Abstract
Chondroitin sulfate proteoglycans (CSPGs) are upregulated in insults to the central nervous system, including multiple sclerosis (MS), an inflammatory demyelinating condition of the central nervous system. CSPGs appear to be detrimental in MS, as they enhance immune responses and act as barriers to oligodendrocyte differentiation and thus remyelination. Despite their deleterious roles, strategies to selectively reduce CSPG production are lacking. The purpose of this study was to develop, screen, and describe a series of glucosamine derivatives and xylosides for their capacity to overcome detrimental CSPGs and inflammatory processes. Specifically, we assess the ability of analogues to interfere with CSPG biosynthesis, promote the outgrowth of oligodendrocyte precursor cells in an inhibitory environment, and lower inflammation by attenuating the proliferation of T lymphocytes. We highlight the beneficial activities of a novel compound, per-O-acetylated 4,4-difluoro-N-acetylglucosamine (Ac-4,4-diF-GlcNAc) in vitro, and report that it reduced inflammation and clinical severity in a mouse model of MS. Thus, this study represents an important advance, as we uncover that targeting CSPG biosynthesis with a potent inhibitor is an effective avenue to ameliorate inflammatory cascades and promote repair processes in MS and other neurological conditions.
Short abstract
To target detrimental inflammatory chondroitin sulfate proteoglycans, we synthesized and screened inhibitory compounds. In vivo, a new potent compound improved outcomes in a multiple sclerosis model.
Introduction
Multiple sclerosis (MS) is an inflammatory disorder of the central nervous system (CNS) accompanied by loss of neurons and oligodendrocytes and prominent demyelination. While several immunomodulators have altered the natural history of relapsing–remitting MS, treatment response in many patients remains inadequate; moreover, there are no current therapies to halt the progression of neurological disabilities of MS. There is a need to develop therapies that not only target the aberrant immune responses but also promote repopulation of oligodendrocytes and remyelination in demyelinated plaques.
As in other tissues, the CNS has an extracellular matrix (ECM) that normally serves important physiological functions; when dysregulated in injury, however, the brain ECM components can directly influence inflammation and repair.1−5 For example, the presence of type I collagen can direct astrocyte fate from reactive to gliotic6 and the laminin composition of the basement membrane dictates where T lymphocytes infiltrate into the CNS.7
An emerging driver of inflammation in the brain is the chondroitin sulfate proteoglycans (CSPGs).2 CSPGs are upregulated in demyelinated plaques in brain specimens in MS8 and in perivascular cuffs where immune cells infiltrate into the brain parenchyma.9 Their presence in MS lesions is associated with enhanced activation and transmigratory capacity of macrophages9 as well as impaired remyelination.10 In both traumatic CNS injuries and MS, CSPGs inhibit regeneration by interfering with the migration of pro-regenerative neural and oligodendrocyte precursor cells (OPCs) into lesions.11−13
Given the above observations, it is pertinent to overcome CSPGs in neurological disorders including MS. In focal traumatic spinal cord injury, the enzyme chondroitinase ABC has been injected directly into the lesion to remove the glycosaminoglycan (GAG) chains of CSPGs, which are a crucial component of their inhibitory action.14−16 The local injection would not be feasible for a condition such as MS, with multifocal lesions throughout the brain and spinal cord. Moreover, we found that, once anchored onto a substrate, the CSPG inhibition of the morphological differentiation of OPCs cannot be overcome by promising pro-remyelinating therapies.17 Thus, preventing the deposition of CSPGs by interfering with their biosynthesis would be an effective approach to countering the problem that CSPG poses.
CSPG synthesis involves the creation of a protein core and covalent attachment of numerous glycosaminoglycan (GAG) side chains (Supporting Information Figure 1).18 The first step of GAG synthesis is the introduction of a β-xylose to a serine or threonine of the core protein. Following extension from the O4-position of the xylose into a trisaccharide linker (xylose, galactose, galactose), chondroitin sulfate GAG chains are elongated with the repeating β-(1 → 3)-linked disaccharides glucuronic acid (GlcA) and N-acetyl-galactosamine (GalNAc) (Supporting Information Figure 1). Uridine-5′-diphosphate-N-acetyl-galactosamine (UDP-GalNAc) is created from UDP-N-acetyl-glucosamine (UDP-GlcNAc) by the enzyme 4-epimerase through an oxidation and reduction process.19 The per-O-acetylated 4-fluorinated glucosamine analogue 3, which we have termed fluorosamine,17 was shown to have a remarkable ability to perturb GAG biosynthesis,20,21 potentially by acting as an inhibitor to 4-epimerase to prevent GAG elongation; fluorosamine may also deplete uridine-5′-triphosphate (UTP) and thus reduce UTP availability for sugar precursors. The per-O-acetylation of fluorosamine 3 was essential because this increases the hydrophobicity of the molecule, allowing it to better cross plasma membranes. After entering the cell, the O-acetates are presumably hydrolyzed by nonspecific esterases, releasing the 4-fluoro-substituted N-acetyl-d-glucosamine, which could be ultimately converted to the UDP-conjugated form and act as an inhibitor of the 4-epimerase.
An aim of this study was to synthesize new analogues that display greater potency and efficacy than fluorosamine.17 One strategy was to modify the substituents on the fluorosamine 3 so that the new derivatives would have improved capacity to cross the plasma membrane or interact with esterases; another strategy was to modify the nature of functionalities introduced to the C-4 position of fluorosamine 3, so that the new derivatives would potentially inhibit the 4-epimerases with improved potency. A third strategy was to create β-xylopyranosides as well as their derivatives substituted at C-4 positions (such as the 4-fluoroxyloses), so the biosynthesis of the GAG chain can be either diverted or inhibited at the attachment point of xylose to the core protein; mechanistically, the 4-fluoroxylose derivatives could inhibit the related xylosyltransferase. We thus synthesized glucosamine analogues 5–18 to target the 4-epimerase and xylosides 19–28 to target the upstream stage of the biosynthesis of CSPGs. Herein, we describe the evaluation of these compounds in various models pertinent to MS. We highlight in vitro screening results and potent in vivo effects of a 4,4-difluoro glucosamine analogue 16 (Ac-4,4-diF-GlcNAc) that attenuates severity of disease in an inflammatory animal model of MS, experimental autoimmune encephalomyelitis (EAE). These results highlight that targeting CSPGs represents a novel and promising therapeutic approach in MS.
Results
Synthesis of Compounds
We synthesized novel acetylated analogues of d-glucosamine that are either monofluorinated (5–13) or difluorinated (16–18) with other substitutions to various carbon positions (Figure 1). We previously described that compound 3 (Ac-4-F-GlcNAc, fluorosamine), our reference compound in the current study, reduced production of CSPGs by astrocytes, promoted remyelination following lysolecithin demyelination of the mouse spinal cord, and attenuated the severity of mice afflicted with EAE.17 Compounds 5 and 6 are analogues of Ac-4-F-GlcNAc 3 with permanent protection at either both the O3- and O6-positions or the O3-position alone via O-methylation; the other GlcNAc derivatives 7–12 are all 4-fluorinated but with removable acyl protecting groups of various lengths at different positions; in particular, compound 9 has a trifluoroacetyl modification on the nitrogen and compounds 10–12 are hemiacetals because they have no acyl group at the anomeric position. Instead of 4-fluorination, the related GlcNAc derivative 14 was also a hemiacetal but with a 4-chlorination. Compound 13 does not have the GlcNAc configuration; instead, it has the N-acetylgalactosamine (GalNAc) configuration with a 4-fluorination. In contrast to all above compounds, three difluorinated derivatives have also been synthesized. Compound 16 (Ac-4,4-diF-GlcNAc) has a 4,4-difluorination, making it unique because it combines the properties of 4-fluorinated derivatives of both GlcNAc and GalNAc series. For comparison, two other difluorinated compounds, 17 (Ac-4,6-diF-GlcNAc) and 18 (Ac-6,6-diF-GlcNAc), were also prepared. In addition, a series of water-soluble novel per-O-acetylated d-xyloside derivatives 19–25 were also produced, along with three 4-fluorinated d-xyloside derivatives 26–28. Compound 19, a nonacetylated benzyl β-xyloside, was known in the literature.22 Derivatives 20 and 21 contain ethylene glycol units of different lengths (neutral), derivatives 22–24 contain alkyl sulfonates of different lengths, and derivative 25 contains an amine functionality at the aglyone which can be protonated under physiological conditions. Additionally, the non-O-acetylated 4-fluorinated xylose and its two per-O-acetylated α-anomer 27 and β-anomer 28 were synthesized. The chemical syntheses of all new derivatives are reported in Supporting Information Figure 2.
Figure 1.
N-Acetyl-d-glucosamine and d-xylose derivatives highlighting their structure, short form in parentheses, and their bolded compound number. (A) Synthesized N-acetyl-d-glucosamine derivatives and (B) synthesized d-xyloside derivatives. Attachments to the oxygens in the skeleton were numbered on the basis of their attachment to the carbons in the skeleton of the monosaccharide. For example, the oxygen atom attached to C4 of xylose is referred to as “O4”.
Sugar Analogues Reduce CSPG Production by Astrocytes
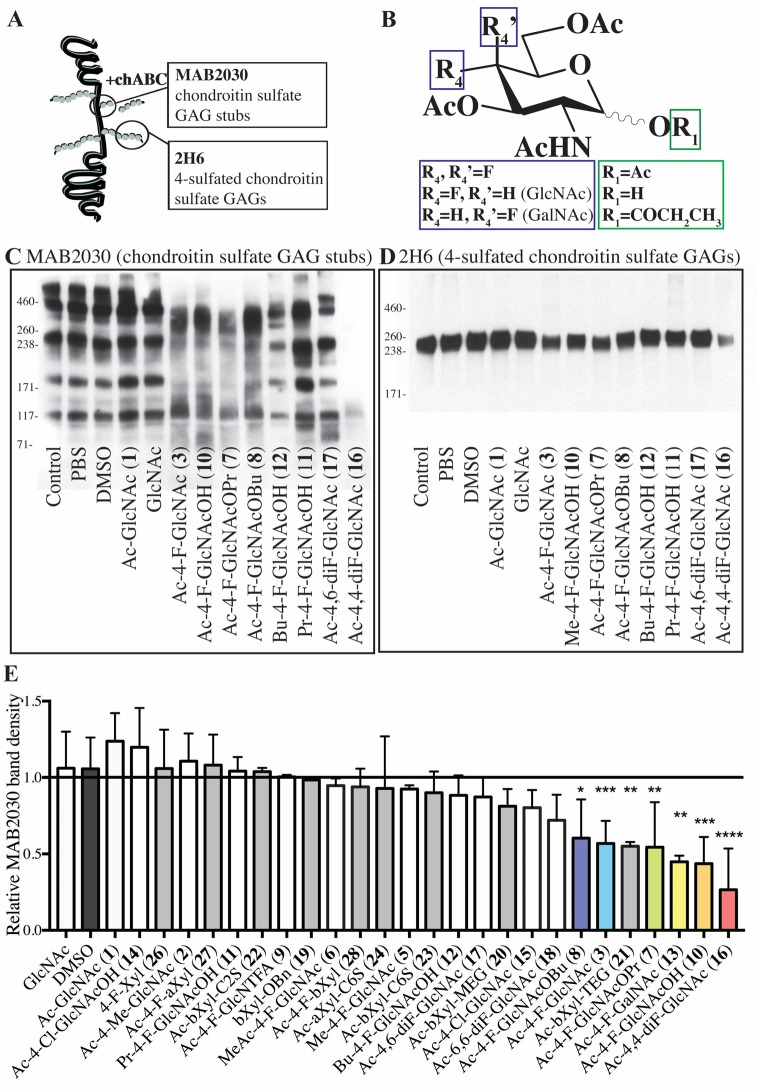
Astrocytes are major producers of CSPGs following injury in the CNS,23−27 and they may help drive progression of disability in a model of progressive MS.28 We therefore used astrocytes as model cells to determine the ability of the sugar analogues to reduce synthesis of CSPGs. Since CSPGs are exported out of cells, the conditioned media from analogue-treated astrocytes were probed by Western blots (Figure 2). We used the 2H6 antibody to label intact 4-sulfated chondroitin sulfate side chains and the MAB2030 antibody to detect the stubs of chondroitin sulfate GAGs attached to the core protein; the latter is a correlate of proteoglycan core proteins based on previous studies that have found that this antibody recognizes only chondroitin GAG chains attached to the core protein, and not native proteoglycans or isolated GAGs.29,30
Figure 2.
Fluorinated glucosamines reduce the synthesis of CSPGs by astrocytes. (A) Schematic binding sites of MAB2030 (after chondroitinase ABC treatment) and 2H6 to CSPGs. (B) Combined chemical structures of the five compounds that most effectively reduced CSPG production (Ac-4,4-diF-GlcNAc 16, Ac-4-F-GlcNAcOH 10, Ac-4-F-GalNAc 13, Ac-4-F-GlcNAcOPr 7, Ac-4-F-GlcNAc 3). (C) Representative Western blot for stub chondroitin-4-sulfate attached to the core protein (MAB2030) showing the effectiveness of certain N-acetyl-d-glucosamine derivatives at reducing CSPG production in astrocytes, as determined by sampling of the astrocyte conditioned medium in treated cells. (D) Representative Western blot of conditioned media for intact chondroitin-4-sulfate (2H6) in astrocytes treated with N-acetyl-d-glucosamine derivatives. (E) Relative band densities of MAB2030 in conditioned media of treated astrocytes versus untreated (control) astrocytes. The column represents the average band densities from three independent Western blots except for the following compounds that were tested in four: GlcNAc, DMSO, Ac-4-Cl-GlcNac 15, Ac-4-F-GlcNAc 3, and Ac-4,4-diF-GlcNAc 16. For each independent experiment, band densities were calculated relative to control band densities. *P < 0.05, **P < 0.01, ****P < 0.0001 one-way analysis of variance (ANOVA) with Dunnett’s post hoc test (respective of DMSO control). Error bars are mean ± s.d.
Using the MAB2030 antibody, we found that fluorinated compounds (Figure 2C) and xylosides (Supporting Information Figure 3A) had a range in their capacity to reduce CSPG production. Figure 2E shows the averaged relative MAB2030 band density of the conditioned media of treated astrocytes over control astrocytes, ranking the compounds on their ability to reduce CSPG production across multiple independent experiments. Cultured astrocytes treated with sugar analogues did not show any distinct morphological changes or toxicity from treatment (Supporting Information Figure 4A,B). The nonacetylated GlcNAc and peracetylated Ac-GlcNAc (1) did not affect CSPG production; CSPG reduction required the 4-fluorinated analogues but not the 4-chlorinated compounds 14 (Ac-4-Cl-GlcNAcOH) and 15 (Ac-4-Cl-GlcNAc), suggesting that the chloride is too bulky to fit in the binding side. The best 4-fluoro glucosamine analogues that significantly reduced chondroitin sulfate GAG stubs by 25% or more were (from best to least) the following: the 4,4-difluorinated 16 (Ac-4,4-diF-GlcNAc), the 4-monofluorinated hemiacetal 10 (Ac-4-F-GlcNAcOH), the anomeric O-propanoate 7 (Ac-4-F-GlcNAcOPr), fluorosamine 3 (Ac-4-F-GlcNAc), and the anomeric O-butanoate 8 (Ac-4-F-GlcNAcOBu). The acetylated 4-fluoro-GalNAc derivative 13 (Ac-4-F-GalNAc) also significantly reduced chondroitin sulfate GAGs. In general, the compounds that reduced chondroitin sulfate GAG stubs by greater than 25% had substitutions on only anomeric carbon (C-1) with the O-acetyl group (3), hydroxyl group (10), or O-propanoate (7) and a fluorine at C-4 or a difluorination at C-4 (16) (Figure 2B). The efficacy of these compounds may be in part due to their similar structure and molecular weight as Ac-GlcNAc (1), allowing them to easily cross the plasma membrane. After esterases remove the O-acyl groups, their O-deacetylated derivatives are likely converted to the corresponding UDP-sugar derivatives that subsequently act as inhibitors to the 4-epimerase, due to their 4-fluorination. The 4-monofluorinated derivatives 10, 7, 3, and 8 are expected to generate the same intermediate after O-deacylations; they indeed exhibited some difference in their inhibitory activities, suggesting other factors may play a role, such as their lipophilicity/hydrophobicity balance which affects their ability to cross the plasma membrane as well as their reactivities toward esterases.
While the above determinations were of the conditioned media of treated astrocytes, we also harvested cell lysates from astrocytes treated with the more potent compounds that reduced secretory CSPG levels. Indeed, after 24 h of treatment, the amount of MAB2030-immunoreactive material in the cell lysates was prominently lowered by the compounds tested (Supporting Information Figure 3E). Thus, the reduction of CSPGs in the conditioned media noted earlier (Figure 2) was also found in the astrocyte cell lysate.
The 2H6 antibody to full length chondroitin sulfate GAGs showed less qualitative changes in sugar analogues; only compounds that were the most effective at reducing MAB2030 levels (i.e., compounds 3 (Ac-4-F-GlcNAc), 10 (Ac-4-F-GlcNAcOH), 16 (Ac-4,4-diF-GlcNAc), and 7 (Ac-4-F-GlcNAcOPr)) showed evidence of reducing total chondroitin sulfate side chains (2H6) (Figure 2D, Supporting Information Figure 3B).
Compounds that had substitutions with multiple bulky groups (e.g., O3,O6-dibutanoate on compound 12 (Bu-4-F-GlcNAcOH) or lacked removable O-acyl protecting groups (e.g., O4,O6-dimethylated compound 5, O3-methylated compound 6) did not affect CSPG synthesis. The presence of multiple large ester protecting groups adds excessive lipophilicity of the molecule; this may slow down the diffusion of the compound from cell membrane, impairing the ability of compounds to enter cells or slowing down the hydrolysis by esterases. The presence of nonhydrolyzable O-methyl group(s) may result in the formation of UDP-sugar derivatives unfit for the binding site of 4-epimerase because of their O-methylations, and thus, the compounds are unable to act as an inhibitor of the enzymes.
Xylosides in general were not as effective at reducing CSPG production as glucosamine analogues. Only the tetraethylene glycol 21 (Ac-bXyl-TEG) was effective at reducing CSPGs (Figure 2E, Supporting Information Figure 3A). CSPG production was not impacted by the benzylated β-d-xyloside 19 and peracetylated analogues that have a substitution at C-1 with different water-solubility-enhancing groups, such as the ethylene glycol 20 (Ac-bXyl-MEG), as well as the anionic 2-sulfoethyl derivative 22 (Ac-bXyl-C2S), and the related β-d-xyloside analogue 23 (Ac-bXyl-C6S) with a 6-sulfohexyl group. Interestingly, the per-O-acetylated 4-fluorinated xylosides 27 (Ac-4-F-aXyl) and 28 (Ac-4-F-bXyl) did not reduce CSPG production. The activity of the tetraethylene glycol derivative 21 (Ac-bXyl-TEG) to reduce CSPG production by astrocytes may be attributed to it acting as an alternate sugar acceptor, diverting CSPG synthesis from the core protein to the soluble xyloside analogue. Our results suggest that the β-anomeric configuration of β-d-xylosides is required for subsequent GAG chain elongation by enzymes, as the analogous α-d-xyloside 24 (Ac-aXyl-C6S) did not act as an inhibitor.
Compounds that were able to reduce chondroitin GAGs were also investigated for their ability to reduce heparan sulfate GAGs. Similar to CSPGs, heparan sulfate proteoglycans (HSPGs) are upregulated in MS lesions31,32 and have detrimental pro-inflammatory capabilities.33 We observed that both Ac-4-F-GlcNAc 3 and Ac-4,4-diF-GlcNAc 16 also reduced HSPG side chains, albeit minimally, as detected by an antibody to intact heparan sulfate GAGs (Supporting Information Figure 3D).
Overcoming the CSPG Inhibition of OPCs
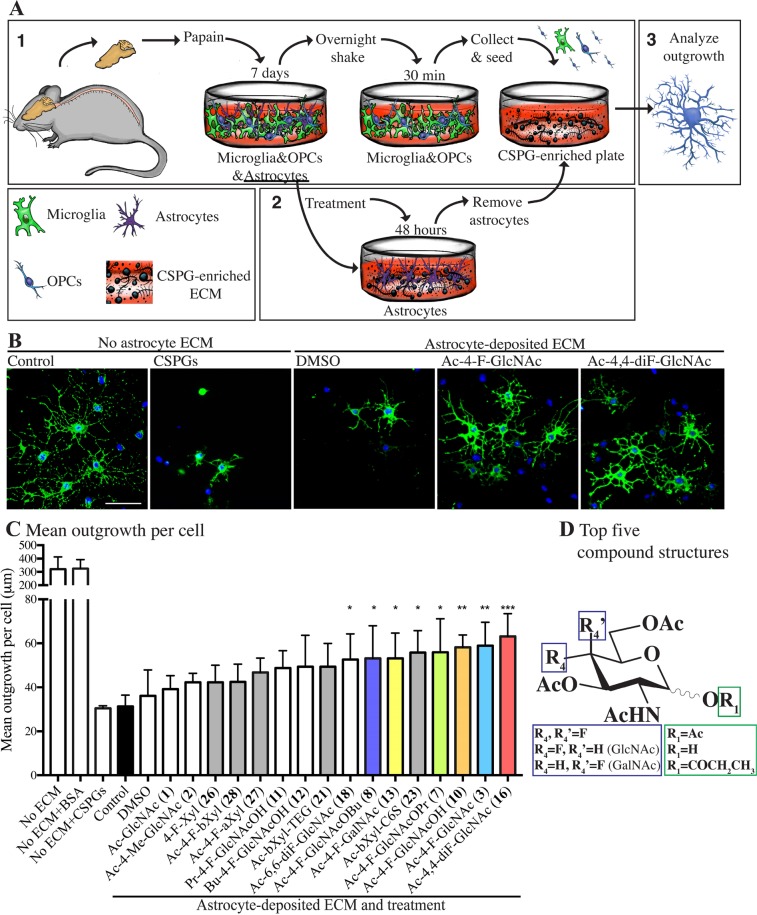
The process of remyelination requires oligodendrocyte lineage cells to undergo process outgrowth prior to their expression of mature myelin proteins for repair. Thus, process outgrowth in culture by cells of the oligodendrocyte lineage has been used as one surrogate for myelinating potential in vivo,17 since an oligodendrocyte needs to elaborate multiple protrusions emanating in several directions to contact many axons, and where these processes then compact around axons to form myelin segments. The presence of CSPGs in culture impairs the process outgrowth of OPCs, and this has been linked to reduced remyelination capacity in vivo.17 We reported previously that astrocytes in culture produce a plate-bound matrix abundant in CSPGs, that is left behind once astrocytes are removed from the plate, and this CSPG-containing matrix inhibits the outgrowth of plated OPCs17 (Figure 3A). Thus, astrocytes were treated with test compounds for 48 h and they were then removed from the cell culture plate, leaving only their secreted ECM behind (Figure 3A). When OPCs were plated on the astrocyte matrix, the extent of their process outgrowth over 2 days of observation was inhibited (Figure 3B). Focusing on selected compounds because of the technical challenges imposed by this test, we found that the treatment with fluorinated compounds exerted a partial rescue of OPC outgrowth on astrocyte ECM. OPCs growing in the absence of astrocyte ECM could reach a mean outgrowth around 300 μm (Figure 3B,C). Addition of CSPGs in the absence of astrocyte ECM exerted a similar inhibitory effect on OPC outgrowth, as when they were cultured on astrocyte ECM (Figure 3B,C). We noted that the majority of fluorinated compounds that significantly reduced CSPG production in astrocytes (Figure 2) were effective at improving mean outgrowth of OPCs onto the astrocyte ECM substrate (Figure 3D). These compounds were Ac-4,4-diF-GlcNAc 16 (Ac-4,4-diF-GlcNAc), fluorosamine 3 and its O1-deacetylated analogue 10 (Ac-4-F-GlcNAcOH), anomeric O-propanoate 7, anomeric O-butanoate 8 (Ac-4-F-GlcNAcOBu), and the 4-fluorinated GalNAc derivative 13 (Ac-4-F-GalNAc) (Figure 3C). Compounds that significantly enhanced OPC outgrowth but did not decrease CSPG production in astrocytes were 6,6-difluorinated compound 18 (Ac-6,6-diF-GlcNAc) and 6-sulfohexyl xyloside 23 (Ac-bXyl-C6S).
Figure 3.
Analogue-treated astrocytes produce a matrix more permissive for OPC growth. (A) Schematic representation of mixed glial cultures (“1”) and enrichment for OPCs and astrocytes. Astrocytes were cultured and treated with glucosamines or xylosides (“2”) and then removed, leaving behind a plate-bound matrix with inhibitory CSPGs. OPCs were seeded on these plates and their outgrowth analyzed (“3”). (B) OPCs plated onto control wells or wells with a CSPG mixture (10 μg/mL) and OPCs cultured on a matrix from astrocytes previously treated with DMSO, Ac-4-F-GlcNAc 3, or Ac-4,4-diF-GlcNAc 16. (C) Quantification of mean process outgrowth of OPCs, showing that some fluorinated analogues can improve OPC outgrowth compared to those grown on matrix from untreated astrocytes (control). Also shown is the mean outgrowth of OPCs grown in plates without astrocyte-deposited ECM that were coated with bovine serum albumin (“No ECM+BSA”), 10 μg/mL CSPGs (“No ECM+CSPGs”), or control (“No ECM”). Results are presented as four replicate wells of an individual experiment that was replicated at least twice. *P < 0.05, **P < 0.01, ***P < 0.001 one-way analysis of variance (ANOVA) with Dunnett’s post hoc test compared treatments with untreated astrocytes (control). Error bars are mean ± s.d. Note that we chose the 2-day time point to analyze the OPCs on the astrocyte matrix because our previous studies11,17 had determined that a CSPG matrix prominently inhibited process outgrowth of OPCs at 1 and 3 days. (D) Combined chemical structures of the five N-acetyl-d-glucosamine derivatives that most effectively reduced CSPG production.
Sugar Analogues Reduce the Proliferation of Splenocytes
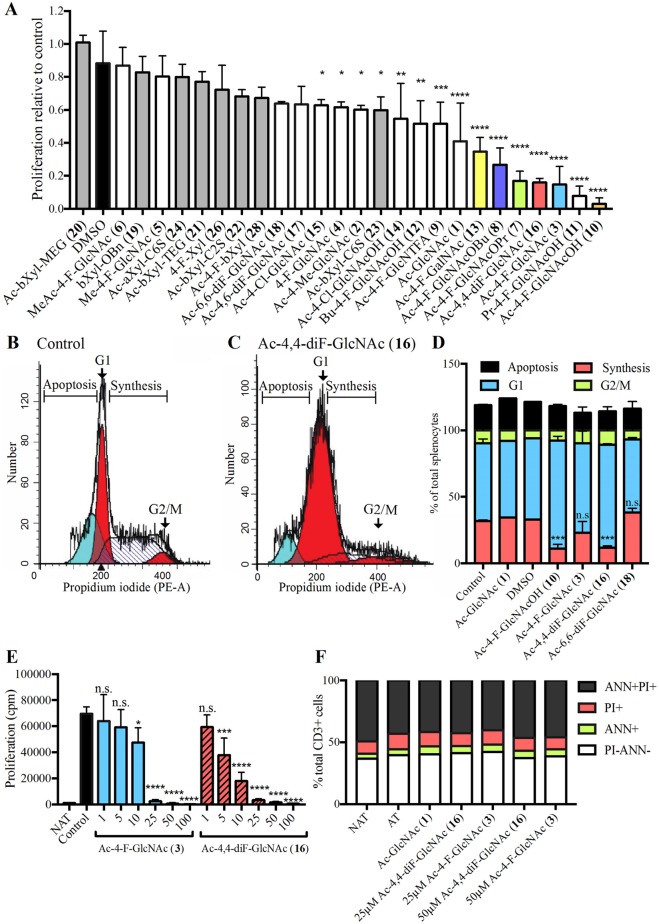
We assessed whether the glucosamine analogues have immunomodulatory properties on splenocytes isolated in culture. T cells within the splenocyte pool were polyclonally activated with anti-CD3 and anti-CD28 antibodies in the presence of compounds for 48 h, and proliferation was determined by the uptake of tritiated thymidine and expressed as counts per minute. We took the relative change in proliferation of treated versus control splenocytes in order to rank the compounds across multiple independent experiments (Figure 4A). The compounds most effective at reducing proliferation by at least 50% include (best to least) 4-monofluorinated Ac-GlcNAc hemiacetals 10 (Ac-4-F-GlcNAcOH) and 11 (Pr-4-F-GlcNAcOH), 3 (Ac-4-F-GlcNAc), the 4,4-difluorinated compound 16 (Ac-4,4-diF-GlcNAc), the O1-propanoate 7 (Ac-4-F-GlcNAcOPr), the O1-butanoate 8 (Ac-4-F-GlcNAcOBu), the 4-fluorinated GalNAc derivative 13 (Ac-4-F-GalNAc), and Ac-GlcNAc 1. The compounds that significantly reduced chondroitin sulfate GAG production in astrocytes were among the top 6 compounds that also reduced proliferation in splenocytes including Ac-4,4-diF-GlcNAc 16, the hemiacetal 10 (Ac-4-F-GlcNAcOH), the O1-propanaote 7 (Ac-4-F-GlcNAcOPr), Ac-4-F-GlcNAc 3, and the O1-butanoate 8 (Ac-4-F-GlcNAcOBu). Intriguingly, compounds that include the per-O-acetylated GlcNAc 1 (Ac-GlcNAc), the hemiacetals 11 (Pr-4-F-GlcNAcOH) and 12 (Bu-4-F-GlcNAcOH), and N-trifluoroacetylated analogue 9 (Ac-4-F-GlcNTFA) reduced splenocyte proliferation but did not lower CSPG production in astrocytes.
Figure 4.
Sugar analogues reduce the proliferation of splenocytes in culture. (A) Proliferation of splenocytes activated with anti-CD3 and anti-CD28 antibodies and treated with 25 μM glucosamine analogues shows that certain compounds significantly reduce proliferation (counts per minute) in [3H]-thymidine incorporation assays. The graph represents the average from three independent experiments, with quadruplicate wells and proliferation normalized to untreated activated splenocytes. Propidium-iodide (PI) cell cycle analysis of (B) control and (C) Ac-4,4-diF-GlcNAc 16-treated splenocytes shows splenocytes are halted in the G1 phase (red curve), with reduced percentage in the synthesis phase (curve with diagonal lines) and G2/M phase (second red curve), with no increase in apoptosis (blue curve). (D) PI cell cycle analysis showing Ac-4-F-GlcNAc 10 and Ac-4,4-diF-GlcNAc 16 reduced the percentage of cells in the S (DNA synthesis)-phase of the cell cycle. (E) Dose–response decrease in proliferation of splenocytes treated with increasing concentrations of Ac-4-F-GlcNAc 3 and Ac-4,4-diF-GlcNAc 16 (25 μM) for 48 h. (F) Isolated CD3+ cells treated with 25 and 50 μM Ac-4-F-GlcNAc 3 and Ac-4,4-diF-GlcNAc 16 with no changes in cells in early cell death (Annexin ANN+), necrosis (PI+), late cell death (ANN+PI+), or healthy (PI-ANN-). *P < 0.05, **P < 0.01, ****P < 0.0001 one-way analysis of variance (ANOVA) with Dunnett’s post hoc test.
Cell-cycle flow cytometry with propidium iodide was used to corroborate the above results and ensure the reduction in proliferation was not due to cell death. The analyses showed that there was an increase in cells halted in the G1 phase of the cell cycle, with a reduction in the percentage of cells in synthesis, and not due to an increase in apoptosis (Figure 4B–D). Due to the efficacy of Ac-4,4-diF-GlcNAc 16 at reducing both CSPG production in astrocytes as well as splenocyte proliferation, we compared the dose–response of Ac-4,4-diF-GlcNAc 16 and Ac-4-F-GlcNAc 3 to reduce proliferation of splenocytes and found that Ac-4,4-diF-GlcNAc 16 was more effective (Figure 4E). This was not due to nonspecific cell death, as evaluated by annexin V and propidium iodide staining that differentiated necrotic (propidium iodide+), apoptotic (annexin V+), and dead (propidium iodide+ and annexin V+) versus live cells (propidium iodide- annexin V-) (Figure 4F).
Testing Fluorinated Glucosamines on Macrophages
While lymphocytes are crucial to the pathogenesis of MS, myeloid cells, particularly macrophages, also have key roles in the disease.34−37 We tested whether the sugar analogues could affect the activity of macrophages, using bone-marrow-derived macrophages (BMDMs) stimulated with lipopolysaccharide (LPS, 100 ng/mL). The sugar analogues were added at 50 μM prior to LPS, and the conditioned media were collected after 24 h and assayed for levels of the secreted cytokine TNFα. In general, the compounds did not reduce TNFα production by LPS-stimulated macrophages. Three compounds tested, including the 4-monofluorinated hemiacetals 10 (Ac-4-F-GlcNAcOH) and 11 (Pr-4-F-GlcNAcOH) that respectively have O3,O6-diacetates, O3,O6-dipropanoates, and the fully acetylated α-xylopyranose 27 (Ac-4-F-aXyl), enhanced TNFα levels (Supporting Information Figure 5). Ac-4-F-GlcNAc 3 and the 4,4-difluorinated compound 16 (Ac-4,4-diF-GlcNAc) did not alter the cytokine level of activated macrophages.
Testing Toxicity of Sugar Analogues
We also studied whether compounds were toxic. Compounds Ac-4,4-diF-GlcNAc 16, Ac-4-F-GlcNAcOH 10, Ac-4-F-GlcNAc 3, and Ac-4-F-GlcNAcOBu 8 did not show detectable cell death on splenocytes with propidium iodide/annexin V staining or cell cycle analysis (Figure 4D,F).
Toxicity on astrocytes was assayed with propidium iodide/calcein AM immunocytochemistry. Live cells convert calcein AM into a green fluorescent product, whereas dying/dead cells are stained with propidium iodide. At the high concentration of 100 μM, the top 6 fluorinated compounds that significantly reduced chondroitin sulfate GAGs from astrocytes (Ac-4,4-diF-GlcNAc 16, Ac-4-F-GlcNAcOH 10, Ac-4-F-GalNAc 13, Ac-4-F-GlcNAcOPr 7, Ac-4-F-GlcNAc 3, Ac-4-F-GlcNAcOBu 8) did not produce toxicity (Supporting Information Figure 4B). As shown with representative staining, there were no morphological changes in treated astrocytes, whereas the positive control of H2O2 caused an increase in propidium iodide-positive staining (Supporting Information Figure 4A).
If the sugar compounds are to be used in neurological disorders, they should not display toxicity to neural cells. Thus, we used human neurons to test the compounds and the ATP luminescence assay as a readout of metabolic stress and a surrogate of toxicity. We tested compounds at a high dose of 100 μM. Neurons had a greater sensitivity to the toxic potential of sugar analogues than astrocytes (Supporting Information Figure 4C). Two compounds that reduced ATP production by more than 50% were the 4-fluorinated hemiacetals that respectively bear O3,O6-diacetates 10 (Ac-4-F-GlcNAcOH) and O3,O6-dipropanoates 11 (Pr-4-F-GlcNAcOH), with the former displaying higher cytotoxicity than the latter (Supporting Information Figure 4D). Interestingly, the homologue 12 (Bu-4-F-GlcNAcOH) bearing slightly longer O3,O6-dibutanoates showed no cytotoxicity.
Summary of Tissue Culture Studies
Table 1 compares the compounds tested in culture in this study. The top 6 most effective fluorinated compounds at reducing CSPG production (Ac-4,4-diF-GlcNAc 16, Ac-4-F-GlcNAcOH 10, Ac-4-F-GalNAc 13, Ac-4-F-GlcNAcOPr 7, Ac-4-F-GlcNAc 3, Ac-4-F-GlcNAcOBu 8) have been assigned colors for easy reference for the assays they have been tested in (see Figures 1–4 and Supporting Information Figures 3–5). Xylosides are color-coded gray to differentiate them from the fluorinated sugar analogues. Across the different tests, Ac-4,4-diF-GlcNAc 16 was the most efficacious, while xylosides were comparatively inactive.
Table 1. Compounds Used in the Studya.
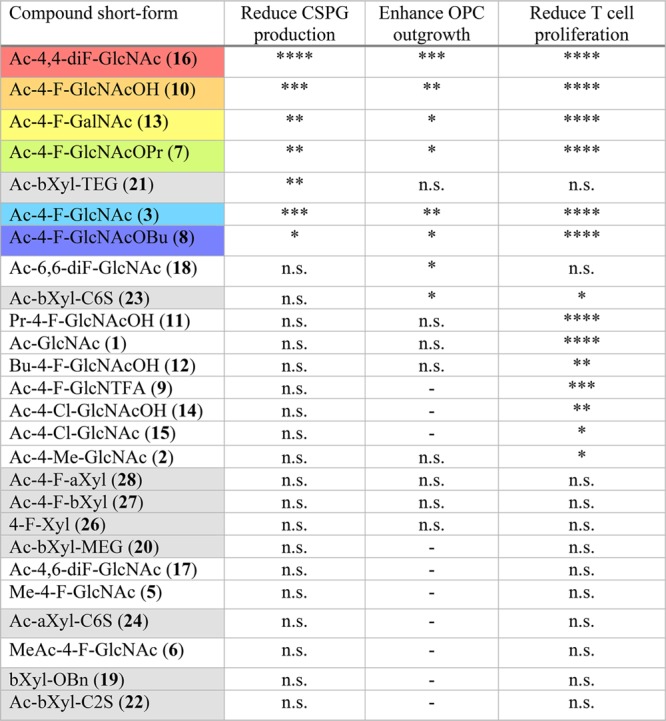
****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, n.s. = nonsignificant, - = not tested.
The Ac-4,4-diF-GlcNAc 16 Novel Sugar Analogue Reduces EAE Disease Activity
The in vitro screens highlight the novel compound 16 (Ac-4,4-diF-GlcNAc) as the most potent drug at reducing CSPG production by astrocytes. Ac-4,4-diF-GlcNAc 16 also maximized OPC outgrowth on an astrocyte inhibitory matrix (Figure 2), had immunomodulatory properties on splenocytes (Figure 4), and showed no obvious toxicity (Supporting Information Figure 4). Thus, we investigated whether these in vitro results translate to a beneficial effect of Ac-4,4-diF-GlcNAc 16 in ameliorating the severity of EAE.
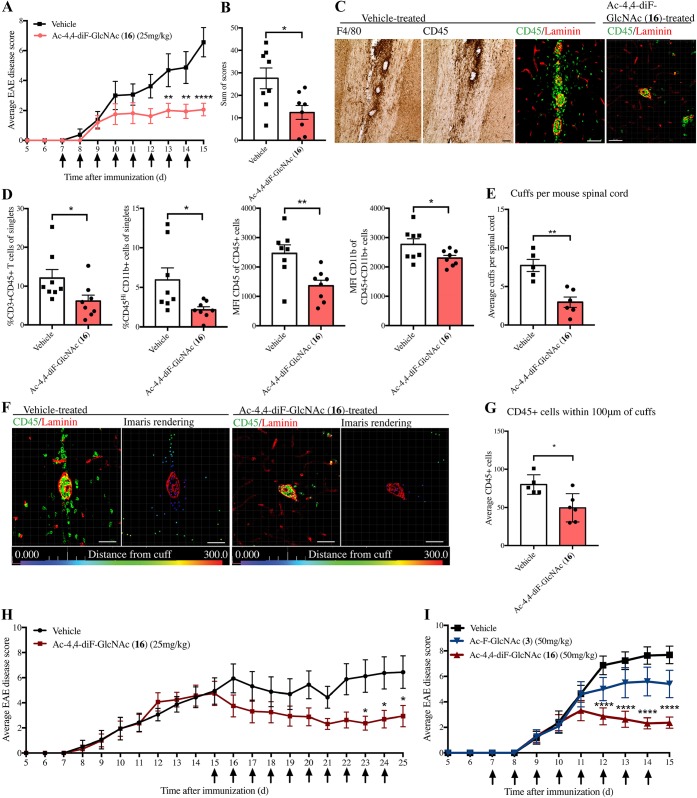
We exposed EAE mice to two dose regimens of Ac-4,4-diF-GlcNAc 16, whereby the drug was initiated prior to the onset of clinical signs, or from peak clinical severity. EAE was induced in mice by myelin oligodendrocyte glycoprotein peptide and associated adjuvants. In the first regimen, treatment began on day 7, a time point just before mice are expected to show clinical signs (“preonset”) but where immune cells are becoming activated and infiltrating into the CNS. Ac-4,4-diF-GlcNAc 16 (25 mg kg–1) or saline vehicle was given intraperitoneally daily until the mice reached peak clinical severity at day 15. Mice treated prophylactically with Ac-4,4-diF-GlcNAc 16 had a significantly lower EAE clinical score than the control group (Figure 5A). The sum of scores (burden of disease), which represents the sum of the daily clinical scores per mouse, was also significantly reduced with Ac-4,4-diF-GlcNAc 16 treatment (Figure 5B).
Figure 5.
Ac-4,4-diF-GlcNAc 16 attenuates EAE. (A) Average daily EAE clinical score of mice treated daily with 25 mg/kg Ac-4,4-diF-GlcNAc 16 or saline vehicle (N = 8) with treatment shown by arrows; mice are analyzed in parts C to G. (B) Sum of scores displaying individual burden of disease. (C) Brightfield images of F4/80 and CD45 and immunofluorescence of CD45 and laminin in vehicle- or Ac-4,4-diF-GlcNAc (16)-treated mice (scale bar 50 μm). (D) Flow cytometry of the spinal cord showing Ac-4,4-diF-GlcNAc 16 treatment reduces %CD3+ T cells and %CD45HiCD11b+ monocytes/macrophages (and median fluorescence intensity). (E) Average perivascular cuffs per spinal cord per mouse in treated and vehicle-treated EAE mice. (F) Immunohistochemistry of perivascular cuffs next to the Imaris-processed image (bar = 50 μm). (G) Number of CD45+ cells within 100 μm of perivascular cuffs, quantified by Imaris. (H) Average daily EAE clinical score of mice treated with 25 mg/kg Ac-4,4-diF-GlcNAc or vehicle from peak clinical severity (N = 8). (I) Average daily EAE clinical score of EAE mice treated with 50 mg/kg Ac-4,4-diF-GlcNAc 16, Ac-4-F-GlcNAc 3, or vehicle from preonset (N = 10); arrows indicate daily injections. *P < 0.05, **P < 0.01, ****P < 0.0001. EAE scores (parts A, H, and I) were analyzed by two-way repeated-measures ANOVA with Sidak’s post hoc test versus vehicle; mean ± s.e.m. Parts B, D, E, and G were analyzed by two-tailed unpaired t test; mean ± s.d.
Notably, flow cytometry of the lumbar/thoracic spinal cord found treatment significantly decreased CD45HiCD11b+ infiltrating monocytes/macrophages and significantly lowered CD45+ CD3+ T lymphocytes within the spinal cord (Figure 5D). Ac-4,4-diF-GlcNAc 16 treatment also significantly reduced the median fluorescence intensity of CD11b and CD45 of CD11b+CD45+ cells (Figure 5D). Flow cytometry of the blood did not show changes in monocyte or lymphocyte populations (Supporting Information Figure 6A).
Routes of entry of immune cells into the CNS include subpial meningeal infiltration, passage across the fenestrated ependymal layer of the choroid plexus, and transmigration through the basement membranes of postcapillary venules.1,38 Through this last route, an inflammatory perivascular cuff forms and is detected as CD45+ cells accumulated within two laminin-positive basement membranes. EAE spinal cords had an abundance of perivascular cuffs, positive for CD45 cells (Figure 5C). There was a significant correlation between the EAE sum of scores or the EAE disease score and the average number of spinal cord perivascular cuffs in EAE mice (Supporting Information Figure 6B,C), suggesting that there is a relationship between EAE severity and number of perivascular cuffs in the spinal cord. There was a significantly reduced average number of perivascular cuffs in Ac-4,4-diF-GlcNAc 16-treated mice compared to vehicle (Figure 5C,E). In contrast to the reduction in clinical score with Ac-4,4-diF-GlcNAc 16, treatment with Ac-4-F-GlcNAcOH 10, the most effective compound at reducing splenocyte proliferation in vitro, did not reduce EAE clinical score when mice were treated from day 7 to day 15 with 50 mg/kg intraperitoneal injections (Supporting Information Figure 6E).
Previously, we have shown that CSPGs are accumulated in perivascular cuffs and may have played a role in activating immune cells and promoting their migration into the CNS.9 For the current study, cervical spinal cord sections from EAE mice treated with Ac-4,4-diF-GlcNAc 16 or vehicle were stained with pan-laminin and CD45. Confocal images were processed by Imaris software to quantify the number of CD45+ cells and their intraparenchymal distances from perivascular cuffs as previously described9 (Figure 5F). In agreement with the flow cytometry findings of reduced infiltrating monocytes and lymphocytes, Ac-4,4-diF-GlcNAc 16 treated EAE mice had significantly fewer CD45+ cells in the vicinity of perivascular cuffs (Figure 5G).
In the second treatment regimen, the difluorinated compound 16 was tested for its ability to lower the disease score after mice had accumulated disease. Treatment (daily, 25 mg kg–1) was initiated after mice reached the peak EAE clinical score (day 15). Over the next 10 days, Ac-4,4-diF-GlcNAc 16 significantly reduced EAE clinical severity (Figure 5H).
We have previously shown that 50 mg kg–1 of fluorosamine (Ac-4-F-GlcNAc 3), with treatment initiated prior to EAE signs or from peak clinical severity, reduced the ensuing EAE clinical disability.17 We thus treated EAE mice with either vehicle, fluorosamine/Ac-4-F-GlcNAc 3 or Ac-4,4-diF-GlcNAc 16, at the higher dose of 50 mg kg–1. Impressively, the difluorinated Ac-4,4-diF-GlcNAc 16 produced a pronounced reduction in EAE clinical severity beyond that seen for the monofluorinated Ac-4-F-GlcNAc 3 (Figure 5I).
Discussion
MS is a common chronic inflammatory degenerative disease of the CNS that presents with profound changes in the ECM.8,31 In particular, the ubiquitous ECM components, the CSPGs, have an impressive capacity to both drive neuroinflammation and also interfere with processes of repair.2,3 Efforts have been made to cleave deposited CSPGs in lesions, but this method will release CSPG GAG chains, which have pro-inflammatory capacities.39 Therefore, we aimed to develop novel compounds to target the synthetic pathway of CSPGs prior to their release into the ECM. Affecting the synthesis of CSPGs should selectively involve members whose synthesis is upregulated during inflammation, such as versican, and not other CSPG members previously laid down in perineuronal nets. Targeting CSPGs represents a therapeutic option to alleviate both neurodegenerative and inflammatory components of MS simultaneously.
In this study, we have investigated fluorinated sugar analogues and have found some of the fluorinated GlcNAc analogues to be effective at both reducing the production of inhibitory CSPGs and their chondroitin sulfate GAGs and attenuating the activity of splenocytes. Compounds were ranked on their capacity to reduce CSPG production in Table 1 and compared for their ability to enhance OPC outgrowth on an inhibitory astrocyte matrix and reduce T cell proliferation. The top 6 most effective fluorinated compounds at reducing CSPG production were Ac-4,4-diF-GlcNAc 16, Ac-4-F-GlcNAcOH 10, Ac-4-F-GalNAc 13, Ac-4-F-GlcNAcOPr 7, Ac-4-F-GlcNAc 3, and Ac-4-F-GlcNAcOBu 8.
The similarity of chemical structures between the most effective compounds highlights the constraints on the modifications of groups on these molecules. The presence of bulky ester protecting groups adds excessive lipophilicity of the molecule; this may impair the ability of compounds to enter cells, slow down hydrolysis by esterases, or impede their ability to interact with 4-epimerase.
While compounds such as Ac-4,4-diF-GlcNAc 16 reduced GAG levels (Figure 2D, Supporting Information Figure 3), since they are targeted at the GAG synthesis pathway, it is intriguing that the amount of the proteoglycan core protein is also lowered (Figure 2C). It is possible that the GAG synthesis pathway requires its conjugation to the core protein prior to synthesis, and the failure to do this leads to recycling of the core protein. Thus, the lack of chondroitin sulfate GAGs may interfere with the sorting process and excretion of the proteoglycans19 where chondroitin (and heparan) sulfate chains were shown to contain the sorting information over the protein core. The lack of association with the proper enzymes in the endoplasmic reticulum may cause the failure of proteoglycans to move to the Golgi and will thus be degraded.
In this study, we have synthesized and investigated the 4,4-difluorinated compound 16 (Ac-4,4-diF-GlcNAc) that has not been previously described in the literature to treat MS. Not only did compound 16 reduce CSPG production in astrocytes more effectively than Ac-4-F-GlcNAc 3, but it also strongly reduced proliferation of splenocytes, and had no signs of toxicity in neurons. When tested in vivo, compound 16 potently reduced the EAE disease score. Notably, prophylactic treatment also decreased the infiltration of monocytes and lymphocytes into the spinal cord. Immunohistochemistry found that there was a reduced number of perivascular cuffs, sites where immune cells can infiltrate into the CNS, as well as lowered CD45+ leukocytes in the parenchyma around perivascular cuffs. That Ac-4,4-diF-GlcNAc 16 did not affect levels of circulating leukocytes was notable, as this indicates that the compound is not a general immunosuppressant. It is intriguing that the prophylactic treatment scheme (beginning at day 7) was more effective at reducing EAE clinical scores versus the therapeutic scheme (day 15) which, although beginning to show a trend in improvement, did not significantly improve disability until 9 days after treatment. Important avenues of future research would be determining the optimal times of dosing for these types of compounds, as well as the long-term clinical effects. This effect may not be solely due to their capacity to reduce splenocyte proliferation, as there was no significant improvement in EAE clinical score when mice were treated with 50 mg/kg of Ac-4-F-GlcNAcOH 10, the most effective compound at reducing splenocyte proliferation. However, Ac-4-F-GlcNAcOH 10 also showed evidence of toxicity on neurons.
This is the first study to detail in vitro and in vivo screening methods of 4-fluorinated analogues to target CSPGs for use in MS; it has implications also for other diseases where CSPGs are upregulated. There is a potential that the activities of these compounds may be due to inhibition of not only chondroitin sulfate GAGs but also heparan sulfate (Supporting Information Figure 3D) and dermatan sulfate synthesis. This is an important question to address comprehensively in future research. Fluorinated analogues have also been shown to directly act on cancer cell lines, suppressing selectin-mediated tumor cell adhesion40 and reducing cancer progression.41 CSPGs are also deposited in traumatic CNS injuries where they are thought to inhibit axonal regeneration; reducing the markedly elevated CSPG production could have long-term favorable outcomes for repair.
In conclusion, we have shown that fluorinated analogues, particularly Ac-4,4-diF-GlcNAc 16 and henceforth named “difluorosamine”, represent a potential effective therapeutic avenue to target CSPGs and reduce inflammation. While this is one step forward for the field, future avenues of research should focus on developing CNS-targeted CSPG-lowering drugs to avoid the risk of peripheral toxicity and off-target effects. Future studies are required to better understand the role of these fluorinated compounds and how CSPG upregulation in MS promotes inflammation and hinders repair. Considering the role of glycosylation in regulating key molecules in the innate and adaptive immune system, this area represents a challenging but promising avenue to target CSPGs, control aberrant inflammatory events, and improve MS treatment.
Acknowledgments
This work was supported by operating grants from the Canadian Institutes of Health Research and the Alberta Innovates – Health Solutions CRIO Team program. E.L.S. and K.S.R. are Canada Vanier Scholars. K.S.R. acknowledges the studentships from the Faculty of Medicine of the University of Calgary and also from the Multiple Sclerosis Society of Canada. V.W.Y. is a Tier 1 Canada Research Chair. C.-C.L. thanks the Alberta Innovates - Technology Futures and the Natural Sciences and Engineering Research Council of Canada (NSERC) for financial support. We thank Janet Wang for skilled technical assistance with tissue culture studies, Dr. Marc François-Heude for the preparation of compounds 26–28, and Dr. Manoj Mishra for helpful advices.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.9b00327.
Materials and methods on chemical, biochemical, immunological, and cell cultural experiments. Supplemental Figures 1–7 including the biosynthesis of CSPGs, synthetic schemes of all glucosamine and xyloside analogues, Western blots results to show the reduction of CSPG and HSPG biosynthesis by astrocytes using different sugar analogues, toxicity studies of sugar analogues, TNFα production in bone-marrow-derived macrophages treated with sugar analogues, average daily EAE clinical scores and flow cytometry analysis of mice treated with Ac-4,4-diF-GlcNAc 16. Details of synthetic procedures of glucosamines and xyloside analogues. Supplemental references (PDF)
Author Contributions
E.L.S. conducted the majority of the experiments and wrote the first draft of the manuscript. P.Z., A.W., and J.G. synthesized the compounds. M.B.K., K.S.R., S.G., and C.S. contributed results to the manuscript. V.W.Y. and C.-C.L. conceived and supervised the study and finalized the manuscript.
The authors declare the following competing financial interest(s): The authors have filed a provisional patent application on this work.
Supplementary Material
References
- Sorokin L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 2010, 10 (10), 712–723. 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- Haylock-Jacobs S.; Keough M. B.; Lau L.; Yong V. W. Chondroitin sulphate proteoglycans: extracellular matrix proteins that regulate immunity of the central nervous system. Autoimmun. Rev. 2011, 10 (12), 766–722. 10.1016/j.autrev.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Pu A.; Stephenson E. L.; Yong V. W. The extracellular matrix: focus on oligodendrocyte biology and targeting CSPGs for remyelination therapies. Glia 2018, 66 (9), 1809–1825. 10.1002/glia.23333. [DOI] [PubMed] [Google Scholar]
- Rolls A.; Shechter R.; London A.; Segev Y.; Jacob-Hirsch J.; Amariglio N.; Rechavi G.; Schwartz M. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS Med. 2008, 5 (8), e171. 10.1371/journal.pmed.0050171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanos N. K.; Piperigkou Z.; Theocharis A. D.; Watanabe H.; Franchi M.; Baud S.; Brézillon S.; Götte M.; Passi A.; Vigetti D.; et al. Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics. Chem. Rev. 2018, 118 (18), 9152–9232. 10.1021/acs.chemrev.8b00354. [DOI] [PubMed] [Google Scholar]
- Hara M.; Kobayakawa K.; Ohkawa Y.; Kumamaru H.; Yokota K.; Saito T.; Kijima K.; Yoshizaki S.; Harimaya K.; Nakashima Y.; et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat. Med. 2017, 23 (7), 818–828. 10.1038/nm.4354. [DOI] [PubMed] [Google Scholar]
- Wu C.; Ivars F.; Anderson P.; Hallmann R.; Vestweber D.; Nilsson P.; Robenek H.; Tryggvason K.; Song J.; Korpos E.; et al. Endothelial basement membrane laminin alpha5 selectively inhibits T lymphocyte extravasation into the brain. Nat. Med. 2009, 15 (5), 519–527. 10.1038/nm.1957. [DOI] [PubMed] [Google Scholar]
- Sobel R. A.; Ahmed A. S. White matter extracellular matrix chondroitin sulfate/dermatan sulfate proteoglycans in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2001, 60 (12), 1198–1207. 10.1093/jnen/60.12.1198. [DOI] [PubMed] [Google Scholar]
- Stephenson E. L.; Mishra M. K.; Moussienko D.; Laflamme N.; Rivest S.; Ling C.-C. C.; Yong V. W. Chondroitin sulfate proteoglycans as novel drivers of leucocyte infiltration in multiple sclerosis. Brain 2018, 141 (4), 1094–1110. 10.1093/brain/awy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A.; Staugaitis S. M.; Dutta R.; Batt C. E.; Easley K. E.; Chomyk A. M.; Yong V. W.; Fox R. J.; Kidd G. J.; Trapp B. D. Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann. Neurol. 2012, 72 (6), 918–926. 10.1002/ana.23693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. W.; Keough M. B.; Haylock-Jacobs S.; Cua R.; Döring A.; Sloka S.; Stirling D. P.; Rivest S.; Yong V. W. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann. Neurol. 2012, 72 (3), 419–432. 10.1002/ana.23599. [DOI] [PubMed] [Google Scholar]
- Silver J.; Miller J. H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Dyck S. M.; Karimi-Abdolrezaee S. Chondroitin sulfate proteoglycans: Key modulators in the developing and pathologic central nervous system. Exp. Neurol. 2015, 269, 169–187. 10.1016/j.expneurol.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Burnside E. R.; Bradbury E. J. Manipulating the extracellular matrix and its role in brain and spinal cord plasticity and repair. Neuropathol. Appl. Neurobiol. 2014, 40 (1), 26–59. 10.1111/nan.12114. [DOI] [PubMed] [Google Scholar]
- Dyck S.; Kataria H.; Alizadeh A.; Santhosh K. T.; Lang B.; Silver J.; Karimi-Abdolrezaee S. Perturbing chondroitin sulfate proteoglycan signaling through LAR and PTPσ receptors promotes a beneficial inflammatory response following spinal cord injury. J. Neuroinflammation 2018, 15 (1), 90. 10.1186/s12974-018-1128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S.; Eftekharpour E.; Wang J.; Schut D.; Fehlings M. G. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J. Neurosci. 2010, 30 (5), 1657–1676. 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keough M. B.; Rogers J. A.; Zhang P.; Jensen S. K.; Stephenson E. L.; Chen T.; Hurlbert M. G.; Lau L. W.; Rawji K. S.; Plemel J. R.; et al. An inhibitor of chondroitin sulfate proteoglycan synthesis promotes central nervous system remyelination. Nat. Commun. 2016, 7, 11312. 10.1038/ncomms11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende M.; Bednarek C.; Wawryszyn M.; Sauter P.; Biskup M. B.; Schepers U.; Bräse S. Chemical synthesis of glycosaminoglycans. Chem. Rev. 2016, 116 (14), 8193–8255. 10.1021/acs.chemrev.6b00010. [DOI] [PubMed] [Google Scholar]
- Prydz K.; Dalen K. T. Synthesis and sorting of proteoglycans. J. Cell Sci. 2000, 113 (2), 193–205. [DOI] [PubMed] [Google Scholar]
- Nigro J.; Wang A.; Mukhopadhyay D.; Lauer M.; Midura R. J.; Sackstein R.; Hascall V. C. Regulation of heparan sulfate and chondroitin sulfate glycosaminoglycan biosynthesis by 4-fluoro-glucosamine in murine airway smooth muscle cells. J. Biol. Chem. 2009, 284 (25), 16832–16839. 10.1074/jbc.M109.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk X. M.; Lawrence R.; Thijssen V. L.; van den Broek S. A.; Troost R.; van Scherpenzeel M.; Naidu N.; Oosterhof A.; Griffioen A. W.; Lefeber D. J.; et al. A common sugar-nucleotide-mediated mechanism of inhibition of (glycosamino)glycan biosynthesis, as evidenced by 6F-GalNAc (Ac3). FASEB J. 2015, 29 (7), 2993–3002. 10.1096/fj.14-264226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Sagredo A.; Fernández-Mayoralas A.; Jiménez-Barbero J.; Martín-Lomas M.; Villanueva D.; Aragón J. J. 4-O-β-spD-Galactopyranosyl-spD-xylose: a new synthesis and application to the evaluation of intestinal lactase. Carbohydr. Res. 1992, 228 (1), 129–135. 10.1016/S0008-6215(00)90554-8. [DOI] [PubMed] [Google Scholar]
- Jones L. L.; Margolis R. U.; Tuszynski M. H. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp. Neurol. 2003, 182 (2), 399–411. 10.1016/S0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- Tang X.; Davies J. E.; Davies S. J. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J. Neurosci. Res. 2003, 71 (3), 427–444. 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- Asher R. A.; Morgenstern D. A.; Fidler P. S.; Adcock K. H.; Oohira A.; Braistead J. E.; Levine J. M.; Margolis R. U.; Rogers J. H.; Fawcett J. W. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J. Neurosci. 2000, 20 (7), 2427–2438. 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon R. J.; Jurynec M. J.; Buck C. R. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J. Neurosci. 1999, 19 (24), 10778–10788. 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C. A.; Rauch U.; Thon N.; Merten T.; Deller T. Entorhinal cortex lesion in adult rats induces the expression of the neuronal chondroitin sulfate proteoglycan neurocan in reactive astrocytes. J. Neurosci. 1999, 19 (22), 9953–9963. 10.1523/JNEUROSCI.19-22-09953.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo L.; Trauger S. A.; Blain M.; Nadeau M.; Patel B.; Alvarez J. I.; Mascanfroni I. D.; Yeste A.; Kivisäkk P.; Kallas K.; et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat. Med. 2014, 20 (10), 1147–1156. 10.1038/nm.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glant T. T.; Buzás E. I.; Finnegan A.; Negroiu G.; Cs-Szabó G.; Mikecz K. Critical roles of glycosaminoglycan side chains of cartilage proteoglycan (aggrecan) in antigen recognition and presentation. J. Immunol. 1998, 160 (8), 3812–3819. [PubMed] [Google Scholar]
- Poole C. A.; Glant T. T.; Schofield J. R. Chondrons from articular cartilage. (IV). Immunolocalization of proteoglycan epitopes in isolated canine tibial chondrons. J. Histochem. Cytochem. 1991, 39 (9), 1175–1187. 10.1177/39.9.1717545. [DOI] [PubMed] [Google Scholar]
- van Horssen J.; Bö L.; Dijkstra C. D.; de Vries H. E. Extensive extracellular matrix depositions in active multiple sclerosis lesions. Neurobiol. Dis. 2006, 24 (3), 484–491. 10.1016/j.nbd.2006.08.005. [DOI] [PubMed] [Google Scholar]
- van Horssen J.; Bö L.; Vos C. M.; Virtanen I.; de Vries H. E. Basement membrane proteins in multiple sclerosis-associated inflammatory cuffs: potential role in influx and transport of leukocytes. J. Neuropathol. Exp. Neurol. 2005, 64 (8), 722–729. 10.1097/01.jnen.0000173894.09553.13. [DOI] [PubMed] [Google Scholar]
- Parish C. R. The role of heparan sulphate in inflammation. Nat. Rev. Immunol. 2006, 6 (9), 633–643. 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- Mishra M. K.; Yong V. W. Myeloid cells - targets of medication in multiple sclerosis. Nat. Rev. Neurol. 2016, 12 (9), 539–551. 10.1038/nrneurol.2016.110. [DOI] [PubMed] [Google Scholar]
- Reich D. S.; Lucchinetti C. F.; Calabresi P. A. Multiple sclerosis. N. Engl. J. Med. 2018, 378 (2), 169–180. 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecher-Allan C.; Kaskow B. J.; Weiner H. L. Multiple sclerosis: Mechanisms and immunotherapy. Neuron 2018, 97 (4), 742–768. 10.1016/j.neuron.2018.01.021. [DOI] [PubMed] [Google Scholar]
- Masuda T.; Prinz M. Microglia: A unique versatile cell in the central nervous system. ACS Chem. Neurosci. 2016, 7 (4), 428–434. 10.1021/acschemneuro.5b00317. [DOI] [PubMed] [Google Scholar]
- Ransohoff R. M.; Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012, 12 (9), 623–635. 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Nagarkatti P.; Zhong Y.; Nagarkatti M. Immune modulation by chondroitin sulfate and its degraded disaccharide product in the development of an experimental model of multiple sclerosis. J. Neuroimmunol. 2010, 223 (1–2), 55–64. 10.1016/j.jneuroim.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marathe D. D.; Buffone A.; Chandrasekaran E. V.; Xue J.; Locke R. D.; Nasirikenari M.; Lau J. T. Y.; Matta K. L.; Neelamegham S. Fluorinated per-acetylated GalNAc metabolically alters glycan structures on leukocyte PSGL-1 and reduces cell binding to selectins. Blood 2010, 115 (6), 1303–1312. 10.1182/blood-2009-07-231480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel S. R.; Antonopoulos A.; Cedeno-Laurent F.; Schaffer L.; Hernandez G.; Patil S. A.; North S. J.; Dell A.; Matta K. L.; Neelamegham S.; et al. Peracetylated 4-fluoro-glucosamine reduces the content and repertoire of N- and O-glycans without direct incorporation. J. Biol. Chem. 2011, 286 (24), 21717–21731. 10.1074/jbc.M110.194597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.