Abstract
Introduction
Cardiovascular diseases impose significant financial impact on countries implementing universal health coverage (UHC). Hypertension is a primary disease that will lead to more severe conditions without adequate clinical care. The quality of its clinical care must be well assessed in order to measure the effective coverage of people with hypertension in UHC. This study aims to identify indicators that can be used to measure the quality of clinical care provided to patients with hypertension in healthcare facilities.
Methods and analysis
This review will be conducted using the six stages of the scoping review method: identifying the research question, searching for relevant studies, selecting studies, charting the data, collating, summarising and reporting the results, and conducting consultation exercises. The review will include all quality indicators used for clinical care of patients with hypertension at any healthcare facility. All research designs will be included. Search strategies are developed using the medical subject headings and keywords related to hypertension and quality indicators. Several electronic databases, that is, MEDLINE, Cochrane, Scopus and Web of Science, including clinical-guideline databases from Agency for Healthcare Research and Quality, National Institute for Health and Care Excellence, National Health Service Evidence and Medical Information Network Distribution Service, and also grey literature will be used. Two researchers will screen the titles and abstracts and review the full text of selected articles to determine the final inclusion. The results will be summarised quantitatively, using numerical counts, and qualitatively, using thematic analysis. The data extraction will include a complete list and detailed profile of all indicators. Stakeholder consultation will be conducted at the beginning and after preliminary results to translate findings to the potential knowledge users.
Ethical considerations and dissemination
Reviews of published articles are considered secondary analysis and do not need ethical approval. The findings will be disseminated through various strategies, such as policy briefs, conferences, peer-reviewed journals, and on selected websites relevant to the subject.
Study status
Data collection for the scoping review will include publications up to May 2019, and the analysis will start in June 2019.
Keywords: quality in health care, hypertension, clinical quality indicator
Strengths and limitations of this study.
This scoping review will inform a full systematic review on the hypertension quality indicators for all healthcare settings and for all types of indicators.
The search strategy is broad and includes both peer-reviewed literature and grey literature.
Stakeholder consultation stage can be used to specifically translate the preliminary scoping study findings and develop effective dissemination strategies.
Since this is a scoping review, the quality assessment of methods and results or grading of evidence will not be performed as is normally done in a systematic review.
By limiting our search to English and Indonesian language documents, we will be excluding some potentially important results in other languages.
Introduction
Implementation of universal health coverage (UHC) in every country faces challenges in expanding coverage of enrolment and coverage of services as well as coverage of financial protection. One of the biggest financial burdens on UHC sustainability is financing for cardiovascular diseases (CVDs).1 2 By 2015, CVD was the leading cause of death. It reached approximately 17.92 million deaths,3 with about three-quarters of them occurring in low-income and middle-income countries.4 Demographic transition due to industrialisation, urbanisation, higher income, education level and technological changes in the society lead to an epidemiological transition involving increases in non-communicable diseases, including CVD.5–8
Hypertension is one of the main risks of CVD and plays a major role in the occurrence of other comorbidities such as stroke, heart disease and kidney failure that entail costly interventions. Accordingly, hypertension should be detected early and managed well through appropriate education and medication. Although a number of studies have shown that various CVD management strategies, including for hypertension, show promising results, there is a lack of agreement on methods of evaluating disease management related to economic evaluation and clinical outcomes.9 Several studies also shown that the quality of care for hypertension was suboptimal.10 11 The quality of clinical care needs to be well assessed to measure the effective coverage of people with hypertension in UHC. This measurement should include how many people with hypertension have received health insurance, used health services and obtained expected results.
Measuring effective coverage requires indicators that are relevant, valid, reliable and applicable.12 Many government associations and professional bodies in the world have developed quality indicators for different regions to improve service quality and detect suboptimal care in structure, process or outcome.13 The development of quality indicators for hypertension can be based on the consensus of experts14 and clinical guidelines.15 Both methods require literature review as an initial stage.
A scoping review is commonly used in the literature review stage for the preparation of clinical service indicators.16–19 However, this method of review has not been applied in the development of quality indicators for the clinical care of patients with hypertension. Through a scoping review, multiple sources, both research and non-research (such as guidelines from professional associations) can be consolidated to produce greater conceptual clarity.20
Objectives
The objectives of this scoping review are to assess and map the range of indicators that can be implemented to measure the quality of clinical care for patients with hypertension in healthcare facilities. This protocol provides the essential procedures for conducting the review, including search methods and article selection, as well as steps in analysing the obtained articles.
Methods
This scoping review method will be conducted using the six stages developed by Arksey and O’Malley with recent advancements by Levac et al.21 22
Step 1: identifying the research question
To meet the objective of the study, the researchers developed the following research question: ‘What are the indicators that can be implemented to measure the quality of clinical care for patients with hypertension in health care facilities?’ In this protocol, the quality indicator is an explicit and measurable criterion in providing clinical care for hypertension.
Step 2: identifying relevant studies
The review will apply approaches from the Joanna Briggs Institute (JBI)20 to identify the suitability of articles (table 1).
Table 1.
Inclusion and exclusion criteria for identifying hypertension and quality indicator-relevant studies
| Population | Concept | Context | Types of sources |
| Clinical care of patients with hypertension Exclusion criteria: hypertension in pregnancy and juvenile hypertension. |
Used or proposed quality indicators. Explain the numerator and denominator or provide a clear description for each quality indicator. No exclusion. |
Any healthcare facilities (hospital, primary care or clinic). Any type of indicators (input, process or output). Any level of indicators (patients, institutions and health systems). Any countries. No exclusion. |
All research designs: observational studies, randomised control trials, systematic reviews, case studies, qualitative studies, clinical guidelines. Exclusion criteria: publication in the form of editorials, letters to the editor, comments and case reports or narrative case reports. |
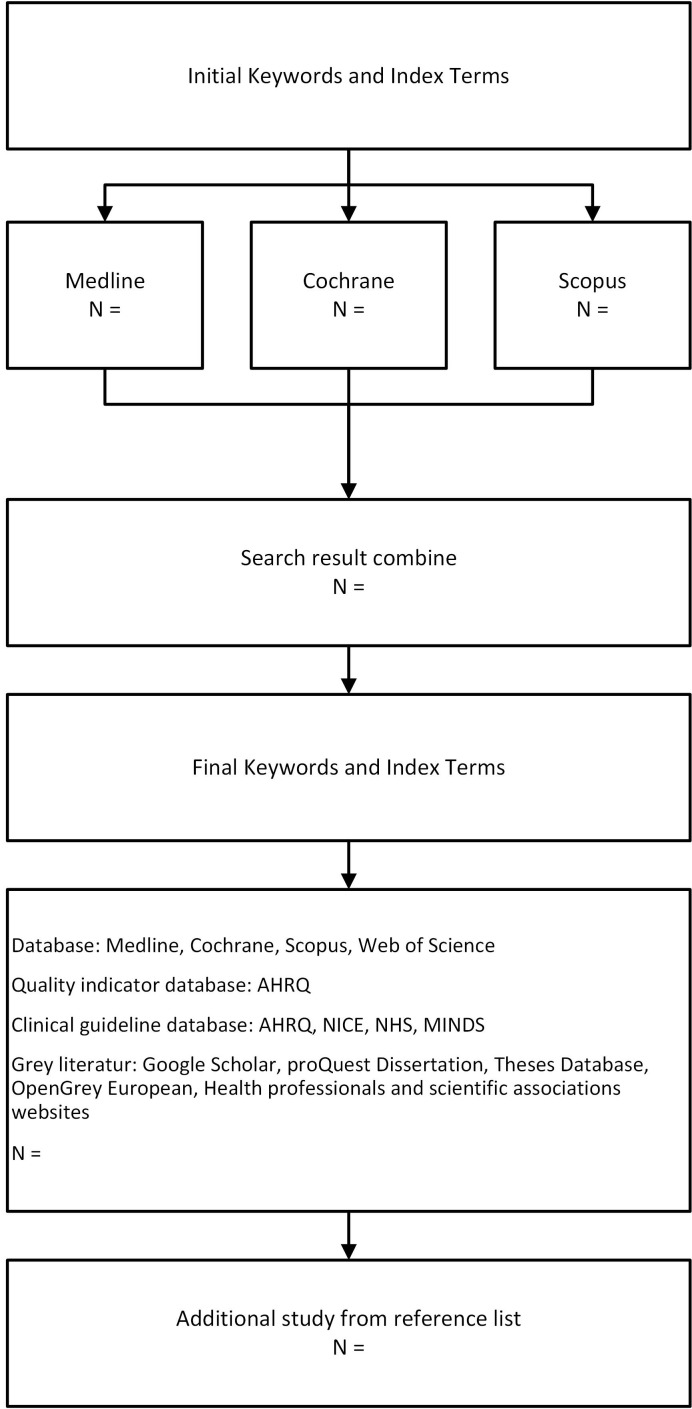
The literature search will include both published and unpublished (grey literature) primary studies as well as reviews. As JBI recommended (21), this review will be conducted through three steps. The first step is conducting an initial search on MEDLINE and Cochrane, using keywords in Medical Subject Headings (MeSH), which are, ‘Hypertension’(MeSH) and ‘Quality Indicators, Health Care’(MeSH) and synonyms of those keywords in the Scopus database (table 2), followed by analysis of the text words contained in the title, abstract, keywords and index terms to find related keywords and index terms (the proposed search strategy in MEDLINE, Cochrane and Scopus is shown in the online supplementary file).
Table 2.
Keywords and query used for hypertension and quality indicator
| Databases | Keywords and query |
| MEDLINE | Keywords: ‘Hypertension’(MeSH), ‘Quality Indicators, Health Care’(MeSH) Query: ‘Hypertension’(MeSH) AND ‘Quality Indicators, Health Care’(MeSH) |
| Cochrane | Keywords: ‘Hypertension’(MeSH), ‘Quality Indicators, Health Care’(MeSH) Query: MeSH descriptor: [Quality Indicators, Health Care] explode all trees AND MeSH descriptor: (Hypertension) explode all trees |
| Scopus | Keywords: Hypertension, High blood pressure, High blood pressures, Hypertensive, Quality indicator, Quality measure, Quality assessment, Clinical indicator, Effectiveness indicator, Outcome indicator, Performance indicator, Structure indicator, Process indicator Query: (KEY ((hypertension OR hypertensive OR ‘high blood pressure*‘) AND (‘quality indicator*’ OR ‘clinical indicator*’ OR ‘quality measure*’ OR ‘outcome indicator*’ OR ‘effectiveness indicator*’ OR ‘performance indicator*’ OR ‘structure indicator*’ OR ‘process indicator*”)) AND TITLE (hypertension OR hypertensive OR ‘high blood pressure*")) |
bmjopen-2018-026167supp001.pdf (399KB, pdf)
The second step is searching articles with all identified keywords and index terms in four databases: MEDLINE, Cochrane, Scopus, and Web of Science. This review will also search one quality indicator database—the National Quality Measures Clearinghouse Agency for Healthcare Research and Quality (AHRQ)—and four clinical guideline databases: AHRQ National Guideline Clearinghouse, National Institute for Health and Care Excellence Find Guidance, National Health Service Evidence and Medical Information Network Distribution Service. The third step is to search additional studies from the reference lists of all identified reports and articles.
We will also search for grey literature to identify unpublished material by using the ProQuest Dissertation and Theses Database, OpenGrey European, and websites of health professionals and scientific associations such as the American Heart Association and the European Society of Cardiology.
This search strategy was assessed by a library information specialist to ensure that the search strategy was accurate and sensitive in capturing the relevant literature. All searches will be performed by the research team, and the literature will then be stored using reference management software (figure 1).
Figure 1.
Flow chart for the search strategy. AHRQ, Agency for Healthcare Research and Quality; NHS, National Health Service; NICE, National Institute for Health and Care Excellence; MINDS, Medical Information Network Distribution Service.
Step 3: study selection
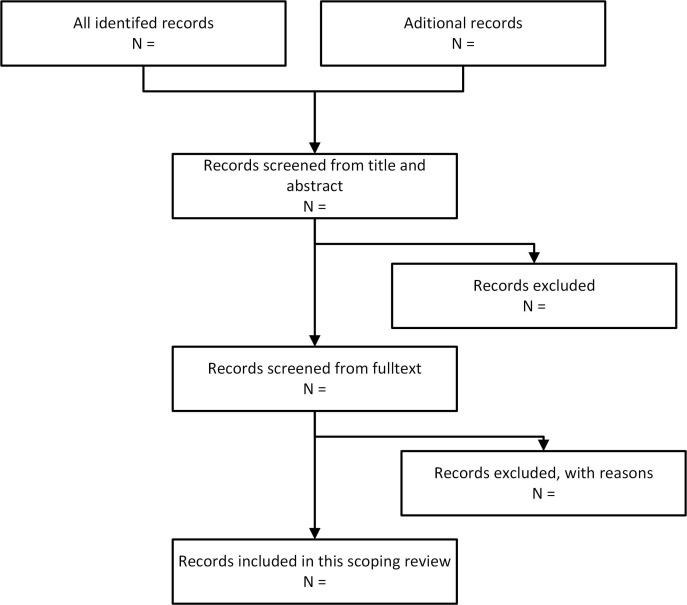
The review will be conducted by two reviewers in two stages. In the first stage, the literature will be screened by title and abstract according to the inclusion and exclusion criteria (table 3). In the second stage, the full text of the articles which passed the first stage will be reviewed (figure 2).
Table 3.
Inclusion and exclusion criteria form
| Criteria | Review result |
| Inclusion: | |
| Clinical care of patients with hypertension | [] Yes [] No |
| Used or proposed quality indicators for hypertension | [] Yes [] No |
| Exclusion: | |
| Subject only in pregnancy population | [] Yes [] No |
| Subject only in juvenile population | [] Yes [] No |
| Publication in the form of editorials, letters to the editor, comments, case reports or narrative case reports | [] Yes [] No |
Figure 2.
Flow chart for study selection.
The articles obtained will be classified into ‘included’, ‘excluded’ and ‘uncertain’ which the two reviewers will discuss for consensus. A third reviewer will be involved if there is no agreement between the two reviewers. Before the study selection, we will conduct a pilot test to measure inter-rater reliability between the two reviewers, using Cohen’s kappa coefficient from 40% of the obtained articles. Values from 0.61 to 0.8 indicate substantial agreement.23
Step 4: data extraction
The data extraction will include several variables as follows: author, year of publication, research location, research design, research objectives, published or grey literature, list of indicators and the authors’ recommendations.
Details of all indicators will be provided, including descriptions of indicators (numerator and denominator if any), indicator objectives, setting of the healthcare facility (hospital, primary care or clinic), type of indicators (input, process or output), level of indicators (patients, institutions and health systems) and validity test (table 4).
Table 4.
Extraction data form
| Author | _______________________________________ |
| Year of publication | _______________________________________ |
| Research location | _______________________________________ |
| Design | _______________________________________ |
| Objective | _______________________________________ |
| Types of sources | _______________________________________ |
| List of indicators used or purposed | |
| 1. Indicator name | _______________________________________ |
| 2. Description of indicators (numerator and denominator if any) | _______________________________________ |
| 3. Indicator objectives | _______________________________________ |
| 4. Setting of healthcare facilities | [] Hospital, [] Primary care, [] Clinic |
| 5. Type of indicators | [] Input, [] Process, [] Output |
| 6. Level of indicators | [] Patients, [] Institutions, [] Health systems |
| 7. Quality domain | _______________________________________ |
| 8. Validity test | [] Done, [] None |
| Author recommendations | _______________________________________ |
| Reviewer’s note | _______________________________________ |
Stage 5: collating, summarising and reporting the results
The results of the scoping review will be summarised quantitatively by using numerical counts and qualitatively by using thematic analysis (qualitative descriptions). This review will also identify gaps in the literature as well as areas of study in the future through implementation studies, consensus meetings or systematic reviews.
Stage 6: consultation
The researchers will contact stakeholder representatives relevant to hypertension, regulators (including ministries of health, health offices, national insurance), managers (hospitals, primary care), clinicians (physicians and nurses) and patient representatives at the beginning of the review process and after the initial results. The purpose of the first consultation is to collect feedback from stakeholders on the researchers’ approach; the second consultation is to validate the preliminary results and to obtain advice on how best to disseminate the results to different stakeholder groups. All meetings will be recorded in audio, and an inductive thematic analysis will be performed.
Supplementary Material
Footnotes
Contributors: HD led the design and conceptualisation of this work and developed the search strategy with an expert librarian from Universitas Gadjah Mada. HD and SL drafted the manuscript. AU served as an expert in designing the study protocol, providing feedback, finalising the manuscript and editing the final manuscript. All authors approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Watkins DA, Nugent RA. Setting priorities to address cardiovascular diseases through universal health coverage in low- and middle-income countries. Heart Asia 2017;9:54–8. 10.1136/heartasia-2015-010690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tolla MT, Norheim OF, Verguet S, et al. Out-of-pocket expenditures for prevention and treatment of cardiovascular disease in general and specialised cardiac hospitals in Addis Ababa, Ethiopia: a cross-sectional cohort study. BMJ Glob Health 2017;2:e000280 10.1136/bmjgh-2016-000280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roth GA, Huffman MD, Moran AE, et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015;132:1667–78. 10.1161/CIRCULATIONAHA.114.008720 [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. A global brief on hypertension. 2013.
- 5. Defo BK. Demographic, epidemiological, and health transitions: are they relevant to population health patterns in Africa? Glob Health Action 2014;7:22443 10.3402/gha.v7.22443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adogu POU, Ubajaka CF, Emelumadu OF, et al. Epidemiologic transition of diseases and health-related events in developing countries: a review. Am J Med Med Sci 2015;5:150–7. [Google Scholar]
- 7. Pradono J, Senewe F, Kristanti CM, et al. Transisi kesehatan di Indonesia: kajian data Surkesnas [Health transition in Indonesia: study of National Health Data]. Ekol Kesehat 2005;4:336–50. [Google Scholar]
- 8. Correa-Rotter R, Naicker S, Katz IJ, et al. Demographic and epidemiologic transition in the developing world: role of albuminuria in the early diagnosis and prevention of renal and cardiovascular disease. Kidney Int Suppl 2004;66:32–7. 10.1111/j.1523-1755.2004.09208.x [DOI] [PubMed] [Google Scholar]
- 9. Ara S. A literature review of cardiovascular disease management programs in managed care populations. J Manag Care Pharm 2004;10:326–44. 10.18553/jmcp.2004.10.4.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kande C, Mash R. Improving the quality of care for patients with hypertension in Moshupa District, Botswana: quality improvement cycle. Afr J Prim Health Care Fam Med 2014;6:1–7. 10.4102/phcfm.v6i1.578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Owolabi EO, Goon DT, Adeniyi OV, et al. Social epidemiology of hypertension in Buffalo City Metropolitan Municipality (BCMM): cross-sectional study of determinants of prevalence, awareness, treatment and control among South African adults. BMJ Open 2017;7:1–12. 10.1136/bmjopen-2016-014349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wollersheim H, Hermens R, Hulscher M, et al. Clinical indicators: development and applications. Neth J Med 2007;65:15–22. [PubMed] [Google Scholar]
- 13. Boulkedid R, Abdoul H, Loustau M, et al. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One 2011;6:e20476 10.1371/journal.pone.0020476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campbell SM, Braspenning J, Hutchinson A, et al. Research methods used in developing and applying quality indicators in primary care. Qual Saf Health Care 2002;11:358–64. 10.1136/qhc.11.4.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kötter T, Blozik E, Scherer M. Methods for the guideline-based development of quality indicators--a systematic review. Implement Sci 2012;7:21 10.1186/1748-5908-7-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van den Driessen Mareeuw FA, Hollegien MI, Coppus AMW, et al. In search of quality indicators for Down syndrome healthcare: a scoping review. BMC Health Serv Res 2017;17:284 10.1186/s12913-017-2228-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zidarov D, Visca R, Gogovor A, et al. Performance and quality indicators for the management of non-cancer chronic pain: a scoping review protocol. BMJ Open 2016;6:e010487 10.1136/bmjopen-2015-010487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pitzul KB, Munce SEP, Perrier L, et al. Quality indicators for hip fracture patients: a scoping review protocol. BMJ Open 2014;4:e006543 10.1136/bmjopen-2014-006543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jolley RJ, Lorenzetti DL, Manalili K, et al. Protocol for a scoping review study to identify and classify patient-centred quality indicators. BMJ Open 2017;7:e013632 10.1136/bmjopen-2016-013632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The Joanna Briggs Institute. Methodology for JBI Scoping Reviews. Adelaide: The Joanna Briggs Institute, 2015. doi. [Google Scholar]
- 21. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 22. Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5:69 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med 2012;22:276–82. 10.11613/BM.2012.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-026167supp001.pdf (399KB, pdf)