract
Purpose
The purpose of this article is to report serous macular detachment (SMD) similar to that seen in central serous chorioretinopathy (CSCR) in patients with nanophthalmos.
Observation
It is a retrospective case series from a tertiary eye care center in India. Multi modal imaging features of eyes with serous macular detachment in patients with nanophthalmos including colour fundus photographs, short wave autofluorescence, fundus fluorescein angiography and optical coherence tomography were studied. In addition axial length, anterior chamber depth, lens thickness and subfoveal choroidal thickness were measured. The eyes were treated with laser photocoagulation to the focal leak seen on fluorescein angiography. The patients were followed up for 12–18 months.
Results
Three eyes of three patients having serous macular detachment in nanophthalmos were identified. All three eyes had axial length <21mm, subfoveal choroidal thickness >450 microns and a focal leak on fluorescein angiography. Two eyes had serous pigment epithelial detachments underneath the SMD as well. Two eyes had peripheral pigmentary changes due to resolved subretinal fluid. The SMD resolved completely in two eyes and partially in one eye following focal laser photocoagulation.
Conclusion and importance
Serous macular detachments bearing features similar to that of CSCR can occur in the setting of nanophthalmos. These may represent manifestation of thick choroid or may represent forme fruste choroidal effusion.
Keywords: Serous macular detachment, Nanophthalmos, Central serous chorioretinopathy, Pachychoroid, Optical coherence tomography
1. Introduction
Nanophthalmos is a subtype of microphthalmos where reduction in size of the eye occurs in the absence of any other congenital ocular anomalies.1 This reduction maybe restricted to the anterior (termed as anterior microphthalmos) or posterior segment (termed as posterior microphthalmos) alone or both (nanophthalmos).2 Clinically they have shallow anterior chamber, high hyperopia and axial length of <21 mm.3 These eyes have been noted to have thicker choroid and sclera as compared to the normal population.4,5
The scleral thickening in nanophthalmos is due to deposition of abnormal collagen leading to scleral inelasticity, which in turn impairs drainage via the vortex veins.6 This may lead to engorgement of choroidal vasculature, thickening of choroid and uveal effusion. It can further get complicated by angle closure glaucoma and exudative retinal detachment.7,8 The choroid is thickened in patients with nanophthalmos because of shorter axial length as well. Thick choroid (pachychoroid) itself is also known to cause subretinal accumulation of fluid which may progress to extensive exudative retinal detachment as is seen in central serous chorioretinopathy (CSCR). However the exact cause of thickened choroid in CSCR is unknown and has been proposed to be inherited9 or because of vortex vein outflow obstruction due to thickened sclera.10 Thus there is some overlap between the pathophysiology of CSCR and nanophthalmos/uveal effusion syndrome. According to Gass as well, CSCR shares some features with the uveal effusion syndrome.11
We hereby describe serous macular detachment (SMD) similar to that of CSCR in three eyes of three patients with nanophthalmos. The pathophysiology of such a SMD in the setting of nanophthalmos is discussed.
2. Methods
This is a retrospective study of three eyes of 3 patients with SMD in the setting of nanophthalmos at a tertiary eye care centre in northern India. The study adheres to the tenets of the declarations of Helsinki and to institutional guidelines for research. Informed consent was obtained from all the patients.
Three patients with SMD in nanophthalmos were identified and their records were reviewed. All the patients had undergone thorough clinical examination including history taking, systemic and ocular examination. The best-corrected visual acuity (BCVA) was assessed using Snellen chart. The multi-modal images of the patients were analysed. The modalities of examination included colour fundus photographs, fundus fluorescein angiography (FFA), swept source optical coherence tomography (SSOCT) and short wave autofluorescence (SWAF, in two patients). The cases are described in detail below:
2.1. Case 1
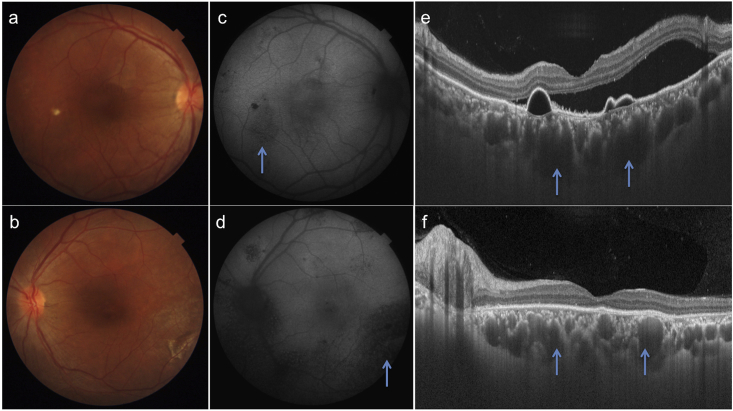
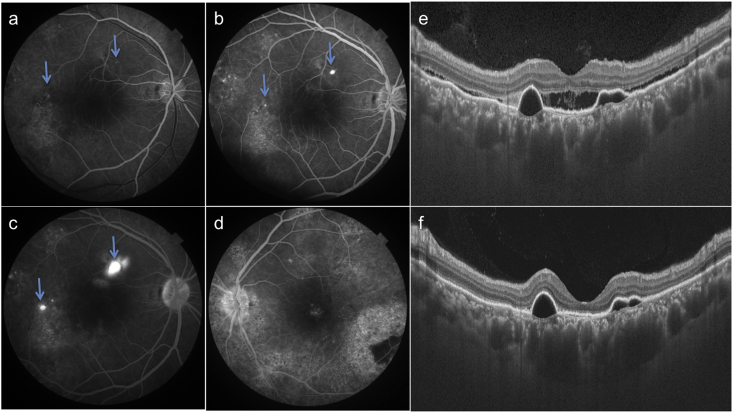
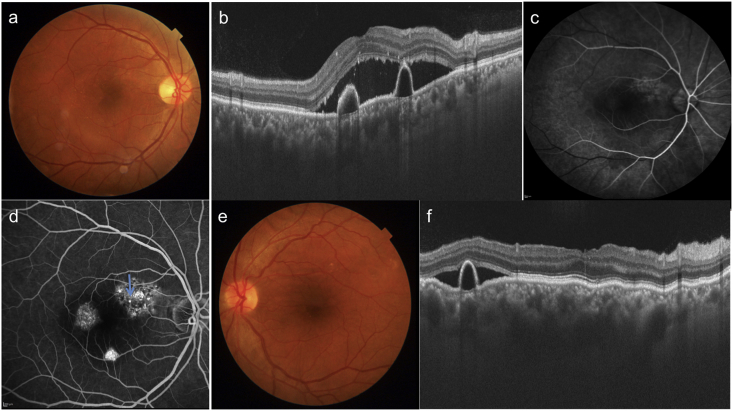
A 32 year-old-male presented with complaints of blurring of vision in OD since two months. Patient gave history of poor vision and use of high-powered convex glasses in OU since many years. Personal and systemic history was unremarkable. On examination, best-corrected visual acuity (BCVA) was 6/60(+10D) and 4/60(+11D) in OD and OS respectively. Axial length, anterior chamber depth and lens thickness are summarized in Table 1. Fundus OD revealed a SMD at the posterior pole with pigmentary changes in the infero-temporal macula (Fig. 1a). The pigmentary changes were better seen on short wave autofluorescence (SWAF) as a hypoautofluorescent area (Fig. 1c). OS fundus revealed altered pigmentation in the macula and peripapillary area with a large infero-temporal area of retinal pigment epithelium (RPE) atrophy (Fig. 1b). RPE changes were delineated better on SWAF imaging (Fig. 1d). The pigmentary changes were extending outside the vascular arcades in OS but not in OD. Swept source optical coherence tomography (SSOCT) in OD showed a SMD with underlying serous retinal pigment epithelium detachments (PED, Fig. 1e). SSOCT in OS showed loss of outer retinal layers. Subfoveal choroidal thickness was increased in both the eyes (488 and 555 microns in OD and OS respectively). The outer choroidal vessels were dilated and obliterated choriocapillaris in OU. Fundus fluorescein angiography (FFA) revealed two ink-blot leaks in OD (arrows, Fig. 2a–c) and widespread window defects corresponding to the RPE changes in OS (Fig. 2d). A diagnosis of OU nanophthalmos with CSCR in OD and resolved subretinal fluid in OS was made. After informed consent, the patient underwent focal laser photocoagulation to the inkblot leaks in OD. The SMD decreased at one month (Fig. 2e) and resolved completely at 3 months follow-up (Fig. 2f). Though the PEDs persisted, BCVA improved to 6/36 in OD. The patient was advised regular follow up and remained stable till one year of follow up.
Table 1.
Axial length, anterior chamber depth and lens thickness of all the three patients.
| Age | Sex | Axial Length (in mm) | Anterior Chamber Depth (in mm) | Lens Thickness (in mm) |
|---|---|---|---|---|
| 32 | M | 20.22 | 3.68 | 3.87 |
| 20.14 | 3.22 | 3.56 | ||
| 26 | M | 15.62 | 1.9 | 3.6 |
| 15.56 | 1.8 | 3.4 | ||
| 45 | M | 20.54 | 2.89 | 4.15 |
| 19.88 | 2.7 | 3.95 |
Fig. 1.
Colour fundus photographs of patient 1 shows serous macular detachment in the right eye (a) and pigmentary changes in both the eyes (a, b) which are better visible on autofluorescence imaging (arrows c, d). SSOCT shows SMD and serous PEDs in the right eye. In addition dilated outer choroidal vessels (pachyvessels) along with obliteration of choriocapillaris and medium choroidal vessels is seen in both eyes (arrows e, f). These pachyvessels are prominent in area beneath the PED.
Fig. 2.
Fluorescein angiograms of patient 1 show two focal leaks in the right eye (arrows a-c) and window defects corresponding to pigmentary changes in both eyes (a–d). SSOCT shows decreased subretinal fluid at one month (e) and complete resolution at 3 months (f) after laser photocoagulation to focal leaks in the right eye.
2.2. Case 2
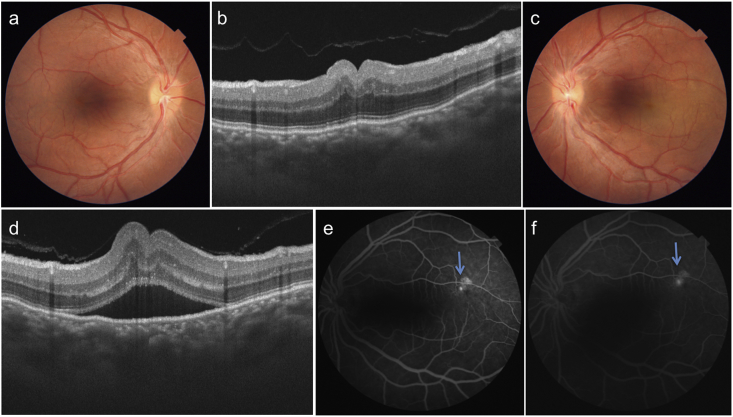
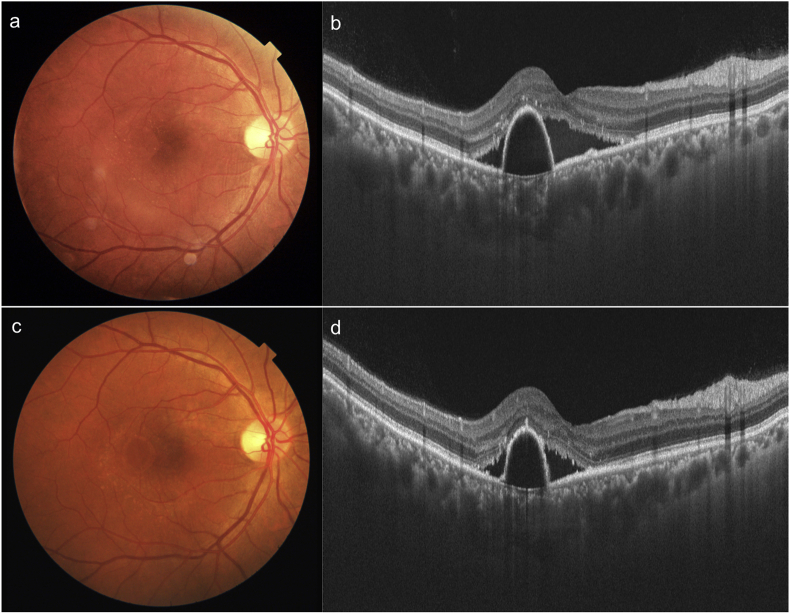
A 26 year-old male patient presented with complaints of metamorphopsia OS since one month. Patient gave history of decreased vision in OU since childhood. Personal and systemic history was unremarkable. On examination, BCVA was 6/60 (+17) in OD and 6/60 (+17D) in OS. Axial length, anterior chamber depth and lens thickness OU are summarized in Table 1. Dilated fundus OU showed a radial fold in the papillomacular bundle (PMB) and crowded optic disc (Fig. 3a,c). The fold in PMB was evident on SSOCT as well (Fig. 3b,d). OS in addition showed a SMD. The subfoveal choroidal thickness was increased in OU (572 and 609 microns in OD and OS respectively. FFA revealed a ink-blot leak in the supero-temporal macula in OS (arrow, Fig. 3e and f). OU in addition showed pigmentary abnormalities in the peripheral retina (Fig. 4a and b). A diagnosis of OU nanophthalmos with OS CSCR was made. After informed consent, focal laser photocoagulation was done at the point of leakage in OS. At six weeks follow up SMD resolved (Fig. 4c and d). Though BCVA was maintained at 6/60, the patient reported subjective improvement and was advised regular follow up. The clinical picture remained unchanged at 18 months follow up.
Fig. 3.
Colour fundus photographs of right (a) and left (c) eye of patient 2 show hypermetropic fundus with radial folds in papillomacular bundle, which are well seen on SSOCT (b, d). SSOCT in left eye also shows a SMD (d). Choroid is diffusely thickened in both the eyes and choroido-scleral junction is not visible. Fluorescein angiograms show inkblot leak in the left eye along supero-temporal arcade (arrows e, f).
Fig. 4.
Wide field colour photographs of patient 2 show peripheral pigmentary changes in both eyes (a,b). Six weeks after laser photocoagulation, colour photo (c) and SSOCT (d) show resolution of SMD in left eye.
2.3. Case 3
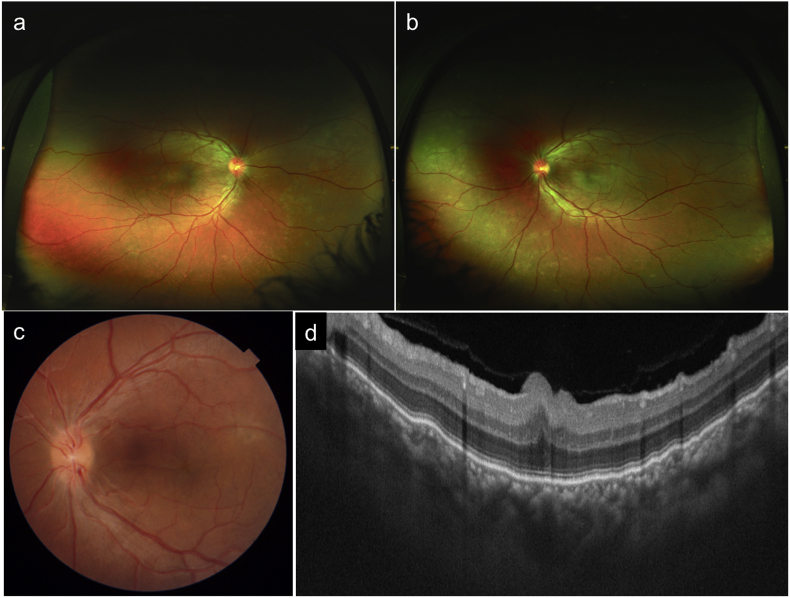
A 45-year-old male patient presented with metamorphopsia in OD since 3 months. The patient reported low vision in OU since many years. Personal and systemic history was unremarkable. On examination, BCVA was 6/36 (+8) in OD and 6/60 (+11D) in OS. Axial length, anterior chamber depth and lens thickness are summarized in Table 1. Dilated fundus examination revealed a SMD and multiple serous PEDs at the posterior pole in OD (Fig. 5a) and extrafoveal SMD, choroidal folds and PED in OS (Fig. 5e). SSOCT confirmed SMD and PEDs in OU (Fig. 5b,f). Subfoveal choroidal thickness was 463 and 529 microns in OD and OS respectively. Outer choroidal vessels were dilated especially in the OS. OD FFA revealed two inkblot leaks in the supero-nasal macula with surrounding window defects, window defects temporal to the fovea and PED in the inferior part of macula (Fig. 5c and d). OS FFA also showed window defects and PEDs. A diagnosis of OU nanophthalmos with OD chronic CSCR was made. After informed consent, focal laser photocoagulation was applied to leakage. Though the fluid decreased at 1 month (Fig. 6a and b) and 3 months follow up (Fig. 6c and d), small amount of residual fluid was still present at six months follow up. BCVA improved to 6/24. The patient was advised regular follow up and clinical picture remained unchanged at one year follow up.
Fig. 5.
Colour fundus photograph of right eye of patient 3 shows SMD and serous PED (a) that is better appreciated on SSOCT (b). Pachvessels along with obliteration of small and medium choroidal vessels is seen that is more pronounced in right eye. Fluorescein angiogram of right eye shows pooling in the PEDs and focal leak (arrow, c-d). Colour fundus photograph of left eye shows choroidal folds due to hypermetropia and serous PED (e) that is better seen on SSCOT (f).
Fig. 6.
Colour fundus photograph (a) and SSOCT (b) of right eye of patient 3 at one month and 3 months (c,d) after laser photocoagulation to the leak point shows decrease in the SMD.
3. Discussion
Pachychoroid spectrum is a group of disorders having thick choroid.12 The spectrum includes pachychoroid pigment epitheliopathy, CSCR, polypoidal choroidal vasculopathy and pachychoroid neovasculopathy.13, 14, 15 Recently, entities like Mactel type 2 and focal choroidal excavation have been included under the same spectrum.16,17 Similar to pachychoroid spectrum, nanophthalmos is also characterized by thickened choroid. The thick choroid in nanophthalmos is secondary to abnormally thick sclera and smaller axial length. Thus manifestations of pachychoroid disorders particularly CSCR may not be uncommon in patients with nanophthalmos.
The pathogenesis of CSCR and nanophthalmos also appear to be similar. In the setting of CSCR, choroidal hyperpermeability and congestion leads to overwhelming of retinal pigment epithelium pump, which leads to serous macular detachment. On the other hand vortex vein outflow obstruction leads to both choroidal effusion as well as serous retinal detachment in nanophthalmos. Recently vortex vein outflow obstruction has been proposed to be the cause of CSCR as well.10 The management is also aimed at reducing choroidal congestion in both CSCR as well nanophthalmos. While in former photodynamic therapy is resorted to, scleral resection is the treatment of choice in latter. In fact CSCR presenting with an exudative retinal detachment, has been reported and treated successfully by scleral resection.18 Therefore, there exist a considerable overlap between these two pathologies.
In our case series, case 2 was a true nanophthalmos with other two probably being heterozygote carriers. Still, they shared similar ocular findings, like hyperopia and axial length of <21 mm. Anterior chamber was shallow in case 2. Lens thickness was high in case 3 and borderline in rest. All patients had SMD (2 also had PED) on SSOCT with thick choroid and dilated large choroidal vessels in the Haller's layer. The presence of PEDs also points towards the possibility of CSCR. FFA showed an inkblot leak typical of CSCR. Since all leaks were extra-foveal, laser photocoagulation of leak was done that led to complete resolution of SMD in two cases and partial resolution in one. There was no evidence of recurrence or progression in any of the cases till last follow up.
CSCR in nanophthalmos has not been described so far in literature. It is our contention that exudative detachment in a case of nanophthalmos may incidentally start as a single pin-point leak with a localized SMD to start with just as noted in our case and later progressing to a full blown detachment. On the contrary the authors have observed leakage in a case with exudative retinal detachment (unpublished data) in nanophthalmos who responded well to scleral resection. The limitations of the study include small number of cases and short follow up. Non-availability of Indocyanine green angiography is another drawback of the study.
To conclude, there is a considerable overlap between pachychoroid disorders (especially CSCR) and nanophthalmos (has thick choroid as well). Serous macular detachment observed in nanophthalmos has features similar to that of CSCR and may represent forme fruste choroidal effusion. Thus, pachychoroid spectrum may be expanded to include nanophthalmos as well.
Patient consent
Consent to publish this case report has been obtained from the patient(s) in writing.
Funding
No funding or grant support.
Conflicts of interest
All the authors have no financial disclosures.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Source(s) of support
Nil.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2019.100522.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Duke-Elder S. Normal and abnormal development. Con- genital deformities. In: Duke-Elder S., editor. vol. 3. CV Mosby; St Louis, MO: 1964. pp. 488–495. (System of Opthalmology). [Google Scholar]
- 2.Park S.H., Ahn Y.J., Shin S.Y., Lee Y.C. Clinical features of posterior microphthalmos associated with papillomacular fold and high hyperopia. Clin Exp Optom. 2016 Nov;99(6):590–593. doi: 10.1111/cxo.12371. [DOI] [PubMed] [Google Scholar]
- 3.Singh O.S., Simmons R.J., Brockhurst R.J., Trempe C.L. Nanophthalmos: a perspective on identification and therapy. Ophthalmology. 1982 Sep;89(9):1006–1012. [PubMed] [Google Scholar]
- 4.Walsh M.K., Goldberg M.F. Abnormal foveal avascular zone in nanophthalmos. Am J Ophthalmol. 2007;143:1067–1068. doi: 10.1016/j.ajo.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 5.Yue B.Y.J.T., Duvall J., Goldberg M.F. Nanophthalmic sclera: morphologic and tissue culture studies. Ophthalmology. 1986;93:534. doi: 10.1016/s0161-6420(86)33704-7. [DOI] [PubMed] [Google Scholar]
- 6.Areiter E., Neale M., Johnson S.M. Spectrum of angle closure, uveal effusion syndrome, and nanophthalmos. J Curr Glaucoma Pract. 2016 Sep-Dec;10(3):113–117. doi: 10.5005/jp-journals-10008-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimbrough R.L., Trempe C.L., Brockhurst R.J. Angle- closure glaucoma in nanophthalmos. Am J Ophthalmol. 1979;88:572. doi: 10.1016/0002-9394(79)90517-8. [DOI] [PubMed] [Google Scholar]
- 8.Brockhurst R.J. Nanophthalmos with uveal effusion: a new clinical entity. Arch Ophthalmol. 1975;93 doi: 10.1001/archopht.1975.01010020923001. 1289-99. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann M., Bousquet E., Beydoun T., Behar-Cohen F. PACHYCHOROID: an inherited condition? Retina. 2015 Jan 1;35(1):10–16. doi: 10.1097/IAE.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 10.Hiroe T., Kishi S. Dilatation of asymmetric vortex vein in central serous chorioretinopathy. Ophthalmology Retina. 2018 Feb 1;2(2):152–161. doi: 10.1016/j.oret.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Gass J.D., Jallow S. Idiopathic serous detachment of the choroid, ciliary body, and retina (uveal effusion syndrome) Ophthalmology. 1982;89 doi: 10.1016/s0161-6420(82)34685-0. 1018e1032. [DOI] [PubMed] [Google Scholar]
- 12.Gallego-Pinazo R., Dolz-Marco R., Gómez-Ulla F., Mrejen S., Freund K.B. Pachychoroid diseases of the macula. Med Hypothesis Discov Innov Ophthalmol. 2014;3(4):111–115. Winter. [PMC free article] [PubMed] [Google Scholar]
- 13.Pachychoroid disease, Cheung C.M.G., Lee W.K. Eye. 2019;33:14–33. doi: 10.1038/s41433-018-0158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warrow D.J., Hoang Q.V., Freund K.B. Pachychoroid pigment epitheliopathy. Retina. 2013 Sep;33(8):1659–1672. doi: 10.1097/IAE.0b013e3182953df4. [DOI] [PubMed] [Google Scholar]
- 15.Pang C.E., Freund K.B. Pachychoroid neovasculopathy. Retina. 2015;35(1):1–9. doi: 10.1097/IAE.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 16.Kumar V., Kumar P., Ravani R., Gupta P. Macular telangiectasia type II with pachychoroid spectrum of macular disorders. Eur J Ophthalmol. 2018 Apr 1 doi: 10.1177/1120672118769527. 1120672118769527. [DOI] [PubMed] [Google Scholar]
- 17.Chung H., Byeon S.H., Freund K.B. FOCAL choroidal excavation and its association with pachychoroid spectrum disorders: a review of the literature and multimodal imaging findings. Retina. 2017 Feb;37(2):199–221. doi: 10.1097/IAE.0000000000001345. [DOI] [PubMed] [Google Scholar]
- 18.Venkatesh P., Chawla R., Tripathy K., Singh H.I., Bypareddy R. Scleral resection in chronic central serous chorioretinopathy complicated by exudative retinal detachment. Eye Vis (Lond) 2016;3(1):23. doi: 10.1186/s40662-016-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.