Abstract
Background
Sulfonamide derivatives are of great attention due to their wide spectrum of biological activities. Sulfonamides conjugated with acetamide fragments exhibit antimicrobial and anticancer activities. The inhibition dihydrofolate reductase (DHFR) is considered as one of the most prominent mechanism though which sulfonamide derivatives exhibits antimicrobial and antitumor activities.
Results
In this study, a new series of 2-(arylamino)acetamides and N-arylacetamides containing sulfonamide moieties were designed, synthesized, characterized and assessed for their antimicrobial activity and screened for cytotoxic activity against human lung carcinoma (A-549) and human breast carcinoma (MCF-7) cell lines. A molecular docking study was performed to identify the mode of action of the synthesized compounds and their good binding interactions were observed with the active sites of dihydrofolate reductase (DHFR).
Conclusion
Most of the synthesized compounds showed significant activity against A-549 and MCF-7 when compared to 5-Fluorouracil (5-FU), which was used as a reference drug. Some of these synthesized compounds are active as antibacterial and antifungal agents.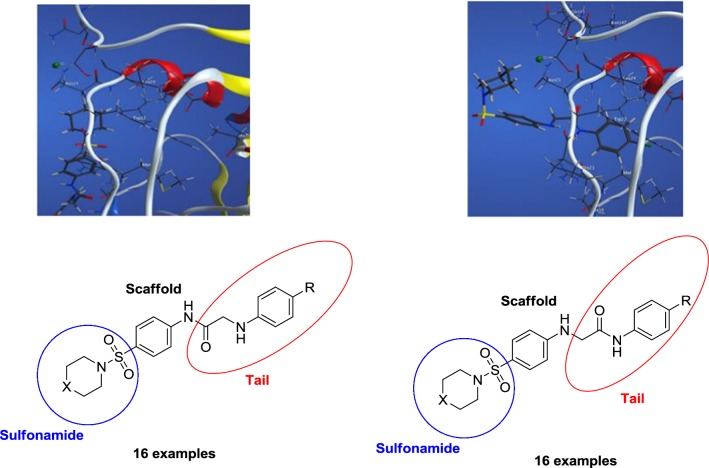
Electronic supplementary material
The online version of this article (10.1186/s13065-019-0603-x) contains supplementary material, which is available to authorized users.
Keywords: Sulfonamide, Anticancer, Antimicrobial, Acetamides, Molecular docking, Structure–activity relationship (SAR), DHFR inhibitors
Introduction
Sulfonamides have attracted considerable deal of interest over past decades due to their broad and wide spectrum of biological activities which includes antibacterial [1], antifungal [2], hypoglycemic [3], anti-thyroid [4], diuretic [5, 6] and anti-HIV properties [7]. Recently, a large number of structurally novel sulfonamides have been reported to show substantial in vitro and in vivo antitumor activity [8–15]. The anticancer activity is exerted by the sulfonamides through a wide range of mechanisms, such as cell cycle arrest in the G1 phase [16], inhibition of carbonic anhydrase (CA) [17], matrix metalloproteinase (MMPs) [18], NADH oxidase [19], cyclin-dependent kinase (CDK) [20], methionine aminopeptidases (MetAPs) [21], histone deacetylases (HDACs) [22], binding to β-Tubulin, and disruption of microtubule assembly [23].
On other hand, compounds with acetamide linkage exhibit variety of applications, which are well documented. The Lewis acid property of acetamides renders them useful as analytical reagents and in the preparation of a number of coordination complexes [24]. The acetamide functional group is responsible for antimicrobial [25, 26], antioxidant [27, 28], narcolepsy treatment [29], anti-inflammatory [30, 31], platelet aggregation inhibitory [32], and urease inhibitory activities [33]. The acetamides and their analogues are also well studied as chemotherapeutic agents [34, 35].
Dihydrofolate reductase (DHFR) is a key enzyme that catalyzes the NADPH-dependent reduction of 7,8-dihydrofolate (DHF) to 5,6,7,8-tetrahydrofolate (THF): DHF + NADPH + H+ → THF + NADP+, which is the precursor of the co-factors required for the biosynthesis of purine nucleotides, thymidine (precursor for DNA replication) and several amino acids [36]. Thus, inhibition of DHFR can lead to the disruption of DNA synthesis and the death of the rapidly proliferating cells [36, 37]. In addition to this, bacteria also need DHFR to grow and multiply and hence inhibitors selective for bacterial against host DHFR have found application as antibacterial agents [38]. These two important aspects render DHFR enzyme as a key target for both antimicrobial and antitumor drug design.
The sulfonamide group conjugated with acetamides possessing different aryl, heteroaryl as well as alkyl substituents exhibits immense pharmacological potential, particularly sulfonamides containing short amine fragments exhibits encouraging anticancer activity [39–41]. This accounts for the growing interest in the synthesis, biological properties and structure activity relationships of sulfonamide-acetamide derivatives.
Based on these prior observations relating to the synthesis of sulfonamide derivatives [42, 43] and bioactive nitrogen-containing heterocyclic agents [44–48] we envisaged that sulfonamides bearing acetamide pharmacophores could be very efficient for antimicrobial and anticancer activity. In the present work, we report the synthesis of some novel sulfonamide-N4-acetamide derivatives and their evaluation of their cytotoxic activity against human lung carcinoma (A-549) and human breast carcinoma (MCF-7) cell lines.
Results and discussion
Chemistry
The present study deals with the design and synthesis of some acetamide derivatives having different aryl substituents (tails) conjugated with biologically active sulfonamide moiety in order to explore their combined effect on the antimicrobial and antitumor activities, and study their structure–activity relationship (SAR).
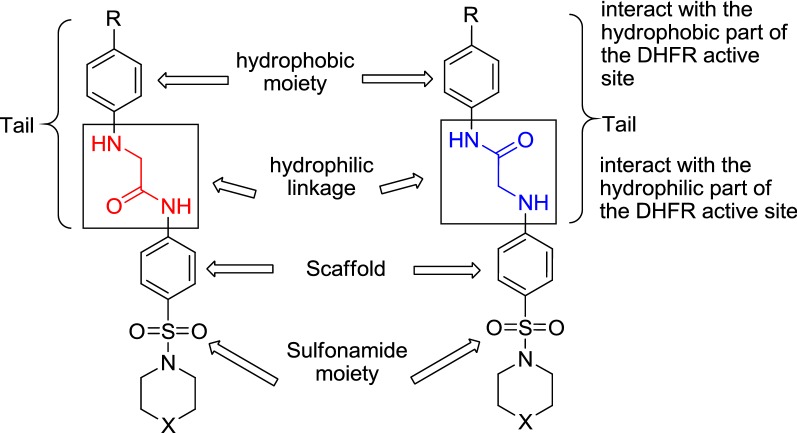
As the DHFR inhibition is considered as one of the most prominent mechanism for the antimicrobial and antitumor activities [49–51], the synthesized compounds were intended to comply with the pharmacophore present in compounds that may act as DHFR inhibitors. The sulfonamide is attached to a scaffold, which is frequently a benzene ring, and a tail comprising of groups such as 2-(arylamino)acetamide or N-arylacetamide is attached to scaffold. The tail possesses a hydrophobic moiety, which is able to interact with the hydrophobic part of the active site and a hydrophilic linker which can interact with the hydrophilic part of the DHFR active site (Fig. 1). This pharmacophore was designed from the analysis of the DHFRs active site and from the structure of inhibitors were described in literature [52, 53].
Fig. 1.
Structural elements of DHFR inhibitors in the DHFR enzymatic active site
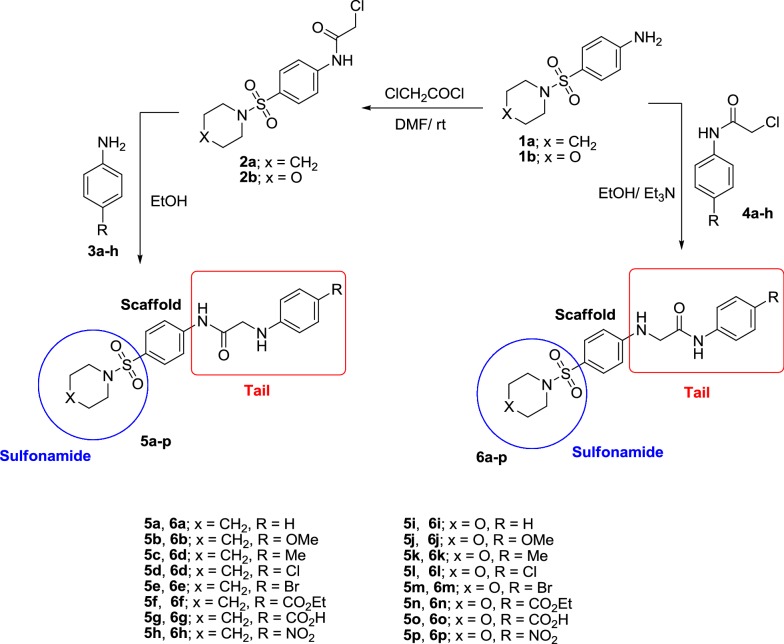
In this work, the starting key materials 4-(piperidin-1-ylsulfonyl)aniline (1a) and 4-(morpholin-4-ylsulfonyl)aniline (1b) were prepared accordingly as the reported method [42], and were converted to the corresponding chloroacetamide derivatives 2a,b in excellent yields (92–95%) by reaction with chloroacetyl chloride in DMF at room temperature.
The target compounds 2-(arylamino)-N-(4-(piperidin-1-ylsulfonyl)phenyl)acetamides 5a–h and 2-(arylamino)-N-(4-(morpholino-sulfonyl)phenyl)acetamides 5i–p, were obtained in moderate to good yields (51–84%) by refluxing chloroacetamide derivatives 2a and 2b, respectively, with arylamines (namely; aniline, 4-methoxyaniline, 4-methylaniline, 4-chloroaniline, 4-bromoaniline, ethyl 4-aminobenzoate, 4-aminobenzoic acid, and 4-nitroaniline) in absolute ethanol for 3–5 h. (Scheme 1).
Scheme 1.
Synthesis of novel N4-substituted sulfonamide derivatives 5a–p and 6a–p
On the other hand, 2-chloro-N-arylacetamides 4a–h were easily prepared by the reaction of arylamines (namely; aniline, 4-methoxyaniline, 4-methylaniline, 4-chloroaniline, 4-bromoaniline, ethyl 4-aminobenzoate, 4-aminobenzoic acid, and 4-nitroaniline) with chloroacetyl chloride in DMF at room temperature.
Reaction of 2-chloro-N-arylacetamides 4a–h with sulfonamide derivatives 1a and 1b in ethanol under refluxing conditions afforded the target compounds N-aryl-2-(4-(piperidin-1-ylsulfonyl)phenylamino)-acetamides 6a–h and N-aryl-2-(4-(morpholinosulfonyl)-phenylamino)-acetamides 6i–p, respectively, in good to excellent yields (57–97%) (Scheme 1). The reactions were performed in the presence of catalytic amount of triethylamine as a basic catalyst with a reaction time of 4-6 h. The structures of all synthesized compounds 2a,b, 5a–p and 6a–p were well-established on the basis of FT-IR, 1H-NMR, 13C-NMR, and DEPT-135 data (c.f. “Experimental” section). The FT-IR spectra of compounds 5a–p displayed the presence of characteristic absorption bands at 3499–3330 and 3365–3191 cm−1 for two NH groups, 1722–1680 cm−1 for (C=O) groups. Furthermore, to entirely confirm the chemical structures of the products, intensive 1D (1H, 13C, and DEPT-135) NMR were conducted in DMSO-d6. For example, analysis of the 13C and 13C-DEPT-135 NMR spectra of 5h indicated the presence of 13 signals (4 aromatic CH’s, 4 aromatic quaternary carbons, 4 methylene carbons, and one carbonyl carbon). Its 1H-NMR spectrum showed two downfield singlet signals at 10.75 and 10.10 ppm for two NH protons. Two doublets at 7.94 and 6.60 ppm (J = 11.0 Hz) for the protons of 4-nitrophenyl moiety and two doublets at 7.84 and 7.70 ppm (J = 10.5 Hz) for aromatic CH’s protons of the scaffold moiety were present. In addition, a singlet signal at 4.32 ppm for the tail methylene protons and three multiplets at 2.85–2.83, 1.52–1.51, and 1.34–1.33 ppm for the piperidinyl ring protons were recorded.
On the other hand, the FT-IR spectra of compounds 6a–p, showed the presence of characteristic absorption bands at 3444–3275 and 3365–3203 cm−1 for two NH groups, 1721–1641 cm−1 for (C=O) groups. As a representative example, the 13C and 13C-DEPT-135 NMR spectra of 6p showed the presence of 12 signals (4 aromatic CH’s, 4 aromatic quaternary carbons, 3 methylene carbons, and one carbonyl carbon). Its 1H-NMR spectrum exhibited two singlet signals at 10.94 and 6.11 ppm for two NH protons. Two doublets at 8.24 and 6.87 ppm (J = 7.5 Hz) for the protons of 4-nitrophenyl moiety and two doublets at 7.84 and 7.35 ppm (J = 8.0 Hz) for aromatic CH’s protons of the scaffold moiety were present. In addition, a singlet signal at 4.35 ppm for the tail methylene protons and two multiplets at 3.60 and 2.78 ppm for the morpholinyl ring protons were recorded.
Antimicrobial activity
The novel compounds were evaluated for antimicrobial activity against two strains of Gram-positive bacteria known as S. aureus (RCMB010010), and B. subtilis RCMB 015 (1) NRRL B-543, as well as two strains of Gram-negative bacteria namely E. coli (RCMB 010052) ATCC 25955, and P. vulgaris RCMB 004 (1) ATCC 13315, in addition to two types of fungi namely A. fumigatus (RCMB 002008), and C. albicans RCMB 005003 (1) ATCC 10231.
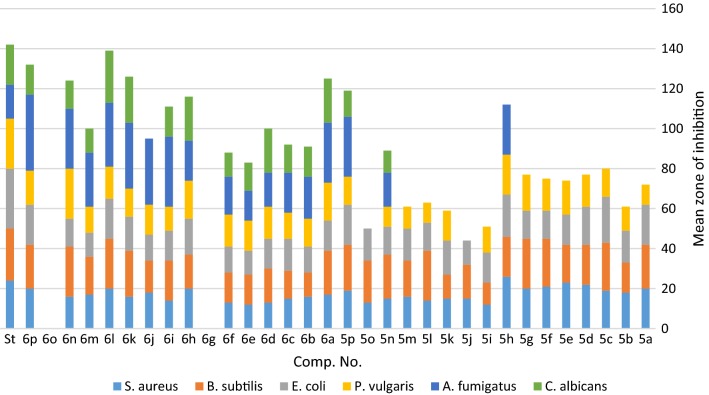
The result of the antimicrobial assay of the synthesized compounds is given in Table 1 and Fig. 2. It is observed that some of the compounds showed higher antimicrobial activity compared to the reference drugs. These compounds have given the best results in the inhibition of different types of bacteria and fungi; compound 5h against the S. aureus, the zone of inhibition with ZOI value 26, compounds 5g, 5l, 6l and 6n against B. Subtili having ZOI value 25, compound 5c against E. coli with ZOI value 23, while, compound 6n against P. vulgaris having ZOI value 25. Moreover, compounds 5h, 5n, 5p, 6a, 6b, 6c, 6d, 6f, 6h, 6i, 6j, 6k, 6l, 6m, 6n and 6p against A. fumigatus, having (ZOI) values 25, 17, 30, 30, 21, 20, 17, 19, 20, 35, 33, 33, 32, 27, 30, 38; respectively. Furthermore, the following compounds 6a, 6d, 6h having ZOI value 22 while, compounds 6k and 6l having ZOI values 23, 26; respectively against C. Albicans. It is clearly evident from the antimicrobial results that the synthesized compounds 6l and 6n exhibit dual activities as promising antibacterial and antifungal agents.
Table 1.
Antimicrobial activity of newly synthesized compounds
| Comp. no. | Gram (+ve) bacteria | Gram (−ve) bacteria | Fungi | |||
|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | P. vulgaris | A. fumigatus | C. albicans | |
| 5a | 20 | 22 | 20 | 10 | – | – |
| 5b | 18 | 15 | 16 | 12 | – | – |
| 5c | 19 | 24 | 23 | 14 | – | – |
| 5d | 22 | 20 | 19 | 16 | – | – |
| 5e | 23 | 19 | 15 | 17 | – | – |
| 5f | 21 | 24 | 14 | 16 | – | – |
| 5g | 20 | 25 | 14 | 18 | – | – |
| 5h | 26 | 20 | 21 | 20 | 25 | – |
| 5i | 12 | 11 | 15 | 13 | – | – |
| 5j | 15 | 17 | 12 | – | – | – |
| 5k | 15 | 12 | 17 | 15 | – | – |
| 5l | 14 | 25 | 14 | 10 | – | – |
| 5m | 16 | 18 | 16 | 11 | – | – |
| 5n | 15 | 22 | 14 | 10 | 17 | 11 |
| 5o | 13 | 21 | 16 | – | – | – |
| 5p | 19 | 23 | 20 | 14 | 30 | 13 |
| 6a | 17 | 22 | 15 | 19 | 30 | 22 |
| 6b | 16 | 12 | 13 | 14 | 21 | 15 |
| 6c | 15 | 14 | 16 | 13 | 20 | 14 |
| 6d | 13 | 17 | 15 | 16 | 17 | 22 |
| 6e | 12 | 15 | 12 | 15 | 15 | 14 |
| 6f | 13 | 15 | 13 | 16 | 19 | 12 |
| 6g | – | – | – | – | – | – |
| 6h | 20 | 17 | 18 | 19 | 20 | 22 |
| 6i | 14 | 20 | 15 | 12 | 35 | 15 |
| 6j | 18 | 16 | 13 | 15 | 33 | – |
| 6k | 16 | 23 | 17 | 14 | 33 | 23 |
| 6l | 20 | 25 | 20 | 16 | 32 | 26 |
| 6m | 17 | 19 | 12 | 13 | 27 | 12 |
| 6n | 16 | 25 | 14 | 25 | 30 | 14 |
| 6o | – | – | – | – | – | – |
| 6p | 20 | 22 | 20 | 17 | 38 | 15 |
| Sta | 24 | 26 | 30 | 25 | 17 | 20 |
Mean zone of inhibition in mm ± standard deviation (S.D.)
— No activity
aReference controls for the microorganisms are “Gentamycin” (for the Gram +ve and Gram −ve bacteria), and “Ketoconazol” for Fungi
Fig. 2.
Comparison of the antimicrobial activity of the newly synthesized compounds
From all the previous data, its be concluded that, the following compounds 5g, 5h, 5l, 6l and 6n are the highly active compounds which have antibacterial activity against strains of Gram-positive bacteria. While, compounds 5c and 6n showing activity against strains of Gram-negative bacteria. All compared to Gentamycin as antibacterial reference drug. Moreover, compounds 5h, 5n, 5p, 6a, 6b, 6c, 6d, 6f, 6h, 6i, 6j, 6k, 6l, 6m, 6n, 6p acted as antifungal agents compared to Ketoconazole as a reference drug.
In vitro anticancer activity
All new tested compounds were screened against human lung carcinoma (A-549) and breast carcinoma (MCF-7). The final result of evaluation were expressed as IC50 (the required concentration which can inhibit 50% of cancer cells viability). Results are explained in Tables 2 and 3. The reference control was 5-Fluorouracil (5-FU).
Table 2.
In vitro anticancer screening of the synthesized compounds against human lung carcinoma cell line (A-549)
| Comp. no. | Validity for sample conc. | IC50 (μg mL−1)a | IC50 (μM)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 500 | 250 | 125 | 62.50 | 31.25 | 15.60 | 7.80 | 3.90 | 2 | 1 | 0 | |||
| 5a | 5.19 | 11.84 | 25.06 | 37.15 | 48.91 | 62.87 | 80.96 | 91.43 | 97.02 | 100 | 100 | 29.90 | 80.06 |
| 5b | 5.38 | 11.97 | 24.02 | 36.59 | 47.38 | 63.20 | 78.15 | 87.56 | 94.03 | 98.76 | 100 | 28.60 | 70.88 |
| 5c | 4.31 | 8.65 | 16.37 | 28.74 | 40.96 | 58.71 | 73.04 | 89.21 | 96.28 | 100 | 100 | 23.20 | 59.87 |
| 5d | 2.65 | 5.34 | 10.26 | 19.45 | 28.73 | 39.04 | 57.26 | 71.89 | 85.22 | 92.34 | 100 | 10.90 | 26.72 |
| 5e | 2.94 | 6.71 | 14.09 | 23.87 | 36.29 | 49.85 | 70.63 | 89.24 | 96.31 | 99.72 | 100 | 15.50 | 34.26 |
| 5f | 4.23 | 9.86 | 20.15 | 32.76 | 45.13 | 59.38 | 75.04 | 82.38 | 91.75 | 97.43 | 100 | 25.80 | 57.91 |
| 5g | 2.75 | 6.39 | 10.42 | 18.65 | 28.91 | 40.74 | 56.26 | 70.89 | 83.11 | 88.62 | 100 | 10.90 | 26.11 |
| 5h | 2.86 | 5.43 | 12.87 | 22.96 | 34.53 | 47.28 | 65.39 | 80.72 | 89.86 | 97.13 | 100 | 14.40 | 34.41 |
| 5i | 4.73 | 10.59 | 18.42 | 28.96 | 40.67 | 59.23 | 73.18 | 85.21 | 92.74 | 97.35 | 100 | 23.30 | 62.06 |
| 5j | 2.98 | 6.74 | 10.85 | 19.73 | 26.8 | 37.28 | 49.06 | 70.31 | 84.15 | 91.38 | 100 | 7.60 | 18.74 |
| 5k | 8.92 | 16.34 | 24.08 | 35.17 | 49.01 | 72.38 | 89.42 | 98.36 | 100 | 100 | 100 | 30.50 | 78.31 |
| 5l | 1.89 | 4.37 | 8.76 | 19.04 | 30.63 | 43.86 | 59.12 | 76.44 | 90.63 | 96.42 | 100 | 12.40 | 30.25 |
| 5m | 6.72 | 10.86 | 22.38 | 31.49 | 46.75 | 69.42 | 86.03 | 92.34 | 99.71 | 100 | 100 | 28.90 | 63.61 |
| 5n | 4.86 | 9.73 | 19.48 | 31.72 | 45.29 | 64.18 | 80.63 | 92.34 | 97.23 | 100 | 100 | 27.30 | 61.00 |
| 5o | 2.89 | 6.54 | 15.93 | 24.06 | 36.41 | 52.97 | 68.02 | 79.19 | 91.42 | 98.76 | 100 | 18.30 | 43.63 |
| 5p | 3.69 | 8.71 | 18.63 | 29.46 | 41.87 | 54.06 | 71.32 | 87.14 | 95.20 | 99.73 | 100 | 20.70 | 49.23 |
| 6a | 3.65 | 8.29 | 16.34 | 25.87 | 37.06 | 46.92 | 58.43 | 80.71 | 87.53 | 93.14 | 100 | 13.50 | 36.15 |
| 6b | 6.14 | 13.65 | 25.38 | 37.25 | 56.41 | 79.84 | 92.36 | 98.6 | 100 | 100 | 100 | 41.70 | 103.35 |
| 6c | 3.74 | 7.46 | 15.28 | 27.4 | 41.35 | 56.79 | 68.42 | 82.37 | 91.43 | 97.60 | 100 | 22.40 | 57.81 |
| 6d | 4.08 | 6.82 | 11.43 | 19.46 | 30.67 | 43.20 | 59.13 | 71.44 | 85.12 | 92.37 | 100 | 12.20 | 29.91 |
| 6e | 4.62 | 11.29 | 20.47 | 34.13 | 45.29 | 62.37 | 80.94 | 89.76 | 97.02 | 100 | 100 | 26.80 | 59.24 |
| 6f | 6.31 | 13.45 | 19.75 | 31.42 | 47.23 | 71.94 | 89.56 | 97.13 | 100 | 100 | 100 | 29.40 | 65.99 |
| 6g | 9.56 | 21.87 | 30.64 | 43.19 | 59.46 | 78.28 | 91.40 | 98.76 | 100 | 100 | 100 | 49.40 | 118.33 |
| 6h | 1.98 | 4.87 | 9.72 | 19.93 | 28.61 | 40.72 | 63.18 | 79.43 | 90.64 | 97.39 | 100 | 12.30 | 29.39 |
| 6i | 7.94 | 11.52 | 24.43 | 36.25 | 49.72 | 71.49 | 89.70 | 97.41 | 100 | 100 | 100 | 30.90 | 82.30 |
| 6j | 3.87 | 7.96 | 16.20 | 27.85 | 41.79 | 54.03 | 71.48 | 88.60 | 96.23 | 100 | 100 | 20.70 | 51.05 |
| 6k | 7.18 | 12.98 | 23.06 | 33.97 | 47.85 | 63.28 | 78.91 | 90.68 | 97.89 | 100 | 100 | 29.00 | 74.46 |
| 6l | 6.74 | 14.35 | 21.88 | 35.46 | 46.29 | 58.17 | 78.03 | 86.36 | 94.12 | 98.78 | 100 | 26.30 | 64.16 |
| 6m | 4.28 | 9.53 | 18.75 | 31.92 | 40.06 | 47.41 | 73.65 | 90.37 | 98.91 | 100 | 100 | 14.80 | 32.57 |
| 6n | 11.82 | 23.48 | 30.51 | 41.3 | 47.42 | 60.31 | 82.42 | 97.14 | 100 | 100 | 100 | 28.07 | 62.72 |
| 6o | 31.96 | 49.72 | 73.68 | 90.63 | 97.46 | 100 | 100 | 100 | 100 | 100 | 100 | 248.00 | 591.25 |
| 6p | 2.79 | 6.28 | 13.91 | 25.04 | 37.18 | 52.94 | 68.29 | 84.67 | 92.84 | 98.25 | 100 | 18.50 | 44.00 |
| 5-FU | 10.28 | 19.45 | 25.39 | 39.48 | 57.21 | 70.82 | 86.19 | 94.36 | 99.25 | 100 | 100 | 43.9 | 337.48 |
IC50 value: concentration causing 50% inhibition of cell viability
aMean of three results obtained from three experiments
Table 3.
In vitro anticancer screening of the synthesized compounds against human breast carcinoma cell line (MCF-7)
| Comp. no. | Validity for sample conc. | IC50 (μg mL−1)a | IC50 (μM)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 500 | 250 | 125 | 62.50 | 31.25 | 15.60 | 7.80 | 3.90 | 2 | 1 | 0 | |||
| 5a | 7.34 | 14.03 | 29.47 | 41.58 | 54.29 | 71.43 | 87.50 | 96.75 | 100 | 100 | 100 | 41.80 | 111.92 |
| 5b | 7.13 | 13.42 | 30.96 | 42.37 | 56.29 | 68.41 | 83.77 | 91.40 | 98.32 | 100 | 100 | 45.40 | 112.52 |
| 5c | 5.28 | 11.32 | 20.79 | 32.65 | 48.51 | 70.38 | 86.42 | 94.03 | 99.71 | 100 | 100 | 30.10 | 77.68 |
| 5d | 3.46 | 7.28 | 15.09 | 27.41 | 36.27 | 45.62 | 63.18 | 80.94 | 93.46 | 99.53 | 100 | 13.70 | 33.59 |
| 5e | 4.02 | 9.56 | 18.48 | 29.67 | 40.82 | 54.61 | 73.28 | 91.42 | 98.70 | 100 | 100 | 20.80 | 45.98 |
| 5f | 6.17 | 14.38 | 27.56 | 39.4 | 53.29 | 68.43 | 81.70 | 93.68 | 99.85 | 100 | 100 | 38.70 | 86.86 |
| 5g | 3.86 | 8.24 | 15.37 | 24.16 | 32.95 | 41.68 | 49.80 | 67.48 | 85.26 | 93.84 | 100 | 7.76 | 18.59 |
| 5h | 3.94 | 7.81 | 16.23 | 31.42 | 39.79 | 48.20 | 67.41 | 82.76 | 91.30 | 98.72 | 100 | 14.90 | 35.61 |
| 5i | 6.28 | 13.47 | 25.13 | 36.78 | 48.50 | 67.41 | 81.67 | 89.43 | 98.16 | 100 | 100 | 30.00 | 79.91 |
| 5j | 4.17 | 9.82 | 18.78 | 27.05 | 35.23 | 46.19 | 54.82 | 69.46 | 87.34 | 94.29 | 100 | 12.10 | 29.84 |
| 5k | 13.49 | 21.86 | 32.75 | 46.23 | 57.18 | 76.94 | 88.60 | 97.41 | 100 | 100 | 100 | 51.70 | 132.74 |
| 5l | 2.73 | 6.46 | 11.38 | 24.95 | 34.89 | 42.67 | 51.53 | 65.76 | 83.20 | 91.42 | 100 | 9.140 | 22.30 |
| 5m | 9.85 | 18.2 | 30.67 | 42.96 | 51.78 | 68.92 | 84.68 | 95.41 | 99.62 | 100 | 100 | 37.50 | 82.54 |
| 5n | 7.63 | 15.26 | 27.39 | 38.04 | 46.15 | 58.20 | 71.36 | 88.42 | 96.28 | 100 | 100 | 26.20 | 58.55 |
| 5o | 3.46 | 9.82 | 20.31 | 29.57 | 43.60 | 56.89 | 70.42 | 83.97 | 92.40 | 97.36 | 100 | 23.60 | 56.26 |
| 5p | 4.98 | 11.75 | 21.42 | 36.75 | 50.38 | 69.41 | 85.26 | 93.02 | 99.76 | 100 | 100 | 32.10 | 76.35 |
| 6a | 4.94 | 10.73 | 23.4 | 32.79 | 45.17 | 56.24 | 71.38 | 85.02 | 92.47 | 98.25 | 100 | 24.40 | 65.33 |
| 6b | 8.71 | 20.42 | 37.53 | 49.81 | 62.97 | 85.46 | 93.04 | 99.32 | 100 | 100 | 100 | 62.00 | 153.66 |
| 6c | 5.31 | 9.14 | 19.56 | 32.71 | 49.82 | 70.38 | 81.6 | 92.88 | 98.76 | 100 | 100 | 31.10 | 80.26 |
| 6d | 6.79 | 13.4 | 19.85 | 27.93 | 36.7 | 48.61 | 63.87 | 76.45 | 88.29 | 96.36 | 100 | 14.90 | 36.53 |
| 6e | 7.46 | 19.53 | 28.65 | 40.37 | 52.91 | 69.42 | 82.36 | 91.73 | 98.6 | 100 | 100 | 38.50 | 85.11 |
| 6f | 9.56 | 17.28 | 28.67 | 36.7 | 50.98 | 67.39 | 82.15 | 93.69 | 98.72 | 100 | 100 | 33.40 | 74.97 |
| 6g | 15.68 | 27.83 | 38.17 | 52.46 | 69.9 | 83.51 | 92.78 | 99.52 | 100 | 100 | 100 | 73.30 | 175.58 |
| 6h | 2.37 | 7.54 | 15.18 | 23.65 | 34.89 | 45.13 | 60.97 | 75.86 | 87.41 | 95.64 | 100 | 13.20 | 31.54 |
| 6i | 11.76 | 21.49 | 34.85 | 43.96 | 58.28 | 77.39 | 91.47 | 97.92 | 100 | 100 | 100 | 49.30 | 131.31 |
| 6j | 5.92 | 11.48 | 23.69 | 34.73 | 47.21 | 68.46 | 80.93 | 92.64 | 98.23 | 100 | 100 | 29.14 | 71.87 |
| 6k | 12.41 | 20.53 | 30.78 | 39.62 | 56.34 | 71.48 | 86.24 | 98.4 | 100 | 100 | 100 | 43.00 | 110.41 |
| 6l | 10.32 | 19.67 | 32.94 | 41.78 | 51.85 | 65.04 | 79.12 | 90.65 | 97.54 | 100 | 100 | 36.90 | 90.02 |
| 6m | 7.64 | 16.29 | 24.31 | 35.17 | 45.04 | 56.28 | 69.46 | 82.78 | 94.13 | 99.2 | 100 | 24.30 | 53.48 |
| 6n | 19.47 | 31.85 | 40.91 | 49.76 | 58.49 | 71.32 | 86.95 | 97.81 | 100 | 100 | 100 | 61.60 | 137.65 |
| 6o | 37.04 | 52.31 | 76.45 | 91.32 | 98.74 | 100 | 100 | 100 | 100 | 100 | 100 | 287.00 | 684.23 |
| 6p | 3.45 | 9.74 | 20.37 | 31.96 | 46.29 | 70.34 | 85.21 | 93.18 | 99.4 | 100 | 100 | 28.70 | 68.26 |
| 5-FU | 9.18 | 17.84 | 28.01 | 35.39 | 47.13 | 60.35 | 71.82 | 86.97 | 95.23 | 98.12 | 100 | 27.80 | 213.71 |
IC50 value: concentration causing 50% inhibition of cell viability
aMean of three results obtained from three experiments
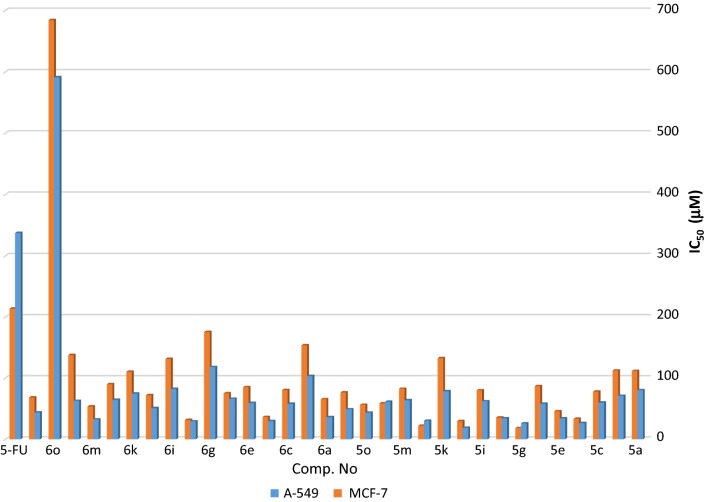
From the obtained results in Tables 2 and 3, we can show the effect of the following compounds on A-549 and MCF-7 cancer cell lines, respectively. Compound 5j possessing 4-methoxyphenylamino acetamide of morpholinosulfonyl moiety exhibited IC50 value of 18.74 µM and 29.84 µM, respectively; Compound 5g having 4-carboxyphenylamino acetamide of piperidinosulfonyl moiety showed IC50 value of 26.11 µM and 18.59 µM, respectively. While, compound 5d having 4-chlorophenylamino acetamide of piperidinosulfonyl moiety showed IC50 value of 26.72 µM and 33.59 µM, respectively. On the other hand, compound 6h having 4-nitrophenyl acetamide of piperidinosulfonyl moiety showed IC50 value of 29.39 µM and 31.54 µM, respectively. Furthermore, compound 6d having 4-chlorophenyl acetamide of piperidinosulfonyl moiety showed IC50 value of 29.91 µM and 36.53 µM, respectively. Finally, compound 5l having 4-chlorophenylamino acetamide of morpholinosulfonyl moiety showed IC50 value of 30.25 µM and 22.30 µM, respectively. From the data represented in Table 2 and Fig. 3 it is clear that, the cytotoxic activity order against cell line (A-549) having the following order: 5j > 5g > 5d > 6h > 6d > 5l > 6m > 5e > 5h > 6a > 5o > 6p > 5p > 6j > 6c > 5f > 6e > 5c > 5n > 5i > 6n > 5m > 6l > 6f > 5b > 6k > 5k > 5a > 6i > 6b > 6g > 5-FU > 6o. However, from data shown in Table 3 and Fig. 3, we can concluded that, the cytotoxic activity order against cell line (MCF-7) is: 5g > 5l > 5j > 6h > 5d > 5h > 6d > 5e > 6m > 5o > 5n > 6a > 6p > 6j > 6f > 5p > 5c > 5i > 6c > 5m > 6e > 5f > 6l > 6k > 5a > 5b > 6i > 5k > 6n > 6b > 6g > 5-FU > 6o. The previous biological screening results of the tested compounds lead to development of potential anticancer agents.
Fig. 3.
Comparison of cytotoxic activity of the tested compounds against (A-549) and (MCF-7) cell lines
Docking and molecular modeling study
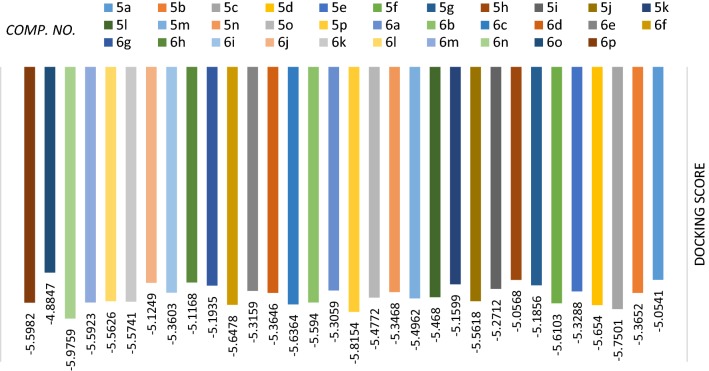
The best enzymes which involved in the improvement of anticancer and antimicrobial activity are thymidylate synthase and dihydrofolate reductase (DHFR) [50, 54]. In the present investigation, Molecular Operating Environment (MOE) [55] module was accomplished to vindicate the cytotoxic potency of all tested compounds. Furthermore, study of Molecular docking help in explanation of how compounds act through their reaction with the enzyme active sites. Docking was performed for the compounds 5a–5p and 6a–6p on the (DHFR) to predict their action as anticancer drugs (c.f. Additional file 1). The synthesized compounds show numerous interactions with DHFR enzyme. It’s important to mention that compounds 6n, 5p, 5c, 5d, 6f and 6c could make their action via inhibition of the DHFR enzyme (Table 4). In Fig. 4, the docking score energy for the newly synthesized compounds was indicated as the following order: 6n > 5p > 5c > 5d > 6f > 6c > 5f > 6p > 6b > 6m > 6k > 6l > 5j > 5m > 5o > 5l > 5b > 6d > 6i > 5n > 5e > 6e > 6a > 5i > 6g > 5g > 5k > 6j > 6h > 5h > 5a > 6o.
Table 4.
Score energy of the tested compounds 5a–p and 6a–p
| Comp. no. | Score | E_conf | E_place | E_score1 | E_score2 | E_refine |
|---|---|---|---|---|---|---|
| 5a | − 5.0541 | 26.6140 | − 31.9343 | − 6.5932 | − 5.0541 | − 24.1143 |
| 5b | − 5.3652 | 15.3242 | − 38.0154 | − 6.0735 | − 5.3652 | − 26.8906 |
| 5c | − 5.7501 | 17.6782 | − 22.8678 | − 6.5044 | − 5.7501 | − 30.5432 |
| 5d | − 5.6540 | 24.5859 | − 31.2672 | − 6.5266 | − 5.6540 | − 27.5442 |
| 5e | − 5.3288 | 23.5121 | − 39.4718 | − 6.5074 | − 5.3288 | − 27.0244 |
| 5f | − 5.6103 | 33.4059 | − 20.9497 | − 6.4211 | − 5.6103 | − 29.1261 |
| 5g | − 5.1856 | − 42.3167 | − 22.2309 | − 6.4648 | − 5.1856 | − 24.4937 |
| 5h | − 5.0568 | 49.2723 | − 4.9075 | − 5.7500 | − 5.0568 | − 23.4996 |
| 5i | − 5.2712 | 59.3063 | − 22.4680 | − 5.4465 | − 5.2712 | − 24.9909 |
| 5j | − 5.5618 | 48.5778 | − 27.9431 | − 6.3215 | − 5.5618 | − 28.8293 |
| 5k | − 5.1599 | 55.1210 | − 25.0222 | − 6.7661 | − 5.1599 | − 25.1400 |
| 5l | − 5.4680 | 52.7716 | − 29.2492 | − 6.7614 | − 5.4680 | − 27.0499 |
| 5m | − 5.4962 | 54.0440 | − 28.8482 | − 5.9473 | − 5.4962 | − 27.2928 |
| 5n | − 5.3468 | 71.7895 | − 10.6748 | − 5.9170 | − 5.3468 | − 25.8020 |
| 5o | − 5.4772 | − 8.5979 | − 36.7758 | − 6.7380 | − 5.4772 | − 29.3019 |
| 5p | − 5.8154 | 93.6886 | − 29.1507 | − 6.7008 | − 5.8154 | − 27.9044 |
| 6a | − 5.3059 | 28.9177 | − 33.5175 | − 6.5957 | − 5.3059 | − 25.2110 |
| 6b | − 5.5940 | 26.3542 | − 40.3486 | − 7.4533 | − 5.5940 | − 27.7459 |
| 6c | − 5.6364 | 21.9008 | − 5.0259 | − 6.0110 | − 5.6364 | − 26.9364 |
| 6d | − 5.3646 | 22.8002 | − 5.5670 | − 6.3649 | − 5.3646 | − 27.1061 |
| 6e | − 5.3159 | 23.2172 | − 28.6743 | − 6.2439 | − 5.3159 | − 26.9349 |
| 6f | − 5.6478 | 35.7362 | − 29.6484 | − 6.7947 | − 5.6478 | − 28.8622 |
| 6g | − 5.1935 | − 37.9832 | − 17.5087 | − 4.9311 | − 5.1935 | − 26.2035 |
| 6h | − 5.1168 | 54.5965 | − 35.3328 | − 7.2622 | − 5.1168 | − 24.2974 |
| 6i | − 5.3603 | 64.5399 | − 47.4900 | − 6.8921 | − 5.3603 | − 26.9684 |
| 6j | − 5.1249 | 55.1297 | − 29.5479 | − 6.4586 | − 5.1249 | − 25.9026 |
| 6k | − 5.5741 | 58.0647 | − 33.7004 | − 6.4011 | − 5.5741 | − 29.5841 |
| 6l | − 5.5626 | 61.7654 | − 25.5315 | − 6.9615 | − 5.5626 | − 30.0500 |
| 6m | − 5.5923 | 61.2420 | − 21.5826 | − 6.5719 | − 5.5923 | − 29.0718 |
| 6n | − 5.9759 | 67.6646 | − 8.4886 | − 6.9574 | − 5.9759 | − 32.6043 |
| 6o | − 4.8847 | − 4.0898 | − 9.1947 | − 5.7962 | − 4.8847 | − 21.8406 |
| 6p | − 5.5982 | 96.4896 | − 35.1380 | − 7.6471 | − 5.5982 | − 28.5998 |
Score lower scores shows more poses that are favorable. The unit is kcal mol−1; E-conf indicate the energy of the conformer; E-place score from the placement stage; E-score 1 score from the first rescoring stage; E-score 2 score from the second rescoring stage; E-refine score from the refinement stage
Fig. 4.
The docking score energy of the tested synthesized compounds
Docking of 5-Fluorouracil (5-FU) into DHFR
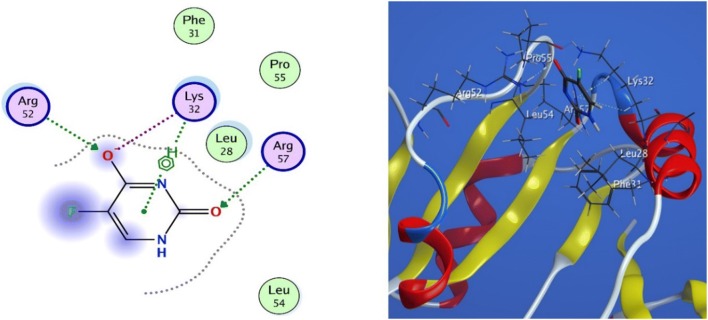
The docking studies at the active site showed presence of two hydrogen bond interactions as two oxygen atoms acted as hydrogen bond acceptors with Arg 52 and Arg 57 (3.13 Å and 2.96 Å) with binding energies of − 2.8 and − 6.5 kcal mol−1, respectively. Moreover, it indicated the presence of two ionic bond interactions that is between oxygen atom and amino acid residues Lys 32 and Arg 52 (3.14 Å and 3.05 Å) with binding energies of − 3.6 and − 4.1 kcal mol−1, respectively. Furthermore, it showed an arene-H interaction between the phenyl ring and Lys 32 (3.81 Å) with binding energy of − 0.9 kcal mol−1. besides, many hydrophobic interactions with: Leu 28, Leu 54, Phe 31 and Pro 55, (Fig. 5).
Fig. 5.
Docking of (5-FU) into DHFR
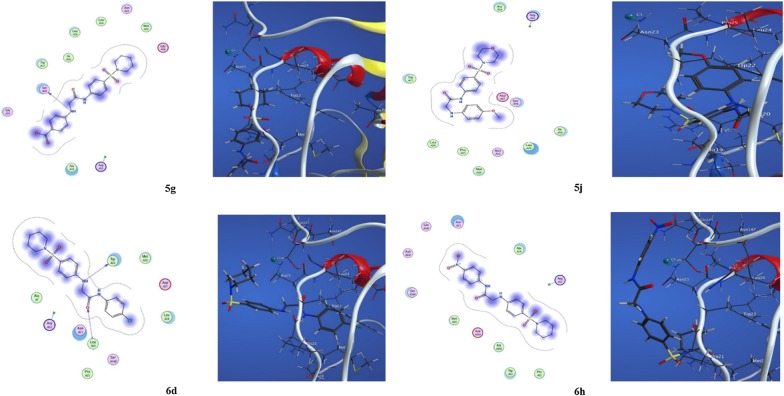
Docking of compounds 5g, 5j, 6d and 6h into DHFR
Methotrexate (PDB ID: 4DFR) used as a template with dihydrofolate reductase co-crystallized in MOE docking studies for the inhibitors. Docking of 5g showed one hydrogen bond interaction as one of the nitrogen atom acted as a hydrogen bond donor with Ser 49 (3.04 Å) with energy − 0.9 kcal mol−1. This is beside several hydrophobic interactions with various amino acid residues: Glu 48, Met 20, Asn 23, Leu 24, Leu 28, Ile 50, Trp 22, Gly 51, Ala 19, Arg 52. In addition, molecular docking study of 5j indicated hydrophobic interactions between various atoms and amino acid residues: Asp 27, Ser 49, Ile 50, Leu 28, Asn 23, Met 20, Pro 25, Leu 24, Trp 22, Ala 19, Arg 52. Furthermore, docking studies of 6d showed that nitrogen acted as a hydrogen bond donor with Trp 22 (2.97 Å) with binding energy of − 1.7 kcal mol−1. Whereas oxygen of carbonyl group acted as a hydrogen bond acceptor with Leu 24 (3.07 Å) with binding energy of − 0.7 kcal mol−1. This is beside hydrophobic interactions different amino acid residues: Met 20, Asp 27, Leu 28, Ser 148, Pro 25, Asn 23, Arg 52, Ala 7. While, docking of 6h showed that there are various hydrophobic interactions among atoms of the compound and different amino acid residues: Arg 52, Pro 21, Trp 22, Ala 145, Asp 144, Met 20, Ser 148, Asn 147, Gln 146, Asn 23, Ala 19 (Fig. 6).
Fig. 6.
Docking of compounds 5g, 5j, 6d and 6h into DHFR
Conclusion
We report herein the synthesis of some new series of 2-(arylamino)acetamides and N-arylacetamides bearing sulfonamide moieties. Most of these new compounds exhibited significant anticancer activity against human lung carcinoma (A-549) and human breast carcinoma (MCF-7) cell lines, when compared to 5-Fluorouracil as a reference drug. In addition, on antimicrobial evaluation; some of these synthesized compounds showed remarkable activity as antibacterial and antifungal agents. To the best of our knowledge, these multi-addressable properties of the new synthesized sulfonamides reported in this work will open a new era in the field of medicinal chemistry and can be considered as pharmacophores.
Experimental
Chemistry
General methods
All solvents used purchased from Sigma-Aldrich are spectroscopic grade and used without further purifications. Melting points were determined on a Stuart SMP3 melting point apparatus and are uncorrected. FT-IR spectra were recorded on a Shimadzu IR-3600 FT-IR spectrometer in KBr pellets. NMR spectra were acquired on a Bruker Avance 500 instrument (500 MHz for 1H, 125 MHz for 13C) in CDCl3 and DMSO-d6 solutions, using residual solvent signals as internal standards.
General procedure for synthesis of 2-chloro-N-(4-((piperidino/morpholino)sulfonyl)-phenyl)acetamides 2a,b
A mixture of 4-(piperidin-1-ylsulfonyl)aniline (1a) or 4-(morpholin-4-ylsulfonyl)aniline (1b) 1 (0.1 mol) and chloroacetyl chloride (8.0 mL, 0.1 mol) in DMF (20 mL) was stirred at room temperature for 2 h. The reaction mixture was poured onto ice-water. The solid obtained was filtered off and crystallized from ethanol to give 2a,b.
2-Chloro-N-(4-(piperidin-1-ylsulfonyl)phenyl)acetamide (2a)
White crystals, yield (98%), m.p. 162–163 °C. FT-IR: 3334 (NH), 3056 (CH arom.), 2945 (CH aliph.), 1696 (C=O) cm−1. 1H NMR (CDCl3): δ = 8.56 (s, 1H, NH), 7.76 (d, J = 5.5 Hz, 2H, Ph-H), 7.73 (d, J = 5.5 Hz, 2H, Ph-H), 4.21 (s, 2H, CH2), 3.00–2.97 (m, 4H, 2CH2), 1.66–1.64 (m, 4H, 2CH2), 1.43–1.42 (m, 2H, CH2) ppm. 13C NMR: δ = 164.4 (C=O), 140.7 (C), 132.0 (C), 128.9 (2CH), 119.7 (2CH), 46.9 (CH2), 42.9 (2CH2), 25.1 (2CH2), 23.4 (CH2) ppm.
2-Chloro-N-(4-(morpholinosulfonyl)phenyl)acetamide (2b)
White crystals, yield (96%), m.p. 189–190 °C. FT-IR: 3336 (NH), 3050 (CH arom.), 2961 (CH aliph.), 1694 (C=O) cm−1. 1H NMR (CDCl3): δ = 8.53 (s, 1H, NH), 7.79 (d, J = 5.0 Hz, 2H, Ph-H), 7.75 (d, J = 5.0 Hz, 2H, Ph-H), 4.24 (s, 2H, CH2), 3.75–3.73 (m, 4H, 2CH2), 3.00–2.90 (m, 4H, 2CH2) ppm. 13C NMR: δ=164.3 (C=O), 141.1 (C), 130.9 (C), 130.0 (CH), 129.1 (CH), 119.8 (CH), 114.0 (CH), 66.1 (CH2), 46.0 (2CH2), 42.8 (2CH2) ppm.
General procedure for the synthesis of 2-chloro-N-arylacetamides 4a–h
A mixture of different aryl amines namely; aniline, 4-methoxyaniline, 4-methylaniline, 4-chloroaniline, 4-bromoaniline, ethyl 4-aminobenzoate, 4-aminobenzoic acid, and 4-nitroaniline (0.05 mol) and chloroacetyl chloride (4.0 ml, 0.05 mol) in DMF (10 mL) was stirred at room temperature for 2 h. The reaction mixture was poured onto ice–water. The solid obtained was filtered off, dried and crystallized from dioxane to give 4a–i. The physical and chemical properties of 4a–i were matched as previously reported [56–61].
General procedure for synthesis of 2-(arylamino)-N-(4-(piperidin-1-ylsulfonyl)-phenyl)acetamides 5a–h and 2-(arylamino)-N-(4-(morpholino-sulfonyl)phenyl)-acetamides 5i–p
A mixture of chloro compound 2a,b (0.001 mol) and different aryl amines namely; aniline, 4-methoxyaniline, 4-methylaniline, 4-chloroaniline, 4-bromoaniline, ethyl 4-aminobenzoate, 4-aminobenzoic acid, and 4-nitroaniline (0.001 mol) in absolute ethanol (20 mL) was refluxed for 3–5 h. The reaction mixture was concentrated under reduced pressure, the solid obtained was filtered, washed with n-hexane, dried and recrystallized from ethanol to give the titled products 5a–h and 5i–p.
2-(Phenylamino)-N-(4-(piperidin-1-ylsulfonyl)phenyl)acetamide (5a)
Beige crystals, yield (60%), m.p. 115–116 °C. FT-IR: 3378 (NH), 3311 (NH), 3090 (CH arom.), 2940 (CH aliph.), 2850 (CH aliph.), 1679 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.81 (s, 1H, NH), 10.51 (s, 1H, NH), 7.88 (d, J = 11.0 Hz, 2H, Ph-H), 7.69 (d, J = 11.0 Hz, 2H, Ph-H), 7.13-7.09 (m, 2H, Ph-H), 6.65–6.61 (m, 3H, Ph-H), 4.33 (s, 2H, CH2), 2.85–2.83 (m, 4H, 2CH2), 1.54–1.52 (m, 4H, 2CH2), 1.32–1.30 (m, 2H, CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.8 (C=O), 143.3 (C), 142.9 (C), 130.2 (C), 129.4 (CH), 129.2 (CH), 129.1 (CH), 119.6 (CH), 119.4 (CH), 113.1 (CH), 47.0 (CH2), 44.0 (CH2), 24.6 (CH2), 22.8 (CH2) ppm.
2-(4-Methoxyphenylamino)-N-(4-(piperidin-1-ylsulfonyl)phenyl)acetamide (5b)
Pale yellow crystals, yield (69%), m.p. 85–87 °C. FT-IR: 3442 (NH), 3299 (NH), 3043 (CH arom.), 2940 (CH aliph.), 2850 (CH aliph.), 1681 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.75 (s, 1H, NH), 10.52 (s, 1H, NH), 7.94 (d, J = 10.5 Hz, 2H, Ph-H), 7.72 (d, J = 10.5 Hz, 2H, Ph-H), 6.77 (d, J = 10.5 Hz, 2H, Ph-H), 6.68 (d, 2H, J = 10.5 Hz, Ph-H), 4.33 (s, 2H, CH2), 3.93 (s, 3H, CH3), 2.85–2.84 (m, 4H, 2CH2), 1.53–1.51 (m, 4H, 2CH2), 1.32–1.30 (m, 2H, CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.8 (C=O), 143.1 (C), 142.9 (C), 132.9 (C), 132.5 (C), 129.2 (CH), 124.2 (CH), 119.6 (CH), 115.1 (CH), 55.7 (CH3), 47.0 (CH2), 43.8 (CH2), 25.1 (CH2), 23.3 (CH2) ppm.
N-(4-(Piperidin-1-ylsulfonyl)phenyl)-2-(p-tolylamino)acetamide (5c)
Beige crystals, yield (67%), m.p. 100–102 °C. FT-IR: 3378 (NH), 3310 (NH), 3080 (CH arom.), 2942 (CH aliph.), 2850 (CH aliph.), 1679 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.75 (s, 1H, NH), 10.40 (s, 1H, NH), 7.86 (d, J = 11.0 Hz, 2H, Ph-H), 7.83 (d, J = 11.0 Hz, 2H, Ph-H), 7.70 (d, J = 10.5 Hz, 2H, Ph-H), 6.66 (d, 2H, J = 10.5 Hz, Ph-H), 4.32 (s, 2H, CH2), 2.86–2.83 (m, 4H, 2CH2), 2.14 (s, 3H, CH3), 1.52–1.51 (m, 4H, 2CH2), 1.34–1.31 (m, 2H, CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.6 (C=O), 146.3 (C), 142.9 (C), 130.3 (C), 130.0 (C), 129.8 (CH), 129.2 (CH), 119.5 (CH), 113.0 (CH), 47.0 (CH2), 44.0 (CH2), 25.1 (CH2), 23.3 (CH2), 20.5 (CH3) ppm.
2-(4-Chlorophenylamino)-N-(4-(piperidin-1-ylsulfonyl)phenyl)acetamide (5d)
White crystals, yield (58%), m.p. 77–79 °C. FT-IR: 3334 (NH), 3329 (NH), 3056 (CH arom.), 2943 (CH aliph.), 2853 (CH aliph.), 1698 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.82 (s, 1H, NH), 10.52 (s, 1H, NH), 7.85 (d, J = 11.0 Hz, 2H, Ph-H), 7.70 (d, J = 11.0 Hz, 2H, Ph-H), 7.12 (d, J = 10.0 Hz, 2H, Ph-H), 6.61 (d, J = 10.0 Hz, 2H, Ph-H), 4.33 (s, 2H, CH2), 2.86–2.84 (m, 4H, 2CH2), 1.53–1.51 (m, 4H, 2CH2), 1.35–1.33 (m, 2H, CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.8 (C=O), 147.7 (C), 143.0 (C), 142.9 (C), 130.2 (C), 129.2 (CH), 119.6 (CH), 119.4 (CH), 114.1 (CH), 47.0 (CH2), 44.0 (CH2), 25.1 (CH2), 23.3 (CH2) ppm.
2-(4-Bromophenylamino)-N-(4-(piperidin-1-ylsulfonyl)phenyl)acetamide (5e)
Pale yellow crystals, yield (50%), m.p. 84–86 °C. FT-IR: 3499 (NH), 3327 (NH), 3056 (CH arom.), 2943 (CH aliph.), 2854 (CH aliph.), 1696 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.88 (s, 1H, NH), 10.56 (s, 1H, NH), 7.85 (d, J = 10.5 Hz, 2H, Ph-H), 7.70 (d, J = 10.5 Hz, 2H, Ph-H), 7.20 (d, J = 10.0 Hz, 2H, Ph-H), 6.58 (d, J = 10.0 Hz, 2H, Ph-H), 4.33 (s, 2H, CH2), 2.86–2.84 (m, 4H, 2CH2), 1.54–1.52 (m, 4H, 2CH2), 1.36–1.34 (m, 2H, CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.4 (C=O), 147.2 (C), 142.5 (C), 132.2 (C), 131.6 (C), 129.8 (CH), 128.7 (CH), 119.1 (CH), 114.8 (CH), 46.6 (CH2), 43.5 (CH2), 24.6 (CH2), 22.8 (CH2) ppm.
Ethyl 4-(2-oxo-2-(4-(piperidin-1-ylsulfonyl)phenylamino)ethylamino)benzoate (5f)
Beige crystals, yield (75%), m.p. 150–151 °C. FT-IR: 3485 (NH), 3349 (NH), 3054 (CH arom.), 2944 (CH aliph.), 2855 (CH aliph.), 1699 (C=O), 1690 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.93 (s, 1H, NH), 10.69 (s, 1H, NH), 7.88 (d, J = 10.5 Hz, 2H, Ph-H), 7.75–7.66 (m, 6H, Ph-H), 4.34 (s, 2H, CH2), 4.21 (q, J = 8.5 Hz, 2H, CH2), 2.85-2.83 (m, 4H, 2CH2), 1.52–1.50 (m, 4H, 2CH2), 1.33–1.29 (m, 2H, CH2), 1.26 (t, J = 8.5 Hz, 3H, CH3) ppm. 13C-NMR (DMSO-d6): δ = 169.8 (C=O), 165.8 (C=O), 152.8 (C), 143.1 (C), 143.0 (C), 131.4 (CH), 130.2 (C), 129.2 (CH), 119.6 (CH), 115.7 (CH), 60.3 (CH2), 47.0 (CH2), 44.0 (CH2), 25.1 (CH2), 23.3 (CH2), 14.7 (CH3) ppm.
4-(2-Oxo-2-(4-(piperidin-1-ylsulfonyl)phenylamino)ethylamino)benzoic acid (5g)
Beige crystals, yield (57%), m.p. 174–175 °C. FT-IR: 3380 (br, OH), 3333 (NH), 3062 (CH arom.), 2941 (CH aliph.), 2852 (CH aliph.), 1700 (C=O), 1680 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.81 (s, 1H, NH), 10.66 (s, 1H, NH), 7.84 (d, J = 10.5 Hz, 2H, Ph-H), 7.72–7.68 (m, 4H, Ph-H), 6.63 (d, J = 10.5 Hz, 2H, Ph-H), 4.85 (s, 1H, OH), 4.33 (s, 2H, CH2), 2.86–2.84 (m, 4H, 2CH2), 1.54–1.52 (m, 4H, 2CH2), 1.35–1.33 (m, 2H, CH2) ppm. 13C-NMR (DMSO-d6): δ = 166.2 (C=O), 165.8 (C=O), 142.9 (C), 142.8 (C), 132.1 (C), 131.9 (CH), 130.2 (C), 129.2 (CH), 119.8 (CH), 113.5 (CH), 47.0 (CH2), 44.0 (CH2), 25.1 (CH2), 23.3 (CH2) ppm.
2-(4-Nitrophenylamino)-N-(4-(piperidin-1-ylsulfonyl)phenyl)acetamide (5h)
Yellow crystals, yield (71%), m.p. 108–110 °C. FT-IR: 3483 (NH), 3363 (NH), 3057 (CH arom.), 2945 (CH aliph.), 2857 (CH aliph.), 1699 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.75 (s, 1H, NH), 10.10 (s, 1H, NH), 7.94 (d, J = 11.0 Hz, 2H, Ph-H), 7.84 (d, J = 10.5 Hz, 2H, Ph-H), 7.70 (d, J = 10.5 Hz, 2H, Ph-H), 6.60 (d, J = 11.0 Hz, 2H, Ph-H), 4.32 (s, 2H, CH2), 2.85–2.83 (m, 4H, 2CH2), 1.52–1.51 (m, 4H, 2CH2), 1.34–1.33 (m, 2H, CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.8 (C=O), 156.1 (C), 142.9 (C), 136.1 (C), 130.3 (C), 129.2 (CH), 126.8 (CH), 119.6 (CH), 112.8 (CH), 47.0 (CH2), 44.0 (CH2), 25.1 (CH2), 23.3 (CH2) ppm.
N-(4-(Morpholinosulfonyl)phenyl)-2-(phenylamino)acetamide (5i)
Beige crystals, yield (59%), m.p. 120–122 °C. FT-IR: 3330 (NH), 3203 (NH), 3051 (CH arom.), 2957 (CH aliph.), 2860 (CH aliph.), 1701 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.85 (s, 1H, NH), 10.50 (s, 1H, NH), 7.88 (d, J = 10.0 Hz, 2H, Ph-H), 7.72 (d, J = 10.0 Hz, 2H, Ph-H), 7.14–7.10 (m, 2H, Ph-H), 6.67–6.63 (m, 3H, Ph-H), 4.34 (s, 2H, CH2), 3.64–3.62 (m, 4H, 2CH2), 2.85–2.83 (m, 4H, 2CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.9 (C=O), 146.3 (C), 143.3 (C), 129.5 (CH), 129.4 (CH), 129.2 (C), 119.6 (CH), 119.4 (CH), 113.2 (CH), 66.7 (CH2), 46.3 (CH2), 44.0 (CH2) ppm.
2-(4-Methoxyphenylamino)-N-(4-(morpholinosulfonyl)phenyl)acetamide (5j)
Pale yellow crystals, yield (67%), m.p. 97–99 °C. FT-IR: 3400 (NH), 3332 (NH), 3099 (CH arom.), 2973 (CH aliph.), 2858 (CH aliph.), 1689 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 11.43 (s, 1H, NH), 10.93 (s, 1H, NH), 7.89 (d, J = 11.0 Hz, 2H, Ph-H), 7.71 (d, J = 11.0 Hz, 2H, Ph-H), 7.04 (d, J = 11.0 Hz, 2H, Ph-H), 6.90 (d, 2H, J = 11.0 Hz, Ph-H), 4.35 (s, 2H, CH2), 4.14 (s, 3H, CH3), 3.65–3.63 (m, 4H, 2CH2), 2.84–2.82 (m, 4H, 2CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.6 (C=O), 142.8 (C), 141.7 (C), 129.7 (CH), 129.2 (C), 119.5 (CH), 115.2 (CH), 65.7 (CH2), 55.8 (CH3), 46.3 (CH2), 44.0 (CH2) ppm.
N-(4-(morpholinosulfonyl)phenyl)-2-(p-tolylamino)acetamide (5k)
Beige crystals, yield (69%), m.p. 86–88 °C. FT-IR: 3332 (NH), 3190 (NH), 3104 (CH arom.), 2973 (CH aliph.), 2859 (CH aliph.), 1698 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.86 (s, 1H, NH), 10.83 (s, 1H, NH), 7.87 (d, J = 10.5 Hz, 2H, Ph-H), 7.72 (d, J = 10.0 Hz, 2H, Ph-H), 7.26–7.00 (m, 4H, Ph-H), 4.33 (s, 2H, CH2), 3.62–3.60 (m, 4H, 2CH2), 2.85–2.83 (m, 4H, 2CH2), 2.34 (s, 3H, CH3) ppm. 13C-NMR (DMSO-d6): δ = 165.9 (C=O), 143.3 (C), 131.7 (C), 129.4 (CH), 129.6 (C), 119.6 (CH), 65.7 (CH2), 46.3 (CH2), 44.0 (CH2), 20.2 (CH3) ppm.
2-(4-Chlorophenylamino)-N-(4-(morpholinosulfonyl)phenyl)acetamide (5l)
White crystals, yield (55%), m.p. 102–104 °C. FT-IR: 3339 (NH), 3191 (NH), 3055 (CH arom.), 2971 (CH aliph.), 2857 (CH aliph.), 1695 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.84 (s, 1H, NH), 10.52 (s, 1H, NH), 7.87 (d, J = 10.5 Hz, 2H, Ph-H), 7.71 (d, J = 10.5 Hz, 2H, Ph-H), 7.12 (d, J = 11.0 Hz, 2H, Ph-H), 6.61 (d, J = 11.0 Hz, 2H, Ph-H), 4.33 (s, 2H, CH2), 3.63–3.61 (m, 4H, 2CH2), 2.84–2.82 (m, 4H, 2CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.9 (C = O), 147.9 (C), 143.4 (C), 143.3 (C), 129.5 (CH), 129.1 (C), 119.6 (CH), 114.2 (CH), 65.7 (CH2), 46.3 (CH2), 44.0 (CH2) ppm.
2-(4-Bromophenylamino)-N-(4-(morpholinosulfonyl)phenyl)acetamide (5m)
Beige crystals, yield (51%), m.p. 126–128 °C. FT-IR: 3341 (NH), 3101 (NH), 3060 (CH arom.), 2970 (CH aliph.), 2854 (CH aliph.), 1700 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.87 (s, 1H, NH), 10.54 (s, 1H, NH), 7.86 (d, J = 11.0 Hz, 2H, Ph-H), 7.71 (d, J = 11.0 Hz, 2H, Ph-H), 7.21 (d, J = 11.0 Hz, 2H, Ph-H), 6.58 (d, J = 11.0 Hz, 2H, Ph-H), 4.34 (s, 2H, CH2), 3.96–3.94 (m, 4H, 2CH2), 2.84–2.82 (m, 4H, 2CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.9 (C=O), 147.4 (C), 143.3 (C), 132.2 (C), 131.9 (CH), 129.4 (C), 129.1 (CH), 119.6 (CH), 114.8 (CH), 65.7 (CH2), 46.3 (CH2), 44.0 (CH2) ppm.
Ethyl 4-(2-(4-(morpholinosulfonyl)phenylamino)-2-oxoethylamino)benzoate (5n)
Beige crystals, yield (78%), m.p. 90–92 °C. FT-IR: 3426 (NH), 3346 (NH), 3040 (CH arom.), 2982 (CH aliph.), 2865 (CH aliph.), 1722 (C=O), 1685 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.84 (s, 1H, NH), 10.65 (s, 1H, NH), 7.87 (d, J = 10.5 Hz, 2H, Ph-H), 7.71 (d, J = 10.5 Hz, 2H, Ph-H), 7.65 (d, J = 10.5 Hz, 2H, Ph-H), 6.59 (d, J = 10.5 Hz, 2H, Ph-H), 4.33 (s, 2H, CH2), 4.19 (q, J = 8.5 Hz, 2H, CH2), 3.62–3.60 (m, 4H, 2CH2), 2.84–2.82 (m, 4H, 2CH2), 1.26 (t, J = 8.5 Hz, 3H, CH3) ppm. 13C-NMR (DMSO-d6): δ = 166.3 (C=O), 165.9 (C=O), 153.2 (C), 143.3 (C), 131.4 (CH), 129.4 (CH), 129.1 (C), 119.6 (CH), 116.9 (C), 113.4 (CH), 65.7 (CH2), 59.9 (CH2), 46.3 (CH2), 44.0 (CH2), 14.8 (CH3) ppm.
4-(2-(4-(Morpholinosulfonyl)phenylamino)-2-oxoethylamino)benzoic acid (5o)
Beige crystals, yield (55%), m.p. 105–108 °C. FT-IR: 3475 (br, OH), 3365 (NH), 3059 (CH arom.), 2977 (CH aliph.), 2858 (CH aliph.), 1699 (C=O.), 1690 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.87 (s, 1H, NH), 10.71 (s, 1H, NH), 7.87 (d, J = 10.0 Hz, 2H, Ph-H), 7.73–7.69 (m, 4H, Ph-H), 6.66 (d, J = 10.5 Hz, 2H, Ph-H), 4.86 (s, 1H, OH), 4.33 (s, 2H, CH2), 3.63–3.61 (m, 4H, 2CH2), 2.84–2.82 (m, 4H, 2CH2) ppm. 13C-NMR (DMSO-d6): δ = 166.3 (C=O), 165.9 (C=O), 143.2 (C), 131.9 (CH), 129.5 (C), 129.4 (CH), 119.6 (C), 119.5 (CH), 113.8 (CH), 65.7 (CH2), 46.3 (CH2), 44.0 (CH2) ppm.
N-(4-(Morpholinosulfonyl)phenyl)-2-(4-nitrophenylamino)acetamide (5p)
Yellow crystals, yield (85%), m.p. 112–114 °C. FT-IR: 3343 (NH), 3362 (NH), 3055 (CH arom.), 2970 (CH aliph.), 2854 (CH aliph.), 1694 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.77 (s, 1H, NH), 7.94 (d, J = 11.0 Hz, 2H, Ph-H), 7.86 (d, J = 10.5 Hz, 2H, Ph-H), 7.71 (d, J = 10.5 Hz, 2H, Ph-H), 6.72 (s, 1H, NH), 6.60 (d, J = 11.0 Hz, 2H, Ph-H), 4.32 (s, 2H, CH2), 3.63-3.61 (m, 4H, 2CH2), 2.84–2.82 (m, 4H, 2CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.9 (C=O), 156.1 (C), 143.3 (C), 136.1 (C), 129.4 (CH), 129.1 (C), 126.8 (CH), 119.6 (CH), 112.8 (CH), 65.7 (CH2), 46.3 (CH2), 44.0 (CH2) ppm.
General procedure for synthesis of N-aryl-2-(4-(piperidin-1-ylsulfonyl)phenyl-amino)acetamides 6a–h and N-aryl-2-(4-(morpholinosulfonyl)phenylamino)- acetamides 6i–p
In a round bottomed flask, 2-chloro-N-arylacetamide 4a–h (0.001 mol), sulfonamide 1a,b (0.001 mol) and triethylamine (0.1 mL) in absolute ethanol (20 mL) was refluxed for 4-6 h. The reaction mixture was cooled to room temperature, the solid obtained was filtered, washed with cold ethanol, dried and recrystallized from ethanol to give the titled products 6a–p and 6i–p.
N-Phenyl-2-(4-(piperidin-1-ylsulfonyl)phenylamino)acetamide (6a)
White crystals, yield (96%), m.p. 122–124 °C. FT-IR: 3437 (NH), 3363 (NH), 3040 (CH arom.), 2950 (CH aliph.), 2836 (CH aliph.), 1674 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.33 (s, 1H, NH), 7.61 (d, J = 10.0 Hz, 2H, Ph-H), 7.36-7.31 (m, 4H, Ph-H), 7.10–7.07 (m, 1H, Ph-H), 6.67–6.65 (m, 2H, Ph-H), 6.00 (br s, 1H, NH), 4.27 (s, 2H, CH2), 2.77 (t, J = 6.5 Hz, 4H, 2CH2), 1.52–1.50 (m, 4H, 2CH2), 1.34-1.32 (m, 2H, CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.1 (C=O), 153.4 (C), 138.9 (C), 129.8 (CH), 129.3 (CH), 124.3 (CH), 120.5 (C), 119.8 (CH), 113.2 (CH), 47.0 (CH2), 44.0 (CH2), 25.1 (CH2), 23.4 (CH2) ppm.
N-(4-Methoxyphenyl)-2-(4-(piperidin-1-ylsulfonyl)phenylamino)acetamide (6b)
White crystals, yield (92%), m.p. 120–121 °C. FT-IR: 3437 (NH), 3340 (NH), 3072 (CH arom.), 2952 (CH aliph.), 2836 (CH aliph.), 1664 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.17 (s, 1H, NH), 7.51 (d, J = 10.5 Hz, 2H, Ph-H), 7.34 (d, J = 10.5 Hz, 2H, Ph-H), 6.91 (d, J = 10.5 Hz, 2H, Ph-H), 6.65 (d, 2H, J = 10.5 Hz, Ph-H), 6.04 (s, 1H, NH), 4.22 (s, 2H, CH2), 3.73 (s, 3H, CH3), 2.78 (t, J = 5.5 Hz, 4H, 2CH2), 1.54–1.52 (m, 4H, 2CH2), 1.34–1.32 (m, 2H, CH2) ppm. 13C-NMR (DMSO-d6): δ = 164.6 (C=O), 156.0 (C), 153.5 (C), 132.0 (C), 129.9 (CH), 121.4 (CH), 120.4 (C), 114.4 (CH), 113.1 (CH), 55.6 (CH3), 47.0 (CH2), 44.0 (CH2), 25.1 (CH2), 23.5 (CH2) ppm.
2-(4-(Piperidin-1-ylsulfonyl)phenylamino)-N-p-tolylacetamide (6c)
White crystals, yield (98%), m.p. 137–138 °C. FT-IR: 3437 (NH), 3363 (NH), 3040 (CH arom.), 2950 (CH aliph.), 2836 (CH aliph.), 1672 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.22 (s, 1H, NH), 7.48 (d, J = 10.5 Hz, 2H, Ph-H), 7.34 (d, J = 10.5 Hz, 2H, Ph-H), 7.13 (d, J = 10.5 Hz, 2H, Ph-H), 6.65 (d, 2H, J = 10.5 Hz, Ph-H), 6.04 (s, 1H, NH), 4.24 (s, 2H, CH2), 2.78 (t, J = 6.0 Hz, 4H, 2CH2), 2.26 (s, 3H, CH3), 1.53–1.51 (m, 4H, 2CH2), 1.34–1.32 (m, 2H, CH2) ppm. 13C-NMR (DMSO-d6): δ = 164.8 (C=O), 153.5 (C), 136.4 (C), 133.3 (C), 129.9 (CH), 129.7 (CH), 120.4 (C), 119.8 (CH), 113.1 (CH), 47.0 (CH2), 44.0 (CH2), 25.1 (CH2), 23.5 (CH2), 20.9 (CH3) ppm.
N-(4-Chlorophenyl)-2-(4-(piperidin-1-ylsulfonyl)phenylamino)acetamide (6d)
White crystals, yield (92%), m.p. 130–131 °C. FT-IR: 3437 (NH), 3362 (NH), 3040 (CH arom.), 2950 (CH aliph.), 2836 (CH aliph.), 1670 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.45 (s, 1H, NH), 7.63 (d, J = 10.5 Hz, 2H, Ph-H), 7.39 (d, J = 10.5 Hz, 2H, Ph-H), 7.33 (d, J = 10.5 Hz, 2H, Ph-H), 6.64 (d, 2H, J = 10.5 Hz, Ph-H), 6.04 (s, 1H, NH), 4.27 (s, 2H, CH2), 2.78 (t, J = 6.0 Hz, 4H, 2CH2), 1.53–1.51 (m, 4H, 2CH2), 1.34–1.32 (m, 2H, CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.2 (C=O), 153.5 (C), 137.9 (C), 129.8 (CH), 129.2 (CH), 127.9 (C), 121.3 (CH), 120.4 (C), 113.1 (CH), 47.0 (CH2), 43.9 (CH2), 25.1 (CH2), 23.4 (CH2) ppm.
N-(4-Bromophenyl)-2-(4-(piperidin-1-ylsulfonyl)phenylamino)acetamide (6e)
White crystals, yield (97%), m.p. 145–146 °C. FT-IR: 3437 (NH), 3362 (NH), 3045 (CH arom.), 2950 (CH aliph.), 2836 (CH aliph.), 1671 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.45 (s, 1H, NH), 7.58 (d, J = 10.5 Hz, 2H, Ph-H), 7.51 (d, J = 10.5 Hz, 2H, Ph-H), 7.34 (d, J = 11.0 Hz, 2H, Ph-H), 6.65 (d, 2H, J = 11.0 Hz, Ph-H), 6.04 (s, 1H, NH), 4.27 (s, 2H, CH2), 2.78 (t, J = 6.5 Hz, 4H, 2CH2), 1.53–1.51 (m, 4H, 2CH2), 1.34–1.32 (m, 2H, CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.2 (C=O), 153.4 (C), 138.3 (C), 132.1 (CH), 129.8 (CH), 121.7 (CH), 120.4 (C), 115.6 (C), 113.1 (CH), 47.0 (CH2), 44.0 (CH2), 25.1 (CH2), 23.4 (CH2) ppm.
Ethyl 4-(2-(4-(piperidin-1-ylsulfonyl)phenylamino)acetamido)benzoate (6f)
White crystals, yield (91%), m.p. 75–76 °C. FT-IR: 3275 (NH), 3203 (NH), 3090 (CH arom.), 2947 (CH aliph.), 2858 (CH aliph.), 1721 (C=O), 1679 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.75 (s, 1H, NH), 10.65 (s, 1H, NH), 7.94 (d, J = 10.5 Hz, 2H, Ph-H), 7.83 (d, J = 10.5 Hz, 2H, Ph-H), 7.74 (d, J = 10.5 Hz, 2H, Ph-H), 7.71 (d, 2H, J = 10.5 Hz, Ph-H), 4.29 (q, J = 6.0 Hz, 4H, 2CH2), 2.85 (t, J = 6.0 Hz, 4H, 2CH2), 1.54–1.52 (m, 4H, 2CH2), 1.34 (t, J = 6.0 Hz, 3H, CH3), 1.33–1.29 (m, 2H, CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.8 (C=O), 165.6 (C=O), 143.2 (C), 142.9 (C), 130.8 (CH), 130.3 (C), 129.2 (CH), 125.6 (C), 119.6 (CH), 119.2 (CH), 60.9 (CH2), 47.0 (CH2), 44.0 (CH2), 25.1 (CH2), 23.3 (CH2), 14.6 (CH3) ppm.
4-(2-(4-(Piperidin-1-ylsulfonyl)phenylamino)acetamido)benzoic acid (6g)
White crystals, yield (81%), m.p. 164–166 °C. FT-IR: 3437 (br OH), 3340 (NH), 3000 (CH arom.), 2950 (CH aliph.), 2836 (CH aliph.), 1680 (C=O), 1641 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 12.77 (s, 1H, OH), 10.94 (s, 1H, NH), 7.92 (d, J = 10.5 Hz, 2H, Ph-H), 7.76 (d, J = 10.5 Hz, 2H, Ph-H), 7.33 (d, J = 10.5 Hz, 2H, Ph-H), 6.65 (d, 2H, J = 10.5 Hz, Ph-H), 6.07 (s, 1H, NH), 4.35 (s, 2H, CH2), 2.79–2.76 (m, 4H, 2CH2), 1.53–1.50 (m, 4H, 2CH2), 1.35–1.33 (m, 2H, CH2) ppm. 13C-NMR (DMSO-d6): δ = 167.1 (C=O), 165.7 (C=O), 153.5 (C), 143.5 (C), 131.4 (CH), 129.8 (CH), 126.4 (C), 120.3 (C), 119.0 (CH), 113.1 (CH), 47.0 (CH2), 44.0 (CH2), 25.1 (CH2), 23.4 (CH2) ppm.
N-(4-Nitrophenyl)-2-(4-(piperidin-1-ylsulfonyl)phenylamino)acetamide (6h)
Yellow crystals, yield (85%), m.p. 142–144 °C. FT-IR: 3437 (NH), 3362 (NH), 3099 (CH arom.), 2947 (CH aliph.), 2836 (CH aliph.), 1685 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.92 (s, 1H, NH), 8.24 (d, J = 11.5 Hz, 2H, Ph-H), 7.85 (d, J = 11.5 Hz, 2H, Ph-H), 7.33 (d, J = 11.0 Hz, 2H, Ph-H), 6.65 (d, 2H, J = 11.0 Hz, Ph-H), 6.04 (s, 1H, NH), 4.35 (s, 2H, CH2), 2.77 (t, J = 6.0 Hz, 4H, 2CH2), 1.53–1.51 (m, 4H, 2CH2), 1.33–1.31 (m, 2H, CH2) ppm. 13C-NMR (DMSO-d6): δ = 166.0 (C=O), 153.4 (C), 145.0 (C), 143.0 (C), 129.8 (CH), 125.5 (CH), 120.4 (C), 119.5 (C), 113.1 (CH), 47.0 (CH2), 44.0 (CH2), 25.1 (CH2), 23.4 (CH2) ppm.
2-(4-(Morpholinosulfonyl)phenylamino)-N-phenylacetamide (6i)
Beige crystals, yield (98%), m.p. 133–134 °C. FT-IR: 3444 (NH), 3365 (NH), 3099 (CH arom.), 2985 (CH aliph.), 2847 (CH aliph.), 1672 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.30 (s, 1H, NH), 7.59 (d, J = 9.5 Hz, 2H, Ph-H), 7.35 (d, J = 10.5 Hz, 2H, Ph-H), 7.32 (d, J = 9.5 Hz, 2H, Ph-H), 7.10–7.06 (m, 1H, Ph-H), 6.68–6.66 (m, 2H, Ph-H), 6.12 (s, 1H, NH), 4.26 (s, 2H, CH2), 3.62–3.60 (m, 4H, 2CH2), 2.77–2.75 (m, 4H, 2CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.1 (C=O), 153.8 (C), 138.9 (C), 130.2 (CH), 129.3 (CH), 124.3 (CH), 119.8 (CH), 119.1 (C), 113.2 (CH), 65.7 (CH2), 46.4 (CH2), 44.0 (CH2) ppm.
N-(4-Methoxyphenyl)-2-(4-(morpholinosulfonyl)phenylamino)acetamide (6j)
Pale yellow crystals, yield (94%), m.p. 127–128 °C. FT-IR: 3444 (NH), 3364 (NH), 3073 (CH arom.), 2957 (CH aliph.), 2844 (CH aliph.), 1665 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.17 (s, 1H, NH), 7.51 (d, J = 11.0 Hz, 2H, Ph-H), 7.35 (d, J = 10.5 Hz, 2H, Ph-H), 6.90 (d, J = 11.0 Hz, 2H, Ph-H), 6.67 (d, J = 10.5 Hz, 2H, Ph-H), 6.13 (s, 1H, NH), 4.22 (s, 2H, CH2), 3.72 (s, 3H, CH3), 3.62–3.60 (m, 4H, 2CH2), 2.77–2.75 (m, 4H, 2CH2) ppm. 13C-NMR (DMSO-d6): δ = 164.6 (C=O), 156.0 (C), 153.8 (C), 132.0 (C), 130.2 (CH), 121.4 (CH), 119.1 (C), 114.4 (CH), 113.2 (CH), 65.7 (CH2), 55.6 (CH3), 46.3 (CH2), 44.0 (CH2), ppm.
2-(4-(Morpholinosulfonyl)phenylamino)-N-p-tolylacetamide (6k)
Beige crystals, yield (55%), m.p. 158–160 °C. FT-IR: 3444 (NH), 3364 (NH), 3035 (CH arom.), 2916 (CH aliph.), 2848 (CH aliph.), 1673 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.21 (s, 1H, NH), 7.48 (d, J = 10.0 Hz, 2H, Ph-H), 7.36 (d, J = 10.5 Hz, 2H, Ph-H), 7.13 (d, J = 10.0 Hz, 2H, Ph-H), 6.68 (d, 2H, J = 10.5 Hz, Ph-H), 6.12 (s, 1H, NH), 4.23 (s, 2H, CH2), 3.61-3.59 (m, 4H, 2CH2), 2.77–2.75 (m, 4H, 2CH2), 2.25 (s, 3H, CH3) ppm. 13C-NMR (DMSO-d6): δ = 164.8 (C=O), 153.8 (C), 136.4 (C), 133.3 (C), 129.6 (CH), 129.8 (CH), 119.1 (CH), 113.2 (CH), 65.7 (CH2), 46.3 (CH2), 44.0 (CH2), 20.9 (CH3) ppm.
N-(4-Chlorophenyl)-2-(4-(morpholinosulfonyl)phenylamino)acetamide (6l)
White crystals, yield (98%), m.p. 166–168 °C. FT-IR: 3444 (NH), 3365 (NH), 3084 (CH arom.), 2984 (CH aliph.), 2847 (CH aliph.), 1670 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.44 (s, 1H, NH), 7.62 (d, J = 10.5 Hz, 2H, Ph-H), 7.39 (d, J = 10.5 Hz, 2H, Ph-H), 7.35 (d, J = 10.5 Hz, 2H, Ph-H), 6.67 (d, 2H, J = 10.5 Hz, Ph-H), 6.12 (s, 1H, NH), 4.26 (s, 2H, CH2), 3.61-3.59 (m, 4H, 2CH2), 2.77–2.75 (m, 4H, 2CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.2 (C=O), 153.8 (C), 137.9 (C), 130.2 (CH), 129.2 (CH), 127.9 (C), 121.3 (CH), 119.1 (C), 113.2 (CH), 65.7 (CH2), 46.3 (CH2), 43.9 (CH2) ppm.
N-(4-Bromophenyl)-2-(4-(morpholinosulfonyl)phenylamino)acetamide (6m)
White crystals, yield (95%), m.p. 173–175 °C. FT-IR: 3444 (NH), 3365 (NH), 3078 (CH arom.), 2954 (CH aliph.), 2847 (CH aliph.), 1671 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.44 (s, 1H, NH), 7.57 (d, J = 10.5 Hz, 2H, Ph-H), 7.52 (d, J = 10.5 Hz, 2H, Ph-H), 7.35 (d, J = 10.5 Hz, 2H, Ph-H), 6.67 (d, 2H, J = 10.5 Hz, Ph-H), 6.12 (s, 1H, NH), 4.26 (s, 2H, CH2), 3.61-3.59 (m, 4H, 2CH2), 2.77–2.75 (m, 4H, 2CH2) ppm. 13C-NMR (DMSO-d6): δ = 165.2 (C=O), 153.8 (C), 138.3 (C), 132.1 (CH), 130.2 (CH), 121.7 (CH), 119.1 (C), 116.4 (C), 113.2 (CH), 65.7 (CH2), 46.4 (CH2), 44.0 (CH2) ppm.
Ethyl 4-(2-(4-(morpholinosulfonyl)phenylamino)acetamido)benzoate (6n)
White crystals, yield (94%), m.p. 97–99 °C. FT-IR: 3327 (NH), 3276 (NH), 3087 (CH arom.), 2971 (CH aliph.), 2863 (CH aliph.), 1720 (C=O), 1676 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.83 (s, 1H, NH), 10.69 (s, 1H, NH), 7.93 (d, J = 10.5 Hz, 2H, Ph-H), 7.87 (d, J = 10.5 Hz, 2H, Ph-H), 7.74 (d, J = 11.0 Hz, 2H, Ph-H), 7.71 (d, J = 11.0, 2H, Ph-H), 4.33–4.25 (m, 4H, 2CH2), 3.63–3.61 (m, 4H, 2CH2), 2.84–2.82 (m, 4H, 2CH2), 1.29 (t, J = 8.5 Hz, 3H, CH3) ppm. 13C-NMR (DMSO-d6): δ = 165.8 (C=O), 165.6 (C=O), 143.2 (C), 143.2 (C), 130.7 (CH), 129.4 (CH), 129.1 (C), 125.2 (C), 119.6 (CH), 119.2 (CH), 65.7 (CH2), 60.9 (CH2), 46.3 (CH2), 44.0 (CH2), 14.6 (CH3) ppm.
4-(2-(4-(Morpholinosulfonyl)phenylamino)acetamido)benzoic acid (6o)
White crystals, yield (83%), m.p. 197–198 °C. FT-IR: 3444 (br OH), 3365 (NH), 3038 (CH arom.), 2984 (CH aliph.), 2847 (CH aliph.), 1682 (C=O),1642 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 12.12 (s, 1H, OH), 10.62 (s, 1H, NH), 7.92 (d, J = 10.5 Hz, 2H, Ph-H), 7.71 (d, J = 10.5 Hz, 2H, Ph-H), 7.35 (d, J = 10.5 Hz, 2H, Ph-H), 6.66 (d, 2H, J = 10.5 Hz, Ph-H), 6.12 (s, 1H, NH), 4.30 (s, 2H, CH2), 3.61–3.59 (m, 4H, 2CH2), 2.77–2.75 (m, 4H, 2CH2) ppm. 13C-NMR (DMSO-d6): δ = 167.3 (C=O), 165.7 (C=O), 153.8 (C), 142.8 (C), 131.0 (C), 130.9 (CH), 130.2 (CH), 126.4 (C), 119.1 (CH), 113.2 (CH), 65.7 (CH2), 46.3 (CH2), 44.0 (CH2) ppm.
2-(4-(Morpholinosulfonyl)phenylamino)-N-(4-nitrophenyl)acetamide (6p)
Pale yellow crystals, yield (96%), m.p. 158–160 °C. FT-IR: 3444 (NH), 3365 (NH), 3097 (CH arom.), 2984 (CH aliph.), 2847 (CH aliph,), 1686 (C=O) cm−1. 1H-NMR (DMSO-d6): δ = 10.91 (s, 1H, NH), 8.24 (d, J = 10.5 Hz, 2H, Ph-H), 7.84 (d, J = 10.5 Hz, 2H, Ph-H), 7.35 (d, J = 10.0 Hz, 2H, Ph-H), 6.67 (d, 2H, J = 10.0 Hz, Ph-H), 6.11 (s, 1H, NH), 4.35 (s, 2H, CH2), 3.61–3.59 (m, 4H, 2CH2), 2.77–2.75 (m, 4H, 2CH2) ppm. 13C-NMR (DMSO-d6): δ = 166.0 (C=O), 153.8 (C), 145.0 (C), 143.0 (C), 130.1 (CH), 125.5 (CH), 119.5 (CH), 119.1 (C), 113.2 (CH), 65.7 (CH2), 46.3 (CH2), 44.0 (CH2) ppm.
Antimicrobial screening
All microbial strains were provided from culture collection of the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, Cairo, Egypt. The antimicrobial activity was evaluated by disc-agar diffusion method [62–64] using Mueller–Hinton Agar. Activation of various strains of bacteria occurred by using a loop that full of bacterial strain in the broth and incubated for 24 h at 37 °C. Furthermore, 0.1 mL of the suspension of strains was poured on the agar, spread well and left to solidify. Moreover, using a sterile cork, about 0.9 cm cut was made and were filled completely with the tested compound solution. The wells were incubated at 37 °C for 24 h. Each assay was done in triplicate. ZOI was determined using the mean value. Tested compound giving high ZOI value reflecting its significant antibacterial activity. Efficacy of the novel compounds was measured against different strains of Gram positive and Gram-negative bacteria, also evaluated against different fungal strains. The Gram-positive organisms that were used for culture sensitivity include S. aureus (RCMB010010), and B. subtilis RCMB 015 (1) NRRL B-543, on the other hand, the Gram-negative organisms that were used for culture sensitivity include E. coli (RCMB 010052) ATCC 25955, and P. vulgaris RCMB 004 (1) ATCC 13315. The fungal strains that were used include A. fumigatus (RCMB 002008), and C. albicans RCMB 005003 (1) ATCC 10231. Different antibiotics were used as a reference for evaluating the antimicrobial activity of novel compounds. Gentamycin and Ketoconazole were used as a reference antibiotic for assessing the antimicrobial activity of the novel compounds against bacterial and fungal strains, respectively.
In vitro anticancer screening
The cell lines were purchased from the American Type Culture collection and their accession number as follows: A-549 (ATCC CCL-185™) lung carcinoma cell line and MCF-7 (ATCC HTB-22™) breast adenocarcinoma cell line.
Cytotoxic activity screening was performed at Regional Center for Mycology and Biotechnology, Al-Azhar University, according to the suggested method of Skehan et al. [65]. Exponentially, cells were placed in 104 cells/well for 24 h, and then add fresh medium which containing different concentration of the tested sample. Serial two-fold dilutions of the tested sample were added using a multichannel pipette. Moreover, all cells were cultivated at 37 °C, 5% CO2 and 95% humidity. Also, incubation of control cells occurred at 37 °C. However, after incubation for 24 h different concentrations of sample (500, 250, 125, 62.50, 31.25, 15.60, 7.80, 3.90, 2, 1 and 0 µg L−1) were added and continued the incubation for 48 h, then, add the crystal violet solution 1% to each well for 0.5 h to examine viable cells. Rinse the wells using water until no stain. After that, add 30% glacial acetic acid to all wells with shaking plates on Microplate reader (TECAN, Inc.) to measure the absorbance, using a test wavelength of 490 nm. Besides, compare the treated samples with the control cell. The cytotoxicity was estimated by IC50 in µM; the concentration that inhibits 50% of growth of cancer cell.
Docking and molecular modeling calculations
Were done in the Department of Pharmaceutical Organic Chemistry, Faculty of Pharmacy (Girls), Al-Azhar University, Egypt, as computational software using Protein Data Bank, 5-Fluorouracil (PDB ID: 1BID) and DHFR (PDB ID: 4DFR).
Additional file
Additional file 1. A molecular docking study was executed to identify their good binding interactions with the active sites of dihydrofolate reductase (DHFR). Most of compounds displayed significant anticancer activity against human lung carcinoma (A-549) and human breast carcinoma (MCF-7) cell lines.
Acknowledgements
Not applicable.
This work is a part of Ph.D thesis of M.M. Al-Rooqi.
Authors’ contributions
EMH and SAA effectively contributed in designing of the experiments, methodology, data analysis and curation, writing and publishing the manuscript. MMA-R, has contributed in data collections and analysis. SMA-G performed the biological activities and molecular docking measurements. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Supporting information with NMR spectra and molecular docking are attached.
Competing interests
The author declares that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Essam M. Hussein, Email: essam.hussein78@yahoo.com
Munirah M. Al-Rooqi, Email: munira900@hotmail.com
Shimaa M. Abd El-Galil, Email: shimaa_pha@yahoo.com
Saleh A. Ahmed, Email: saahmed@uqu.edu.sa, Email: saleh_63@hotmail.com
References
- 1.Drews J. Drug discovery: a historical perspective. Science. 2000;287:1960–1964. doi: 10.1126/science.287.5460.1960. [DOI] [PubMed] [Google Scholar]
- 2.Krátký M, Vinsová J, Volková M, Buchta V, Trejtnar F, Stolaríková J. Antimicrobial activity of sulfonamides containing 5-chloro-2-hydroxy-benzaldehyde and 5-chloro-2-hydroxybenzoic acid scaffold. Eur J Med Chem. 2012;50:433–440. doi: 10.1016/j.ejmech.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 3.Anjaneyulu R, Anjaneyulu K, Couturier E, Malaisse WJ. Opposite effects of hypoglycemic and hyperglycemic sulfonamides upon ionophore-mediated calcium transport. Biochem Pharmacol. 1980;29:1879–1882. doi: 10.1016/0006-2952(80)90097-0. [DOI] [PubMed] [Google Scholar]
- 4.Thornber CW. Isosterism and molecular modification in drug design. Chem Soc Rev. 1979;8:563–580. doi: 10.1039/cs9790800563. [DOI] [Google Scholar]
- 5.Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors and their therapeutic potential. Expert Opin Ther Pat. 2000;10:575–600. doi: 10.1517/13543776.10.5.575. [DOI] [Google Scholar]
- 6.Jaiswal M, Khadikar PV, Supuran CT. Topological modeling of lipophilicity, diuretic activity, and carbonic inhibition activity of benzene-sulfonamides: a molecular connectivity approach. Bioorg Med Chem Lett. 2004;14:5661–5666. doi: 10.1016/j.bmcl.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 7.Ogden RC, Flexner CW. In protease inhibitors in AIDS therapy. Basel, New York: Marcel Dekker; 2001. pp. 200–250. [Google Scholar]
- 8.Casini A, Scozzafava A, Mastrolorenzo A, Supuran CT. Sulfonamides and sulfonylated derivatives as anticancer agents. Curr Cancer Drug Targ. 2002;2:55–75. doi: 10.2174/1568009023334060. [DOI] [PubMed] [Google Scholar]
- 9.Abbate F, Casini A, Owa T, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: E7070, a sulfonamide anticancer agent, potently inhibits cytosolic isozymes I and II, and trans-membrane, tumor-associated isozyme IX. Bioorg Med Chem Lett. 2004;14:217–223. doi: 10.1016/j.bmcl.2003.09.062. [DOI] [PubMed] [Google Scholar]
- 10.Ismail MM, Ghorab MM, Noaman E, Ammar YA, Heiba HI, Sayed MY. Novel synthesis of pyrrolo[2,3-d]pyrimidines bearing sulfonamide moieties as potential antitumor and radioprotective agents. Arzneim Forsch. 2006;56:301–308. doi: 10.1055/s-0031-1296725. [DOI] [PubMed] [Google Scholar]
- 11.Al-Said MS, Ghorab MM, Al-qasoumi SI, El-Hossary EM, Noaman E. Synthesis and in vitro anticancer screening of some novel 4-[2-amino-3-cyano-4-substituted-5,6,7,8-tetrahydroquinolin-1-(4H)-yl]Benzenesulfonamides. Eur J Med Chem. 2010;45:3011–3018. doi: 10.1016/j.ejmech.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Alqasoumi SI, Al-Taweel AM, Alafeefy AM, Noaman E, Ghorab MM. Novel quinolines and pyrimido[4,5-b]quinolines bearing biologically active sulfonamide moiety as a new class of antitumor agents. Eur J Med Chem. 2010;45:738–744. doi: 10.1016/j.ejmech.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Alqasoumi SI, Al-Taweel AM, Alafeefy AM, Ghorab MM, Noaman E. Discovering some novel tetrahydroquinoline derivatives bearing the biologically active sulfonamide moiety as a new class of antitumor agents. Eur J Med Chem. 2010;45:1849–1853. doi: 10.1016/j.ejmech.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Ghorab MM, Ragab FA, Heiba HI, El-Hazek RM. Anticancer and radio-sensitizing evaluation of some new thiazolopyrane and thiazolopyranopyrimidine derivatives bearing a sulfonamide moiety. Eur J Med Chem. 2011;46:5120–5126. doi: 10.1016/j.ejmech.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Ghorab MM, Ragab FA, Heiba HI, Agha HM, Nissan YM. Novel 4-(4-substituted-thiazol-2-ylamino)-N-(pyridin-2-yl)-benzenesulfonamides as cytotoxic and radiosensitizing agents. Arch Pharm Res. 2012;35:59–68. doi: 10.1007/s12272-012-0106-y. [DOI] [PubMed] [Google Scholar]
- 16.Fukuoka K, Usuda J, Iwamoto Y, Fukumoto H, Nakamura T, Yoneda T, Narita N, Saijo N, Nishio K. Mechanisms of action of the novel sulfonamide anticancer agent E7070 on cell cycle progression in human non-small cell lung cancer cells. Invest New Drugs. 2001;19:219–227. doi: 10.1023/A:1010608317361. [DOI] [PubMed] [Google Scholar]
- 17.Supuran CT, Briganti F, Tilli S, Chegwidden WR, Scozzafava A. Carbonic anhydrase inhibitors: sulfonamides as antitumor agents. Bioorg Med Chem. 2001;9:703–714. doi: 10.1016/S0968-0896(00)00288-1. [DOI] [PubMed] [Google Scholar]
- 18.Casini A, Scozzafava A, Supuran CT. Sulfonamide derivatives with protease inhibitory action as anticancer, anti-inflammatory and antiviral agents. Expert Opin Ther Pat. 2002;12:1307–1327. doi: 10.1517/13543776.12.9.1307. [DOI] [Google Scholar]
- 19.Villar R, Encio I, Migliaccio M, Gil MJ, Martinez-Merino V. Synthesis and cytotoxic activity of lipophilic sulphonamide derivatives of the benzo[b]thiophene 1,1-dioxide. Bioorg Med Chem. 2004;12:963–968. doi: 10.1016/j.bmc.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Huang S, Connolly PJ, Lin R, Emanuel S, Middleton SA. Synthesis and evaluation of N-acyl sulfonamides as potential prodrugs of cyclin-dependent kinase inhibitor JNJ-7706621. Bioorg Med Chem Lett. 2006;16:3639–3641. doi: 10.1016/j.bmcl.2006.04.071. [DOI] [PubMed] [Google Scholar]
- 21.Kawai M, BaMaung NY, Fidanze SD, Erickson SA, Tedrow JS, Sanders WJ, Vasudevan A, Park C, Hutchins C, Comess KM, Kalvin D, Wang J, Zhang Q, Lou P, Tucker-Garcia L, Bouska J, Bell RL, Lesniewski R, Henkin J, Sheppard GS. Development of sulfonamide compounds as potent methionine aminopeptidase type II inhibitors with antiproliferative properties. Bioorg Med Chem Lett. 2006;16:3574–3577. doi: 10.1016/j.bmcl.2006.03.085. [DOI] [PubMed] [Google Scholar]
- 22.Payne JE, Bonnefous C, Hassig CA, Symons KT, Guo X, Nguyen PM, Annable T, Wash PL, Hoffman TZ, Rao TS, Shiau AK, Malecha JW, Noble SA, Hager JH, Smith ND. Identification of KD5170: a novel mercaptoketone-based histone deacetylase inhibitor. Bioorg Med Chem Lett. 2008;18:6093–6096. doi: 10.1016/j.bmcl.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 23.Kenneth R, Hande KR, Hagey A, Berlin J, Cai Y, Meek K, Kobayashi H, Lockhart AC, Medina D, Sosman J, Gordon GB, Rothenberg ML. The pharmacokinetics and safety of ABT-751, a novel, orally bioavailable sulfonamide antimitotic agent: results of a phase 1 study. Clin Cancer Res. 2006;12:2834–2840. doi: 10.1158/1078-0432.CCR-05-2159. [DOI] [PubMed] [Google Scholar]
- 24.Nagendrappa G, Subramanya Raj Urs S, Madhava MS, Somashekar R. Phase stabilization in cinnarizine complexes using X-ray profile analysis. Pramana J Phys. 2001;56:797–808. doi: 10.1007/s12043-001-0080-2. [DOI] [Google Scholar]
- 25.Berest GG, Voskoboynik OY, Kovalenko SI, Antypenko OM, Nosulenko IS, Katsev AM, Shandrovskaya OS. Synthesis and biological activity of novel N-cycloalkyl-(cycloalkylaryl)-2-[(3-R-2-oxo-2H-[1,2,4]triazino[2,3-c]quinazoline-6-yl)thio]acetamides. Eur J Med Chem. 2011;46:6066–6074. doi: 10.1016/j.ejmech.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Shams HZ, Mohareb RM, Helal MH, Mahmoud Ael S. Design and synthesis of novel antimicrobial acyclic and heterocyclic dyes and their precursors for dyeing and/or textile finishing based on 2-N-acylamino-4,5,6,7-tetrahydro-benzo[b]thiophene systems. Molecules. 2011;26:6271–6305. doi: 10.3390/molecules16086271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Autore G, Caruso A, Marzocco S, Nicolaus B, Palladino C, Pinto A, Popolo A, Sinicropi MS, Tommonaro G, Saturnino C. Acetamide derivatives with antioxidant activity and potential anti-inflammatory activity. Molecules. 2010;15:2028–2038. doi: 10.3390/molecules15032028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ley JP, Bertram H-J. Synthesis of polyhydroxylated aromatic mandelic acid amides and their antioxidative potential. Tetrahedron. 2001;57:1277–1282. doi: 10.1016/S0040-4020(00)01136-4. [DOI] [Google Scholar]
- 29.Zhu X, Zhou J, Zhu Y, Hu X, Bian Y, Hu X, Tao Z, Gao C, Huang W. Synthesis and biological activities of sulfinyl acetamide derivatives for narcolepsy treatment. Lett Drug Des Discov. 2013;10:266–270. [Google Scholar]
- 30.Dogruer DS, Kupeli E, Yesilada E, Sahin MF. Synthesis of new 2-[1(2H)-phthalazinon-2-yl]acetamide and 3-[1(2H)-phthalazinon-2-yl]propanamide derivatives as antinociceptive and anti-inflammatory agents. Arch Pharm. 2004;337:303–310. doi: 10.1002/ardp.200200719. [DOI] [PubMed] [Google Scholar]
- 31.Raghavendra NM, Jyothsna A, Venkateswara Rao A, Subrahmanyam CV. Synthesis, pharmacological evaluation and docking studies of N-(benzo[d]thiazol-2-yl)-2-(piperazin-1-yl)acetamide analogs as COX-2 inhibitors. Bioorg Med Chem Lett. 2012;22:820–823. doi: 10.1016/j.bmcl.2011.12.062. [DOI] [PubMed] [Google Scholar]
- 32.Xiang Y, Wang X-H, Yang Q, Tan J-L, Jang H-J, Zuo H, Shin D-S. Rational design, synthesis, and biological activity of N-(1,4-Benzoxazinone)acetamide derivatives as potent platelet aggregation inhibitors. Bull Korean Chem Soc. 2018;39:146–155. doi: 10.1002/bkcs.11359. [DOI] [Google Scholar]
- 33.Gull Y, Rasool N, Noreen M, Altaf AA, Musharraf SG, Zubair M, Nasim FH, Yaqoob A, DeFeo V, Zia-Ul-Haq M. Synthesis of N-(6-Arylbenzo[d]thiazole-2-acetamide derivatives and their biological activities: an experimental and computational approach. Molecules. 2016;21:266–282. doi: 10.3390/molecules21030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mccarthy O, Musso-Buendia A, Kaiser M, Brun R, Ruiz-Perez LM, Johansson NG, Pacanowska DG, Gilbert IH. Design, synthesis and evaluation of novel uracil acetamide derivatives as potential inhibitors of Plasmodium falciparum dUTP nucleotidohydrolase. Eur J Med Chem. 2009;44:678–688. doi: 10.1016/j.ejmech.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Liu Z, Zhou Z, Tian W, Fan X, Xue D, Yu L, Yu Q, Long Y-Q. Discovery of novel 2-N-aryl-substituted benzenesulfonamidoacetamides: orally bioavailable tubulin polymerization inhibitors with marked antitumor activities. Chem Med Chem. 2012;7:680–693. doi: 10.1002/cmdc.201100529. [DOI] [PubMed] [Google Scholar]
- 36.Blakley RL. Dihydrofolate reductase. In: Blakley RL, Benkovic SJ, editors. Folates and pteridines. New York: Wiley; 1984. pp. 191–253. [Google Scholar]
- 37.Brown KA, Kraut J. Exploring the molecular mechanism of dihydrofolate reductase. Faraday Discuss. 1992;93:217–224. doi: 10.1039/fd9929300217. [DOI] [PubMed] [Google Scholar]
- 38.Hawser S, Lociuro S, Islam K. Dihydrofolate reductase inhibitors as antibacterial agents. Biochem Pharmacol. 2006;71:941–948. doi: 10.1016/j.bcp.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 39.Ghorab MM, Ragab FA, Hamed MM. Design, synthesis and anticancer evaluation of novel tetrahydroquinoline derivatives containing sulfonamide moiety. Eur J Med Chem. 2009;44:4211–4217. doi: 10.1016/j.ejmech.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Alsaid MS, Ghorab MM, Al-Dosari MS, Hamed MM. Synthesis and in vitro anticancer evaluation of some novel hexahydroquinoline derivatives having a benzenesulfonamide moiety. Eur J Med Chem. 2011;46:201–207. doi: 10.1016/j.ejmech.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Alsaid MS, Bashandy MS, Alqasomi SI, Ghorab MM. Anti-breast cancer activity of some novel 1,2-dihydropyridine, thiophene and thiazole derivatives. Eur J Med Chem. 2011;46:137–141. doi: 10.1016/j.ejmech.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 42.Hussein EM. Ammonium chloride-catalyzed four-component sonochemical synthesis of novel hexahydroquinolines bearing a sulfonamide moiety. Russ J Org Chem. 2015;51:54–64. doi: 10.1134/S1070428015010091. [DOI] [Google Scholar]
- 43.Hussein EM, Ahmed SA. An efficient and green synthesis of polyfunctionalized spirothiazolidin-4-ones using sulfonated mesoporous silica as a reusable catalyst. Chem Heterocycl Comp. 2017;53:1148–1155. doi: 10.1007/s10593-017-2185-7. [DOI] [Google Scholar]
- 44.Hussein EM, Abdel-Monem MI. Regioselective synthesis and anti-inflammatory activity of novel dispiro[pyrazolidine-4,3′-pyrrolidine-2′,3″-indoline]-2″,3,5-triones. Arkivoc. 2011;10:85–98. [Google Scholar]
- 45.Abdel-Mohsen SA, Hussein EM. A green synthetic approach to the synthesis of Schiff bases from 4-amino-2-thioxo-1,3-diazaspiro[5.5]undec-4-ene-5-carbonitrile as potential anti-inflammatory agents. Russ J Bioorg Chem. 2014;40:343–349. doi: 10.1134/S1068162014030029. [DOI] [PubMed] [Google Scholar]
- 46.Hussein EM, Masaret GS, Khairou KS. Efficient synthesis and antimicrobial evaluation of some Mannich bases from 2-arylidine-1-thia-4-azaspiro[4.5]decan-3-ones. Chem Cent J. 2015;9:25. doi: 10.1186/s13065-015-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hussein EM, Al-Shareef HF, Aboellil AH, Elhady HA. Synthesis of some novel 6′-(4-chlorophenyl)-3,4′-bipyridine-3′-carbonitriles: assessment of their antimicrobial and cytotoxic activity. Z Naturforsch. 2015;70b:783–795. doi: 10.1515/znb-2015-0065. [DOI] [Google Scholar]
- 48.Al-Shareef HF, Elhady HA, Aboellil AH, Hussein EM. Ammonium chloride catalyzed synthesis of novel Schiff bases from spiro[indoline-3,4′-pyran]-3′-carbonitriles and evaluation of their antimicrobial and anti-breast cancer activities. SpringerPlus. 2016;5:887. doi: 10.1186/s40064-016-2458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bush K, Freudenberger JS, Slusarchyk DS, Sykes RB, Meyers E. Activity of sulfa drugs and dihydrofolate reductase inhibitors against Candida albicans. Experientia. 1982;38:436–437. doi: 10.1007/BF01952625. [DOI] [PubMed] [Google Scholar]
- 50.Rao KN, Venkatachalam SR. Dihydrofolate reductase and cell growth activity inhibition by the β-carboline-benzoquinolizidine plant alkaloid deoxytubulosine from Alangium lamarckii: its potential as an antimicrobial and anticancer agent. Bioorg Med Chem. 1999;7:1105–1110. doi: 10.1016/S0968-0896(98)00262-4. [DOI] [PubMed] [Google Scholar]
- 51.Patel TS, Vanparia SF, Patel UH, Dixit RB, Chudasama CJ, Patel BD, Dixit BC. Novel 2,3-disubstituted quinazoline-4(3H)-one molecules derived from amino acid linked sulphonamide as a potent malarial antifolates for DHFR inhibition. Eur J Med Chem. 2017;129:251–265. doi: 10.1016/j.ejmech.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 52.Schormann N, Senkovich O, Walker K, Wright DL, Anderson AC, Rosowsky A, Ananthan S, Shinkre B, Velu S, Chattopadhyay D. Structure-based approach to pharmacophore identification, in silico screening, and three-dimensional quantitative structure-activity relationship studies for inhibitors of Trypanosoma cruzi dihydrofolate reductase function. Proteins. 2008;73:889–901. doi: 10.1002/prot.22115. [DOI] [PubMed] [Google Scholar]
- 53.Bennett BC, Wan Q, Ahmad MF, Dealwis CG. X-ray structure of the ternary MTX•NADPH complex of the anthrax dihydrofolate reductase: a pharmacophore for dual-site inhibitor design. J Struct Biol. 2009;166:162–171. doi: 10.1016/j.jsb.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vilar S, Cozza G, Moro S. Medicinal chemistry and the molecular operating environment (MOE): application of QSAR and molecular docking to drug discovery. Curr Top Med Chem. 2008;8:1555–1572. doi: 10.2174/156802608786786624. [DOI] [PubMed] [Google Scholar]
- 55.Du QR, Li DD, Pi YZ, Li JR, Sun J, Fang F, Zhong WQ, Gong HB, Zhu HL. Novel 1,3,4-oxadiazole thioether derivatives targeting thymidylate synthase as dual anticancer/antimicrobial agents. Bioorg Med Chem. 2013;21:2286–2297. doi: 10.1016/j.bmc.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Gowda BT, Usha KM, Jayalakshmi KL. 1H and 13C NMR spectral studies on N-(Aryl)-substituted acetamides, C6H5NHCOCH3-iXi and 2/4 XC6H4NHCOCH3-iXi (where X = Cl or CH3 and i = 0, 1, 2 or 3) Z Naturforsch. 2003;58a:801–806. [Google Scholar]
- 57.Katke SA, Amrutkar SV, Bhor RJ, Khairnar MV. Synthesis of biologically active 2-chloro-N-alkyl/aryl acetamide derivatives. Int J Pharm Sci Res. 2011;2:148–156. [Google Scholar]
- 58.Patel RV, Kumari P, Rajani DP, Chikhalia KH. Synthesis of coumarin-based 1,3,4-oxadiazol-2ylthio-N-phenyl/benzothiazolylacetamides as antimicrobial and antituberculosis agents. Med Chem Res. 2013;22:195–210. doi: 10.1007/s00044-012-0026-x. [DOI] [Google Scholar]
- 59.Behbehani H, Ibrahim HM. 4-Thiazolidinones in heterocyclic synthesis: synthesis of novel enaminones, azolopyrimidines and 2-arylimino-5-arylidene-4-thiazolidinones. Molecules. 2012;17:6362–6385. doi: 10.3390/molecules17066362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu J, Xu B, Huang Z, Pan W, Cao P, Liu C, Hao X, Song B, Liang G. Synthesis and biological evaluation of Matijing-Su derivatives as potent anti-HBV agents. Bioorg Med Chem. 2011;19:5352–5360. doi: 10.1016/j.bmc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Kumar R, Kaur M, Bahia MS, Silakari O. Synthesis, cytotoxic study and docking based multidrug resistance modulator potential analysis of 2-(9-oxoacridin-10(9H)-yl)-N-phenyl acetamides. Eur J Med Chem. 2014;80:83–91. doi: 10.1016/j.ejmech.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 62.Hay RJ, Cleland MM, Durkin S, Reid YA (2000) Cell line preservation and authentication in animal cell culture. In: Masters JRW, editor. Oxford University Press, New York
- 63.Wilson AP (2000) Cytotoxicity and viability assays in animal cell culture: a practical approach. 3rd ed. In: Masters JRW, editor. Oxford University Press, New York
- 64.Saintigny Y, Makienko K, Swanson C, Emond MJ, Monnat JR. Homologous recombination resolution defect in werner syndrome. Mol Cell Biol. 2002;22:6971–6978. doi: 10.1128/MCB.22.20.6971-6978.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skehan P, Storegng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1999;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. A molecular docking study was executed to identify their good binding interactions with the active sites of dihydrofolate reductase (DHFR). Most of compounds displayed significant anticancer activity against human lung carcinoma (A-549) and human breast carcinoma (MCF-7) cell lines.
Data Availability Statement
Supporting information with NMR spectra and molecular docking are attached.