Abstract
The angiotensin converting enzyme 2 (ACE2) catalyzes the degradation of Angiotensin II (Ang II) to generate Angiotensin-(1-7), which reduces inflammation and oxidative stress stimulated by Ang II. ACE2 has been shown to be protective in cardiovascular and metabolic diseases including diabetes and its complications. However, the challenge for its clinical application is large-scale production of high-quality ACE2 with sufficient target tissue bioavailability. We developed an expression and delivery system based on the use of probiotic species Lactobacillus paracasei (LP) to serve as a live vector for oral delivery of human ACE2. We show that codon-optimized ACE2 can be efficiently expressed in LP. Mice treated with the recombinant LP expressing the secreted ACE2 in fusion with the non-toxic subunit B of cholera toxin, which acts as a carrier to facilitate transmucosal transport, showed increased ACE2 activities in serum and tissues. ACE2-LP administration reduced the number of acellular capillaries, blocked retinal ganglion cell loss, and decreased retinal inflammatory cytokine expression in two mouse models of diabetic retinopathy. These results provide proof of concept for feasibility of using engineered probiotic species as live vector for delivery of human ACE2 with enhanced tissue bioavailability for treating diabetic retinopathy, as well as other diabetic complications.
Keywords: renin angiotensin system, ACE2, diabetes, diabetic retinopathy, probiotics, Lactobacillus, drug delivery
Introduction
The renin-angiotensin system (RAS) plays an important role in cardiovascular physiology and body homeostasis through the regulation of electrolyte balance, blood pressure, and vascular tone. In addition to circulating RAS, local tissue RAS exists in all organs, including the eye.1, 2, 3 Dysfunction of the RAS, resulting in elevated concentrations of Angiotensin II (Ang II), contributes to increased oxidative stress, inflammation, and development of metabolic syndrome, diabetes, and its complications,4, 5, 6, 7, 8, 9, 10, 11, 12 including diabetic retinopathy (DR).13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 The discovery of ACE2,27, 28 which counteracts the effects of angiotensin-converting enzyme (ACE) through degradation of Ang II and generation of Angiotensin-(1-7) (Ang-(1-7)), established a protective axis for the RAS (i.e., the ACE2/Ang-(1-7)/Mas axis). This axis has been shown to be effective in producing beneficial effects on metabolic, immune, and cardiovascular dysfunctions by blocking apoptosis, fibrosis, oxidative stress, and inflammation, thus improving metabolic dysfunction and diabetic complications in a large number of animal models.29, 30, 31, 32, 33 Our recent studies have also established the protective effects of ACE2/Ang-(1-7) on retinal inflammation and DR.34, 35, 36, 37 However, it is challenging to translate this fundamental knowledge into clinical applications for management and treatment of these diseases. Current strategies to deliver therapeutic proteins and peptides face a large number of obstacles, from production, formulation, and administration to limited bioavailability and potential immunogenicity.38, 39, 40
Recent evidence also implicates gut dysbiosis, an altered composition and function of the gut microbiome, in the pathogenesis of both type 1 and type 2 diabetes41, 42, 43 and supports the beneficial effects of probiotics in managing diabetes and other metabolic diseases.44, 45, 46, 47, 48, 49, 50 Since increased ACE2 has been shown to be beneficial in multiple organs and recent evidence links its role in gut and gut microbiome,51, 52 it would be ideal to increase ACE2 function both systemically and locally at target tissues. In this study, we sought to develop an expression and delivery system based on the use of recombinant probiotic species Lactobacillus paracasei (L. paracasei) to serve as a live vector for the oral delivery of human ACE2. L. paracasei is a gram-positive, facultative heterofermentative bacteria of the Lactobacillus genus that are the components of the normal gut microbiota53 and are also commonly used in the food industry for production of fermented food and beverages; thus, L. paracasei bacteria are generally recognized as safe (GRAS). Moreover, Lactobacillus bacteria are usually used as probiotic supplements with beneficial effects in humans.54, 55, 56 As a result, engineering of Lactobacilli is emerging as a promising approach for delivery of therapeutics.57, 58, 59 Since the codon usage and codon preferences in Lactobacillus bacteria vary significantly from mammalian organisms,60 we generated different gene constructs to express human ACE2 protein with codon usage that was optimized for expression in Lactobacillus. One of the constructs showed higher level expression of ACE2 in L. paracasei. This construct was then selected to build a secreted ACE2 protein fused with the non-toxic subunit B of cholera toxin (CTB) as a carrier to facilitate transmucosal transport into the circulation and target tissue uptake. We show that oral administration of recombinant L. paracasei expressing human ACE2 in mice increased both serum and tissue levels of ACE2 activity. We further evaluated the efficacy of the recombinant L. paracasei in two different mouse models of DR and showed that oral administration of recombinant L. paracasei expressing human ACE2 significantly reduced diabetes-induced retinal capillary and retinal ganglion cell loss and decreased inflammatory cytokine expression in the retina. These results provide proof of concept for feasibility of using engineered probiotic species as live vectors for delivery of human ACE2 protein with enhanced tissue bioavailability for treating DR as well as other cardiovascular, metabolic diseases and diabetic complications. This unconventional approach provides a more efficient and cost-effective strategy to enhance this protective axis at both circulating and target tissues for clinical application.
Results
Expression Vector Construction and Codon Optimization
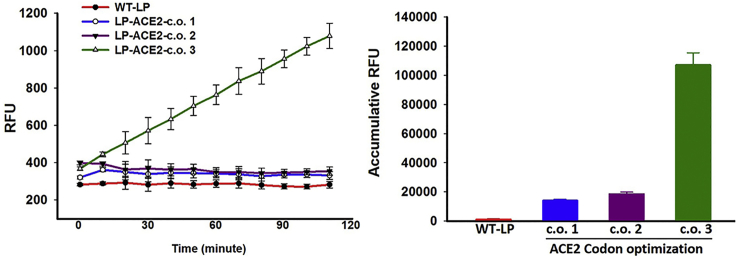
The original Lactobacillus shuttle plasmid containing a GFP reporter gene driven by the lactate dehydrogenase (LDH) promoter from Lactobacillus acidophilus was from Addgene (plasmid #27167).61 As human ACE2 cDNA has very low-level expression in Lactobacillus (data not shown), codon optimization was performed based on preference of codon usage of L. paracasei, and three synthetic genes with different optimization options were cloned into this vector to replace the GFP. The expression level in Lactobacillus bacteria was determined by ACE2 enzymatic activity assay using self-quenching fluorescent substrate as previously reported.34 As shown in Figure 1, only one of the three constructs showed higher level ACE2 activity, and thus this construct (see Figure S1 for complete sequence) was selected for subsequent studies.
Figure 1.
ACE2 Activities in Lactobacillus paracasei Expressing Human ACE2 Protein with Three Different Options for Codon Optimization
Error bars represent SD of three separate assays, each run duplicate. LP, Lactobacillus paracasei; c.o., codon optimization; RFU, relative fluorescent unit.
In Vivo Expression Characterization in Mice Orally Administered with L. paracasei Expressing Codon-Optimized Human ACE2
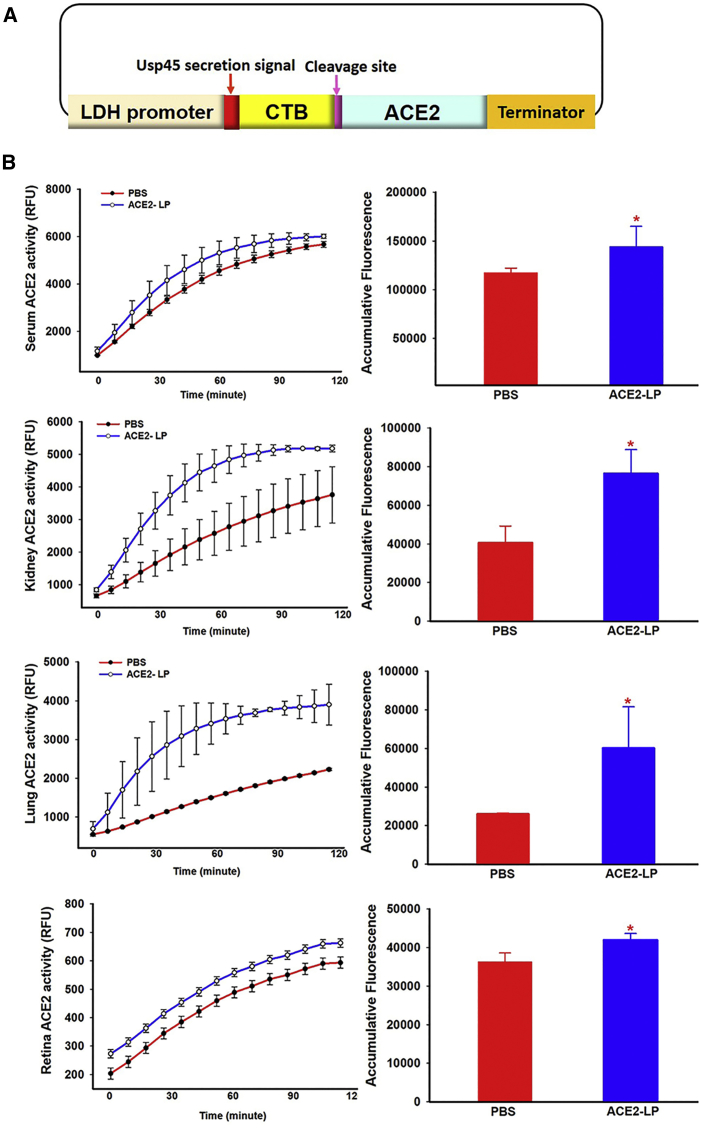
To enhance in vivo secretion into the circulation and target tissue uptake, we designed a construct to express secreted ACE2 fused with a carrier protein to facilitate transport into circulation. As shown in Figure 2A, ACE2 expression is under the control of the LDH promoter and is expressed as a fusion protein with the non-toxic CTB as a carrier to facilitate transmucosal transport into the circulation and target tissue uptake. CTB is separated by a furin cleavage site to release ACE2 once it is expressed. The signal peptide from the usp45 gene of Lactococcus lactis, slightly modified from Kajikawa et al.,62 was used. We next confirmed that human ACE2 protein expressed from recombinant L. paracasei can be delivered into circulation and taken up by different tissues following oral administration in vivo. Adult wild-type C57BL/6J mice (6–8 weeks old) were gavaged with either the same volume of vehicle (PBS) or recombinant L. paracasei expressing ACE2 (ACE2-LP) at 1 × 1010 colony-forming units (CFUs)/mouse daily for 3 days. Mice were then sacrificed, and serum and tissue samples were collected for ACE2 enzymatic activity assay. As shown in Figure 2B, oral administration of recombinant ACE2-LP not only significantly increased serum levels of ACE2 activity, an ∼20% increase compared to control mice, but also ACE2 activities in kidney, lung, and retina.
Figure 2.
Construction of Lactobacillus Vector for Expression of Secreted Human ACE2 Protein Fused with CTB and Evaluation of In Vivo ACE2 Activities
(A) Diagram of the Lactobacillus vector expressing codon optimized human ACE2. ACE2 is expressed as a secreted fusion protein with the CTB, which is separated by a furin cleavage site to release ACE2 once it is expressed. (B) ACE2 activities in different tissues in mice administered with LP-ACE2. RFU, relative fluorescent unit. N = 4/group.
In Vivo Evaluation of Recombinant L. paracasei Expressing ACE2 in Animal Models of Diabetic Retinopathy
To determine the efficacy of recombinant L. paracasei expressing ACE2 in protection against DR, two type 1 models of diabetes were used. The first model was generated by using streptozotocin (STZ) in eNOS−/− mice and shows an accelerated time course and increased DR severity.36, 63 The second model is the Akita mouse, which carries a mutation in the insulin 2 gene resulting in mice exhibiting reduced β cell mass and reduced insulin secretion.64 Mice heterozygous for the Akita spontaneous mutation (Ins2Akita) develop progressive retinal abnormalities including increased vascular permeability, apoptosis, and inner retinal thinning as early as 12 weeks after the onset of hyperglycemia.65 For both models, mice were gavaged with either 1 × 1010 CFU of wild-type L. paracasei (WT-LP), LP expressing human ACE2 (ACE2-LP), or vehicle (PBS) at three times/week. The diabetic eNOS−/− mice were treated for 8 weeks after STZ-induced diabetes. This duration of treatment for diabetic eNOS−/− mice was based on a previous characterization when severe retinopathy was developed.36, 63 As the progression of retinopathy in Akita mice is slower, these mice were treated for 12 weeks beginning at 6 weeks of age.
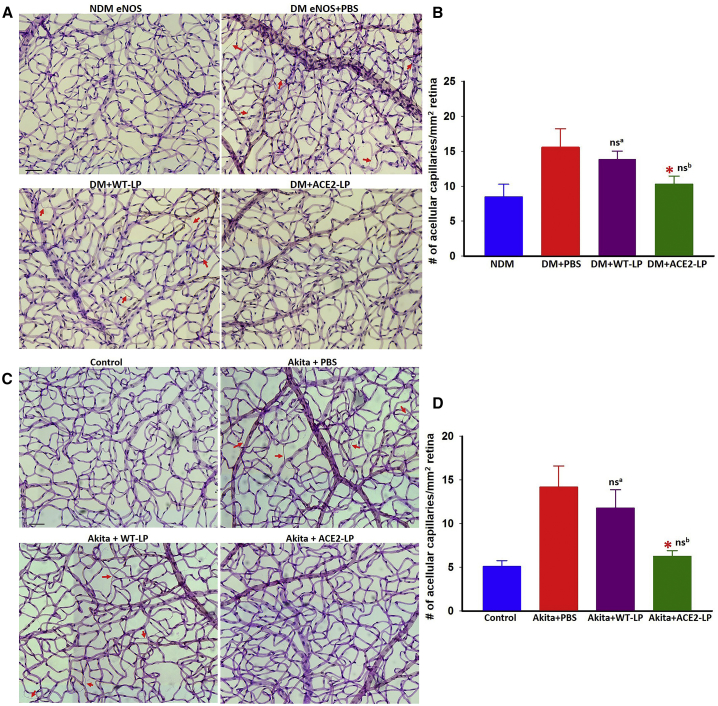
Diabetes in eNOS−/− mice resulted in severe capillary loss, as previously reported.36, 63 Mice treated with ACE2-LP demonstrated reduced capillary loss (Figure 3A). Similar retinal protection was seen in Akita mice treated with ACE2-LP (Figure 3B). Treatment with WT-LP showed slightly but insignificant improvement compared to vehicle-treated mice in both models.
Figure 3.
Evaluation of Retinal Acellular Capillary in Diabetic eNOS–/– and Akita Mice
(A and C) Representative images of trypsin-digested retinal vascular preparations from non-diabetic (NDM) and diabetic (DM) eNOS−/− mice treated with vehicle (PBS), WT-LP, and ACE2-LP (A); wild-type littermate control and Akita mice (C) treated with PBS, WT-LP, and ACE2-LP; and quantitative measurements of acellular capillaries of eNOS−/− (B) and Akita (D) mice. Arrows indicate the acellular capillaries. *p < 0.01 (versus PBS-treated groups). nsa, not significant (versus PBS-treated group). nsb, not significant (versus control group). Error bars represent SD (n = 8/group). Treatments with ACE2-LP significantly reduced acellular capillaries in both eNOS−/− and Akita mice. LP alone showed slight but not statistically significant reduction of capillary loss in both diabetic eNOS−/− and Akita mice. Scale bar, 50 μm.
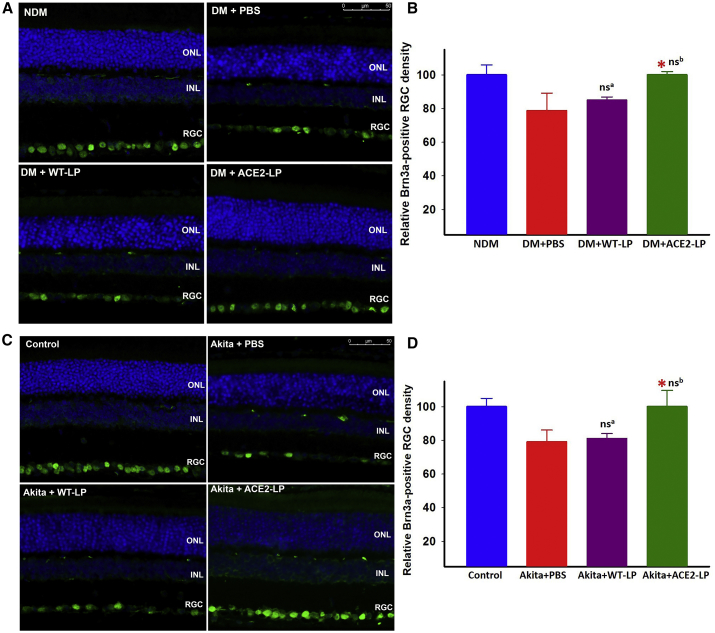
Untreated or vehicle-treated diabetic eNOS−/− and Akita mice showed an ∼20% loss of Brn3a-positive retinal ganglion cells (RGCs), which was completely prevented by ACE2-LP in both diabetic eNOS−/− and Akita models, whereas WT-LP did not show any effect (Figure 4).
Figure 4.
Evaluation of Retinal Ganglion Cell (RGC) Density Detected by Brn3a Immunostaining in Diabetic eNOS–/– and Akita Mice
Representative immunofluorescence images of Brn3a staining from non-diabetic (NDM) and diabetic (DM) eNOS−/− mice treated with vehicle (PBS), WT-LP, and ACE2-LP (A); wild-type littermate control and Akita mice (C) treated with PBS, WT-LP, and ACE2-LP; and quantification of Brn3a-positive cells of eNOS−/− (B) and Akita (D) mice. *p < 0.01 (versus PBS-treated groups). nsa, not significant (versus PBS-treated group). nsb, not significant (versus control group). Error bars represent SD (n = 6/group). Scale bar, 50 μm. Treatments with ACE2-LP prevented diabetes-induced RGC loss in both eNOS−/− and Akita mice.
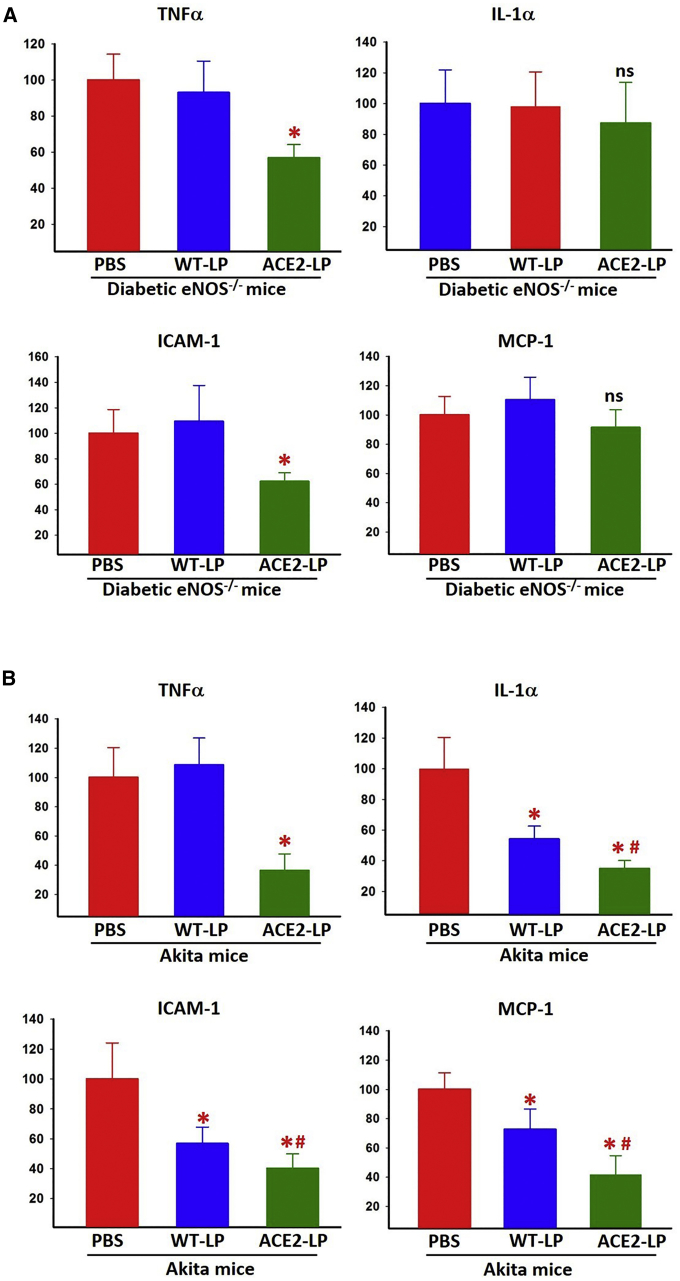
Real-time RT-PCR was used to evaluate the expression level of pro-inflammatory cytokines and chemokines in the retina from each experimental group. In diabetic eNOS−/− mice, ACE2-LP treatment significantly reduced retinal expression of tumor necrosis factor alpha (TNF-α) and intercellular adhesion molecule 1 (ICAM-1) but did not have any effect on the expression level of interleukin-1α (IL-1α) and moocyte chemoattractant protein-1 (MCP-1) (Figure 5A). WT-LP treatment did not affect the expression of any of the cytokines in diabetic eNOS−/− mouse retina. In contrast, the retinal expression of all of these genes was significantly reduced in ACE2-LP-treated Akita mice (Figure 5B), and unexpectedly, WT-LP treated Akita mice also showed significantly reduced expression of IL-1α, ICAM-1, and MCP-1 but no significant difference in TNF-α level compared to the vehicle-treated group. The expression levels of IL-1α, ICAM-1, and MCP-1 in ACE2-LP-treated mice were also significantly reduced compared to WT-LP-treated Akita mice.
Figure 5.
Real-Time RT-PCR Analysis of Retinal mRNA Levels of Inflammatory Cytokines in Diabetic eNOS–/– and Akita Mice
Retinal mRNA levels of inflammatory cytokines in diabetic eNOS−/− (A) and Akita mice (B). Values on y axis represent relative expression levels compared to PBS-treated group for each gene. NDM, non-diabetic; DM, diabetic. *p < 0.01 (versus PBS groups). #p < 0.05 (versus WT-LP group). ns, not significant (versus PBS groups). Error bars represent SD (n = 4/group).
Discussion
In this study, we developed an expression and delivery system based on the use of recombinant probiotic species L. paracasei to serve as a live vector for the oral delivery of human ACE2. We show that codon-optimized human ACE2 can be efficiently expressed in L. paracasei with enzymatic activity. Oral administration of recombinant L. paracasei expressing the secreted ACE2 in fusion with CTB in mice increased both serum and tissue ACE2 activities. More importantly, oral administration of recombinant ACE2-LP significantly reduced diabetes-induced retinal neurovascular degeneration in two mouse models of DR.
Human ACE2 is a large protein (805 amino acid residues) with several predicted glycosylation sites.66 The original human cDNA has a very low expression level in L. paracasei and several other species of Lactobacillus (data not shown). Even with different codon optimization, only one construct showed higher level expression, suggesting that codon optimization is critical to achieve high expression levels in Lactobacillus species. Since the ACE2 expressed by Lactobacillus bacteria is enzymatically active and functional in vivo, the potential glycosylation or other protein modifications of ACE2 may not be critical for its function.
In order to increase both serum and tissue level of ACE2 in vivo, the construct with the highest expression level of ACE2 in L. paracasei was selected to create the final fusion gene of ACE2 with a secretion signal peptide and CTB, which facilitates transmucosal transport into the circulation and tissue uptake by GM1 receptor-mediated endocytosis. Oral administration of recombinant L. paracasei expressing the secreted ACE2 in mice resulted in increased ACE2 activities not only in serum but also several tissues including kidney, lung, and retina, suggesting that bacteria-expressed ACE2 can be efficiently secreted into circulation and target tissues following oral gavage. Oral administration of ACE2-LP bacteria significantly reduced diabetes-induced retinal capillary loss compared to WT-LP or vehicle-treated animals in both models, consistent with our previous results using AAV viral vector for ACE2 local delivery.36, 37 Treatment with ACE2-LP bacteria prevented retinal ganglion cell loss to the same extent in both models. However, the effect of ACE2-LP treatment on retinal cytokine and chemokine gene expression was different in the two models; the expression of all four cytokines measured was significantly reduced in Akita mice treated with ACE2-LP, but only TNF-α and ICAM-1 expression was significantly reduced in diabetic eNOS−/− mice. Surprisingly, treatment with WT-LP also significantly reduced retinal expression of IL-1α and ICAM-1 in Akita mice but had no effects in diabetic eNOS−/− mice. Future studies will be required to further elucidate the mechanisms responsible for the different responses between the two models. Although WT-LP showed some effects on retinal cytokine expression in Akita mice, WT-LP treatment did not show any protective effects on diabetes-induced retinal capillary and RGC loss in either model. The species L. paracasei has been used as probiotic supplement;67, 68, 69 recent studies also indicate L. paracasei and its related species are beneficial in diabetes; however, such an effect is likely strain specific.
A wide range of doses of different probiotic Lactobacillus species, varying from 107 to 1011 CFU, has been reported in different mouse disease models. In this study, we have chosen a higher dose (1010 CFU/mouse) to establish the feasibility and efficacy of recombinant LP-ACE2 in protection against DR in mouse models. The frequency of oral administration used in this study (three times/week) was based on the observation that increased serum and tissue ACE2 activities can be detected 48 h after a single oral gavage (data not shown). Nevertheless, future studies will be required to further determine optimal doses and frequency for oral administration.
Overall, these results provide proof of concept for feasibility of using engineered probiotic species as live vectors for delivery of human ACE2 protein with enhanced tissue bioavailability for treating DR, as well as other cardiovascular and metabolic diseases and diabetic complications. The probiotic-based delivery of ACE2 offers advantages also. Probiotics are generally recognized as safe with many beneficial effects on their own, including anti-diabetes effects. As ingested probiotics can survive both gastric acid and bile to reach the small intestine and colon, where they exert their effects, they are ideal vehicles for delivery of protein drugs. Conventionally, protein therapeutics has to be administered intravenously with limited bioavailability to target tissues; by using receptor-mediated transmucosal oral delivery via CTB as a carrier, probiotics-based oral delivery of protein therapeutics enables efficient transmucosal transport into the circulation and target tissue uptake, thus enhancing their bioavailability. Moreover, oral delivery of therapeutics is more patient friendly and cost effective. Due to their safety profile, long history of use in food industry, and probiotic beneficial health effects, genetic engineering of lactic acid bacteria has been used to deliver several therapeutic proteins.57, 58, 59 The novel aspect of our strategy is that we use the CTB fusion as a carrier to enhance the bioavailability of our therapeutic protein, ACE2. Furthermore, because a large number of probiotic species and strains are known to have different beneficial effects, specific probiotics species or strains can be selected for oral delivery of therapeutics to optimally target specific patient populations, thus achieving the precision medicine paradigm.
The prevalence of diabetes has been continuously increasing for the last few decades70, 71, 72, 73 and so is the prevalence of DR, the most common diabetic complication and the leading cause of severe vision loss in people under the age of 60.74, 75, 76, 77, 78 Growing evidence implicates gut dysbiosis as one of the key factors contributing to the incidence of diabetes and obesity;41, 42, 43, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88 probiotics-based delivery of ACE2 may provide a promising and more cost-effective approach for therapy and management for this devastating condition.
However, there are questions that remain unanswered. First, unlike conventional protein drug delivery, in which the concentration of therapeutic protein spikes following systemic delivery and is quickly cleared from circulation, probiotic bacteria-expressed therapeutic protein is constitutively secreted into circulation and target tissues; therefore, the therapeutic dose is influenced not only by the expression level and the amount of bacteria, but also survival and persistence of orally delivered probiotics. Detailed analysis of factors affecting the survival and persistence of recombinant probiotics will be critical for future clinical application. Second, the impact of recombinant bacteria expressing human ACE2 on gut microbiome, intestinal function, and immune system will also require further investigation. Despite these challenges, our results demonstrate the feasibility of using engineered probiotic species as live vector for delivery of human ACE2 protein with enhanced tissue bioavailability, and this approach may hold important therapeutic potential for treating DR, as well as other cardiovascular and metabolic diseases and diabetic complications.
Materials and Methods
Bacterial Strains and Growth Conditions
The Lactobacillus bacteria L. paracasei (ATCC 27092) used in this study were from American Type Culture Collection (ATCC; Manassas, VA, USA) and were cultured in de Man, Rogosa, and Sharpe (MRS) broth (Thermo Fisher Scientific, #DF0881-17-5) at 37°C for 18 h without shaking. The plasmid pTRKH3-ldhGFP (Addgene, plasmid #27170) was used as a backbone for cloning of human ACE2 gene constructs, which were synthesized by Genscript (Piscataway, NJ, USA). The resulting plasmids were electroporated into Lactobacillus bacteria by electroporation as described by Welker et al.89. Recombinant Lactobacillus bacteria expressing ACE2 were grown in the MRS media supplemented with 5 μg/mL erythromycin (Sigma-Aldrich, St. Louis, MO, USA). For oral gavage of mice, bacteria were harvested by centrifugation at 5,000 × g for 20 min and resuspended in sterile PBS.
Animals and Experimental Procedures
Wild-type C57BL/6J (stock number 000664), eNOS−/− (stock number 002684), and Akita mice (stock number 003548) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and maintained in the Animal Care Service at the University of Florida. Akita mice were maintained by breeding C57BL/6J inbred females with heterozygous males and confirmed by genotyping using protocol provided by the Jackson Laboratory. All procedures adhered to the Association for Research in Vision and Opthalmology (ARVO) statement for the use of Animals in Ophthalmic and Vision Research, and the protocol was approved by the Animal Care and Use Committee of the University of Florida. The animals were fed standard laboratory chow and allowed free access to water in an air-conditioned room with a 12-12-hr light-dark cycle. Diabetes in adult eNOS−/− mice (8–10 weeks old) was induced by STZ injection as reported previously.63 Gavage of diabetic eNOS−/− mice was performed with either 1 × 1010 CFU of WT-LP, ACE2-LP, or vehicle (PBS), three times/week for 8 weeks. Akita mice were gavaged with the same dose for 12 weeks.
ACE2 Activity Assay
To measure ACE2 activity in L. paracasei, bacterial protein was extracted using the lysozyme treatment method described by Sieo et al.90 In brief, the cell pellet was suspended in 0.15 M Tris/HCl buffer (pH 6.8). Lysozyme (10 mg/mL) was added and incubated at 37°C for 90 min, followed by sonication and centrifugation at 8,000 rpm for 10 min at 4°C. The protein concentration in the supernatant was determined using a BCA protein assay kit (Pierce, Thermo Fisher Scientific, Rockford, IL, USA). To measure mouse tissue ACE2 activity, proteins were extracted by sonication in ACE2 assay buffer and centrifugation at 8,000 rpm for 10 min at 4°C as described before.35 The ACE2 activity assay was performed using 50 μg of extracted protein or 10 μL of serum diluted in assay buffer (75 mM Tris, 1 M NaCl, 0.5 μM ZnCl2 [pH 7.5]) in black 96-well opaque plates with 50 μM ACE2-specific fluorogenic peptide substrate (R&D Systems, Minneapolis, MN) in a final volume of 100 μL per well reaction mixture. The fluorescent intensity was measured using SpectraMax M3 fluorescence microplate reader (Molecular Devices, Sunnyvale, CA, USA) for every 90 s with excitation at 340 nm and emission at 400 nm at 37°C for 2 h, as described previously.35 Experiments were carried out in duplicate, and results were expressed as relative fluorescent units (RFU).
Retinal Vascular Preparation
Retinal vasculature was prepared using trypsin digest as described previously.63 In brief, eyes were fixed in 4% paraformaldehyde freshly made in PBS overnight. Retinas were dissected out from the eyecups and digested in 3% trypsin (Gibco-BRL) for 2–3 h at 37°C. Retinal vessels were separated from other retinal neuronal cells by gentle shaking and manipulation under a dissection microscope. The vessels were then mounted on a clean slide, allowed to dry, and stained with PAS-H&E (periodic acid solution-hematoxylin, Gill No.3, Sigma, St. Louis, MO, USA) according to the instruction manual. After staining and washing in water, the tissue was dehydrated and mounted using Permount mounting media (Sigma, St. Louis, MO, USA). The prepared retinal vessels were imaged using the Leica LAS X widefield systems. At minimum, 10 representative, non-overlapping fields from each quadrant of the retina were imaged. Acellular capillaries are counted from images for each retina and expressed as number of acellular vessels per mm2.
Immunofluorescence
For immunofluorescence studies, eyes were fixed in 4% paraformaldehyde overnight at 4°C and subsequently processed for paraffin embedding. Four μm-thick paraffin sections were cut and mounted on Superfrost Plus slides. The paraffin sections were first deparaffinized followed by antigen retrieval in low-pH citric acid buffer for 20 min. The sections were then incubated in blocking solution (5% BSA + 0.3% Triton X-100 in PBS) for 1 h. This was followed by incubation overnight at 4°C with primary mouse anti-Brn3a (1:200, MAB1585; Millipore, Billerica, MA, USA). After washing, secondary antibody conjugated to Alexa 488 (Molecular Probes/Invitrogen, Carlsbad, CA, USA) was incubated for 1 h at room temperature. Sections were washed in PBS containing the nuclear counterstain DAPI and mounted in Dako mounting media. The images were captured with a Leica fluorescence microscope LAS X system (Leica Microsystems, Buffalo Grove, IL, USA). Brn3a-positive cells in each eye were quantified from at least 15 sections from at least six animals for each experimental group.
Real-Time RT-PCR Analysis of Inflammatory Cytokines
Total RNA was isolated from freshly enucleated eyes using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instructions. Reverse transcription was performed using an enhanced avian HS RT-PCR kit (Sigma-Aldrich, St. Louis, MO, USA) following manufacturer’s instructions. Real-time PCR was carried out on a real-time thermal cycler (iCycler; Bio-Rad Life Sciences, Hercules, CA, USA) using iQTM Sybr green supermix (Bio-Rad Life Sciences, Hercules, CA, USA), and relative quantification was determined as reported previously.35 Each reaction was run in duplicate. All the reactions were repeated at least twice. Primer sequences used in this study are shown in Table S1.
Statistical Analysis
Data are expressed as the mean + SD of at least two independent experiments. Differences between mean values of multiple groups were analyzed by one-way ANOVA with Dunnett’s test for post hoc comparisons. A p value less than 0.05 was considered statistically significant.
Author Contributions
Q.L., M.B.G., and M.K.R. conceived and designed the experiments; A.V., Z.L., and S.L. performed experiments in construction and evaluation of Lactobacillus expression; A.V., K.X., T.D., P.Z., and J.Z. performed experiments in animal models, tissue processing, and analysis; A.V., M.B.G., and Q.L. prepared and revised the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported in part by NIH grants EY021752 and EY024564, the American Diabetes Association, and the BrightFocus Foundation (to Q.L.); EY028858 (to M.B.G.); and HL102033 (to M.K.R.). Core facilities were supported by NIH grant P30 EY02172 and Research to Prevent Blindness to University of Florida.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2019.06.007.
Supplemental Information
References
- 1.Paul M., Poyan Mehr A., Kreutz R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 2.Bader M. Tissue renin-angiotensin-aldosterone systems: Targets for pharmacological therapy. Annu. Rev. Pharmacol. Toxicol. 2010;50:439–465. doi: 10.1146/annurev.pharmtox.010909.105610. [DOI] [PubMed] [Google Scholar]
- 3.Bader M., Ganten D. Update on tissue renin-angiotensin systems. J. Mol. Med. (Berl.) 2008;86:615–621. doi: 10.1007/s00109-008-0336-0. [DOI] [PubMed] [Google Scholar]
- 4.Underwood P.C., Adler G.K. The renin angiotensin aldosterone system and insulin resistance in humans. Curr. Hypertens. Rep. 2013;15:59–70. doi: 10.1007/s11906-012-0323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das U.N. Renin-angiotensin-aldosterone system in insulin resistance and metabolic syndrome. J. Transl. Int. Med. 2016;4:66–72. doi: 10.1515/jtim-2016-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabandugama P.K., Gardner M.J., Sowers J.R. The Renin Angiotensin Aldosterone System in Obesity and Hypertension: Roles in the Cardiorenal Metabolic Syndrome. Med. Clin. North Am. 2017;101:129–137. doi: 10.1016/j.mcna.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernardi S., Michelli A., Zuolo G., Candido R., Fabris B. Update on RAAS Modulation for the Treatment of Diabetic Cardiovascular Disease. J. Diabetes Res. 2016;2016:8917578. doi: 10.1155/2016/8917578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dikalov S.I., Nazarewicz R.R. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid. Redox Signal. 2013;19:1085–1094. doi: 10.1089/ars.2012.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goossens G.H. The renin-angiotensin system in the pathophysiology of type 2 diabetes. Obes. Facts. 2012;5:611–624. doi: 10.1159/000342776. [DOI] [PubMed] [Google Scholar]
- 10.Ramalingam L., Menikdiwela K., LeMieux M., Dufour J.M., Kaur G., Kalupahana N., Moustaid-Moussa N. The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1106–1114. doi: 10.1016/j.bbadis.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Kalupahana N.S., Moustaid-Moussa N. The renin-angiotensin system: a link between obesity, inflammation and insulin resistance. Obes. Rev. 2012;13:136–149. doi: 10.1111/j.1467-789X.2011.00942.x. [DOI] [PubMed] [Google Scholar]
- 12.Catalá-López F., Macías Saint-Gerons D., González-Bermejo D., Rosano G.M., Davis B.R., Ridao M., Zaragoza A., Montero-Corominas D., Tobías A., de la Fuente-Honrubia C. Cardiovascular and Renal Outcomes of Renin-Angiotensin System Blockade in Adult Patients with Diabetes Mellitus: A Systematic Review with Network Meta-Analyses. PLoS Med. 2016;13:e1001971. doi: 10.1371/journal.pmed.1001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjølie A.K., Dodson P., Hobbs F.R. Does renin-angiotensin system blockade have a role in preventing diabetic retinopathy? A clinical review. Int. J. Clin. Pract. 2011;65:148–153. doi: 10.1111/j.1742-1241.2010.02552.x. [DOI] [PubMed] [Google Scholar]
- 14.Ghattas A., Lip P.L., Lip G.Y. Renin-angiotensin blockade in diabetic retinopathy. Int. J. Clin. Pract. 2011;65:113–116. doi: 10.1111/j.1742-1241.2010.02592.x. [DOI] [PubMed] [Google Scholar]
- 15.Wright A.D., Dodson P.M. Diabetic retinopathy and blockade of the renin-angiotensin system: new data from the DIRECT study programme. Eye (Lond.) 2010;24:1–6. doi: 10.1038/eye.2009.189. [DOI] [PubMed] [Google Scholar]
- 16.Perkins B.A., Aiello L.P., Krolewski A.S. Diabetes complications and the renin-angiotensin system. N. Engl. J. Med. 2009;361:83–85. doi: 10.1056/NEJMe0904293. [DOI] [PubMed] [Google Scholar]
- 17.Sjølie A.K. Prospects for angiotensin receptor blockers in diabetic retinopathy. Diabetes Res. Clin. Pract. 2007;76(Suppl 1):S31–S39. doi: 10.1016/j.diabres.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Byon I.S., Jeon H.S., Kim H.W., Lee S.J., Lee J.E., Oum B.S. The effect of a systemic angiotensin receptor blocker on vascular endothelial growth factor in the vitreous of patients with proliferative diabetic retinopathy. Curr. Eye Res. 2013;38:774–780. doi: 10.3109/02713683.2013.772206. [DOI] [PubMed] [Google Scholar]
- 19.Cheema B.S., Kohli H.S., Sharma R., Shah V.N., Bhansali A., Khullar M. Angiotensin-converting enzyme gene variants interact with the renin-angiotensin system pathway to confer risk and protection against type 2 diabetic retinopathy. J. Diabetes Investig. 2013;4:103–104. doi: 10.1111/jdi.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher E.L., Phipps J.A., Ward M.M., Vessey K.A., Wilkinson-Berka J.L. The renin-angiotensin system in retinal health and disease: Its influence on neurons, glia and the vasculature. Prog. Retin. Eye Res. 2010;29:284–311. doi: 10.1016/j.preteyeres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Harindhanavudhi T., Mauer M., Klein R., Zinman B., Sinaiko A., Caramori M.L., Renin Angiotensin System Study (RASS) group Benefits of Renin-Angiotensin blockade on retinopathy in type 1 diabetes vary with glycemic control. Diabetes Care. 2011;34:1838–1842. doi: 10.2337/dc11-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller A.G., Zhu T., Wilkinson-Berka J.L. The renin-angiotensin system and advanced glycation end-products in diabetic retinopathy: impacts and synergies. Curr. Clin. Pharmacol. 2013;8:285–296. doi: 10.2174/1574884711308040004. [DOI] [PubMed] [Google Scholar]
- 23.Prasad T., Roksnoer L.C., Zhu P., Verma A., Li Y., Batenburg W.W., de Vries R., Danser A.H., Li Q. Beneficial Effects of Combined AT1 Receptor/Neprilysin Inhibition (ARNI) Versus AT1 Receptor Blockade Alone in the Diabetic Eye. Invest. Ophthalmol. Vis. Sci. 2016;57:6722–6730. doi: 10.1167/iovs.16-20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batenburg W.W., Verma A., Wang Y., Zhu P., van den Heuvel M., van Veghel R., Danser A.H., Li Q. Combined renin inhibition/(pro)renin receptor blockade in diabetic retinopathy--a study in transgenic (mREN2)27 rats. PLoS ONE. 2014;9:e100954. doi: 10.1371/journal.pone.0100954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeganathan V.S. The therapeutic implications of renin-angiotensin system blockade in diabetic retinopathy. Curr. Pharm. Biotechnol. 2011;12:392–395. doi: 10.2174/138920111794480615. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson-Berka J.L. Angiotensin and diabetic retinopathy. Int. J. Biochem. Cell Biol. 2006;38:752–765. doi: 10.1016/j.biocel.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 28.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-dos-Santos A.J., da Costa J., Zhang L., Pei Y. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 29.Santos S.H., Andrade J.M. Angiotensin 1-7: a peptide for preventing and treating metabolic syndrome. Peptides. 2014;59:34–41. doi: 10.1016/j.peptides.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Patel V.B., Parajuli N., Oudit G.Y. Role of angiotensin-converting enzyme 2 (ACE2) in diabetic cardiovascular complications. Clin. Sci. (Lond.) 2014;126:471–482. doi: 10.1042/CS20130344. [DOI] [PubMed] [Google Scholar]
- 31.Padda R.S., Shi Y., Lo C.S., Zhang S.L., Chan J.S. Angiotensin-(1-7): A Novel Peptide to Treat Hypertension and Nephropathy in Diabetes? J. Diabetes Metab. 2015;6:6. doi: 10.4172/2155-6156.1000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rein J., Bader M. Renin-Angiotensin System in Diabetes. Protein Pept. Lett. 2017;24:833–840. doi: 10.2174/0929866524666170728144357. [DOI] [PubMed] [Google Scholar]
- 33.Santos R.A.S., Sampaio W.O., Alzamora A.C., Motta-Santos D., Alenina N., Bader M., Campagnole-Santos M.J. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7) Physiol. Rev. 2018;98:505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu Y., Shil P.K., Zhu P., Yang H., Verma A., Lei B., Li Q. Angiotensin-converting enzyme 2 (ACE2) activator diminazene aceturate ameliorates endotoxin-induced uveitis in mice. Invest. Ophthalmol. Vis. Sci. 2014;55:3809–3818. doi: 10.1167/iovs.14-13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shil P.K., Kwon K.C., Zhu P., Verma A., Daniell H., Li Q. Oral delivery of ACE2/Ang-(1-7) bioencapsulated in plant cells protects against experimental uveitis and autoimmune uveoretinitis. Mol. Ther. 2014;22:2069–2082. doi: 10.1038/mt.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma A., Shan Z., Lei B., Yuan L., Liu X., Nakagawa T., Grant M.B., Lewin A.S., Hauswirth W.W., Raizada M.K., Li Q. ACE2 and Ang-(1-7) confer protection against development of diabetic retinopathy. Mol. Ther. 2012;20:28–36. doi: 10.1038/mt.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dominguez J.M., 2nd, Hu P., Caballero S., Moldovan L., Verma A., Oudit G.Y., Li Q., Grant M.B. Adeno-Associated Virus Overexpression of Angiotensin-Converting Enzyme-2 Reverses Diabetic Retinopathy in Type 1 Diabetes in Mice. Am. J. Pathol. 2016;186:1688–1700. doi: 10.1016/j.ajpath.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown L.R. Commercial challenges of protein drug delivery. Expert Opin. Drug Deliv. 2005;2:29–42. doi: 10.1517/17425247.2.1.29. [DOI] [PubMed] [Google Scholar]
- 39.Rehman K., Hamid Akash M.S., Akhtar B., Tariq M., Mahmood A., Ibrahim M. Delivery of Therapeutic Proteins: Challenges and Strategies. Curr. Drug Targets. 2016;17:1172–1188. doi: 10.2174/1389450117666151209120139. [DOI] [PubMed] [Google Scholar]
- 40.Chirmule N., Jawa V., Meibohm B. Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. AAPS J. 2012;14:296–302. doi: 10.1208/s12248-012-9340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blandino G., Inturri R., Lazzara F., Di Rosa M., Malaguarnera L. Impact of gut microbiota on diabetes mellitus. Diabetes Metab. 2016;42:303–315. doi: 10.1016/j.diabet.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Hartstra A.V., Bouter K.E., Bäckhed F., Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38:159–165. doi: 10.2337/dc14-0769. [DOI] [PubMed] [Google Scholar]
- 43.Needell J.C., Zipris D. The Role of the Intestinal Microbiome in Type 1 Diabetes Pathogenesis. Curr. Diab. Rep. 2016;16:89. doi: 10.1007/s11892-016-0781-z. [DOI] [PubMed] [Google Scholar]
- 44.Akbari V., Hendijani F. Effects of probiotic supplementation in patients with type 2 diabetes: systematic review and meta-analysis. Nutr. Rev. 2016;74:774–784. doi: 10.1093/nutrit/nuw039. [DOI] [PubMed] [Google Scholar]
- 45.Samah S., Ramasamy K., Lim S.M., Neoh C.F. Probiotics for the management of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2016;118:172–182. doi: 10.1016/j.diabres.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 46.Sáez-Lara M.J., Robles-Sanchez C., Ruiz-Ojeda F.J., Plaza-Diaz J., Gil A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016;17:E928. doi: 10.3390/ijms17060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li C., Li X., Han H., Cui H., Peng M., Wang G., Wang Z. Effect of probiotics on metabolic profiles in type 2 diabetes mellitus: A meta-analysis of randomized, controlled trials. Medicine (Baltimore) 2016;95:e4088. doi: 10.1097/MD.0000000000004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J., Buys N.J. Glucose- and glycaemic factor-lowering effects of probiotics on diabetes: a meta-analysis of randomised placebo-controlled trials. Br. J. Nutr. 2016;115:1167–1177. doi: 10.1017/S0007114516000076. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Q., Wu Y., Fei X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina (Kaunas) 2016;52:28–34. doi: 10.1016/j.medici.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Razmpoosh E., Javadi M., Ejtahed H.S., Mirmiran P. Probiotics as beneficial agents in the management of diabetes mellitus: a systematic review. Diabetes Metab. Res. Rev. 2016;32:143–168. doi: 10.1002/dmrr.2665. [DOI] [PubMed] [Google Scholar]
- 51.Perlot T., Penninger J.M. ACE2 - from the renin-angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013;15:866–873. doi: 10.1016/j.micinf.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cole-Jeffrey C.T., Liu M., Katovich M.J., Raizada M.K., Shenoy V. ACE2 and Microbiota: Emerging Targets for Cardiopulmonary Disease Therapy. J. Cardiovasc. Pharmacol. 2015;66:540–550. doi: 10.1097/FJC.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heeney D.D., Gareau M.G., Marco M.L. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr. Opin. Biotechnol. 2018;49:140–147. doi: 10.1016/j.copbio.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lebeer S., Vanderleyden J., De Keersmaecker S.C. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 2008;72:728–764. doi: 10.1128/MMBR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Cerbo A., Palmieri B., Aponte M., Morales-Medina J.C., Iannitti T. Mechanisms and therapeutic effectiveness of lactobacilli. J. Clin. Pathol. 2016;69:187–203. doi: 10.1136/jclinpath-2015-202976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salvetti E., O’Toole P.W. The Genomic Basis of Lactobacilli as Health-Promoting Organisms. Microbiol. Spectr. 2017;5 doi: 10.1128/microbiolspec.bad-0011-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bermúdez-Humarán L.G., Aubry C., Motta J.P., Deraison C., Steidler L., Vergnolle N., Chatel J.M., Langella P. Engineering lactococci and lactobacilli for human health. Curr. Opin. Microbiol. 2013;16:278–283. doi: 10.1016/j.mib.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 58.Cano-Garrido O., Seras-Franzoso J., Garcia-Fruitós E. Lactic acid bacteria: reviewing the potential of a promising delivery live vector for biomedical purposes. Microb. Cell Fact. 2015;14:137. doi: 10.1186/s12934-015-0313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plavec T.V., Berlec A. Engineering of lactic acid bacteria for delivery of therapeutic proteins and peptides. Appl. Microbiol. Biotechnol. 2019;103:2053–2066. doi: 10.1007/s00253-019-09628-y. [DOI] [PubMed] [Google Scholar]
- 60.Pouwels P.H., Leunissen J.A. Divergence in codon usage of Lactobacillus species. Nucleic Acids Res. 1994;22:929–936. doi: 10.1093/nar/22.6.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lizier M., Sarra P.G., Cauda R., Lucchini F. Comparison of expression vectors in Lactobacillus reuteri strains. FEMS Microbiol. Lett. 2010;308:8–15. doi: 10.1111/j.1574-6968.2010.01978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kajikawa A., Ichikawa E., Igimi S. Development of a highly efficient protein-secreting system in recombinant Lactobacillus casei. J. Microbiol. Biotechnol. 2010;20:375–382. [PubMed] [Google Scholar]
- 63.Li Q., Verma A., Han P.Y., Nakagawa T., Johnson R.J., Grant M.B., Campbell-Thompson M., Jarajapu Y.P., Lei B., Hauswirth W.W. Diabetic eNOS-knockout mice develop accelerated retinopathy. Invest. Ophthalmol. Vis. Sci. 2010;51:5240–5246. doi: 10.1167/iovs.09-5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J., Takeuchi T., Tanaka S., Kubo S.K., Kayo T., Lu D., Takata K., Koizumi A., Izumi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J. Clin. Invest. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barber A.J., Antonetti D.A., Kern T.S., Reiter C.E., Soans R.S., Krady J.K., Levison S.W., Gardner T.W., Bronson S.K. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest. Ophthalmol. Vis. Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 66.Towler P., Staker B., Prasad S.G., Menon S., Tang J., Parsons T., Ryan D., Fisher M., Williams D., Dales N.A. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J. Biol. Chem. 2004;279:17996–18007. doi: 10.1074/jbc.M311191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maldonado Galdeano C., Novotny Nuñez I., Carmuega E., de Moreno de LeBlanc A., Perdigón G. Role of probiotics and functional foods in health: gut immune stimulation by two probiotic strains and a potential probiotic yoghurt. Endocr. Metab. Immune Disord. Drug Targets. 2015;15:37–45. doi: 10.2174/1871530314666141216121349. [DOI] [PubMed] [Google Scholar]
- 68.Mizock B.A. Probiotics. Dis. Mon. 2015;61:259–290. doi: 10.1016/j.disamonth.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 69.Sanders M.E. Probiotics in 2015: Their Scope and Use. J. Clin. Gastroenterol. 2015;49(Suppl 1):S2–S6. doi: 10.1097/MCG.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 70.Rathmann W., Giani G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:2568–2569. doi: 10.2337/diacare.27.10.2568. author reply 2569. [DOI] [PubMed] [Google Scholar]
- 71.Lam D.W., LeRoith D. The worldwide diabetes epidemic. Curr. Opin. Endocrinol. Diabetes Obes. 2012;19:93–96. doi: 10.1097/MED.0b013e328350583a. [DOI] [PubMed] [Google Scholar]
- 72.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 73.Menke A., Casagrande S., Geiss L., Cowie C.C. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988-2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 74.Cheung N., Mitchell P., Wong T.Y. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 75.Klein B.E. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14:179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- 76.Yau J.W., Rogers S.L., Kawasaki R., Lamoureux E.L., Kowalski J.W., Bek T., Chen S.J., Dekker J.M., Fletcher A., Grauslund J., Meta-Analysis for Eye Disease (META-EYE) Study Group Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pascolini D., Mariotti S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 78.Nentwich M.M., Ulbig M.W. Diabetic retinopathy - ocular complications of diabetes mellitus. World J. Diabetes. 2015;6:489–499. doi: 10.4239/wjd.v6.i3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paun A., Danska J.S. Modulation of type 1 and type 2 diabetes risk by the intestinal microbiome. Pediatr. Diabetes. 2016;17:469–477. doi: 10.1111/pedi.12424. [DOI] [PubMed] [Google Scholar]
- 80.Han J.L., Lin H.L. Intestinal microbiota and type 2 diabetes: from mechanism insights to therapeutic perspective. World J. Gastroenterol. 2014;20:17737–17745. doi: 10.3748/wjg.v20.i47.17737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vaarala O. Human intestinal microbiota and type 1 diabetes. Curr. Diab. Rep. 2013;13:601–607. doi: 10.1007/s11892-013-0409-5. [DOI] [PubMed] [Google Scholar]
- 82.Zipris D. The interplay between the gut microbiota and the immune system in the mechanism of type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2013;20:265–270. doi: 10.1097/MED.0b013e3283628569. [DOI] [PubMed] [Google Scholar]
- 83.Slyepchenko A., Maes M., Machado-Vieira R., Anderson G., Solmi M., Sanz Y., Berk M., Köhler C.A., Carvalho A.F. Intestinal Dysbiosis, Gut Hyperpermeability and Bacterial Translocation: Missing Links Between Depression, Obesity and Type 2 Diabetes. Curr. Pharm. Des. 2016;22:6087–6106. doi: 10.2174/1381612822666160922165706. [DOI] [PubMed] [Google Scholar]
- 84.Bibbò S., Dore M.P., Pes G.M., Delitala G., Delitala A.P. Is there a role for gut microbiota in type 1 diabetes pathogenesis? Ann. Med. 2017;49:11–22. doi: 10.1080/07853890.2016.1222449. [DOI] [PubMed] [Google Scholar]
- 85.Scott F.W., Pound L.D., Patrick C., Eberhard C.E., Crookshank J.A. Where genes meet environment-integrating the role of gut luminal contents, immunity and pancreas in type 1 diabetes. Transl. Res. 2017;179:183–198. doi: 10.1016/j.trsl.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 86.Paun A., Yau C., Danska J.S. Immune recognition and response to the intestinal microbiome in type 1 diabetes. J. Autoimmun. 2016;71:10–18. doi: 10.1016/j.jaut.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 87.Paun A., Yau C., Danska J.S. The Influence of the Microbiome on Type 1 Diabetes. J. Immunol. 2017;198:590–595. doi: 10.4049/jimmunol.1601519. [DOI] [PubMed] [Google Scholar]
- 88.Brunkwall L., Orho-Melander M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia. 2017;60:943–951. doi: 10.1007/s00125-017-4278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Welker D.L., Hughes J.E., Steele J.L., Broadbent J.R. High efficiency electrotransformation of Lactobacillus casei. FEMS Microbiol. Lett. 2015;362:1–6. doi: 10.1093/femsle/fnu033. [DOI] [PubMed] [Google Scholar]
- 90.Sieo C.C., Wong B.T., Abdullah N., Ho Y.W. Effects of Extraction Methods and Age of Cells on the Whole-cell Protein Patterns of Lactobacillus. Research Journal of Microbiology. 2007;2:727–734. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.