Abstract
2-keto-d-gluconic acid (2-KGA) is a key precursor for synthesising vitamin C and isovitamin C. However, phage contamination is as constant problem in industrial production of 2-KGA using Pseudomonas fluorescens. Gluconobacter holds promise for producing 2-KGA due to impressive resistance to hypertonicity and acids, and high utilisation of glucose. In this study, the 2-KGA synthesis pathway was regulated to enhance production of 2-KGA and reduce accumulation of the by-products 5-keto-d-gluconic acid (5-KGA) and d-gluconic acid (D-GA) in the 2-KGA producer Gluconobacter japonicus CGMCC 1.49. Knocking out the ga5dh-1 gene from a competitive pathway and overexpressing the ga2dh-A gene from the 2-KGA synthesis pathway via homologous recombination increased the titre of 2-KGA by 63.81% in shake flasks. Additionally, accumulation of 5-KGA was decreased by 63.52% with the resulting G. japonicas-Δga5dh-1-ga2dh-A strain. Using an intermittent fed-batch mode in a 3 L fermenter, 2-KGA reached 235.3 g L−1 with a 91.1% glucose conversion rate. Scaling up in a 15 L fermenter led to stable 2-KGA titre with productivity of 2.99 g L−1 h−1, 11.99% higher than in the 3 L fermenter, and D-GA and 5-KGA by-products were completely converted to 2-KGA.
Keywords: 2-Keto-d-gluconic acid, Gluconobacter, Dehydrogenase, Homologous recombination, Fed-batch fermentation
1. Introduction
The 2-keto-d-gluconic acid (2-KGA) is used widely as a food additive, detergent, and photographic developer [1,2]. Importantly, it is the key precursor for the synthesis of isovitamin C, and most 2-KGA produced in industry is used to synthesise isovitamin C and its salts. Additionally, 2-KGA is a pivotal precursor of 2-keto-l-gulonic acid, the direct precursor of vitamin C [3]. Currently, 2-KGA is mainly produced by chemical synthesis and biotechnological routes [4,5]. The chemical synthesis route commonly uses Pt/Pb as catalyst, oxidising d-glucose to d-glucuronic acid or D-glucolipid with oxygen, followed by conversion to 2-KGA. However, many drawbacks exist with this route including high cost, formation of by-products, and low product selectivity [6]. Consequently, biotechnological routes are an economically and ecologically attractive choice, in which glucose is converted into 2-KGA by replacing the metal catalyst with enzymes or microbial cells. Approaches can involve enzyme catalysis, whole cell catalysis and biological fermentation methods. Due to advantages including high selectivity, high titre and high conversion, biological fermentation is the mostly commonly employed method [7,8].
Biological fermentation of 2-KGA for industrial production is receiving increasing attention [9,10]. Previous research has shown that several microorganisms can accumulate 2-KGA using glucose as substrate, including Pseudomonas, Arthrobacter, Serratia, Gluconobacter and Erwinia [4,11,12]. Pseudomonas is the most widely employed strain for industrial-scale fermentative production of 2-KGA. In this strain, glucose is oxidised to gluconic acid, then further oxidised to 2-KGA by gluconic acid dehydrogenase, rather than feeding directly into the glycolysis pathway [13,14]. A series of studies have been performed to enhance 2-KGA production in Pseudomonas, including multi-phase process optimisation and metabolic engineering strategies [15,16]. However, phage contamination is a constant problem in the industrial production of 2-KGA, leading to large-scale economic losses. Using a more resistant strain with suitable properties could potentially solve this problem.
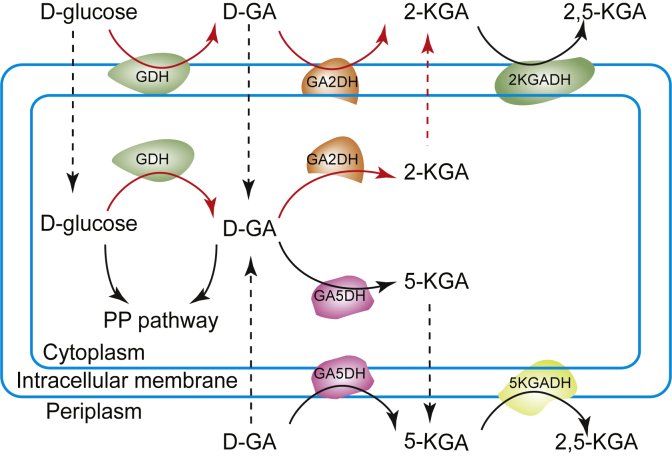
Gluconobacter, a typical Gram negative bacterium, has a complex redox enzyme system that includes membrane-bound dehydrogenase and soluble dehydrogenase enzymes [17,18]. These dehydrogenases can rapidly and efficiently oxidise alcohols/sugars/ketones to corresponding sugars/ketones/acids. Strains have been widely used in the production of 1,3-dihydroxyacetone [19], gluconic acid [20] and vitamin C [21]. Regarding 2-KGA accumulation, there are two synthesis pathways in Gluconobacter (Fig. 1) [9,22]. One is catalysed by a membrane-bound dehydrogenase in the periplasm, in which glucose is directly oxidised to d-gluconic acid (D-GA) then further oxidised to 2-KGA and 2,5-diketo-d-gluconic acid (2,5-KGA). The other is catalysed by an intracellular dehydrogenase, in which glucose is transported into the cytoplasm, oxidised to D-GA, then further oxidised to 2-KGA or 5-KGA or metabolised by the pentose phosphate pathway. D-GA or 5-KGA is the main by-products during 2-KGA production in Gluconobacter [23].
Fig. 1.
Pathways for 2-KGA synthesis from glucose in Gluconobacter. There exist two synthesis pathways for 2-KGA accumulation. One is catalysed by a membrane-bound dehydrogenase in the periplasm, in which glucose is directly oxidized to D-GA and further oxidised to 2-KGA and 2,5-KGA. The other is catalysed by an intracellular dehydrogenase, in which glucose transported into the cytoplasm is oxidised to D-GA, and further oxidised to 2-KGA or 5-KGA, or metabolised by the PP pathway. GDH, glucose dehydrogenase; GA2DH, gluconate-2-dehydrogenase; GA5DH, gluconate-5-dehydrogenase; 2KGADH, 2-keto-gluconate dehydrogenase; 5KGADH, 5-keto-gluconate dehydrogenase; PP, pentose phosphate.
In the present study, the wild-type Gluconobacter japonicus strain CGMCC 1.49 was selected for the efficient production of 2-KGA with minimal accumulation of by-products. Based on whole-genome sequencing information from previous work, gene sequences of dehydrogenases associated with the synthesis of 2-KGA were analysed. The ga2dh gene comprises three sequences encoding large and small subunits of gluconate-2-dehydrogenase (GA2DH), and an associated cytochrome C subunit. Gluconate-5-dehydrogenase (GA5DH), related to 5-KGA synthesis in a competitive pathway, is encoded by two ga5dh genes. We engineered the G. japonicas-Δga5dh-1-ga2dh-A strain using homologous recombination to knock out the ga5dh gene and enhance expression of the ga2dh gene. Using this strain, fed-batch optimisation in a 3 L fermenter resulted in a 2-KGA titre of 235.3 g L−1 with a 91.1% glucose conversion rate. Subsequent scale-up in a 15 L fermenter further increased the product yield and accumulation of 2-KGA was stable.
2. Materials and methods
2.1. Strains and plasmids
The wild-type strain used in this study, Gluconobacter japonicus CGMCC 1.49, was purchased from the China General Microbiological Culture Collection Center (CGMCC). Escherichia coli JM109 was used as the host for plasmid construction. Details of strains and plasmids are listed in Table 1.
Table 1.
Strains and plasmids used in this study.
| Strain and plasmids | Descriptions | Source |
|---|---|---|
| G. japonicus CGMCC 1.49 | Wild-type strain | Preserved in lab |
| E. coli JM109 | Cloning host | Preserved in lab |
| pMD19-T | Cloning vector | Sangon biotech |
| pBBR-MCS | Host for Kana gene | Sangon biotech |
| pMD19-ga5dh-1-sy-Kana-ga5dh-1-xy | Plasmid for gene knocking out | This study |
| pMD19-ga5dh-2-sy-Kana- ga5dh-2-xy | Plasmid for gene knocking out | This study |
| pMD19-ga5dh-1-sy-ga2dh-A-Kana-ga5dh-1-xy | Plasmid for gene knocking out | This study |
| pMD19-ga5dh-1-sy-ga2dh-B-Kana-ga5dh-1-xy | Plasmid for gene knocking out | This study |
| G. japonicus-Δga5dh-1 | Engineered strain | This study |
| G. japonicus-Δga5dh-2 | Engineered strain | This study |
| G. japonicus-Δga5dh-1-ga2dh-A | Engineered strain | This study |
| G. japonicus-Δga5dh-1-ga2dh-B | Engineered strain | This study |
2.2. Media and culture conditions
The medium for seed and slant cultures contained 10 g L−1 yeast extract and 50 g L−1 sorbitol, and 20 g L−1 agar was added to the slant medium. The fermentation medium contained 50 g·L−1glucose (in shake flasks), 100 g L−1 initial glucose (in fermenters), 20 g L−1 corn syrup, 0.1 g L−1 CaCO3, 0.2 g L−1 MgSO4·7H2O, 2 g L−1 (NH4)2SO4, and 3 g L−1 KH2PO4. The pH was maintained at 6.0 in fermenters, which was obtained by optimizing different pH values in previous study (data not shown).
Cells preserved in glycerol stocks were inoculated onto slants after thawing and incubated at 30 °C for 24 h. Seed and flask cultures were grown in 500 mL flasks containing 50 mL culture medium at 30 °C on a reciprocal shaker at 220 rpm. Fermentation was performed in a 3 L fermenter (T&J Bio-engineering, Shanghai, China) containing a 1 L initial working volume with stirring at 600 rpm and a volume air per volume (vvm) of 4. Fermentation was performed in a 15 L fermenter (T&J Bio-engineering) containing an 8 L initial working volume with stirring at 600 rpm, 4 vvm and 0.05 MPa pressure. The inoculation volume was 10% (v/v) and all cultivations were performed at 30 °C. All fermentation processes were performed in triplicate and the results are presented as mean values.
2.3. Plasmid and strain construction
For knocking out ga5dh, the upstream and downstream homologous arms of ga5dh were amplified by PCR from the G. japonicus CGMCC 1.49 genome (Table 2). The kanamycin resistance gene (kana) was obtained by PCR amplification from plasmid pBBR-MCS, and it was fused with the upstream and downstream homologous arms of ga5dh. Two fused fragments (ga5dh-1-sy-kana-xy and ga5dh-2-sy-kana-xy) corresponding to ga5dh-1 and ga5dh-2 were obtained. After purifying and ligating to the T-Vector pMD19, plasmids in which ga5dh-1 or ga5dh-2 were knocked out were obtained and verified by DNA sequencing. Homologous recombinant fragments were obtained from the above two plasmids by PCR. Strains in which ga5dh-1 or ga5dh-2 was knocked out were generated by integrating the obtained homologous recombinant fragments into the G. japonicus CGMCC 1.49 parental strain (see Table 1).
Table 2.
Primers used in this study.
| Primers | Sequence (5′–3′) |
|---|---|
| ga5dh-1-sy-F | TTACCATCACCTGCCCGGTCATTCTTATGCTGATCGGATCGG |
| ga5dh-1-sy-R | ACCTCCGCAAAATCTTCTCCCTGTCC |
| ga5dh-1-xy-F | GAAAGGAAGAGCATTACCGGCGCAG |
| ga5dh-1-xy-R | CTGTGGCGATCTATTGGGGATCTGGACGGCTGCTGTGG |
| ga5dh-2-sy-F | CCGCCGCCTTCTATGAAAGGTCCTTCCAACCCTCCGACCCGAAAAG |
| ga5dh-2-sy-R | ATCGACGCTGTCATCGGTCAG |
| ga5dh-2-xy-F | TGACCTTCCGGCAGGCCTACGTCTTCGCCGCAGGTTTTG |
| ga5dh-2-xy-R | TTGCATCTATTCAGTTCCCGATATATGGAAGC |
| Kana-F | GGAGAAGATTTTGCGGAGGTATTTGGAATGAGTCGCC |
| Kana-R | CCGGTAATGCTCTTCCTTTCATAGAAGGCGGCG |
| ga2dh-A-F | ATGACCAAAAAACATGCAGATGCCATTGTT |
| ga2dh-A-R | TCATGCCTGCACCAGAGGTCCGGGAGATTT |
| ga2dh-B-F | ATGAAGATTTTCCCATTCCTGGCCTTTGCG |
| ga2dh-B-R | GTTGCCCCAGGCGTGACGGATGAACGTCAC |
For overexpressing ga2dh, the ga2dh sequence was amplified by PCR from the G. japonicus CGMCC 1.49 genome. Fragments of kana, ga2dh, and upstream and downstream homologous arms of ga5dh-1 were fused. Two fused fragments (ga5dh-1sy-ga2dh-A-kana-ga5dh-1xy and ga5dh-1sy-ga2dh-B-kana-ga5dh-1xy) corresponding to ga2dh-A and ga5dh-B were obtained. After purifying and ligating to the T-Vector pMD19, plasmids containing ga2dh-A or ga2dh-B were obtained and verified by DNA sequencing. Homologous recombinant fragments were obtained from the above two plasmids by PCR, and strains lacking ga5dh-1 but containing the ga2dh-A or ga2dh-B genes were generated by integrating the obtained homologous recombinant fragments into the G. japonicus CGMCC 1.49 parental strain.
2.4. Analytical methods
The optical density of the culture broth was measured at 600 nm after appropriate dilution using a Biospe-1601 spectrophotometer (Shimadzu Co., Kyoto, Japan). The concentrations of 2-KGA, 5-KGA, D-GA and glucose were determined by high-performance liquid chromatography using an Agilent 1260 series instrument (Agilent, CA, USA) equipped with an Aminex HPX-87H column (Bio-Rad, Richmond, CA, USA). A 10 μL sample was injected and analysed using 5 mM H2SO4 as the mobile phase. The flow rate was 0.6 mL min−1 and the temperature was 40 °C. 2-KGA, 5-KGA and D-GA were detected using a UV absorbance detector at 210 nm, and glucose was determined using a refractive index detector.
3. Results
3.1. Effect of knocking out ga5dh on 2-KGA production in G. japonicus
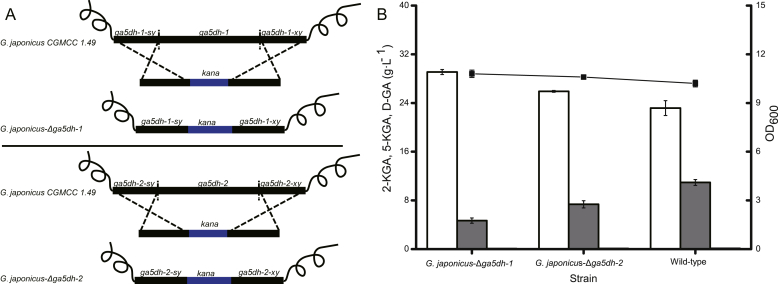
GA5DH, associated with 5-KGA synthesis in a competitive pathway, is encoded by two genes (ga5dh-1 and ga5dh-2). The upstream and downstream homologous arms of each of the ga5dh genes was fused to the kana gene and ligated to the T-Vector pMD19. Strains in which either ga5dh-1 or ga5dh-2 were knocked out were generated by homologous recombination, resulting in G. japonicus-Δga5dh-1 and G. japonicus-Δga5dh-2 (Fig. 2A). These engineered strains were fermented in the presence of 50 g L−1 glucose (initial concentration) alongside the wild-type strain as a control. G. japonicus-Δga5dh-1 and G. japonicus-Δga5dh-2 achieved titres of 28.3 g L−1 and 25.8 g L−1, respectively, representing an increase of 26.91% and 15.70% compared with the wild-type strain (22.3 g L−1). Furthermore, accumulation of by-product 5-KGA was decreased by 58.51% and 32.82% compared with the wild-type strain, and accumulation of 5-GA in the fermentation broth was minimal for all three strains (Fig. 2B).
Fig. 2.
Effect of knocking out ga5dh on 2-KGA production. Compared with the wild-type strain, the 2-KGA titre of G. japonicus-Δga5dh-1 and G. japonicus-Δga5dh-1 strains was increased by 26.91% and 15.70%, respectively, and accumulation of the by-product 5-KGA was decreased by 58.51% and 32.82%, respectively. Almost no D-GA accumulation was detected in the fermentation broth. (A) Construction of G. japonicus-Δga5dh-1 and G. japonicus-Δga5dh-2 by knocking out the ga5dh-1 gene in the wild-type strain. (B) Comparison of the fermentation efficiency of strains. White columns = 2-KGA, grey columns = 5-KGA, black columns = D-GA. The line indicates the OD600 value.
3.2. Effect of replacing ga5dh-1 with ga2dh on 2-KGA production in G. japonicus
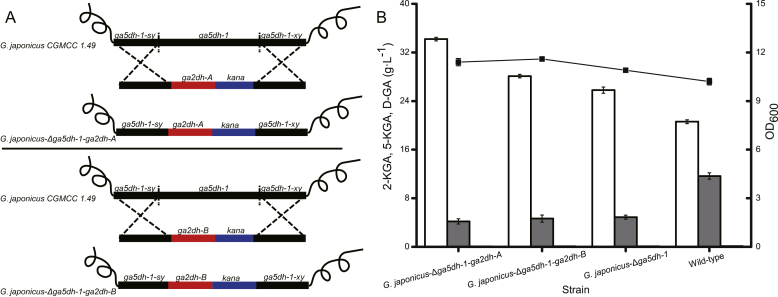
The ga2dh gene consists of three fragments, ga2dh-A, ga2dh-B and ga2dh-C, encoding large and small subunits of GA2DH, and the associated cytochrome C subunit, respectively. Based on the above results, we attempted to overexpress ga2dh by replacing the natural ga5dh-1 sequence with ga2dh-A or ga2dh-B. Upstream and downstream homologous arms of ga5dh-1 were fused to the kana gene, the product was ligated into the T-Vector pMD19, and the corresponding fragments were integrated into the parental strain, yielding strains japonicus-Δga5dh-1-ga2dh-A and G. japonicus-Δga5dh-1-ga2dh-B lacking ga5dh-1 and ga5dh-2, respectively (Fig. 3A). Compared with the wild-type strain, the 2-KGA titre was enhanced by 63.81%, and accumulation of 5-KGA was decreased by 63.52%. Compared with the G. japonicus-Δga5dh-1 strain, the 2-KGA titre of G. japonicus-Δga5dh-1-ga2dh-A was enhanced by 32.56%, and accumulation of 5-KGA was decreased by 14.41%. Accumulation of 5-GA in the fermentation broth was also minimal (Fig. 3B).
Fig. 3.
Effect of knocking out the ga5dh-1 and overexpressing the ga2dh on 2-KGA production. Compared with the wild-type strain, the 2-KGA titre of G. japonicus-Δga5dh-1-ga2dh-A and G. japonicus-Δga5dh-1-ga2dh-B was increased by 63.81% and 36.44%, respectively. Compared with G. japonicus-Δga5dh-1, the 2-KGA titre was increased by 32.56% and 9.76%, respectively. (A) Construction of G. japonicus-Δga5dh-1-ga2dh-A and G. japonicus-Δga5dh-1-ga2dh-B from the wild-type strain. (B) Comparison of the fermentation efficiency of strains. White columns = 2-KGA, grey columns = 5-KGA, black columns = D-GA. The line indicates the OD600 value.
3.3. Comparison of engineered and wild-type strains in a 3 L fermenter
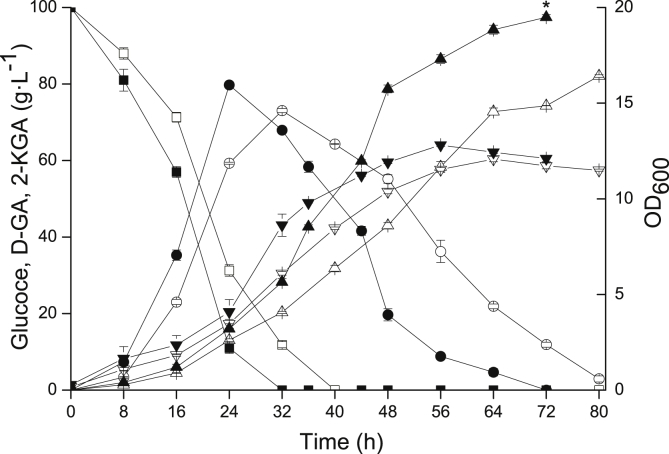
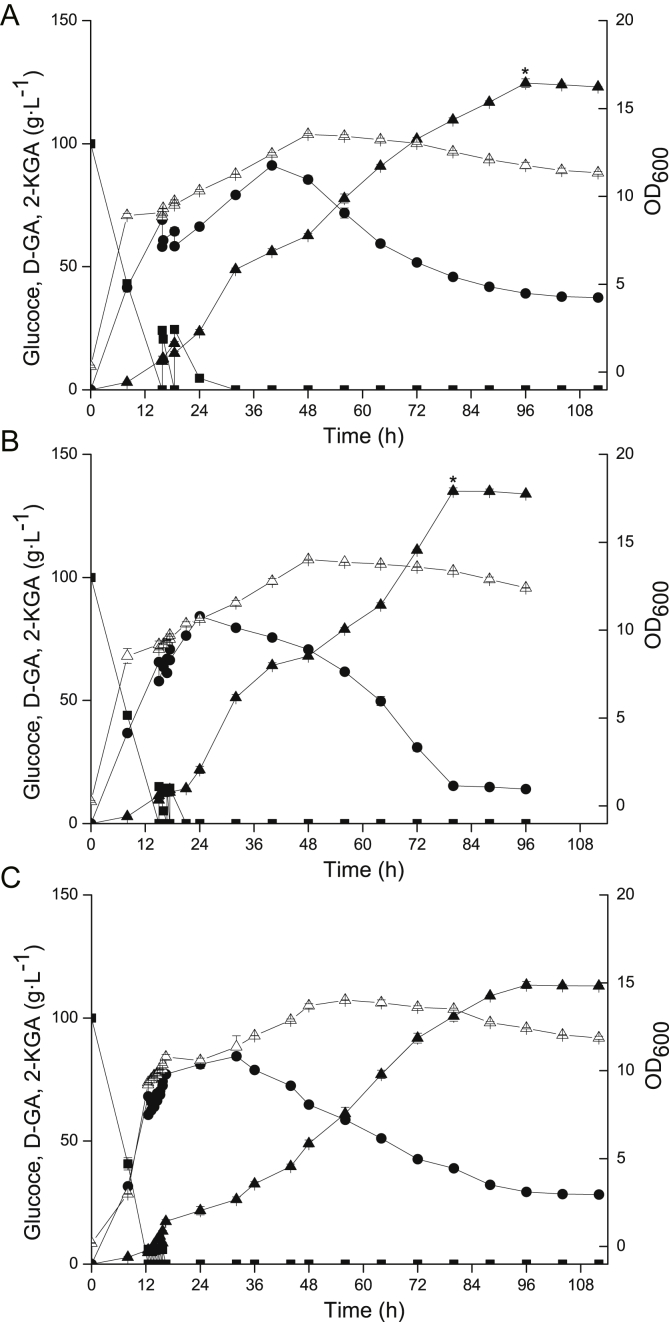
Batch fermentation of the engineered G. japonicus-Δga5dh-1-ga2dh-A and wild-type strains was conducted in a 3 L fermenter with 100 g L−1 initial glucose under the same culture conditions for 80 h. The results are shown in Fig. 4. The growth rate of the engineered strain was faster than that of the wild-type strain. Unlike the results of shake flask experiments, the 5-KGA by-product was not detected in the fermentation broth, and D-GA was the main by-product. 2-KGA production by G. japonicus-Δga5dh-1-ga2dh-A was increased by 18.76%, and the D-GA intermediate was completely converted to 2-KGA, whereas 2.9 g L−1 was retained in the fermentation broth of the wild-type strain. The glucose conversion rate with engineered strain reached 90.5%, which was increased by 15.80% compared with the wild-type strain. More importantly, the fermentation period of the engineered strain was 8 h shorter than that of the wild-type strain and the productivity of the engineered strain was increased by 31.07% than that of the wild-type strain. The highest 2-KGA titre of engineered strain presented significant difference compared to that of wild-type strain.
Fig. 4.
Batch culture of 2-KGA production using engineered and wild-type strains in a 3 L fermenter. Batch mode was employed in a 3 L fermenter with 100 g L−1 initial glucose concentration at 30 °C, 600 rpm, and 4.0 vvm. The growth rate of the engineered strain was faster than that of the wild-type strain. The 2-KGA production by G. japonicus-Δga5dh-1-ga2dh-A was increased by 18.76%, the glucose conversion rate was increased by 15.80%, the productivity of the engineered strain was increased by 31.07%, and the fermentation period was 8 h shorter than that of the wild-type strain. The significant difference about highest 2-KGA titre of engineered strain compared to that of wild-type strain was analysed with T-test, p ≤ 0.01 was considered significant difference marked with a "*". Black = G. japonicus-Δga5dh-1-ga2dh-A strain, white = Wild-type strain, squares = glucose, circles = D-GA, down triangles = OD600 values, up triangles = 2-KGA.
3.4. Development of a fed-batch system for efficient production of 2-KGA in a 3 L fermenter
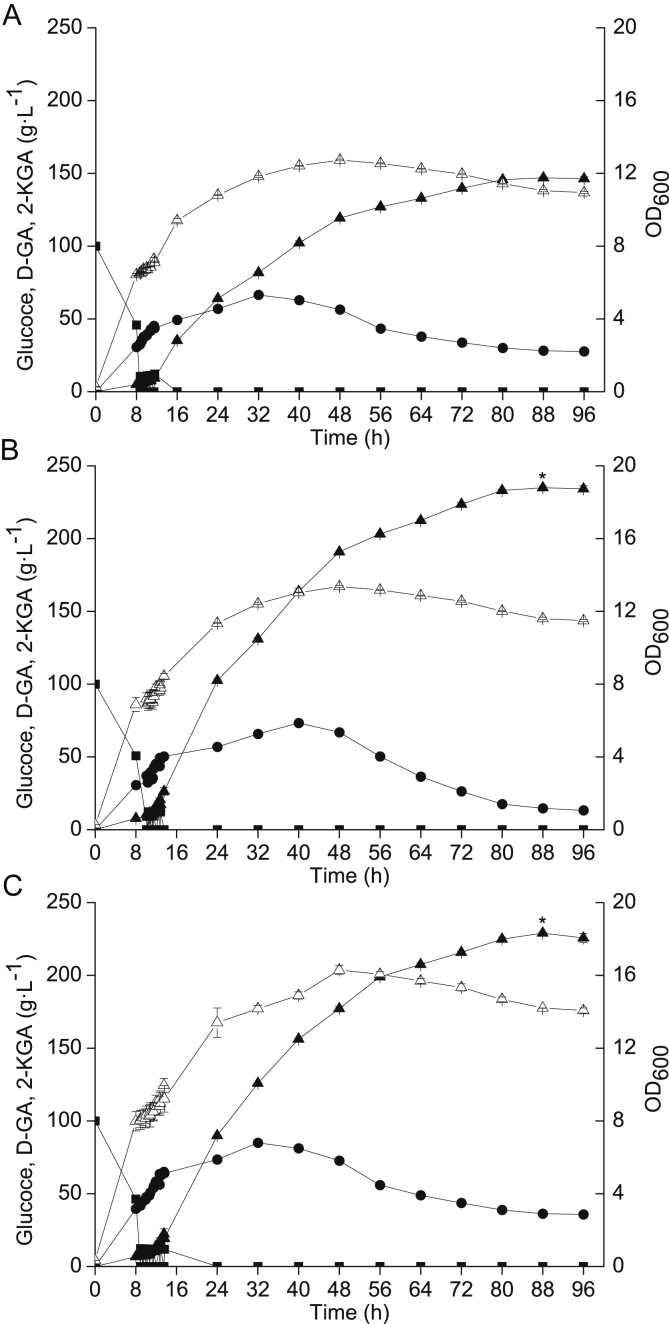
Based on the batch fermentation results, some feeding strategies tried have no significant effects on both of the 2-KGA production and the glucose conversion rate, including single dose fed-batch mode, constant rate feeding mode and mode of maintaining glucose concentration by adjusting feeding rate. An intermittent fed-batch mode was further investigated to improve the titre of 2-KGA. With a total glucose concentration of 160 g L−1 and an initial glucose concentration of 100 g L−1, glucose was intermittently fed two, three or six times, corresponding to 30 g L−1 each time, 20 g L−1 each time and 10 g L−1 each time, once glucose in the fermentation broth was depleted. Three intermittent glucose additions yielded the best results, with a highest 2-KGA titre of 134.0 g L−1, a glucose conversion efficiency of 77.7%, and productivity of 1.67 g L−1 h−1. The highest 2-KGA titre of modes of two/three intermittent additions presented significant difference compared to that of mode of six intermittent additions with T-test analysis (Fig. 5). Less substrate concentration fluctuation fed with 20 g L−1 each time was seemed to be more suitable for maintaining high cell activity to accumulate 2-KGA.
Fig. 5.
Effects of intermittent glucose addition on 2-KGA production. With an initial glucose concentration of 100 g L−1, 60 g L−1 glucose was intermittently fed several times when glucose was depleted. The titre of 2-KGA was 134.0 g L−1 with a 77.7% conversion efficiency with intermittent feeding three times, higher than with two feeds (123.2 g L−1 with a 71.4% conversion efficiency) and six feeds (113.5 g L−1 with a 65.8% conversion efficiency). The significant differences about highest 2-KGA titre of modes of two/three intermittent additions compared to that of mode of six intermittent additions were analysed with T-test, p ≤ 0.01 was considered significant difference marked with a "*". (A) Two additions of 30 g L−1 glucose each time. (B) Three additions of 20 g L−1 glucose each time. (C) Six additions of 10 g L−1 glucose each time. Squares = glucose, circles = D-GA, white triangles = OD600 values, black triangles = 2-KGA.
We subsequently explored the effect of total glucose concentration, and tested 220 g L−1, 240 g L−1 and 260 g L−1 (corresponding to six, seven or eight additions of 20 g L−1 each time. The results showed that the highest 2-KGA titre of modes of seven/eight times additions presented significant difference compared to mode of six time additions. At a total glucose concentration of 240 g L−1 after seven glucose additions, the titre of 2-KGA was 235.3 g L−1, the glucose conversion efficiency was 91.1%, and the productivity was 2.67 g L−1 h−1 (Fig. 6). Under these conditions, 11.6 g L−1 D-GA remained in the fermentation broth and was not converted into 2-KGA. The retention of D-GA was further increased under the mode of eight additions totalling 160 g L−1 glucose, which showed that the cell have no enough activity to convert D-GA to accumulate 2-KGA.
Fig. 6.
Effects of total glucose concentration on 2-KGA production. With an initial glucose concentration of 100 g L−1, different amounts of total glucose were fed intermittently (20 g L−1 each time). The titre of 2-KGA was 235.3 g L−1 with a 91.1% conversion efficiency after intermittently feeding seven times, higher than with six feeds (147.4 g L−1 with a 62.1% conversion efficiency) and eight feeds (229.0 g L−1 with an 81.7% conversion efficiency). The significant differences about highest 2-KGA titre of modes of seven/eight times additions compared to that of mode of six time additions were analysed with T-test, p ≤ 0.01 was considered significant difference marked with a "*". (A) Six additions totalling 120 g L−1 glucose. (B) Seven additions totalling 140 g L−1 glucose (C) Eight additions totalling 160 g L−1 glucose. Squares = glucose, circles = D-GA, white triangles = OD600 values, black triangles = 2-KGA.
3.5. Scale-up of 2-KGA production in a 15 L fermenter
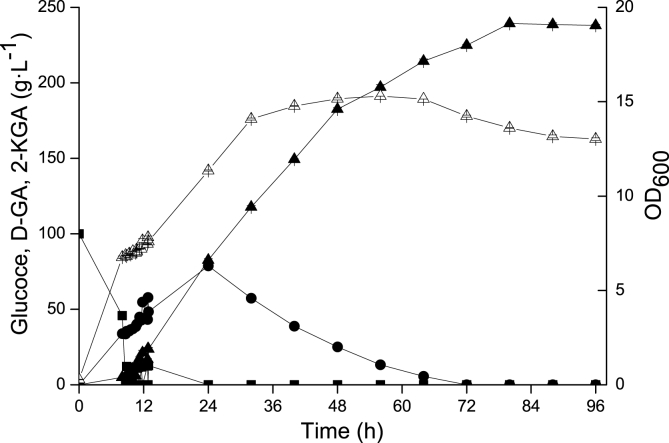
In order to test the intermittent fed-batch mode under conditions closer to those of industrial production, the process was scaled up to a 15 L fermenter. The results showed that compared with the intermittent fed-batch fermentation mode in the 3 L fermenter, accumulation of 2-KGA remained stable, while productivity was increased to 2.99 g L−1 h−1, representing an enhancement of 11.99% (Fig. 7). More importantly, the by-product was completely converted to 2-KGA, and the fermentation period was reduced to 80 h for peak production of 2-KGA. Compared to the 3 L fermenter, the significant difference about highest 2-KGA titre and productivity in 15 L fermenter was analysed with T-test. Results showed the difference in productivity were significant (p ≤ 0.01 was considered significant difference marked with a "*"). The specific fermentation parameters for the intermittent fed-batch mode in 3 L and 15 L fermenters are listed in Table 3. The comparison of various strains for 2-KGA production in recent decade was presented in Table 4.
Fig. 7.
Time courses of 2-KGA production by intermittent fed-batch culturing in a 15 L fermenter. G. japonicus-Δga5dh-1-ga2dh-A was cultivated in a 15 L fermenter at 30 °C, 600 rpm, 4 vvm and 0.05 MPa. The initial glucose concentration was 100 g L−1, and 140 g L−1 glucose was intermittently fed in seven steps. The by-product D-GA was completely converted into 2-KGA, maximum 2-KGA production reached 239.4 g/L, and the fermentation period was reduced to 80 h. Squares = glucose, circles = D-GA, white triangles = OD600 values, black triangles = 2-KGA.
Table 3.
Comparison of the fermentation characteristics of engineered strains in 3 L and 15 L fermenters.
| Parameters/Fermenter | 3 L (A) | 15 L (B) | Change (B/A-1, 100%) |
|---|---|---|---|
| Time (h) | 88 | 80 | −9.09 |
| Max of OD600 | 13.5 | 16.1 | +19.26 |
| 2-KGA (g·L−1) | 235.3 | 239.4 | +1.74 |
| 5-KGA (g·L−1) | ND | ND | / |
| D-GA (g·L−1) | 13.2 | 0 | −100.00 |
| Y (mol·mol−1, 100%) | 91.1 | 92.5 | +1.54 |
| P (g·L−1·h−1) | 2.67 | 2.99* | +11.99 |
ND indicates that the substance was not detected in the fermentation broth; * means difference was considered as significant.
Table 4.
Comparison of various strains for 2-KGA production.
| Strain | Material (g·L−1) | Process (Bioreactor) | 2-KGA (g·L−1) | Y (g·g−1) | P (g·L−1·h−1) | Reference |
|---|---|---|---|---|---|---|
| G. japonicus-Δga5dh-1-ga2dh-A | Glucose (240.0) | Fermentation (15 L fermenter) | 239.4 | 0.998 | 2.99 | This study |
| Pseudomonas fluorescens K1005 | Glucose (180.0) | Fermentation (shaking flasks) | 159.9 | 0.89 | 2.00 | [15] |
| Pseudomonas fluorescens AR4 | Glucose (181.8) | Bioconversion (shaking flasks) | 195.0 | 1.07 | 3.05 | [16] |
| Pseudomonas fluorescens AR4 | Corn starch hydrolysate | Fermentation (20 L fermenter) | 444.9 | 0.93 | 6.74 | [6] |
| Pseudomonas fluorescens AR4 | Corn starch hydrolysate | Fermentation (20 L fermenter) | 135.9 | 0.95 | 1.42 | [7] |
| Pseudomonas aeruginosa IFO 3448 | Cassava | Bioconversion (bioreactor) | 35.0 | 0.65 | 0.11 | [13] |
| Arthrobacter globiformis JUIM02 | Glucose (163.4) | Bioconversion (5 L bioreactor) | 172.9 | 1.06 | 5.41 | [44] |
| Arthrobacter globiformis C224 | Rice starch hydrolysate | Fermentation (50 L fermenter) | 124.7 | 0.97 | 1.04–1.30 | [45] |
| G. oxydans_tufB_ga2dh | Glucose (270.0) | Bioconversion (7 L bioreactor) | 321.0 | 1.19 | 17.83 | [22] |
| G. oxidans/pBBR-3510-ga2dh | Gluconic acid (480.0) | Fermentation (7 L fermenter) | 486 | 1.01 | 10.80 | [9] |
| Serratia sp. FMME043 | Glucose (258.1) | Fermentation (30 L fermenter) | 268.5 | 1.04 | 6.10 | [46] |
| Serratia sp. BK-98 | Glucose (180.0) | Fermentation (100 L fermenter) | 211.2 | 1.06 | 7.82 | [47] |
| Klebsiella pneumoniae ΔbudA | Glucose | Fermentation (5 L fermenter) | 186.0 | 1.05 | 7.15 | [48] |
4. Discussion
The 2-KGA accumulates through two synthetic pathways in Gluconobacter, but the synthetic efficiency is limited due to the existence of a competitive pathway, as well as low activity of a key dehydrogenase, resulting in the accumulation of 5-KGA and D-GA by-products. Based on whole-genome sequences determined in our previous research, we decided to knock out the ga5dh-1 gene encoding GA5DH from a competitive pathway, and overexpress the ga2dh-A gene encoding the large subunit of GA2DH. The 2-KGA titre of the resulting engineered strain was enhanced by 63.81% in shake flasks, while accumulation of the 5-KGA by-product was reduced by 63.52%. Using the strain, the 2-KGA titre was increased to 235.3 g L−1 with an intermittent fed-batch culture mode in a 3 L fermenter, and scale-up to a 15 L fermenter enhanced productivity by a further 11.99% without affecting the 2-KGA titre. These results demonstrated that Gluconobacter is a promising species for industrial production of 2-KGA.
Membrane-bound dehydrogenases associated with the respiratory chain are mainly responsible for rapid oxidation of substrates in Gluconobacter, while soluble dehydrogenases mainly participate in cell growth and maintenance [24]. The activity of membrane-bound dehydrogenases is much higher than that of soluble dehydrogenases [25,26]. In the present study, a high 2-KGA production strain was obtained by overexpressing ga2dh-A and knocking out the ga5dh-1 gene. GA5DH, encoded by ga5dh-1, appears to be a membrane-associated dehydrogenase based on its higher efficiency. 2-KGA produced from glucose via GA in Gluconobacter was catalysed by the two membrane-bound enzymes, glucose dehydrogenase (GDH) and GA2DH. A number of metabolic engineering modifications have been conducted on these membrane-bound dehydrogenases [27,28]. In one study, gldh (encoding glycerol dehydrogenase) and sdh (encoding sorbitol dehydrogenase) replaced by gdh (encoding GDH) and ga2dh in the chromosome enhanced 2-KGA titre and glucose conversion rate [29], and overexpression of ga2dh encoding membrane-bound GA2DH under the control of a strong plasmid-based promoter enhanced the titre of 2-KGA [22]. In another study, membrane-bound and soluble G5DH were manipulated to increase the 5-KGA titre [30]. Thus, enhancing the expression and activity of key dehydrogenases clearly boosted the production of target molecules in Gluconobacter.
Gluconobacter can efficiently oxidise glucose, sorbitol and glycerol as substrates to produce a large number of functional compounds [31,32]. The oxidation process is typically catalysed by membrane-bound dehydrogenases under suitable dissolved oxygen conditions [33,34]. In the present work, fermentation in 15 L performed better than in 3 L, possibly due to differences in dissolved oxygen. Gluconobacter is subject to low-oxygen stress conditions in the natural habitat, due to the rapid oxygen consumption by native metabolism [34]. Report showed that genes involved in respiration and energy metabolism were down-expressed under oxygen limitation conditions, including genes for the membrane-bound PQQ containing GDH, the PQQ-containing major polyol dehydrogenase and the type II NADH dehydrogenase [35]. Based on this feature, several optimisation strategies for dissolved oxygen have been employed to improve target product production. For example, high efficiency cell proliferation and high l-sorbose production were achieved by applying a high oxygen tension supply strategy [36], and a dissolved oxygen control strategy was established for enhancement of 5-KGA production [37]. In other studies, a dissolved oxygen control strategy and fed-batch fermentation mode were combined for production of dihydroxyacetone [38] and xylonic acid [39]. Oxygen consumption is also an important factor that could offer a fermentation control approach for maximum enhancement of 2-KGA production.
Although the titre of 2-KGA was significantly improved in the present work by knocking out ga5dh-1 encoding the probable membrane-bound GA5DH and overexpressing the ga2dh-A encoding the large subunit of GA5DH, other approaches could be implemented to further enhance the titre. Knocking out ga5dh-2, encoding an apparently soluble GA5DH enzyme, and replacing it with ga2dh-A or ga2dh-B could increase 2-KGA production, and this should be further investigated. A pipeline was previously established for screening strong promoters by proteomics analysis, and the production of l-sorbose was enhanced using a stronger promoter from G. oxydans [40]. The key dehydrogenase GA2DH could also be overexpressed to enhance 2-KGA production via a promoter replacement strategy [22,41]. Additionally, Gluconobacter contains complex membrane-bound dehydrogenases, while the space on the cytoplasmic side of the membrane available for dehydrogenase binding is limited [29,42], and the presence of most membrane-associated dehydrogenases is not necessary for the synthesis of target products [27,43]. Regulating the synergistic expression of membrane-bound dehydrogenases could therefore enhance the activity of key dehydrogenases involved in 2-KGA synthesis.
5. Conclusions
In this study, the G. japonicus-Δga5dh-1-ga2dh-A strain was engineered for 2-KGA production by knocking out ga5dh-1, involved in a competitive pathway, and overexpressing ga2dh-A from the 2-KGA synthesis pathway. The production of 2-KGA was enhanced by 63.81% in shake flasks, compared with the wild-type strain. Production reached 235.3 g L−1 in a 3 L fermenter employing an intermittent fed-batch mode. Scale-up to a 15 L fermenter resulted in even higher productivity, and the main by-product was completely converted to 2-KGA. The findings indicated that Gluconobacter could be applied for 2-KGA production to solve the problems that exist in current industrial production processes.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2017YFC1600403), the National Natural Science Foundation of China (31830068, 21822806), the Fundamental Research Funds for the Central Universities (JUSRP51701A), the National First-class Discipline Program of Light Industry Technology and Engineering (LITE2018-08), and the Distinguished Professor Project of Jiangsu Province.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Stottmeister U. White biotechnology for green chemistry: fermentative 2-oxocarboxylic acids as novel building blocks for subsequent chemical syntheses. J Ind Microbiol Biotechnol. 2005;32(11–12):651–664. doi: 10.1007/s10295-005-0254-x. [DOI] [PubMed] [Google Scholar]
- 2.Wang D. A membrane-bound gluconate dehydrogenase from 2-keto-D-gluconic acid industrial producing strain Pseudomonas plecoglossicida JUIM01: purification, characterization, and gene identification. Appl Biochem Biotechnol. 2019;188(4):897–913. doi: 10.1007/s12010-019-02951-0. [DOI] [PubMed] [Google Scholar]
- 3.Pappenberger G., Hohmann H.P. Industrial production of L-ascorbic acid (vitamin C) and D-isoascorbic acid. Biotechnol Food Feed Additiv. 2014:143–188. doi: 10.1007/10_2013_243. [DOI] [PubMed] [Google Scholar]
- 4.Zhuan W. Research progress on fermentation prcduction of 2-keto-D-gluconic acid. Food Sci (N Y) 2008;29(8):636–639. [Google Scholar]
- 5.Wang D. Purification, characterization and gene identification of a membrane-bound glucose dehydrogenase from 2-keto-D-gluconic acid industrial updettas producing strain Pseudomonas plecoglossicida JUIM01. Int J Biol Macromol. 2018;118:534–541. doi: 10.1016/j.ijbiomac.2018.06.097. [DOI] [PubMed] [Google Scholar]
- 6.Sun W. Semi-continuous production of 2-keto-gluconic acid by Pseudomonas fluorescens AR4 from rice starch hydrolysate. Bioresour Technol. 2012;110:546–551. doi: 10.1016/j.biortech.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 7.Sun W. Continuous 2-keto-gluconic acid (2KGA) production from corn starch hydrolysate by Pseudomonas fluorescens AR4. Biochem Eng J. 2013;77:97–102. [Google Scholar]
- 8.Pal P. Manufacture of gluconic acid: a review towards process intensification for green production. Chem Eng Process. 2016;104:160–171. [Google Scholar]
- 9.Shi Y. Engineered expression vectors significantly enhanced the production of 2-keto-D-gluconic acid by Gluconobacter oxidans. J Agric Food Chem. 2015;63(22):5492–5498. doi: 10.1021/acs.jafc.5b01652. [DOI] [PubMed] [Google Scholar]
- 10.Umezawa K. A novel pyrroloquinoline quinone-dependent 2-keto-D-glucose dehydrogenase from Pseudomonas aureofaciens. J Bacteriol. 2015;197(8):1322–1329. doi: 10.1128/JB.02376-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei D. 2-ketogluconic acid production by Klebsiella pneumoniae CGMCC 1.6366. J Ind Microbiol Biotechnol. 2013;40(6):561–570. doi: 10.1007/s10295-013-1261-y. [DOI] [PubMed] [Google Scholar]
- 12.Xue Q. 2-keto-D-gluconate-yielding membrane-bound D-glucose dehydrogenase from Arthrobacter globiformis C224: purification and characterization. Molecules. 2015;20(1):846–862. doi: 10.3390/molecules20010846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chia M. DO-stat fed-batch production of 2-keto-D-gluconic acid from cassava using immobilized Pseudomonas aeruginosa. Appl Microbiol Biotechnol. 2008;78(5):759–765. doi: 10.1007/s00253-008-1374-9. [DOI] [PubMed] [Google Scholar]
- 14.De Werra P. Role of gluconic acid production in the regulation of biocontrol traits of Pseudomonas fluorescens CHA0. Appl Environ Microbiol. 2009;75(12):4162–4174. doi: 10.1128/AEM.00295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun W. A novel bacteriophage KSL-1 of 2-keto-gluconic acid producer Pseudomonas fluorescens K1005: isolation, characterization and its remedial action. BMC Microbiol. 2012;12 doi: 10.1186/1471-2180-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun W. Non-sterile and buffer-free bioconversion of glucose to 2-keto-gluconic acid by using Pseudomonas fluorescens AR4 free resting cells. Process Biochem. 2015;50(4):493–499. [Google Scholar]
- 17.De Muynck C. The genus Gluconobacter oxydans: comprehensive overview of biochemistry and biotechnological applications. Crit Rev Biotechnol. 2007;27(3):147–171. doi: 10.1080/07388550701503584. [DOI] [PubMed] [Google Scholar]
- 18.Tkac J. Membrane-bound dehydrogenases from Gluconobacter sp.: interfacial electrochemistry and direct bioelectrocatalysis. Bioelectrochemistry. 2009;76(1–2):53–62. doi: 10.1016/j.bioelechem.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Hu Z. Repeated biotransformation of glycerol to 1,3-dihydroxyacetone by immobilized cells of Gluconobacter oxydans with glycerol- and urea-feeding strategy in a bubble column bioreactor. Bioresour Technol. 2017;233:144–149. doi: 10.1016/j.biortech.2017.02.096. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y. Gluconic acid production from potato waste by Gluconobacter oxidans using sequential hydrolysis and fermentation. ACS Sustain Chem Eng. 2017;5(7):6116–6123. [Google Scholar]
- 21.Wang P. Current challenges facing one-step production of L-ascorbic acid. Biotechnol Adv. 2018;36(7):1882–1899. doi: 10.1016/j.biotechadv.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Li K. Overexpression of membrane-bound gluconate-2-dehydrogenase to enhance the production of 2-keto-D-gluconic acid by Gluconobacter oxydans. Microb Cell Factories. 2016;15 doi: 10.1186/s12934-016-0521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toyama H. Membrane-bound, 2-keto-D-gluconate-yielding D-gluconate dehydrogenase from "Gluconobacter dioxyacetonicus" IFO 3271: molecular properties and gene disruption. Appl Environ Microbiol. 2007;73(20):6551–6556. doi: 10.1128/AEM.00493-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoelscher T. Glucose oxidation and PQQ-dependent dehydrogenases in Gluconobacter oxydans. J Mol Microbiol Biotechnol. 2009;16(1–2):6–13. doi: 10.1159/000142890. [DOI] [PubMed] [Google Scholar]
- 25.Yakushi T. Improved heterologous expression of the membrane-bound quinoprotein quinate dehydrogenase from Gluconobacter oxydans. Protein Expr Purif. 2018;145:100–107. doi: 10.1016/j.pep.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhu J. Identification of the enzymes responsible for 3-hydroxypropionic acid formation and their use in improving 3-hydroxypropionic acid production in Gluconobacter oxydans DSM 2003. Bioresour Technol. 2018;265:328–333. doi: 10.1016/j.biortech.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H. Enhancement of cell growth and glycolic acid production by overexpression of membrane-bound alcohol dehydrogenase in Gluconobacter oxydans DSM 2003. J Biotechnol. 2016;237:18–24. doi: 10.1016/j.jbiotec.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Mientus M. Characterization of membrane-bound dehydrogenases of Gluconobacter oxydans 621H using a new system for their functional expression. Appl Microbiol Biotechnol. 2017;101(8):3189–3200. doi: 10.1007/s00253-016-8069-4. [DOI] [PubMed] [Google Scholar]
- 29.Yi X. Production of 2-keto-D-gluconic acid by metabolically engineered Gluconobacter suboxydans. China Biotechnol. 2014;34(12):97–106. [Google Scholar]
- 30.Merfort M. High-yield 5-keto-D-gluconic acid formation is mediated by soluble and membrane-bound gluconate-5-dehydrogenases of Gluconobacter oxydans. Appl Microbiol Biotechnol. 2006;73(2):443–451. doi: 10.1007/s00253-006-0467-6. [DOI] [PubMed] [Google Scholar]
- 31.Deppenmeier U. Biochemistry and biotechnological applications of Gluconobacter strains. Appl Microbiol Biotechnol. 2002;60(3):233–242. doi: 10.1007/s00253-002-1114-5. [DOI] [PubMed] [Google Scholar]
- 32.Bertokova A. Gluconobacter sp cells for manufacturing of effective electrochemical biosensors and biofuel cells. Chem Pap. 2015;69(1):27–41. [Google Scholar]
- 33.Macauley S. The genus Gluconobacter and its applications in biotechnology. Crit Rev Biotechnol. 2001;21(1):1–25. doi: 10.1080/20013891081665. [DOI] [PubMed] [Google Scholar]
- 34.Meyer M. Effects of membrane-bound glucose dehydrogenase overproduction on the respiratory chain of Gluconobacter oxydans. Appl Microbiol Biotechnol. 2013;97(8):3457–3466. doi: 10.1007/s00253-012-4265-z. [DOI] [PubMed] [Google Scholar]
- 35.Hanke T. Influence of oxygen limitation, absence of the cytochrome bc(1) complex and low pH on global gene expression in Gluconobacter oxydans 621H using DNA microarray technology. J Biotechnol. 2012;157(3):359–372. doi: 10.1016/j.jbiotec.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X. Continuous co-production of biomass and bio-oxidized metabolite (sorbose) using Gluconobacter oxydans in a high-oxygen tension bioreactor. Bioresour Technol. 2019;277:221–224. doi: 10.1016/j.biortech.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 37.Yuan J. Enhancement of 5-keto-D-gluconate production by a recombinant Gluconobacter oxydans using a dissolved oxygen control strategy. J Biosci Bioeng. 2016;122(1):10–16. doi: 10.1016/j.jbiosc.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Poljungreed I., Boonyarattanakalin S. Dihydroxyacetone production by Gluconobacter frateurii in a minimum medium using fed-batch fermentation. J Chem Technol Biotechnol. 2017;92(10):2635–2641. [Google Scholar]
- 39.Zhou X. Improvement of fermentation performance of Gluconobacter oxydans by combination of enhanced oxygen mass transfer in compressed-oxygen-supplied sealed system and cell-recycle technique. Bioresour Technol. 2017;244:1137–1141. doi: 10.1016/j.biortech.2017.08.107. [DOI] [PubMed] [Google Scholar]
- 40.Hu Y. Enhanced production of L-sorbose in an industrial Gluconobacter oxydans strain by identification of a strong promoter based on proteomics analysis. J Ind Microbiol Biotechnol. 2015;42(7):1039–1047. doi: 10.1007/s10295-015-1624-7. [DOI] [PubMed] [Google Scholar]
- 41.Peters B. Expression of membrane-bound dehydrogenases from a mother of vinegar metagenome in Gluconobacter oxydans. Appl Microbiol Biotechnol. 2017;101(21):7901–7912. doi: 10.1007/s00253-017-8479-y. [DOI] [PubMed] [Google Scholar]
- 42.Peters B. Deletion of pyruvate decarboxylase by a new method for efficient markerless gene deletions in Gluconobacter oxydans. Appl Microbiol Biotechnol. 2013;97(6):2521–2530. doi: 10.1007/s00253-012-4354-z. [DOI] [PubMed] [Google Scholar]
- 43.Peters B. Characterization of membrane-bound dehydrogenases from Gluconobacter oxydans 621H via whole-cell activity assays using multideletion strains. Appl Microbiol Biotechnol. 2013;97(14):6397–6412. doi: 10.1007/s00253-013-4824-y. [DOI] [PubMed] [Google Scholar]
- 44.Sun L. A novel 2-keto-D-gluconic acid high-producing strain Arthrobacter globiformis JUIM02. Appl Biochem Biotechnol. 2018;185(4):947–957. doi: 10.1007/s12010-018-2707-5. [DOI] [PubMed] [Google Scholar]
- 45.Teng W. Continuous conversion of rice starch hydrolysate to 2-keto-D-gluconic acid by Arthrobacter globiformis C224. Biotechnol Bioproc E. 2013;18(4):709–714. [Google Scholar]
- 46.Luo Q. Optimization of the fermentation condition for 2-keto-D-gluconic acid producing strain. J Food Sci Biotechnol (China) 2019;38(2):22–27. [Google Scholar]
- 47.Zhang W. Fermentation process optimization and kinetics studies of 2-keto-D-gluconic acid production by Serratia sp. BK-98. J Chem Ind Eng (China) 2011;62(5):1371–1376. [Google Scholar]
- 48.Sun Y. Two-stage fermentation for 2-ketogluconic acid production by Klebsiella pneumoniae. J Microbiol Biotechnol. 2014;24(6):781–787. doi: 10.4014/jmb.1401.01038. [DOI] [PubMed] [Google Scholar]