Abstract
Objective
Chronic obstructive pulmonary disease (COPD) is mainly treated pharmaceutically with bronchodilators. The purpose of this study was to evaluate the clinical benefits of two-times-per-day aclidinium bromide (Acli-BID) compared with once-a-day tiotropium bromide hydrate (Tio-QD) in patients with COPD.
Design
This study was a multicentre, open-label, randomised study.
Setting
Fourcentres in Kagawa prefecture, Japan.
Participant
Patients who were diagnosed to have COPD Grade 2–3 according to the Global Initiative for Chronic Obstructive Lung Disease 2015 criteria were enrolled.
Interventions
Patients were randomly assigned to receive Acli-BID or Tio-QD at a 1:1 ratio, and followed for 8 weeks. Acli-BID was administered in the morning and night, and Tio-QD was administered in the night.
Primary and secondary outcome measures
Primary outcome was forced expiratory volume in one second area under the curve (FEV1AUC0-3), and secondary outcomes were pulmonary function, physical activity, St George’s Respiratory Questionnaire (SGRQ), modified Medical Research Council (mMRC), the 8-item Short-Form Health Survey (SF-8) and COPD exacerbations. Adverse events were evaluated during the study.
Results
44 patients were included in this study. FEV1AUC0-3 at week 8 was 4.62±1.43 L·hour in Acli-BID and 4.73±1.60 L·hour in Tio-QD (mean difference (MD) −0.11 L·hour; 95% CI), −1.04 to 0.83). Significant improvement was observed in activity-related subscales of SGRQ (MD −7.78; 95% CI −14.61 to −0.94) and SF-8 (MD 4.01; 95% CI 0.37 to 7.65), mMRC (MD −0.66; 95% CI −1.19 to −0.13) and rate ratio (0.52, 95% CI 0.27 to 0.99) of exacerbations in the Acli-BID compared with the Tio-QD. Acli-BID and Tio-QD significantly improved sedentary behaviour (MD −35.20 min; 95% CI −67.41 to −2.94 and MD −55.40 min; 95% CI −98.15 to −12.77) within each group, but there was no significant difference between the two groups.
Conclusion
Acli-BID as with Tio-QD could be one of the therapeutic options for patients with COPD to improve pulmonary function. Also, our results suggest that intervention with bronchodilators enhanced physical activity in patients with COPD.
Trial registration number
UMIN 000020020.
Keywords: respiratory medicine (see thoracic medicine), clinical trials, thoracic medicine
Strengths and limitations of this study.
This was the first study to evaluate the clinical benefit of two-times-per-day aclidinium bromide compared with once-a-day tiotropium bromide hydrate, assessed through evaluation of pulmonary function and physical activity.
Physical activity was evaluated objectively using the triaxial accelerometer as well as subjectively using self-reported questionnaires.
Because we conducted this study in a real-life setting, the inclusion and exclusion criteria were not very stringent.
This was an exploratory study, and as such, it is a small open-label study with a short period of follow-up and without placebo.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major chronic respiratory disease. It is a progressive and irreversible pathological condition and is among the leading causes of death worldwide. To improve the quality of life (QoL) of patients with COPD, particularly in ageing societies such as Japan, Europe and the USA, inhibiting disease progression by early therapeutic intervention with bronchodilators is considered to be increasingly important.1 The recommendation of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) was changed in the 2017 report to select treatment plans for individual patients considering the severity based on pulmonary function and the evaluation of subjective symptoms, including COPD assessment test, and the frequency of acute exacerbation, reflecting the increasing importance of achieving symptomatic control and increasing QoL in patients with COPD.2 GOLD 2017 also recommends increasing the level of physical activity, because reduced physical activity is a strong predictor of fatality.3
Inhalation therapy, including long-acting β2 agonists (LABA) and long-acting muscarinic antagonists (LAMA), is one of the main treatment options for COPD. Aclidinium, a novel, long-acting, inhaled muscarinic agent, for which a Genuair inhaler is used as an inhalation device, has been approved as a COPD treatment drug for two-times-per-day administration in Japan, Europe and the USA. Aclidinium demonstrated equal efficacy to tiotropium for pulmonary function after 6 weeks of inhalation in phase IIIb study comparing aclidinium and tiotropium (LAMA).4 Post hoc analysis of this study reported that aclidinium improved COPD symptoms in the early morning and at night.5
Our speculation was that providing two peaks of forced expiratory volume in one second (FEV1) with two-times-per-day administration might be more beneficial compared with one peak of FEV1 with once-a-day administration for the improvement of morning symptoms and daytime physical activity. Although once a day is the preferred frequency of inhalation from the point of view of treatment adherence, it is also important to consider administering inhalation therapy when it is needed the most (ie, during time of day when the COPD symptoms are worse and when physically most active). However, it has not yet been clarified whether there is a difference in the therapeutic effect of LAMAs on early morning symptoms and physical activity, depending on the difference in the number of doses per day.
Objective
Given this background, we compared once-a-day inhalation therapy of tiotropium bromide hydrate and two-times-per-day inhalation therapy of aclidinium bromide in terms of pulmonary function, health-related QoL (HRQoL), and dyspnoea, and also studied their effects on physical activity measured by a validated GT3X-BT (ActiGraph) triaxial accelerometer, as recommended in the European Respiratory Society statement.6
Methods
Study design
This study was a multicentre, open-label, randomised, parallel-group, controlled study, named ‘Command Study’.
Setting
This study was conducted across four centres in Kagawa prefecture, Japan.
Participants
Eligible patients were outpatients over the age of 40, with disease severity of Grade 2–3 defined by GOLD 2015. Patients were eligible if they had had spirometry performed in the previous year and had predicted FEV1 (≧30% and <80%) and FEV1/forced vital capacity <70%. Patients were also only eligible if they had a smoking history of at least 10 pack-years and had been controlled by medicines other than LAMA for at least 4 weeks before enrolment. Patients were excluded if they had inhaled corticosteroid (ICS); if their ICS/LABA treatment was changed in the previous 4 weeks; had concurrent bronchial asthma; if they were clearly diagnosed as having pulmonary fibrosis by CT or high-resolution (HR) CT; had contracted upper or lower airway infection in the previous 4 weeks; had been hospitalised due to exacerbation of COPD in the previous 3 months; or if they were judged ineligible by the investigator or subinvestigators. Other eligibility criteria, including treatment transition criteria, are shown in the study protocol (see online supplementary study protocol).
bmjopen-2018-024114supp003.pdf (280.6KB, pdf)
Participants were recruited from four study centres in Kagawa prefecture between December 2015 and March 2016. The study was conducted in compliance with the Declaration of Helsinki (revised in October 2013), Ethical Principles for Medical Research Involving Human Subjects. Ethical principles, selection of study sites, storing of documents, monitoring and audit were conducted based on the International Conference on Harmonization-Good Clinical Practice.
Intervention
At visit 1 (week −4), informed consent was obtained. Enrolment and randomisation were conducted at visit 2 (week 0), and the investigator enrolled patients who fulfilled the criteria. The patients were randomly assigned either to an aclidinium group (Acli-BID) or a tiotropium group (Tio-QD) using block randomisation, comprising four patients per block using a 1:1 allocation scheme. The block randomisation was conducted with the support of a clinical research organisation, Mebix. A third-party blinding committee was commissioned by the study organisation. The patients were sequentially assigned from visit 2, with stratification according to the centre from which they were recruited. For 8 weeks, aclidinium bromide 400 µg was administered two times per day (in the morning between 9:00 and 11:00 hours and in the night between 21:00 and 23:00 hours), and tiotropium bromide hydrate 5 µg was administered once-a-day (in the night between 20:00 and 22:00 hours). Aclidinium was inhaled using a Genuair, and tiotropium was inhaled using a Respimat inhaler. A clinical research organisation (Mebix) conducted data collection, statistical analysis and data centre operations. The investigators enrolled patients who visited the centres for the treatment of their respiratory symptoms if they met the inclusion and exclusion criteria at visit 1. At visit 1, baseline characteristics assessment, clinical examinations and respiratory function test were conducted by investigators in each centre. Also, the investigators provided the subjects with GT3X-BT and explained how to use it. The compliance to treatment was checked by investigators in each centre at visits 3 (week 4) and 4 (week 8) based on the study protocol. The endpoints and evaluation schedule were shown in the online supplementary table 1.
bmjopen-2018-024114supp002.pdf (221.3KB, pdf)
Primary and secondary outcome measures
The area under the curve (AUC) of FEV1 in the continuous respiratory function test (FEV1AUC0-3, L·hour), the primary outcome, was evaluated at week 8. The participants were asked to visit their respective study centres between 9:00 and 11:00 hours. In the Acli-BID group, continuous respiratory function tests were performed before inhaling the study drug and 1, 2 and 3 hours after inhalation, while in the Tio-QD group, continuous respiratory function tests were performed at the time of the patients’ arrival and 1, 2 and 3 hours later. The secondary endpoints, which were the patient-reported HRQoL based on St George’s Respiratory Questionnaire (SGRQ), which was a disease-specific quality of life questionnaire, the 8-item Short-Form Health Survey (SF-8), which was general health questionnaire and modified Medical Research Council (mMRC) questionnaire, which was a scale of dyspnoea in daily living, were assessed at visit 2, 3 and 4. COPD exacerbations were confirmed by investigators according to the Guidelines for the Diagnosis and Treatment of COPD (fourth edition) issued by the Japanese respiratory society. The degree of exacerbation was evaluated at the investigator’s interviews at visits 2, 3 and 4 and calculated the rate ratio of exacerbation (person-years). The radiologists and pulmonologists made the diagnosis of emphysema using HRCT. The compliance to treatment was checked by investigators in each centre at visit 3 and 4 based on study protocol. Also, the adverse events were evaluated through the investigator’s interviews at visit 2, 3 and 4.
Physical activity assessment with accelerometer
Patients wore a validated triaxial accelerometer GT3X-BT (ActiGraph), which is a physiological sensor that provides an indicator of activities. Physical activity was categorised based on the amount of counts per minute (cpm), recorded by the GT3X-BT device during the study period. The physical activity was categorised into an intensity-related indicator depending on the measured cpm as ‘sedentary’ (<100 cpm) or ‘light’ (100–1951 cpm). To determine the activity indicator values at visit 2, 3 and 4, the mean score over the previous 7 consecutive days before each visit (days −7 to −1) was used. The average duration of each intensity-related indicator during the morning (6:00–12:00 hours), afternoon (12:00–22:00 hours) and over the day (6:00–22:00 hours) were calculated and expressed in minutes.7 The data from GT3X-BT was downloaded to a personal computer and was calculated using the ActiLife 6 Data Analysis Software. The data was independently checked by the two of the authors.
Statistical analyses
To calculate the number of patients needed to evaluate the difference between once-a-day administration and two-times-per-day administration of the inhaled muscarinic agents, we estimated FEV1AUC of Acli-BID to be 186 mL·hour and that of Tio-QD to be 93 mL·hour (SD was 80% for both) based on a paper by Beier et al.4 With α=0.05 and for a power of 80%, 52 patients were needed to have statistically significant differences on both sides. With the dropout rate set at 10%, 30 patients in each group, a total of 60 patients were needed. But, it was calculated at the first enrolment of up to 15 patients that the SD value of AUC0-3 would be smaller than 80%. SD was predicted to be 30%. Given this was the first study comparing aclidinium bromide and tiotropium bromide hydrate in Japan, we recalculated the sample size as follows; When FEV1AUC was 186±56 in Acli-BID, 93±28 mL・hour in Tio-QD with α=0.05 and a power of 80%, 40 patients were needed to have statistically significant differences with effect size, allocation ratio and dropout rate are 1, 1 and 10%, respectively. Data were expressed as mean±SD deviation, mean difference (MD) and 95% CI. Statistical significance was defined as p<0.05 in all analyses. FEV1AUC in the continuous respiratory function test was calculated using the trapezoidal method to obtain FEV1AUC0-3. Analysis of covariance was used to estimate the difference in FEV1AUC0-3 adjusting for baseline measures. Paired t-test was used for intragroup comparison of outcomes between different evaluation time points, and Student’s t-test was used for intergroup comparison. For data of outcomes that followed a non-normal distribution, the Wilcoxon signed rank test or Wilcoxon rank sum test was performed. Poisson analysis of the number of COPD exacerbations during the study period was performed to compare the frequency in terms of person-years. The linear correlation between physical activities indicators was evaluated using the Pearson product–moment correlation coefficient. The factors affecting the physical activities from baseline were investigated by a simple linear regression and stepwise multiple linear regression with covariates using backward elimination. All analyses were conducted with SAS V.9.4 and JMP V.13 (SAS Institute).
Patient and public involvement
In this randomised study, patients attending the clinics of either of the four participating centres were recruited. The burden of participating in the study was explained to the patients when informed consent was sought from all patients. The results of the study were registered in the University hospital Medical Information Network Center, which is accessible to patients. Patients were not involved in the development of the research question, study aim and design of this study.
Results
Participants
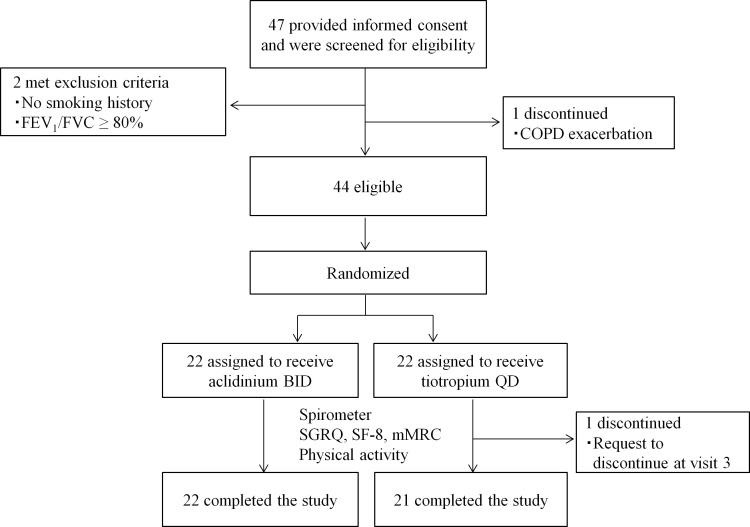
Of the 47 participants enrolled, three participants dropped out before administration of the study drug; two patients were found to be ineligible (no smoking history, spirometry results did not meet the selection criteria), and one patient was hospitalised with exacerbation of COPD. Participants were registered at four centres in Kagawa prefecture. The remaining 44 patients were randomised to Acli-BID or Tio-QD. One patient requested to discontinue during the study period because of difficulties in using the device to self-administer tiotropium. This patient discontinued treatment at visit 3 and was not included in the calculations for the primary and secondary outcomes (visit 4). Forty-three patients completed the study (figure 1). Characteristics of the patients at baseline were similar between the Acli-BID group and the Tio-QD group (table 1). The patients enrolled in this study had never experienced the use of tiotropium. The compliance to treatment was >80% in all patients. The baseline patient characteristics for each study centre are shown in the online supplementary table 2.
Figure 1.
Study flow. COPD, chronic obstructive pulmonary disease; BID, two times per day; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; mMRC, Medical Research Council; QD, once a day; SF-8, 8-item Short-Form Health Survey; SGRQ, St George’s Respiratory Questionnaire.
Table 1.
Patient characteristics at baseline
| Characteristics | Aclidinium BID (n=22) |
Tiotropium QD (n=22) |
|
| Gender (male), n (%) | 20 (90.9) | 20 (90.9) | |
| Age (years), mean (SD) | 72.3 (6.7) | 70.9 (7.4) | |
| BMI (kg/m2), mean (SD) | 22.8 (2.9) | 22.5 (3.4) | |
| Duration of smoking (years), mean (SD) | 41.9 (10.5) | 40.5 (12.9) | |
| Current smoker, n (%) | 6 (27.3) | 6 (27.3) | |
| COPD duration (years), mean (SD) | 7.2 (6.0) | 5.3 (3.3) | |
| COPD severity*, n (%) | |||
| Moderate | 15 (68.2) | 13 (59.1) | |
| Severe | 7 (31.8) | 9 (40.9) | |
| Emphysema type, n (%) | 21 (95.5) | 22 (100) | |
| Pulmonary fibrosis by HRCT, n (%) | 0 (0) | 1 (0.5) | |
| Previous exacerbation, n (%) | 7 (31.8) | 10 (45.5) | |
| Exacerbation (n/year), mean (SD) | 0.4 (0.7) | 0.6 (0.7) | |
| Previous medicine, n (%) | |||
| Long-acting β2 agonists | Indacaterol | 12 (54.5) | 14 (63.6) |
| Formoterol | — | 1 (4.5) | |
| Tulobuterol | — | 1 (4.5) | |
| Inhaled corticosteroid /long-acting β2 agonists |
Fluticasone/Salmeterol | 2 (9.1) | 3 (13.6) |
| Working, n (%) | 7 (31.8) | 6 (27.3) | |
| Working time (hours), mean (SD) | 9.9 (1.2) | 6 (3.5) | |
| Working days, mean (SD) | 4.9 (0.9) | 5.7 (1.2) | |
| SGRQ total score, mean (SD) | 28.9 (10.9) | 35.0 (12.4) | |
| SGRQ symptom, mean (SD) | 51.6 (14.8) | 54.6 (10.8) | |
| SGRQ activity, mean (SD) | 35.1 (21.4) | 44.6 (17.8) | |
| SGRQ impact, mean (SD) | 19.0 (8.9) | 23.6 (13.1) | |
| mMRC, mean (SD) | 1.0 (1.0) | 1.1 (1.1) | |
| SF-8 physical component summary, mean (SD) | 46.7 (8.2) | 45.7 (4.7) | |
| SF-8 mental component summary, mean (SD) | 50.2 (4.8) | 48.6 (8.3) | |
| SF-8 physical functioning | 45.1 (8.8) | 44.7 (7.1) | |
| SF-8 role physical, mean (SD) | 46.8 (8.1) | 45.0 (8.5) | |
| FEV1 (L), mean (SD) | 1.6 (0.4) | 1.5 (0.5) | |
| %FEV1 predicted (%), mean (SD) | 60.1 (14.6) | 57.6 (15.5) | |
| FEV1/FVC (%), mean (SD) | 56.3 (8.6) | 56.8 (8.9) | |
| IC (L), mean (SD) | 2.2 (0.6) | 2.0 (0.7) | |
*COPD severity defined by Global Initiative for Chronic Obstructive Lung Disease.
BID, two times per day; BMI, body mass index; COPD, Chronic Obstructive Pulmonary Disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.; HRCT, high-resolution CT; IC, inhaled corticosteroid; mMRC, modified medical research council; QD, once a day; SF-8, 8-item Short-Form Health Survey; SGRQ, St George’s Respiratory Questionnaire.
Primary and secondary outcomes
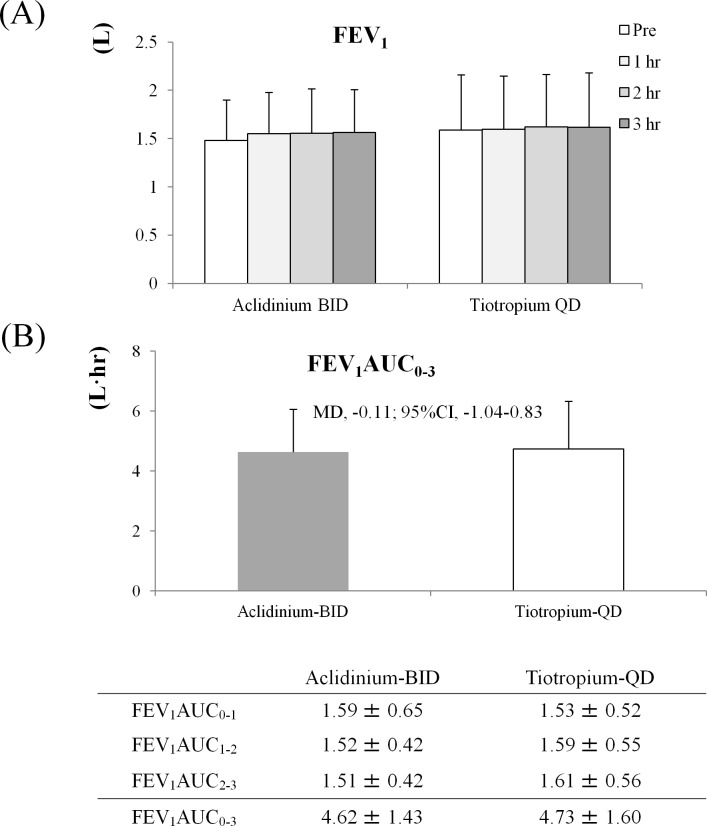
FEV1AUC0-3 (L·hour) of primary outcome was 4.62±1.43 L·hour in the Acli-BID group and 4.73±1.60 L·hour in the Tio-QD group, showing no statistically significant difference between the two groups (MD −0.11 L·hour; 95% CI −1.04 to 0.83) (figure 2). FEV1 in Acli-BID and Tio-QD were determined at 0 hour, 1 hour, 2 hours and 3 hours. Although 0 hour for the Acli-BID group was preinhalation, 0 hour for the Tio-QD group was about 12 hours after night inhalation. FEV1 values after 0 hour, 1 hour, 2 hours and 3 hours at week 8 were 1.48±0.42, 1.55±0.43, 1.56±0.46 and 1.56±0.44 L in the Acli-BID group and 1.59±0.57, 1.60±0.55, 1.62±0.55 and 1.62±0.56 L in the Tio-QD group, respectively. Each value of FEV1 AUC was shown in the figure 2. Change in the SGRQ activity score from week 0 to week eight significantly improved in the Acli-BID group (−4.11±11.77) compared with the Tio-QD group (3.67±10.33) (MD −7.78; 95% CI −14.61 to −0.94), and the change reached a minimal clinically important difference, which was defined as an improvement of 4 points. Although the difference in improvement in physical functioning was not significant between the two groups, there was a significant improvement in the role physical domain of SF-8 at weeks 4 and 8 in the Acli-BID group (3.91±6.32, 2.41±6.33) compared with the Tio-QD group (−2.11±5.54,–1.60±5.43) (MD 6.01; 95% CI 2.40 to 9.63, MD 4.01; 95% CI 0.37 to 7.65, respectively). The mMRC score also demonstrated significant improvement at week 8 in the Acli-BID group (−0.18±0.85) compared with the Tio-QD group (0.48±0.87) (MD −0.66; 95% CI −1.19 to −0.13) (table 2). The scores at week 0, week 4 and week 8 are detailed in the online supplementary table 3. Interpretation and range of questionnaires are detailed in the online supplementary table 4.
Figure 2.
(A) FEV1 measured at week 8. (B) FEV1AUC0-3 at week 8. The values of FEV1 were shown as mean±SD, and FEV1AUC were shown as mean±SD, MD and 95% CI. FEV1AUC0-3 was calculated as follows; FEV1AUC0-1=(FEV1 (0 hour)+FEV1 (1 hour))×(measurement time (1 hour)– measurement time (0 hour))÷2 FEV1AUC1-2, and FEV1AUC2-3 were calculated as well. Finally, FEV1AUC0-3=FEV1AUC0-1+FEV1AUC1-2+FEV1AUC2-3. ANCOVA was used for intergroup comparison of FEV1AUC0-3. Statistical significance, p<0.05. ANCOVA, analysis of covariance;AUC, area under the curve; BID, two times per day; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; QD, once a day.
Table 2.
Mean change of SGRQ, HRQoL (SF-8) and mMRC scores from week 0
| N | Change in score Week 4 week 0 |
Change in score Week 8 week 0 |
||||
| Aclidinium-BID Mean (SD) |
Tiotropium-QD Mean (SD) |
Mean differences (95% CI) |
Aclidinium-BID Mean (SD) | Tiotropium-QD Mean (SD) | Mean differences (95% CI) |
|
| 22 | 22 | 22 | 21 | |||
| SGRQ | ||||||
| Total score | 0.92 (6.33) | 2.94 (6.20) | −2.01 (-5.83 to 1.80) | 0.09 (8.37) | 2.69 (5.26) | −2.59 (-6.92 to 1.73) |
| Symptom | 0.09 (9.18) | 4.07 (15.56) | −3.98 (-11.75 to 3.79) | 0.00 (16.74) | 2.80 (10.47) | −2.80 (-11.45 to 5.85) |
| Activity | 1.54 (10.52) | 0.26 (9.48) | 1.28 (-4.82 to 7.37) | −4.11 (11.77) | 3.67 (10.33) | −7.78 (-14.61 to 0.94) |
| Impact | 0.77 (8.28) | 4.15 (8.93) | −3.39 (-8.63 to 1.85) | 2.59 (8.62) | 2.26 (6.17) | 0.33 (-4.30 to 4.97) |
| SF-8 | ||||||
| Physical component summary | 1.97 (5.70) | −1.53 (4.53) | 3.50 (0.36 to 6.63) | 1.59 (7.12) | −1.49 (4.03) | 3.09 (-0.50 to 6.67) |
| Mental component Summary | 1.52 (5.37) | 0.80 (8.25) | 0.71 (-3.52 to 4.95) | 1.16 (5.38) | 0.32 (7.96) | 0.84 (-3.33 to 5.01) |
| General health | 0.77 (6.32) | −0.25 (5.72) | 1.02 (-2.65 to 4.69) | 1.64 (4.84) | 0.42 (7.85) | 1.21 (-2.78 to 5.21) |
| Physical functioning | 1.65 (6.31) | −0.23 (4.67) | 1.88 (-1.50 to 5.26) | 1.31 (7.52) | −1.78 (5.56) | 3.09 (-1.00 to 7.18) |
| Role physical | 3.91 (6.32) | −2.11 (5.54) | 6.01 (2.40 to 9.63) | 2.41 (6.33) | −1.60 (5.43) | 4.01 (0.37 to 7.65) |
| Bodily pain | 1.62 (6.04) | 0.09 (8.51) | 1.53 (-2.96 to 6.02) | 2.34 (8.04) | −1.11 (7.95) | 3.45 (-1.47 to 8.38) |
| Vitality | 0.69 (4.47) | −3.07 (6.08) | 3.76 (0.51 to 7.01) | 0.12 (5.73) | −1.17 (7.57) | 1.29 (-2.83 to 5.41) |
| Social functioning | 1.98 (6.03) | 0.80 (10.65) | 1.18 (-4.08 to 6.45) | 0.07 (7.89) | 0.96 (10.38) | −0.89 (-6.55 to 4.78) |
| Mental health | 2.03 (5.63) | 1.07 (6.26) | 0.96 (-2.66 to 4.58) | 2.57 (5.36) | −0.30 (6.14) | 2.87 (-0.68 to 6.41) |
| Role emotional | 2.20 (5.43) | 0.22 (8.31) | 1.98 (-2.29 to 6.25) | 1.65 (6.19) | −0.86 (8.51) | 2.51 (-2.06 to 7.07) |
| mMRC | −0.14 (0.71) | 0.36 (0.85) | −0.50 (-0.98 to 0.02) | −0.18 (0.85) | 0.48 (0.87) | −0.66 (-1.19 to 0.13) |
Data within-treatment change from week 0 presented as mean (SD) and between-treatment difference in change presented as mean (95% CI).
BID, two times per day; HRQoL, health-related quality of life; mMRC, modified medical research council dyspnoea scale; QD, once a day; SF-8, 8-item Short-Form Health Survey; SGRQ, St George’s Respiratory Questionnaire.
During the study period, the total number of COPD exacerbation was two in the Acli-BID group and four in the Tio-QD group. Frequency of exacerbation was 0.64/person-year in the Acli-QD group and 1.23/person-year in the Tio-QD group. Rate ratio of exacerbation was 0.52 (95% CI 0.27 to 0.99) with Acli-BID relative to Tio-QD.
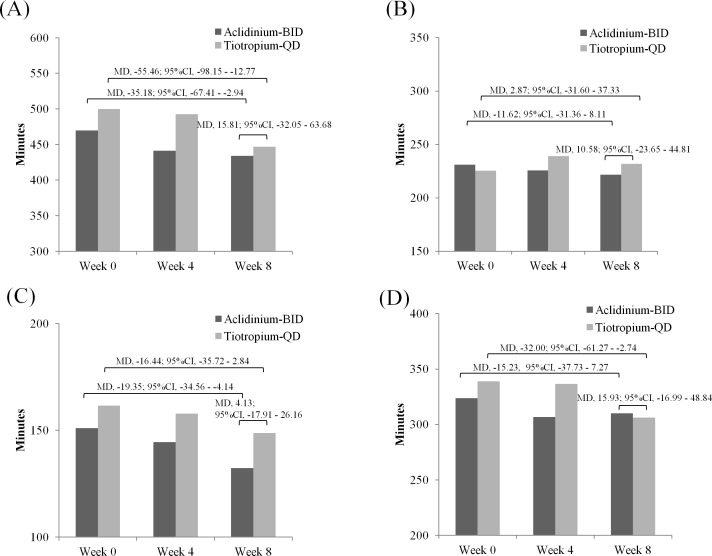
Objectively measured physical activity showed no significant differences in sedentary behaviour (MD 15.81 min; 95% CI −32.05 to 63.68) or in light behaviour (MD 10.58 min; 95% CI −23.65 to 44.81) between the two groups. Significant difference over the treatment period was observed within each group in sedentary behaviour in Acli-BID (MD −35.18 min; 95% CI −67.41 to −2.94) and Tio-QD (MD −55.46 min; 95% CI −98.15 to −12.77) (figure 3A) but not observed with regards to light behaviour between the two groups (figure 3B). Duration of sedentary behaviour in the morning hours significantly reduced in the Acli-BID group (MD −19.35; 95% CI −34.56 to −4.14) (figure 3C), while that in the afternoon hours significantly reduced in the Tio-QD group (MD −32.00; 95% CI −61.27 to −2.74) (figure 3D). A strong negative correlation (Acli-BID; r=−0.75, Tio-QD; r=−0.85, p<0.0001) was found between the mean changes in the duration of sedentary behaviour and light behaviour (see online supplementary figure 1A,B, respectively). In fact, 88% of patients showing the increase in light behaviour showed the decrease of sedentary behaviour. The factors influencing the sedentary behaviour were age, duration of smoking, comorbidities and %FEV1 (see online supplementary table 5).
Figure 3.
Objectively measured physical activity parameters. (A) Mean duration (in minutes) of sedentary behaviour at day 1. (B) Mean duration (in minutes) of light behaviour at day 1. (C) Mean duration (in minutes) of sedentary behaviour in the morning hours. (D) Mean duration (in minutes) of sedentary behaviour in the afternoon hours. The values were shown as MD and 95% CI. Paired t-test was used for intragroup comparison. Student’s t-test was used for intergroup comparison (assessed as change from week 0). Statistical significance, p<0.05. BID, two times per day; MD, mean difference; QD, once a day.
In the Acli-BID group, nine adverse events occurred, and in the Tio-QD group, seven adverse events occurred. There were no serious adverse events. Adverse events were as follows: in the Acli-BID group, there were two events of skin rash and one event each of dizziness, pollinosis, bronchitis, dry mouth, pneumonia, vertigo, and senile dementia; in the Tio-QD group, there were two events of bronchitis and one event each of upper airway inflammation, cramp, herpes zoster, acute exacerbation of COPD and allergic rhinitis.
Discussion
We evaluated the clinical benefit of two-times-per-day therapy of aclidinium bromide (at morning and night-time administration) comparing with once-a-day therapy of tiotropium (at night-time administration) in patients with moderate-to-severe COPD. There was no significant difference between Acli-BID and Tio-QD in terms of FEV1AUC0-3, the primary outcome. These results could lead to conclusion that the bronchodilating effect of Acli-BID and Tio-QD was similar from the viewpoint of improving FEV1AUC0-3. We chose FEV1AUC0-3 as the primary outcome instead of ΔFEV1AUC0-3, given the different timing of administration between the two treatment groups. As the pre FEV1 value (0 hour) at visit 4 in the Acli-BID group was a trough value, it was lower in comparison with that in the Tio-QD group, in which the drug was administered at the previous night between 20:00 and 22:00. Thus, ΔFEV1AUC0-3 (mL/hour) in the Acli-BID group could be more favourable compared with that in the Tio-QD group.
Both Acli-BID and Tio-QD reduced objectively measured sedentary behaviour, with neither treatment demonstrating superiority over the other. However, Acli-BID improved physical activity-related subscales of self-administered questionnaires such as the SGRQ and SF-8 when compared with Tio-QD. Thus, even though Acli-BID demonstrated equivalence to Tio-QD in terms of improving the objective parameters, it was considered superior to Tio-QD in terms of the subjective but pertinent measures of activity in patients with COPD. This study is the first to show the decrease of sedentary behaviour in COPD patients with the use of LAMAs. Decreasing sedentary behaviour in COPD patients is an important issue, given that it is an independent factor related to fatality.8 Moreover, sedentary behaviour is more common in patients with COPD, as was demonstrated in a large-scale health survey conducted in the USA, which demonstrated that the proportion of sedentary behaviour was higher in patients with COPD.9 Most importantly, decreasing the sedentary behaviour and increasing the light behaviour are thought to be more feasible than increasing the moderate or more intense behaviour in patients with COPD, thereby hopefully improving the prognosis of patients.10 A recent study showed that sedentary behaviour was reduced by comprehensive pulmonary rehabilitation, and strong correlation between sedentary behaviour and light behaviour was also found in patients with COPD by using a triaxial accelerometer.11 Likewise, we were also able to demonstrate a correlation between sedentary behaviour and light behaviour through the administration of LAMAs in patients with COPD.
COPD symptoms frequently occurred in the morning, resulting in limitations of morning activities.12 In another study, an association between morning symptoms and physical activity was demonstrated.13 Morning symptoms of COPD are related to deterioration of QoL and impaired activity.14 15 Although we anticipated that the night-time administration of tiotropium would improve the morning symptoms, there was no improvement in HRQoL. Meanwhile, the sedentary behaviour in the morning hours significantly reduced and HRQoL improved in the Acli-BID group.
The VESUTO study16 (Efficacy of tiotropium/olodaterol on lung volume, exercise capacity, and physical activity) set up certain criteria when measuring exercise tolerance and physical activity. That study, as well as ours, will provide important information for evaluating physical activity in patients with COPD in the future.
Some of the limitations of this study include the fact that we did not set up the study to have stringent inclusion/exclusion criteria. As such, the baseline activity levels and the lifestyles of the participants were heterogeneous and may have been reflected in the wide variation of objective activity levels measured by the accelerometer.
Although we did not determine the FEV1 over a 24-hour period, we inferred from previous reports that an important change in FEV1 occurred 12–15 hours after Tio-QD inhalation.4 17 It was reported that FEV1 was not significantly different when Tio-QD was inhaled in the morning or evening, and FEV1 in Acli-BID compared with Tio-QD, which was inhaled in the evening, was not significantly different.18 In the present study, aclidinium was administered two times per day at 9:00–11:00 hours and 21:00–23:00 hours, while tiotropium was administered at 20:00–22:00 hours, intending to improve all morning COPD symptoms. Tiotropium was administered at night, with the intention of improving the morning COPD symptoms, given that the drug needs time to exert its therapeutic effects.
This was an exploratory study in a small-scale conducted in a single prefecture in Japan with a short follow-up duration, being open labelled and without a placebo control. Although the participants were randomly assigned to the treatment arms, the patients might have assumed that, for example, two-times-a-day administration would be better than once-a-day administration, and through such placebo effect, improvement might have been achieved in some of the results for Acli-BID. Finally, because we did not determine FEV1 AUC0-3 at visit 2, the difference between visit 2 and visit 4 could not be compared between the inhalants. These limitations might have made the study insufficient in evaluation of clinical benefit such as pulmonary function, exacerbation and physical activity.
Conclusions
Acli-BID as with Tio-QD could be one of the therapeutic options for patients with COPD to improve pulmonary function, HRQoL and physical activity. In particular, this study suggests that intervention with bronchodilators may enhance physical activity by decreasing sedentary behaviour and increasing light behaviour.
bmjopen-2018-024114supp001.jpg (404KB, jpg)
Supplementary Material
Acknowledgments
The authors thank all the participants and study investigators involved in the Command study. Assistance in data collection, data management, statistical analyses and the preparation of this manuscript was supported by a clinical research organization, Mebix Inc. The authors also thank the clinical research coordinators working with clinical principal investigators in each of the study centers.
Footnotes
Contributors: TK, HN, NN and YMo were members of the Command Study group and contributed to patient enrolment. YMi and KM were the advisers for this study. All members participated in the concept, study protocol and data analysis plan of this study. The manuscript was drafted by TK YMi and KM and finalised by the first author, with all members involved in data analysis, data interpretation, editing and revisions and the decision to submit the manuscript. Tables and figures were constructed by TK. The literature search was conducted by HN, NN and YMo. All the authors assumed responsibility for data and analysis, and vouched for the fidelity of the study according to the protocol.
Funding: This study was supported by Kyorin Pharmaceutical Co, Ltd.
Competing interests: YMi has reported grants from Kyorin Pharmaceutical during the conduct of the study; personal fees from Boehringer Ingelheim outside the submitted work; TK has reported grants from Kyorin Pharmaceutical during the conduct of the study; KM has reported grants from Kyorin Pharmaceutical during the conduct of the study; YMo has reported grants from Kyorin Pharmaceutical during the conduct of the study; personal fees from Novartis Pharma, personal fees from GlaxoSmithKline Pharmaceutical, personal fees from AstraZeneca pharmaceutical, personal fees from Boehringer Ingelheim Pharmaceutical outside the submitted work; HN has reported grants from Kyorin Pharmaceutical during the conduct of the study; NN has reported grants from Kyorin pharmaceutical company during the conduct of the study.
Ethics approval: Central Ethics Committee, Medical Corporation Kyoyukai, Riverside Clinic.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The additional unpublished data are available from the corresponding author on request.
Presented at: Data included in this paper were presented at the American Thoracic Society International Conference, 19-24 May 2017, Washington, DC as a poster presentation, and the abstract was published in "American Thoracic Society International Conference Abstracts", Am J Respir Crit Care Med 2017;195:A5471.
Patient consent for publication: Not required.
References
- 1. Drummond MB, Hansel NN, Connett JE, et al. Spirometric predictors of lung function decline and mortality in early chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;185:1301–6. 10.1164/rccm.201202-0223OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Woodruff PG, Barr RG, Bleecker E, et al. Paine R 3rd, Rennard S, Tashkin DP, Han MK; SPIROMICS Research Group. Clinical Significance of Symptoms in Smokers with Preserved Pulmonary Function. N Engl J Med 2016;374:1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 2011;140:331–42. 10.1378/chest.10-2521 [DOI] [PubMed] [Google Scholar]
- 4. Beier J, Kirsten AM, Mróz R, et al. Efficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderate-to-severe chronic obstructive pulmonary disease: results from a 6-week, randomized, controlled Phase IIIb study. COPD 2013;10:511–22. 10.3109/15412555.2013.814626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGarvey L, Morice AH, Smith JA, et al. Effect of aclidinium bromide on cough and sputum symptoms in moderate-to-severe COPD in three phase III trials. BMJ Open Respir Res 2016;3:e000148 10.1136/bmjresp-2016-000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watz H, Pitta F, Rochester CL, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J 2014;44:1521–37. 10.1183/09031936.00046814 [DOI] [PubMed] [Google Scholar]
- 7. Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30:777–81. 10.1097/00005768-199805000-00021 [DOI] [PubMed] [Google Scholar]
- 8. Furlanetto KC, Donária L, Schneider LP, et al. Sedentary behavior is an independent predictor of mortality in subjects with COPD. Respir Care 2017;62:579–87. 10.4187/respcare.05306 [DOI] [PubMed] [Google Scholar]
- 9. Park SK, Richardson CR, Holleman RG, et al. Physical activity in people with COPD, using the National Health and Nutrition Evaluation Survey dataset (2003-2006). Heart Lung 2013;42:235–40. 10.1016/j.hrtlng.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hill K, Gardiner PA, Cavalheri V, et al. Physical activity and sedentary behaviour: applying lessons to chronic obstructive pulmonary disease. Intern Med J 2015;45:474–82. 10.1111/imj.12570 [DOI] [PubMed] [Google Scholar]
- 11. Mesquita R, Meijer K, Pitta F, et al. Changes in physical activity and sedentary behaviour following pulmonary rehabilitation in patients with COPD. Respir Med 2017;126:122–9. 10.1016/j.rmed.2017.03.029 [DOI] [PubMed] [Google Scholar]
- 12. van Buul AR, Kasteleyn MJ, Chavannes NH, et al. Association between morning symptoms and physical activity in COPD: a systematic review. Eur Respir Rev 2017;26:160033 10.1183/16000617.0033-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buul ARvan, Kasteleyn MJ, Chavannes NH, et al. The association between objectively measured physical activity and morning symptoms in COPD. Int J Chron Obstruct Pulmon Dis 2016;11:2931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roche N, Small M, Broomfield S, et al. Real world COPD: association of morning symptoms with clinical and patient reported outcomes. COPD 2013;10:679–86. 10.3109/15412555.2013.844784 [DOI] [PubMed] [Google Scholar]
- 15. Roche N, Chavannes NH, Miravitlles M. COPD symptoms in the morning: impact, evaluation and management. Respir Res 2013;14:112 10.1186/1465-9921-14-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ichinose M, Minakata Y, Motegi T, et al. Study Design of VESUTO®: Efficacy of Tiotropium/Olodaterol on Lung Hyperinflation, Exercise Capacity, and Physical Activity in Japanese Patients with Chronic Obstructive Pulmonary Disease. Adv Ther 2017;34:1622–35. 10.1007/s12325-017-0554-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fuhr R, Magnussen H, Sarem K, et al. Efficacy of aclidinium bromide 400 μg twice daily compared with placebo and tiotropium in patients with moderate to severe COPD. Chest 2012;141:745–52. 10.1378/chest.11-0406 [DOI] [PubMed] [Google Scholar]
- 18. Calverley PM, Lee A, Towse L, et al. Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary disease. Thorax 2003;58:855–60. 10.1136/thorax.58.10.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-024114supp003.pdf (280.6KB, pdf)
bmjopen-2018-024114supp002.pdf (221.3KB, pdf)
bmjopen-2018-024114supp001.jpg (404KB, jpg)