Abstract
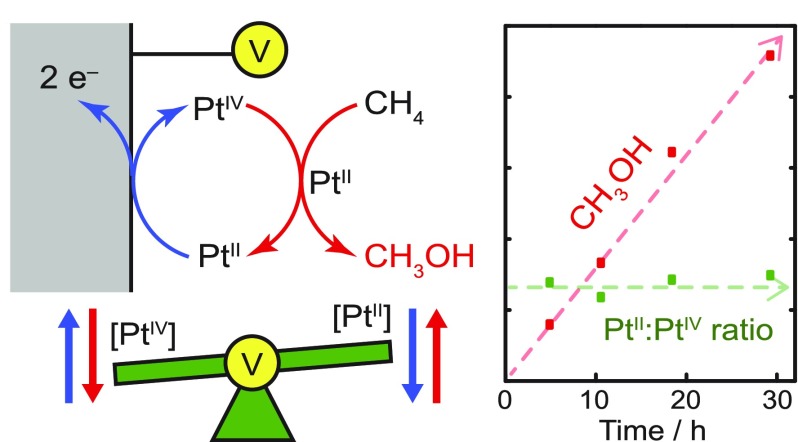
The direct conversion of methane to methanol would enable better utilization of abundant natural gas resources. In the presence of stoichiometric PtIV oxidants, PtII ions are capable of catalyzing this reaction in aqueous solutions at modest temperatures. Practical implementation of this chemistry requires a viable strategy for replacing or regenerating the expensive PtIV oxidant. Herein, we establish an electrochemical strategy for continuous regeneration of the PtIV oxidant to furnish overall electrochemical methane oxidation. We show that Cl-adsorbed Pt electrodes catalyze facile oxidation of PtII to PtIV at low overpotential without concomitant methanol oxidation. Exploiting this facile electrochemistry, we maintain the PtII/IV ratio during PtII-catalyzed methane oxidation via in situ monitoring of the solution potential coupled with dynamic modulation of the electric current. This approach leads to sustained methane oxidation catalysis with 70% selectivity for methanol.
Short abstract
PtII ions catalyze the oxidation of methane to methanol but consume PtIV ions as the oxidant. Now, with electrochemical oxidation, the PtIV ions can be regenerated to achieve sustained catalysis.
Introduction
Methane is an abundant hydrocarbon resource that is often underutilized because of its low boiling point and chemical inertness. Thus, technologies for converting methane to high-demand liquid chemicals such as methanol would enable better utilization of this low-carbon resource.1−3 Current methane valorization technologies rely on an indirect process involving initial steam reforming to H2 and CO. The reforming step requires capital-intensive facilities that are not amenable to remote deployment.4 Consequently, spontaneously released natural gas at oil wells is being flared at massive scales.5,6 The development of mild, direct methane-to-methanol processes (eq 1) that can operate portably is expected to stem flaring as well as expand the versatility of natural gas.7,8
| 1 |
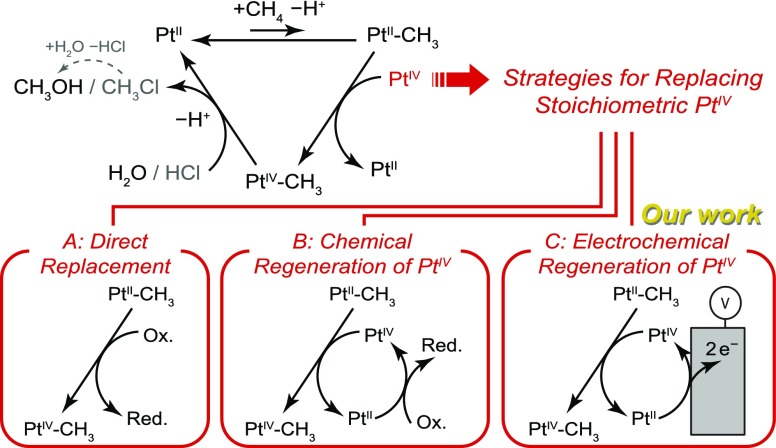
While many homogeneous and heterogeneous systems have been investigated for methane-to-methanol conversion,1,8 simple PtII chloride salts in water, PtIIClx(H2O)(4–x)(2–x) (denoted collectively as PtII), offer unique advantages.9 The catalytic cycle (Scheme 1) is initiated by PtII ions, which carry out reversible C–H activation of CH4 to yield a PtII−CH3 intermediate. This intermediate is then oxidized by PtIVClx(H2O)(6–x)(4–x) (denoted collectively as PtIV) to generate a PtIV−CH3 species that undergoes rapid reductive elimination to produce CH3OH or CH3Cl, which can be hydrolyzed to CH3OH. This system has the following advantages: First, the organometallic activation of methane offers superior selectivity for mono-oxidation compared to catalysts that operate via radical intermediates.8,10−12 Second, while most homogeneous catalysts that do organometallic activation require impractical8,13 concentrated acid media for boosting the catalytic rate and selectivity,14,15 PtII operates in dilute aqueous acids. Along with the relatively low reaction temperature (>100 °C), these advantages position PtII chloride salts, often referred to as “Shilov’s catalyst,” as privileged agents for methane-to-methanol conversion under mild conditions.
Scheme 1. Catalytic Cycle for the Functionalization of Methane by Aqueous Pt Salts (Shilov’s Catalyst) and Distinct Strategies To Overcome the Stoichiometric Use of PtIV.
A critical drawback of Shilov’s catalyst, as originally reported, is its requirement for a stoichiometric PtIV oxidant, which is economically impracticable.9 The key to developing an alternative oxidation strategy for this catalytic system is to achieve precise control over the driving force (thermodynamics) and/or rate (kinetics) of the oxidation reaction. In view of the catalytic cycle, there are two distinct approaches to the problem. First, PtIV may be directly replaced by an alternative oxidant that can oxidize the PtII−CH3 intermediate (Scheme 1, Strategy A). Success of this strategy requires an oxidant that (i) rapidly oxidizes the fleeting PtII−CH3 intermediate before it can undergo protonation back to PtII + CH4 and (ii) possesses a low enough redox potential to avoid oxidizing the PtII catalyst to PtIV, which is unreactive toward CH4. The conflicting requirement for fast rates and low driving force places an inherent constraint on the oxidants that are viable. Second, one may employ PtIV itself, which is an efficient oxidant for PtII−CH3, as a redox mediator for the overall reaction (Scheme 1, Strategy B). Success of this strategy hinges on carefully matching the rate of PtIV regeneration by PtII oxidation to the rate of PtIV consumption by methane functionalization. Rapid PtII oxidation will progressively deplete the pool of PtII, retarding catalysis, whereas slow oxidation will deplete PtIV and induce irreversible decomposition of the PtII to metallic Pt0 via, inter alia, disproportionation of PtII.9,16 Thus, a viable alternate oxidant must achieve good control over the oxidation driving force and/or rate.
The inherent difficulty of fine-tuning oxidation using chemical reagents has, presumably, contributed to the limited success in replacing stoichiometric PtIV. Notably, oxidants such as heteropoly acids, CuCl2, FeCl3, and Br2 were identified as kinetically competent toward the oxidation of PtII−CH3 (Scheme 1, Strategy A).17 These oxidants have achieved PtII-mediated oxidation of methane or other aliphatic substrates,18−21 and some of them, being air-regenerable, have been employed in concert with O2 to effect overall aerobic methane functionalization. However, none of these studies established long-term stability. For example, the combination of CuCl2 and O2 ultimately resulted in complete oxidation of PtII to PtIV,9 highlighting the difficulty of controlling the oxidation driving force. Studies aimed at mediating turnover via the PtII/IV redox couple (Scheme 1, Strategy B) showed that Cl222 and H2O216 are viable oxidants. However, the PtII oxidation rate was not actively modulated, and thus, continuous operation was not demonstrated. Furthermore, neither of these oxidants are air-regenerable or affordable for methanol production. In sum, there exists yet no suitable alternative to stoichiometric PtIV for sustained aqueous PtII-catalyzed methane-to-methanol conversion.
We show that electrochemistry affords a unique solution to this problem. Unlike all stoichiometric chemical oxidations, electrochemical oxidation allows for unparalleled control over the rate and driving force for electron transfer. Furthermore, the rate and driving force can be toggled instantaneously for real-time, dynamic modulation. While direct electro-oxidation of the fleeting PtII−CH3 intermediate is unfeasible due to the small fraction of reaction solution volume in contact with the electrode surface, electrochemistry is well-suited to regenerate PtIV via reoxidation of PtII (Scheme 1, Strategy C). As noted above, the success of this approach relies on maintaining a constant PtII:PtIV ratio; electrochemistry allows for simultaneous measurement and fine-tuning of this ratio in real-time. In addition, coupling the methane oxidation half-reaction with an oxygen reducing cathode would render the overall process aerobic (eq 1).
Despite its attractiveness, there exist a paucity of examples of this approach. One report applied electrochemical oxidation in the presence of PtII, heteropoly acids, and O2 to achieve 1.4 turnovers for methanol production, but no information about the mechanism or stability of the system was provided.23 Earlier, a similar scheme was employed to oxidize a test substrate, p-toluenesulfonic acid; while 11 turnovers of the PtII catalyst were attained, deposition of Pt0 was observed with increasing reaction times.24 A particular impediment to electrochemical turnover of the aqueous PtII catalyst is the general sluggishness of two-electron PtII/IV oxidation at an electrode.25,26 Previously, we used electrochemical oxidation of PdII salts to generate a highly electrophilic Pd2III,III species that effects methane conversion to methanol precursors.27 While this system showed exceptional rates and high selectivity, it required concentrated acid media that restrict practical utility. Herein, we combine Pt electrodes that catalyze facile electrochemical oxidation of PtII to PtIV ions28 with in situ modulation of electric current to achieve continuous, steady-state methane oxidation over the course of 30 h. We observe the generation of methanol and methyl chloride as the principal products with >80% combined selectivity, demonstrating continuous PtII-catalyzed electrochemical methane oxidation.
Results and Discussions
Identification of a Suitable Electrode for the PtII-Catalyzed Electrochemical Methane Oxidation Reaction (EMOR)
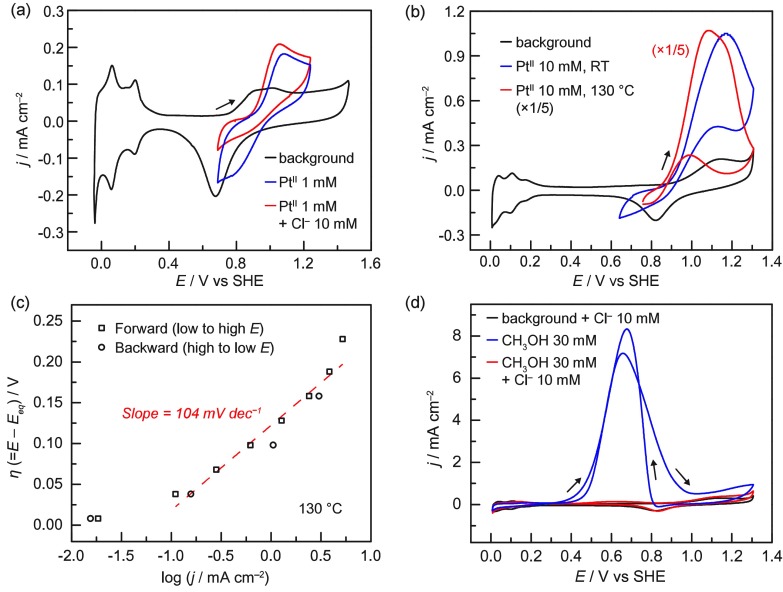
The electrochemical mediation scheme put forward above (Scheme 1, Strategy C) requires an electrode capable of oxidizing PtII to PtIV. In view of the high PtII/IV oxidation potential (E0 = 0.68 V versus SHE for PtIICl42–/PtIVCl62–)29 and the acidic environment required for stability of the Pt ions,30 we focused our investigations on carbon, fluorine-doped tin oxide (FTO), and Pt electrodes as possible candidates. Whereas carbon and FTO electrodes displayed progressive deactivation and/or sluggish PtII oxidation kinetics (see the SI, Section S2), Pt electrodes showed facile oxidation of PtII at modest potentials. In 0.5 M H2SO4, the Pt electrode displays the typical voltammetric features associated with hydrogen underpotential deposition (H UPD) and oxide formation at low and high potentials, respectively (Figure 1a, black; also Figure S1).31,32
Figure 1.
(a) Cyclic voltammograms obtained on a Pt disk electrode at room temperature in 0.5 M H2SO4; (black) background, (blue) 1 mM K2PtIICl4, and (red) 1 mM K2PtIICl4 with 10 mM NaCl. (b) Cyclic voltammograms obtained on a Pt wire electrode in 10 mM NaCl, 0.5 M H2SO4; (black) background, (blue) 10 mM K2PtIICl4 at room temperature, and (red) 10 mM K2PtIICl4 at 130 °C. (c) Tafel plot at 130 °C for PtII electro-oxidation. The solution contained 5 mM each of K2PtIICl4 and Na2PtIVCl6 in 10 mM NaCl, 0.5 M H2SO4. Eeq (= 0.829 V vs SHE) was obtained from the open-circuit potential. (d) Cyclic voltammograms obtained on a Pt wire electrode in 10 mM NaCl, 0.5 M H2SO4 at 130 °C; (black) background, (blue) 30 mM CH3OH without the 10 mM NaCl, and (red) 30 mM CH3OH. All scan rates = 100 mV s–1.
Upon addition of 1 mM PtIICl42–, a reversible wave appears at Ep,a = 1.1 V and Ep,c = 0.8 V (Figure 1a, blue). The appearance of this wave is accompanied by a suppression in the background Pt oxide wave, which we ascribe to inhibition by surface-adsorbed Cl– that has dissociated from the PtIICl42– ions (Figure S14).33 As sustained methanol production requires Cl– ions (see below), we also examined the voltammetric response of PtII in the presence of 10 mM Cl– (Figure 1a, red). Whereas the PtII oxidation wave is largely unaffected by the additional Cl–, the cathodic wave associated with PtIV back-reduction is significantly suppressed. These observations are in line with the previous literature on PtII/IV oxidation at Pt electrodes that invokes an inner-sphere electron transfer mechanism involving transfer of a surface-adsorbed Cl– to PtII during the oxidation reaction.28 Indeed, the reported Cl-adsorption isotherm at 2 mM Cl– stretches from 0 to 0.8 V versus SHE (Figure S15),34 showing near-saturation at the potential for PtIV reduction. These observations suggest that Cl– surface coverage at this potential may be incomplete at low [Cl–] but complete at 10 mM Cl–. Thus, higher surface coverage of Cl– induced by higher [Cl–] has a negligible impact on PtII oxidation, but the back-reduction of PtIV, which requires Cl– transfer back to the electrode surface, is inhibited (see the SI, Section S2, for additional explanation). This inner-sphere mechanism explains why Pt electrodes display superior PtII electro-oxidation kinetics compared to carbon or FTO.
Having identified a suitable electrode material, we then investigated PtII/IV electro-oxidation at the elevated temperatures required for methane activation by PtII. These experiments were conducted above the boiling point of water and were, therefore, carried out in a home-built high-pressure electrochemical cell (see the SI and below). As shown in Figure 1b, red, high PtII oxidation current flowed at 130 °C; the 5-fold enhancement in current and approximately 100 mV negative shift in Ep,a compared to room temperature (Figure 1b, blue) reflect faster mass transport and electrode kinetics. The decrease in current at E > 1.1 V is attributed to the formation of surface oxides that inhibit the inner-sphere PtII oxidation. This inhibition is particularly pronounced at high [PtII] and high temperatures (see the SI, Section S2, for details). We also examined the dependence of PtII oxidation current on electrochemical driving force (Figure 1c). Keeping the potential below Pt oxide formation, <1.1 V, the steady-state current increased 10-fold per 104 mV of additional overpotential (η = E – Eeq). This Tafel slope at 130 °C corresponds to a rate-limiting one-electron transfer with a transfer coefficient of 0.77, in agreement with the aforementioned mechanism.35 These results show that Pt electrodes are capable of facile oxidation of PtII at elevated temperatures.
Pt electrodes were also capable of sustained and efficient PtII/IV oxidation. We carried out bulk electrolyses of a stirred solution at 130 °C by applying a constant potential below 1.1 V. After chronoamperometry at 0.874, 0.924, and 0.974 V for 77, 40, and 17 min, respectively, half of the PtII ions in the initial solution were converted to PtIV ions as determined by UV−Vis analysis. At all three potentials examined, PtIV was generated with 100% Faradaic efficiency (Table S1).
Sustained methane oxidation catalysis will lead to a progressive rise in methanol concentration in the reactor over time. Thus, in addition to supporting facile PtII/IV oxidation, the electrode must be inert toward further oxidation of the CH3OH product. This is a particular concern for Pt, which is the standard electrocatalyst for oxidation of CH3OH to CO2.36 Indeed, in 0.5 M H2SO4 at 130 °C, addition of 30 mM CH3OH gives rise to the well-known anodic features associated with CH3OH electro-oxidation (Figure 1d, blue).37 Remarkably, upon addition of 10 mM Cl–, this CH3OH oxidation feature is almost completely suppressed (Figure 1d, red) over the entire potential range examined. This suppression is ascribed to surface adsorption of Cl– ions.38 An additional control experiment confirmed that the non-electrochemical oxidation of CH3OH catalyzed on metallic Pt39 is also negligible under our conditions (see the SI, Section S2). These data indicate that, fortuitously, the presence of Cl– serves to simultaneously promote PtII/IV oxidation and suppress surface-catalyzed oxidation of the methanol product. Together, these studies establish that Pt electrodes are suitable for EMOR.
Sustained Methane Oxidation Catalysis via Dynamic Electrochemical Control of the PtII:PtIV Ratio
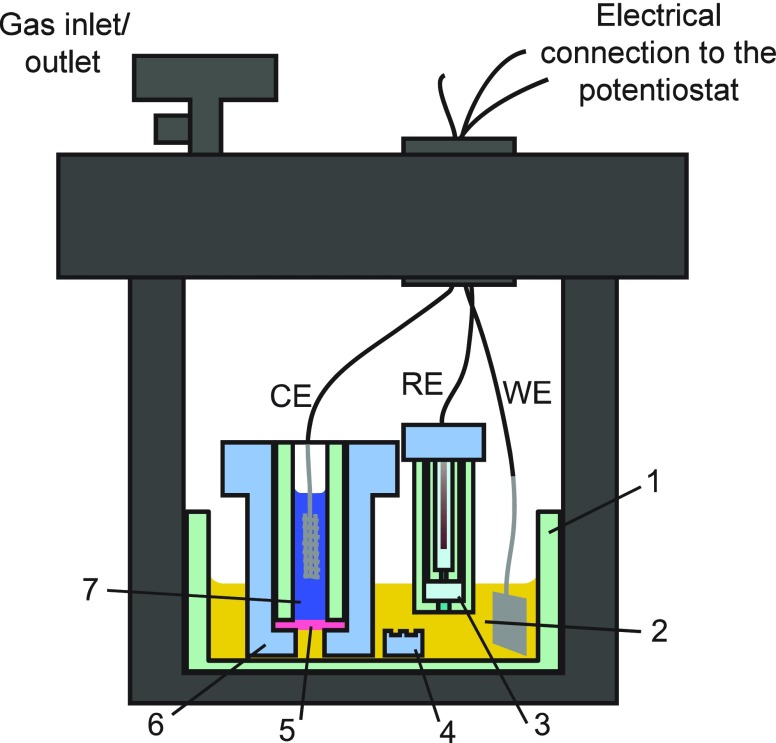
The above studies provide the basis for carrying out continuous methane-to-methanol oxidation catalysis via electrochemical regeneration of PtIV (Scheme 1, Strategy C). The EMOR was carried out in a home-built high-pressure cell which consisted of a modified Parr reactor with electrical feedthroughs (Figure 2; see the SI, Section S1, for full details). The working compartment was charged with 3 mM PtII and 7 mM PtIV in 10 mM NaCl, 0.5 M H2SO4 (see the SI, Section S4, for details of electrolyte optimization). The counter compartment, separated by a H+-conducting membrane stack, contained 3 M vanadyl sulfate ((VIVO)(SO4)) as a sacrificial oxidant to be reduced at the cathode. In a practical device, oxygen could be supplied to the cathode, but given the low solubility of O2 and complications of co-pressurizing the cell with O2, we opted to use the vanadyl ion as a surrogate. The highly soluble and fairly inert vanadyl ions enabled examination of long-term electrolysis. This counter reaction prevented H2 evolution, which must be avoided in this configuration due to the irreversible reduction of PtII to Pt0 by H2; however, in a well-engineered system with good gas stream separation, H2 may be deliberately generated as a useful byproduct. The solutions and the cell were purged to remove O2 prior to pressurization with 500 psi of methane. Following heating and temperature stabilization at 130 °C, electrolysis was initiated to continuously reoxidize PtII during methane functionalization catalysis. The electrolysis was carried out with control of the current instead of the potential, which is the preferred method in industrial electrolysis.40
Figure 2.
High-pressure, three-electrode, two-compartment electrochemical cell for the EMOR. WE, Pt foil working electrode; RE, Ag/AgCl reference electrode; CE, Pt mesh counter electrode. 1, glass cell; 2, working solution containing the Pt ions; 3, fritted tubes for housing the RE; 4, PTFE stir bar; 5, H+-conducting membrane separating the counter compartment; 6, PTFE body holding the membrane stack; 7, counter compartment solution containing (VIVO)(SO4) as the sacrificial electron acceptor.
Careful choice of the applied current is critical for sustained catalysis. To maintain a constant PtII:PtIV ratio over the course of the reaction, the rate of PtII oxidation at the electrode must match the rate of methane oxidation catalysis in the solution. A simple mathematical derivation shows that, at a fixed rate of PtII/IV oxidation, any small difference between the two rates will cause the PtII:PtIV ratio to deviate from the initial value exponentially over time (SI, Section S6). Thus, the applied current must be constantly readjusted to match the rate of catalysis to maintain a steady ratio of PtII:PtIV. To achieve this, we employed the open-circuit potential (OCP) of the working compartment as an in situ probe of the instantaneous PtII:PtIV ratio in solution and adjusted the current (i) accordingly. In our reactor, the PtII and PtIV ions exist in various ligated states (PtIIClx(H2O)(4–x)(2–x) and PtIVClx(H2O)(6–x)(4–x)), each pair of which has different redox potentials. Assuming that [Cl–] is constant, the following modified form of the Nernst equation may be derived:
| 2 |
where E0″ and n represent the weighted average of the redox potentials and Cl– stoichiometries, respectively. Thus, using eq 2, we can estimate the instantaneous PtII:PtIV ratios potentiometrically. EC can be determined from the initial OCP reading and the known initial PtII:PtIV ratio.
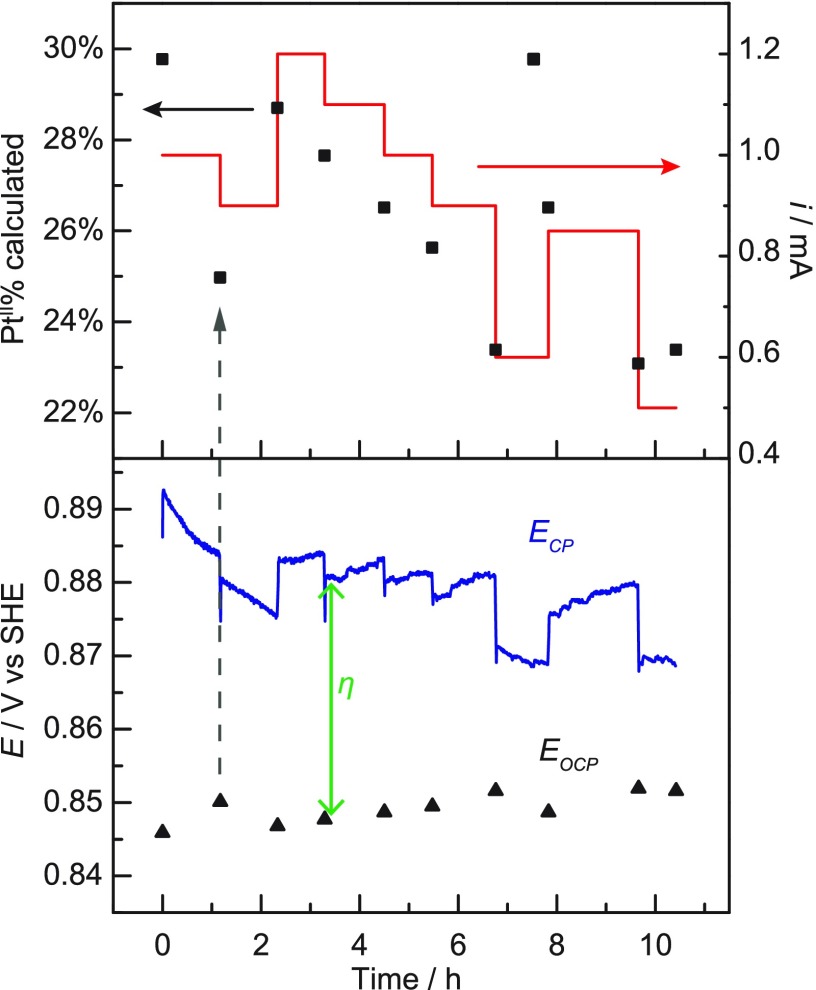
Figure 3 shows the electrochemical data recorded during a typical EMOR trial with periodic OCP monitoring and adjustment of the current. To aid the interpretation, the PtII:PtIV ratio was converted to the percentage of PtII ions (PtII%), defined as [PtII]/([PtII] + [PtIV]). In a representative reaction, after 1.0 mA of current was passed for 1 h, the PtII% decreased from 30% to 25%. This led us to adjust the current to 0.9 mA, and after another 1 h, the PtII% rose to 29%. This process of quantifying the PtII% in the solution and adjusting the current to maintain a roughly constant PtII% was repeated periodically until the reaction was terminated. Incidentally, while our test reactor was too congested to conveniently add a fourth electrode, incorporation of a separate sensing electrode could allow, in principle, for real-time feedback modulation of i.
Figure 3.
Representative electrochemical data recorded during an EMOR trial (the 10.5 h long trial in Table 1). The open-circuit potential (EOCP) reading at approximately 1 h time intervals (bottom, black triangles) was used to calculate the PtII% in the solution (top, black squares). This was in turn used to determine how much current to pass (top, red line), and the electrode potential during the electrolysis (ECP) was recorded (bottom, blue line).
The potential required for electrolysis (ECP, CP = chronopotentiometry) equals the equilibrium electrode potential (EOCP) plus the magnitude of overpotential (η) applied. By definition, η is the difference between the applied potential (ECP) and EOCP, as marked with green arrows in Figure 3. Over multiple trials, we consistently observed a steady decrease in η during the initial 2−3 h of each electrolysis, which we attribute to a slow initial electrode activation process. After stabilization of the electrode activity, η was ca. 20−40 mV, at an average current of around 0.9 mA. Normalizing by the electrode surface area, we estimate an average current density of 0.09 mA cm–2. This is in line with the previously obtained Tafel plot (Figure 1c) after considering the difference in [PtII] (5 mM in the Tafel plot, approximately 3 mM in the EMOR trials). While the required η in our system is an extrinsic parameter that depends on the reactor configuration (see the SI, Section S6), we emphasize that the fast PtII oxidation kinetics on the Pt electrode enable such a low η.
Independent quantification of the PtII:PtIV ratio at the end of the EMOR experiment confirmed the power of in situ current modulation. At the end of each reactor trial, [PtII] and [PtIV] in the working compartment were measured by UV−Vis spectroscopy. Despite a wide variation in reaction time (5−29 h) and consequently turnover number (see below), UV−Vis analysis confirmed that the final PtII% (19−23%) values were all similar (Table 1). These values are somewhat lower than the initial PtII% (30%), reflecting our preference to err on the side of lower PtII% to prevent irreversible Pt0 deposition (see below). Interestingly, despite the agreement in final PtII% values, ΔOCP (= OCPlast – OCPfirst), which should reflect the final PtII% according to eq 2, was more negative for longer reactions by up to 14 mV. We postulate that this may be due to decreasing [Cl–] in the reaction solution as a result of CH3Cl formation. Despite this additional long-term effect, changes in the OCP between constant-current intervals provided a faithful indication of whether the PtII% was increasing or decreasing, allowing for appropriate adjustment of i. Together, these results demonstrate that the PtII% can indeed be maintained over long time durations of catalysis through dynamically-controlled electrochemical oxidation.
Table 1. Results of EMOR Trials at T = 130 °C and PCH4 = 675 psia.
| product [μmol (relative fraction)] |
approximate
TONb |
approximate
TOFb (h–1) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| timec (h) | iaved (mA) | ΔOCPe (mV) | final [PtII%] | CH3OH | CH3Cl | CH2(OH)2f | HCOOH | CO2 | CH3X | total | CH3X | total |
| 4.9 | 1.19 | 7.9 | 22% | 60.5 (72%) | 20.1 (24%) | 2.2 (3%) | 0.1 (0%) | 1.1 (1%) | 1.4 | 1.6 | 0.29 | 0.32 |
| 10.5 | 0.88 | 5.7 | 19% | 93.7 (71%) | 27.9 (21%) | 5.1 (4%) | 1.2 (1%) | 4.4 (3%) | 2.3 | 2.9 | 0.21 | 0.27 |
| 18.4 | 1.00 | –2.8 | 22% | 205.4 (72%) | 44.8 (16%) | 21.9 (8%) | 2.9 (1%) | 12.2 (4%) | 4.5 | 6.3 | 0.24 | 0.34 |
| 29.3 | 0.91 | –6.0 | 23% | 268.0 (69%) | 52.0 (13%) | 36.4 (9%) | 7.2 (2%) | 24.1 (6%) | 5.8 | 9.3 | 0.20 | 0.32 |
Initial [PtII] and [PtIV] in the working solution were 3 mM and 7 mM, respectively, and the solution volume was 23 mL. The electrochemically active surface area of the Pt working electrode was 10.3 cm2.
The TONs were determined from dividing the moles of product by the average of the initial and final moles of PtII for each reaction. The TOFs were obtained by dividing the TON by the time duration of each reaction. The total number of turnovers was calculated by assuming that all oxidation reactions were catalyzed by PtII: (μmolCH3OH + μmolCH3Cl + 2 × μmolCH2(OH)2 + 3 × μmolHCOOH + 4 × μmolCO2) was divided by the average μmolPtII to determine total TON. For CH3X-specific turnovers, only (μmolCH3OH + μmolCH3Cl) was divided by μmolPtII.
The reaction time is the length of time the reactor was at the designated temperature, which spanned from ∼80 min after the start of heating to the time at which the reactor was removed from the oil bath.
iave was calculated by dividing the total charge passed by the reaction time.
ΔOCP is the difference between the first and last OCP readings (= OCPlast – OCPfirst).
The hydrated form of formaldehyde, which is the predominant form of formaldehyde in the acidic pH employed.
Careful control of the PtII:PtIV ratio during the reaction is essential for another reason: PtIV ions suppress the irreversible decomposition of PtII to Pt0.9,16 Indeed, at the end of all of our EMOR trials, the bulk reaction solutions contained no visible Pt0 precipitates. Only a few adventitious Pt0 deposits were observed on the reactor surfaces and crevices where mass transport was restricted and replenishment of PtIV was impeded (see the SI, Section S5). One of the Pt0 deposition mechanisms is disproportionation of PtII.16 While the solution composition is thermodynamically inclined to deposit Pt0 (Figure S19),41 our results demonstrate that, under sufficiently high [PtIV], nucleation of Pt0 may be inhibited (see the SI, Section S5 and Table S3). Although an extensive discussion of Pt0 deposition mechanisms is beyond the scope of the current work, these considerations highlight the importance of maintaining a stable PtII:PtIV ratio.
Analysis of Methane Oxidation Products from the EMOR Reactor
Operation of the EMOR reactor using the feedback modulation procedure described above allowed for continuous functionalization of methane (Table 1 and Figure 4). In all cases, we observe CH3OH as the majority product in 69−72% yield (Table 1). We also observe appreciable quantities of CH3Cl with a yield that decreases from 24% to 13% as the reaction time increases. Small amounts of overoxidized products (CH2(OH)2, HCOOH, and CO2) were observed in less than 20% combined yield. Taking these overoxidized products to represent PtII-catalyzed oxidation of CH3OH by 1, 2, and 3 equivalents of PtIV, respectively, the overall Faradaic efficiencies were in excess of 90% in all cases (Table S2). The per-PtII turnover numbers (TON) could not be rigorously determined due to minor fluctuations in [PtII] over the course of the reaction (see above), but approximate values were calculated from the known initial and final PtII amounts. For the longest trial, TON values of 6 and 9 for monofunctionalized products (CH3X = CH3OH and CH3Cl) and total oxidation events were obtained, respectively (Table 1). The TOF for CH3X, estimated to be 0.2−0.3 h–1, showed a decreasing trend with increasing reaction time due to the overoxidation of CH3OH. In contrast, the TOF for total oxidation events was relatively constant at ca. 0.3 h–1 for different reaction times. Together, these observations demonstrate that electrochemical reoxidation effectively sustains PtII-based methane functionalization catalysis.
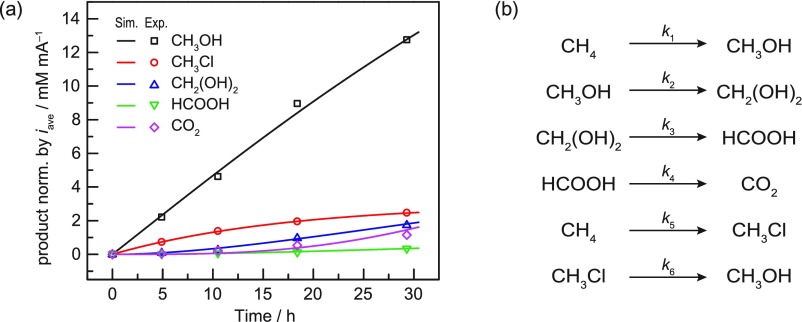
Figure 4.
(a) Amounts of methane oxidation products generated in the EMOR reactor versus reaction time. Each point represents a different trial in Table 1, and the product concentrations were normalized by iave of each trial (see the SI, Section S6, for explanation). The lines represent fitting with the (b) set of putative reactions.
Combining the four trials in Table 1, Figure 4a visualizes the temporal progression of EMOR. We fit these data to the set of reactions suggested earlier: oxidation of CH4 to CH3OH and CH3Cl, hydrolysis of CH3Cl to CH3OH, and subsequent overoxidation of CH3OH to CH2(OH)2, HCOOH, and CO2 (Figure 4b). While the fitted apparent rate constants (Table S6) for CH2(OH)2 and HCOOH oxidation show deviation from values separately determined outside the reactor (Table S7), the fitted values for CH4 and CH3OH oxidation are in good agreement with those independent measurements (Table S8). Thus, this simple model provides a reasonable description of the methane oxidation processes taking place during the EMOR.
All of the EMOR experiments shown in Table 1 were performed with identical reaction solution compositions with a low (3 mM) catalyst concentration. When the concentrations of PtII, PtIV, and Cl– were increased, CH3OH and CH3Cl output increased while the fraction of CO2 decreased (see the SI, Section S6 and Table S5). These results suggest that there is ample room for optimization of the solution composition to maximize yield and selectivity.
Outlook for Practical Methane Oxidation
Our studies establish that electrochemical oxidation endows Shilov’s catalyst with a sustainable mechanism for turnover and an inherent stability against deactivation through either complete oxidation of PtII to PtIV or Pt0 deposition. However, we acknowledge that the PtII catalyst displays a relatively low reaction rate and moderate selectivity.9 Our work does not directly address these inherent limitations of the catalyst; furthermore, our proof-of-concept reactor was not designed to demonstrate optimal TON, TOF, or selectivity for methanol. However, the EMOR approach developed here opens the door toward a broader exploration of reaction conditions and reactor configurations that may overcome these rate and selectivity limitations. For example, higher temperatures and catalyst concentrations could be employed to enhance the reaction rate, but these conditions would lower the kinetic barrier to deactivation by Pt0 deposition. The EMOR can be used to maintain an optimal PtII/IV ratio that is matched to these conditions (e.g., Figure S19, red square) and thereby sustain catalysis at higher volumetric productivity. Additionally, since the PtIIClx(H2O)(4–x)(2–x) catalyst displays modest selectivity for methane versus methanol oxidation (∼1:1) (SI, Table S8),11 strategies for continuous product removal would be needed to minimize overoxidation. As opposed to a volatile chemical oxidant that may be released at a similar rate as the methanol product, electrochemical oxidation could allow for independent control of oxidant delivery and product release. While many challenges remain, EMOR offers new opportunities for developing practical Shilov-type systems for methane-to-methanol conversion.
Safety Statement
No unexpected or unusually high safety hazards were encountered.
Conclusions
We have established an electrochemical approach for continuous methane-to-methanol conversion using aqueous PtII catalysts. Cl-adsorbed Pt surfaces were shown to be competent for the inner-sphere two-electron oxidation of PtII to PtIV while inert toward parasitic oxidation of the methanol product. In situ potential measurements and current modulation allowed us to carry out continuous steady-state catalysis by maintaining the PtII:PtIV ratio. While our test reactors were run up to 30 h, further reactor engineering to automatically modulate the current in real-time, enhance solution mixing, and rigorously separate the anode and cathode compartments should allow for extended operation. Moreover, integration of an oxygen-consuming counter electrode will enable net aerobic methane-to-methanol conversion (eq 1). While many additional challenges remain to realize viable PtII-catalyzed methane conversion,9 we envision that the electrochemical approach developed here will stimulate continued progress toward practical technologies for aerobic methane valorization.
Acknowledgments
We thank Travis Marshall-Roth, Patrick Smith, Michael Pegis, Thejas Wesley, Jaeyune Ryu, Bing Yan, Randall Field, and Sahag Voskian for helpful discussions. We thank Marcel Schreier and Jianbo Wang for assistance with GC analysis. This work was supported by Eni S.p.A. through the MIT Energy Initiative. Y.S. acknowledges the Sloan Foundation, Research Corporation for Science Advancement (Cottrell Scholar), and the Canadian Institute for Advanced Research (CIFAR Azrieli Global Scholar).
Glossary
Abbreviations
- EMOR
electrochemical methane oxidation reaction
- FE
Faradaic efficiency
- OCP
open-circuit potential
- SHE
standard hydrogen electrode
- FTO
fluorine-doped tin oxide
- TON
turnover numbers
- TOF
turnover frequencies
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.9b00273.
Full experimental details, description of the high-temperature electrochemistry, additional electrochemical data, additional EMOR reactor data, electrolyte optimization, and substrate oxidation experiments (PDF)
The authors declare the following competing financial interest(s): R.S.K. and Y.S. are inventors on provisional patent application 62/819,046 filed by the Massachusetts Institute of Technology that covers the electrochemical regeneration method reported in this work.
Supplementary Material
References
- da Silva M. J. Synthesis of Methanol from Methane: Challenges and Advances on the Multi-Step (Syngas) and One-Step Routes (DMTM). Fuel Process. Technol. 2016, 145, 42–61. 10.1016/j.fuproc.2016.01.023. [DOI] [Google Scholar]
- Wang B.; Albarracín-Suazo S.; Pagan-Torres Y.; Nikolla E. Advances in Methane Conversion Processes. Catal. Today 2017, 285, 147–158. 10.1016/j.cattod.2017.01.023. [DOI] [Google Scholar]
- Olah G. A. Beyond Oil and Gas: The Methanol Economy. Angew. Chem., Int. Ed. 2005, 44, 2636–2639. 10.1002/anie.200462121. [DOI] [PubMed] [Google Scholar]
- Holmen A. Direct Conversion of Methane to Fuels and Chemicals. Catal. Today 2009, 142, 2–8. 10.1016/j.cattod.2009.01.004. [DOI] [Google Scholar]
- Bank W.Zero Routine Flaring by 2030. www.worldbank.org/en/programs/zero-routine-flaring-by-2030 (accessed Sept 24, 2016).
- Promoppatum P.; Viswanathan V. Identifying Material and Device Targets for a Flare Gas Recovery System Utilizing Electrochemical Conversion of Methane to Methanol. ACS Sustainable Chem. Eng. 2016, 4, 1736–1745. 10.1021/acssuschemeng.5b01714. [DOI] [Google Scholar]
- Wogan T.Methane to methanol catalyst could end gas flaring. www.chemistryworld.com/news/methane-to-methanol-catalyst-could-end-gas-flaring/3007247.article (accessed Feb 1, 2019).
- Ravi M.; Ranocchiari M.; van Bokhoven J. A. The Direct Catalytic Oxidation of Methane to Methanol—A Critical Assessment. Angew. Chem., Int. Ed. 2017, 56, 16464–16483. 10.1002/anie.201702550. [DOI] [PubMed] [Google Scholar]
- Labinger J. A.; Bercaw J. E. Mechanistic Studies on the Shilov System: A Retrospective. J. Organomet. Chem. 2015, 793, 47–53. 10.1016/j.jorganchem.2015.01.027. [DOI] [Google Scholar]
- Latimer A. A.; Kakekhani A.; Kulkarni A. R.; Nørskov J. K. Direct Methane to Methanol: The Selectivity-Conversion Limit and Design Strategies. ACS Catal. 2018, 8, 6894–6907. 10.1021/acscatal.8b00220. [DOI] [Google Scholar]
- Owen J. S.; Labinger J. A.; Bercaw J. E. Kinetics and Mechanism of Methane, Methanol, and Dimethyl Ether C-H Activation with Electrophilic Platinum Complexes. J. Am. Chem. Soc. 2006, 128, 2005–2016. 10.1021/ja056387t. [DOI] [PubMed] [Google Scholar]
- Labinger J. A.; Bercaw J. E.; Luinstra G. A.; Lyon D. K.; Herring A. M. Organometallic Methane Activation: Functionalization by Aqueous Platinum Complexes. In. Stud. Surf. Sci. Catal. 1994, 81, 515–520. 10.1016/S0167-2991(08)63922-1. [DOI] [Google Scholar]
- Michalkiewicz B. Assessment of the Possibility of the Methane to Methanol Transformation. Pol. J. Chem. Technol. 2008, 10, 20–26. 10.2478/v10026-008-0023-5. [DOI] [Google Scholar]
- Labinger J. A.Alkane Functionalization via Electrophilic Activation. In Catalysis by Metal Complexes: Alkane C-H Activation by Single-Site Metal Catalysis; Pérez P. J., Ed.; Catalysis by Metal Complexes; Springer Netherlands: Dordrecht, 2012; Vol. 38, Chapter 2, pp 17–71. [Google Scholar]
- Gunsalus N. J.; Koppaka A.; Park S. H.; Bischof S. M.; Hashiguchi B. G.; Periana R. A. Homogeneous Functionalization of Methane. Chem. Rev. 2017, 117, 8521–8573. 10.1021/acs.chemrev.6b00739. [DOI] [PubMed] [Google Scholar]
- DeVries N.; Roe D. C.; Thorn D. L. Catalytic Hydroxylation Using Chloroplatinum Compounds. J. Mol. Catal. A: Chem. 2002, 189, 17–22. 10.1016/S1381-1169(02)00194-2. [DOI] [Google Scholar]
- Weinberg D. R.; Labinger J. A.; Bercaw J. E. Competitive Oxidation and Protonation of Aqueous Monomethylplatinum(II) Complexes: A Comparison of Oxidants. Organometallics 2007, 26, 167–172. 10.1021/om060763g. [DOI] [Google Scholar]
- Lin M.; Shen C.; Garcia-Zayas E. A.; Sen A. Catalytic Shilov Chemistry: Platinum Chloride-Catalyzed Oxidation of Terminal Methyl Groups by Dioxygen. J. Am. Chem. Soc. 2001, 123, 1000–1001. 10.1021/ja001926+. [DOI] [PubMed] [Google Scholar]
- Bar-Nahum I.; Khenkin A. M.; Neumann R. Mild, Aqueous, Aerobic, Catalytic Oxidation of Methane to Methanol and Acetaldehyde Catalyzed by a Supported Bipyrimidinylplatinum-Polyoxometalate Hybrid Compound. J. Am. Chem. Soc. 2004, 126, 10236–10237. 10.1021/ja0493547. [DOI] [PubMed] [Google Scholar]
- Kreutz J. E.; Shukhaev A.; Du W.; Druskin S.; Daugulis O.; Ismagilov R. F. Evolution of Catalysts Directed by Genetic Algorithms in a Plug-Based Microfluidic Device Tested with Oxidation of Methane by Oxygen. J. Am. Chem. Soc. 2010, 132, 3128–3132. 10.1021/ja909853x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.; Sanford M. S. Platinum-Catalyzed, Terminal-Selective C(sp3)-H Oxidation of Aliphatic Amines. J. Am. Chem. Soc. 2015, 137, 12796–12799. 10.1021/jacs.5b09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth I. T.; Cook R. A.; Millar J. M.; Kiss G. Low-Temperature Methane Chlorination with Aqueous Platinum Chlorides in the Presence of Chlorine. Organometallics 1993, 12, 8–10. 10.1021/om00025a004. [DOI] [Google Scholar]
- Liu S. F.; Nusrat F. Electrocatalytic Shilov Chemistry for the Oxidation of Aliphatic Groups. Mol. Catal. 2019, 463, 16–19. 10.1016/j.mcat.2018.11.008. [DOI] [Google Scholar]
- Freund M. S.; Labinger J. A.; Lewis N. S.; Bercaw J. E. Electrocatalytic Functionalization of Alkanes Using Aqueous Platinum Salts. J. Mol. Catal. 1994, 87, L11–L15. 10.1016/0304-5102(93)E0230-E. [DOI] [Google Scholar]
- Lappin G.Redox Mechanisms in Inorganic Chemistry; Ellis Horwood: New York, 1994. [Google Scholar]
- Jude H.; Krause Bauer J. A.; Connick W. B. An Outer-Sphere Two-Electron Platinum Reagent. J. Am. Chem. Soc. 2003, 125, 3446–3447. 10.1021/ja034003y. [DOI] [PubMed] [Google Scholar]
- O’Reilly M. E.; Kim R. S.; Oh S.; Surendranath Y. Catalytic Methane Monofunctionalization by an Electrogenerated High-Valent Pd Intermediate. ACS Cent. Sci. 2017, 3, 1174–1179. 10.1021/acscentsci.7b00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing J. P.; Hubbard A. T. Study of the Kinetics of Electrochemical Reactions By Thin Layer Voltammetry. II. Electro-Oxidation of Platinum (II) Complexes. J. Electroanal. Chem. Interfacial Electrochem. 1969, 23, 183–203. 10.1016/S0022-0728(69)80209-3. [DOI] [Google Scholar]
- Vanysek P.Thermo, Electro & Solution Chemistry: Electrochemical Series. In CRC Handbook of Physics and Chemistry, 99th ed.; CRC Press, 2018. [Google Scholar]
- Elding L. I. Preparation and Properties of the Tetra-Aquaplatinum(II) Ion in Perchloric Acid Solution. Inorg. Chim. Acta 1976, 20, 65–69. 10.1016/S0020-1693(00)94092-1. [DOI] [Google Scholar]
- Scortichini C. L.; Reilley C. N. Surface Characterization of Pt Electrodes Using Underpotential Deposition of H and Cu. V. Characterization of BD Pt Catalyst Surface. J. Catal. 1983, 79, 138–146. 10.1016/0021-9517(83)90296-8. [DOI] [Google Scholar]
- Jerkiewicz G.; Vatankhah G.; Lessard J.; Soriaga M. P.; Park Y. S. Surface-Oxide Growth at Platinum Electrodes in Aqueous H2SO4 Reexamination of Its Mechanism through Combined Cyclic-Voltammetry, Electrochemical Quartz-Crystal Nanobalance, and Auger Electron Spectroscopy Measurements. Electrochim. Acta 2004, 49, 1451–1459. 10.1016/j.electacta.2003.11.008. [DOI] [Google Scholar]
- Novak D. M.; Conway B. E. Competitive Adsorption and State of Charge of Halide Ions in Monolayer Oxide Film Growth Processes at Pt Anodes. J. Chem. Soc., Faraday Trans. 1 1981, 77, 2341–2359. 10.1039/f19817702341. [DOI] [Google Scholar]
- Balashova N. A.; Kazarinov V. E. Study of the Structure of the Electrical Double Layer on Platinum by the Radioactive Tracer Method. Russ. Chem. Rev. 1965, 34, 730–736. 10.1070/RC1965v034n10ABEH001557. [DOI] [Google Scholar]
- Compton R. G.; Banks C. E.. Understanding Voltammetry, 2nd ed.; World Scientific Publishing: Singapore, 2007. [Google Scholar]
- Zhao X.; Yin M.; Ma L.; Liang L.; Liu C.; Liao J.; Lu T.; Xing W. Recent Advances in Catalysts for Direct Methanol Fuel Cells. Energy Environ. Sci. 2011, 4, 2736. 10.1039/c1ee01307f. [DOI] [Google Scholar]
- Chung D. Y.; Lee K. J.; Sung Y. E. Methanol Electro-Oxidation on the Pt Surface: Revisiting the Cyclic Voltammetry Interpretation. J. Phys. Chem. C 2016, 120, 9028–9035. 10.1021/acs.jpcc.5b12303. [DOI] [Google Scholar]
- Snell K. D.; Keenan A. G. Chloride Inhibition of Ethanol Electrooxidation at a Platinum Electrode in Aqueous Acid Solution. Electrochim. Acta 1981, 26, 1339–1344. 10.1016/0013-4686(81)85119-5. [DOI] [Google Scholar]
- Sen A.; Lin M.; Kao L. C.; Hutson A. C. C-H Activation in Aqueous Medium. The Diverse Roles of Platinum(II) and Metallic Platinum in the Catalytic and Stoichiometric Oxidative Functionalization of Organic Substrates Including Alkanes. J. Am. Chem. Soc. 1992, 114, 6385–6392. 10.1021/ja00042a014. [DOI] [Google Scholar]
- Cardoso D. S. P.; Šljukić B.; Santos D. M. F.; Sequeira C. A. C. Organic Electrosynthesis: From Laboratorial Practice to Industrial Applications. Org. Process Res. Dev. 2017, 21, 1213–1226. 10.1021/acs.oprd.7b00004. [DOI] [Google Scholar]
- Gammons C. H. Experimental Investigations of the Hydrothermal Geochemistry of Platinum and Palladium: V. Equilibria between Platinum Metal, Pt(II), and Pt(IV) Chloride Complexes at 25 to 300°C. Geochim. Cosmochim. Acta 1996, 60, 1683–1694. 10.1016/0016-7037(96)00048-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.