Abstract
Ethiopia is one of the sub-Saharan African countries contributing to the highest number of maternal and neonatal deaths. Coverage of maternal and neonatal health (MNH) interventions has remained very low in Ethiopia. We examined the cost-effectiveness of selected MNH interventions in an Ethiopian setting. We analysed 13 case management and preventive MNH interventions. For all interventions, we used an ingredients-based approach for cost estimation. We employed a static life table model to estimate the health impact of a 20% increase in intervention coverage relative to the baseline. We used disability-adjusted life years (DALYs) as the health outcome measure while costs were expressed in 2018 US$. Analyses were based on local epidemiological, demographic and cost data when available. Our finding shows that 12 out of the 13 interventions included in our analysis were highly cost-effective. Interventions targeting newborns such as neonatal resuscitation (institutional), kangaroo mother care and management of newborn sepsis with injectable antibiotics were the most cost-effective interventions with incremental cost-effectiveness ratios of US$7, US$8 and US$17 per DALY averted, respectively. Obstetric interventions (induction of labour, active management of third stage of labour, management of pre-eclampsia/eclampsia and maternal sepsis, syphilis treatment and tetanus toxoid during pregnancy) and safe abortion cost between US$100 and US$300 per DALY averted. Calcium supplementation for pre-eclampsia and eclampsia prevention was the least cost-effective, with a cost per DALY of about US$3100. Many of the MNH interventions analysed were highly cost-effective, and this evidence can inform the ongoing essential health services package revision in Ethiopia. Our analysis also shows that calcium supplementation does not appear to be cost-effective in our setting.
Keywords: Maternal and neonatal health, cost-effectiveness analysis, Ethiopia
Key Messages
Most neonatal and obstetric interventions are highly cost-effective in an Ethiopian setting.
Neonatal resuscitation and kangaroo mother care were the most cost-effective interventions while calcium supplementation was the least cost-effective among the 13 interventions included in our analysis.
The evidence can inform the ongoing essential health services package revision in Ethiopia.
Introduction
With 11 000 women dying during pregnancy or childbirth, Ethiopia is one among the 10 countries that contribute to 60% of the global maternal deaths estimated in 2015 (Trends in maternal mortality: 1990 to 2015, 2015). Even though Ethiopia has witnessed a significant decline in under-five mortality (Central Statistical Agency (CSA) [Ethiopia] and ICF, 2016); the proportion of under-five deaths that occur during the first 28 days of life is increasing. Neonatal deaths accounted for 30% and 43% of under-five deaths in the 2000 and 2016 Demographic and Health Surveys (DHS), respectively (Central Statistical Agency (CSA) [Ethiopia] and ICF, 2001, 2016). Ethiopia is among the five sub-Saharan African countries contributing to the highest number of neonatal deaths, with >90 000 deaths in 2016 (Levels and Trends in Child Mortality, 2017).
Maternal and neonatal deaths arise from the risks attributable to pregnancy and childbirth as well as from low coverage and poor-quality health services (Freedman et al., 2005). Effective interventions during the antenatal period, the time around birth, and the first week of life that can significantly decrease maternal and neonatal deaths are available (Trends in maternal mortality: 1990 to 2015, 2015; Levels and Trends in Child Mortality, 2017), but their coverage levels have remained very low in Ethiopia: coverage of skilled birth attendance and post-natal visits (within 2 days of birth), were in 2015 28% and 13%, respectively (Central Statistical Agency (CSA) [Ethiopia] and ICF, 2016).
Maternal and child health has been the focus of both the Millennium Development Goals and sustainable development goals (SDGs) (Sustainable Development Knowledge Platform, 2018). SDG targets 3.1 and 3.2 for maternal and child mortality represent a transformative new agenda towards ending preventable maternal and child deaths globally; all countries aiming to reduce maternal deaths to 70 per 100 000 live births and neonatal mortality to 12 per 1000 live births by 2030 (Sustainable Development Knowledge Platform, 2018). The Ministry of Health of Ethiopia intends to achieve population health levels commensurate with the average of lower middle-income countries in 2025 and upper middle-income countries in 2035 (Admasu et al., 2014). The national targets set are in line with the SDGs targets for maternal and neonatal deaths. Achievement of these targets requires scale-up of high impact and cost-effective maternal and neonatal interventions in Ethiopia. This is particularly important given the resource scarcity in Ethiopia as evidenced by the sixth National Health Accounts, which reported Ethiopia’s total health expenditure per capita at about US$30 for the years 2013/14 (Federal Democratic Republic of Ethiopia Ministry of Health, 2017).
Cost-effectiveness analysis (CEA) in the evaluation of health delivery has become an increasingly accepted component of health policy and planning (World Health Organization, 2010). Experience from several countries, however, has shown that CEA results are rarely used to inform policy decision-making largely due to lack of contextualized evidence (Tan-Torres Edejer et al., 2003). Absence of local data on estimates of cost and effectiveness of health interventions leads health planners to use evidence generated elsewhere, often from a setting different to the local one. However, studies have shown that several factors may alter the actual cost-effectiveness of a given intervention across settings (Hutubessy et al., 2003). Evidence on cost-effectiveness of interventions to improve maternal and neonatal health (MNH) outcomes in developing countries is scarce and to our knowledge non-existent in Ethiopia.
In this article, we examine the cost-effectiveness of selected MNH interventions in an Ethiopian setting. The evidence generated could inform policymakers on how to efficiently use scarce financial resources.
Methods
Interventions
We evaluate 13 MNH interventions that are provided during pregnancy, childbirth and the neonatal period (Table 1). Interventions were selected based on expert recommendations and scientific evidence of benefit to maternal and neonatal survival (Blencowe et al., 2010; Cousens et al., 2010; Lawn et al., 2010; Mwansa-Kambafwile et al., 2010; Blencowe et al., 2011; Hussain et al., 2011; Imdad et al., 2011; Jabeen et al., 2011; Lee et al., 2011; Pollard et al., 2013; Ronsmans and Campbell, 2011; Zaidi et al., 2011). The interventions included were: safe abortion; interventions during pregnancy [tetanus toxoid, syphilis detection and treatment, calcium supplementation, management of pre-eclampsia and eclampsia,1 antibiotics for preterm pre-labour rupture of membrane (pPRoM), antenatal corticosteroids for preterm labour, induction of labour]; intrapartum interventions (active management of the third stage of labour); post-partum interventions (maternal sepsis case management, newborn sepsis management, neonatal resuscitation and kangaroo mother care). These interventions were selected because they had the most data available, target the most important maternal and neonatal disease conditions in Ethiopia and were among the national priorities of the Ethiopia’s ministry of health (Federal Democratic Republic of Ethiopia Ministry of Health, 2015). Most of these interventions, except calcium supplementation, are part of the essential services rendered in public health facilities in Ethiopia though their coverage is very low (Table 1). For safe abortion services and tetanus toxoid baseline coverage data were extracted from the Health Sector Transformation Plan and the 2016 Ethiopia Health and Demographic Survey, respectively (Federal Democratic Republic of Ethiopia Ministry of Health, 2015; Central Statistical Agency [CSA] [Ethiopia] and ICF, 2016), but for the other interventions, we used baseline coverage rates from the Lives Saved Tool (LiST) (Avenir Health, 2017). Given the low coverage of interventions, we set modest target coverage with a 20 percentage points increase from the baseline of all the interventions.
Table 1.
Interventions included in the analysis, their description, target population, baseline and target coverage rates
| Intervention | Description of the intervention (number of visits) | Population in need | Baseline coverage (2017), % | Target coverage (Post 2017),a % |
|---|---|---|---|---|
| Safe abortion | Medical abortion using 800 μg vaginal misoprostolb (two visits) | Women seeking abortion (20% of births) | 37 | 57 |
| Tetanus toxoid | Two tetanus toxoid injectionsb (two visits) | All pregnant women during ANC visit | 49 | 69 |
| Syphilis detection and treatment | Screening of all pregnant women by RPR test and treatment of identified cases with benzathine penicillinb (one visit) | All pregnant women during ANC visit | 31 | 51 |
| Calcium supplementation | Routine calcium supplementation during pregnancyb (one visit) | All pregnant women during ANC visit | 0 | 20 |
| Management of pre-eclampsia and eclampsia | Package of care including anti-hypertensives and magnesium sulfatesb (three hospital bed days) | Women with pre-eclampsia (2.8% of births) and eclampsia (1%) | 3 | 23 |
| Antibiotics for pPRoM | Administration of oral antibiotics to women with pPRoMb (two hospital bed days) | Women with pPRoM (4% of births) | 3 | 23 |
| Antenatal corticosteroids for preterm labour | Administration of steroids and inpatient care of women with suspected preterm labourb (two hospital bed days) | Women with premature labour (10.1% of births) | 0 | 20 |
| Active management of the third stage of labour | Administration of prophylactic oxytocin, cord clamping and delivery of the placenta by controlled cord tractionb | All pregnant women | 23 | 43 |
| Induction of labour (beyond 41 weeks) | Induction of labour at 41 weeks gestation with 200 μg misoprostolb | Women at gestational age 41+ weeks (5% of births) | 3 | 23 |
| Maternal sepsis case management | Development of sepsis within the 42 days following delivery, requiring inpatient care including treatment with antibioticsb (four hospital bed days) | Women with infection within 42 days of giving birth (4.1% of births) | 22 | 42 |
| Neonatal resuscitation (institutional) | Detection of breathing problems and resuscitation if required | Births with asphyxia (1% of births) | 26 | 46 |
| Newborn sepsis | Administration of IV/IM antibiotics for neonatal sepsis, meningitis, or pneumoniab | Neonates with infection (10% of births) | 26 | 46 |
| Kangaroo mother care | Thermal care for newborn babies weighing <2000 g through continual skin to skin contact (1 visit) | Low birth weight babies (15% of births) | 22 | 42 |
Target coverage is an increase in 20% coverage points from the baseline.
Details of the drug regimen, other supplies and laboratory cost are provided in Web Appendix I.
IV/IM, intravenous/intramuscular; pPRoM, preterm pre-labour rupture of membrane; RPR, rapid plasma reagin.
Intervention effectiveness and health impacts
Efficacy data were based on available evidence in accordance with a recent update for LiST (Blencowe et al., 2010; Cousens et al., 2010; Lawn et al., 2010; Mwansa-Kambafwile et al., 2010; Blencowe et al., 2011; Hussain et al., 2011; Imdad et al., 2011; Jabeen et al., 2011; Lee et al., 2011; Ronsmans and Campbell, 2011; Zaidi et al., 2011; Pollard et al., 2013). We used LiST to determine cause-specific neonatal and maternal deaths associated with diseases/conditions for the baseline coverage levels. LiST combines national inputs on age-specific population size, disease incidence, prevalence and mortality to calculate the number of maternal and neonatal lives saved for a given set of interventions at a specified increase in coverage (Boschi-Pinto et al., 2010). The number of cause-specific deaths that would be prevented for a change in the coverage of a given intervention would be calculated as (Winfrey et al., 2011):
| (1) |
where, N is the number of deaths at baseline coverage, I is the intervention effectiveness, P0 is the baseline intervention coverage, P1 is the intervention target coverage, AF is the attributable fraction (fraction of the adverse condition such as death that is attributable to a specific exposure). Table 2 presents cause-specific neonatal and maternal outcomes, related baseline deaths, attributable fractions and intervention effectiveness. Once the number of deaths prevented by each intervention was estimated, the disability-adjusted life years (DALYs) averted were also computed, based on the life table for Ethiopia adjusted by health state valuations from the World Health Organization cost-effectiveness and strategic planning (WHO-CHOICE) data for the African region (World Health Organization, 2018a and 2018b). DALYs averted by each intervention were then calculated as the sum of deaths preventable at each age, multiplied by disability-adjusted life expectancy at that age, which were discounted at 3% per year (Tan-Torres Edejer et al., 2003).
Table 2.
Intervention effectiveness on neonatal and maternal mortality and attributable fractions (of deaths)
| Intervention | Neonatal outcome (number of deaths at baseline coverage) | Risk reduction on neonatal mortality (%) | Attributable fraction | Maternal outcome (number of deaths at baseline coverage) | Risk reduction on maternal mortality (%) | Attributable fraction |
|---|---|---|---|---|---|---|
| Safe abortion | Deaths from unsafe abortion (905) | 95 | 1 | |||
| Tetanus toxoid (pregnant women) | Deaths from tetanus (1684) | 94 | 1 | Deaths from indirect causes (2887) | 98 | 0.0125 |
| Syphilis detection and treatment (pregnant women) | Deaths from severe infection (18 168) | 97 | 0.04 | |||
| Deaths from congenital abnormality (10 227) | 80 | 0.027 | ||||
| Calcium supplementation | Deaths from hypertensive disorders during pregnancy (1734) | 20 | 1 | |||
| Management of pre-eclampsia and eclampsia | Deaths from hypertensive disorders during pregnancy (1734) | 59 | 1 | |||
| Antibiotics for pPRoM | Deaths from neonatal sepsis (12 292) | 39 | 0.198 | |||
| Deaths from prematurity (15 450) | 12 | 0.33 | ||||
| Antenatal corticosteroids for preterm labour | Deaths from prematurity (15 450) | 31 | 0.33 | |||
| Active management of the third stage of labour | Deaths from post-partum haemorrhage (1716) | 70 | 1 | |||
| Induction of labour (beyond 41 weeks) | Deaths from asphyxia (22 611) | 69 | 0.03 | |||
| Maternal sepsis case management | Deaths from sepsis (1171) | 80 | 1 | |||
| Neonatal resuscitation (institutional) | Deaths from asphyxia (22 611) | 40 | 1 | |||
| Newborn sepsis—injectable antibiotics | Deaths from neonatal sepsis (12 292) | 65 | 1 | |||
| Deaths from neonatal pneumonia (5876) | 75 | 1 | ||||
| Kangaroo mother care | Deaths from prematurity (15 450) | 40 | 1 |
Costs
Costs are estimated from the provider’s perspective and only direct medical costs borne by the provider at the point of service delivery were included. Costs were divided into patient- and programme-level costs. Patient-level costs included: drugs, supplies, laboratory, outpatient visits, inpatient stays and individual health education messages. Programme-level costs included resources to establish and maintain an intervention: administration, publicity, training and delivery of supplies.
To calculate costs, we used an ingredients approach that requires information on the quantities of inputs needed and their unit price (details of cost computation are presented in Supplementary Data). Costs were computed by multiplying quantities of inputs with their unit price. The quantities of resources used for the interventions were based on WHO-CHOICE assumptions (Table 1). Unit costs for hospital and health centre visits and hospital bed-day costs were extracted from WHO-CHOICE (World Health Organization, 2018c). Costs for laboratory tests were collected from several government health facilities. We used the average value of the laboratory cost depending on the level of service delivery. For drugs and supplies that are traded internationally, we used the median supplier price available internationally in 2015 (Table 3) with adjustment to include transportation costs (Joint Medical Store, 2014; Management Science for Health, 2016). We estimated programme-level costs to be 10% of patient-level costs, based on a previous study in Ethiopia (Mathewos et al., 2017). We used the same programme-level costs across interventions, which is 10% of the average patient-level cost of all interventions included in our analysis. Direct non-medical costs (e.g. transportation) and indirect costs to patients and caregivers such as lost productivity were not included in the analysis. Total cost was the sum of patient- and programme-level costs. Costs were reported in 2018 US$.
Table 3.
Unit cost inputs and socio-demographic data
| Description |
Unit prices, median (US$) |
Description |
Unit prices, median (US$) |
|
|---|---|---|---|---|
| Drugs and supplies (source: Management Science for Health, 2016) | ||||
| Misoprostol 200 µg (TAB/CAP) | 0.1810 | Amoxicillin suspension, 1 BOTT | 0.4600 | |
| Paracetamol, 500 mg (TAB/CAP) | 0.0044 | Betamethasone 4 mg/ml (INJ) | 0.2503 | |
| Tetanus toxoid vial (INJ) per dose | 0.6869 | Oxytocin 10 IU AMP (INJ) | 0.1557 | |
| Benzanthine Pn 2.4 MU vial (INJ) | 0.2612 | Ampicillin 500 mg vial (INJ) | 0.1507 | |
| Calcium Lactate 300 mg (TAB/CAP) | 0.0129 | Gentamicin 80 mg AMP (INJ) | 0.0600 | |
| Sodium lactate solution, 500 ml (IV) | 0. 5000 | Gentamicin 20 mg AMP (INJ) | 0.1760 | |
| MgSO4 5 g/10 ml vial (INJ) | 0.7340 | Metronidazole 500 mg vial (INJ) | 0.5000 | |
| Lidocaine 2 ml AMP (INJ) | 0.0972 | Ceftriaxone 250 mg vial (INJ) | 0.3538 | |
| Hydralazine, 20 mg AMP (INJ) | 3.8578 | Foley catheter, ch 14a | 0.5662 | |
| Erythromycin 250 mg (TAB/CAP) | 0.0306 | Urine bag, 2 La | 0.3700 | |
| Amoxicillin 500 mg (TAB/CAP) | 0.0300 | Infant Bag and Maskb | 20.44 | |
| Syphilis RPR (DIAG) per test | 0.1250 | |||
| Healtd service delivery costs (source: World Health Organization, 2018c) | ||||
| Hospital (cost per visit) | 1.59 | Health centre (cost per visit) | 1.96 | |
| Hospital (cost per bed day) | 4.04 | |||
| Laboratory tests and procedures | ||||
| Complete blood count | 1.98 | CSFc | 8.42 | |
| Blood culture and sensitivity | 1.5 | |||
| Socio-demographic data | ||||
| Total population in 2018 | 107.5 million | United Nations Population Division (2017) | ||
| Live births in 2018 | 3.34 million | FMoH (2017) d | ||
| GDP per capita ($US) | 707 | The World Bank (2016) | ||
| GDP per capita, PPP (Int.$) | 1735 | The World Bank (2016) | ||
| Official exchange rate (ETB per $US) | 27.5 | NBE (2018) e | ||
Source: Joint Medical Store (2014).
Includes the cost of lumbar puncture, cerebrospinal fluid analysis, culture and sensitivity.
Source: National Bank of Ethiopia (2018).
Estimation of cost-effectiveness
We assessed the cost and health effects on the population of the interventions scale-up compared with the current coverage scenario. We opted to use the current coverage levels for the following reasons: (1) most of the interventions were part of the health services package though their coverage rates were very low, ranging from 0% to 49%; (2) For the current coverage levels, baseline mortality data were available from the LiST tool; (3) The current coverage level could impact the magnitude of incremental health benefits of intervention scale-up and subsequently the incremental cost-effectiveness ratios (ICERs); and (4) The policy relevance is likely higher when ICERs are estimated from the current coverage rates. ICERs were estimated dividing the incremental cost by incremental health effects of each intervention for the 20 percentage points coverage increase. Comparing the cost of the intervention with the gain in health could then be used to identify the most cost-effective sets of interventions. ICERs are reported in US$ per DALY averted for the year 2018.
Uncertainty analysis
Given the uncertainty surrounding intervention costs and effectiveness inputs, we conducted a probabilistic uncertainty analysis with Monte Carlo simulations to assess the robustness of our findings (Baltussen et al., 2002). We used a truncated normal distribution for both costs and effects with 15–25% standard deviations and proceeded to n = 1000 simulation runs. We also conducted one-way sensitivity analyses where we varied the inputs as follows: applying 50% of the effectiveness estimates; doubling the price of drugs, supplies and laboratory tests; no discounting of health benefits; and 75% adherence to treatment.
Results
Annual intervention costs, DALYs averted, and ICERs associated with a 20% coverage increase are shown in Table 4. Kangaroo mother care, neonatal resuscitation (institutional), antibiotics for preterm pre-labour rupture of membrane, antenatal corticosteroids for preterm labour and injectable antibiotics for newborn sepsis cost <US$100 per DALY averted. Induction of labour, safe abortion, management of pre-eclampsia/eclampsia, maternal sepsis case management, syphilis detection and treatment during pregnancy, active management of third stage of labour and tetanus toxoid during pregnancy cost between US$100 and US$300 per DALY averted. The ICER for Calcium supplementation is 3100 per DALY averted, which is 4.4 times the gross domestic product (GDP) per capita in Ethiopia. For all other interventions, the ICERs are less than US$300 per DALY averted, which is <0.4 times the GDP per capita in Ethiopia. The ICERs suggest that interventions for newborn care are highly cost-effective. Neonatal resuscitation, Kangaroo mother care and newborn sepsis management are all estimated to provide 1 DALY averted for a cost of less than US$20.
Table 4.
Cost, effects and cost-effectiveness for a 20% points increase in coverage of maternal and neonatal interventions in Ethiopia in 2018
| No. | Intervention | Total cost US$ (millions) | DALYs averted (millions) |
ICER (US$ per DALYs averted) | |
|---|---|---|---|---|---|
| Discounted | Undiscounted | ||||
| 1 | Kangaroo mother care | 0.29 | 0.037 | 0.074 | 8 |
| 2 | Neonatal resuscitation (institutional) | 0.36 | 0.055 | 0.110 | 7 |
| 3 | Induction of labour (beyond 41 weeks) | 0.39 | 0.003 | 0.005 | 152 |
| 4 | Management of pre-eclampsia and eclampsia | 0.52 | 0.005 | 0.008 | 108 |
| 5 | Antibiotics for preterm pre-labour rupture of membrane (pPRoM) | 0.59 | 0.009 | 0.017 | 69 |
| 6 | Safe abortion | 0.74 | 0.007 | 0.011 | 108 |
| 7 | Antenatal corticosteroids for preterm labour | 0.84 | 0.009 | 0.017 | 98 |
| 8 | Newborn sepsis—injectable antibiotics | 0.91 | 0.052 | 0.105 | 17 |
| 9 | Maternal sepsis case management | 1.15 | 0.005 | 0.009 | 220 |
| 10 | Syphilis detection and treatment (pregnant women) | 1.52 | 0.007 | 0.014 | 224 |
| 11 | Active management of the third stage of labour | 1.62 | 0.007 | 0.011 | 245 |
| 12 | Tetanus toxoid (pregnant women) | 2.69 | 0.016 | 0.032 | 168 |
| 13 | Calcium supplementation | 4.95 | 0.002 | 0.003 | 3081 |
Implementation of all 12 individual interventions (except calcium supplementation) for a 20% point increase in coverage would avert nearly 8000 neonatal deaths (11% reduction from baseline) and >1000 maternal deaths (10% reduction from baseline) at the cost of nearly US$12 million annually. Neonatal resuscitation, newborn sepsis management and kangaroo mother care would contribute to 80% of the neonatal deaths averted (nearly 6400 deaths) at a cost of nearly US$1.6 million.
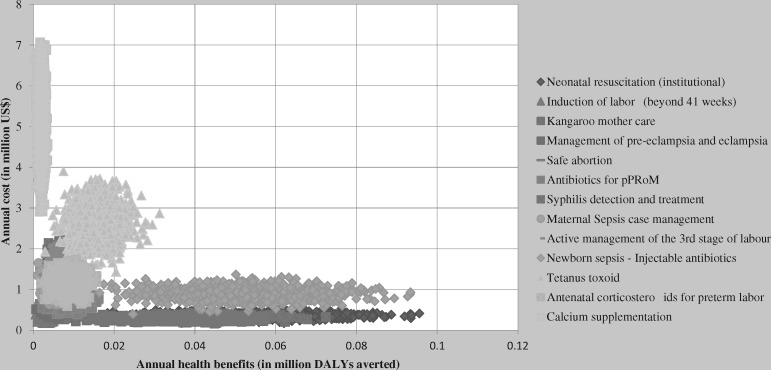
Table 5 summarizes the results of the one-way sensitivity analyses of individual interventions and shows the substantial uncertainty that resides within the cost-effectiveness estimates. Including undiscounted health benefits halves the ICER of all interventions making all interventions more cost-effective. However, decreasing the intervention effectiveness by 50% doubles the ICERs of interventions. Doubling the cost of drugs, supplies and laboratory increased the ICERs 1.6 to 2 times higher as compared with the base-case. Despite such variations, all the interventions except calcium supplementation, remain at an ICER <US$500 per DALY averted. The most important cost driver for calcium supplementation was the drug cost, which accounted to 77% of the intervention’s total cost. Figure 1 shows the result of probabilistic sensitivity analysis. It illustrates the considerable uncertainty surrounding our cost-effectiveness estimates, with wide and overlapping cost and effectiveness ranges.
Table 5.
ICERs for maternal and neonatal interventions under multiple scenarios
| No. | Intervention | Base-case | Undiscounted health benefits | 75% adherence to treatment | Double cost | 50% effectiveness |
|---|---|---|---|---|---|---|
| 1 | Induction of labour (beyond 41 weeks) | 152 | 76 | 229 | 256 | 306 |
| 2 | Kangaroo mother care | 8 | 4 | 12 | 12 | 16 |
| 3 | Neonatal resuscitation (institutional) | 7 | 3 | 10 | 11 | 13 |
| 4 | Management of pre-eclampsia and eclampsia | 108 | 65 | 162 | 189 | 217 |
| 5 | Antibiotics for preterm pre-labour rupture of membrane (pPRoM) | 69 | 35 | 104 | 124 | 139 |
| 6 | Safe abortion | 108 | 70 | 162 | 197 | 216 |
| 7 | Antenatal corticosteroids for preterm labour | 98 | 49 | 147 | 181 | 196 |
| 8 | Newborn sepsis—injectable antibiotics | 17 | 9 | 26 | 32 | 35 |
| 9 | Maternal sepsis case management | 220 | 133 | 330 | 415 | 440 |
| 10 | Syphilis detection and treatment (pregnant women) | 224 | 107 | 336 | 430 | 449 |
| 11 | Active management of the third stage of labour | 245 | 148 | 367 | 470 | 489 |
| 12 | Tetanus toxoid (pregnant women) | 168 | 84 | 252 | 328 | 336 |
| 13 | Calcium supplementation | 3081 | 1925 | 4770 | 6087 | 6167 |
Figure 1.
Probabilistic sensitivity analysis of MNH interventions in Ethiopia.
Discussion
Our analysis showed that 12 out of the 13 MNH interventions were highly cost-effective. All interventions that are included in our analysis (except calcium supplementation that is not yet implemented in public health facilities) are not entirely new to the Ethiopian health system. However, most of the interventions have a very low coverage rate. Therefore, assessing the cost-effectiveness of interventions that are currently implemented as well as adding those that could potentially be introduced can assist policymakers and planners in Ethiopia to prioritize the unfinished agenda of MNH. All interventions, besides calcium supplementation, were found to be very cost-effective (<US$300 per DALY) in Ethiopia. Adam et al. (2005) have reported a similar pattern to what we found in their CEA of strategies for MNH in developing countries (calcium supplementation and safe abortion were not included in their analysis however). A study conducted on the cost-effectiveness of calcium supplementation in Colombia concluded that varying the cost of calcium tablets or the incidence of pre-eclampsia renders the intervention no longer cost-effective for a threshold of three times Colombia’s GDP per capita (Becerra et al., 2012).
Our results demonstrate that the interventions that could be delivered at the primary health care (PHC) level were very cost-effective. In the last decade, Ethiopia has introduced a health extension programme with the training and deployment of >38 000 health extension workers (HEWs) which represents an opportunity to scale-up MNH interventions at the community level (Federal Democratic Republic of Ethiopia Ministry of Health 2015; Federal Ministry of Health of Ethiopia 2007). HEWs could indeed play important roles such as identifying pregnant women and teaching them to identify complications that arise during pregnancy and responding immediately; or providing post-delivery follow-up care for the mother infant pair. Management of neonatal infection with antibiotics and community care of newborns were found to be effective in reducing neonatal mortality and scalable at the community level (Baqui et al., 2009; Traa et al., 2010). Furthermore, a programme intervention study conducted in the Southern part of Ethiopia (using a ‘continuum of care’ approach) to evaluate the delivery of essential antenatal and obstetric services in communities through HEWs resulted in a significant decline in both maternal deaths and stillbirths highlighting the feasibility and effectiveness of maternal and neonatal interventions in Ethiopia (Lindtjørn et al., 2017, 2018). Delivery of services at the community level using HEWs has the additional benefit of bringing care to all women and infants, particularly to those socio-economically disadvantaged and marginalized rural residents. In our analysis, kangaroo mother care that was offered in neonatal facilities was very cost-effective (US$8 per DALY averted). Perhaps the introduction of KMC along with breastfeeding support for preterm/low birth weight newborns at the community level may even be a more cost-effective alternative than providing this service in neonatal wards.
Safe abortion care is one of the cost-effective services that can be delivered effectively at PHC facilities. The cost of treatment from the provider perspective to provide safe abortion using the medical method (vaginal misoprostol) for a single case was US$10 in Ghana, which is comparable to our estimate of an average cost per patient close to US$8 (Hu et al., 2010). Safe abortion at the health centre level, such as manual vacuum aspiration or medical abortion using misoprostol resulted in substantial cost savings as compared with dilatation and curettage that is often hospital based. Most public health centres in Ethiopia are not currently providing safe abortion services (Federal Democratic Republic of Ethiopia Ministry of Health, 2015). The broadening of legal indications for abortion (the 2005 revised family law of Ethiopia) and the issuance of safe abortion technical guideline in 2006 created a favourable environment to scale-up delivery of safe abortion services in Ethiopia.
Despite the fact that most of the interventions delivered at the community level and in PHC facilities are very cost-effective, prevention of most maternal and neonatal deaths requires access to quality clinical care services. Improving quality of care is often considered very costly. However, a research project by the prevention of maternal mortality network in West Africa found that renovation or upgrading of essential obstetric care services in district hospitals and health centres was not as expensive as often assumed. Most developing countries have extensive health systems that are often under-utilized. With inputs such as opening operating rooms with a supply of electricity and blood banks, for less than $15 000, improvement in the provision of quality delivery care services with significant impact on maternal mortality have been seen (Prevention of Maternal Mortality Network, 1997). Ethiopia has undertaken an accelerated expansion of PHC facilities since 2003 and currently there are >3300 functional health centres that could serve as important inputs in the scale-up of obstetric care in Ethiopia (Federal Democratic Republic of Ethiopia Ministry of Health, 2015). Additionally, delivery of quality obstetric and neonatal services requires a reliable supply of medicines, functioning equipment and respectful provider attitude (Kruk et al., 2010). Cultural factors also influence utilization of facility delivery care services. According to 2014 Ethiopian Mini DHS, 34% of rural women reported that facility deliveries were not customary highlighting the need for enhanced community mobilization (Central Statistical Agency, 2014).
The study has several limitations. First, our analysis does not include all possible maternal and neonatal interventions that could be considered in Ethiopia. Additional analysis may therefore be warranted. Second, the efficacy data we used were derived from studies conducted in more developed countries with higher quality of services and may not translate directly to Ethiopia. In the analysis of cost, we have included patient- and programme-level intervention costs that are incremental to the current coverage levels and fail to address the cost required for facility expansion. Data on perinatal mortality were not included in the analysis that may result in under estimation of the cost-effectiveness of interventions such as syphilis case detection and treatment. Due to the possibility of interactions in both cost and health impacts of implementing several single interventions at once; it is likely that the deaths averted could have been overestimated.
Despite these limitations, our findings can guide future health sector planning in Ethiopia. Along with the government’s vision to become a middle-income country, the Ministry of Health of Ethiopia has set ambitious health targets (Admasu et al., 2014). The key strategy is ensuring universal access of basic health interventions for all Ethiopians mainly through strengthening PHC. The base-case scenario targets in Ethiopia for 2025 includes; a maternal mortality ratio of 260 per 100 000 live births, neonatal mortality rate of 28 per 1000 live births and 77% coverage for four antenatal care visits and skilled birth attendance. Even though our analysis considered a 20% point increase in coverage in a year, further scale-up of all individual interventions (excluding calcium supplementation) to the target coverage rates as set in the envisioning document will likely contribute to the achievement of maternal and neonatal mortality targets. The annual budget required to implement a 20% increase in coverage of all the 12 individual interventions costs an additional 0.11 US$per capita which is 0.46% of the 2013/14 annual total health expenditure (2.52 billion US$) for Ethiopia. In comparison, according to a study on CEA of cardiovascular diseases interventions in Ethiopia, combination of drug treatment for absolute risk of cardiovascular diseases >35% (which had the lowest ICER, 67 US$per DALYs averted) was estimated to cost 0.4% of the 2010/11 annual total health expenditure (1.6 billion US$) and averts about 107 000 DALYs (Tolla et al., 2016). Similarly, a CEA of mental health interventions showed a 2.1% increase in annual total health budget (2012) would be required to implement the mental health interventions in Ethiopia with an expected health gain of 197 000 healthy life years (Strand et al., 2015).
While evidence on cost-effectiveness is an important tool in prioritizing health interventions, it should not be the only consideration in the selection of interventions for implementation in a country. The priority setting process should also consider other socially desirable goals and major health system objectives such as equity and financial risk protection (World Health Organization, 2014). For example, most interventions included in our analysis could be further evaluated in terms of the gains in financial risk protection they bring to Ethiopian families (e.g. reduction in medical impoverishment related to maternal and child health conditions) (Johansson et al., 2015; Pecenka et al. 2015; Verguet et al., 2016; Memirie et al., 2017). In this respect, many of the interventions examined in this article could be delivered at PHC facilities bringing care nearer to rural women and infants where most of the poorer Ethiopians reside (Moges, 2013).
Conclusions
Given the substantial health dividend from investing in universal coverage of the intervention included in our analysis, this evidence could inform national policymakers to prioritize scale-up of maternal and child health interventions in Ethiopia. It can also inform the ongoing essential health services package revision in Ethiopia. Our analysis also suggests that calcium supplementation may not be prioritized for inclusion in Ethiopia’s essential MNH intervention package.
Supplementary Material
Footnotes
Even though the management of pre-eclampsia/eclampsia could start during pregnancy it will usually continue during labour and delivery.
Authors’ contributions
STM and KAJ initiated and conceptualized the study. STM co-ordinated the research and did the analysis with KAJ, OFN and MTT. MH and DD helped with the acquisition of data. STM wrote the first draft of the manuscript. KAJ, MTT, MH, DD, SV, and OFN reviewed the manuscript and provided advice and suggestions. STM had final responsibility to submit for publication.
Funding
This work was supported by NORAD/the Norwegian Research Council through the project Priorities 2020 as well as the Bill & Melinda Gates Foundation through the Disease Control Priorities-Ethiopia project [OPP1162384].
Conflict of interest statement. None declared.
References
- Adam T, Lim SS, Mehta S. et al. 2005. Cost effectiveness analysis of strategies for maternal and neonatal health in developing countries. The BMJ 331: 1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admasu K, Tamire A, Tsegaye S.. 2014. Envisioning the future of the health sector: an update. Federal Democratic Republic of Ethiopia Ministry of Health Quarterly Health Bulletin 6: 3–12. [Google Scholar]
- Avenir Health. 2017. OneHealth Tool. v4, 5th edn. Glastonbury, CT: Avenir Health. [Google Scholar]
- Baltussen RM, Hutubessy RC, Evans DB, Murray CJ.. 2002. Uncertainty in cost-effectiveness analyses: probabilistic uncertainty analysis and stochastic league tables. International Journal of Technology Assessment in Health Care 18: 112–9. [PubMed] [Google Scholar]
- Baqui AH, Arifeen SE, Williams EK. et al. 2009. Effectiveness of home-based management of newborn infections by community health workers in rural Bangladesh. The Pediatric Infectious Disease Journal 28: 304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra LC, Molina MG, Oviedo S. et al. 2012. Cost-Effectiveness of Calcium Supplement in Reducing Preeclampsia Related Maternal Mortality https://papers.ssrn.com/sol3/papers.cfm? abstract_id=2194574, accessed 2 November 2014. [DOI] [PubMed]
- Blencowe H, Cousens S, Kamb M, Berman S, Lawn JE.. 2011. Lives saved tool supplement detection and treatment of syphilis in pregnancy to reduce syphilis related still births and neonatal mortality. BMC Public Health 11 Suppl 3: S9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H, Lawn J, Vandelaer J, Roper M, Cousens S.. 2010. Tetanus toxoid immunization to reduce mortality from neonatal tetanus. International Journal of Epidemiology 39: i102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschi-Pinto C, Young M, Black RE.. 2010. The child health epidemiology reference group reviews of the effectiveness of interventions to reduce maternal, neonatal and child mortality. International Journal of Epidemiology 39: i3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Central Statistical Agency. 2014. Ethiopia Mini Demographic Health Survey 2014. Addis Ababa, Ethiopia: Central Statistics Agency.
- Central Statistical Agency (CSA) [Ethiopia] and ICF. 2001. Ethiopia Demographic and Health Survey 2000. Addis Ababa, Ethiopia, and Rockville, MD: CSA and ICF. [Google Scholar]
- Central Statistical Agency (CSA) [Ethiopia] and ICF. 2016. Ethiopia Demographic and Health Survey 2016. Addis Ababa, Ethiopia, and Rockville, MD: CSA and ICF. [Google Scholar]
- Cousens S, Blencowe H, Gravett M, Lawn JE.. 2010. Antibiotics for pre-term pre-labor rupture of membranes: prevention of neonatal deaths due to complications of pre-term birth and infection. International Journal of Epidemiology 39 Suppl 1: i134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Democratic Republic of Ethiopia Ministry of Health. 2015. Health Sector Transformation Plan (HSTP) (2015/2016-2019/2020). Addis Ababa, Ethiopia: Federal Ministry of Health.
- Federal Democratic Republic of Ethiopia Ministry of Health. 2017. Ethiopia’s Sixth National Health Accounts, 2013/2014. Addis Ababa, Ethiopia: Federal Ministry of Health.
- Federal Ministry of Health of Ethiopia. 2007. Guideline for Health Extension Program. Addis Ababa, Ethiopia: Federal Ministry of Health.
- Freedman LP, Waldman RJ, de Pinho H. et al. 2005. Transforming health systems to improve the lives of women and children. Lancet (London, England) 365: 997–1000. [DOI] [PubMed] [Google Scholar]
- Hu D, Grossman D, Levin C, Blanchard K, Adanu R, Goldie SJ.. 2010. Cost-effectiveness analysis of unsafe abortion and alternative first-trimester pregnancy termination strategies in Nigeria and Ghana. African Journal of Reproductive Health 14: 85–103. [PubMed] [Google Scholar]
- Hussain AA, Yakoob MY, Imdad A, Bhutta ZA.. 2011. Elective induction for pregnancies at or beyond 41 weeks of gestation and its impact on still births: a systematic review with meta-analysis. BMC Public Health 11 Suppl 3: S5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutubessy R, Chisholm D, Edejer TT.. 2003. Generalized cost effectiveness analysis for national level priority setting in the health sector. Cost Effectiveness and Resource Allocation 1: 8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imdad A, Jabeen A, Bhutta ZA.. 2011. Role of calcium supplementation during pregnancy in reducing risk of developing gestational hypertensive disorders: a meta-analysis of studies from developing countries. BMC Public Health 11 Suppl 3: S18.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabeen M, Yakoob MY, Imdad A, Bhutta ZA.. 2011. Impact of interventions to prevent and manage preeclampsia and eclampsia on still births. BMC Public Health 11 Suppl 3: S6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson KA, Memirie ST, Pecenka C. et al. 2015. Health gains and financial protection from pneumococcal vaccination and pneumonia treatment in Ethiopia: results from an extended cost-effectiveness analysis. PLoS One 10: E0142691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint Medical Store. 2014. Catalogue and Price indicator http://www.jms.co.ug, accessed 1 February 2018.
- Kruk ME, Paczkowski MM, Tegegn A. et al. 2010. Women’s preferences for obstetric care in rural Ethiopia: a population-based discrete choice experiment in a region with low rates of facility delivery. Journal of Epidemiology and Community Health 64: 984–8. [DOI] [PubMed] [Google Scholar]
- Lawn JE, Mwansa-Kambafwile J, Barros FC, Horta BL, Cousens S.. 2010. Kangaroo mother care to prevent neonatal deaths due to preterm birth complications. International Journal of Epidemiology 39: i144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Cousens S, Wall SN. et al. 2011. Neonatal resuscitation and immediate newborn assessment and stimulation for the prevention of neonatal deaths: a systematic review, meta-analysis and Delphi estimation of mortality effect. BMC Public Health 11 Suppl 3: S12.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levels and Trends in Child Mortality. 2017. Estimates Developed by the UN Inter-agency Group for Child Mortality Estimation. Report 2017. New York, NY: UNICEF.
- Lindtjørn B, Mitiku D, Zidda Z, Yaya Y.. 2017. Reducing maternal deaths in Ethiopia: results of an intervention programme in southwest Ethiopia. PLoS One 12: e0169304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindtjørn B, Mitike D, Zidda Z, Yaya Y.. 2018. Reducing stillbirths in Ethiopia: results of an intervention programme. PLoS One 13: e0197708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Management Science for Health. 2016. International Drug Price Indicator Guide 2015 Edition. http://www.msh.org/blog/2014/07/30/2013-international-drug-price-indicator-guide-now-available, accessed 1 February 2018.
- Mathewos B, Owen H, Sitrin D. et al. 2017. Community-based interventions for newborns in Ethiopia (COMBINE): cost-effectiveness analysis. Health Policy and Planning 32: i21–32. [DOI] [PubMed] [Google Scholar]
- Memirie ST, Metaferia ZS, Norheim OF. et al. 2017. Household expenditures on pneumonia and diarrhoea treatment in Ethiopia: a facility-based study. BMJ Global Health 1: e000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moges GA. 2013. The challenges and policies of poverty reduction in Ethiopia. Ethiopian e-Journal for Research and Innovation Foresight 5: 94–117. [Google Scholar]
- Mwansa-Kambafwile J, Cousens S, Hansen T, Lawn JE.. 2010. Antenatal steroids in preterm labor for the prevention of neonatal deaths due to complications of preterm birth. International Journal of Epidemiology 39 Suppl 1: i122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Bank of Ethiopia. 2018. Commercial Banks’ Exchange Rate https://www.nbe.gov.et/market/banksexchange.html, accessed 25 May 2018.
- Pecenka CJ, Johansson KA, Memirie ST. et al. 2015. Health gains and financial risk protection: an extended cost-effectiveness analysis of treatment and prevention of diarrhea in Ethiopia. BMJ Open 5: e006402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard SL, Mathai M, Walker N.. 2013. Estimating the impact of interventions on cause specific maternal mortality: a Delphi approach. BMC Public Health 13 Suppl 3: S12. [Google Scholar]
- Prevention of Maternal Mortality Network. 1997. Abstracts from the PMM Results Conference. New York, NY: Center for Population and Family Health, School of Public Health, Faculty of Medicine, Columbia University. [Google Scholar]
- Ronsmans C, Campbell O.. 2011. Quantifying the fall in mortality associated with interventions related to hypertensive diseases of pregnancy. BMC Public Health 11 Suppl 3: S8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand KB, Chisholm D, Fekadu A, Johansson KA.. 2016. Scaling-up essential neuropsychiatric services in Ethiopia: a cost-effectiveness analysis. Health Policy and Planning 31: 504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sustainable Development Knowledge Platform. 2018. Sustainable Development Goal 3; Ensure Healthy Lives and Promote Wellbeing for All at All Ages https://sustainabledevelopment.un.org/sdg3, accessed 29 May 2018.
- Tan-Torres Edejer T, Baltussen R, Adam T. et al. 2003. WHO Guide to Cost-Effectiveness Analysis. Geneva: World Health Organization. [Google Scholar]
- The Government of Federal Democratic Republic of Ethiopia. 2017. Proposal for Support Submitted to the Global Alliance for Vaccines and Immunization (GAVI) and The Vaccine Fund. Fe Addis Ababa, Ethiopia: Federal Ministry of Health.
- The World Bank. 2016. GDP per Capita (Current US$) https://data.worldbank.org/indicator/NY.GDP.PCAP.CD, accessed 22 May 2018.
- Tolla MT, Norheim OF, Memirie ST. et al. 2016. Prevention and treatment of cardiovascular disease in Ethiopia: a cost-effectiveness analysis. Cost Effectiveness and Resource Allocation 14: 10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traa BS, Walker CF, Munos M, Black RE.. 2010. Antibiotics for the treatment of dysentery in children. International Journal of Epidemiology 39 Suppl 1: i70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trends in maternal mortality: 1990 to 2015: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva: World Health Organization, 2015.
- United Nations Population Division. 2017. Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2017 Revision, United Nations: DVD Edition. [Google Scholar]
- Verguet S, Memirie ST, Norheim OF.. 2016. Assessing the burden of medical impoverishment by cause: a systematic breakdown by disease in Ethiopia. BMC Medicine 14: 164.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey W, McKinnon R, Stover J.. 2011. Methods used in the Lives Saved Tool (LiST). BMC Public Health 11: 3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2010. The World Health Report: Health Systems Financing: The Path to Universal Coverage. Geneva: World Health Organization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2014. Making Fair Choices on the Path to Universal Health Coverage. Geneva: World Health Organization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2018a. Cost-effectiveness and Strategic Planning (WHO-CHOICE): Health State Valuation http://www.who.int/choice/demography/health_valuations/en/, accessed 22 May 2018.
- World Health Organization. 2018b. Global Health Observatory Data Repository: Life-Table by Country Ethiopia http://apps.who.int/gho/data/view.main.60550? lang=en, accessed 22 May 2018.
- World Health Organization. 2018c. Health Services Delivery Costs http://www.who.int/choice/cost-effectiveness/inputs/health_service/en/, accessed 22 May 2018.
- Zaidi AKM, Ganatra HA, Syed S. et al. 2011. Effect of case management on neonatal mortality due to sepsis and pneumonia. BMC Public Health 11 Suppl 3: S13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.