Abstract
Background:
Endometriosis is a common gynecological disease in which oxidative stress is a potential factor. Caffeine and caffeic acid are present in various foods and beverages with anti-oxidant, anti-inflammatory, and anti-carcinogenic properties. In this study, we aimed to investigate the ameliorative effects of caffeine, caffeic acid, and caffeine+caffeic acid treatments on oxidative stress in ectopic endometrial cells taken from patients and eutopic ones from women without endometriosis.
Methods:
In this experimental study, eutopic and ectopic endometrial cells were obtained from biopsies of women free of disease (n=10) and patients with endometriosis (n=10) who referred to Shiraz reference hospitals (2017-2018). Both eutopic and ectopic endometrial cells were divided into four groups: Treated with caffeine, with caffeic acid, with caffeine+caffeic acid, and the control. Also, antioxidant enzyme activities and the levels of glutathione (GSH) and malondialdehyde (MDA) were determined in each group. The data were analyzed using independent sample t test and one-way ANOVA followed by Tukey post-hoc test.
Results:
Caffeic acid, but not caffeine treatment demonstrated a decrease in MDA level (P<0.001) as well as an increase in GSH level (P<0.001) and antioxidant enzyme activities in ectopic endometrial cells. Also, the treatment of the cells with caffeine+caffeic acid caused similar effects as those ectopic cells treated with caffeic acid.
Conclusion:
According to the findings of the present study, caffeic acid reduced oxidative stress which may alleviate the complications associated with endometriosis. However, more investigations are needed for evaluating the efficiency and safety of caffeic acid.
Keywords: Endometriosis , Oxidative stress , Caffeine , Caffeic acid
What’s Known
Endometriosis is a common and multifactorial gynecological disorder and recent studies have suggested that oxidative stress is a potential factor in the development and progression of endometriosis.
What’s New
This is the first study that investigates the ameliorative effects of caffeine, caffeic acid, and their combination treatments on oxidative stress in ectopic and eutopic endometrial cells.
Caffeic acid reduces oxidative stress which may alleviate the complications associated with endometriosis.
Introduction
Endometriosis, defined as extrauterine presence of the endometrial tissue, is a gynecologic medical disorder.1 It causes dysmenorrhea, dyspareunia, pelvic pain, and subfertility in 10-15% of women of reproductive age.2
Despite enormous investigation, its pathogenesis and etiology remain unclear;3 however, several studies suggest that the genetic, hormonal, environmental, immunological, and anatomical factors are crucial in the pathogenesis of endometriosis.2 Moreover, recent studies have suggested that oxidative stress, which is caused by the disturbance between the generation of reactive oxygen species (ROS) and antioxidant defense system, is related to various diseases and is a potential factor in the pathogenesis of endometriosis.4-6 Also, ectopic endometrial cells, as well as follicular fluid and serum of women with endometriosis, display elevated levels of Malondialdehyde (MDA) and ROS levels compared to ones from women free of diseases.7,8 ROS may alter the traits of endothelial cells including the permeability and adhesion molecule expression of the endothelial cells, leading to inflammatory processes.9
Caffeine (1, 3, 7-trimethylxanthine) is abundant in nature and is found in numerous plant species and beverages. It is used as a natural stimulant in beverages such as coffee, chocolate, tea, and soft drinks.10 Caffeic acid (3, 4-dihydroxy cinnamic acid) is one of the most prominent phenolic acids, which can be found in fruits, grains, dietary supplements, coffee, and honeybee propolis.11,12 It has been reported that caffeic acid reduces the risk of chronic disorders such as inflammation, cardiovascular disease, and cancer.13 Caffeic acid is well-known for its pharmacological properties such as antiviral,14 anti-inflammatory,15 anti-carcinogenic,16 and immunomodulatory activities.17 It has also been revealed that the administration of caffeic acid can protect rats from cisplatin-induced oxidative stress and gastrointestinal toxicity and reverse the activities of enzymes superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GPx) near to their normal level.15
Caffeine and caffeic acid are present in various foods and widely consumed beverages, such as coffee, with different distributions. To date, there is no study that has investigated the possible ameliorative effects of single and combination treatments of such substances on oxidative stress associated with endometriosis. Therefore, the objectives of the present study were to reconfirm the higher level of oxidative stress in ectopic endometrial cells in comparison with the eutopic ones and evaluate the ameliorative effects of caffeine, caffeic acid, and/or the combination treatments of them on the eutopic and ectopic endometrial cells in order to find out if they can be used, accompanied by the current drugs, to decrease the oxidative stress and the related problems in these patients.
Patients and Methods
Tert-butyl hydroperoxide (t-BuOOH), nicotinamide adenine dinucleotide phosphate (NADPH), bovine serum albumin (BSA), di-methyl sulfoxide (DMSO), caffeine, caffeic acid, GPx, GR, GSH, and Oxidized glutathione (GSSG) were purchased from Sigma Chemical Co (Poole, Dorset, UK). Sodium chloride, sodium azide, Ethylenediaminetetraacetic acid (EDTA), and magnesium chloride were obtained from Merck (Darmstadt, Germany). DMEM-F12, fetal bovine serum (FBS), penicillin, and streptomycin were obtained from Gibco-BRL (Paisley, UK).
Sample Collection
Our investigation was an experimental study, and tissue samples were collected from Ghadir Mother and Child Hospital as well as Shahid Faghihi Hospital, Shiraz University of Medical Sciences (Shiraz, Iran), between 2017 and 2018. Ectopic and eutopic endometrial tissues were obtained from 10 women with ovarian endometriosis (cases) and 10 women with benign gynecological conditions (controls) such as tubal infertility, non-endometriotic ovarian cysts, or uterine myoma and without any evidence of endometriosis. Ectopic and eutopic endometrial tissues were obtained through laparoscopy and hysteroscopy, respectively.
Only cases with the following criteria were included in the study: (1) Patients with reproductive age (20-42 years old), (2) patients with severe endometriosis (stage III-IV according to the revised American Fertility Society system);18 for the confirmation of endometriosis, a piece of endometriotic tissues was sent to pathology laboratory.
Members of the control group were selected from individuals who fulfilled the following inclusion criteria: (1) They were 20-42 years old (2) with normal menstrual cycles, and (3) individuals who were in the proliferative phase.
Participants who had received hormonal medication or contraception at least 3 months prior to the study or who had a history of malignancy, pelvic inflammatory disorders or autoimmune diseases were excluded from the study. The study was approved by Ethics Committee for subjects of Shiraz University of Medical (ethics code: 1395.S278) and written informed consent was obtained from each participant who enrolled in this study.
Purification and Culture of Eutopic and Ectopic Endometrial Stromal Cells
The tissue biopsies were immersed into DMEM-F12 medium which contained falcon immediately after the collection and were put into the refrigerator at most for 1 day. We isolated endometrial cells according to Totonchi et al.19 Briefly, tissues were rinsed and minced into small pieces of about 1-3 mm3. Then, the tissues were soaked in DMEM/10% FBS containing collagenase type I and 100 U/mL penicillin/Streptomycin for 2 h at 37 °C with intermittent vortexing every 20 minutes. Following the filtration of the cell suspension using a 40-mm sieve, stromal cells were passed through a filter while the intact glands were trapped. Finally, staining of the cells with trypan blue was done in order to count the viable cells.
Cell Culture
Endometrial stromal cells were suspended in DMEM-F12, supplemented with FBS, 100 mg/ml streptomycin, and 100 U/mL penicillin at 37 °C in the humidified atmosphere with 5% CO2 until 70-80% confluency and culture medium was changed every 3-4 days.
Immunocytochemistry
Cells were cultured at a cell density of 105 cells in a 4-well chamber slide. After washing cultured cells with PBS, they were fixed with cold acetone for 10 minutes at -20 °C. The cells were rehydrated in PBS, and blocking of nonspecific binding was done with 5% goat serum in PBS for 2 h. In the next step, incubation with primary mouse anti-vimentin antibody (Sigma Aldrich, 1:100) was performed at 4 °C overnight. The negative control slide was treated with PBS and mouse immunoglobulin (Sigma Aldrich). Following the incubation with primary antibody, the slide was rinsed with PBS; 1 h incubation under the dark condition with secondary FITC-conjugated donkey anti-mouse IgG antibody (Sigma Aldrich) was also done. After incubation with Hoechst (Thermo scientific), the mounting of slides was performed with Vectashield (Vector Lab., Burlingame, USA).20
MTT Assay
MTT assay is a colorimetric test that is based on the reduction of yellow 3-(4, 5-dimethythiazol-2-yl)-2, 5-diphenyl tetrazolium bromide to dark purple formazan product by mitochondrial succinate dehydrogenase in viable cells as described previously.21 Briefly, endometrial cells were incubated at a cell density of 5x104 cells in 96-well plate with different doses of caffeine (1-50000 μ mol/l), caffeic acid (0.1-50000 μ mol/l), and caffeine (0-6000 μ mol/l)+caffeic acid (0-3000 μ mol/l) for 24 h at 37 ºC. At the next step, 100 μl of MTT solution in PBS (0.50 mg/mL) was added to each well and incubated for 3.5 h at 37 °C. Finally, for dissolving formazan crystals, 100 μl of DMSO was added and the absorbance was determined in an ELISA reader at 570 nm. Well containing untreated cells was served as control and treated cells were compared to the control. The experiment was repeated three times.21
Treatment of Ectopic and Eutopic Endometrial Stromal Cells
Both ectopic and eutopic endometrial cells were divided into four separate groups: 1) Control (non-treated cells), 2) 1200 μM caffeine-treated cells, 3) 600 μM caffeic acid and 4) 600 μM caffeine+300 μM caffeic acid treated cells. Finally, after 24 h treatment of 70% confluent endometrial cells of mentioned groups with caffeine, caffeic acid, and caffeine+caffeic acid, the cells were lysed and antioxidant enzyme activities, GSH, and MDA levels were measured.
Cell Lysate Preparation
Following 24 h treatment of cells with caffeine, caffeic acid, and caffeine+caffeic acid, the cell lysate was prepared as described previously with some modifications.22 First, cells were harvested from the flasks and 700 μl lysis buffer was added to the pellet of each flask while it was kept on ice. Then, the cell suspension was transferred to 1.50 ml microtube; next, the cell suspension in microtubes was sonicated on ice for 2 times as to lyse the cells (the sonication condition: Amplitude 25%, palse 0.50 second, cycle 30 seconds). Afterward, microtubes were centrifuged at 10000 ×g and 4 ºC for 20 min and, finally, microtubes were gently removed from the centrifuge and placed on ice. The supernatant was aspirated and aliquot to the new tubes on ice. An aliquot was taken for protein determination. Samples were frozen at −20 ºC until use.
Protein Determination of Cell Lysate
Protein concentration in cell lysate was measured by bicinchoninic acid (BCA) Protein Assay Kit (Pierce, Rockford, IL) using bovine serum albumin as a standard. The absorbance of the purple-colored product of this assay was measured at 562 nm.
Determination of CAT Activity
The enzyme activity of CAT was determined based on the method as explained previously with minor modifications.23 Briefly, 100μl of endometrial cell lysate was added to a cuvette containing 100 μl H2O2 solution in 200 μl phosphate buffer in order to assess the decomposition of H2O2 that was followed by the reduction in absorbance at 240 nm. CAT enzyme activity in the clear supernatant of the endometrial cell lysates was expressed as mmol of H2O2 consumed/min/mg cell protein, using a molar extinction coefficient of 43.60 L/mol per cm for H2O2.
Determination of GPx Activity
The procedures as described previously (24), monitor continuous conversion of GSSG to GSH in the presence of GR and salt of reduced NADPH were used to determine the enzyme activity of GPx in the lysates of endometrial cell. The enzyme activity was calculated as μmol of NADPH oxidized/min/ mg of cell protein, using a molar extinction coefficient of 6.22×106 M-1cm-1 for NADPH.24
Determination of GR Activity
The enzyme activity of GR was assayed using the method as reported by Mostafavi-Pour et.al with minor modifications.21 GR assay was performed in a cuvette in a total volume of 1 ml that contained 60 μM buffer, 5 mM EDTA (pH 8.0), 0.03 M GSSG, 2 mM NADPH, and a sample in a final volume of 1 ml. The decrease in absorbance was measured spectrophotometrically at 340 nm for 3 minutes.
Determination of GSH
The GSH assay with 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) was done based on the previously described method with some modifications.25 The clear supernatant of cell lysate was assayed for GSH level. 2.30 ml of potassium phosphate buffer (0.20 M, pH 7.60) was mixed with 0.50 ml of DTNB (0.001 M) and 0.20 ml of cell lysate supernatant. After 5 minutes of incubation at room temperature, the absorbance was read at 412 nm.
Determination of MDA
MDA level was analyzed according to a colorimetric method that was described previously.26 Briefly, 0.50 mL supernatant of cell lysate was added to 2 mL of 2-thiobarbituric acid (TBA) reagent containing 0.37% TBA, 15% trichloroacetic acid, and 0.25 mol/L HCl. The mixture was heated in a boiling water bath for 15 minutes, cooled, and centrifuged at 8000 x g for 15 minutes at 4 °C. The absorbance of the supernatant was measured at 532 nm. Tetraethoxypropane (TEP) was used as the standard for the calculation of MDA concentration. The results were represented as nmol/mg protein.
Data Analysis
After checking the normality and homogeneity of the variance test, the data were analyzed with the SPSS software (version 16.0). The analyses between the data from control groups of ectopic and eutopic endometrial cells were done using independent sample t test. However, statistical analyses between treated groups and their related controls were done using one-way ANOVA followed by the Tukey post-hoc test. All the values were reported as mean±SEM of the three independent experiments (n=3) and P<0.05 was considered statistically significant (*P<0.05, **P<0.01, ***P<0.001).
Results
The Effect of Caffeine, Caffeic Acid, and Caffeine+Caffeic Acid on Ectopic and Eutopic Endometrial Cell Viability
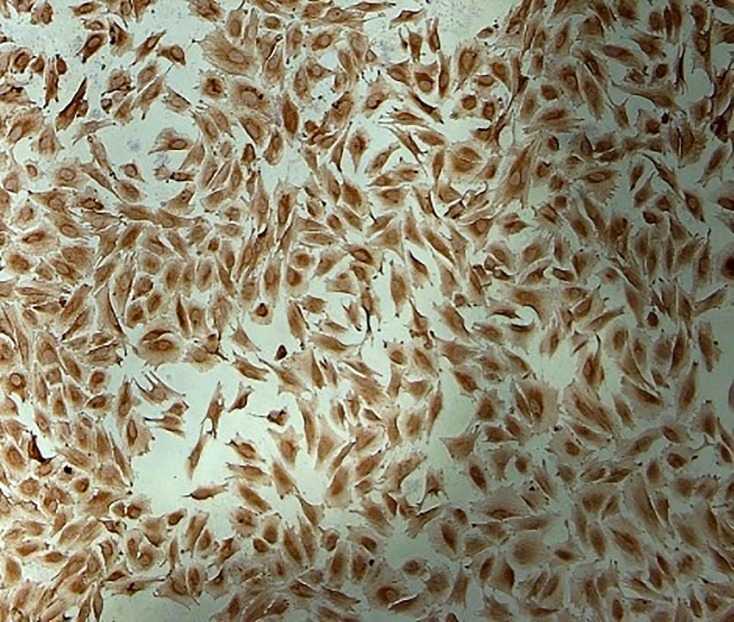
As shown in figure 1, the isolation of endometrial stromal fibroblasts was confirmed by vimentin staining. To evaluate the cytotoxic effects of caffeine and caffeic acid on endometrial cells survival, the cells were treated with 1-50000 µM of caffeine or caffeic acid for 24 h separately. After the determination of proper single concentration, the effects of different combined concentrations of caffeine and caffeic acid on cell viability were assessed.
Figure1.
Immunocytochemistry analysis shows endometrial stromal fibroblasts. The cells were isolated from ectopic and eutopic endometrial tissues followed by staining with primary antibody against vimentin (10x magnification). Cells were observed under a phase-contrast microscope, and the representative field was photographed.
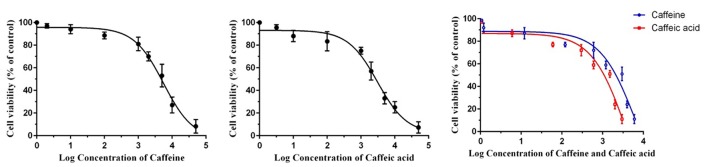
As shown in figure 2, caffeine and caffeic acid treatments decreased cell viability in a dose-dependent manner; an IC50 of 5713±215.55 μM for caffeine, IC50 of 3192±72.31 μM for caffeic acid, and IC50 for caffeine+caffeic acid were 3000±52.30 μM for caffeine and 1500±34.80 μM for caffeic acid. Based on these findings, the subtoxic concentrations of caffeine (1200 µM), caffeic acid (600 µM), and caffeine+caffeic acid (600 µM+300 µM) were selected to investigate the effects of caffeine and caffeic acid and their combination on the oxidative stress parameters and antioxidant enzyme activities in endometrial cells.
Figure2.
Cytotoxicity assessment was done after 24 h treatment of 70% confluent endometrial cells with various concentrations of caffeine, caffeic acid, and their combination. Values are presented as mean±SEM of three independent experiments. Sample size (n=10).
The Effects of Caffeine, Caffeic Acid and Caffeine+Caffeic Acid Treatments on MDA Level in Eutopic and Ectopic Endometrial Cells
As displayed in figure 3A, the basal level of MDA in ectopic endometrial cells was significantly (P<0.001) higher than that of the eutopic ones. There were no significant changes in the MDA level between groups in eutopic endometrial cells. Although the amount of MDA in caffeine-treated cells did not significantly differ from the control group, treatment of mentioned cells with caffeic acid or caffeine+caffeic acid significantly diminished MDA levels by 48.83% and 52.21%, respectively (P<0.001).
Figure3.
Effects of caffeine, caffeic acid, and caffeine+caffeic acid treatments were assessed on MDA (A) and GSH (B) levels in eutopic and ectopic endometrial cells. Eutopic and ectopic endometrial cells at 70% confluence were treated with caffeine 1200 µM (C 1200), caffeic acid 600 µM (CA 600), and caffeine 600 µM+caffeic acid 300 µM (C600+CA300). Control cells were treated with media only. Values are presented as mean±SEM of three independent experiments. Sample size (n=10 for eutopic endometrial cells, and n=10 for ectopic endometrial cells) (*P <0.05, **P<0.01 and P<0.001*** indicate significant differences between the groups). GSH, glutathione, MDA, malondialdehyde.
The Effect of Caffeine, Caffeic Acid and Caffeine+Caffeic Acid Treatments on GSH Level in Eutopic and Ectopic Endometrial Cells
As shown in figure 3B, the GSH level in ectopic endometrial cells was much lower, by 63.20% (P<0.001), than that of the eutopic endometrial cells, without any treatments. In eutopic endometrial cells, we did not find any significant changes between treated groups and related controls. In ectopic endometrial cells, only caffeic acid- and caffeine+caffeic acid-(but not caffeine) treated cells showed increased levels of GSH by 90.74% and 85.53%, respectively (P<0.001).
The Effects of caffeine, Caffeic Acid and Caffeine+Caffeic Acid Treatments on CAT Enzyme Activity in Ectopic and Eutopic Endometrial Cells
Our data showed that CAT activity in ectopic endometrial cells was notably lower than that of eutopic endometrial cells by 40.71% (P<0.001) without any treatments. As demonstrated in figure 4A, there were no significant differences in CAT enzyme activity among the groups in eutopic endometrial cells. In ectopic endometrial cells, the treatment with caffeine did not change enzyme activity; however, caffeic acid- or caffeine+caffeic acid-treated ectopic cells showed increased enzyme activity by 56% (P=0.002) and 57% (P=0.003), respectively, compared to the control.
Figure4.
Effects of caffeine, caffeic acid, and caffeine+caffeic acid treatments were determined on (A) CAT, (B) GPx, and (C) GR specific activities in eutopic and ectopic endometrial cells. Eutopic and ectopic endometrial cells at 70% confluence were treated with caffeine 1200 µM (C 1200), caffeic acid 600 µM (CA 600), and caffeine 600 µM+caffeic acid 300 µM (C600+CA300). Control cells were treated with media only. Values are presented as mean±SEM of three independent experiments. Sample size (n = 10 for eutopic endometrial cells, and n=10 for ectopic endometrial cells) (*P<0.05, **P<0.01 and P<0.001*** indicate significant differences between the groups). CAT, catalase, GPx, Glutathione Peroxidase, GR, Glutathione Reductase.
The Effects of Caffeine, Caffeic Acid and Caffeine+Caffeic Acid Treatments on GPx Activity in Ectopic and Eutopic Endometrial Cells
As demonstrated in figure 4B, GPx enzyme activity in ectopic endometrial cells dropped significantly by 42% (P<0.001) as compared to that of eutopic cells. We did not find any significant change between groups in the eutopic endometrial cells; however, the treatment of ectopic endometrial cells with caffeic acid or caffeine+caffeic acid increased GPx activity by 81% (P<0.001) and 91% (P<0.001), respectively, as compared to the related control.
The Effects of Caffeine, Caffeic Acid and Caffeine+Caffeic Acid Treatments on GR Activity in Ectopic and Eutopic Endometrial Cells
As shown in figure 4C, GR enzyme activity in the ectopic endometrial cells diminished remarkably by 42% (P<0.001) in comparison with that of eutopic cells without any treatment. In eutopic endometrial cells, the enzyme activity of treated groups did not differ from the related controls significantly. Although the treatment of ectopic endometrial cells did not change the enzyme activity of the mentioned cells notably, caffeic acid- or caffeine+caffeic acid-treated cells exhibited an increased enzyme activity by 59.52% (P=0.009) and 69% (P=0.008), respectively, as compared to the controls.
Discussion
The data presented here show that the MDA level in the ectopic endometrial cells without any treatment increased significantly when compared to the eutopic cells. Moreover, the level of GSH and the activities of CAT, GPx, and GR enzymes in the ectopic endometrial cells significantly reduced in comparison with those of the the eutopic cells.
In ectopic endometrial cells, unlike eutopic cells, caffeic acid- and caffeic acids+caffeine-treated cells showed a significant increase in the level of GSH and a significant decrease in the level of MDA. Also, in the treated ectopic endometrial cells either by caffeic acid or caffeic acid+caffeine, increased CAT, GPx, and GR activity was seen in each group compared with the controls.
Our results reconfirm the findings of a previous study which found increased H2O2 level, as well as decreased GSH, in the ectopic endometrial cells in women with endometriosis compared to the eutopic cells; the oxidative status of ectopic endometrial cells are already higher than that of the eutopic cells.8
Indeed, based on Sampson’s theory of retrograde menstruation, menstrual reflux transports red blood cells and endometrial cells into the peritoneal cavity, and increased menstrual reflux results in the release and accumulation of pro-oxidant factors such as heme and iron. This iron overload induces the generation of ROS and disturbs the balance between ROS formation and antioxidant defense system which results in oxidative stress associated with the pathogenesis of endometriosis.5
In contrast to our data, Ngo et al. demonstrated that the activity of CAT enzyme in the ectopic endometrial cells was greater than that of the eutopic cells.8 This could be explained by the fact that their study focused on some stages of the disease which were different from the stages we focused on in our study. In the early stages of the disease and following increased oxidative stress, the body attempts to cope with the oxidative condition by increasing the activity of the antioxidant enzyme system. Following the development and progression of the disease and the increase in the inflammatory and oxidative factors, the antioxidant defense system is incapacitated against the existing conditions and this leads to a decrease in the activity of the antioxidant enzymes. In addition, our findings are in line with those of of Kao et al.’s who reported the indicators of oxidative damage such as mitochondrial DNA rearrangement, 8-OH-deoxyguanosine, and lipoperoxide components in the ectopic endometrial tissue, which were significantly higher than those of the eutopic cells.27
Furthermore, the results of our study demonstrated that the levels of GSH and MDA, as well as the activities of CAT, GPx, and GR antioxidant enzymes in the eutopic endometrial cells treated with caffeine, caffeic acid, and caffeine+caffeic acid, did not significantly differ from the same data that were obtained for the related controls. Contrary to our expectation, the treatment of ectopic cells with caffeine revealed no significant difference in the oxidative stress parameters, GSH and MDA level, and the activities of the antioxidant enzymes in comparison with those of the controls. Interestingly, based on our data, caffeine+caffeic acid-treated ectopic cells indicated similar results as caffeic acid alone, proving that there existed no synergistic or additive effects between these two substances.
Lv et al. reported that the administration of caffeine in rats with alcohol-induced liver damage reduced MDA level and increased the hepatic antioxidant capacity including superoxide dismutase (SOD) and GPx enzymes.28 Demirtas et al. have reported that caffeine in rat’s diet could decrease the level of MDA and increase the activity of SOD, CAT, GPx, and glutathione S transferase enzymes in their liver tissue and the attenuated oxidative stress. 29 Contrary to what they reported, we did not observe any antioxidant effect of caffeine in endometrial cells. One possible explanation for this controversy may be due to the metabolism of caffeine within the body and the production of its derivatives by liver enzymes. Moreover, our data are based on in vitro experiments.
In agreement with our results, Lee et al. revealed that caffeine had dose-dependent antioxidant activity with optimal occurrence at 0.16 mol/l and not at concentrations less than 0.02 mol/l (physiological concentrations). They also found that observed antioxidant property in the physiological concentrations was related to major metabolites of caffeine such as 1-methylxanthine and 1-methyluric acid as effective and potent antioxidants.30
In line with the results of the present study, Pang et al. reported that the treatment of HepG2 and L-02 cell lines with caffeic acid could enforce both a protective effect and the reduction of ROS level on aspirin-induced liver cell injury. Moreover, caffeic acid reduced the expression of keap1 which, in turn, inhibited the binding of keap1 to Nrf2, resulting in the activation of Nrf2 followed by the expression of antioxidative signals such as NAD(P)H quinone oxidoreductase 1 (NQO1) and heme oxygenase 1 (HO-1).31 It has also been demonstrated that cell treatment with chitosan-caffeic acid (50–400 μg/ml) was able to significantly increase survival in the damaged liver cells by H2O2. They reported that chitosan-caffeic acid reduced the production of ROS and lipid peroxidation and increased GSH level in cultured liver cells. The authors also found that treatment with chitosan-caffeic acid increased the nuclear translocation of Nrf2 transcription factor, which, in turn, increased the expression of SOD, GR, HO-1, and NQO1 enzymes.32
Alternatively, the findings of Marcellin et al. suggested that the expression of Nrf2 transcription factor and its target gene, glutamate cysteine ligase, in the endometriotic tissue of the affected women reduced compared to the endometrial tissue of healthy subjects. The authors also showed that the gene the expression level of Nrf2 significantly reduced in the ectopic endometrial cells.33 Nrf2 regulates oxidation-reduction status of the cells and controls the expression of GPx and GR enzymes.34
Therefore, it seems that caffeic acid probably shows antioxidant activity via increasing both the nuclear translocation of Nrf2 and the expression of antioxidant enzymes, GPx and GR, which in turn decreasing the oxidative stress.
Collectively, our study revealed that although caffeine did not indicate any antioxidant activities in either ectopic or eutopic endometrial cells separated from women with and without endometriosis, respectively, caffeic acid treatment had antioxidant properties on the ectopic endometrial cells and could reverse the normal enzyme activities and improve the antioxidant status of these cells. Furthermore, no evidence was found for a synergistic or additive effect of the combination of caffeine+caffeic acid in this study.
Although the pathophysiology of endometriosis is understood well, oxidative stress is a potential element in the development and progression of endometriosis.35 Also, oxidative stress markers, such as 8-OH-deoxyguanosine and lipid hydroperoxides, may rise in women with endometriosis. The total antioxidant status of these patients is lower than that of women without endometriosis.36 These findings manifest that the diminished antioxidant levels may be associated with the pathogenesis of endometriosis. The findings of the previous studies suggest that supplementation with antioxidants may reduce endometriosis-related symptoms and oxidative damage. For example, the treatment of mice with resveratrol as an antioxidant decreased the number and volume of the endometriotic lesion in mouse model of endometriosis, and supplementation with the combination of vitamin C and E in women with the disease decreased endometriosis-related pelvic pain.37,38 Unfortunately, since caffeine and caffeic acid had not received FDA drug approval, we did not evaluate their therapeutic effects along and in combination with the current drugs and medications.
Conclusion
Our data demonstrated that caffeic acid could, on one hand, reduce oxidative stress and, on the other hand, improve the antioxidant status of ectopic endometrial cells. However, further investigations on animal and human models are needed to evaluate the efficiency and safety of the treatment with caffeic acid, as well as figuring out the molecular and genetic mechanisms underlying the antioxidant effect of caffeic acid.
Acknowledgement
The present study was part of an MSc student thesis by Navid Jamali (Department of Biochemistry, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran), who received grant (grant no. 94-01-01-11178) from the office of Vice Chancellor for Research and the Committee for Advanced Biomedical Sciences, Shiraz University of Medical Sciences.
Conflict of Interest:None declared.
References
- 1.Gonzalez-Foruria I, Santulli P, Chouzenoux S, Carmona F, Chapron C, Batteux F. Dysregulation of the ADAM17/Notch signalling pathways in endometriosis: from oxidative stress to fibrosis. Mol Hum Reprod. 2017;23:488–99. doi: 10.1093/molehr/gax028. [DOI] [PubMed] [Google Scholar]
- 2.Sekhon LH, Agarwal A. Endometriosis and Oxidative Stress. Studies on Women’s Health: Springer; 2013. [Google Scholar]
- 3.Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27:441–7. doi: 10.1007/s10815-010-9436-1. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Lima CB, Cordeiro FB, Camargo M, Zylbersztejn DS, Cedenho AP, Bertolla RP, et al. Follicular fluid lipid peroxidation levels in women with endometriosis during controlled ovarian hyperstimulation. Hum Fertil (Camb) 2017;20:48–54. doi: 10.1080/14647273.2016. [DOI] [PubMed] [Google Scholar]
- 5.Donnez J, Binda MM, Donnez O, Dolmans MM. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil Steril. 2016;106:1011–7. doi: 10.1016/j.fertnstert.2016.07.1075. [DOI] [PubMed] [Google Scholar]
- 6.Signorini L, Granata S, Lupo A, Zaza G. Naturally Occurring Compounds: New Potential Weapons against Oxidative Stress in Chronic Kidney Disease. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18071481. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasiri N, Moini A, Eftekhari-Yazdi P, Karimian L, Salman-Yazdi R, Arabipoor A. Oxidative Stress Statues in Serum and Follicular Fluid of Women with Endometriosis. Cell J. 2017;18:582–7. doi: 10.22074/cellj.2016.4724. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngo C, Chereau C, Nicco C, Weill B, Chapron C, Batteux F. Reactive oxygen species controls endometriosis progression. Am J Pathol. 2009;175:225–34. doi: 10.2353/ajpath.2009.080804. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambrinoudaki IV, Augoulea A, Christodoulakos GE, Economou EV, Kaparos G, Kontoravdis A, et al. Measurable serum markers of oxidative stress response in women with endometriosis. Fertil Steril. 2009;91:46–50. doi: 10.1016/j.fertnstert.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Cauli O, Morelli M. Caffeine and the dopaminergic system. Behav Pharmacol. 2005;16:63–77. doi: 10.1097/00008877-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Castellari M, Sartini E, Fabiani A, Arfelli G, Amati A. Analysis of wine phenolics by high-performance liquid chromatography using a monolithic type column. J Chromatogr A. 2002;973:221–7. doi: 10.1016/s0021-9673(02)01195-0. [DOI] [PubMed] [Google Scholar]
- 12.Genaro-Mattos TC, Mauricio AQ, Rettori D, Alonso A, Hermes-Lima M. Antioxidant Activity of Caffeic Acid against Iron-Induced Free Radical Generation--A Chemical Approach. PLoS One. 2015;10:e0129963. doi: 10.1371/journal.pone.0129963. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JB. 5-Caffeoylquinic acid and caffeic acid orally administered suppress P-selectin expression on mouse platelets. J Nutr Biochem. 2009;20:800–5. doi: 10.1016/j.jnutbio.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Utsunomiya H, Ichinose M, Ikeda K, Uozaki M, Morishita J, Kuwahara T, et al. Inhibition by caffeic acid of the influenza A virus multiplication in vitro. Int J Mol Med. 2014;34:1020–4. doi: 10.3892/ijmm.2014.1859. [DOI] [PubMed] [Google Scholar]
- 15.Arivarasu NA, Priyamvada S, Mahmood R. Oral administration of caffeic acid ameliorates the effect of cisplatin on brush border membrane enzymes and antioxidant system in rat intestine. Exp Toxicol Pathol. 2013;65:21–5. doi: 10.1016/j.etp.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Rosendahl AH, Perks CM, Zeng L, Markkula A, Simonsson M, Rose C, et al. Caffeine and Caffeic Acid Inhibit Growth and Modify Estrogen Receptor and Insulin-like Growth Factor I Receptor Levels in Human Breast Cancer. Clin Cancer Res. 2015;21:1877–87. doi: 10.1158/1078-0432.CCR-14-1748. [DOI] [PubMed] [Google Scholar]
- 17.Traves PG, Luque A, Hortelano S. Macrophages, inflammation, and tumor suppressors: ARF, a new player in the game. Mediators Inflamm. 2012;2012:568783. doi: 10.1155/2012/568783. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts CP, Rock JA. The current staging system for endometriosis: does it help? Obstet Gynecol Clin North Am. 2003;30:115–32. doi: 10.1016/s0889-8545(02)00056-6. [DOI] [PubMed] [Google Scholar]
- 19.Totonchi H, Miladpour B, Mostafavi-Pour Z, Khademi F, Kasraeian M, Zal F. Quantitative analysis of expression level of estrogen and progesterone receptors and VEGF genes in human endometrial stromal cells after treatment with nicotine. Toxicol Mech Methods. 2016;26:595–600. doi: 10.1080/15376516.2016.1218578. [DOI] [PubMed] [Google Scholar]
- 20.Raheem K, Fouladi-Nashta A. Isolation and characterization of endometrial luminal epithelial and stromal cells in vitro. Sokoto Journal of Veterinary Sciences. 2014;12:1–8. doi: 10.4314/sokjvs.v12i3.1. [DOI] [Google Scholar]
- 21.Mostafavi-Pour Z, Khademi F, Zal F, Sardarian AR, Amini F. In Vitro Analysis of CsA-Induced Hepatotoxicity in HepG2 Cell Line: Oxidative Stress and alpha2 and beta1 Integrin Subunits Expression. Hepat Mon. 2013;13:e11447. doi: 10.5812/hepatmon.11447. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon YC, Jewett MC. High-throughput preparation methods of crude extract for robust cell-free protein synthesis. Sci Rep. 2015;5:8663. doi: 10.1038/srep08663. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarahmadi A, Zal F, Bolouki A. Protective effects of quercetin on nicotine induced oxidative stress in ‘HepG2 cells’. Toxicol Mech Methods. 2017;27:609–14. doi: 10.1080/15376516.2017. [DOI] [PubMed] [Google Scholar]
- 24.Fecondo JV, Augusteyn RC. Superoxide dismutase, catalase and glutathione peroxidase in the human cataractous lens. Exp Eye Res. 1983;36:15–23. doi: 10.1016/0014-4835(83)90085-4. [DOI] [PubMed] [Google Scholar]
- 25.Sardarian A, Andisheh Tadbir A, Zal F, Amini F, Jafarian A, Khademi F, et al. Altered oxidative status and integrin expression in cyclosporine A-treated oral epithelial cells. Toxicol Mech Methods. 2015;25:98–104. doi: 10.3109/15376516.2014.990595. [DOI] [PubMed] [Google Scholar]
- 26.Zal F, Mahdian Z, Zare R, Soghra B, Mostafavi-Pour Z. Combination of vitamin E and folic acid ameliorate oxidative stress and apoptosis in diabetic rat uterus. Int J Vitam Nutr Res. 2014;84:55–64. doi: 10.1024/0300-9831/a000193. [DOI] [PubMed] [Google Scholar]
- 27.Kao SH, Huang HC, Hsieh RH, Chen SC, Tsai MC, Tzeng CR. Oxidative damage and mitochondrial DNA mutations with endometriosis. Ann N Y Acad Sci. 2005;1042:186–94. doi: 10.1196/annals.1338.021. [DOI] [PubMed] [Google Scholar]
- 28.Lv X, Chen Z, Li J, Zhang L, Liu H, Huang C, et al. Caffeine protects against alcoholic liver injury by attenuating inflammatory response and oxidative stress. Inflamm Res. 2010;59:635–45. doi: 10.1007/s00011-010-0176-6. [DOI] [PubMed] [Google Scholar]
- 29.Demirtas C, Ebru O, Ahmed H, Hatice P. Effects of caffeine on oxidant-antioxidant mechanism in the rat liver. Gazi Med J. 2012;23:13–8. doi: 10.5152/gmj.2012.04. [DOI] [Google Scholar]
- 30.Lee C. Antioxidant ability of caffeine and its metabolites based on the study of oxygen radical absorbing capacity and inhibition of LDL peroxidation. Clin Chim Acta. 2000;295:141–54. doi: 10.1016/s0009-8981(00)00201-1. [DOI] [PubMed] [Google Scholar]
- 31.Pang C, Zheng Z, Shi L, Sheng Y, Wei H, Wang Z, et al. Caffeic acid prevents acetaminophen-induced liver injury by activating the Keap1-Nrf2 antioxidative defense system. Free Radic Biol Med. 2016;91:236–46. doi: 10.1016/j.freeradbiomed.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Ahn CB, Je JY, Kim YS, Park SJ, Kim BI. Induction of Nrf2-mediated phase II detoxifying/antioxidant enzymes in vitro by chitosan-caffeic acid against hydrogen peroxide-induced hepatotoxicity through JNK/ERK pathway. Mol Cell Biochem. 2017;424:79–86. doi: 10.1007/s11010-016-2845-4. [DOI] [PubMed] [Google Scholar]
- 33.Marcellin L, Santulli P, Chouzenoux S, Cerles O, Nicco C, Dousset B, et al. Alteration of Nrf2 and Glutamate Cysteine Ligase expression contribute to lesions growth and fibrogenesis in ectopic endometriosis. Free Radic Biol Med. 2017;110:1–10. doi: 10.1016/j.freeradbiomed.2017.04.362. [DOI] [PubMed] [Google Scholar]
- 34.Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Menezo Y, Dale B, Cohen M. DNA damage and repair in human oocytes and embryos: a review. Zygote. 2010;18:357–65. doi: 10.1017/S0967199410000286. [DOI] [PubMed] [Google Scholar]
- 36.Gupta S, Sinha A. Potential Markers of Endometriosis: Latest Update. J Genit Syst Disord. 2016;5 doi: 10.4172/2325-9728.1000157. [DOI] [Google Scholar]
- 37.Ricci AG, Olivares CN, Bilotas MA, Baston JI, Singla JJ, Meresman GF, et al. Natural therapies assessment for the treatment of endometriosis. Hum Reprod. 2013;28:178–88. doi: 10.1093/humrep/des369. [DOI] [PubMed] [Google Scholar]
- 38.Santanam N, Kavtaradze N, Murphy A, Dominguez C, Parthasarathy S. Antioxidant supplementation reduces endometriosis-related pelvic pain in humans. Transl Res. 2013;161:189–95. doi: 10.1016/j.trsl.2012.05.001. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]