Abstract
Use of chiral solvents in asymmetric synthesis as a sole source of enantioselection remains largely unexplored in organic synthesis. We found that the use of a helical macromolecular catalyst of which helical chirality is dynamically formed in chiral solvents allowed several mechanistically different reactions to proceed with high enantioselectivity. In this system, the chirality of the solvent, such as limonene, induces a configurational imbalance to the helical macromolecular scaffold of the catalyst, and in turn to the reaction products through palladium-catalyzed asymmetric reactions including Suzuki-Miyaura cross-coupling (up to 98% ee), styrene hydrosilylation (up to 95% ee), and silaboration (up to 89% ee). Not only enantiomerically pure limonene but also limonene with low enantiomeric excesses induce single-handed helical structures with majority-rule-based amplification of homochirality. The helical conformation of the macromolecular catalyst was retained even in the absence of limonene in the solid state, enabling asymmetric cross-coupling in achiral solvent with high enantioselectivity.
Short abstract
Single-handed helical structures of a macromolecular catalyst were dynamically induced in chiral solvents, such as limonene, allowing asymmetric reactions to proceed with high enantioselectivity.
The use of chiral reaction media in asymmetric reactions as the sole source of enantioselection constitutes a long-standing challenge in organic synthesis.1,2 Given that the chirality of the final products can generally be traced back to the chirality of natural resources via steps of stereoselective/specific reactions as well as optical resolution, asymmetric synthesis can therefore be regarded as “the relay of chirality”, where original natural chirality is transferred into the chirality of the target molecules. From this perspective, employing abundant, naturally occurring chiral solvents as sources of enantioselection in asymmetric reactions is highly desirable, in a sense that the transfer of natural chirality to the final product is accomplished within a single step. Several reports have shown that organic transformations in chiral solvents proceed enantioselectively. For example, in the electrochemical dimerization of acetophenone, the use of (S,S)-1,4-bis(dimethylamino)-2,3-dimethoxybutane as a cosolvent with pentane afforded the product with 23% ee.3 A photochemical cyclization to give a helicene derivative in (S)-ethyl mandelate4 and the photoisomerization of nitrones to oxaziridines in (S)-2,2,2-trifluoro-1-phenylethanol4 afford the corresponding products with 2% and 31% ee, respectively. In addition to these remarkable precedents, which use nonionic chiral organic liquids as solvents, the use of chiral borate-based ionic liquids that contain two malic acid units affords 84% ee in a phosphine-catalyzed aza-Baylis-Hillman reaction.5 The high selectivity is rationalized in terms of a two-point interaction of the anionic charge on the borate center and the carboxylic hydrogen atom with the zwitterionic Baylis-Hillman intermediate, in which the enantio-discrimination occurs. It should be noted that all these reaction systems employ molecules bearing densely located polar functional groups and/or acidic groups to differentiate diastereomeric transition states or intermediates that are formed by interaction with chiral media (Figure 1a). This system design makes it difficult to use nonpolar, abundant chiral organic liquids such as monoterpene hydrocarbons as the sole determinant of enantioselectivity in asymmetric reactions, as abundant chiral organic liquids engage only in weak molecular interactions, based on, e.g., van der Waals forces.
Figure 1.
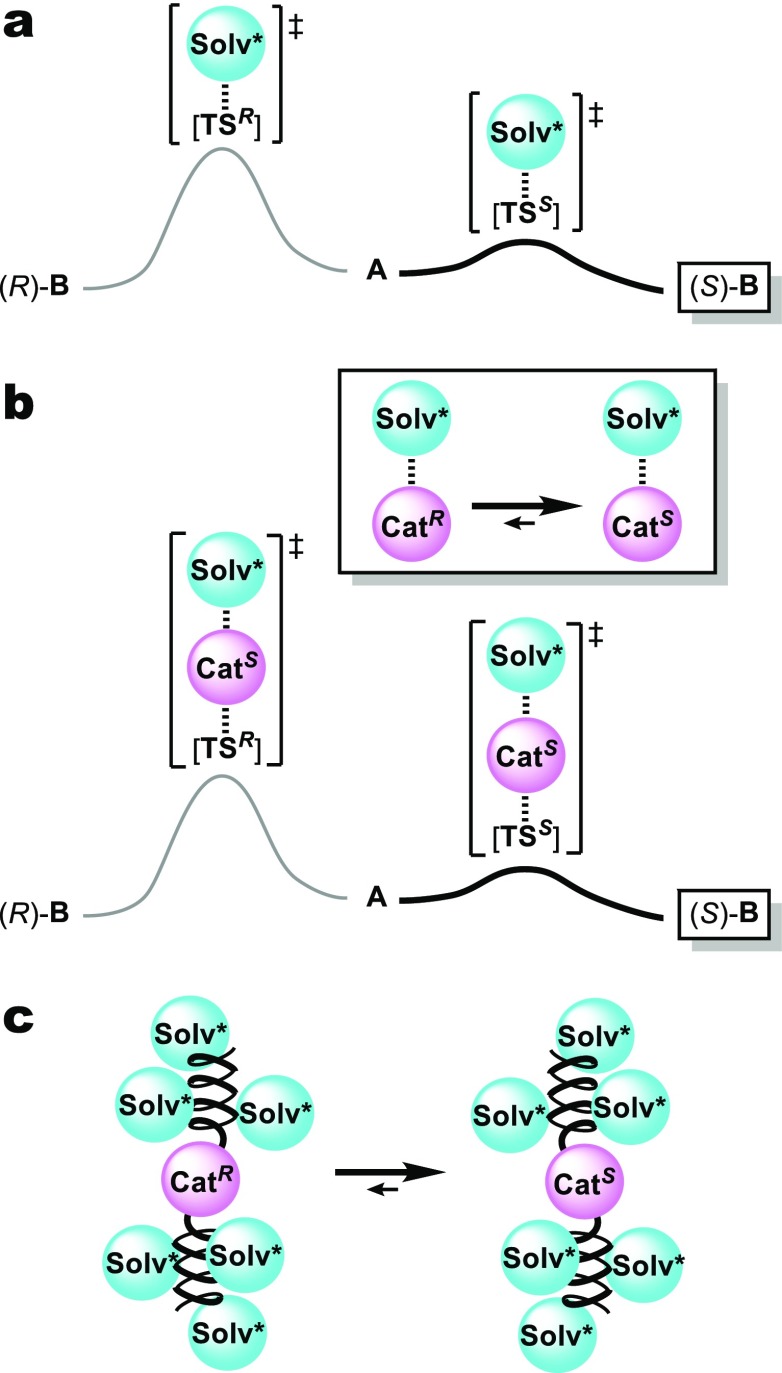
Possible effect of chiral solvents on asymmetric reactions, in which the chiral solvent serves as the sole source of enantioselection. (a) Differentiation of two enantiomeric reaction pathways via the direct interaction between the chiral solvent (Solv*) with the transition structures (TSR and TSS). (b) The use of a catalyst with dynamic chirality, whose interactions with a chiral solvent induce one predominant enantiomeric chiral conformation (CatS). (c) Control of the dynamic chirality of a catalyst based on the induction of a chiral conformation in a helical macromolecule by a chiral solvent.
Our idea to circumvent this obstacle is based on the use of chiral catalysts whose chiral conformation is induced by a chiral solvent (Figure 1b). We expect that this catalyst-based strategy can be extended to various reactions in which the same catalyst is applied, given that the mechanism of stereochemical induction is independent of the individual reaction mechanism. As the induction power is very weak, it is essential to multiply or amplify it in order to gain a sufficiently high difference in free energy difference between stable chiral conformations. To satisfy this requirement, we are focusing on helical macromolecular architectures (Figure 1c).6,7 Inducing chiral conformations in helical macromolecules has been attempted using poly(n-hexyl isocyanate)s8 or polyacetylenes bearing 2,2′-biphenol-derived pendants,9 whose right- and left-handed helical conformations are in equilibrium. Although the degree of helix induction is not sufficient, deracemization to induce helical structures is observed in (S)-1-chloro-2-methylbutane and (S)-5-ethyl-5-propylundecane. Moreover, the formation of helical supramolecular architectures has been reported in chiral solvents such as limonene, although their degree of helix induction could not be quantified.10,11
We have extensively studied poly(quinoxaline-2,3-diyl)s (hereafter: PQXs), which adopt rigid but dynamic helical structures. Single-handed helical conformations of PQXs can be easily obtained by introduction of various chiral side chains.12,13 The key characteristics of PQXs that bear chiral side chains are the solvent-dependent, bidirectional induction of right- and left-handed helix structures.14 For instance, PQXs with (S)-3-octyloxymethyl side chains exhibit exclusive left- or right-handed helicity in n-octane or cyclooctane.15 Furthermore, PQXs serve as highly enantioselective chirality-switchable macromolecular catalysts upon incorporation of catalytically active pendants.16−20 The high sensitivity of the main-chain chirality toward the nature of the solvent inspired us to use chiral solvents to induce helical screw-sense in PQX-based catalysts that bear achiral side chains.
Herein, we report the successful use of a helically dynamic macromolecular catalyst for this purpose. We discovered that an achiral PQX that bears 2-(diphenylphosphino)phenyl pendants forms a purely single-handed helical structure in enantiomerically enriched limonene, which is a natural organic compound. In limonene, this helically chiral PQX can serve as a highly effective chiral catalyst in several different asymmetric catalytic reactions with enantioselectivities of up to 98% ee. The use of limonene with low enantiomeric purity still afforded high enantioselectivity thanks to the amplification of the homochirality. The chirality of the PQX was fixed in the solid state, and this “memory of chirality”21,22 also allowed an enantioselective cross-coupling reaction in achiral solvents. All these key aspects arise from the characteristic features of macromolecules, where even subtle energy differences in nonbonding molecular interactions can be accumulated and thus multiplied along the rigid macromolecular scaffold. We anticipate that this aspect might make our system applicable to a wide variety of catalytic asymmetric reactions.
Results and Discussion
Helical Screw-Sense Induction to PQXs by Chiral Solvents
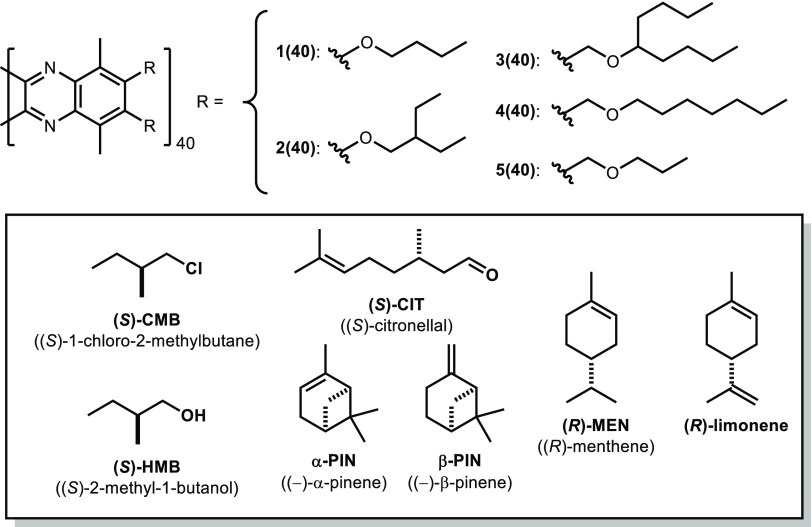
Initially, achiral PQXs 40mers 1(40)–5(40), which differ in their achiral side chains, were dissolved in readily available, naturally occurring chiral solvents. As judged from their circular dichroism (CD) spectra,23 PQXs 5(40), which bears n-propoxymethyl side chains, exhibited the most effective screw-sense induction in the chiral solvents (Table 1). In particular, (R)-limonene induced a right-handed (P) helical structure of 5(40) with 72% screw-sense excess (se). Among the seven chiral solvents used, (R)-limonene generally exhibited the most effective induction of a right-handed screw-sense: its comparison with (R)-menthene ((R)-MEN) suggested that the terminal carbon–carbon double bond of (R)-limonene may play an important role in the screw-sense induction. It should be noted that (S)-2-methyl-1-butanol ((S)-HMB) showed in general a moderate but noticeable screw-sense induction. These results prompted us to use PQXs 5(DP) with n-propoxymethyl side chains and (R)-limonene as a chiral solvent for further experiments in this study.24
Table 1. Structures and Screw-Sense Induction Properties of Achiral PQXs 1(40)–5(40) Based on Kuhn’s CD-Derived Dissymmetry Factor gabsa in Chiral Solvents.
| se (%)b |
|||||||
|---|---|---|---|---|---|---|---|
| polymer | (S)-CMB | (S)-HMB | (S)-CIT | α-PIN | β-PIN | (R)-MEN | (R)-limonene |
| 1(40) | c | c | c | 2 (P) | c | c | 51 (P) |
| 2(40) | 5 (P) | c | 3 (P) | 6 (M) | 4 (P) | 4 (P) | 30 (P) |
| 3(40) | 5 (M) | 31 (M) | 20 (P) | 14 (P) | 24 (P) | 21 (P) | 44 (P) |
| 4(40) | 3 (M) | 29 (M) | 8 (P) | 12 (P) | 17 (P) | 33 (P) | 59 (P) |
| 5(40) | 10 (P) | 34 (M) | 11 (P) | 20 (P) | 27 (P) | 40 (P) | 72 (P) |
Amplification of Homochirality in the Screw-Sense Induction by Chiral Solvents
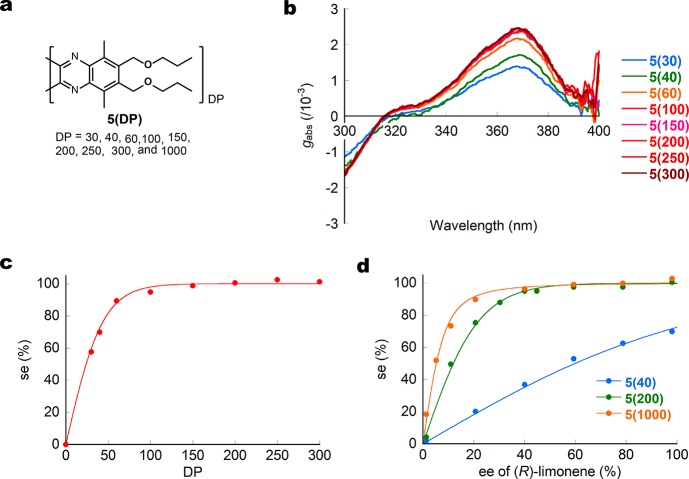
PQXs 5(DP) bearing n-propoxymethyl side chains with varying degrees of polymerization (DP = 30–1000) (Figure 2a) exhibited identical CD signals, albeit with a different intensity (Kuhn’s dissymmetry factor, gabs), which nonlinearly increases with increasing DP (Figure 2b). The application of Green’s theory afforded an estimate of ΔGh = 0.10 kJ/mol, which corresponds to the free energy difference between P- and M-helices per monomer unit.23 Homochiral (>99% se) screw-sense was induced in PQX 5(DP) of which DP ≥ 120 (Figure 2c). In (R)-limonene of varying enantiopurity (Figure 2d), the se of 5(DP) with varying DP (40, 200, and 1000) increased nonlinearly with increasing enantiomeric excess of (R)-limonene. The nonlinearity depended on DP: 5(DP)s with higher DP exhibited sharper positive nonlinear increases in screw-sense induction. This plot clearly indicated that (R)-limonene with 65% ee is enough to induce an exclusively homochiral right-handed helical structure in 5(1000).23,25
Figure 2.
Determination of se values of 5(DP) in (R)-limonene. (a) Structure of 5(DP). (b) CD spectra of 5(DP) (DP = 30–300) in (R)-limonene (98.1% ee). (c) Correlation between DP and the se of 5(DP) (DP = 30–300) in (R)-limonene (98.1% ee). (d) Correlation between the ee of (R)-limonene and the se of 5(40), 5(200), and 5(1000).
Asymmetric Reaction in Chiral Solvents
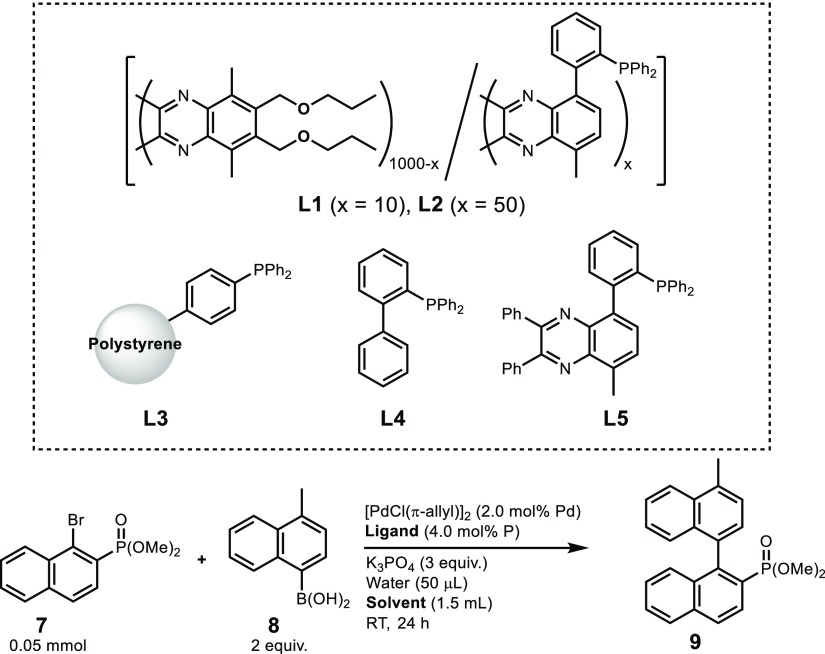
We then prepared the random co-1000mers L1 and L2, which contain on average 10 and 50 coordination units, respectively, with 2-(diphenylphosphino)phenyl pendants, by living copolymerization for their use in asymmetric Suzuki-Miyaura coupling (SMC) reactions.18,26 The use of L1 in THF resulted in the formation of a racemic coupling product (entry 1, Table 2). Subsequently, (R)-limonene was used as the reaction solvent together with THF in these coupling reactions. Before adding substrates (7 and 8) and reagents, solutions of L1 in the designated solvents were allowed to stand for 12 h at room temperature to allow completion of the helical screw-sense induction. The reactions in (R)-limonene/THF afforded coupling product 9 in high enantioselectivity (96–98% ee) (entries 2–4). (S)-Limonene, which is also readily available from natural sources, afforded an enantiomeric coupling product with identical enantiomeric excess (entry 5). The use of L2, which contains more coordination units, delivered products with a little lower enantioselectivity (entry 6). The fact that PQX 5(1000) failed to afford any reaction product clearly suggests that the asymmetric reaction occurs at the palladium center, which is coordinated by the phosphorus atom (entry 7). For comparison, triphenylphosphine and its polystyrene-embedded derivative L3 were used instead of L1, giving no or merely trace amounts of the product (entries 8 and 9). (Biaryl)diphenylphosphine L4 and 5-(diphenylphosphino)phenylquinoxaline L5, which can be regarded as a low-molecular-weight model of L1, afforded racemic products (entries 10 and 11). These results suggest that the limonene is not exerting its effect directly on the palladium center but on the main chain of the PQX, to which a single-handed helical structure is induced. This mechanism of chirality transfer also allowed the use of (S)-HMB as a chiral solvent that gave high enantiomeric excess (entry 12). High enantioselectivity was also attained with the use of orange oil, which contains (R)-limonene as a major component (entry 13). When (R)-limonene with 63% ee was used as a solvent, the cross-coupling product was obtained with 93% ee (entry 16). The degree of homochirality amplification in catalysis using L1 can be directly correlated to the nonlinear screw-sense induction in PQX 5(1000) (Figure 2d). We also carried out SMC reactions in achiral solvents using L1*, i.e., L1 that was dissolved in (R)-limonene prior to addition of Pd precursor in order to induce a P-helical conformation. The catalyst L1* was reprecipitated into MeOH, washed, and dried under reduce pressure to completely remove (R)-limonene. When L1* was used in THF, which is a good solvent for L1*, the SMC reaction proceeded with 45% ee, which suggests a memory effect with respect to the helical screw-sense (entry 17).21,22 The use of solvents that dissolve L1* less readily rendered the catalyst heterogeneous and produced more pronounced memory effects. Indeed, the SMC reaction in 1-PrOH resulted in the formation of 9 with 88% ee (entry 18).
Table 2. SMC Reactions Using Various Achiral Ligands in Chiral Solvents.
| entry | ligand | solventa | yield (%)b | ee (%) |
|---|---|---|---|---|
| 1 | L1 | THF | 70 | <1 |
| 2 | L1 | (R)-limonene/THF (70/30) | 61 | 96 (S) |
| 3 | L1 | (R)-limonene/THF (95/5) | 66 | 98 (S) |
| 4 | L1 | (R)-limonene/THF (98/2) | 56 | 97 (S) |
| 5 | L1 | (S)-limonene/THF (95/5) | 54 | 98 (R) |
| 6 | L2 | (R)-limonene/THF (95/5) | 55 | 85 (S) |
| 7 | 5(1000) | (R)-limonene/THF (95/5) | NDd | |
| 8 | PPh3 | (R)-limonene/THF (95/5) | trace | |
| 9 | L3 | (R)-limonene/THF (95/5) | NDd | |
| 10 | L4 | (R)-limonene/THF (95/5) | 19 | <1 |
| 11 | L5 | (R)-limonene/THF (95/5) | 59 | <1 |
| 12 | L1 | (S)-HMB | 50 | 92 (R) |
| 13 | L1 | orange oil/THF (95/5) | 55 | 97 (S) |
| 14 | L1 | (R)-limonene (6.7% ee)/THF (95/5) | 68 | 36 (S) |
| 15 | L1 | (R)-limonene (12.6% ee)/THF (95/5) | 69 | 70 (S) |
| 16 | L1 | (R)-limonene (63.4% ee)/THF (95/5) | 61 | 93 (S) |
| 17 | L1*c | THF | 62 | 45 (S) |
| 18 | L1*c | 1-PrOH | 64 | 88 (S) |
Unless otherwise noted, (R)-limonene: 98.1% ee; (S)-limonene: 95.5% ee; (S)-HMB: > 99.9% ee.
Isolated yield.
L1 was dissolved in (R)-limonene at RT for 24 h in order to induce a (P)-helical structure prior to the formation of the palladium catalyst.
Not detected.
Applications to Other Catalytic Asymmetric Reactions
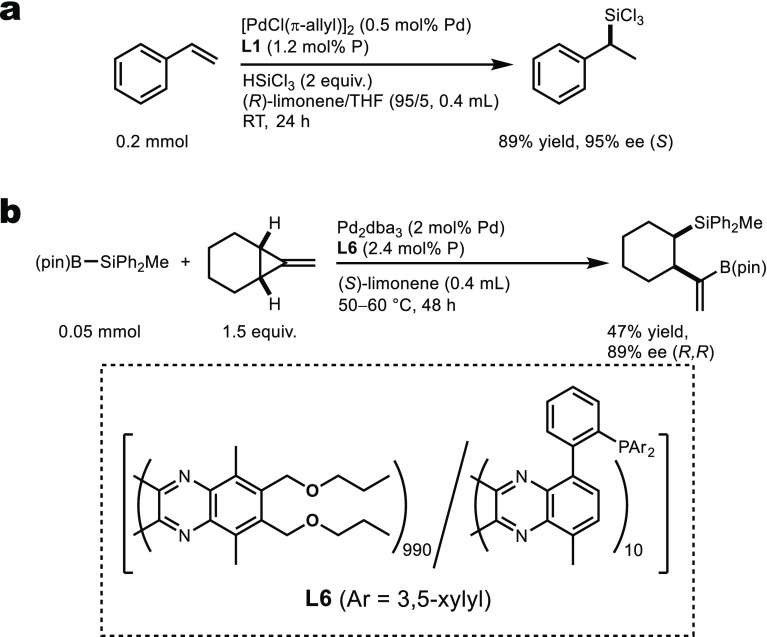
Finally, we examined the generality of our chirality transfer system by applying it to other asymmetric catalytic reactions, taking advantage of indirect participation of chiral solvents to the reaction center through noncovalent molecular interactions at the polymer backbone. For instance, the hydrosilylation of styrene using L1 in (R)-limonene afforded the corresponding product with 95% ee in good yield (Figure 3a).16,17,27 A PQX-based ligand (L6), in which the phosphorus atom carries 3,5-xylyl groups, exhibited high levels of enantioselectivity in the asymmetric silaborative C–C bond cleavage of a methylenecyclopropane19,28 even at elevated temperatures (Figure 3b).
Figure 3.
Other asymmetric reactions using the PQX-based ligands in enantiomerically enriched limonene. (a) Hydrosilylation reaction of styrene in (R)-limonene. (b) Asymmetric silaborative C–C bond cleavage of a methylenecyclopropane in (S)-limonene.
Safety Statement
No unexpected or unusually high safety hazards were encountered in this research.
Conclusions
In summary, we have achieved a highly efficient chirality transfer from solvent to the macromolecular main chain of PQXs. The induced helical screw-sense resulted in high levels of stereodifferentiation in asymmetric reactions by attaching catalytically active pendants onto the macromolecular scaffold in a modular manner. Overall, our system represents the first example for the use of solvent chirality as the exclusive source of enantioselection in catalytic asymmetric reactions. The most important aspect of our macromolecular system is the accumulation of the small solvent effect in the macromolecular scaffold without relying upon direct interactions between the chiral solvent and the reaction center. The induced chirality can be successfully memorized in the solid state, even after removal of the chiral solvent, which allowed highly enantioselective SMC reactions in achiral solvents. These characteristic aspects of helical macromolecular scaffolds should open up new highly attractive opportunities for catalytic asymmetric transformations, where abundant chiral solvents serve as the determinant of enantioselectivity.
Supporting Information Available
These materials are available free of charge via the Internet at The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.9b00330.
Experimental procedure and spectral data for the new compounds (PDF)
Author Contributions
Y.N. and M.S. designed and directed the project. R.T. and Y.N. performed the experiments and analyzed the data. Y.N. and M.S. cowrote the paper.
Financial support for this research was provided by CREST, JST (M.S., Establishment of Molecular Technology toward the Creation of New Function, JPMJCR14L1) and JSPS KAKENHI Grants JP16K05796 (Y.N.), JP15H00994 (Y.N.), JP17H05368 (Y.N.), and JP15H05811 (M.S.).
The authors declare no competing financial interest.
Supplementary Material
References
- Gruttadauria M.; Giacalone F.. Catalytic Methods in Asymmetric Synthesis: Advanced Materials, Techniques, and Applications; John Wiley & Sons, Inc.: New Jersey, USA, 2011. [Google Scholar]
- Bredig G.; Balcom R. W. Kinetik der kohlendioxyd-abspaltung aus camphocarbonsäure. Ber. Dtsch. Chem. Ges. 1908, 41, 740–751. 10.1002/cber.190804101137. [DOI] [Google Scholar]
- Seebach D.; Oei H. A. Mechanism of electrochemical pinacolization - 1st asymmetric synthesis in a chiral medium. Angew. Chem., Int. Ed. Engl. 1975, 14, 634–636. 10.1002/anie.197506342. [DOI] [Google Scholar]
- Laarhoven W. H.; Cuppen T. J. H. M. Chiral solvent-induced asymmetric synthesis; photosynthesis of optically enriched hexahelicene. J. Chem. Soc., Chem. Commun. 1977, 47a. 10.1039/c3977000047a. [DOI] [Google Scholar]
- Gausepohl R.; Buskens P.; Kleinen J.; Bruckmann A.; Lehmann C. W.; Klankermayer J.; Leitner W. Highly enantioselective Aza-Baylis-Hillman reaction in a chiral reaction medium. Angew. Chem., Int. Ed. 2006, 45, 3689–3692. 10.1002/anie.200600327. [DOI] [PubMed] [Google Scholar]
- Yashima E.; Maeda K.; Iida H.; Furusho Y.; Nagai K. Helical Polymers: Synthesis, Structures, and Functions. Chem. Rev. 2009, 109, 6102–6211. 10.1021/cr900162q. [DOI] [PubMed] [Google Scholar]
- Yashima E.; Ousaka N.; Taura D.; Shimomura K.; Ikai T.; Maeda K. Supramolecular helical systems: Helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem. Rev. 2016, 116, 13752–13990. 10.1021/acs.chemrev.6b00354. [DOI] [PubMed] [Google Scholar]
- Green M. M.; Khatri C.; Peterson N. C. A macromolecular conformational change driven by a minute chiral solvation energy. J. Am. Chem. Soc. 1993, 115, 4941–4942. 10.1021/ja00064a086. [DOI] [Google Scholar]
- Maeda K.; Hirose D.; Okoshi N.; Shimomura K.; Wada Y.; Ikai T.; Kanoh S.; Yashima E. Direct detection of hardly detectable hidden chirality of hydrocarbons and deuterated isotopomers by a helical polyacetylene through chiral amplification and memory. J. Am. Chem. Soc. 2018, 140, 3270–3276. 10.1021/jacs.7b10981. [DOI] [PubMed] [Google Scholar]
- George S. J.; Tomovic Z.; Schenning A. P. H. J.; Meijer E. W. Insight into the chiral induction in supramolecular stacks through preferential chiral solvation. Chem. Commun. 2011, 47, 3451–3453. 10.1039/c0cc04617e. [DOI] [PubMed] [Google Scholar]
- Kawagoe Y.; Fujiki M.; Nakano Y. Limonene magic: noncovalent molecular chirality transfer leading to ambidextrous circularly polarised luminescent pi-conjugated polymers. New J. Chem. 2010, 34, 637–647. 10.1039/b9nj00733d. [DOI] [Google Scholar]
- Suginome M.; Yamamoto T.; Nagata Y.; Yamada T.; Akai Y. Catalytic asymmetric synthesis using chirality-switchable helical polymer as a chiral ligand. Pure Appl. Chem. 2012, 84, 1759–1769. 10.1351/PAC-CON-11-08-23. [DOI] [Google Scholar]
- Suginome M.; Yamamoto T.; Nagata Y. Poly(quinoxaline-2,3-diyl)s: A fascinating helical macromolecular scaffold for new chiral functions. Yuki Gosei Kagaku Kyokaishi 2015, 73, 1141–1155. 10.5059/yukigoseikyokaishi.73.1141. [DOI] [Google Scholar]
- Nagata Y.; Nishikawa T.; Suginome M.; Sato S.; Sugiyama M.; Porcar L.; Martel A.; Inoue R.; Sato N. Elucidating the solvent effect on the switch of the helicity of poly(quinoxaline-2,3-diyl)s: a conformational analysis by small-angle neutron scattering. J. Am. Chem. Soc. 2018, 140, 2722. 10.1021/jacs.7b11626. [DOI] [PubMed] [Google Scholar]
- Nagata Y.; Nishikawa T.; Suginome M. Poly(quinoxaline-2,3-diyl)s bearing (S)-3-octyloxymethyl side chains as an efficient amplifier of alkane solvent effect leading to switch of main-chain helical chirality. J. Am. Chem. Soc. 2014, 136, 15901–15904. 10.1021/ja509531t. [DOI] [PubMed] [Google Scholar]
- Yamamoto T.; Suginome M. Helical poly(quinoxaline-2,3-diyl)s bearing metal-binding sites as polymer-based chiral ligands for asymmetric catalysis. Angew. Chem., Int. Ed. 2009, 48, 539–542. 10.1002/anie.200803719. [DOI] [PubMed] [Google Scholar]
- Yamamoto T.; Yamada T.; Nagata Y.; Suginome M. High-molecular-weight polyquinoxaline-based helically chiral phosphine (PQXphos) as chirality-switchable, reusable, and highly enantioselective monodentate ligand in catalytic asymmetric hydrosilylation of styrenes. J. Am. Chem. Soc. 2010, 132, 7899–7901. 10.1021/ja102428q. [DOI] [PubMed] [Google Scholar]
- Yamamoto T.; Akai Y.; Nagata Y.; Suginome M. Highly enantioselective synthesis of axially chiral biarylphosphonates: Asymmetric suzuki-miyaura coupling using high-molecular-weight, helically chiral polyquinoxaline-based phosphines. Angew. Chem., Int. Ed. 2011, 50, 8844–8847. 10.1002/anie.201103792. [DOI] [PubMed] [Google Scholar]
- Akai Y.; Yamamoto T.; Nagata Y.; Ohmura T.; Suginome M. Enhanced catalyst activity and enantioselectivity with chirality-switchable polymer ligand pqxphos in pd-catalyzed asymmetric silaborative cleavage of meso-methylenecyclopropanes. J. Am. Chem. Soc. 2012, 134, 11092–11095. 10.1021/ja303506k. [DOI] [PubMed] [Google Scholar]
- Yamamoto T.; Murakami R.; Suginome M. Single-handed helical poly(quinoxaline-2,3-diyl)s bearing achiral 4-aminopyrid-3-yl pendants as highly enantioselective, reusable chiral nucleophilic organocatalysts in the steglich reaction. J. Am. Chem. Soc. 2017, 139, 2557–2560. 10.1021/jacs.6b12349. [DOI] [PubMed] [Google Scholar]
- Furusho Y.; Kimura T.; Mizuno Y.; Aida T. Chirality-memory molecule: A D2-symmetric fully substituted porphyrin as a conceptually new chirality sensor. J. Am. Chem. Soc. 1997, 119, 5267–5268. 10.1021/ja970431q. [DOI] [Google Scholar]
- Yashima E.; Maeda K.; Okamoto Y. Memory of macromolecular helicity assisted by interaction with achiral small molecules. Nature 1999, 399, 449–451. 10.1038/20900. [DOI] [Google Scholar]
- Lifson S.; Andreola C.; Peterson N. C.; Green M. M. Macromolecular stereochemistry: Helical sense preference in optically active polyisocyanates. Amplification of a conformational equilibrium deuterium isotope effect. J. Am. Chem. Soc. 1989, 111, 8850–8858. 10.1021/ja00206a013. [DOI] [Google Scholar]
- Degree of screw-sense induction of 5(100) by (R)-limonene in cyclohexane was investigated. 5(100) adopted P-helical conformation regardless of the amount of (R)-limonene in cyclohexane. The screw-sense excess of 5(100) showed a positive nonlinear relationship to the ratio of (R)-limonene in cyclohexane. The detailed results are as follows. ((R)-limonene/cyclohexane (v/v), screw-sense excess): (20/80, 48% se); (40/60, 75% se); (60/40, 84% se); (80/20, 96% se).
- Ke Y.-Z.; Nagata Y.; Yamada T.; Suginome M. Majority-rules-type helical poly(quinoxaline-2,3-diyl)s as highly efficient chirality-amplification systems for asymmetric catalysis. Angew. Chem., Int. Ed. 2015, 54, 9333–9337. 10.1002/anie.201502209. [DOI] [PubMed] [Google Scholar]
- Shen X. Q.; Jones G. O.; Watson D. A.; Bhayana B.; Buchwald S. L. Enantioselective synthesis of axially chiral biaryls by the pd-catalyzed suzuki miyaura reaction: Substrate scope and quantum mechanical investigations. J. Am. Chem. Soc. 2010, 132, 11278–11287. 10.1021/ja104297g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T. Chiral monodentate phosphine ligand MOP for transition metal-catalyzed asymmetric reactions. Acc. Chem. Res. 2000, 33, 354–362. 10.1021/ar990080f. [DOI] [PubMed] [Google Scholar]
- Ohmura T.; Taniguchi H.; Kondo Y.; Suginome M. Palladium-catalyzed asymmetric silaborative C-C cleavage of meso-methylenecyclopropanes. J. Am. Chem. Soc. 2007, 129, 3518–3519. 10.1021/ja0703170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.