Abstract
Introduction
Colorectal cancer (CRC) is preventable, as screening leads to the identification and removal of precancerous polyps. African-American men consistently have the highest CRC mortality rates, and their CRC-screening uptake remains low for complex reasons. Culture-specific masculinity barriers to care may contribute to the low uptake among African-American men. Examining these barriers to care is vital as CRC screening may challenge cultural role expectations of African-American men, whose tendency is to delay help-seeking medical care. Barbershops provide a pathway for reaching African-American men with masculinity barriers to care who are not regularly receiving healthcare services and CRC screening. This study aims to develop and pilot test a theory-driven, culture-specific, barbershop-based intervention targeting masculinity barriers to care and CRC-screening uptake among African-American men ages 45–75.
Methods and analysis
Guided by the theory of planned behaviour and the behaviour change wheel, we will use a multistage mixed-methods study design, beginning with an exploratory sequential approach to validate items for subsequent use in a pilot mixed-methods intervention. First, we will collect and analyse qualitative data from focus groups, cognitive interviews and expert item review to validate and test a culture-specific Masculinity Barriers to Care Scale (MBCS) among African-American men. Next, we will administer the MBCS to our target population as an online quantitative survey and evaluate the association between scores and CRC-screening uptake. Then, we will consider existing evidence-based approaches, our integrated results (qualitative +quantitative), and community input to design a culture-specific, behavioural intervention aimed at increasing CRC-screening uptake among African-American men and feasible for barbershop delivery. We will test the peer intervention in a pilot study with a two-arm cluster randomised design (six barbershops, randomised by site) to reduce contamination and account for barbershop culture differences. Our primary outcomes for the pilot are recruitment, sample size estimation, preliminary efficacy and acceptability.
Ethics and dissemination
Ethics approval was obtained from the University of Utah Institutional Review Board (00113679), who will also be responsible for receiving communication updates regarding important protocol modifications. To ensure confidentiality, data dispersed to project team members will be blinded of any identifying participant information. Study results will be disseminated through publications in peer-reviewed journals, community dialogue sessions, and presentations at conferences.
Trial registration number
ClinicalTrials.gov identifier: NCT03733197 (Pre-results); https://clinicaltrials.gov/ct2/show/NCT03733197
Keywords: african-americans, colonic neoplasms, community-based participatory research, men’s health, minority health
Strengths and limitations of this study.
By drawing on constructs of the theory of planned behaviour and the behaviour change wheel, our study will be among the first to offer a structured approach to designing a behavioural change focused, culture-specific arm for our pilot intervention, while considering a range of psychosocial factors associated with colorectal cancer (CRC) screening among African-American men.
Our study proposes a new, culture-specific Masculinity Barriers to Care Scale for understanding and reducing CRC-screening disparities among African-American men.
Given the rising CRC burden among young adults, our study engages African-American men starting at age 45 years.
Though self-report questionnaires are a common behavioural science methodology, social desirability and non-response bias are potential concerns that we will offset by testing the reliability and validity of the data, while collecting it electronically and securely.
Additional research will be needed to ascertain the generalisability of the findings to other settings, since this study limits involvement to African-American men from two metropolitan areas in Utah and Minnesota.
Introduction
Colorectal cancer (CRC) is one of the most treatable and preventable cancers. Despite CRC screening’s (CRCS) life-saving potential, however, nearly 28% of Americans aged 50–75 years have not received timely screening.1 Across all gender and racial/ethnic groups, African-American men have the highest CRC mortality and shortest survival.2 In 2010, national CRCS uptake rates among African Americans (56%) were significantly lower than among non-Hispanic whites (62%).3–5 CRC incidence and mortality rates are 27% and 52% higher, respectively, among African-American men than among non-Hispanic white men.2 6
Recommendations for CRCS
The US Preventive Services Task Force endorses a CRC-screening age range of 50–75 years for average-risk men and screening initiation at age 40 years for those with a family history of CRC.7 Because African-American men are more likely than non-Hispanic white men to be diagnosed at both a younger age and a more advanced disease stage,3 the American College of Gastroenterology has lowered its recommended age of screening initiation to 45 years for African-American men.2 3 8 The proportion of CRC cases diagnosed in individuals aged under 55 years has doubled in the past two decades, and CRC incidence among younger adults (aged 35–49 years), including African-American men, is predicted to increase 28%–46% by 2030.9
Masculinity and psychosocial factors may contribute to low CRCS
Masculinity is an important aspect of gendered and cultural identity for men10–12 and plays a critical role in African-American men’s healthcare use, health behaviours and mortality.13–17 Because CRCS challenges some cultural role expectations of African-American men, who tend to delay seeking medical care, examination of masculinity barriers to care is perilous. However, the specific influence of cultural masculinity perceptions on African-American men’s CRCS rates is not well studied. An unacknowledged sense of vulnerability that conflicts with culturally accepted gender norms is also often inherent in men’s experience of CRCS. Previous research suggests that inadequate existing validated measures and biases towards Western culture (norms, values, customs, etc, associated with Europe and European descent) may explain the absence of a significant association between masculinity and CRCS attitudes among African-American men.18–23 In a systematic review of the literature examining connections between masculinity, racism, social support and CRCS uptake among African-American men,7 few studies have examined how masculinity relates to poor CRCS uptake and, of these, none used validated measures. Further, no validated masculinity measures have been developed for African-American men in the context of CRCS uptake or medical care.18 24 25
Consideration of how psychosocial factors relate to CRCS uptake is also critical. Previous research18–22 with African-American men has documented the influence of factors such as attitudes, knowledge, racism and perceived barriers (eg, embarrassment, fear) on CRCS. Medical mistrust is another widely cited attitudinal barrier to CRCS and treatment seeking21 26 27 and is related to the low health services utilisation among African-American men,21 yet it is unclear whether trust-related barriers are related to CRCS.18 26–31 Since each of the aforementioned factors represents deeply intricate aspects of the social milieu in which African-American men make health decisions, the first author leads the creation and psychometric evaluation of the reliability and validity of his Male Role Norms, Knowledge, Attitudes, and Perceptions associated with CRCS (MKAP-CRCS) survey.21 On average, our sample of young adult African-American men (ages 19–45) disagreed with traditional masculinity ideology—as measured by the 21-item Male Role Norms Inventory-Short Form (MRNI-SF) scale.32 Our principal component analysis revealed the MKAP-CRCS measure was psychometrically sound, but some participants may have withdrawn prematurely from the MRNI-SF portion as they found the measures’ norms offensive/taboo, or felt awkward sharing beliefs about the roles expected of men. We concluded that research focused on developing a scale explicitly considering African-American men’s masculinity beliefs in the medical care context and with more rigorous psychometric assessments (eg, exploratory factor analysis) is needed.
Barbershops as a site for interventions to improve CRCS
Barbershops serving African-American men are favourable settings for reaching our target population.33 Previous multicomponent, barbershop-based trials have been conducted with African-American men on HIV risk reduction, prostate cancer education, heart disease control and hypertension detection.34–36 Few trials of CRCS uptake among African-American men have found significant results. The MISTER B study, the first and to date only barbershop-based CRCS trial, tested a phone-based patient-navigation intervention to urge CRCS among older (mean age 57 years), low-income African-American men with uncontrolled hypertension.37 Intervention completion was associated with a 16-fold increase in the odds of CRCS uptake by 6 months; however, although nearly 70% of participants voiced the intent to obtain colonoscopy screening in the next 6 months, only 17% in the intervention groups and 8% in the control group did so. Our study will help fill this gap between uptake and intention by creating a new, culture-specific intervention that directly addresses masculinity barriers to care, psychosocial factors and CRCS uptake among African-American men beginning at age 45, then test its feasibility and acceptability in a cluster randomised pilot intervention (at the barbershop level).
Study objectives
Disparities associated with CRCS uptake for African-American men, the failure of previous interventions to significantly increase screening rates, and the novel idea of using the barbershop as an intervention setting led to the current study, with the following objectives: (1) validate and test a culture-specific Masculinity Barriers To Care Scale (MBCS) relative to psychosocial factors and CRCS uptake among African-American men and (2) develop and pilot test a theory-driven, culture-specific peer intervention that targets masculinity barriers to care, psychosocial factors and uptake of CRCS (specifically, of the fecal immunochemical test (FIT)) among African-American men. Culture-specific refers to the embodiment of ‘an (African-American male’s) real-life experiences within a given cultural context (eg, neighbourhood) and his understanding of those experiences.’38
Methods and analysis
Overall study design
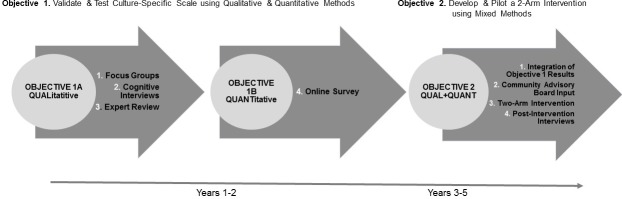
The Standard Protocol Items: Recommendations for Interventional Trials checklist was used while developing this manuscript.39 For the proposed study, a multistage mixed-methods design that is shown in figure 1 will be employed. We will begin with an exploratory sequential approach intended to validate items for subsequent use in a pilot mixed-methods intervention. For Objectives 1A and 1B (years 1–2), we will collect and analyze qualitative data from focus groups, cognitive interviews and expert item review to validate and test a culture-specific MBCS among African-American men. Questions for the MCBS will stem from modifications to the (1) the Barriers to Help-Seeking Scale developed by Mansfield et al,38 (2) the Group-Based Medical Mistrust Scale developed by Thompson et al,40 (3) Mincey et al Masculinity Inventory Scale,41 (4) the MRNI-SF by Levant et al,31 Bowleg et al Black Men’s Experiences Scale,42 and the Masculinity Salience scale developed by Hammond et al.13 Six factors are expected for the underlying structure of the 21 items in the MBCS: (1) need for control and self-reliance, (2) minimising health problems and resignation (3) medical mistrust, (4) privacy, (5) emotional control and (6) black masculinity. For all factors, individual items will be assessed on a Likert-type scale. Higher scores will indicate a greater degree of endorsement of masculinity barriers to care. Next, we will administer the MBCS as an online quantitative survey of our target population to evaluate the association between scale scores and CRCS uptake.
Figure 1.
Exploratory sequential intervention design.
For objective 2 (years 3–5), we will consider existing evidence-based approaches (eg, motivational interviewing, MI), our integrated results (qualitative +quantitative) from objectives 1A and 1B regarding masculinity barriers to care, and community input to design a novel, culture-specific, behavioural intervention that is (1) aimed at increasing CRCS uptake (via FIT) among African-American men and (2) feasible for delivery in barbershops. To reduce contamination and account for differences in barbershop culture, we will pilot test the peer intervention in a two-arm cluster randomised intervention (six barbershops, with participants randomised by site). Our primary outcomes for the pilot are recruitment, sample size estimation, preliminary efficacy and acceptability. We will also conduct postintervention interviews with participants from both arms to evaluate acceptability (ie, why and how each arm was or was not successful). To ensure confidentiality, data dispersed to project team members will be blinded of any identifying participant information.
Patient and public involvement
Neither patients nor the public were involved in the design of the study.
Theoretical foundation
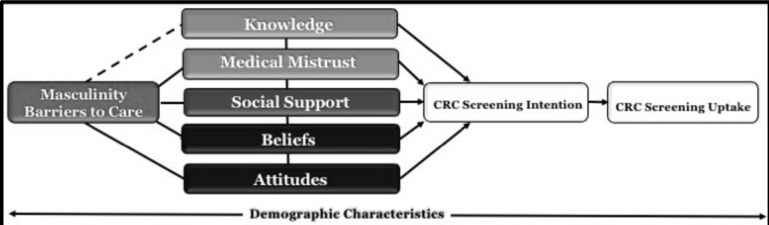
A conceptual framework integrating constructs of the theory of planned behaviour (TPB) will guide our work. The TPB posits that behaviour is a function of intention, which is influenced by attitudes and beliefs.43 Figure 2 illustrates how masculinity barriers to care and other psychosocial factors may influence CRCS intention and uptake among African-American men. We will also assess demographic characteristics (eg, age, marital status and health insurance status) that are known to influence African-American men’s masculinity, and CRCS perceptions and behaviours.6 19 21 22
Figure 2.
Conceptual model of factors influencing CRC-screening uptake among African-American men. CRC, colorectal cancer.
Evidence-based cultural grounding to facilitate understanding of African-American men’s culture and engage community stakeholders as trial-development partners is the best achieved by an iterative, participatory and reflexive research process.44 45 Hence, we will use the behaviour change wheel (BCW) as the conceptual framework driving our study’s intervention-development phase. Developed from 19 behavioural change frameworks, the BCW offers a structured approach to inclusively analysing available intervention options and designing behavioural change interventions.46
Setting
We will conduct this research in the Salt Lake City, Utah and Minneapolis–St. Paul (Twin Cities), Minnesota, metro areas, regions notable for having the largest populations of African Americans in their respective states.47 48 Moreover, in both states, CRCS rates among African Americans are well below statewide averages (53% vs 72% for all ethnic and racial groups combined in Utah; 57% vs 73% for both non-Hispanic whites and all ethnic and racial groups combined in Minnesota).12–14 Nationally, African-American men exhibit a lower screening likelihood than African-American women.3 5 49–51
Focus groups
Participants and procedures
To inform MBCS development, we will conduct twelve 2-hour focus groups (a sufficient number to reach saturation),52 53 each involving eight men who (1) self-identify as non-Hispanic black/African American; (2) were born in the USA; (3) are aged 45–75 years; (4) have a working telephone; (5) speak English and (6) reside in the Salt Lake City or Twin Cities metro area. Six focus groups will be conducted in each metro area. Because participants may be more comfortable with other African-American men of similar age who either have or have not completed CRCS, each group will be clustered by age and CRCS status (table 1). Men aged 45–49 years will be included because African-American men are diagnosed with CRC at both an earlier age and a more advanced disease stage.3 7 8
Table 1.
Focus group composition
| Groups | Age range | Colorectal cancer screening status |
| 1–2 | 45–49 | Never completed |
| 3–4 | 50–65 | Not current |
| 5–6 | 66–75 | Not current |
| 7–8 | 45–49 | Completed/current |
| 9–10 | 50–65 | Completed/current |
| 10–12 | 66–75 | Completed/current |
We will use culture-specific marketing materials to promote the study through existing social networks, including newspaper advertisements, social media, predominantly African-American churches, air time on two radio stations (one in Minneapolis, one in Salt Lake City) with a predominantly African-American male audience and African-American male serving barbershops. The principal investigator (PI), CRR, has a record of success in recruiting African-American men using these strategies.19 21 22
Potential participants will be encouraged to visit www.cuttingCRC.com to express interest in focus group participation. Basic demographic information will be collected (and kept confidential) to enable research team members to contact participants by phone to confirm eligibility and discuss participation arrangements. Food and drink will be provided during each session. Each participant will receive a US$20 target gift card and participants may choose to be entered into a random drawing to win one of three incentives: (1) a US$100 tatget gift card, (2) two tickets to a Utah Jazz or Minnesota Timberwolves basketball game in 2019-20 season (respective of their home state), or (3) a Samsung 55’ 4K UHDTV.
Data collection and analyses
CRR will facilitate the focus groups, using an interview guide stemming from modifications to existing measures13 40–42 that examine masculinity as well as attitudes and practices precluding men from seeking healthcare access. Another team member will assist with consenting and note taking. The 2-hour sessions will be audio-recorded with two voice recorders, transcribed, and checked for accuracy. De-identified transcripts will be imported into NVivo V.11 software (QSR International, Melbourne, Australia). Our NVivo-proficient coding team (TNR, CRR, and the research assistant) will use constant comparative and content analysis methods to independently code transcripts for themes.54 55 After identifying themes relevant to our research questions from a sample reading and initial coding, we will automate term searches, code all documents and run reports to ascertain the code text related to study themes. NVivo can organise data by participant characteristics, allowing us to compare the responses of participants who have or have not undergone CRCS. To interpret and discuss findings and develop a codebook depicting how our codes inter-relate, we will track coding decisions in NVivo and adjudicate them at team meetings. MDF and/or SZ will referee coding deviations, as needed. Key themes will be identified and incorporated into the new MBCS.56 57
Cognitive interviews
Participants and procedures
We will pilot test the MBCS with 10 CRC advocates and survivors from across the USA (men and women who speak English, have a working telephone and are aged 18–75 years), using 1-hour cognitive interviews (conducted in person or by phone) to elicit input as participants respond to the survey in real time.58 59 Interviews will probe (1) how participants understand each question and response option; (2) whether the questions are likely to elicit an honest response; (3) the clarity of question wording; (4) the user-friendliness of the online survey set-up and (5) the questions’ cultural specificity. Participants will engage in a thinking-aloud process with follow-up probes such as ‘How did you arrive at that answer?’ These approaches will improve feasibility, reduce response error and enhance face validity (an estimate of the degree to which the scale is clearly tapping the desired construct we aim to assess, that is, culture-specific masculinity barriers to care).60–62 Cognitive interviews also allow us to assess participants’ comfort with online survey completion via PsychData (PsychData, State College, Pennsylvania, USA), a secure, web-based application that supports data capture for research objective 1B. Our MBCS will be modified as a result of the cognitive interviews when necessary. Interviewees will receive a US$20 Amazon gift card. RJT and two additional leaders in African-American men’s health will provide expert item review of the final MBCS using a questionnaire appraisal system.63
Online survey
Participants and procedures
During year 2, we will recruit 400 African-American men to complete an online survey, administered via smartphone, to test the relationship between masculinity barriers to care and CRCS. Eligible respondents are men who (1) self-describe as non-Hispanic black/African American; (2) were born in the USA; (3) are aged 45–75 years; (4) reside in the Salt Lake City or Twin Cities metro area; (5) have a telephone with internet access and (6) speak English. With the aid of barbers and culture-specific marketing materials, we will recruit participants from African-American male serving barbershops. Survey participants will have the opportunity to participate in drawings for one of five incentives: (1) a US$50 visa gift card, (2) a US$75 gas station gift card, (3) a US$100 grocery store gift card, (4) an Apple iPad or (5) Samsung 43’ 4K UHDTV.
Barbershops are cultural hubs of trust essential in the growth and development of African-American men. Men usually spend at least 30 min waiting for or getting a haircut or chatting with others in the barbershop. Using PsychData, participants will be able to complete our survey within 15 min on their smartphones while waiting for or getting a haircut in participating barbershops. PsychData prevents survey alterations and eliminates transcription errors.64 The PI has a successful record of recruiting African-American men to complete surveys using mobile technology,19 21 22 and African Americans outpace all groups for smartphone use.65 For men who want to complete the survey but do not own a smartphone, each participating barbershop will be provided one smartphone courtesy of the study.
Dependent variables
We will use two Behavioral Risk Factor Surveillance System (BRFSS) questions to assess CRC S uptake: (1) ‘A blood stool test is a test that may use a special kit at home to determine whether the stool contains blood. Have you ever had this test using a home kit?”’ and (2) ‘Sigmoidoscopy and colonoscopy are examinations in which a tube is inserted in the rectum to view the colon for signs of cancer or other health problems. Have you ever had either of these examinations?’66
Independent variables
In accordance with our conceptual model illustrated in figure 2 and the PI’s MKAP-CRCS tool,1 18–22 our independent variables will be masculinity barriers to care from our new scale and five factors known to influence CRCS uptake among African-American men: knowledge, social support, beliefs and attitudes towards CRC and two CRCS exams (FIT, colonoscopy).13 18 21 27
Demographic covariates
Age, educational level, marital status, employment status and other covariates will be included as previous studies by the PI and others have found these factors to be related to CRCS among African-American men.2 18–22 67 68
Sample size and power considerations
With a sample of 400 African-American men, we will have 80% power at the 0.05 level to detect a masculinity barriers to care effect on the odds of having had CRCS, assuming 35% of men with a masculinity barriers to care index equal to the mean have had CRCS compared with 25% of men with a masculinity barriers to care index 1 SD above the mean. We estimate that the average screening rate will be 35%, as the screening rate for African Americans is 53.1% in Utah and 52% in Minnesota and African-American men in both states tend to have lower CRCS rates than women.4 6 12 45 This assumes a moderately strong relationship between the masculinity barriers to care index and confounders (ie, R2=0.25 for the linear model that regresses the masculinity barriers to care index on the confounders). If the relationship between the confounders and the masculinity index is weaker, the power will be higher: 82% power with R2=0.2% and 87% power with R2=0.1. Power calculations were performed using PASS V.15.
Data collection and analyses
We will test for associations between masculinity barriers to care, psychosocial factors and CRCS uptake. Our central hypothesis is that masculinity barriers to care will be negatively associated with CRC S uptake. The masculinity barriers to care items emerging from objective 1 will be used to create a latent variable that represents the construct being measured. The CRCS uptake outcome will be a binary variable that indicates whether a participant self-reported CRCS uptake (ie, answered yes to either BRFSS dependent-variable question). We will fit a structural equation model with CRCS uptake as the outcome and masculinity barriers to care as the predictor. We will adjust for potential confounders (eg, age, educational level). We will also present the estimate and 95% CI for the OR comparing the odds of CRCS uptake between participants with a one-point difference in masculinity barriers to care scores. Descriptive statistics will summarise the participants’ characteristics.
Two-arm intervention
Integration
In an exploratory sequential designed study, a key step is to apply the qualitative data captured in objective 1A to assist with building objective 1B’s quantitative phase. As described by Fetters et al,69 we will ‘merge’ qualitative and quantitative data from objective 1 to identify content areas for contrasting, comparing and synthesising results. During the first 6 months of year 3, two team members (MDF and CRR) will determine to what degree and how the results from the combined qualitative and quantitative datasets yield a richer and more comprehensive understanding of the impact of masculinity barriers to care on CRCS uptake among African-American men. Through this process, we will apply what we learn about the role of our variable of interest on CRCS to develop a pilot intervention for overcoming these barriers.
Development
During the first 6 months of year 3, we will adopt the BCW approach, working with community advisory board members (2 hours small-group discussions via conference call and/or in person, three members per meeting) to develop the culture-specific intervention arm of our pilot intervention. We will use information from (1) our integrated objective 1e results, (2) existing CRCS intervention evidence and (3) study team expertise to apply the Acceptability, Practicability, Effectiveness/cost-effectiveness, Affordability, Safety/side effects, Equity (APEASE criteria) (table 2). Our hypothesis is that CRCS uptake will be higher in the culture-specific arm than in the control arm.
Table 2.
Behaviour change wheel (BCW) activities to drive development of culture-specific trial arm
| 11. Behavioural diagnosis | Use the BCW to determine what needs to change for colorectal cancer (CRC) screening uptake to increase among African-American men. |
| 22. Intervention strategy selection | Use1 to decide which intervention functions to apply (eg, education, persuasion, enablement). |
| 33. Behaviour change technique Identification | Develop a detailed culture-specific arm plan by selecting from among a range of specific, evidence-based behaviour change techniques (eg, intervention components such as barbers as motivational interviewers plus barbers distributing fecal immunochemical test kits; info about health consequences related to negating CRC screening). |
| 44. Draft full intervention specifications | Create the detailed intervention specifications covering all aspects of content and delivery of the intervention structured around.3 |
We anticipate that the culture-specific arm will include at least two core components: barbers as motivational interviewers and InSure FIT kits distributed by barbers. MI is ‘a collaborative conversation style for strengthening a person’s own motivation and commitment to change.’69 Telephone-based MI is an effective way to improve cancer screening among under-represented groups. Community-member–led MI has proved successful, but it is unknown if barbers as motivational interviewers can assist with reducing CRCS inequalities among African-American men.70–73 Also, randomised trials have shown that the FIT is the first-choice fecal occult blood test for CRCS, is less invasive, less costly and may be better accepted than other CRCS tests.74 If we choose this route for the culture-specific arm, preliminary data from our barbers suggest that the PI may teach the barbers the MI technique using content stems from objective 1 findings. Additional components for this arm may be developed during the APEASE process.
Based on the PI’s research18–22 and evidence-based strategies,75 the control arm will include an informational CRCS brochure developed by the American Cancer Society76 plus an FIT kit distributed by the barbers. Since the FIT kits will be free and the study will cover postage and processing fees, participants will be able to complete screening regardless of whether they have health insurance. Participants will mail the completed FIT kits to our local laboratory for processing. We will refer participants with positive FIT results to Huntsman Cancer Institute for a colonoscopy.
Participants and procedures
Intervention participants will be non-Hispanic black/African-American men (n=60) who (1) have never completed CRCS; (2) are aged 45–75 years; (3) were born in the USA; (4) reside in the Salt Lake City metro area; (5) have a telephone with internet access and (6) speak English. As a feasibility intervention, sample size is not based on the power to detect a certain effect size.77 Rather, n=60 (30 per arm) was chosen based on practical considerations (eg, cost, recruitment).
The intervention will comprise a recruitment phase and an implementation phase. The recruitment phase will occur during the last 6 months of year 3. With the assistance of barbers and culture-specific marketing materials, we will enrol 10 eligible African-American men at each of 6 barbershops. At baseline, participants will complete the demographic portion of our online survey. Once total enrolment is reached and consent obtained, the six barbershops will be randomised to the culture-specific or control arm using a permuted block size of 6. Then the implementation phase will begin. These distinct phases provide advantages in a cluster randomised design. First, we eliminate recruitment bias as we blind participants to the intervention at enrolment.77 Second, the distinct phases allow each man to be exposed to the intervention for the same amount of time. We foresee the recruitment phase lasting 3 months and the implementation phase 7 months, resulting in a 10-month intervention, allowing ample time for participants to obtain CRCS.
During the last 6 months of year 4, our coding team will conduct exit interviews. Prior literature documents that 6–12 individual interviews per homogeneous group are sufficient to reach data saturation.78 79 Thus, 18 in-depth, 60 min participant interviews (2 participants from each of the 6 barbershops plus 3 barbers from each arm) will permit us to obtain rigorous outcomes data as well as participant accounts of what worked well and what did not for our two-arm intervention’s implementation.
Analyses
Descriptive statistics will summarise participants’ baseline characteristics. Continuous variables will be summarised by mean (SD) or median (IQR), and categorical variables in contingency tables. Feasibility of the study protocol will be evaluated as follows:
Recruitment
We will calculate the number of days needed to reach full enrolment at each barbershop, the percentage of men meeting eligibility criteria and, of those, the percentage who chose to enrol.
Sample size estimation
The intraclass correlation coefficient will be estimated from our study data and inflated (due to the expected downward bias) to estimate the necessary sample size for the full trial.80
Preliminary efficacy
By treatment arm, we will calculate the percentage for whom we can ascertain FIT uptake. We will assess intervention adherence 7 months after the recruitment phase by FIT kits returned to our laboratory for processing. Because we will have only three barbershops per arm, no formal statistical analysis of FIT uptake will be performed. Instead, percentages for these outcomes will be calculated by barbershop. We will perform this as intention to treat, with men included based in the arm to which their shop was randomised. A per-protocol analysis will also be performed. Many more barbershops are needed to accurately account for the correlation of African-American men within the same barbershop and achieve a cluster-level confounding balance.81 In our pilot trial, a logistic mixed-effects model with a random intercept for each barbershop will be used to estimate the ORs comparing CRCS uptake between our control and culture-specific arms.
Acceptability
After the 7-month intervention phase, we will conduct 18 postintervention interviews to obtain rigorous outcomes data. The audio-recorded and transcribed postintervention interviews will be analysed by our coding team using NVivo64 and Creswell’s methods.82 To increase our findings’ internal validity, data will be triangulated or compared from the perspectives of the two study arms.83
Conclusion
African-American men have the highest CRC mortality across all gender and racial/ethnic groups. Moreover, national findings predict a 28%–46% increase in CRC incidence among adults ages 35–49 years, including African-American men, by 2030.9 Our study aims to aid in reducing CRCS inequities among African-American men by creating a new, culture-specific intervention that directly addresses masculinity barriers to care, psychosocial factors, and CRCS uptake among African-American men beginning at age 45 years. Subsequently, we will test its feasibility and acceptability in a cluster randomised pilot intervention.
Completing our objective will provide the preliminary data needed for an R01 application to test the new intervention’s efficacy in a large-scale, well-powered, cluster randomised controlled trial. More broadly, this research will demonstrate that decisions regarding CRCS uptake are not detached from cultural and other influences. We will use the culture-specific survey instrument we create to more rigorously assess the association between masculinity barriers to care and CRCS uptake. This will strengthen our scale’s predictive utility while endorsing optimal health for African-American men as warranted by Healthy People 2020.82 Additionally, our scale could be adapted for use in research on other types of cancer (eg, prostate cancer) that disproportionately affect African-American and other under-represented men.
Overall, our efforts will serve as a model for more culture-specific tailored approaches to prevention, diagnosis and treatment, a goal aligned with the National Cancer Institute’s Cancer Moonshot initiative.
Supplementary Material
Acknowledgments
The research team extends gratitude to Eleanor Mayfield for editorial assistance.
Footnotes
Contributors: KO, EDP, MH, SZ, RJT and MDF serve as data monitoring committee (DMC) members for this study, while additional data monitoring, harms and auditing logistics are available from the University of Utah Institutional Review Board. Only the DMC will have access to the full pilot trial dataset in order to ensure that the overall results are not disclosed by an individual study site prior to the main publication. CRR is the study PI, wrote the first draft of the study protocol and edited every draft thereafter. KO is a primary mentor for the study and edited the study protocol with the PI. EDP is a secondary mentor of the study and edited the study protocol with the PI. Study comentors MH, SZ and RJT edited the study protocol with the PI. CRR and TNR are coinvestigators of the study and edited the study protocol with the PI. In addition to being a mixed-methods expert, MDF is a comentor on the study who edited the study protocol extensively with the PI as a senior coauthor. All authors agreed to be accountable for all aspects of the study in ensuring that questions related to the accuracy or integrity of any part of the study are appropriately investigated and resolved. Lastly, all authors read and approved the final manuscript.
Funding: This work was supported by the National Cancer Institute of the National Institutes of Health (NIH) under Award Number K01CA234319. RJT was supported by the National Institute on Aging (K02AG059140) and the National Institute on Minority Health and Health Disparities (U54MD000214-6867).
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Competing interests: None declared.
Ethics approval: This study protocol has received ethics approval from the University of Utah Institutional Review Board (00113679), who will also be responsible for receiving communication updates regarding important protocol modifications.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Centers for Disease Control and Prevention (US). Vital signs: colorectal cancer screening test use–United States. 62 Atlanta, GA: U.S. Centers for Disease Control MMWR Morb Mortal Wkly Rep, 2012:881–8. [Google Scholar]
- 2. American Cancer Society. Colorectal cancer facts & figures 2017–2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf (accessed 7 Feb 2019).
- 3. Agrawal S, Bhupinderjit A, Bhutani MS, et al. Colorectal cancer in African Americans. Am J Gastroenterol 2005;100:515–23. 10.1111/j.1572-0241.2005.41829.x [DOI] [PubMed] [Google Scholar]
- 4. Agrawal J, Syngal S. Colon cancer screening strategies. Curr Opin Gastroenterol 2005;21:166–70. 10.1097/00132980-200504000-00010 [DOI] [PubMed] [Google Scholar]
- 5. Gwede CK, Ward BG, Luque JS, et al. Application of geographic information systems and asset mapping to facilitate identification of colorectal cancer screening resources. Online J Public Health Inform 2010;2:2893 10.5210/ojphi.v2i1.2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Cancer Society. Cancer facts & figures for African Americans 2016-2018. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036921.pdf (accessed 7 Feb 2019).
- 7. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. US Preventive Services Task Force. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564–75. 10.1001/jama.2016.5989 [DOI] [PubMed] [Google Scholar]
- 8. Dominic OG, McGarrity T, Dignan M, et al. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008. Am J Gastroenterol 2009;104:2626–7. 10.1038/ajg.2009.419 [DOI] [PubMed] [Google Scholar]
- 9. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 2015;150:17–22. 10.1001/jamasurg.2014.1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. David D, Brannon R. The male sex role: our culture’s blueprint for manhood, and what it’s done for us lately In: David D, Brannon R, eds The Forty-Nine Percent Majority: The Male Sex Role. Reading, MA: Addison-Wesley, 1976:1–48. [Google Scholar]
- 11. Levant RF. The male role: an investigation of contemporary norms. J Ment Health Couns 1992;14:325–37. [Google Scholar]
- 12. Mahalik JR, Locke BD, Ludlow LH, et al. Development of the conformity to masculine norms inventory. Psychol Men Masc 2003;4:3–25. [Google Scholar]
- 13. Hammond WP, Matthews D, Mohottige D, et al. Masculinity, medical mistrust, and preventive health services delays among community-dwelling African-American men. J Gen Intern Med 2010;25:1300–8. 10.1007/s11606-010-1481-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Courtenay WH. Constructions of masculinity and their influence on men’s well-being: a theory of gender and health. Soc Sci Med 2000;50:1385–401. [DOI] [PubMed] [Google Scholar]
- 15. Griffith DM, Gunter K, Allen JO. A systematic approach to developing contextual, culturally, and gender-sensitive interventions for African-American men: The example of men 4 health. Cancer disparities: Causes and evidence-based solutions. 2012 New York, NY: Springer, 2012:193–206. [Google Scholar]
- 16. Harrison J. Warning: The male sex role may be dangerous to your health. J Soc Issues 1978;34:65–86. [Google Scholar]
- 17. Marcell AV, Ford CA, Pleck JH, et al. Masculine beliefs, parental communication, and male adolescents’ health care use. Pediatrics 2007;119:966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rogers CR, Mitchell JA, Franta GJ, et al. Masculinity, Racism, Social Support, and Colorectal Cancer Screening Uptake Among African American Men: A Systematic Review. Am J Mens Health 2017;11:1486–500. 10.1177/1557988315611227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rogers CR, Goodson P, Obidike OJ. Measuring Factors Associated with Colorectal Cancer Screening among Young Adult African American Men: A Psychometric Study. J Immigr Minor Health 2018;20:101–6. 10.1007/s10903-016-0523-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rogers CR, Goodson P, Foster MJ. Factors Associated with Colorectal Cancer Screening among Younger African American Men: A Systematic Review. J Health Dispar Res Pract 2015;8:133–56 http://digitalscholarship.unlv.edu/jhdrp/vol8/iss3/8. [PMC free article] [PubMed] [Google Scholar]
- 21. Rogers CR, Goodson P. Male Role Norms, Knowledge, Attitudes, and Perceptions of Colorectal Cancer Screening among Young Adult African American Men. Front Public Health 2014;2:1–12. 10.3389/fpubh.2014.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rogers CR, Goodson P, Dietz LR, et al. Predictors of intention to obtain colorectal cancer screening among African American men in a state fair setting. Am J Mens Health 2016:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levant RF, Hall RJ, Rankin TJ. Male Role Norms Inventory-Short Form (MRNI-SF): development, confirmatory factor analytic investigation of structure, and measurement invariance across gender. J Couns Psychol 2013;60:228–38. 10.1037/a0031545 [DOI] [PubMed] [Google Scholar]
- 24. Winterich JA, Quandt SA, Grzywacz JG, et al. Masculinity and the body: how African American and White men experience cancer screening exams involving the rectum. Am J Mens Health 2009;3:300–9. 10.1177/1557988308321675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beeker C, Kraft JM, Southwell BG, et al. Colorectal cancer screening in older men and women: qualitative research findings and implications for intervention. J Community Health 2000;25:263–78. [DOI] [PubMed] [Google Scholar]
- 26. McKinstry B, Ashcroft RE, Car J, et al. Interventions for improving patients’ trust in doctors and groups of doctors. Cochrane Database Syst Rev 2006;3:1–26. [DOI] [PubMed] [Google Scholar]
- 27. LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev 2000;57(Suppl 1):146–61. 10.1177/1077558700057001S07 [DOI] [PubMed] [Google Scholar]
- 28. Adams LB, Richmond J, Corbie-Smith G, et al. Medical Mistrust and Colorectal Cancer Screening Among African Americans. J Community Health 2017;42:1044–61. 10.1007/s10900-017-0339-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Griffith DM, Johnson JL. Implications of racism for African American men’s cancer risk, morbidity, and mortality : Treadwell HM, Xantos C, Holden CB, Social determinants of health among African-American men. San Francisco, CA: Jossey-Bass, 2013:21–38. [Google Scholar]
- 30. Henry J, Foundation KF. Fact sheet: the health status of African American men in the United States. http://www.kff.org/minorityhealth/upload/7630.pdf (accessed 7 Feb 2019).
- 31. Griffith DM, Gunter K, Watkins DC. Measuring masculinity in research on men of color: findings and future directions. Am J Public Health 2012;102(S2):S187–S194. 10.2105/AJPH.2012.300715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Airhihenbuwa CO. Health and Culture: Beyond the Western Paradigm. Thousand Oaks, CA: SAGE Publications, 1995. [Google Scholar]
- 33. Linnan LA, D’Angelo H, Harrington CB. A literature synthesis of health promotion research in salons and barbershops. Am J Prev Med 2014;47:77–85. 10.1016/j.amepre.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luque JS, Ross L, Gwede CK. Qualitative systematic review of barber-administered health education, promotion, screening and outreach programs in African-American communities. J Community Health 2014;39:181–90. 10.1007/s10900-013-9744-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hood S, Hall M, Dixon C, et al. Organizational-level recruitment of barbershops as health promotion intervention study sites. Health Promot Pract 2018;19:377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teo CH, Ling CJ, Ng CJ. Improving Health Screening Uptake in Men: A Systematic Review and Meta-analysis. Am J Prev Med 2018;54:133–43. 10.1016/j.amepre.2017.08.028 [DOI] [PubMed] [Google Scholar]
- 37. Cole H, Thompson HS, White M, et al. Community-Based, Preclinical Patient Navigation for Colorectal Cancer Screening Among Older Black Men Recruited From Barbershops: The MISTER B Trial. Am J Public Health 2017;107:1433–40. 10.2105/AJPH.2017.303885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mansfield AK, Addis ME, Courtenay W. “Measurement of Men’s Help Seeking: Development and Evaluation of the Barriers to Help Seeking Scale”. Psychology of Men & Masculinity 2005;6:95–108. [Google Scholar]
- 39. Campbell MK, Piaggio G, Consort EDR. statement: extension to cluster randomized trials. BMJ 2010;2012:e5661. [DOI] [PubMed] [Google Scholar]
- 40. Thompson HS, Valdimarsdottir HB, Winkel G, et al. The Group-Based Medical Mistrust Scale: psychometric properties and association with breast cancer screening. Prev Med 2004;38:209–18. [DOI] [PubMed] [Google Scholar]
- 41. Mincey K, Alfonso M, Hackney A, et al. Being a Black Man: Development of the Masculinity Inventory Scale (MIS) for Black Men. J Mens Stud 2014;22:167–79. 10.3149/jms.2203.167 [DOI] [Google Scholar]
- 42. Bowleg L, English D, Del Rio-Gonzalez AM, et al. Measuring the Pros and Cons of What It Means to Be a Black Man: Development and Validation of the Black Men’s Experiences Scale (BMES). Psychol Men Masc 2016;17:177–88. 10.1037/men0000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nastasi BK, Varjas K, Bernstein R, et al. Conducting participatory culture-specific consultation: a global perspective on multicultural consultation. School Psych Rev 2000;29:401–13. [Google Scholar]
- 44. Ajzen I, Fishbein M. Understanding Attitudes and Predicting Social Behavior. Englewood Cliffs, NJ: Prentice Hall Publishing, 1980. [Google Scholar]
- 45. Nastasi BK, Hitchcock JH, Research MM. and Culture-Specific Interventions: Program Design and Evaluation. Thousand Oaks, CA: SAGE Publications 2016. [Google Scholar]
- 46. Glandon D, Paina L, Alonge O, et al. 10 Best resources for community engagement in implementation research. Health Policy Plan 2017;32:1457–65. 10.1093/heapol/czx123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6:42 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Office of Health Disparities. Moving forward in 2016: fifteen years of health data for Blacks/African Americans in Utah. Salt Lake City, UT: Utah Department of Health. https://health.utah.gov/disparities/data/race-ethnicity-report/MovingForwardAA2016.pdf (accessed 7 Feb 2019).
- 49. United State Census Bureau. QuickFacts Salt Lake City city, Utah; United State. https://www.census.gov/quickfacts/fact/table/saltlakecitycityutah,US/PST045217 (accessed 7 Feb 2019).
- 50. Agrawal J, Syngal S. Colon cancer screening strategies. Curr Opin Gastroenterol 2005;21:59–63. [PubMed] [Google Scholar]
- 51. Wallace PM, Regional SR. racial, and gender differences in colorectal cancer screening in middle-aged African-Americans and Whites. J Cancer Educ 2012;27:703–8. [DOI] [PubMed] [Google Scholar]
- 52. Martinez KA, Pollack CE, Phelan DF, et al. Gender differences in correlates of colorectal cancer screening among black Medicare beneficiaries in Baltimore. Cancer Epidemiol Biomarkers Prev 2013;22:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krueger RA, Casey MA. Focus group interviewing : Wholey J, Hattry H, Newcomer K, The Handbook of Practical Program Evaluation. 3rd ed San Francisco, CA: Jossey-Bass, 2010:378–403. [Google Scholar]
- 54. Krueger RA, Casey MA. Focus Groups: A Practical Guide for Applied Research. 5th ed: SAGE Publications, 2014. [Google Scholar]
- 55. Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. New York: Aldine De Gruyter, 1967. [Google Scholar]
- 56. Strauss A, Corbin J. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. Newbury Park, CA: Sage Publications, 1990. [Google Scholar]
- 57. Miles MB, Huberman AM, Analysis QD. Thousand Oaks. 2nd ed CA: Sage Publications, 1994. [Google Scholar]
- 58. Saldana J. The Coding Manual for Qualitative Researchers. Thousand Oaks, CA: Sage Publications, 2009. [Google Scholar]
- 59. Willis GB. Cognitive Interviewing: A Tool for Improving Questionnaire Design. Thousand Oaks, CA: Sage Publications, 2005. [Google Scholar]
- 60. Sudman S, Bradburn NM, Schwarz N. Thinking about Answers: The Application of Cognitive Processes to Survey Methodology. San Francisco, CA: Jossey-Bass Publishers, 1996. [Google Scholar]
- 61. Bornstein RF, Rossner SC, Hill EL, et al. Face validity and fakability of objective and projective measures of dependency. J Pers Assess 1994;63:363–86. [DOI] [PubMed] [Google Scholar]
- 62. Willis G, Lessler J. Questionnaire Appraisal System-1999. Research Triangle Institute: Research Triangle Park, NC, 1999. [Google Scholar]
- 63. Messick S. Validity of psychological assessment: Validation of inferences from persons' responses and performances as scientific inquiry into score meaning. Am Psychol 1995;50:741–9. [Google Scholar]
- 64. Nevo B. Face validity revisited. J Educ Meas 1985;22:287–93. [Google Scholar]
- 65. Andrews D, Nonnecke B, Preece J. Electronic survey methodology: a case study in reaching hard to involve Internet Users. Int J Hum Comput Interact 2003;16:185–210. [Google Scholar]
- 66. The Nielsen Company. Multifaceted connections: African-American media usage outpaces across platforms. http://www.nielsen.com/us/en/insights/news/2015/multifaceted-connections-african-american-media-usage-outpaces-across-platforms.html (accessed 7 Feb 2019).
- 67. Centers for Disease Control and Prevention. behavioral risk factor surveillance system questionnaire. 2016. https://www.cdc.gov/brfss/questionnaires/pdf-ques/2016_brfss_questionnaire_final.pdf (accessed 7 Feb 2019).
- 68. Gimeno García AZ. Factors influencing colorectal cancer screening participation. Gastroenterol Res Pract 2012;2012:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fetters MD, Curry LA, Creswell JW. Achieving integration in mixed methods designs – principles and practices. Health Services Research 2013;48:2134–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Miller SJ, Foran-Tuller K, Ledergerber J, et al. Motivational interviewing to improve health screening uptake: a systematic review. Patient Education and Counseling 2017;100:190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Miller WR, Rollnick S. Motivational Interviewing Helping People Change. New York: Guilford Press 2013. [Google Scholar]
- 72. Spencer JC, Wheeler SB. A systematic review of Motivational Interviewing interventions in cancer patients and survivors. Patient Education and Counseling 2016;99:1099–105. [DOI] [PubMed] [Google Scholar]
- 73. Resnicow K, Jackson A, Wang T, et al. A motivational interviewing intervention to increase fruit and vegetable intake through black churches: results of the eat for life trial. Am J Public Health 2001;91:1686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US multi-society task force on colorectal cancer. Gastroenterol 2017;152:1217–37. [DOI] [PubMed] [Google Scholar]
- 75. National Cancer Institute. Colorectal cancer screening intervention programs. 2018. https://rtips.cancer.gov/rtips/topicPrograms.do?topicId=102265&choice=default (accessed 7 Feb 2019).
- 76. Society AC. They know how to prevent colon cancer – and you can. Too 2017. https://www.cancer.org/content/dam/cancer-org/cancer-control/en/booklets-flyers/they-know-how-to-prevent-colon-cancer-handout.pdf (accessed 7 Feb 2019). [Google Scholar]
- 77. Eldridge S, Kerry S, Torgerson D. Bias in identifying and recruiting participants in cluster randomized trials: what can be done?”. BMJ 2009;339:b4006. [DOI] [PubMed] [Google Scholar]
- 78. Crabtree BF, Miller MW. Using codes and code manuals : Crabtree BF, Miller MW, Doing Qualitative Research. 2nd ed Thousand Oaks, CA: Sage, 1999. [Google Scholar]
- 79. Creswell JW. Analyzing and validating data 30 Essential Skills for the Qualitative Researcher. Thousand Oaks, CA: SAGE Publications, 2015:151–65. [Google Scholar]
- 80. Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psych Res 2011;45:626–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Creswell JW. Sampling and Integration Issues. A Concise Introduction to Mixed Methods Research. Thousand Oaks, CA: Sage, 2015:74–5. [Google Scholar]
- 82. Secretary’s Advisory Committee on Health Promotion and Disease Prevention Objectives for 2020. Healthy people 2020: an opportunity to address the societal determinants of health in the United States. http://www.healthypeople.gov/2010/hp2020/advisory/SocietalDeterminantsHealth.htm (accessed 7 Feb 2019).
- 83. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.