Abstract
Glioblastoma multiform (GBM) is a heterogeneous group of primary neoplasm resistant to conventional therapies. Due to their infiltrative nature it not fully isolated by aggressive surgery, radiation and chemotherapy showing poor prognosis in glioma patients. Unfortunately, diagnosed patients die within 1.5-2 year treatment schedule. Currently temozolomide (TMZ) is the first choice for the prognosis of GBM patients. TMZ metabolites methyl triazen imidazol carboxamide form complex with alkyl guanine alkyl transferase (O6 MGMT- DNA repair protein) induced DNA damage following resistance properties of TMZ and inhibit the overall survival of the patients. Last few decades different TMZ conjugated strategy is developed to overcome the resistance and enhance the chemotherapy efficacy. The main aim of this review is to introduce the new promising pharmaceutical candidates that significantly influence the therapeutic response of the TMZ in context of targeted therapy of glioblastoma patients. It is hoped that this proposed strategy are highly effective to overcome the current resistance limitations of TMZ in GBM patients and enhance the survival rate of the patients.
Key words: Glioblastoma, temozolomide, resistance, targeted therapy
Introduction
Glioblastoma multiform (GBM) is a primary malignant brain neoplasia occurring in the intracranial tissue/glial cell these cells are responsible for the supplying of functional nutrient and O2 to the neurons. The GBM causes premature mortality under the age of 60 in adults and 15 years old in children’s with higher mortality rate and median survival time of about 12.1 to 14.6 month and 2-3% patients survive up to two years after aggressive resection of tumor (surgery), radiation therapy and chemotherapy.1 Reason behind unsuccessful prognosis of these malignancies is infiltrative nature of the glioma cells. This result in not only poor prognosis but their direct impact on neurologic function of the brain, psychological health and quality of life, cause serious consequential problems in GBM patients.2 Better understandings are required relating to epidemiology, physicochemical characteristics of the drug, and microenvironment and resistance properties of glioma cell, novel carrier systems and their delivery techniques. Therefore, there is a widely need of new targeted therapy to overcome the drug response issues faced during past few decades in the field of pharmaceutical clinical research. Moreover, several alternative effective molecules are also to be investigated for enhancing the responsiveness of TMZ and inhibit the resistance in brain glioma region to achieve better outcome.3 Some of the promising agents to enhance the therapeutic efficacy of the TMZ are mentioned in Figure 1.4-26 They include:
Figure 1.
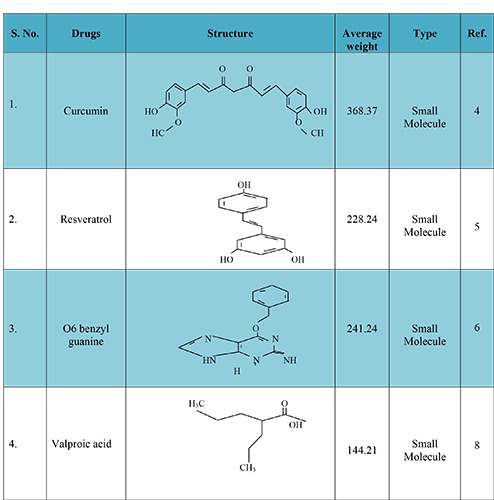
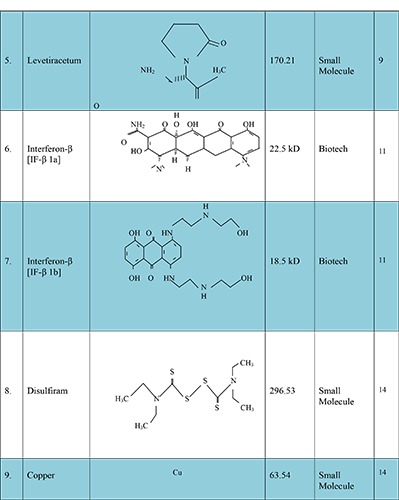
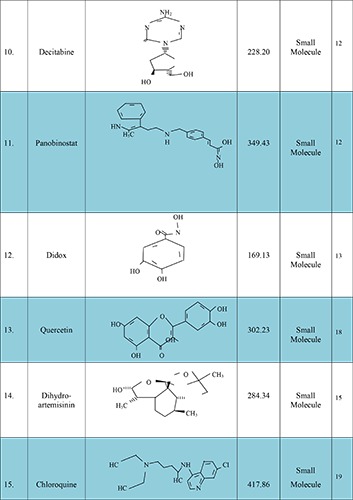
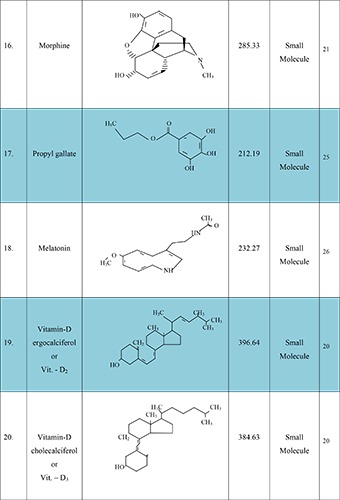
New molecules, with their structure, average weight and type, which have potential to enhance temozolomide response.
Filho et al. (2015) evaluated the efficacy of TMZ in GBM when combined with curcumin. The mechanisms involved autophagy lead to suppress the apoptosis. The in vitro and in vivo studies in U251 MG, U87 MG and C6 cell suggested that autophagy occurred with G2/M arrest and proceeds apoptosis and blocking this response using autophagy inhibitors such as threemethyl- adenine, autophagy-7 (protein coding gene) siRNA and chloroquine. In addition, curcumin susceptible to retard the signal transducer and activator of transcription-3 (STAT3), nuclear factor cappa light-chain-enhancer of activated B cell (NFκB) and intracellular signaling pathway (PI3K/Akt) to affect cell cycle/survival while TMZ promote autophagy and repair components such as ataxia telangiectasia mutate kinase (ATM), DNA mismatch repair protein (MSH6), mitogen activated protein kinase (p38) and c-jun N-terminal kinase (JNK1/2). Both TMZ and curcumin required phospho-extracellular signal regulated protein kinase (p-ERK1/2) to promote autophagy. These findings suggested that small molecule autophagy inhibitors like combined form of TMZ and curcumin is highly susceptible to cross the blood brain barrier (BBB) and could be optimize the synergistic effect of the TMZ therapy due to the redundant mechanisms.4
Yuan et al. (2012) investigated the impact of resveratrol (3, 5, 4 -Trihydroxy Trans Stilbene) on antitumor effects of TMZ in GBM via ROS dependent AMPK-TSC-m TOR signaling pathway. The additive effect of resveratrol (10μM) and temozolomide (100μM) is analyzed by FMC analyzer after 72 hours on SHG 44 cell showing increased amount of apoptosis. The treatment with TMZ at 100 μM strongly induced the cell cycle arrest in G2/M phase at 36.67%, while resveratrol at 10 μM showed aggregation of the S-phase cell population and their adjuvant form resulted 61.62% G2/M phase arrest in SHG44 cell line. The continued therapy over 72 hours shown to be cell migration expressed in MMP-9 were be analyzed by in vitro scratch assay at the beginning of 24 hours there was no any difference between temozolomide and resveratrol individual alone on cell migration but over this time it shown to be better effective inhibitory effects. The cell differentiation studies showed proved data that resveratrol and TMZ combination having property to differentiate the GBM cell from normal cell which is confirmed by the western blotting assays and in orthotropic model by immunohistochemistry. Orthotropic implanted GBM cell (SHG44) in nude mice at the concentration range 68 mg/kg of TMZ for 3 days and 40 mg/kg resveratrol daily for 28 days treatment cycle showed tumor growth inhibition consistently and no any toxicity were be observed. Their combined forms are induced AMPK activation and inhibit the m-TOR signaling in TSC dependent manner. Additionally, mTOR inhibition caused Bcl-2 down regulation and cell apoptosis in GBM cell.5
Quinn et al. (2009) studied the maximum tolerated dose and toxicity of 3 different 5 days dosing regimen of TMZ in combination with O6-benzylguanine (O6-BG). This study was conducted on phase-I clinical trial. The study of maximum tolerance dose for TMZ was conducted in 29 patients with O6-BG. Dosage regimen is described in Table 1 for better understanding. Schedule –1 on day first at a dose of 200 mg/m2, 50 mg/m2/day on days 2. Schedule-2 at a dose of 50 mg/m2/day on days 1-5, and Schedule- 3 at a dose of 50 mg/m2/day on days 1-5 along with O6-BG at a dose of 30 mg/m2/day of a 28 days treatment cycle. In consequences, in schedule-1 seventeen patients showed continued growth of malignancies, in schedule-3 three patients showed steady state and 7 patients in schedule-2 only one patient showed partial response in schedule-2. In toxicity study the 5 patients access toxicity on schedule-1. The dose limiting toxicity like neutropenia, leucopenia and thrombocytopenia were observed in grade 4 (GBM). Data indicated that particular strategy is not suitable for maintaining sensitivity of TMZ in GBM apoptosis. This study provided the base for phase II trial of O6-BG in combination with TMZ in TMZ-resistant MG in 5-day dosing schedule.6
Table 1.
TMZ and O6-BG dosing schedules according to Quinn et al.
| Dosage regime | Dosing schedule of TMZ | Dosing schedule of O6- BG |
|---|---|---|
| I | 200 mg/m2 on day 1 | 120 mg/m2 i.v. bolus |
| Dose augmented from 25 mg/m2/day on day 2 to 5 | on day 1, 2 and 3 | |
| II | Dose augmented from 75 mg/m2/day on day 1 to 5 | 30 mg/m2/day i.v. |
| III | Dose augmented from 75 mg/m2/day on day 1 to 5 with pagfilgrastim between days 7 to 14 | infusion on days 1 to 5 |
Quinn et al. (2009) studied the TMZ sensitivity in malignant glioma and evaluated the safety and dose related toxicity of O6- BG in combination with TMZ especially to the formation of blood cellular components (hematopoietic system) in phase-II clinical trial studies. Anaplastic astrocytoma bearing male patients were enrolled for this study. The patients were treated with conjugated form of O6-BG (30 mg/m2/day bolus infusion) and TMZ was administered in fasting condition at a dose of (472 mg/m2 orally) for 28 days treatment cycle. The combination of O6-BG with TMZ was able to alteration the resistance in minority of patients confirmed in phase-II clinical trial studies. Increased dose of TMZ with O6-BG of five days dosing regimen more effective than one day dosing regimen but no any such evidence are reported relating to dosing regimen to suppress the resistance level in malignant brain tumor. The authors observed slight response in GBM patients. This investigation helped in exploring further research in a direction to fix dosing regimen of TMZ and O6-BG. Additional investigation in combining TMZ and O6-BG in a five days dosing schedule was undertaken in a phase-1 clinical trial.7
Nasser et al. (2015) studied the inhibitory potency of valproic acid (VPA) in human brain glioblastoma. The in vitro retrospective analyses indicated that combination of TMZ, VPA and radiotherapy improve the survival rate in GBM patients from 13.96 to 17.35 months than TMZ alone and in vivo experiments in mouse glioma model suggested that significant reduction in resistance at MGMT was observed. In addition, it plays critical role as seizure prophylaxis agent and provided two months excess survival than chemoradiation alone. Further, studies necessary relating to the various concentration of VPA with TMZ to overcome the dose related limitations. This analysis is very important to evaluate and build the right concentration for ideal apoptosis of tumor cells.8
Marutani et al. (2017) evaluated the efficacy of levetiracetum (antiepileptic drug) when combined with TMZ in cell proliferation rate in GBM cell. The main purpose of this work was to identify the levetiracetum activity in MGMT resistance. The experiment was performed in T 98/A172 GBM cell and the inhibitory effect was observed after 72 hours. Experiment-1 conducted in both T98 and A172 tumor cell four variable concentration of levetiracetum (0, 80,160 and 320 μg/ml) were be applied and harvested at 72 h for cell proliferation assays. Experiment-2 temozolomide (0, 18.75, 37.5,75 and 150 μg/ml) were be applied and harvested at 72 h for cell proliferation assays. Experiment-3 cells were treated daily with (0, 80,160 and 320 μg/ml) for 72 h and then treated with TMZ (0, 18.75, 37.5,75 and 150 μg/ml) for another 72 h before assays were performed. The combined high dose of levetiracetum (80 μg/ml) and low dose of TMZ (18.75 μg/ml) treatment showed significantly greater inhibitory effect than the high dose of TMZ (75 μg/ml) treatment alone on cell proliferation rate in A172 GBM cell line. Thus further investigations are needed to improve the TMZ sensitivity when combined with levetiracetum in tumor suppression effect and vital prognosis of glioma patients.9
Scicchitano et al. (2018) studied the effect of levetiracetum in GBM cell proliferation and apoptosis rate when combined with TMZ. The purpose of their work was to evaluate the MGMT resistance of TMZ and enhanced the TMZ sensitivity by combining with levetiracetum. Jurkat cell was used for this study. Results demonstrate that levetiracetum enhances the effect of TMZ on GBM cancer stem cells (GCSC) by HDAC4 dependent down regulation of MGMT and by the activation of apoptotic pathways while peritumoral cancer stem cells (PCSC) showing resistance against MGMT and less effective. Although further investigation are needed to evaluate the role of levetiracetum and its molecular mechanism of action in GBM cell apoptosis.10
Park et al. (2015) investigated new approaches to enhance the TMZ efficacy in gliomas suppression. In which alternative brain tumor treatments was studied instead of TMZ alone when combined with interferon β. The in vitro study accessed in the GL26 cell line and MGMT resistance was analyzed by western blot analysis (WBA) and (RTPCR). The combination of catholic master cells (MSCs), interferon β (IF-β) and TMZ significantly reduces the disease progression in vitro and enhance the survival rate (p<0.05) of patients and follows the synergistic effects in vivo. Hence, the combination of MSCs, IF- β and TMZ could be considered as a new option for the treatment of malignant brain tumor.11
Xia C. et al. (2014) evaluated the decitabine and panobinostat potency to overcome the resistance issues in metastatic melanoma when combined with TMZ. In cycle-2 these epigenetic adjuvant therapy in metastatic melanoma significantly enhance the survival rate of the patients and useful in reducing resistance of TMZ at DNA guanine bases and enhance the TMZ response in the malignant melanoma therapy. Patients treated with decitabine and panobinostat subcutaneous injection for continuous 6-week treatment cycle shown to be significant inhibitory effects on disease progression. This triple treatment strategy is shown to be promising targeted therapy and safe confirmed in a Phase II clinical trial.12
Figul et al. (2003) investigated the new alternative brain targeted approach. The purpose of this approach is to evaluate the toxicity of the TMZ and rate-limiting enzyme of DNA synthesis such as ribonucleotide reductase inhibitors (didox and trimidox) in malignant brain gliomas. The proposed experiment was performed in human malignant glioma cell lines U87MG, T98G, LNZ308, and wt1119. In U87MG cells, IC50 values for didox and trimidox and temozolomide were 252 l μM, 203 μM and195 μM, respectively. In T98G cells, the IC50 values for didox, trimidox and temozolomide were 35 μM, 198 μM and 1446 μM, and in LNZ308 cells, 98 μM, 147 μM and 988 μM, respectively. Finally, in wt1119 p53-positive cells, the IC50 values for didox, trimidox and temozolomide were 132 μM, 173 μM and 2498 μM, and in the corresponding wt1119 p53-null cells, 122 μM, 133 μM and 2936 μM. In T98G cells combination of TMZ and trimidox was showed synergistic effects at low dose levels (ED50) or at high levels (ED90 and ED95) for TMZ/didox. In LNZ308 and wt1119 p53-ve- and p53-ve+ cells, synergistic effects were recorded at all dose levels for both combinations.13
Lun et al. (2016) investigated the new approaches (Disulfiram- Copper) to overcome the resistance occurred during TMZ therapy in brain glioma. The main purpose of this study is to enhance the TMZ therapy against brain tumor initiating cells (BTIC) and reduces resistance. In vivo efficacy studies were determined by stereotactically implanting 1×105 brain tumor initiating cells (BTIC) into the right striatum of SCID mice. Dose administered to treatment groups by oral gavage at a dose of [Disulfiram, 100 mg/kg/daily, copper (Copper) 2 mg/kg/daily]; TMZ (50 mg/kg/day). Animals were treated for 3 cycles with each cycle consisting of 5 days of treatment followed by two non-treatment days. Therapeutic benefit were be recorded both reduces temozolomide tissue toxicity and progression related mutator phenotypes.14
Zhang et al (2015) investigate the new agent such as dihydroartemisinin (antimalarial medicine) that enhance the sensitivity of TMZ in GBM therapy. The purpose of these studies is to enhance the antitumor effect of TMZ and reduces resistance occurred during long-term therapy. The proposed studies were performed in ten glioma cell lines namely MGR1, MGR3, SF295, SKMG-1, SKMG-4, T98G, U251, U251/CP2, U373, U373 and U87. They reported that particular agent is highly applicable to inhibit the glioma cell proliferation rate through the induction of autophagy. The adjuvant therapy of TMZ and dihydroartemisinin significantly enhance the TMZ efficacy both in-vitro and in-vivo studies. So further, investigations are required relating to autophagy mechanism and signaling pathways.15
Fujimaki et al. (2007) studied the efficacy of interferon-β (IF- β) in primary anaplastic astrocytoma. The purpose of these studies is to evaluate the IF-β affinity in malignant gliomas resistance. A 51-year-old patient (man) suffering from brain tumor in the bilateral frontal lobe and right thalamus was enrolled for this study. To differentiate radiation necrosis from recurrence, a fluorodeoxy glucose (FDG) PET study was performed. As the FDG-PET findings strongly suggested recurrence, TMZ chemotherapy was started. The dosing schedule is shown in Table 2. The patient treated with the 5-days protocol and cycles repeated in every 28 days at a dose of 150 mg/m2 for the first 5 days and dose increases to 200 mg/m2 in the proposed treatment cycles. IF-β was administered by intravenous infusion followed by five days of 200 mg/m2 TMZ (days 2-6). The patient’s neurological symptoms were improved after first cycle and MRI scanning images showed shrinkage of tumor size after treatment cycle-2. IF-β has been used as adjuvant therapy for glioma patients in Japan. It acts as a tumor suppressive agents, immunomodulator and antiangiogenic agent. So, further investigations are required to evaluate the efficacy of IF-β in brain gliomas.16
Table 2.
Conjugated drug TMZ and IF-β dosing schedules.
| Dosing schedule of TMZ | Dosing Schedule of IF-β |
|---|---|
| 150 mg/m2 for 5 days protocol (treatment cycles repeated in every 28 days) | 3 * 106 IU body IF-β (feron) i.v. followed by 5 days of |
| 200 mg/m2 dose augmented from day 6 through-out the 28 days treatment cycle | 200 mg/m2 TMZ on days 2 to 6 |
Chinot et al. (2014) evaluated the TMZ sensitivity by means of overcome the resistance of TMZ when combined with bevacizumab. The dose administered to the treatment group is bevacizumab (10 mg/kg i.v. for 2 weeks), radiotherapy (2 Gy 5 days a week) and oral TMZ (75 mg/m2/day) for 6 weeks. After 28 days treatment cycle changed and maintenance dose were administered as follows bevacizumab (10 mg/kg i.v. every 2 weeks) plus temozolomide (150-200 mg/m2 /day for 5 days) was continued for 6 week cycle followed by bevacizumab monotherapy (15 mg/kg i.v. every 3 week) these adjuvant therapy was not improve survival rate in patients with GBM. The bevacizumab plus TMZ shown to be median progression free survival 12.6 months was observed across multiple sub groups including patients methylated and those unmethylated MGMT status. Further investigations are needed related to survival of the patients.17
Nezami et al. (2005) proposed new concept for successful treatment of GBM. The proposed approaches were based on combined form of IV quercetin and edge gamma knife in the treatment of GBM. 70 years old white female were enrolled for experiments. Concomitant edge gamma knife radiotherapy and TMZ (75 mg/m2) was continued for 8 weeks. Administration of quercetin alone or combination with edge gamma knife plus radiation therapy. Increased cellular apoptosis and inhibition of angiogenesis was observed. Data indicated that particular approaches are safe, effective and enhances the quality of life of patients. Further, investigations are required to evaluate the efficacy of these combined therapies in GBM.18
Hsu et al. (2018) studied the new alternative targeted therapy for proliferation of GBM cell and maintaining sensitivity of TMZ. The autophagy inducer-sirolimus (Rapamicin, Rapa), autophagy inhibitor-chloroquine and DNA alkylating drug-TMZ could give synergistic effects in GBM treatment. This triple agent combination therapy is to inhibit the lysosomal function, prevention of cholesterol extraction from low density lipoprotein, causes clumping of lysosome associated membrane protein (LAMP-1), prevention of cholesterol extraction from low density lipoprotein and lipid droplets accumulation. These all are the responsible for GBM cell survival and invasion. The proposed experiment was performed in GBM8401 cell, U87 MG cell, M059K cell and HS683 cell. The animal was treated by administering the TMZ alone (50 mg/kg intraperitonial), chloroquine (50 mg/kg intraperitonial) plus rapamicin (5 mg/kg intraperitonial). Each group was treated for 6 days and TMZ was administered from day 3 to 6. This triple agent combination therapy was significantly inhibited mitochondrial function and induces lysosome induces apoptotic cell death in vitro and in vivo. Further, clinical investigations are necessary to evaluate their potency in GBM cell apoptosis, their safety evaluation and validation.19
Bak et al (2015) studied the synergistic effects of vitamin D. in brain tumor. The main purpose of these studies is to enhance the TMZ based autophagy in glioma therapy. The proposed experiment was performed in C6 rat glioma cell line. Male sprague dawley rats were used for the experiment and animals were anaesthetized by using ketamine 40-90 mg/kg (intraperitonially) and xylazine 5-10 mg/kg (subcutaneously). After incision of the skin small hole was made on the right side of the skull at the point of 2 mm lateral and 2 mm anterior to the bregma. 1* 106 C6 cell was resuspended in 10 μl saline solution. After that, cells were injected by using 25 gauge Hamilton syringe at 3 mm depth of dura at a rate of 2 μl/min. After 7 days (post operative time) the animals were divided into 3 groups. Group-1 treated with 200 μl dimethyl sulfoxide, Group-2 treated with 10 mg/kg/day TMZ intraorally and Group-3 treated with 0.2 μg/kg/day subcutaneous injection of vitamin- D. The total treatment schedule was monitored over 7 days. TMZ and vitamin-D showed decreases on colony formation (2.6±2.2 vs.5.9±0.8 colonies, respectively; P<0.01) and inhibited wound healing (wound distance, 0.56±0.05 vs 0.28± 0.03 mm; P<0.001) in vitro. The median survival rate in rat 4 weeks alone TMZ therapy while ≥5 weeks when treated with TMZ plus vitamin D. Further, investigations are necessary to investigate their potency in brain GBM treatment.20
Iorio et al. (2017) studied the efficacy of morphine in human glioma cell prognosis. The purpose of this hypothesis was to enhance the potency of TMZ in GBM treatment when combined with morphine. The U87 MG-luc2 human GBM cell bearing nude mice were assigned for the experiment. Received 10 animals in each group. Step-1 experiment the drug TMZ and morphine was administered to the animal in the ratio of (1.77 mg/kg and 10 mg/kg) daily for 5 weeks. Step-2 experiment treatment was continued for 12 weeks and animals were observed from day +42 (end of the treatment) until the +84 for long term response evaluation. Cotreatment therapy was showed less susceptible to inhibit the tumor cell growth on day 5 but maximal tumor cell apoptosis response was observed after day +42 to end of treatment schedule. These adjuvant chemotherapy approaches was showing significant GMB cell apoptosis. So, further evaluation was needed to evaluate the potency and safety of morphine for successful strategies to reduce the side effects of TMZ and enhance their therapeutic applications in malignant tumor treatments.21
Yang et al. (2014) proposed the apoptosis mechanism involved of shikonin (quinine containing natural product) in human glioma cell. This study provides the new promising glioma cell targeted strategies. The U87MG and Hs683 human glioma cell lines were assigned for these studies. In conclusion, shikonin (antioxidant) induces reactive oxygen species (ROS) production in glioma cell in the mitochondria. The cell apoptosis mechanism of the shikonin was determined through the caspase activity assays. The pancaspase inhibitor and other caspase inhibitor (caspase 3, caspase 8 and caspase 9 inhibitors) were inhibiting the apoptosis induced by shikonin through intrinsic and extrinsic apoptosis mechanism. This hypothesis provides new promising investigations of oxidative mechanisms and apoptosis related reactive oxygen species pathway in shikonin treatment in human glioma cell.22
Francisco et al. (2018) investigated the efficacy of neuronal nitric oxide synthase enzyme (nNOS) in astrocytic tumor cell. The main purpose of this study was to enhance the TMZ apoptosis response in glioma cell. The presence of tumor cells in high metabolic activity created oxidative stress. This phenomena causes complex problem for cancer treatment. The increased concentration of reactive oxygen species (ROS) results in cell damage and apoptosis while the complementary form of nitric oxide and ROS results in increased survival rate in brain tumors. In addition, nonreactive NOS inhibitor N (ω)-nitro-L-arginine –methyl-ester (LNAME) showed inhibition of brain tumor growth in rat model. A highest degree of cancer cell inhibition was observed after 72 hours of post dose administration in U138 MG cell at a dose of TMZ (250 μM) and ROS in increased parallel way (p<0.05). Instead of these cells U251 cell showed increased oxidative stress response over 72 hours of post administration. In other hand highest cell damage were observed when TMZ was given alone in U251 cell line. The nNOS 7 NI is mainly expressed in astocytic tumor region act as a tumor cell inhibitor. The experiment was performed in U87MG cell and U138MG cell. The nNOS inhibitory response in glioma cell was confirmed by the RNAi experiments. nNOS enzymes causes effects on TMZ cell damage at 48 hrs and decreases the viability of temozolomide damaged cells by 9.6 and 12.8 respectively (p<0.05). Additionally, it enhance the reactive oxygen species production (p<0.05) caused by TMZ. Thus this hypothesis was highly applicable to enhance the TMZ response in prognosis of Astrocytic tumor cell, decreased their side effects and increased the overall survival of patients. Further, clinical investigations are necessary to optimize their safe use in glioma cell prognosis.23
Teng et al. (2017) investigated the efficacy of hydroxyurea (HU) in GBM cell proliferation and maintaining TMZ sensitivity in malignant brain tumor therapy. The main purpose of this investigation is to evaluate the TMZ related resistance at MGMT DNA enzyme and their response failure. The Hs683, U87 and LNZ308 human GBM cell line was assigned for the study. The ability of HU and TMZ adjuvant therapy in cell apoptosis was increased in MGG8 cell and U87 cell line from (39.4%±2.5% TMZ) to (72.0%±5.45 HU and TMZ; p < 0.01). In U87R1 and U87R2 subline temozolomide cause minor apoptosis (~10%) while adjuvant therapy of temozolomide and HU enhanced apoptosis rate upto (~25%) (P<0.01). 5 mg/kg body weight of TMZ and 50 mg/kg of HU was intraperitoneally given for 4 days/week for 2 weeks in U87 cell bearing mice. The comparative study showed median survival duration 37.5 days (P=0.0391 vs control) in case TMZ alone while combined form of TMZ and HU showed median survival duration 56.5 days (P=0.0165 vs TMZ) in vivo study. The conjugated form of TMZ (30 μM) and HU (30 μM) in MGG8 cell line showed increased apoptosis from 39.4%±2.5% in case of TMZ alone while in combined therapy of (TMZ and HU) showed 72.0±5.4% apoptosis of cancer cells in vitro. In addition, the triplicate therapy of 30 mg/kg body weight of TMZ, 50 mg/kg body weight of HU and 6 Gy radiation for 4 days/week for 2 week showed significant reduction of tumor growth (P<0.001 vs TMZ, HU or radiation only) with enhanced survival duration upto 53.5 days in mice bearing U87R1 cell line. The HU sensitivity test was determined by administering the (5 mg/kg body weight of TMZ plus 50 mg/kg HU intraperitoneally-4 days/week for 2 weeks). Significant tumor cell proliferation was observed in U87 tumor cell model and enhance the median survival 56.5 days in mice. Thus the reported data was indicated that adjuvant therapy is promising strategy to overcome the resistance of TMZ in recurrent brain glioma. HU is FDA approved inhibitor of RNR in brain tumor. So, further studies necessary to identify the maximum tolerated dose and explore the maximum therapeutic dose (MDT) and toxicity profile of that particular compound.24
Yang et al. (2017) studied the antimigration effect of propyl gallate in malignant glioma cell. The purpose of this research work was to increase the inhibition of migration in human U87 MG glioma cell using adjuvant chemotherapy of TMZ and propyl gallate. The cell viability of 85% was reported when treated with TMZ (200 μM) alone, propyl gallate (50 and 100 μM) alone and adjuvant therapy after 48 hrs and that proposed concentration was used in further studies. The propyl gallate alone or combination was quite effective in suppressing the constitutive activation of the NF- κB pathway including p-IKK, p- IκB and p-p65 in U87 MG cell. Further clinical investigations are required relating to the concentration of propyl gallate to optimize the safety profile of that particular compounds.25
Martin et al. (2013) studied the new alternative approaches to overcome the multidrug resistance in brain tumor stem cells. In this study melatonin induced methylation based ABCG2/BCRP promoter was investigated. This experiment was accessed in A172, U87 and U373 cell line. Melatonin toxicity of chemotherapeutic drugs was confirmed by the MTT assays. The IC50 values for each drug were decreases when it was combined with indolamine. The co-administration of melatonin plus temozolomide, DOX or mitoxantrone showed synergistic toxic effect in the A172, U87 and U373 brain tumor stem cell line this was confirmed by IC values interpretation. The author studied the multi drug resistance potency of melatonin in A 172 cell line with TMZ (0-1 mM), DOX (0-10 μM) and mitoxantrone (0-20 μM) alone or combined form of melatonin (0-1 mM) for 48 hours and viability was determined by MTT assay. Resulted melatonin notably reduces the IC50 values of TMZ, DOX and mitoxantrone, which results in a synergistic toxic response. This response was decreases when expression and function of the ABCG2/BCRP transporter by melatonin. The author also suggested that this strategy showed high potential to enhance the efficacy of chemotherapeutic agent. Also, targeting efficiency increases for both brain tumor stem cells and regulation of expression and function of the ABCG2/BCRP transporter. Melatonin significantly inhibits the cell proliferation rate confirmed by the in vivo and in vitro studies. Further, studied are necessary to elucidate the potency/role of these compounds in epigenetic control of gene expression and mechanism involved by which it enhances the toxicity of chemotherapeutic drugs.26
Naziroglu et al. (2019) studied the efficacy of alpha lipoic acid (anti-oxidant) in hypoxia induced apoptosis, inflammation and mitochondrial oxidative stress in human glioma cell. The main aim of this study was to evaluate the alpha lipoic acid efficacy with hypoxia through TAPRA1 channel activation in the DBTRG cell. The author stated that hypoxia is a condition in which the entry of Ca2+ ions and oxidative stress is high in neurons. Cancer cells that resist the chemotherapeutic agent causes cell death due to the insufficient supply of oxygen. Alpha lipoic acid showed antioxidant properties with pro-antioxidant activity. When the oxidative stresses occur then ARPA1 channel was open and it acts as oxygen regulator for the entry of alpha lipoic acid to the neurons. The mean standard deviation demonstrated the statistical stastical significances (P≤0.05) by using mann-whiteney test. In conclusion, the alpha lipoic acid induces the anti-apoptotic, anti-inflammatory and anti-oxidant effects through inhibition of the TRPA-1 channel. Stimulation of TRPA1 channel showed enhanced apoptotic effect of hypoxia in the DBTRG cell. However, inhibition of TRPA-1 mediated Ca2+ entry through alpha lipoic acid treatment was may not be potential treatment for killing the glioma cancer cell.27
Shih et al. (2012) studied the potency of resveratrol in activity of TMZ in brain glioma treatment by inhibiting the autophagy response. The U87 MG, GBM 8401 and SKH-GBM cell lines were chooses for experiments. Cell death autophagy was observed in mesangial cell on human GBM. The combined dose ratio of (TMZ-10 mg/kg and resveratrol-12.5 mg/kg) was showed drastically reduced tumor size with significant value (P<0.0001). Its combined therapy reduces Ras-Raf ERK activity and autophagy related protein light chain (LC-3 II) levels and enhance the breakdown of poly (ADP-ribose) polymerase chain proved in in vitro and in vivo studies. In addition, resveratrol posse’s antioxidant activity which is dose dependent and type of cell.28
Chen et al. (2015) developed TMZ loaded lipid nanocarriers. The main purpose of this investigation is to enhance the delivery of TMZ and DNA cells in glioblastoma therapy. The U87 MG cell line and mice were be used for the in vitro and in vivo studies respectively. Its co-loaded therapy in gliomas cerebri demonstrated targeted delivery and increased survival rate of the patients.29
Sobol et al. (2011) investigated new strategies for enhancing TMZ stability in physiological condition wshere two simultaneous pathways are reported that significantly enhances the TMZ response. His demonstrated its combined approaches such as base excision repair (BER) and nicotinamide adenine dinucleotide (NAD+) significantly enhance the TMZ potency in glioblastoma therapy. Interestingly its combined therapy was prevent the DNA resistance mechanism and increased the TMZ response in the glioma treatments.30
Cohen et al. (2014) demonstrated that withaferin (steroidal lactones) enhances sensitivity of the TMZ and prevent MGMT resistance by using Akt/mTOR pathway. Depleting of heat shock protein (HSP90) was also modulated Raf-1 and glomerular filtration rate (EGFR). This activity of withaferin is a positive key point for the prohibition of MGMT resistance level associated by TMZ. In addition, it is showed antioxidant properties that lead to antiproliferative and proapoptotic effects this is also positive point for the tissue targeted delivery.31
Sheng et al. (2016) hypothesized new strategies for overcoming resistance issue of TMZ as well as biomarker for tracking phenomena of GBM survival patients. The protein connexin (Cx43) was used as targeted agents to GBM through the αCT1 signaling pathway including antisense properties. Interestingly, Cx43 showed anti-healing properties both for diabetic foot and venous leg ulcers.32
Zhang et al. (2015) developed TMZ loaded peptide chlorotoxin nanocarriers. The main aim of this investigation is to reduction of TMZ degradation, systemic toxicity and enhancement of the targeting efficiency of the TMZ in glioblastoma therapy. In consequences, its stability in cell culture media upto 2 weeks, half life (t1/2) 7 fold increased than TMZ alone, 6 fold increased uptake efficiency. In addition, IC50 was 50-90% reduced at 72 hours.33
Bandi et al. (2017) designed TMZ loaded solid lipid nanoparticles through the w/o/w micro emulsification method. The evaluation studies demonstrated that the zeta potential (-20.3 mV), particle size (119.34 nm) and diffusion studies showed 79.16% drug release at 8 hr from carrier system followed by peppas and fickian diffusion (n=0.3808).34
Khan et al. (2016) developed TMZ encapsulated nanostructured lipid carriers (NLCs) for targeted delivery to the brain through nasal route. The main purpose of this strategy was to enhance the therapeutic efficacy of the chemotherapeutic agent with minimization of degradation of drug in physiological pH. The author reported that the steady state flux of TMZ intravenous (i.v.) after 24 hours was 4.23 μg/cm2/h while TMZ-NLCs was 9.815 μg/cm2/h noted ex vivo because of surfactant present in outer layer of NLCs which acts as a penetration enhancer to the porcine mucosa. In other hand, the enhanced brain targeted delivery was observed at a dose of 35 μl (equivalent to 5 mg/ml TMZ) in case of TMZ-NLCs i.e., 457% while TMZ (i.v.) as the same dose the response was reported i.e., 169.7% in vivo. Additionally, the author claimed these developments showed zero order kinetic, residence time of drug in brain was enhanced, and low dose of drug was required.34
Wu et al. (2012) developed TMZ/PLGA/Hydroxyapetite nanosystem by the solid/aqueous/oil method for the targeted delivery of TMZ in U87 glioma cell. The main purpose of this investigation is to differentiation between TMZ/PLGA nanocarrier and TMZ/PLGA/Hydroxyapetite nanocarrier and drug release properties from system. The lowest amount of drug (50μM) were be added in formulation and its combination was showed high glioma cell proliferation rate for 35 days with sustain release property in 44.01% after 3 days. The higher apoptosis was observed after 24 hrs with (p=0.01). Additionally, the activity of αVβ3 integrin receptor (responsible for phagocytosis) was also be decreased.35
Huang et al. (2016) hypothesized new natural molecules (withaferin) for enhanced therapy of malignant gliomas having antiangiogenic properties. The delivery of withferin at a dose of (30 ng/ml) in BALB 5023 cell line showed significant glioma cell proliferation rate. Pathways involved in delivery of the withferin into endothelial cell proliferation rate follows the fas-fasl pathway. The combined therapy of soluble sFasL (0.16 ng/ml) and withaferin (0.25 ng/ml) showed significant cell proliferation rate (P<0.05).36
Ellis (2015) hypothesized new strategies for prohibiting the resistance issue of TMZ glioma therapy. His proposed two strategies first is used DP68 as (crosslinker) and DP86 as (mono alkylating agent) that suppresses the MGMT DNA damage and resistance activity of TMZ followed by prevent the recovery of cell proliferation rate and second strategies is gamma (ϒ) secretase inhibitor showed notch inhibition in gliomas cell proliferation. Human GBM cell line U87MG, U251, U118MG and T98G were be used for the experiment. The U87NS tumor cell bearing Nu/Nu male mice animals were treated with TMZ, DP68 and DP86 at the same concentration range (5mg/ml). The in vitro and in vivo studies demonstrated that agents are highly potent to overcome the resistance induced by TMZ metabolites and enhanced the overall survival of the patients.37
Zhang et al. (2015) developed dual targeting TMZ loaded miR-218 mimics nanogel for glioma targeted delivery. U87MG human tumor xenograft female BALB/C nude mice were chosen for proposed experiment. AuCOO-miR-218 mimic and TMZ at a concentration (2 mg/ml, 1 ml) was loaded to the chitosan nanogels (co-treatment group). The animals treated with TMZ dispersed in saline solution (control group) and a developed nanogel was administered via tail intravenously every 3 days for 3 weeks at a dose of 10 mg/kg of TMZ. The comparative studies on both of them showed 1/20 times small tumor size in case of co-treatment group with decreased tumor weight 1/40 as compare to control group 1/4. In vitro study in U87 cell line was suggested that delivery systems are great potential for inhibiting proliferation of the GBM cell with minimum toxicity. In this regard, in vivo animal studies are needed for estimation of the dynamic and kinetic properties and related phenomena of that hypothesized strategies.38
Orza et al. (2013) hypothesized new alternative strategies to overcome the resistance properties of TMZ. The purpose of this hypothesis is to development of TMZ loaded gold nanocarriers thereby rendering the chemoresistance in the treatment of malignant glioma. Enhanced tumor cell proliferation was observed as 82.7% compared to that of the TMZ alone.39
Athawale et al. (2013) developed TMZ loaded PLA nanocomplex and evaluated in C6 glioma cell. The nanoparticles were be formulated using solvent evaporation method. The author reported the cell toxicity studies of pure TMZ showed IC50 value was 150 μg/ml the pure form of drug response was based on concentration dependent but not time dependent while TMZ loaded nanoparticles showed IC50 was 175 μg/ml after 72 and 96 hr. The developed nanoparticles showed decreased IC50 with increase in exposure time and concentration of TMZ the delayed release property of the developed nanoparticles was noted approximately 96 hr. Also blank nanoparticle showed higher IC50 values it confirms the safety profile of the polymer and excipient used in nanoparticle formulation. On other hand clonogenic assay showed cancer cell inhibitory effect was 70±10 at a concentration of 75 μg/ml and 150 μg/ml for pure TMZ while developed nanoparticles showed 87±8 and 70±11 cell inhibition at a concentration 75 μg/ml and 150 μg/ml this observation was confirms the potency of TMZ loaded nanoparticles. In wound scratch assay studied the cell motility treated with pure drug TMZ showed 64.133±7.066 and 70.415± 3.255 for TMZ nanoparticles the motility of the glioma cells was significantly decreases with (p<0.05). The potency of decreases the motility of cells was found to be 24 hr for pure TMZ and 96 hr for developed TMZ nanoparticles. Finally the higher drug distribution to the diseased cell decreased the cell toxicity to the nearby tissues. In consequences, the particle size 200 nm was achieved. In-vitro determination was suggested that formulation showed sustained release properties and half-life was also enhanced.40
Gao et al. (2013) hypothesized new approaches interleukin-13 nanoparticles to enhance the peptide drug uptake via interleukin- 13Ra2 receptor into glioma cell. In-vitro studies on U87 cell line demonstrated that conjugated strategy significantly enhanced the peptidal drug uptake in glioma region. The mechanism involved macrophage or microvessels.41
Conclusions
GBM is aggressive deadly malignant brain tumors, which mainly originates in glial cell region in brain and causes many serious consequential problems. The neurosurgery, radiotherapy and chemotherapy are current treatment options for GBM patient. The problem with this type of tumor treatment is early recurrence after therapy because of infiltrative nature of glioma cell to other tissues. TMZ is the highly potent chemotherapeutic agent, which is mainly used for the care of malignant gliomas (GBM). Their metabolites (MTIC) direct deplete on the O6 methyl guanine bases of DNA cause DNA damage and alter their regulations. Long-term administration of TMZ causes resistance against malignant glioma cell prognosis and hampers the efficacy of these drugs. Thus adjuvant administration of above described agents is having potency to overcome the resistance of TMZ in malignant gliomas and enhance the survival rate in patients and improve the outcome. So further, investigations are needed for better understandings of GBM environment, pharmacodynamic parameter, delivery technique of chemotherapeutic agent, safety profile of the new molecules and mechanisms involved to proliferate the tumor cell and its prognosis, etc.
References
- 1.Simpson L, Galanis E. Recurrent glioblastoma multiforme : advances in treatment and promising drug candidates. Expert Rev Anticancer Ther 2006;6:1593-607. [DOI] [PubMed] [Google Scholar]
- 2.Reardon D, Wen P. Therapeutic advances in the treatment of glioblastoma: rationale and potential role of targeted agents. Oncologist 2006;11:152-64. [DOI] [PubMed] [Google Scholar]
- 3.Nakada M. The strategy for enhancing temozolomide against malignant glioma. Front Oncol 2012; 2:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filho AZ. Autophagy inhibition improves the efficacy of curcumin/ temozolomide combination therapy in glioblastomas. Cancer Lett 2015;358:220-31. [DOI] [PubMed] [Google Scholar]
- 5.Yuan Y. Resveratrol enhances the antitumor effects of temozolomide in glioblastoma via ROS-dependent AMPK-TSCmtor signaling pathway. CNS Neurosci Ther 2012;00:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinn JA. Phase I trial of temozolomide plus O6-benzylguanine 5-day regimen with recurrent malignant glioma. Neuro Oncol 2009;556-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quinn JA. Phase II trial of temozolomide plus O6 benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. J Clin Oncol 2009;27:1262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosein AN. The effect of valproic acid in combination with irradiation and temozolomide on primary human glioblastoma cells. J Neuro-Oncol 2015;1-9. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura A. Tumor-inhibition effect of levetiracetam in combination with temozolomide in glioblastoma cells. J Neurochem 2017;11:43-9. [Google Scholar]
- 10.Scicchitanno BM. Levetiracetam enhances the temozolomide effect on glioblastoma stem cell proliferation and apoptosis. Cancer Cell Int 2018;18:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JH. Combination therapy for gliomas using temozolomide and interferon-beta secreting human bone marrow derived mesenchymal stem cells. J Corean Neurol 2015;57:323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia C. Treatment of resistant metastatic melanoma using sequential epigenetic therapy (decitabine and panobinostat) combined with chemotherapy (temozolomide). Cancer Chemother Pharmacol 2014;74:691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figul M. Combined effects of temozolomide and the ribonucleotide reductase inhibitors didox and trimidox in malignant brain tumor cells. Cancer Chemother Pharmacol 2003;52:41-6. [DOI] [PubMed] [Google Scholar]
- 14.Lun X. Disulfiram when combined with copper enhances the therapeutic effects of temozolomide for the treatment of glioblastoma. Clin Cancer Res 2016;1-16. [DOI] [PubMed] [Google Scholar]
- 15.Zhang ZS. Dihydroartemisinin increases temozolomide efficacy in glioma cells by inducing autophagy. Oncol Lett 2015;10:379-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimaki T. Effectiveness of interferon-beta and temozolomide combination therapy against temozolomide-refractory recurrent anaplastic astrocytoma. World J Surg Oncol 2007;5:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinot OL. Bevacizumab plus radiotherapy temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014;8:709-22. [DOI] [PubMed] [Google Scholar]
- 18.Nezami MA. Proof of concept in a case study of glioblastoma multiforme successfully treated with IV Quercetin in combination with leading edge gamma knife and standard treatments. J Cancer Ther 2018;9:522-8. [Google Scholar]
- 19.Hsu SPC, Kuo JS. Temozolomide, sirolimus and chloroquine is a new therapeutic combination that synergizes to disrupt lysosomal function and cholesterol homeostasis in GBM cells. Oncotarget 2018;9:6883-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bak DH. Autophagy enhancement contributes to the synergistic effect of vitamin D in temozolomide-based glioblastoma chemotherapy. Exp Ther Med 2016;11:2153-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iorio AL. Tumor response of temozolomide in combination with morphine in a xenograft model of human glioblastoma. Oncotarget 2017;8:89595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang JT. An oxidative stress mechanism of shikonin in human glioma cells. PLoS One 2013;9:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Resende FFB, Almeida SSTD. Function of neuronal nitric oxide synthase enzyme in temozolomide-induced damage of astrocytic tumor cells. Oncol Lett 2018;15:4891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hejazi A, Tannous BA. Recycling drug screen repurposes hydroxyurea as a sensitizer of glioblastomas to temozolomide targeting de novo DNA synthesis, irrespective of molecular subtype. Neuro-Oncol 2017;1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang JT. Propyl gallate exerts an antimigration effect on temozolomide- treated malignant glioma cells through inhibition of ROS and the NF-κB pathway. Hindawi J Immunol Res 2017;1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin V. Melatonin-induced methylation of the ABCG2/BCRP promoter as a novel mechanism to overcome multidrug resistance in brain tumour stem cells. Br J Cancer 2013;108:2005-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naziroglu M. Alpha lipoic acid attenuates hypoxia-induced apoptosis, inflammation andmitochondrial oxidative stress via inhibition of TRPA1 channel in human glioblastoma cell line. Biomed Pharmacother 2019;111:292-304. [DOI] [PubMed] [Google Scholar]
- 28.Shih CM. Resveratrol enhances the therapeutic effect of temozolomide against malignant glioma in vitro and in vivo by inhibiting autophagy. Free Radical Biol Med 2012;52:377-91. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z. Nanostructured lipid carriers based temozolomide and gene co-encapsulated nanomedicine for gliomatosis cerebri combination therapy. Drug Deliv 2016;23:1369-73. [DOI] [PubMed] [Google Scholar]
- 30.Sobol RW, Goellner EM. Overcoming temozolomide resistance in glioblastoma via dual inhibition of NAD+ biosynthesis and base excision repair. Cancer Res 2011;71:2308-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen MS. Oxidative cytotoxic agent withaferin A resensitizes temozolomide-resistant glioblastomas via MGMT depletion and induces apoptosis through Akt/mtor pathway inhibitory modulation. Invest New Drugs 2014;32:604-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheng Z, Gourdie RG. Connexin 43 inhibition sensitizes chemoresistant glioblastoma cells to temozolomide. Cancer Res 2016;76:139-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang M, Fang C. Temozolomide nanoparticles for targeted glioblastoma therapy. ACS Appl Mater Interf 2015;1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan K, Khan A. Brain targeting of temozolomide via the intranasal route using lipid-based nanoparticles: brain pharmacokinetic and scintigraphic analyses. Mol Pharm 2016;13:3773-82. [DOI] [PubMed] [Google Scholar]
- 35.Wu A, Xue X. The effect of temozolomide/poly(lactide-coglycolide) (plga)/nano-hydroxyapatite microspheres on glioma u87 cells behavior. Int J Mol Sci 2012;13:1109-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang LA. Withaferin a promotes proliferation and migration of brain endothelial cells. Trop J Pharm Res 2016;15:1487-92. [Google Scholar]
- 37.Ellis YP. Exploring new strategies to overcome resistance in glioblastoma multiforme: Degree Dissertation., University of Medical School Massachusetts; 2015. [Google Scholar]
- 38.Zhang Y, Wu H. Dual loading mir-218 mimics and Temozolomide using Aucooh@FA-CS drug delivery system: promising targeted antitumor drug delivery system with sequential release functions. J Exp Clin Cancer Res 2015;34:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Orza A. reversing chemoresistance of malignant glioma stem cells using gold nanoparticles. Int J Nanomed 2013;8:689-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Athawale RB, Gude RP. Poly lactic acid (PLA) nanoparticles sustain the cytotoxic action of temozolomide in C6 Glioma cells. Biomed Aging Pathol 2013;3:201-8. [Google Scholar]
- 41.Gao H. Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Sci Rep 2013;3:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


