Abstract
The utility of oxazole as intermediates for the synthesis of new chemical entities in medicinal chemistry have been increased in the past few years. Oxazole is an important heterocyclic nucleus having a wide spectrum of biological activities which drew the attention of researchers round the globe to synthesize various oxazole derivatives and screen them for their various biological activities. The present review article aims to review the work reported on therapeutic potentials of oxazole scaffolds which are valuable for medical applications during new millennium.
Keywords: Oxazole derivatives, Antimicrobial, Anticancer, Antitubercular
Background
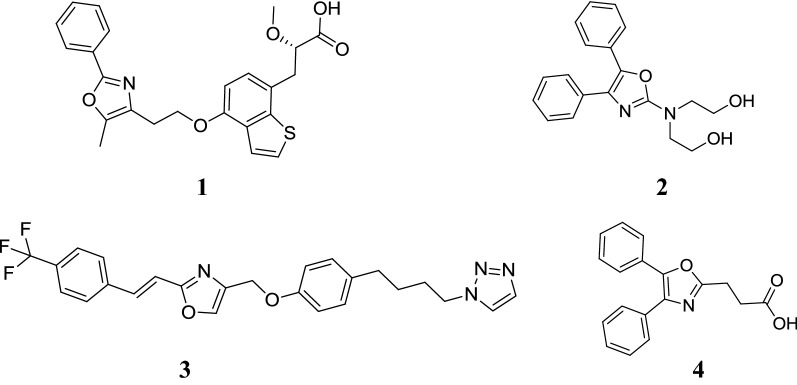
Heterocyclic systems are a part of large number of drugs and biologically relevant molecules. Often the presence of hetero atoms or groupings imparts preferential specificities in their biological responses. The chemistry and biological study of heterocyclic compounds has been interesting field for a long time [1] and oxazole is one such moiety which has gained attention in recent times due to its increasing importance in the field of medicinal chemistry. Oxazoles is a doubly unsaturated 5-membered ring having one oxygen atom at position 1 and a nitrogen at position 3 separated by a carbon in-between. It was first prepared in 1947, has a boiling point of 69 °C and is a stable liquid at room temperature [2]. Substitution pattern in oxazole derivatives play a pivotal role in delineating the biological activities like antimicrobial [3], anticancer [4], antitubercular [5] anti-inflammatory [6], antidiabetic [7], antiobesity [8] and antioxidant [9] etc. Oxazoles and its derivatives are a part of number of medicinal compounds (Fig. 1) which includes aleglitazar (1, antidiabetic), ditazole (2, platelets aggregation inhibitor), mubritinib (3, tyrosine kinase inhibitor), and oxaprozin (4, COX-2 inhibitor) [10].
Fig. 1.
Marketed preparations containing oxazole
From the literature, it was found that various types of review articles have been written on synthesized/natural oxazole compounds which are focused on their pharmacological significance in medicinal filed. Some of the reported review articles on oxazole moiety includes the work done by Joshi et al. who have presented a review on systematic scientific study of 1, 3-oxazole derivatives as a useful lead for pharmaceuticals [11], Swellmeen, prepared a review on 1,3-oxazole derivatives exhibiting their biological activities as antipathogenic [2] whereas Singh and Tilvi, have presented a review on synthesis of oxazole, oxazoline and isoxazoline derived marine natural products [12]. The current review is concentrates on the diverse biological potential of oxazole derivatives in the new millennium, as no such extensive review article is reported recently.
Biological activities of oxazole
Pharmacological interventions of oxazole derivatives are voluminous, but this article covers the most relevant ones.
Antimicrobial activity
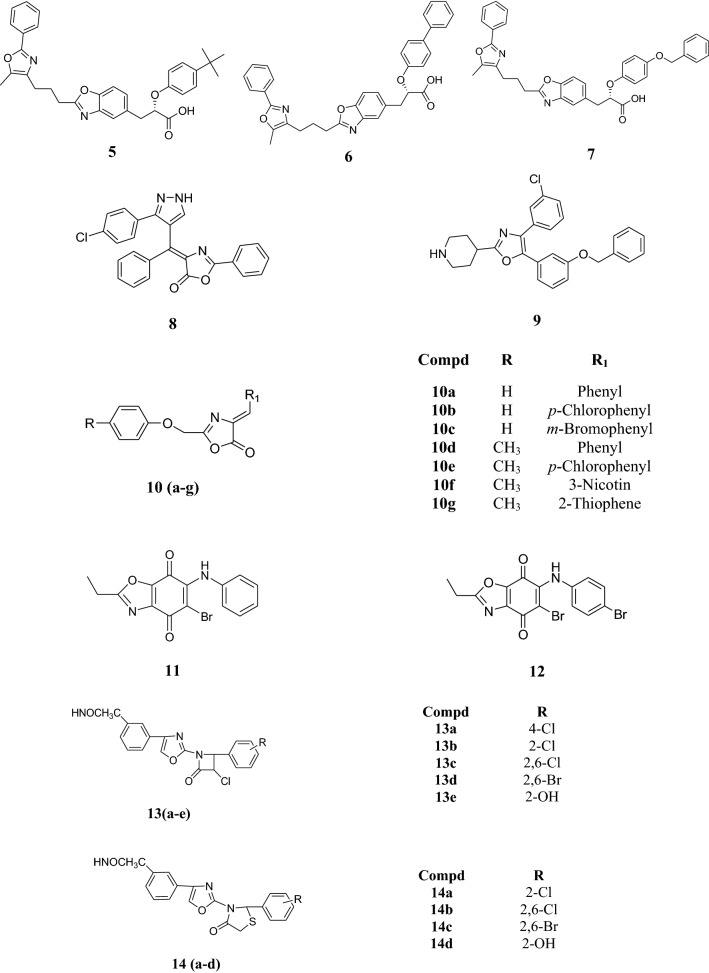
Zhang et al. synthesized a chain of some propanoic acid derivatives and examined them for antibacterial and antifungal potential against various strains using different reference drugs as mentioned in Table 1. Compounds 5, 6 and 7 exhibited most potent antibacterial activities but poor antifungal activity (Table 1) [3].
Table 1.
Minimal inhibition concentration (µg/ml) of compounds 5, 6 and 7
| Compd. | MIC (µg/ml) | ||||
|---|---|---|---|---|---|
| EC | SA | MRSA | BS | CA | |
| 5 | 3.12 | 1.56 | 1.56 | 3.12 | > 200 |
| 6 | 3.12 | 1.56 | 1.56 | 3.12 | > 200 |
| 7 | 6.25 | 1.56 | 1.56 | 1.56 | > 200 |
| Ceftazidime | 200 | 0.78 | 12.5 | 6.25 | – |
| Cefradine | 25 | 25 | 50 | 50 | – |
| Sodium penicillin | 0.78 | 3.12 | 3.12 | < 0.39 | – |
| Ketoconazole | – | – | – | – | < 0.39 |
EC, Escherichia coli; SA, Staphylococcus aureus; MRSA, Methicillin resistant Staphylococcus aureus, BS, Bacillus subtilis; CA, Candida albicans
A series of pyrazole linked to oxazole-5-one moiety was synthesized and assessed for their antimicrobial potential against S. aureus, E. coli, P. aeruginosa and C. albicans. Ampicillin and streptomycin (10 and 25 µg/ml) were used as reference drugs for antibacterial activity and fluconazole, ketaconazole and clotrimazole (10, 20 and 30 µg/ml) were used for antifungal activity. Compound 8 showed highest activity amongst all the synthesized derivatives (Table 2) [13].
Table 2.
Biological activities of compound 8
| Compd. | Conc. | Inhibition zone (mm) for antimicrobial activity | |||
|---|---|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | C. albicans | ||
| 8 | 15 | – | – | – | − |
| 20 | – | – | – | NA | |
| 25 | 9.4 | 7.4 | 8.3 | NA | |
| 30 | 13.7 | 8.5 | 10.6 | − | |
| 45 | NA | NA | NA | +++ | |
| 60 | NA | NA | NA | +++ | |
| Ampicillin | 10 | 10 | – | 08 | − |
| 25 | 18 | 08 | 13 | NA | |
| Streptomycin | 10 | 18 | 06 | 8 | NA |
| 25 | 20 | 18 | 9 | NA | |
| Fluconazole | 10 | NA | NA | NA | − |
| 20 | NA | NA | NA | ++ | |
| 30 | NA | NA | NA | ++ | |
| Ketaconazole | 10 | NA | NA | NA | − |
| 20 | NA | NA | NA | + | |
| 30 | NA | NA | NA | +++ | |
| Clotrimazole | 10 | NA | NA | NA | ++ |
| 20 | NA | NA | NA | +++ | |
| 30 | NA | NA | NA | +++ | |
Tanitame et al. prepared a range of novel pyrazole, oxazole and imidazole derivatives and checked for its antibacterial potential against various strains such as Staphylococcus aureus FDA 209P, S. aureus KMP 9, Escherichia Coli NIHJ JC-2 and, E. coli W3110 ∆acrA. Sparfloxacin and novobiocin have been used as reference drugs. Among the tested oxazole derivatives, compound 9 was found to possess maximum antibacterial activity but was less potent as compared to pyrazole and imidazole derivatives (Table 3) [14].
Table 3.
Minimal inhibition concentration (µg/ml) of compound 9
| Compd. | MIC (µg/ml) | |||
|---|---|---|---|---|
| S. aureus | E. coli | |||
| FDA 209P | KMP 9 | NIHJ JC-2 | W3110 ∆acrA | |
| 9 | 2 | 2 | 64 | 4 |
| Sparfloxacin | 0.125 | 128 | 0.032 | 0.004 |
| Novobiocin | 0.25 | 0.25 | 64 | 0.5 |
Aagalwe et al. carried out the preparation of4-substituted aryl 2–4-disubstituted phenoxy methyl 4-oxazol-5-one derivatives (10) and screened their antibacterial potential against E. coli and Xanthomonas citri using cup-plate method against the standard drug streptomycin. Amongst all the compounds, 10b, 10c, 10e, 10f showed highest activity against E. coli and compounds 10a, 10b, 10c, 10d, 10e, 10g showed highest activity against X. citri (Table 4) [15].
Table 4.
Antibacterial activity data of compound 10
| Compd. | Zone of inhibition (mm) | |
|---|---|---|
| E. coli | X. citri | |
| 10a | 08 | 13 |
| 10b | 12 | 15 |
| 10c | 13 | 12 |
| 10d | 10 | 13 |
| 10e | 12 | 14 |
| 10f | 12 | 08 |
| 10g | 07 | 13 |
| Streptomycin | 12 | 14 |
Ryu et al. performed the synthesis of series of benzo[d]oxazoles and evaluated its antifungal potential against various strains using 5-flourocytosine as a reference drug. The activity of compound 11 and 12 was found to be superior or comparable to reference drug (Table 5) [16].
Table 5.
Antifungal activity of compounds 11 and 12
| Compd. | MIC (µg/ml) | |||||
|---|---|---|---|---|---|---|
| Candida albicans | Candida tropicalis | Candida krusei | Candida neoformans | Aspergillus niger | Aspergillus flavus | |
| 11 | 1.6 | 3.2 | 3.2 | 1.6 | 1.6 | 3.2 |
| 12 | 0.8 | 3.2 | 3.2 | 1.6 | 0.8 | 1.6 |
| 5-Flourocytosine | 3.2 | 3.2 | 3.2 | 3.2 | 1.6 | 1.6 |
Singh et al. carried out the synthesis of substituted oxa/thiazoles and evaluated its antibacterial potential against various bacterial strains using the reference drugs ampicillin and ciprofloxacin. Antibacterial activity of the compound (13) revealed that 13a had good activity against E. coli (20 mm); 13b, 13d and 13e had equipotent activity as standard compound and 13c exhibited good antibacterial potential. In case of antibacterial activity of compound 14, the derivatives 14a, 14c, 14d showed good antibacterial activity and 14b exhibited better antibacterial activity than standard drugs. Results are presented in Table 6 [17].
Table 6.
Bacterial growth inhibition of compounds 13 and 14
| Compd. | Bacterial growth inhibition (diameter in mm) | |||
|---|---|---|---|---|
| S. aureus | E. coli | P. vulgaris | K. pneumonia | |
| 13a | – | 20 | – | – |
| 13b | 19 | – | – | – |
| 13c | 23 | – | 22 | – |
| 13d | – | – | – | 21 |
| 13e | 19 | 21 | – | – |
| 14a | – | 20 | – | 21 |
| 14b | 25 | – | – | 23 |
| 14c | – | – | 22 | – |
| 14d | 20 | – | – | 21 |
| Ampicillin | 20 | 18 | 18 | 15 |
| Ciprofloxacin | 20 | 22 | 20 | 21 |
Kamble et al. synthesized various oxazole-2-amine and its analogues and used S. aureus and E. coli for examining their antibacterial activity using amoxicillin as standard drug. The compounds, (E)-4-(benzofuran-2-yl)-N-benzylideneoxazol-2-amine (15) and (E)-N-(4-nitrobenzylidene)-4-(benzofuran-2-yl)oxazol-2-amine (16) showed appreciable activity as compared to standard drug (Table 7) [18].
Table 7.
Antibacterial activity data of compounds 15 and 16
| Compd. | Bacterial growth inhibition in mm | |
|---|---|---|
| S. aureus | E. coli | |
| 15 | 20 | 17 |
| 16 | 18 | 15 |
| Amoxicillin | 30 | 27 |
Benzoxazole-5-carboxylatederivatives were prepared and their antimicrobial activity was evaluated by Chilumula et al. against Gram positive and Gram negative bacterial (S. typhi, E. coli, S. aureus and B. subtilis) and fungal strains (C. albicans and A. niger). The results were evaluated using ampicillin and clotrimazole as a reference drugs for antimicrobial activity. Compound 17 showed the highest activity whereas compound 18 had much higher potency than other tested compounds. Results are mentioned in Table 8 [19].
Table 8.
Antimicrobial activity data of compounds 17 and 18
| Compd. | Inhibition zone in mm | |||||
|---|---|---|---|---|---|---|
| BS | SA | EC | ST | CA | AN | |
| 17 | 23 | 21 | 20 | 18 | 28 | 20 |
| 18 | 24 | 22 | 21 | 20 | 30 | 21 |
| Ampicillin | 22 | 20 | 18 | 17 | – | – |
| Clotrimazole | – | – | – | – | 27 | 19 |
BS, Bacillus subtilis; SA, Staphylococcus aureus; EC, Escherichia coli; ST, Salmonella typhi; CA, Candida albicans; AN, Aspergillus niger
Synthesis of series of heterocyclic derivatives and its antibacterial potential against various organisms such as B. subtilis, S. aureus, E. coli and K. pneumonia using standard drug ampicillin was done by Kaspady et al. 2-tert-Butyl-4-(4-chlorophenyl)oxazole (19) and 4-(4-bromophenyl)-2-tert-butyloxazole (20) were found to be the most active compounds (Table 9) [20].
Table 9.
Zone of inhibition in mm of compound 19 and 20
| Compd. | B. subtilis | S. aureus | E. coli | K. pneumonia |
|---|---|---|---|---|
| 19 | *** | *** | ** | ** |
| 20 | *** | *** | *** | ** |
| Ampicillin | ***** | ***** | ***** | ***** |
* Less than 12 mm; **12–15 mm; ***15–21 mm; ****21–27 mm; *****> 27 mm
Shamsuzzaman et al. synthesized a series of 2ˈ-amino-5α-cholest-6-eno [6,5-d] oxazole derivatives (21). Disk diffusion assay was used to examine the antimicrobial activity using various bacterial and fungal strains against chloramphenicol and nystatin which were used as reference drugs for the study. Out of all the compounds, 21b was found to be the most active one. Results are presented in Tables 10 and 11 [21].
Table 10.
Antifungal activity of synthesized derivatives
| Compd. | Inhibition zone (mm) at 100 µg/ml | ||||
|---|---|---|---|---|---|
| Ca | Cg | Psp | Fo | An | |
| 21a | 20.1 ± 0.2 | 10.1 ± 0.2 | 15.1 ± 0.2 | 12.1 ± 0.2 | 11.2 ± 0.5 |
| 21b | 21.5 ± 0.5 | 15.2 ± 0.5 | 16.2 ± 0.5 | 13.1 ± 0.5 | 12.5 ± 0.2 |
| 21c | 19.1 ± 0.5 | 09.2 ± 0.2 | 14.5 ± 0.2 | 10.1 ± 0.2 | 10.1 ± 0.5 |
| Nystatin | 29.0 ± 0.5 | 29.0 ± 0.5 | 24.5 ± 0.5 | 19.5 ± 0.5 | 19.5 ± 0.5 |
Ca, Candida albicans; Cg, Candida glabrata; Psp, Penicillium spp.; Fo, Fusarium oxyporium; An, Aspergillus niger
Table 11.
Antibacterial activity of synthesized derivatives
| Compd. | Inhibition zone (mm) at 100 µg/ml | |||||
|---|---|---|---|---|---|---|
| Bs | Sp | Sa | Pa | St | Ec | |
| 21a | 32 | 128 | 128 | 64 | 128 | 128 |
| 21b | 64 | 128 | 64 | 64 | 64 | 128 |
| 21c | 128 | 256 | 128 | 64 | 128 | 256 |
| Chloramphenicol | 32 | 32 | 32 | 32 | 32 | 32 |
Bs, Bacillus subtilis; Sp, Streptococcus pyogenes; Sa, Staphylococcus aureus; Pa, Pseudomonas aeruginosa; Ec, Escherichia coli; St, Salmonella typhimurium
Tomi et al. synthesized new derivatives of five membered heterocyclic compounds containing oxazole and benzothiazole rings and then screened them for their antimicrobial activity using ofloxacin and ketoconazole as standard drugs. Amongst the tested oxazole derivatives (22), three compounds, 22a, 22b, 22c came out to be active against bacterial and fungal strains (Table 12) [22].
Table 12.
Antimicrobial activity of oxazole derivatives
| Compd. | N | Inhibition zone in mm | ||||
|---|---|---|---|---|---|---|
| E. coli | S. aureus | P. aeruginosa | A. niger | C. albicans | ||
| 22a | 4 | 12 | 9 | 11 | 10 | 12 |
| 22b | 7 | 11 | 8 | 11 | 9 | 16 |
| 22c | 8 | 12 | 9 | 13 | 11 | 13 |
| Ofloxacin | – | 17 | 16 | 16 | – | – |
| Ketoconazole | – | – | – | – | 20 | 30 |
A chain of 1,3-oxazole derivatives was prepared and examined for microbial inhibition potential against various bacterial and fungal strains by Sadek et al. Ofloxacin and ketoconazole were used as reference drugs for antimicrobial study. The 1,3oxazole derivative (23) showed notable activity at higher concentration (200 µg/ml) (Table 13) [23].
Table 13.
Antimicrobial activity of compound 23
| Compd. | MIC in µg/ml | ||
|---|---|---|---|
| S. aureus | E. coli | A. niger | |
| 23 | 200 | 200 | 200 |
| Ofloxacin | 10 | 12.5 | – |
| Ketoconazole | – | – | 12.5 |
Synthesis of a number of multi-substituted oxazoles containing a heterocyclic moiety was carried out and checked for antibacterial activity by Babulreddy et al. against different bacterial strains (S. aureus, E. coli, B. subtilis, K. pneumonia). Ampicillin was used as reference drug for antibacterial activity. Out of all the derivatives investigated, 24, 25, 26 and 27 showed pronounced antibacterial activity whose results are mentioned in Table 14 [24].
Table 14.
Antibacterial activity of multi-substituted oxazoles
| Compd. | Inhibition zone (MIC in µg/ml) | |||
|---|---|---|---|---|
| B. subtilis | S. aureus | E. coli | K. pneumonia | |
| 24 | +++ (258) | ++++ (294) | +++ (276) | +++ (266) |
| 25 | ++++ (264) | ++++ (298) | +++ (254) | ++ (277) |
| 26 | ++++ (255) | ++++ (312) | +++ (284) | ++++ (291) |
| 27 | ++++ (310) | ++++ (285) | ++++ (289) | ++++ (273) |
| Ampicillin | +++++ (3.28) | +++++ (3.36) | +++++ (3.88) | +++++ (4.00) |
Dabholkar et al. carried out the synthesis of 2, 4-disubstituted oxazoles and checked their antibacterial activity against Gram negative bacteria, E. coli and P. aeruginosa and Gram-positive bacteria S. aureus and C. diphtheriae. Ampicillin trihydrate was the standard drug used and inhibition zone was measured in mm. Compound 28 showed convincing activity against the various bacterial strains. Results are presented in Table 15 [25].
Table 15.
Antibacterial activity of compounds 28a and 28b
| Compd. | Zone of inhibition (in mm) | |||
|---|---|---|---|---|
| S. aureus | C. diphtheriae | P. aeruginosa | E. coli | |
| 28a | 13 | 16 | 18 | 14 |
| 28b | 14 | 18 | 18 | 15 |
| Ampicillin trihydrate | 26 | 28 | 24 | 21 |
Some new aryl oxazoles were prepared by Dawood et al. and then assessed its antimicrobial potential. Reference drugs used were chloramphenicol and fluconazole. Compound 29 was found to have the highest antibacterial and antifungal activity (Table 16) [26].
Table 16.
Minimum inhibitory concentration of compound 29
| Compd. | MIC in µg/ml | |||||||
|---|---|---|---|---|---|---|---|---|
| E.c | S.a | B.s | P.a | S.r | A.f | C.a | G.c | |
| 29 | 250 | 31.25 | 125 | 62.5 | 125 | 31.25 | 62.5 | 62.5 |
| Chloramphenicol | 15.60 | 31.25 | 31.25 | 31.25 | – | – | – | – |
| Fluconazole | – | – | – | – | 250 | 125 | 250 | 250 |
E.c, Escherichia coli; S.a, Staphylococcus aureus; B.s, Bacillissubtillis; P.a, Pseudomonas aeruginosa; S.r, Syncephalastrumracemosum; A.f, Aspergillusfumigatus; C.a, Candidaalbicans; G.c, Geotrichumcandidum
Synthesis of a chain of oxazole derivatives was done by Singh et al. and were checked for its antimicrobial potential and compared with reference drugs ciprofloxacin, gatifloxacin, fluconazole. Among the tested compounds, 3-(2-(4-methoxybenzylideneamino)oxazol-4-ylamino)-2H-chromen-2-one (30) showed potent antibacterial activity, 3-(2-(2-hydroxybenzylideneamino)oxazol-4-ylamino)-2H-chromen-2-one (31) exhibited moderate antifungal activity, 3-chloro-4-(4-methoxyphenyl)-1-(4-(2-oxo-2H-chromen-3-ylamino)oxazol-2-yl)azetidin-2-one (32) showed potent antibacterial activity, and 3-chloro-4-(2-hydroxyphenyl)-1-(4-(2-oxo-2H-chromen-3-ylamino)oxazol-2-yl)azetidin-2-one (33) exhibited most potent antifungal activity. Results are mentioned in Table 17 [27].
Table 17.
Antimicrobial activity of compounds 30, 31, 32 and 33
| Compd. | Bacterial growth inhibition (mm) | Fungal growth inhibition (mm) | |||
|---|---|---|---|---|---|
| S. aureus | E. coli | P. vulgaris | K. pneumoniae | C. albicans | |
| 30 | 19 | 22 | 16 | 20 | 8 |
| 31 | 14 | – | 12 | 18 | 16 |
| 32 | 28 | 30 | 21 | 22 | – |
| 33 | – | 9 | – | – | 30 |
| Ciprofloxacin | 20 | 22 | 20 | 20 | – |
| Gatifloxacin | 25 | 22 | 20 | 20 | – |
| Fluconazole | – | – | – | – | 29 |
Taile et al. prepared a series of oxazol-5-ones and screened its antibacterial potential against various pathogenic bacteria using ciprofloxacin and sulphacetamide as reference drugs. The prepared derivatives were also examined for their antifungal potential against Aspergillus niger and Candida albicans. The zone of inhibition was checked in comparison with gentamycin and clotrimazole. Compounds 34 and 35 exhibited good antibacterial activity whereas the compounds 36 and 37 showed good antifungal activity. Results are given in Table 18 [28].
Table 18.
Antimicrobial activity of compounds 34, 35, 36 and 37
| Compd. | Diameter of Bacterial growth inhibition | Diameter of Fungal growth inhibition | ||||
|---|---|---|---|---|---|---|
| SA | BS | EC | KA | CA | AN | |
| 34 | 29 | 28 | 24 | 18 | 16 | 24 |
| 35 | 30 | 26 | 29 | 22 | 17 | 17 |
| 36 | 19 | 24 | 16 | 17 | 21 | 22 |
| 37 | 23 | 15 | 23 | 19 | 22 | 21 |
| Ciprofloxacin | 34 | 29 | 35 | 22 | – | – |
| Sulphacetamide | 31 | 26 | 29 | 21 | – | – |
| Gentamycin | – | – | – | – | 21 | 25 |
| Clotrimazole | – | – | – | – | 23 | 24 |
SA, Staphylococcus aureus; BS, Bacillus subtilis; EC, Escherichia coli; KA, Klebsiella aerogenes; CA, Candida albicans; AN, Aspergillus niger
Prasad et al. carried out the synthesis of compounds 38 and 39 and evaluated their antimicrobial activity by disk diffusion method against various bacterial strains using ciprofloxacin and ketoconazole as reference drugs. Both the derivatives exhibited good antimicrobial activity and the results are presented in Table 19 [29].
Table 19.
Antimicrobial data of the compounds 38 and 39
| Compd. | Zone of inhibition (mm) by disk diffusion method | |||||
|---|---|---|---|---|---|---|
| SA | BC | EC | PA | AN | AF | |
| 38 | 24 | 25 | 28 | 27 | 27 | 27 |
| 39 | 25 | 24 | 24 | 28 | 24 | 25 |
| Ciprofloxacin | 38 | 39 | 40 | 40 | – | – |
| Ketoconazole | – | – | – | – | 40 | 39 |
SA, Staphylococcus aureus; BC, Bacillus cereus, PA, Pseudomonas aeruginosa; EC, Escherichia coli; AN, Asperigillusniger; AF, Aspergillus fumigates
Various oxazole derivatives were prepared and assessed for their antimicrobial potential by Patel et al. against various Gram positive (S. aureus and S. pyogenes), Gram negative (P. aeruginosa and E. coli) and fungal strains (C. albicans, A. niger and A. clavatus). Ampicillin, chloramphenicol, ciprofloxacin, nystatin and griseofulvin have been used as reference drugs. Compound 40 was found to be most potent antibacterial agent whereas compound 41 was the most potent antifungal agent (Table 20) [30].
Table 20.
Minimum inhibitory concentration for compounds 40 and 41
| Compd. | MIC in µg/ml | ||||||
|---|---|---|---|---|---|---|---|
| Ec | Pa | Sa | Sp | An | Af | Ac | |
| 40 | 50 | 100 | 50 | 250 | 1000 | > 1000 | > 1000 |
| 41 | 200 | 500 | 200 | 200 | 500 | 500 | 500 |
| Ampicillin | 100 | 100 | 250 | 100 | – | – | – |
| Chloramphenicol | 50 | 50 | 50 | 50 | – | – | – |
| Ciprofloxacin | 25 | 25 | 50 | 50 | – | – | – |
| Nystatin | – | – | – | – | 100 | 100 | 100 |
| Griseofulvin | – | – | – | – | 500 | 100 | 100 |
Ec, Escherichia Coli; Pa, Pseudomonas aeruginosa; Sa, Staphylococcus aureus; Sp, Streptococcus pyogenes; Ca, Candida albicans; An, Aspergillus niger; Ac, Aspergillus clavatus
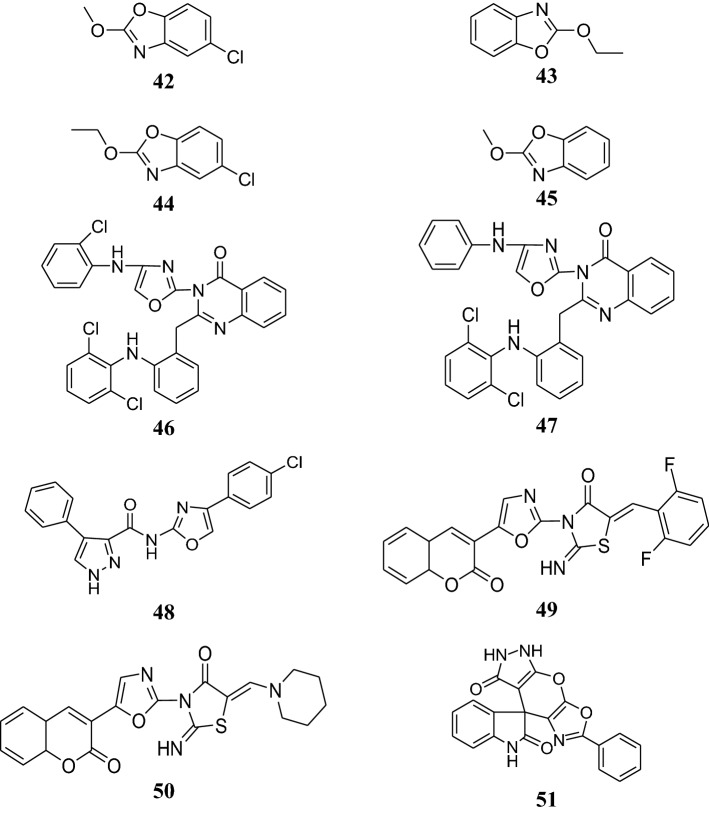
Anand et al. synthesized various substituted benzoxazoles and evaluated their antimicrobial potential against S. aureus, E. coli, C. albicans and C. glabrata using trimethoprim and miconazole as standard drug. Among the investigated compounds, 2-methoxy-5-chlorobenzo[d]oxazole (42) and 2-ethoxybenzo[d]oxazole (43) had excellent antibacterial activity whereas 2-ethoxy-5-chlorobenzo[d]oxazole (44) and 2-methoxybenzo[d]oxazole (45) had excellent antifungal activity (Table 21) [31].
Table 21.
Antimicrobial activity of compounds 42, 43, 44 and 45
| Compd. | Zone of inhibition (mm) | |||
|---|---|---|---|---|
| SA | EC | CA | CG | |
| 42 | 18 | 16 | 19 | 16 |
| 43 | 18 | 15 | 14 | 16 |
| 44 | 17 | 14 | 19 | 18 |
| 45 | 16 | 15 | 18 | 20 |
| Trimethoprim | 25 | 23 | – | – |
| Miconazole | – | – | 26 | 15 |
SA, Staphylococcus aureus; EC, Escherichia coli; CA, Candida albicans; CG, Candida glabrata
Patel et al. synthesized a series of 2-[2-(2,6-dichloro-phenylamino)-phenyl methyl]-3-{4-[(substituted phenyl) amino]-1,3-oxazol-2-yl-}quinazolin-4(3H)ones and examined its antibacterial potential against S. aureus and S. pyogenes, P. aeruginosa and E. coli and C. albicans, A. niger and A. clavatus using chloramphenicol, gentamycin, ampicillin, ciprofloxacin and norfloxacin as reference drugs for antibacterial activity and nystatin and griseofulvin for antifungal activity. 2-(2-(2,6-Dichlorophenylamino)benzyl)-3-(4-(2-chlorophenylamino)oxazol-2-yl)quinazolin-4(3H)-one (46) was found to possess good activity against all the bacterial strains and Candida albicans but not against Aspergillus niger and Aspergillus clavatus whereas 2-(2-(2,6-dichlorophenylamino)benzyl)-3-(4-(phenylamino)oxazol-2-yl)quinazolin-4(3H)-one (47) was found to be active against Aspergillus niger and Aspergillus clavatus. Results of antimicrobial study are shown in Table 22 [32].
Table 22.
Antimicrobial activities of the compounds 46 and 47
| Compd. | MIC (µg/ml) | ||||||
|---|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | S. pyogenes | C. albicans | A. niger | A. clavatus | |
| 46 | 100 | 100 | 100 | 100 | 500 | 1000 | 500 |
| 47 | 100 | 1000 | 1000 | 500 | 100 | 100 | 100 |
| Gen | 0.05 | 1 | 0.25 | 0.5 | – | – | – |
| Amp | 100 | 100 | 250 | 100 | – | – | – |
| Chlorl | 50 | 50 | 50 | 50 | – | – | – |
| Cipro | 25 | 25 | 50 | 50 | – | – | – |
| Nor | 10 | 10 | 10 | 10 | – | – | – |
| Nys | – | – | – | – | 100 | 100 | 100 |
| Gri | – | – | – | – | 500 | 100 | 100 |
Gen Gentamycin, Amp Ampicillin, Chlor Chloramphenicol, Cipro Ciprofloxacin, Nor Norfloxacin, Nys Nystatin, Gri Griseofulvin
Padmavathi et al. synthesized a new class of amido linked bis heterocycles and checked them for antibacterial and antifungal activity against S. aureus, B. subtilis, P. aeruginosa, K. pneumonia, A. niger and P. chrysogenum using chloramphenicol and ketoconazole as standard drugs. Among the prepared oxazole derivatives, 48 was found to possess most effective antimicrobial activity at 100 µg/ml (Table 23) [33].
Table 23.
Antibacterial and antifungal potential of the compound 48
| Compd. | Inhibition zone in mm | |||||
|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | P. aeruginosa | K. pneumoniae | A. niger | P. chrysogenum | |
| 48 | 23 | 22 | 21 | 24 | 27 | 29 |
| Std. | 35* | 38* | 30* | 42* | – | – |
| Std | – | – | – | – | 36** | 38** |
Std. Chloramphenicol*; Ketoconazole**
A series of new oxazole derivatives were prepared and assayed for their antibacterial activity against Gram-positive bacteria and Gram-negative bacteria by Reddy et al. using penicillin and streptomycin as reference drugs. The compounds 49 and 50 were found to possess good antibacterial activity as compared to standard drugs. Results are shown in Table 24 [34].
Table 24.
Antibacterial activity of the compound 49 and 50
| Compd. | Minimum inhibitory concentration in µg/ml | |||||
|---|---|---|---|---|---|---|
| BS | BSph | SA | PA | KA | CV | |
| 49 | 7 ± 0.7 | 8 ± 0.4 | 10 ± 0.4 | 8 ± 0.4 | 8 ± 0.5 | 16 ± 0.3 |
| 50 | 8 ± 0.4 | 8 ± 0.4 | 9 ± 0.4 | 10 ± 0.4 | 12 ± 0.8 | 20 ± 0.8 |
| Penicillin | 10 ± 0.5 | 19 ± 0.8 | 16 ± 0.8 | 18 ± 0.5 | 20 ± 1.0 | 18 ± 0.3 |
| Streptomycin | 10 ± 0.6 | 14 ± 0.9 | 14 ± 1.1 | 18 ± 1.0 | 20 ± 0.8 | 16 ± 1.2 |
BS, Bacillus subtilis; BSph, Bacillus sphaericus; SA, Staphylococcus aureus; PA, Pseudomonas aeruginosa; KA, Klebsiella aerogenes; CV, Chromobacterium violaceum
Several new spiroindoline-based heterocycles were made by Rahman et al. and examined for their antimicrobial potential. Among the tested derivatives, compound 51 was found to be the most effective against Bacillus subtilis, Bacillus megatherium, E. coli, Aspergillus niger and Aspergillus oryzae. Ampicillin, chloramphenicol and fluconazole were used as reference drugs (Table 25) [35].
Table 25.
Inhibition zone (in mm) of new spiroindoline-based heterocycles
| Compd. | Inhibition zone (in mm) | ||||
|---|---|---|---|---|---|
| B. subtilis | B. megatherium | E. coli | A. niger | A. oryzae | |
| 51 | 87 | 86 | 45 | 80 | 86 |
| Ampicillin | 41 | 29 | 26 | 33 | – |
| Chloramphenicol | 28 | 55 | 48 | 35 | – |
| Fluconazole | – | – | – | 22 | 16 |
The structures of the most active antimicrobial compounds (5–51) are shown in Figs. 2, 3, 4, 5.
Fig. 2.
Structures of the most active antimicrobial compounds
Fig. 3.
Structures of the most active antimicrobial compounds
Fig. 4.
Structures of the most active antimicrobial compounds
Fig. 5.
Structures of the most active antimicrobial compounds
Anticancer activity
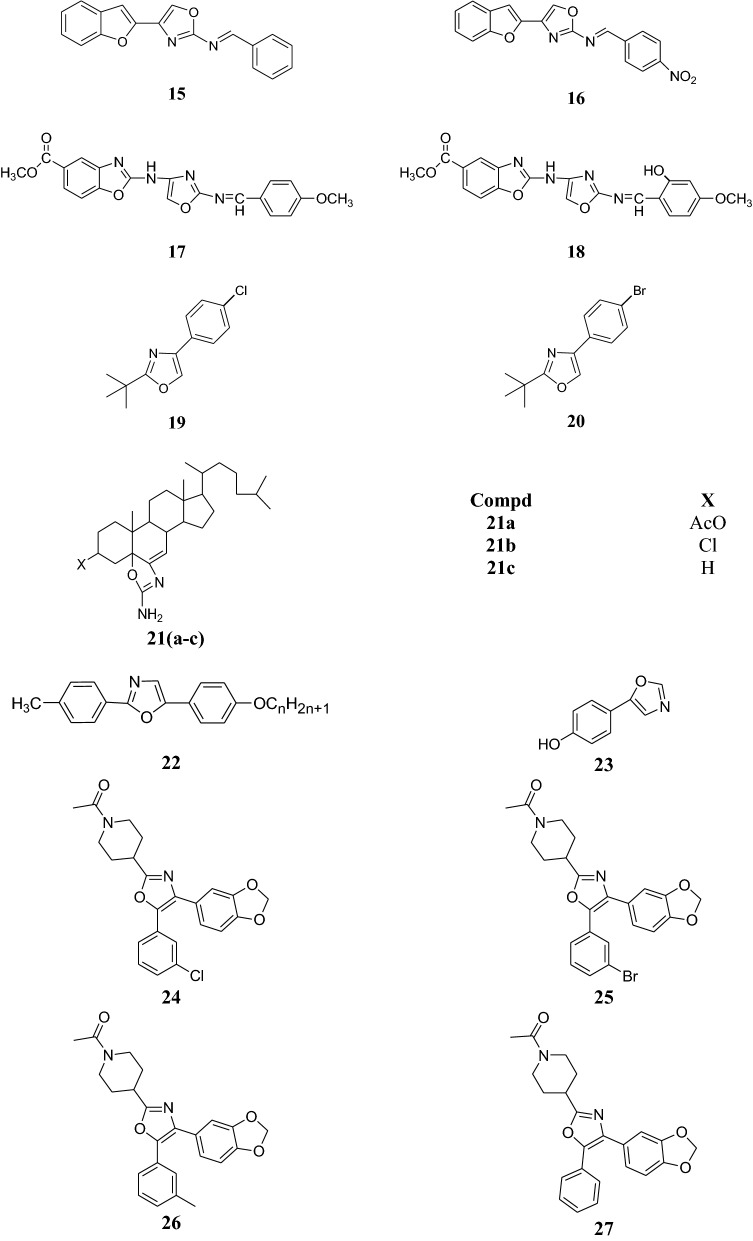
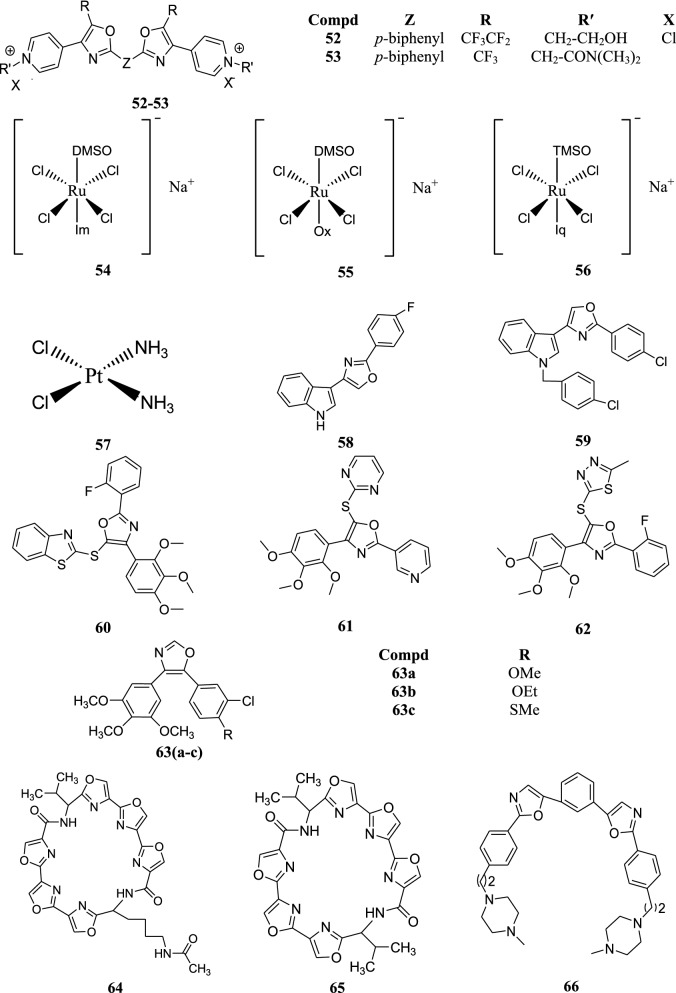
Cantalejo et al. synthesized bisoxazoles and evaluated their anticancer activity against the cancer cell line HT-29. As well as tested in an ex vivo system using recombinant human choline kinase (ChoK) to assess the inhibitory potency of the derivatives towards ChoK. Compound 52 was found to possess the maximum antiproliferative activity with an IC50 value of 0.84 ± 0.005 whereas compound 53 was found to be most active in case of ex vivo study (IC50 = 0.30 ± 0.003) [36].
The molecular interactions of three ruthenium complexes were studied by Barca et al. in isolated mammalian nuclei. The complexes were chemotherapeutic agents that are effective in reducing metastatic tumours in vivo and were compared with antitumour drug cis-diamminedichloroplatinum (CDDP) (57). Na trans-RuCl4 (DMSO) imidazole (NAMI) (54), Na trans-RuCl4 (DMSO) oxazole (NAOX) (55) and Na trans-RuCl4(TMSO) isoquinoline (TEQU) (56) were the complexes under investigation. The Ru complexes were screened for toxicity on V79 cells which showed that NAMI and NAOX did not reduce the cloning efficiency, only TEQU reduced the cloning efficiency as well as induced a number of mutants in V79 cells in culture [37].
Kumar et al. carried out the synthesis of a series of oxazole derivatives and evaluated its antitumour activity using various cell lines. Among all the screened derivatives, compounds 58 and 59 were found to have potent cytotoxic action against tested cell lines (Table 26) [4].
Table 26.
Cytotoxicity profile of compounds 58 and 59
| Compd. | Cancer cell lines | |||||
|---|---|---|---|---|---|---|
| PC3 | DU145 | LnCaP | MCF7 | MDA231 | PaCa2 | |
| 58 | 42.8 | 31.8 | 59.8 | 28 | 90.4 | 40.6 |
| 59 | 349.8 | 80.5 | 181.6 | 14.1 | 216.3 | 26 |
Liu et al. carried out the preparation of various trisubstituted oxazole derivatives and checked their antitumour potential against two cancer cells, PC-3 (human prostate cancer) and A431(human epidermoid carcinoma)using 5-flourouracil as reference. Among the investigated compounds, 60, 61 and 62 were the most effective (Table 27) [38].
Table 27.
Antiproliferative potential of the synthesized derivatives
| Compd. | IC50 (µM) | |
|---|---|---|
| PC-3 | A431 | |
| 60 | 0.0030 | 0.0031 |
| 61 | 0.0047 | 0.0076 |
| 62 | 0.0035 | 0.0026 |
| 5 Flouro-uracil | 0.016 | 0.018 |
Mahal et al. studied the antitumoral properties of a metabolite of the South-African bush willow Combretum caffrum, cis-stilbene combretastatin A-4 (CA-4). However the conversion of CA-4 into the trans-isomer and its poor solubility limits its use in anticancer therapy. In order to overcome these drawbacks different heterocycles were integrated with CA-4 which led to the formation of CA-4 analogues having imidazole and oxazole rings. The halogen substituted oxazoles showed enhanced anticancer activity and showed antivascular activity as well. Different cell lines used were human HT-29 colon carcinoma, human 518A2 melanoma and Ea.hy926 endothelial hybrid cells. The oxazole derivatives 63 (a–c) were found to be active whose IC50 values are given in Table 28 [39].
Table 28.
Cytotoxicity profile of compound 63
| Compd. | IC50 (nM) | ||
|---|---|---|---|
| HT-29 | 518A2 | Ea.hy926 | |
| 63a | 6 ± 1 | 3 ± 2 | 9 ± 1 |
| 63b | 11 ± 1 | 2 ± 1 | 31 ± 3 |
| 63c | 76 ± 3 | 50 ± 15 | 77 ± 4 |
Pilch et al. characterized two synthetic hexaoxazole-containing macrocyclic compounds, HXLV-AC (64) and HXDV (65) and evaluated its antiproliferative potential against various cell lines. Cytotoxicity was evaluated using MTT assay and the IC50 values are shown in Table 29 [40].
Table 29.
Cytotoxicity of HXDV and HXLV-AC
| Compd. | IC50 (µM) | |
|---|---|---|
| RPMI 8402 | KB3-1 | |
| HXLV-AC | 0.8 ± 0.3 | 0.9 ± 0.2 |
| HXDV | 0.4 ± 0.1 | 0.4 ± 0.1 |
Ohnmacht et al. reported some bisoxazole derivatives and evaluated them for anticancer potential. The analogue 66 was found to be the most effective in the series having high selectivity for the HSP90A over HSP90B quadruplexes. The compound 66 was evaluated for anticancer activity against various cell lines and the IC50 values are mentioned in Table 30 [41].
Table 30.
Cytotoxicity of compound 66
| Cancer cell lines | IC50in µmol |
|---|---|
| A549 | 1.02 |
| MCF7 | 1.32 |
| RCC4 | 0.94 |
| 786-o | 1.33 |
| Mia-Pa-Ca2 | 1.25 |
| W138 | 2.59 |
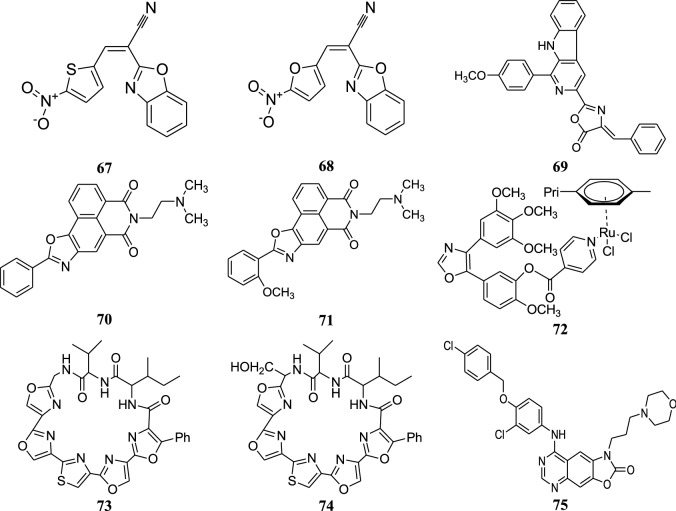
Various new oxazole derivatives were synthesized and examined for their antitumour activity by Sączewski et al. Among the synthesized derivatives, compounds 67 and 68 were evaluated against a number of different cell lines using nitrofurantoin, cisplatin, melphalan and thiotepa as reference drugs and the results are mentioned in Table 31 [42].
Table 31.
IC50 values (µM) in human cancer cell lines
| Compd. | RT-4 | RT-112 | 5637 | KYSE-70 | KYSE-510 | DAN-G | SISO | LCLC-103H | MCF-7 | A-427 |
|---|---|---|---|---|---|---|---|---|---|---|
| 67 | 6.57 | 3.88 | 3.91 | 5.30 | 22.63 | 12.62 | 14.12 | 12.06 | 5.69 | 2.33 |
| 68 | 3.98 | 1.41 | 1.65 | 2.91 | 7.00 | 3.00 | 2.86 | 1.33 | 2.87 | 1.13 |
| NTF | 7.00 | Nf | 21.3 | 22.8 | 29.0 | 6.74 | 7.27 | 2.34 | 4.44 | 1.86 |
| CP | 1.61 | 1.22 | 0.35 | 0.63 | 0.44 | 0.73 | 0.24 | 0.90 | 1.38 | 1.96 |
| Mph | 14.25 | 4.69 | 0.31 | 16.16 | 8.18 | 2.65 | 1.00 | 4.00 | 3.71 | 5.13 |
| Ttp | 18.27 | 3.40 | 2.0 | 5.40 | 4.31 | 1.66 | 1.40 | 6.97 | 3.23 | 1.58 |
nf not found, NTF Nitrofurantoin, CP Cisplatin, Mph Melphalan, Ttp Thiotepa
Savariz et al. prepared a range ofoxazol-5-one derivatives and carried out the in vitro antitumor evaluation. Doxorubicin was used as a positive control. Among all the synthesized compounds, 69 was found to possess maximum activity against prostate (PC-3) and ovarian (OVCAR-03) cancer cell lines with IC50values of 1.50 and 1.07 µM respectively [43].
Three series of novel oxo-heterocyclic fused naphthalimide derivatives were made by Tan et al. and were evaluated for antiproliferative potential using various tumor cell lines. Among the synthesized oxazole derivatives, 70 and 71 were found to be the most active ones (Table 32) [44].
Table 32.
IC50 (µM) of active compounds 70 and 71
| Compd. | A549 (Human lung cancer cell) | P388 (Murine Leukemia Cell) | LO2 (Human Liver Cell) |
|---|---|---|---|
| 70 | 0.53 | 2.50 | 3.0 |
| 71 | 0.89 | 1.30 | 1.9 |
| Amonafide | 1.10 | 0.20 | 5.0 |
Biersack et al. reported that oxazole-linked combretastatin A-4 analogues (possessing anti-vascular and anti-angiogenic activity) when linked to Ru(η6-arene) complex fragments shows additional cytotoxic activity. MTT tests with the oxazoles and their ruthenium complexes revealed them to be effective against cells of human518A2 melanoma and HL-60 leukaemia. Compound 72 showed the highest activity [45].
Hernández et al. did the synthesis of several analogues of the cytotoxic thiopeptide IB-01211 or mechercharmycin A. The cytotoxicity of synthesized analogues was checked against three human tumour cell lines. The peptide heterocycles 73 and 74 were found to be the most active ones (Table 33) [46].
Table 33.
In vitro cytotoxicity of peptide derivatives
| Compd. | Cytotoxicity (GI50, µM) | ||
|---|---|---|---|
| A-549 lung carcinoma NSCL | HT-29 colon carcinoma | MDA-MB-231 231breast adenocarcinoma | |
| 73 | 0.17 | 0.12 | 0.10 |
| 74 | 0.12 | 0.13 | 0.12 |
A series of oxazole derivatives were prepared by Lin et al. and the EGFR and Src inhibition activities were checked using gefitinib as reference compound. In vitro cell cytotoxicity of the synthesized derivatives was evaluated against KB and A498 cells using MTT assay. Among all the screened compounds, 75 was found to be the most effective with IC50values 0.82 and 3.0 µM against KB and A498 cells respectively [47].
The structures of the most active anticancer compounds (52–75) are shown in Fig. 6, 7.
Fig. 6.
Structures of the most active anticancer compounds
Fig. 7.
Structures of the most active anticancer compounds
Antitubercular activity
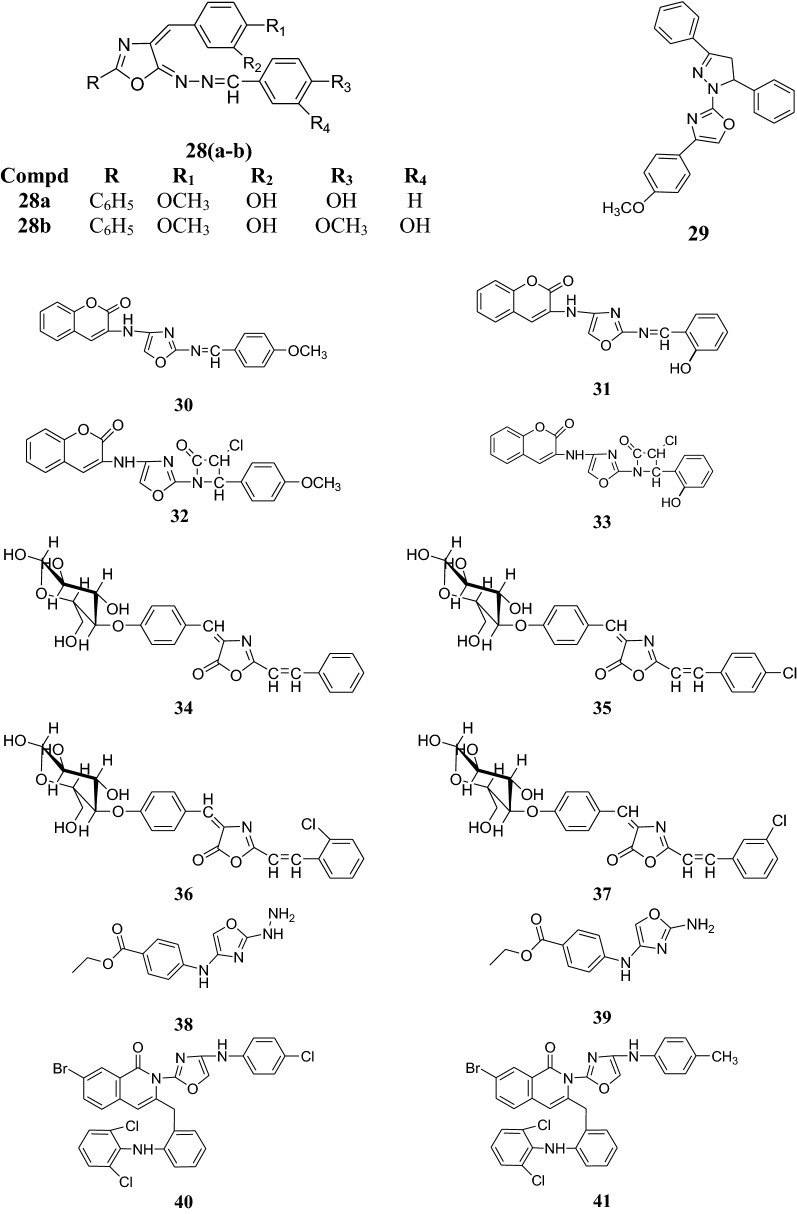
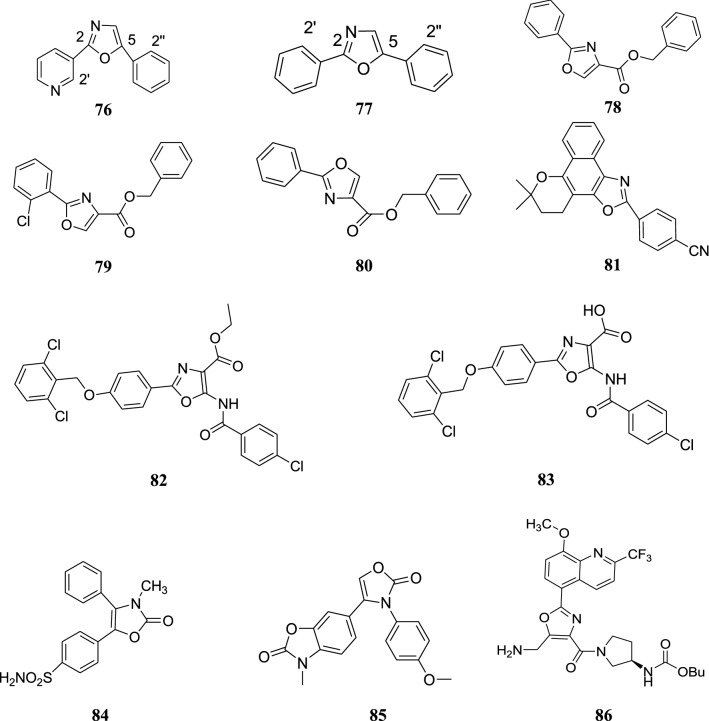
Texaline is an antitubercular oxazole-containing alkaloid which is obtained from Amyris texana and Amyris elemifera. Several analogues of it, namely 2-(3´-pyridyl)-5-phenyloxazole (76) and 2,5-diphenyloxazole (77) were synthesized and checked for their antimycobacterial activity by Giddens et al. Both the compounds were found to be effective antitubercular agents. Results are shown in Table 34 [48].
Table 34.
Antimycobacterial activity of compounds 76 and 77
| Compd. | MIC (µg/ml) for M. tuberculosis H37Rv | |
|---|---|---|
| MABA | Microbroth | |
| 76 | 30.1 | 31.25 |
| 77 | 29.0 | 31.25 |
Moraski et al. carried out the synthesis of several oxazoline- and oxazole-containing compounds, which were tested for inhibition of Mycobacterium tuberculosis H37Rv in two different culture media, GAS and GAST using rifampicin as a positive control. Tween 80 is present in GAST but not in GAS whereas GAST is more iron deficient medium than GAS. Among all the synthesized oxazole derivatives, 78 and 79 were found to be most potent against MtbH37Rv whose results are presented in Table 35 [5].
Table 35.
Anti tubercular activity of compound 78 and 79
| Compd. | MIC for M. tuberculosis H37Rv | |
|---|---|---|
| GAS (µM) | GAST (µM) | |
| 78 | 0.47 | 0.49 |
| 79 | 0.73 | 1.69 |
Moraski et al. reported various classes of compound sand their antitubercular potential was evaluated against MtbH37Rv. Among the investigated oxazole derivatives, benzyl 2-phenyloxazole-4-carboxylate (80) was found to possess the highest activity against MtbH37Rv with MIC value of 5.7 ± 2.3 µM [49].
Moura et al. synthesized a number of naphthoimidazoles and naphthoxazoles and evaluated them against susceptible and rifampicin- and isoniazid-resistant strains of M. tuberculosis. The study was carried out using M. tuberculosis H37Rv, RIFr with a His-526 → Tir mutation in the rpoB gene and INHR with a Ser-315 → Tir mutation in the katG gene. Among the synthesized naphthoxazoles, compound 81 came out to be most potent. MIC (minimum inhibitory concentration) of the compound 81 against M. tuberculosis H37Rv, rifampicin-resistant M. tuberculosis (RIFr) and isoniazid resistant M. tuberculosis (INHr) is given in Table 36 [50].
Table 36.
MIC values for compound 81
| Compd. | MIC (µg/ml) | ||
|---|---|---|---|
| H37Rv | RIFr | INHr | |
| 81 | 6.25 | 1.56 | 3.12 |
| Rifampicin | ≤ 0.125 | > 4 | ≤ 0.125 |
| Isoniazid | ≤ 0.06 | ≤ 0.06 | 1 |
Lu et al. carried out the synthesis of a series of substituted thiazole, oxazole and imidazole derivatives. The derivatives were examined for in vitro antitubercular potential using M. tuberculosis, and were also evaluated for antibacterial activities. The results for the antimycobacterial activity of oxazole derivatives 82, 83 are shown in Table 37 [51].
Table 37.
In vitro antitubercular activities of compound 82 and 83
| Compd. | MABA MIC (µM) |
|---|---|
| 82 | > 128 |
| 83 | > 128 |
The structures of the most active antitubercular compounds (76–83) are shown in Fig. 8.
Fig. 8.
Structures of the most active antitubercular and anti-inflammatory compounds
Anti-inflammatory activity
Dündar et al. prepared a range of oxazole derivatives and evaluated them for COX-2 inhibition. Homeostasis and gastro protective effects involve COX-1 which is the constitutive form, whereas inflammatory sites involve COX-2. Among the synthesized compounds, 84 was found to possess the highest selective COX-2 inhibition (70.14% ± 1.71) [52].
Eren et al. synthesized a chain of diaryl heterocyclic derivatives and carried out the evaluation of in vitro inhibitory activities against COX-1 and COX-2 isoforms. Among the oxazole derivatives, compound 85 was found to possess the maximum COX-2 inhibition of 47.10% ± 1.05 against the standard drug indomethacin and rofecoxib [6].
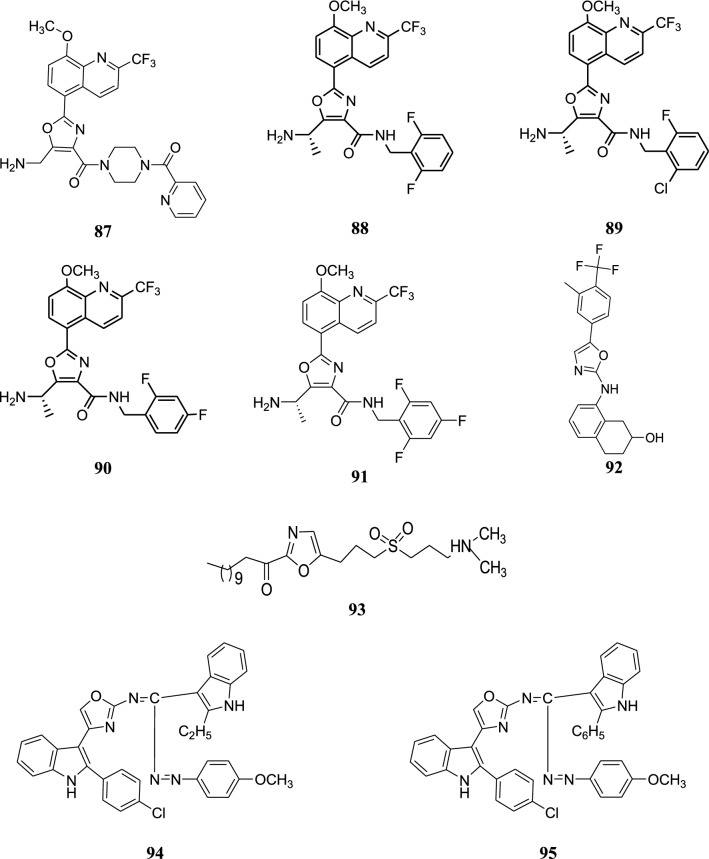
Kuang et al. discovered the substituted quinolyl oxazoles as highly effective phosphodiesterase 4 (PDE4) inhibitors. Inflammatory and immune cells involve the expression of PDE4 which is one of the cAMP specific PDE enzymes. Among the investigated compounds, 86 and 87 were found to be most effective with PDE4 IC50 values of 1.4 nm and 1 nm, respectively [53].
Kuang et al. carried out the synthesis of series of oxazole derivatives. Among the potent carboxamides, the N-benzylcarboxamide was found to exhibit good selectivity for phosphodiesterase 4 over phosphodiesterase 10 and phosphodiesterase 11. Further optimization of this series of potent compounds was carried out which led to the discovery of highly selective PDE4 inhibitors with picomolar potency. Compounds 88, 89, 90 and 91 were found to be the most effective PDE4 inhibitors whose IC50 values are given in Table 38 [54].
Table 38.
Anti-inflammatory activity of compounds 88, 89, 90 and 91
| Compd. | PDE4 IC50 (nm) |
|---|---|
| 88 | 0.05 |
| 89 | 0.03 |
| 90 | 0.06 |
| 91 | 0.04 |
Perner et al. carried out the synthesis of series of oxazole derivatives and tested for its TRPV1 receptor inhibition. The TRPV1 receptor is responsible for transmission of pain signaling. Among the synthesized compounds, 92 was discovered as a novel TRPV1 antagonist with IC50 value of 15 ± 3 nm [55].
Rusch et al. carried out the synthesis of 2-α-keto oxazoles and evaluated them for fatty acid amide hydrolase (FAAH) inhibition. FAAH is a membrane-bound serine hydrolase and is responsible for pain and inflammation. Out of all the tested compounds, 93 was found to be the most effective having an IC50 value of 290 nm [56].
Singh et al. prepared some oxazole derivatives and evaluated them for anti-inflammatory potential against carrageenan induced oedema in albino rats. Out of all the screened oxazole derivatives, 94 and 95 were found to be the most potent compounds (Table 39) [57].
Table 39.
Biological data of compound 94 and 95
| Compd. | Mean increase in paw volume ± SE | Anti-inflammatory activity % | Analgesic activity % |
|---|---|---|---|
| 94 | 0.56 ± 0.015 | 25.3 | 23.7 |
| 95 | 0.49 ± 0.015 | 27.9 | 26.3 |
The structures of the most active anti-inflammatory compounds (84–95) are shown in Figs. 8 and 9.
Fig. 9.
Structures of the most active anti-inflammatory compounds
Antidiabetic activity
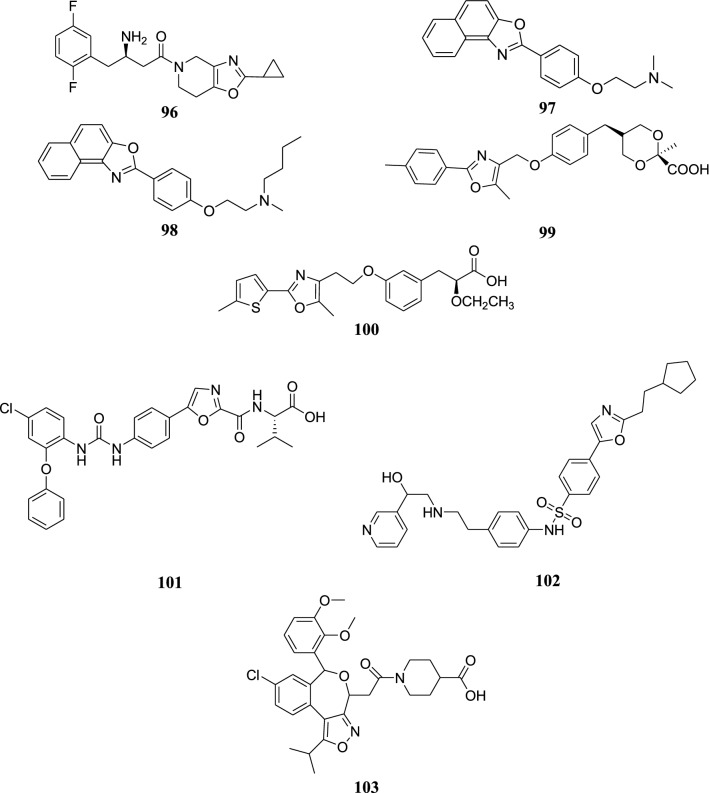
Ashton et al. synthesized a range of β-aminoacylpiperidines with fused five-membered heterocyclic rings (thiazole, oxazole, isoxazole, or pyrazole) as dipeptidyl peptidase IV inhibitors. Out of all the screened oxazole derivatives, (R)-3-amino-1-(2-cyclopropyl-6,7-dihydrooxazolo[4,5-c]pyridin-5(4H)-yl)-4-(2,5-difluorophenyl)butan-1-one (96) was found to possess considerable DPP-IV inhibition (IC50 = 0.18 µM) [7].
A chain of oxazole derivatives were synthesized by Kumar et al. and checked for PTP-1B inhibitory activity. Protein tyrosine phosphatase-1B (PTP-1B) has been found important for the treatment of diabetes and obesity. Out of all compounds, 97 and 98 exhibited the most promising activity (Table 40) [58].
Table 40.
Biological data of compounds 97 and 98
| Compd. | PTP-1B inhibitory activity (%) |
|---|---|
| 97 | 89.4 |
| 98 | 95.0 |
Pingali et al. designed and synthesized 1,3-dioxane carboxylic acid derivatives and combined this with substituted oxazole and evaluated them for in vitro PPAR agonistic potential and in vivo sugar lowering and lipid lowering efficacy in animal models using rosiglitazone and tesaglitazar as standard compounds. Compound 99 was found to be the most active (EC50 = 0.0015 µM) [59].
Raval et al. designed and synthesized novel thiophene substituted oxazole containing α-alkoxy-phenylpropanoic acid derivatives as highly potent PPAR α/γ dual agonists. Peroxisome proliferator-activated receptors (PPARs) play a very important role in metabolic syndrome whose major manifestations are hyperglycemia, dyslipidemia and obesity. Compound 100 was found to be the most efficacious PPAR α/γ dual agonist and showed the glucose reduction of 72% [60].
The structures of the most active antidiabetic compounds (96–100) are shown in Fig. 10.
Fig. 10.
Structures of the most active antidiabetic and antiobesity compounds
Antiobesity activity
Jadhav et al. prepared and checked a range of derivative shaving oxazole units for their hDGAT1 inhibition. Diacylglycerol acyltransferase (DGAT1) is an enzyme in obesity which is involved in triglyceride synthesis. Among all the tested oxazole derivatives, 101 was found to possess maximum in vivo plasma triglyceride reduction (91%) [8].
Ok et al. found a range of substituted oxazole derivatives that are effective β3 agonists. Compound 102 was found to be the best β3AR agonist (EC50 = 14 nM, 84% activation) [61].
Griebenow et al. prepared a range of novel squalene synthase inhibitors and evaluated them for lipid lowering activity. Squalene synthase is an enzyme which is involved in one of the steps of cholesterol biosynthesis. Compound 103 was found to be most effective. Results are mentioned in Table 41 [62].
Table 41.
Biological data of compound 103
| Compd. | IC50 (nm) | Sterol biosynthesis (%) |
|---|---|---|
| 103 | 112 | 79 |
The structures of the most active antiobesity compounds (101–103) are shown in Fig. 10.
Antioxidant activity
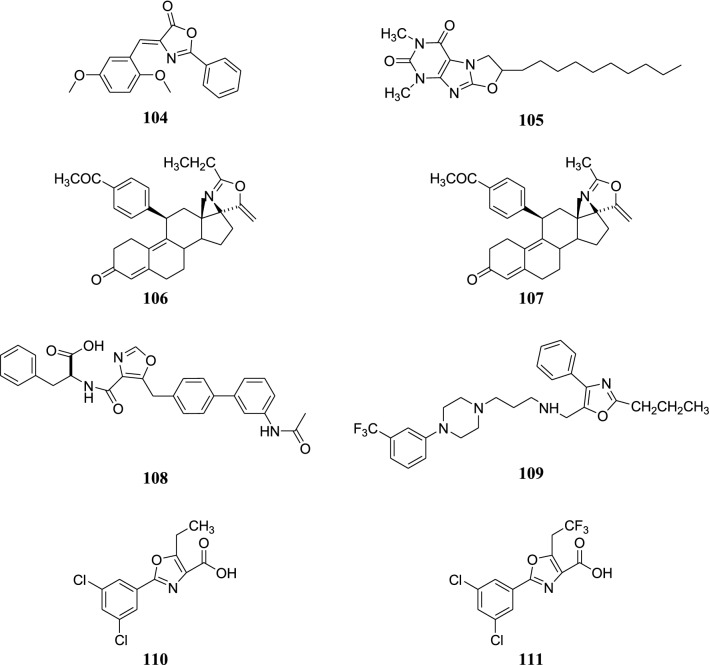
Parveen et al. synthesized several 4-arylidene-2-phenyl-5(4H)-azlactones and evaluated their antioxidant potential which revealed that compound 104 showed the highest IC50 value of 5.15 [9].
Adrenergic receptor ligand
Drabczyńska et al. prepared a chain of oxazole derivatives and evaluated their affinity at adenosine A1 and A2A receptors and anticonvulsant potential. 7-Decyl-1,3-dimethyl-6,7-dihydrooxazolo[3,2-a]purine-2,4(1H,3H)-dione (105) was found to possess the maximum affinity towards the A2A receptor but had poor anticonvulsant activity (A2Aversus[3H]MSX-2b % inhibition = 90%) [63].
Anti progesterone activity
Synthesis of novel oxazole analogs was done by Jin et al. and assessed their antagonist hormonal properties using mifepristone as standard drug. Compounds 106 and 107 showed highly potent antiprogestational activity. Results are mentioned in Table 42 [64].
Table 42.
Anti-hormonal property of compound 106 and 107
| Compd. | T47D IC50 (nM) |
|---|---|
| 106 | 0.34 |
| 107 | 0.59 |
| Mifepristone | 0.054 |
Prostacyclin receptor antagonist
Brescia et al. carried out the synthesis and evaluated the prostacyclin (IP) receptor antagonistic activity of oxazole derivatives. Prostacyclin (PGI2), which is an eicosanoid, plays an important role in inhibition of platelet aggregation, vasodilatation, and also acts as an antagonist of thromboxane A2. Out of all the tested compounds, 108 was found to be the most effective one. Results are shown in Table 43 [65].
Table 43.
Biological activity of compound 108
| Compd. | IC50 (µM) | |
|---|---|---|
| IPR | HEL cAMP | |
| 108 | 0.476 ± 0.193 | 0.016 ± 0.001 |
T-type calcium channel blocker
Lee et al. synthesized a number of oxazole derivatives substituted with arylpipera-zinylalkylamines and biologically evaluated against α1G (Cav3.1) T-type calcium channel. Out of all the synthesized derivatives the most active one was 109 with an IC50 value of 0.65 µM, which was found to be comparable with the reference drug mibefradil [66].
Transthyretin (TTR) amyloid fibril inhibitors
Razavi et al. carried out the synthesis of few oxazole derivatives and assessed as transthyretin (TTR) amyloid fibril inhibitors. 2-(3,5-Dichlorophenyl)-5-ethyloxazole-4-carboxylic acid (110) and 2-(3,5-dichlorophenyl)-5-(2,2,2-trifluoroethyl)oxazole-4-carboxylic acid (111) were found to possess the maximum activity. Results are mentioned in Table 44 [67].
Table 44.
In vitro transthyretin binding selectivity assay
| Compd. | Binding selectivity to transthyretin in human blood plasma |
|---|---|
| 110 | 0.49 ± 0.07 |
| 111 | 0.68 ± 0.04 |
The structures of the most active antioxidant compound (104), adrenergic receptor ligand (105), antiprogesterone compounds (106–107), prostacyclin receptor antagonist (108), T-type calcium channel blocker (109) and transthyretin (TTR) amyloid fibril inhibitors (110–111) are shown in Fig. 11.
Fig. 11.
Structure of the most active antioxidant compound, adrenergic receptor ligand, antiprogesterone compounds, prostacyclin receptor antagonist, T-type calcium channel blocker and transthyretin (TTR) amyloid fibril inhibitors
Conclusion
In summary, the present article aims to review the work reported on therapeutic potentials of oxazole derivatives which are valuable for medical applications during new millennium. This review article is based on synthesized oxazole derivatives which displays wide spectrum of biological potentials i.e. antibacterial, analgesic, anti-inflammatory, antidepressant, anticancer, antimicrobial, antidiabetic, antiobesity, antioxidant, adrenergic receptor ligand, antiprogesterone activity, prostacyclin receptor antagonist, T-type calcium channel blocker and transthyretin amyloid fibril inhibitory. The heterocyclic moiety being so versatile in nature offers the medicinal chemist to explore more about it in medicinal field and the data mentioned in this article will be a great help to prospective researchers working in this area for further study of this scaffold.
Oxazole moiety is an important heterocyclic compound as they are being an essential constituent of large number of marketed drugs. Having such diverse spectrum of biological activities, oxazoles has immense potential to be investigated for newer therapeutic possibilities and is an important class of lead compounds for development of new chemical entities (NCE) to treat various diseases of clinical importance.
Authors’ contributions
Authors BN and SK have designed and prepared the manuscript. Both authors read and approved the final manuscript.
Acknowledgements
The authors are thankful to Head, Department of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak, for providing necessary facilities to carry out this work.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Saloni Kakkar, Email: salonikakkar2007@gmail.com.
Balasubramanian Narasimhan, Email: naru2000us@yahoo.com.
References
- 1.Patel NB, Shaikh FM. New 4-thiazolidinones of nicotinic acid with 2-amino-6-methyl benzothiazole and their biological activity. Sci Pharm. 2010;78:753–765. doi: 10.3797/scipharm.1009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swellmeen L. 1,3-Oxazole derivatives: a review of biological activities as antipathogenic. Der Pharma Chemica. 2016;8(13):269–286. [Google Scholar]
- 3.Zhang W, Liu W, Jiang X, Jiang F, Zhuang H, Fu L. Design, synthesis and antimicrobial activity of chiral 2-(substituted-hydroxyl)-3-(benzo[d]oxazol-5-yl)propanoic acid derivatives. Eur J Med Chem. 2011;46(9):3639–3650. doi: 10.1016/j.ejmech.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Kumar D, Kumar NM, Sundaree S, Johnson EO, Shah K. An expeditious synthesis and anticancer activity of novel 4-(3´-indolyl)oxazole. Eur J Med Chem. 2010;45(3):1244–1249. doi: 10.1016/j.ejmech.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Moraski GC, Chang M, Villegas-Estrada A, Franzblau SG, Möllmann M, Miller MJ. Structure-activity relationship of new anti-tuberculosis agents derived from oxazoline and oxazole benzyl esters. Eur J Med Chem. 2010;45(5):1703–1716. doi: 10.1016/j.ejmech.2009.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eren G, Ünlü S, Nuñezv MT, Labeaga L, Ledo F, Entrena A, Ea Banoğlu, Costantino G, Şahin MF. Synthesis, biological evaluation, and docking studies of novel heterocyclic diaryl compounds as selective COX-2 inhibitors. Bioorg Med Chem. 2010;18:6367–6376. doi: 10.1016/j.bmc.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Ashton WT, Sisco RM, Dong H, Lyons KA, He H, Doss GA, Leiting B, Patel RA, Wu JK, Marsilio F, Thornberry NA, Weber AE. Dipeptidyl peptidase IV inhibitors derived from β-aminoacylpiperidines bearing a fused thiazole, oxazole, isoxazole, or pyrazole. Bioorg Med Chem Lett. 2005;15(9):2253–2258. doi: 10.1016/j.bmcl.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Jadhav RD, Kadam KS, Kandre S, Guha T, Reddy MMK, Brahma MK, Deshmukh NJ, Dixit A, Doshi L, Potdar N, Enose AA, Vishwakarma RA, Sivaramakrishnan H, Srinivasan S, Nemmani KVS, Gupte A, Gangopadhyay AK, Sharma R. Synthesis and biological evaluation of isoxazole, oxazole, and oxadiazole containing heteroaryl analogs of biarylureas as DGAT1 inhibitors. Eur J Med Chem. 2012;54:324–342. doi: 10.1016/j.ejmech.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Parveen M, Ali A, Ahmed S, Malla AM, Alam M, Silva PSP, Silva MR, Lee DU. Synthesis, bioassay, crystal structure and ab initio studies of Erlenmeyer azlactones. Spectrochim Acta A Mol Biomol Spectrosc. 2013;104:538–545. doi: 10.1016/j.saa.2012.11.054. [DOI] [PubMed] [Google Scholar]
- 10.Kakkar S, Kumar S, Lim SM, Ramasamy K, Mani V, Shah SAA, Narasimhan B. Design, synthesis and biological evaluation of 3-(2-aminooxazol-5-yl)-2H-chromen-2-one derivatives. Chem Cent J. 2018;12(130):1–13. doi: 10.1186/s13065-018-0499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi S, Bisht AS, Juyal D. Systematic scientific study of 1, 3-oxazole derivatives as a useful lead for pharmaceuticals: a review. Pharm Innov J. 2017;6(1):109–117. [Google Scholar]
- 12.Tilvi S, Singh KS. Synthesis of oxazole, oxazoline and isoxazoline derived marine natural products: a review. Curr Org Chem. 2016;20(8):898–929. doi: 10.2174/1385272819666150804000046. [DOI] [Google Scholar]
- 13.Argade ND, Kalrale BK, Gill CH. Microwave assisted improved method for the synthesis of pyrazole containing 2,4,-disubstituted oxazol-5-one and their antimicrobial activity. E-J Chem. 2008;5(1):120–129. doi: 10.1155/2008/265131. [DOI] [Google Scholar]
- 14.Tanitame A, Oyamada Y, Ofuji K, Fujimoto M, Suzuki K, Ueda T, Terauchi H, Kawasaki M, Nagai K, Wachi M, Yamagishi J. Synthesis and antibacterial activity of novel and potent DNA gyrase inhibitors with azole ring. Bioorgan Med Chem. 2004;12(21):5515–5524. doi: 10.1016/j.bmc.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Aaglawe MJ, Dhule SS, Bahekar SS, Wakte PS, Shinde DB. Synthesis and antibacterial activity of some oxazolone derivatives. J Kor Chem Soc. 2003;47(2):133–136. doi: 10.5012/jkcs.2003.47.2.133. [DOI] [Google Scholar]
- 16.Ryu CK, Lee RY, Kim NY, Kim YH, Song AL. Synthesis and antifungal activity of benzo[d]oxazole-4,7-diones. Bioorg Med Chem Lett. 2009;19(20):5924–5926. doi: 10.1016/j.bmcl.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 17.Singh I, Kaur H, Kumar S, Lata S, Kumar A, Kumar A. Synthesis and antibacterial activity of 3-chloro 4-(substituted phenyl) azetidinonyl/thiazolidinonyl-4-(3-acetanilido) oxa/thiazoles. Int J Pharm Sci Res. 2010;1(2):148–168. [Google Scholar]
- 18.Kamble VS, Habade BM, Patil GK, Agasimundin Y. Synthesis and evaluation of 4-(1-benzofuran-2-yl)-1,3-oxazole-2-amine and its derivatives. Int J Res Pharm Chem. 2012;2(1):32–36. [Google Scholar]
- 19.Chilumula NR, Gudipati R, Ampati S, Manda S, Gadhe D. Synthesis of some novel methyl-2-(2-(arylideneamino)oxazol-4-ylamino)benzoxazole-5-carboxylate derivatives as antimicrobial agents. Int J Chem Res. 2010;1(2):1–6. [Google Scholar]
- 20.Kaspady M, Narayanaswamy VK, Raju M, Rao GK. Synthesis, antibacterial activity of 2,4-disubstituted oxazoles and thiazoles as bioisosteres. Lett Drug Des Discov. 2009;6(1):21–28. doi: 10.2174/157018009787158481. [DOI] [Google Scholar]
- 21.Shamsuzzaman Khan MS, Alam M, Tabassum Z, Ahmad A, Khan AU. Synthesis, antibacterial and antifungal activities of 6,5 fused steroidal oxazoles in cholestane series. Eur J Med Chem. 2010;45:1094–1097. doi: 10.1016/j.ejmech.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Tomi IHR, Tomma JH, Al-Daraji AHR, Al-Dujaili AH. Synthesis, characterization and comparative study the microbial activity of some heterocyclic compounds containing oxazole and benzothiazole moieties. J Saudi Chem Soc. 2015;19:392–398. doi: 10.1016/j.jscs.2012.04.010. [DOI] [Google Scholar]
- 23.Sadek B, Fahelelbom KMS. Synthesis, characterization, and antimicrobial evaluation of oxadiazole congeners. Molecules. 2011;16(6):4339–4347. doi: 10.3390/molecules16064339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy AB, Hymavathi RV, Swamy GN. A new class of multi-substituted oxazole derivatives: synthesis and antimicrobial activity. J Chem Sci. 2013;125(3):495–509. doi: 10.1007/s12039-013-0417-7. [DOI] [Google Scholar]
- 25.Dabholkar VV, Ali Syed SAS. Synthesis of novel oxazoles and their hydrazones. Rasayan J Chem. 2010;3(4):761–765. [Google Scholar]
- 26.Dawood NTA. Synthesis and antimicrobial activity of 1-(4-aryl-2-thiazolyl) - and 1-(4-aryl-2-oxazolyl)-3, 5-diaryl ∆2-pyrazoline derivatives. J Chem Pharm Res. 2011;3(4):111–121. [Google Scholar]
- 27.Singh I, Kaur H, Kumar S, Kumar A, Lata S, Kumar A. Synthesis of new coumarin derivatives as antibacterial agents. Int J Chem Tech Res. 2010;2(3):1745–1752. [Google Scholar]
- 28.Taile V, Hatzade K, Gaidhane P, Ingle V. Synthesis and biological activity of -(4-hydroxybenzylidene)-2-(substituted styryl) oxazol-5-ones and their o-glucosides. Turk J Chem. 2009;33(2):295–305. [Google Scholar]
- 29.Ram Prasad S, Saraswathy T, Niraimathi V, Indhumathi B. Synthesis, characterization and antimicrobial activity of some hetero benzocaine derivatives. Int J Pharm Pharm Sci. 2012;4(5):285–287. [Google Scholar]
- 30.Patel NB, Shaikh AR. Synthesis, characterization and in vitro antimicrobial studies of new 2,3-disubstituted quinazolin-4(3H)ones of 2-[2-(2,6-dichlorophenyl)amino]phenyl acetic acid. Indian J Chem. 2010;49B:929–936. [Google Scholar]
- 31.Anand M, Ranjitha A, Himaja M. Silica sulfuric acid catalyzed microwave-assisted synthesis of substituted benzoxazoles and their antimicrobial activity. Int Res J Pharm. 2011;2(4):211–213. [Google Scholar]
- 32.Patel NB, Shaikh AR. In vitro antimicrobial studies of newly synthesized 1, 3-oxazolylquinazolin-4(3H) ones. Farmacia. 2011;59(4):531–538. [Google Scholar]
- 33.Padmavathi V, Kumara CP, Venkatesh BC, Padmaja A. Synthesis and antimicrobial activity of amido linked pyrrolyl andpyrazolyl-oxazoles, thiazoles and imidazoles. Eur J Med Chem. 2011;46(11):5317–5326. doi: 10.1016/j.ejmech.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 34.Reddy CS, Rao LS, Vani Devi M, Kumar GR, Nagaraj A. Synthesis of some new 3-[5-(2-oxo-2H-3-chromenyl)-1,3-oxazol-2-yl]-1,3-thiazolan-4-ones as antimicrobials. Chinese Chem Lett. 2010;21:1045–1048. doi: 10.1016/j.cclet.2010.03.018. [DOI] [Google Scholar]
- 35.Rahman AHA, Keshk EM, Hanna MA, El-Bady ShM. Synthesis and evaluation of some new spiroindoline-based heterocycles as potentially active antimicrobial agents. Bioorg Med Chem. 2004;12(9):2483–2488. doi: 10.1016/j.bmc.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 36.Cantalejo YM, Sáez B, Monterde MI, Murillo MT, Braña MF. Synthesis and biological activity of new bispyridinium salts 4,4´ bispyridyl-5-5´-perflouroalkyl-2,2´-bisoxazoles. Eur J Med Chem. 2011;46:5662–5667. doi: 10.1016/j.ejmech.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 37.Barca A, Pani B, Tamaro M, Russo E. Molecular interactions of ruthenium complexes in isolated mammalian nuclei and cytotoxicity on V-79 cells in culture. Mutat Res. 1999;423(1–2):171–181. doi: 10.1016/S0027-5107(98)00240-1. [DOI] [PubMed] [Google Scholar]
- 38.Liu XH, Lv PC, Xue JY, Song BA, Zhu HL. Novel 2,4,5-trisubstituted oxazole derivatives: synthesis and antiproliferative activity. Eur J Med Chem. 2009;44(10):3930–3935. doi: 10.1016/j.ejmech.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Mahal K, Biersack B, Schobert R. New oxazole-bridged combretastatin A-4 analogues as potential vascular-disrupting agents. Int J Clin Pharmacol Ther. 2013;15(1):41–43. doi: 10.5414/CPP51041. [DOI] [PubMed] [Google Scholar]
- 40.Pilch DS, Barbieri CM, Rzuczek SG, LaVoi EJ, Rice JE. Targeting human telomeric G-quadruplex DNA with oxazole-containing macrocyclic compounds. Biochimie. 2008;90(8):1233–1249. doi: 10.1016/j.biochi.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohnmacht SA, Micco M, Petrucci V, Todd AK, Reszka AP, Gunaratnam M, Carvalho MA, Zloh M, Neidle S. Sequences in the HSP90 promoter form G-quadruplex structures with selectivity for disubstituted phenyl bis-oxazole derivatives. Bioorg Med Chem Lett. 2012;22(18):5930–5935. doi: 10.1016/j.bmcl.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 42.Sączewski F, Stencel A, Bieńczak AM, Langowska KA, Michaelis M, Werel W, Hałasa R, Reszka P, Bednarski PJ. Structure activity relationships of novel heteroaryl-acrylonitriles as cytotoxic and antibacterial agents. Eur J Med Chem. 2008;43(9):1847–1857. doi: 10.1016/j.ejmech.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 43.Savariz FC, Foglio MA, de Carvalho JE, Ruiz ALTG, Duarte MCT, da Rosa MF, Meyer E, Sarragiotto MH. Synthesis and evaluation of new β-carboline-3-(4-benzylidene)-4H-oxazol-5-one derivatives as antitumor agents. Molecules. 2012;17:6100–6113. doi: 10.3390/molecules17056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan S, Yin H, Chen Z, Qian X, Xu Y. Oxo-heterocyclic fused naphthalimides as antitumor agents: synthesis and biological evaluation. Eur J Med Chem. 2013;62:130–138. doi: 10.1016/j.ejmech.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 45.Biersack B, Effenberger K, Knauer S, Ocker M, Schobert R. Ru(η6-arene) complexes of combretastatin-analogous oxazoles with enhanced anti-tumoral impact. Eur J Med Chem. 2010;45:4890–4896. doi: 10.1016/j.ejmech.2010.07.061. [DOI] [PubMed] [Google Scholar]
- 46.Hernández D, Altuna M, Cuevas C, Aligué R, Albericio F, Alvarez M. Synthesis and antitumor activity of mechercharmycin a analogues. J Med Chem. 2008;51(18):5722–5730. doi: 10.1021/jm800513w. [DOI] [PubMed] [Google Scholar]
- 47.Lin J, Shen W, Xue J, Sun J, Zhang X, Zhang C. Novel oxazolo[4,5-g]quinazolin-2(1H)-ones: dual inhibitors of EGFR and Srcprotein tyrosine kinases. Eur J Med Chem. 2012;55:39–48. doi: 10.1016/j.ejmech.2012.06.055. [DOI] [PubMed] [Google Scholar]
- 48.Giddens AC, Boshoff HIM, Franzblau SG, Barry CE, III, Copp BR. Antimycobacterial natural products: synthesis and preliminary biological evaluation of the oxazole-containing alkaloid texaline. Tetrahedron Lett. 2005;46(43):7355–7357. doi: 10.1016/j.tetlet.2005.08.119. [DOI] [Google Scholar]
- 49.Moraski GC, Markley LD, Chang M, Cho S, Franzblau SG, Hwang CH, Boshoff H, Miller MJ. Generation and exploration of new classes of antitubercular agents: the optimization of oxazolines, oxazoles, thiazolines, thiazoles to imidazo[1,2-a]pyridines and isomeric 5,6-fused scaffolds. Bioorg Med Chem. 2012;20(7):2214–2220. doi: 10.1016/j.bmc.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moura KCG, Carneiro PF, Maria do Carmo FR Pinto, da Silva JA, Malta VRS, de Simone CA, Dias GG, Jardim GAM, Cantos J, Coelho TS, da Silva PEA, da Silva EN., Jr 1,3-Azoles from ortho-naphthoquinones: synthesis of aryl substituted imidazoles and oxazoles and their potent activity against Mycobacterium tuberculosis. Bioorg Med Chem. 2012;20:6482–6488. doi: 10.1016/j.bmc.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 51.Lu X, Liu X, Wan B, Franzblau SG, Chen L, Zhou C, You Q. Synthesis and evaluation of anti-tubercular and antibacterial activities of new4-(2,6-dichlorobenzyloxy)phenyl thiazole, oxazole and imidazole derivatives. Part 2. Eur J Med Chem. 2012;49:164–171. doi: 10.1016/j.ejmech.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Dündar Y, Ünlü S, Banoğlu E, Entrena A, Costantino G, Nunez MT, Ledo F, Şahin MF, Noyanalpan N. Synthesis and biological evaluation of 4,5-diphenyloxazolone derivatives on route towards selective COX-2 inhibitors. Eur J Med Chem. 2009;44:1830–1837. doi: 10.1016/j.ejmech.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 53.Kuang R, Shue HJ, Blythin DJ, Shih NY, Gu D, Chen X, Schwerdt J, Lin L, Ting PC, Zhu X, Aslanian R, Piwinski JJ, Xiao L, Prelusky D, Wu P, Zhang J, Zhang X, Celly CS, Minnicozzi M, Billah M, Wang P. Discovery of a highly potent series of oxazole-based phosphodiesterase 4 inhibitors. Bioorg Med Chem Lett. 2007;17:5150–5154. doi: 10.1016/j.bmcl.2007.06.092. [DOI] [PubMed] [Google Scholar]
- 54.Kuang R, Shue HJ, Li Xiao, Blythin DJ, Shih NY, Chen X, Gu D, Schwerdt J, Lin L, Ting PC, Cao J, Aslanian R, Piwinski JJ, Prelusky D, Wu P, Zhang J, Zhang X, Celly CS, Billah M, Wang P. Discovery of oxazole-based PDE4 inhibitors with picomolar potency. Bioorg Med Chem Lett. 2012;22(7):2594–2597. doi: 10.1016/j.bmcl.2012.01.115. [DOI] [PubMed] [Google Scholar]
- 55.Perner RJ, Koenig JR, Di Domenico S, Gomtsyan A, Schmidt RG, Lee CH, Hsu MC, McDonald HA, Gauvin DM, Joshi S, Turner TM, Reilly RM, Kym PR, Kort ME. Synthesis and biological evaluation of 5-substituted and 4,5-disubstituted-2-arylamino oxazole TRPV1 antagonists. Bioorg Med Chem. 2010;18(13):4821–4829. doi: 10.1016/j.bmc.2010.04.099. [DOI] [PubMed] [Google Scholar]
- 56.Rusch M, Zahov S, Vetter IR, Lehr M, Hedberg C. Design, synthesis and evaluation of polar head group containing 2-keto-oxazole inhibitors of FAAH. Bioorg Med Chem. 2012;20:1100–1112. doi: 10.1016/j.bmc.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 57.Singh N, Bhati SK, Kumar A. Thiazolyl/oxazolyl formazanyl indoles as potent anti-inflammatory agents. Eur J Med Chem. 2008;43(11):2597–2609. doi: 10.1016/j.ejmech.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 58.Kumar A, Ahmad P, Maurya RA, Singh AB, Srivastava AK. Novel 2-aryl-naphtho[1,2-d]oxazole derivatives as potential PTP-1B inhibitors showing antihyperglycemic activities. Eur J Med Chem. 2009;44(1):109–116. doi: 10.1016/j.ejmech.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 59.Pingali H, Jain M, Shah S, Makadia P, Zaware P, Goel A, Patel M, Giri S, Patel H, Patel P. Design and synthesis of novel oxazole containing 1,3-Dioxane-2-carboxylic acid derivatives as PPAR α/γ dual agonists. Bioorg Med Chem. 2008;16(15):7117–7127. doi: 10.1016/j.bmc.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 60.Raval P, Jain M, Goswami A, Basu S, Gite A, Godha A, PingalI H, Raval S, Giri S, Suthar D, Shah M, Patel P. Revisiting glitazars: thiophene substituted oxazole containing α-ethoxy phenylpropanoic acid derivatives as highly potent PPAR α/γ dual agonists devoid of adverse effects in rodents. Bioorg Med Chem Lett. 2011;21(10):3103–3109. doi: 10.1016/j.bmcl.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 61.Ok HO, Reigle LB, Candelore MR, Cascieri MA, Colwell LF, Deng L, Feeney WP, Forrest MJ, Hom GJ, MacIntyre DE, Strader CD, Tota L, Wang P, Wyvratt MJ, Fisher MH, Weber AE. Substituted oxazole benzenesulfonamides as potent human β3 adrenergic receptor agonists. Bioorg Med Chem Lett. 2000;10(14):1531–1534. doi: 10.1016/S0960-894X(00)00277-8. [DOI] [PubMed] [Google Scholar]
- 62.Griebenow N, Buchmueller A, Kolkhof P, Schamberger J, Bischoff H. Identification of 4H,6H-[2]benzoxepino[4,5-c][1, 2]oxazoles as novel squalene synthase inhibitors. Bioorg Med Chem Lett. 2011;21(12):3648–3653. doi: 10.1016/j.bmcl.2011.04.092. [DOI] [PubMed] [Google Scholar]
- 63.Drabczyńska A, Müller CE, Schumacher B, Hinz S, Wojciechowska JK, Michalak B, Pękala E, Kieć-Kononowicz K. Tricyclic oxazolo[2,3-f]purinediones: potency as adenosine receptor ligands and anticonvulsants. Bioorg Med Chem. 2004;12(18):4895–4908. doi: 10.1016/j.bmc.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 64.Jin C, Manikumar G, Kepler JA, Cook CE, Allan GF, Kiddoe M, Bhattacharjee S, Linton O, Lundeen SG, Sui Z. Synthesis and identification of novel 11β-aryl-4´,5´-dihydrospiro[estra-4,9-diene-17β,4´-oxazole] analogs with dissociated antiprogesterone activities. Bioorg Med Chem Lett. 2007;17:5754–5757. doi: 10.1016/j.bmcl.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 65.Brescia MR, Rokosz LL, Cole AG, Stauffer TM, Lehrach JM, Auld DS, Henderson I, Webb ML. Discovery and preliminary evaluation of 5-(4-phenylbenzyl)oxazole4-carboxamides as prostacyclin receptor antagonists. Bioorg Med Chem Lett. 2007;17:1211–1215. doi: 10.1016/j.bmcl.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 66.Lee JE, Koh HY, Seo SH, Baek YY, Rhim H, Cho YS, Choo H, Pae AN. Synthesis and biological evaluation of oxazole derivatives as T-type calcium channel blockers. Bioorg Med Chem Lett. 2010;20(14):4219–4222. doi: 10.1016/j.bmcl.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 67.Razavi H, Powers ET, Purkey HE, Adamski-Werner SL, Chiang KP, Dendle MTA, Kelly JW. Design, synthesis, and evaluation of oxazole transthyretin amyloidogenesis inhibitors. Bioorg Med Chem Lett. 2005;15(4):1075–1078. doi: 10.1016/j.bmcl.2004.12.022. [DOI] [PubMed] [Google Scholar]