Abstract
Background:
Colorectal cancer (CRC) is the third most common cancer worldwide. Studies have indicated that immune cells and soluble factors play a key role in maintaining the balance between tumor-promoting inflammation and anti-tumor immunity. It has been shown that secreted cytokines from CRC cell lines could affect peripheral blood mononuclear cells (PBMCs), monocytes, and macrophages phenotypes. Macrophage infiltration has been associated with good prognosis in some cancers, but with poor prognosis in others. The present study aimed to evaluate the effect of conditioned media from CRC cells (Caco-2) on immune responses produced by PBMCs.
Methods:
The present study was performed at the Gastroenterology and Liver Diseases Research Institute, Shahid Beheshti University of Medical Sciences (Tehran, Iran) in 2017. Human monocytes were isolated from PBMCs by Ficoll gradient media. The co-culture of monocytes and Caco-2 conditioned media was carried out. RNA extraction and cDNA synthesis of monocytes were performed after 96 hours. Gene expression of pro- and anti-inflammatory cytokines was evaluated by real-time PCR. Statistical analysis was performed using the SPSS software (version 21.0) with the independent sample t test. P<0.05 was considered statistically significant.
Results:
Compared to the control group, the treated monocytes showed increased levels of interleukin-6 (P=0.001), interleukin-12b (P=0.001), and interferon-gamma (P=0.02), as well as decreased amounts of interleukin-4 (P=0.01), interleukin-10 (P=0.01), and tumor necrosis factor-alpha (P=0.01).
Conclusion:
Secreted cytokines and soluble factors from Caco-2 induced the differentiation of PBMCs, particularly the monocytes, toward inflammatory phenotype according to the altered gene expression of inflammatory and anti-inflammatory cytokines.
Keywords: Monocytes , Macrophages , Cytokines , Colorectal neoplasms , Caco-2 cells
What’s Known
It is assumed that some secreted factors from different cell lines can affect pro- and anti-inflammatory cytokines profile in immune cells.
What’s New
The impact of colorectal cancer cells conditioned media on the peripheral blood mononuclear cells without using colorectal cancer cells in co-culture condition was evaluated.
Monocytes induced by secreted factors from colorectal cancer cells acquired pro-inflammatory phenotype due to alteration in gene expression of pro- and anti-inflammatory cytokines.
Introduction
The digestive system is exposed to many risk factors leading to the development and progression of malignancies.1 According to the World Cancer Research Fund International (WCRF), CRC is the third most common cancer in the general population.2 In addition, it is the second and third diagnosed malignancy in women and men, respectively.3 CRC occurs mainly in individuals aged ≥50 years and is caused by sporadic, genetics, and epigenetics changes.4
The tumor microenvironment (TME) is a complex system consisting of various cell types, such as smooth muscle cells, myeloid-derived suppressor cells (MDSCs), and leukocytes (mainly inflammatory monocytes and macrophages).4,5 The TME secrets various soluble factors, cytokines, chemokines, and cancer-derived exosomes; all of which have a critical impact on the recruitment of different immune cells.4,6,7 The immune system plays a critical role in the maintenance of intestinal homeostasis.8 Infiltration by monocytes and secreted anti-inflammatory cytokines from these cells are the main factors in tumor progression and metastasis in prostate and breast cancers, resulting in poor prognosis. On the other hand, in skin and CRCs, activation of the immune system through the inflammatory pathways correlates with a better prognosis.9,10 Such interaction between tumor cells and monocytes-macrophages is considered as one of the important issues in cancer research.11
Based on the above-mentioned findings, monocytes are the main players in CRC. The transition of monocytes into tissue-specific macrophages occurs in response to different factors and cytokines of the tissue microenvironment.11,12 Monocytes are recruited to the tumor site by cancer cell-derived chemotactic cytokines, such as monocyte chemoattractant protein-1 (MCP-1) and vascular endothelial growth factor (VEGF).13 The macrophages have an important role in innate and adaptive immune responses. 7,14 In terms of their plasticity feature, they are divided into two main categories, classically activated (M1) macrophages with tumor-preventing properties and alternatively activated (M2) macrophages with tumor-promoting properties. Each of these subtypes play different roles in different types of cancers.9,11
The bacterial antigens, Toll-like receptor (TLR) ligands, lipopolysaccharides (LPS), and interferon-gamma (IFN-ɣ) induce the polarization of monocytes into M1 phenotype.10,15 M1-macrophages induce adaptive immune responses by producing pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-12b (IL-12b) with anti-tumoral properties.7,16 On the other hand, M2-macrophages participate in tissue homeostasis and wound healing. They suppress the immune system by inducing angiogenesis and tumor progression. M2 subtypes are characterized by immune suppressive cytokines like interleukin-4 (IL-4), interleukin-10 (IL-10), and tumor growth factor-beta (TGF-β).7,12
In the present study, we investigated the impact of Caco-2 conditioned media on PBMCs. Additionally, the impact of CRC-conditioned media on the differentiation of monocytes in acquiring either an inflammatory or anti-inflammatory phenotype was evaluated through the expression level of pro- and anti-inflammatory genes. This is the first study that evaluated the impact of CRC-conditioned media on PBMCs without using CRC cells under co-culture condition.
Materials and Methods
Cell Line and in Vitro Cell Culture
The colon cancer cell line was purchased from Shahid Beheshti University of Medical Sciences (Tehran, Iran) in 2017. The Caco-2 cell line was cultured in RPMI 1640 (Life Technologies, Carlsbad, CA, USA) and enriched with 10% fetal bovine serum (FBS) (Life Technologies, Carlsbad, CA, USA), 100 U/ml penicillin (Invitrogen, Carlsbad, CA, USA), and 100 mg/ml streptomycin (Invitrogen, Carlsbad, CA, USA). The culture was incubated at 37 °C, 5% CO2, and 90% relative humidity. The Caco-2 cell line was cultured in six T-75 cell culture flasks at a density of 1~1.5×106 cells/ml. The medium was changed every 2 or 3 days until the confluency reached 50-60%. Then, the medium was replaced with 5% completed media and the cells were incubated under the same conditions for 4 days until the confluency reached 70-80%. The conditioned media were collected every 2 days after filtrating through 0.22 µm sterilized syringe-driven filters (Jet Biofile, Guangzhou, China). The filtered media were stored at -70 °C till further use.
Patients’ Demographic Data
The participants were 12 healthy donors (6 men and 6 women) with a similar physical condition. The age range of the donors was between 24 and 28 years with a normal body mass index range between 18.5 and 24.9 kg/m2. The donors were in good physical condition without any family history of gastrointestinal disorders and inflammatory diseases, such as inflammatory bowel disease. Demographic data of the donors are presented in table 1.
Table 1.
Demographic data of all healthy donors
| Participants’ codes | Sex | Age (year-month) | BMI | Inflammatory disease | Gastrointestinal disorders | IBD | Celiac | Region (country) |
|---|---|---|---|---|---|---|---|---|
| P01 | F | 26-02 | 20.51 | No | Constipation (mild) | No | No | Tehran (Iran) |
| P02 | F | 25-01 | 21.04 | No | No | No | No | Tehran (Iran) |
| P03 | F | 25-06 | 20.90 | No | No | No | No | Gilan (Iran) |
| P04 | F | 26-03 | 23.37 | No | No | No | No | Tehran (Iran) |
| P05 | F | 24-09 | 21.85 | No | No | No | No | Yazd (Iran) |
| P06 | F | 25-11 | 22.43 | No | No | No | No | Tehran (Iran) |
| P07 | M | 26-08 | 22.81 | No | No | No | No | Tehran (Iran) |
| P08 | M | 27-02 | 23.79 | No | Constipation (mild) | No | No | Tehran (Iran) |
| P09 | M | 27-08 | 24.04 | No | No | No | No | Isfahan (Iran) |
| P10 | M | 24-06 | 23.87 | No | No | No | No | Tehran (Iran) |
| P11 | M | 25-02 | 22.34 | No | No | No | No | Ardebil (Iran) |
| P12 | M | 26-04 | 21.50 | No | No | No | No | Kermanshah (Iran) |
P: Participant; F: Female; M: Male
The participants were informed of the study procedures and a written informed consent was obtained prior to participation. The study was approved (number: IR.IAU.TMU.REC.1395.384) by the Ethics Committee of Shahid Beheshti University of Medical Sciences (Tehran, Iran).
Isolation of Peripheral Blood Mononuclear Cells
Peripheral blood was obtained from the 12 healthy donors and collected in heparinized tubes. The blood was diluted with 1×Dulbecco’s phosphate-buffered saline (D-PBS) (Life Technologies, Carlsbad, CA, USA). The human PBMCs were obtained using Ficoll gradient media (Sigma-Aldrich, St. Louis, MO, USA). Briefly, the diluted blood was layered on Ficoll and centrifuged at 100×g for 20 minutes at room temperature. Then, the PBMCs layer located between the plasma and Ficoll layer was separated. PBMCs were washed in D-PBS before resuspension in the medium by centrifugation at 100×g for 10 minutes at room temperature.17
Expansion of Human Monocytes
Isolated PBMCs were cultured in two different groups as a test and control group (2×106 cells/ml) in RPMI-1640 (Life Technologies, Carlsbad, CA, USA) supplemented with 10% FBS (Life Technologies, Carlsbad, CA, USA), 100 U/ml penicillin (Invitrogen, Carlsbad, CA, USA), and 100 mg/ml streptomycin (Invitrogen, Carlsbad, CA, USA). PBMCs were cultured in six T-25 cell culture flasks: three test flasks and three control flasks. All experiments were performed in triplicate. After 2 hours, the medium was removed and PBMCs were washed twice with D-PBS to eliminate the lymphocytes and to maintain the monocytes in the flasks. The viability of the cultured monocytes was evaluated by Trypan blue staining and counted using a hemocytometer.
Treatment of Monocytes with CRC-Conditioned Media
After 24 hours, the test flasks of cultured PBMCs were treated with Caco-2 collected conditioned media. The controls were cultured in RPMI complete medium. Both the test and control flasks were incubated for 96 hours at 37 °C, 5% CO2, and 90% relative humidity. All experiments were performed in triplicate.
RNA Extraction and Quantitative Reverse Transcription PCR (RT-PCR)
After 96 hours, the test and control monocytes were detached using Trypsin-EDTA (Invitrogen, Carlsbad, CA, USA). The total RNA of detached monocytes from both the test and control flasks was extracted using the YTA total RNA purification mini kit (FavorGen, Taiwan). Briefly, 350 µl of RB Buffer (25 ml) and 3.5 µl of β-Mercaptoethanol (β-ME) were added to detach the monocytes (1~5×106 cells). Moreover, DNase I digestion was performed to eliminate genomic DNA contamination. RNA was eluted by adding 50 μl of RNase-free ddH2O. The purified total RNA was quantified using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, USA). The RNA was stored at -70 °C till further use.
The extracted RNAs were used as the substrate in the qPCRBIO cDNA synthesis kit in accordance with the manufacturer’s protocol (PCR Biosystems Ltd., London, UK) in a final volume of 20 μl. mRNA expression was analyzed by quantitative PCR (ABI 7500; Life Technologies, Carlsbad, CA, USA) containing SYBR Green I in a final volume of 20 μl (qPCRBIO SyGreen Mix High-ROX, PCR Biosystems Ltd., London, UK). Forward and reverse primers were added to the 1×PCR buffer under the following conditions: denaturation at 95 °C (1 min), 35 cycles at 95 °C (5 seconds), annealing/extension at 60 °C (34 seconds). ABL-1 was used as an internal reference in each reaction. All experiments were performed in triplicate.
Primers for individual inflammatory/anti-inflammatory monocytes and M1/M2 macrophages markers were designed by Primer3 and Gene Runner software version 6.4.08 Beta (Hastings Software Inc., NY, US). High-performance primers for individual M1/M2 markers are shown in table 2. The data were analyzed as a relative expression in the treated group compared to the control group using the ΔΔCT method with the 7500 software version 2.3 (Life Technologies, Carlsbad, CA, USA) (figures 1 and 2). The 2-ΔΔCT method was used to calculate the relative expression levels of the targeted mRNA compared to the reference values.
Table 2.
Primer sets sequence for quantitative reverse transcription PCR
| Gene name | Sense and anti-sense sequence primers | Amplicon length (bp) | Temperature (°C) | |
|---|---|---|---|---|
| IL-4 | Forward | 5΄-TAGCAGGAAGAACAGAGGGG-3΄ | 81 | 58 |
| Reverse | 5΄-TTGCCTCACATTGTCACTGC-3΄ | |||
| IL-6 | Forward | 5΄-TACATCCTCGACGGCATCTC-3΄ | 80 | 58 |
| Reverse | 5΄-AGTGCCTCTTTGCTGCTTTC-3΄ | |||
| IL-10 | Forward | 5΄-TTCCATTCCAAGCCTGACCA-3΄ | 115 | 59 |
| Reverse | 5΄-ATTTGTAGCAGTTAGGAAGCCC-3΄ | |||
| IL-12b | Forward | 5΄-AAGAATTCTCGGCAGGTGG-3΄ | 86 | 57 |
| Reverse | 5΄-ACGCAGAATGTCAGGGAG-3΄ | |||
| TNF-α | Forward | 5΄-TCTGGGCAGGTCTACTTTGG-3΄ | 116 | 59 |
| Reverse | 5΄-GGTTGAGGGTGTCTGAAGG-3΄ | |||
| IFN-ɣ | Forward | 5΄-TGAATGTCCAACGCAAAGC-3΄ | 80 | 58 |
| Reverse | 5΄-TCGCTTCCCTGTTTTAGCTG-3΄ | |||
| ABL-1 | Forward | 5΄-TTCAGCGGCCAGTAGCATCTGAC-3΄ | 201 | 58 |
| Reverse | 5΄-TGTGATTATAGCCTAAGACCCGGAGC-3΄ | |||
IL-4: interleukin-4; IL-6: interleukin-6; IL-10: interleukin-10; IL-12b: interleukin-12b; TNF-α: tumor necrosis factor-alpha; IFN-ɣ: interferon-gamma; ABL: Abelson murine leukemia viral oncogene homolog 1
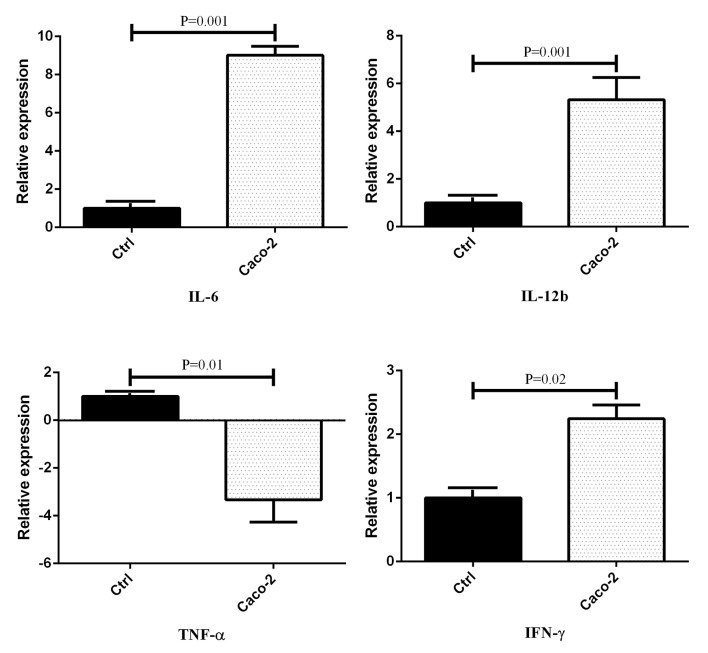
Figure1.
The expression level of inflammatory cytokines in cultured monocytes after treatment with Caco-2 conditioned media. Data presented as mean±SEM. Ctrl: Control group
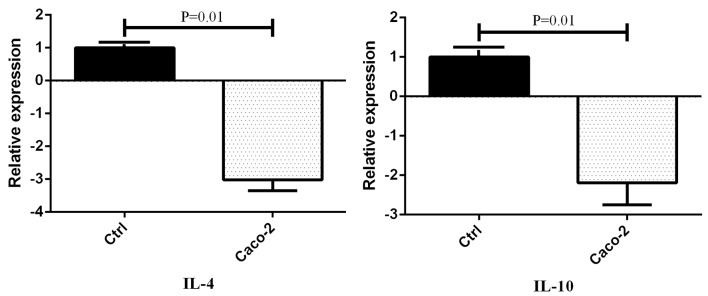
Figure2.
The expression level of anti-inflammatory cytokines in cultured monocytes after treatment with Caco-2 conditioned media. Data presented as mean±SEM. Ctrl: Control group
Statistical Analysis
The analysis was performed using the SPSS software, version 21.0, (SPSS Inc., Chicago, Illinois). The treated and control groups were compared using the independent sample t test. A two-tailed test was used for analysis and P<0.05 was considered statistically significant.
Results
The treated groups with Caco-2 conditioned media showed an increase in the expression level of IL-6, IL-12b, and IFN-ɣ genes compared to the control groups (figure 1). Moreover, the expression of TNF-α was decreased in the Caco-2 conditioned media of the treated cells. On the other hand, the expression level of IL-4 and IL-10 was decreased in the groups treated with Caco-2 conditioned media (figure 2). There was a considerable augmentation in the gene expression of IL-6 (P=0.001), IL-12b (P=0.001), and IFN-ɣ (P=0.02) in the Caco-2 treated groups by an expression change of 9.016, 5.325, and 2.243, respectively. In addition, there was a decrease in the gene expression of TNF-α (P=0.01), IL-4 (P=0.01), and IL-10 (P=0.01) by an expression change of -3.333, -3.019, and -2.19, respectively.
Discussion
CRC is still a severe disease and one of the threats to global health.18,19 The present study was instigated (17, 18)to better understand the interactions between tumors and the immune system. Monocytes treated with the conditioned media of the Caco-2 cell line showed an increase in the gene expression of inflammatory cytokines, contrary to anti-inflammatory cytokines.
The evaluation of secreted inflammatory cytokines (IL-6, IL-12b, TNF-α, IFN-ɣ) using quantitative RT-PCR from monocytes treated with the Caco-2 conditioned media showed a significant influence on monocytes by inducing inflammatory phenotype to enhance the functioning of the immune system.12 Solinas and colleagues, and Sica and others demonstrated that pro-inflammatory monocytes produced cytotoxic products, serine proteases, and reactive oxygen intermediates in TME. Therefore, monocytes-derived macrophages increased the ability of processing and presenting tumor antigens to T cells and activated T helper (Th) type-1 cells. They showed that pro-inflammatory monocytes and macrophages were associated with the anti-tumor activity of the immune system.20,21 However, we demonstrated a decrease in the gene expression of anti-inflammatory cytokines secreted from monocytes (e.g. IL-4 and IL-10). Such discrepancies in the reported gene expression level of secreted cytokines are probably due to the autocrine and paracrine impact of secreted soluble factors, exosomes, and cytokines released by tumor cells.22,23 Secreted factors from tumor cells recruited monocytes from peripheral blood toward TME. Hence, the level of secreted inflammatory cytokines is related to the increase of the inflammatory monocytes.6,24
Considering the fact that the inflammatory and anti-inflammatory genes are associated with inflammatory and anti-inflammatory monocytes, we showed that mononuclear cells, particularly the monocytes, were differentiated toward inflammatory phenotype. Hence, we hypothesized that CRC-conditioned media had an impact on the differentiation of monocytes isolated from PBMCs toward the inflammatory state. Additionally, inflammatory monocytes secreted more inflammatory cytokines. We also demonstrated an exception in the mRNA expression level of TNF-α. It decreased in the treated monocytes with the conditioned media of Caco-2 cell line. Some studies indicated that such a decline is probably due to the VEGF production by the tumor cells.25,26 Mulligan and colleagues, and Roland and others showed that VEGF exerted tumor-promoting effects by decreasing TNF-α secretion from immune cells, such as monocytes-macrophages and natural killer cells. Moreover, VEGF promoted the infiltration of MDSCs and regulatory T cells into TME and facilitated tumor growth.25,27
Various studies have demonstrated that TME is a critical hallmark of cancer consisting of different immune cells. Monocytes and macrophages represented the major components of the immune cells recruited to TME and had opposed roles in different types and stages of cancers.28 In some human cancers, they exhibited an anti-inflammatory phenotype and promoted tumor growth (e.g. prostate cancer). But, in other types of cancer (e.g., CRC, skin, stomach) they presented their pro-inflammatory phenotypes and suppressed tumor growth in the first stages of tumor formation.20,21 Although, the exact mechanisms of tumor-suppressive properties of inflammatory monocytes in CRC remain unclear, a high infiltration of inflammatory monocytes was associated with good prognosis in patients at an early stage of CRC.14,29 Algars and colleagues demonstrated that higher inflammatory monocytes and a higher M1/M2 macrophage ratio in CRC correlated with good prognosis, whereas a lower ratio resulted in more recurrent and progressive disease.30 Lundholm and colleagues indicated that secreted factors from CRC cells contained higher levels of inflammatory cytokines (e.g. IL-6, IL-12b, TNF-α, IFN-ɣ) contrary to anti-inflammatory cytokines and chemokines.12 Inflammatory cytokines and chemokines have an effect on monocytes enabling them to acquire inflammatory phenotypic features. A higher level of inflammatory monocytes in CRC microenvironment caused the immune system to acquire anti-tumoral activity.12,31 Some studies have suggested that higher levels of both anti-inflammatory monocytes and M2-macrophages caused a poor prognosis and more progression in CRC patients.32 Zhang and colleagues showed that anti-inflammatory monocytes increased the level of Th2 cytokines such as IL-4 and IL-10. These cytokines contributed to increased differentiation of anti-inflammatory monocytes and induced more immunosuppression.32,33 In contrast, Zhang and colleagues and Fridman and others indicated that infiltration of inflammatory monocytes not only promoted anti-tumoral responses in patients at the early stages of CRC, but also contributed to tumor progression and metastasis by inducing more inflammation in TME.34,35 Edin and colleagues showed that M1-macrophages could be phenotypically altered to M2-macrophages and could increase the M2/M1 ratio due to the complex immune components and cells in TME.10 There were different secreted soluble factors, some inflammatory and anti-inflammatory cytokines, chemokines, exosomes and various immune cells, such as immune-suppressive M2-macrophages and MDSCs in TME. These factors could all contribute to immune suppression, tumor promotion, and metastasis.36,37
In the present study, the impact of CRC-conditioned media on PBMCs was evaluated without using CRC cells in co-culture condition. We demonstrated that tumor cells secrete soluble and non-soluble factors, both having an impact on mononuclear cells and monocytes, and activated them against tumor cells. The main limitation of the study was the non-use of cytokines level or animal models to confirm our findings. It is worth investigating the described mechanism in animal models in order to verify our results in vivo. This would allow the development of various strategies to manipulate the immune system to activate anti-tumoral immunity.
Conclusion
The secretion of CRC cell line plays a crucial role in recruiting monocytes from the peripheral blood to TME as well as the differentiation of mononuclear cells, particularly the monocytes, toward inflammatory phenotype. Therefore, CRC cells can stimulate the immune system through secreting soluble factors, cytokines, and chemokines. In addition, CRC cells recruited monocytes and contributed to more inflammatory monocytes polarization. Inflammatory mononuclear cells and monocytes were significantly activated against tumor cells. These inflammatory cells assisted the immune system to acquire tumoricidal activity through the activation of acute inflammation, which was a critical part of a host defense. Likewise, infiltration of inflammatory monocytes in the CRC microenvironment has been associated with good prognosis, contrary to its poor prognosis in other types of cancer.
Acknowledgement
The present manuscript was extracted from the thesis by Bahareh Mohebbi. The study was supported (grant number: 810) by the Research Center for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences (Tehran, Iran) and the Department of Genetics, Tehran Medical Sciences Branch, Islamic Azad University (Tehran, Iran).
Conflict of Interest:None declared.
References
- 1.Alteri R, Bandi P, Brooks D, Cokkinides V, Doroshenk M, Gansler T, et al. Colorectal cancer facts & figures 2011-2013. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 2.Palaghia M, Prelipcean C, Cotea E, Vlad N, Leneschi L. Metastatic colorectal cancer: review of diagnosis and treatment options. Jurnalul de Chirurgie/J Surg. 2015;10:249–56. doi: 10.7438/1584-9341-10-4-2. [DOI] [Google Scholar]
- 3.Ren BJ, Zhou ZW, Zhu DJ, Ju YL, Wu JH, Ouyang MZ, et al. Alisertib Induces Cell Cycle Arrest, Apoptosis, Autophagy and Suppresses EMT in HT29 and Caco-2 Cells. Int J Mol Sci. 2015;17 doi: 10.3390/ijms17010041. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baj-Krzyworzeka M, Mytar B, Szatanek R, Surmiak M, Weglarczyk K, Baran J, et al. Colorectal cancer-derived microvesicles modulate differentiation of human monocytes to macrophages. J Transl Med. 2016;14:36. doi: 10.1186/s12967-016-0789-9. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi N, Kataoka H, Yano S, Tanaka M, Moriwaki K, Akashi H, et al. A novel photodynamic therapy targeting cancer cells and tumor-associated macrophages. Mol Cancer Ther. 2015;14:452–60. doi: 10.1158/1535-7163.MCT-14-0348. [DOI] [PubMed] [Google Scholar]
- 6.Kano A. Tumor cell secretion of soluble factor(s) for specific immunosuppression. Sci Rep. 2015;5:8913. doi: 10.1038/srep08913. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edin S, Wikberg ML, Rutegard J, Oldenborg PA, Palmqvist R. Phenotypic skewing of macrophages in vitro by secreted factors from colorectal cancer cells. PLoS One. 2013;8:e74982. doi: 10.1371/journal.pone.0074982. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao T, Evers BM. Colorectal cancer: A new innate immune sensor - functions from inside the colonic epithelium. Nat Rev Gastroenterol Hepatol. 2017;14:199–200. doi: 10.1038/nrgastro.2017.10. [DOI] [PubMed] [Google Scholar]
- 9.Ong SM, Tan YC, Beretta O, Jiang D, Yeap WH, Tai JJ, et al. Macrophages in human colorectal cancer are pro-inflammatory and prime T cells towards an anti-tumour type-1 inflammatory response. Eur J Immunol. 2012;42:89–100. doi: 10.1002/eji.201141825. [DOI] [PubMed] [Google Scholar]
- 10.Edin S, Wikberg ML, Dahlin AM, Rutegard J, Oberg A, Oldenborg PA, et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7:e47045. doi: 10.1371/journal.pone.0047045. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda T, Inagawa H, Yamamoto I. Differential expression of mRNA in human monocytes following interaction with human colon cancer cells. Anticancer Res. 2011;31:2493–7. [PubMed] [Google Scholar]
- 12.Lundholm M, Hagglof C, Wikberg ML, Stattin P, Egevad L, Bergh A, et al. Secreted Factors from Colorectal and Prostate Cancer Cells Skew the Immune Response in Opposite Directions. Sci Rep. 2015;5:15651. doi: 10.1038/srep15651. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey C, Negus R, Morris A, Ziprin P, Goldin R, Allavena P, et al. Chemokine expression is associated with the accumulation of tumour associated macrophages (TAMs) and progression in human colorectal cancer. Clin Exp Metastasis. 2007;24:121–30. doi: 10.1007/s10585-007-9060-3. [DOI] [PubMed] [Google Scholar]
- 14.Hedbrant A, Wijkander J, Seidal T, Delbro D, Erlandsson A. Macrophages of M1 phenotype have properties that influence lung cancer cell progression. Tumour Biol. 2015;36:8715–25. doi: 10.1007/s13277-015-3630-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhong Y, Yi C. MicroRNA-720 suppresses M2 macrophage polarization by targeting GATA3. Biosci Rep. 2016;36 doi: 10.1042/BSR20160105. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia X, Li X, Shen Y, Miao J, Liu H, Li G, et al. MiR-16 regulates mouse peritoneal macrophage polarization and affects T-cell activation. J Cell Mol Med. 2016;20:1898–907. doi: 10.1111/jcmm.12882. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panda SK, Ravindran B, Histopaque F. Isolation of human PBMCs. Bio Protocol. 2013;3:e323. doi: 10.21769/BioProtoc.323. [DOI] [Google Scholar]
- 18.Diogo V, Teixeira J, Silva PM, Bousbaa H. Spindle Assembly Checkpoint as a Potential Target in Colorectal Cancer: Current Status and Future Perspectives. Clin Colorectal Cancer. 2017;16:1–8. doi: 10.1016/j.clcc.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Klampfer L. Cytokines, inflammation and colon cancer. Curr Cancer Drug Targets. 2011;11:451–64. doi: 10.2174/156800911795538066. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 21.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–55. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Chen JQ, Liu JL, Tian L. Exosomes in tumor microenvironment: novel transporters and biomarkers. J Transl Med. 2016;14:297. doi: 10.1186/s12967-016-1056-9. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roma-Rodrigues C, Fernandes AR, Baptista PV. Exosome in tumour microenvironment: overview of the crosstalk between normal and cancer cells. Biomed Res Int. 2014;2014:179486. doi: 10.1155/2014/179486. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurotaki D, Sasaki H, Tamura T. Transcriptional control of monocyte and macrophage development. Int Immunol. 2017;29:97–107. doi: 10.1093/intimm/dxx016. [DOI] [PubMed] [Google Scholar]
- 25.Mulligan JK, Lathers DM, Young MR. Tumors skew endothelial cells to disrupt NK cell, T-cell and macrophage functions. Cancer Immunol Immunother. 2008;57:951–61. doi: 10.1007/s00262-007-0425-x. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camus M, Tosolini M, Mlecnik B, Pages F, Kirilovsky A, Berger A, et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 2009;69:2685–93. doi: 10.1158/0008-5472.CAN-08-2654. [DOI] [PubMed] [Google Scholar]
- 27.Roland CL, Lynn KD, Toombs JE, Dineen SP, Udugamasooriya DG, Brekken RA. Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PLoS One. 2009;4:e7669. doi: 10.1371/journal.pone.0007669. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen SR, Schmid MC. Macrophages as Key Drivers of Cancer Progression and Metastasis. Mediators Inflamm. 2017;2017:9624760. doi: 10.1155/2017/9624760. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 30.Algars A, Irjala H, Vaittinen S, Huhtinen H, Sundstrom J, Salmi M, et al. Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer. 2012;131:864–73. doi: 10.1002/ijc.26457. [DOI] [PubMed] [Google Scholar]
- 31.Burmeister K, Quagliata L, Andreozzi M, Eppenberger-Castori S, Matter MS, Perrina V, et al. Vascular endothelial growth factor A amplification in colorectal cancer is associated with reduced M1 and M2 macrophages and diminished PD-1-expressing lymphocytes. PLoS One. 2017;12:e0175563. doi: 10.1371/journal.pone.0175563. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva VL, Al-Jamal WT. Exploiting the cancer niche: Tumor-associated macrophages and hypoxia as promising synergistic targets for nano-based therapy. J Control Release. 2017;253:82–96. doi: 10.1016/j.jconrel.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Sime W, Juhas M, Sjolander A. Crosstalk between colon cancer cells and macrophages via inflammatory mediators and CD47 promotes tumour cell migration. Eur J Cancer. 2013;49:3320–34. doi: 10.1016/j.ejca.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 36.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–58. doi: 10.1053/j.gastro.2010.01.054. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khaled YS, Ammori BJ, Elkord E. Myeloid-derived suppressor cells in cancer: recent progress and prospects. Immunol Cell Biol. 2013;91:493–502. doi: 10.1038/icb.2013.29. [DOI] [PubMed] [Google Scholar]