Obesity is a metabolic disease of ever-increasing prevalence characterized by excess accumulation of white adipose tissue resulting from a combination of overnutrition, energy imbalance, and genetics. In contrast to its original characterization as an inert tissue depot of triglycerides, adipose tissue has since been recognized as a dynamic organ orchestrating metabolic, endocrine, and immune responses.[1] Accumulating evidence from recent decades has linked obesity to chronic low-grade inflammation, which underlies obesity-associated insulin resistance, diabetes mellitus, metabolic syndrome, and cardiovascular diseases. It has become clear that the role of white adipose tissue has exceeded its original notion for energy storage. In addition to adipokines, adipose tissue also produces a variety of cytokines and chemokines and plays an important function as an endocrine organ for orchestrating systemic physiology. Chronic overnutrition-induced obesity is also characterized by two hallmark responses, insulin and metainflammation, which are the causal factors for obesity-associated metabolic syndromes and other health risks.
Adipose tissue macrophages (ATMs) represent the largest immune population in the adipose tissue stroma and contribute essential support for tissue remodeling, metabolic homeostasis, as well as inflammatory responses under obesity stress. In healthy lean adipose tissues, ATMs exert crucial functions for maintaining immunological and metabolic homeostasis, including immune regulation, efferocytosis, lipid buffering, and angiogenesis.[2, 3] Obesity is associated with a 10-fold increase in macrophages in adipose tissue due to recruitment of circulating monocytes and/or local proliferation of tissue macrophages.[4, 5] Within expanding adipose tissue, macrophage release pro-inflammatory cytokines such as TNF-α and form crown-like structures that surround dying adipocytes, the latter of which is a histologic hallmark of inflammation within adipose tissue.[6, 7] Such pro-inflammatory environments orchestrated by macrophages contribute significantly to long-term high-fat diet-induced insulin resistance in mice.[8] In addition, multiple studies support that pro-inflammatory macrophages inhibit adipogenitor cell proliferation and differentiation.[9, 10, 11, 12] Through these mechanisms, ATMs profoundly alter adipose tissue functions, impact local and systemic metabolism, and orchestrate pathological changes during obesity stress.
Tissue-residing macrophages including ATMs are highly plastic, allowing them to adopt diverse functions in response to various stimuli, such as cytokines, infections, and chemicals.[13] Researchers have broadly classified macrophages by their activation states as M1 “classically” activated or M2 “alternatively” activated, and also have defined macrophage responses with this classification. In the case of ATMs, obese conditions induce a switch from an anti-inflammatory M2 to a pro-inflammatory M1 activation state. M2 ATMs contribute to homeostasis maintenance and tissue remodeling, whereas M1 ATMs promote insulin resistance through the activation of pro-inflammatory pathways (such as JNK, ERK, p38, and NF-κB) that target insulin receptor signaling.[14] Macrophages regulate tissue functions mainly through the release of secreted products, including cytokines, reactive molecules, and extracellular RNAs. Cytokines released by M1 macrophages are usually pro-inflammatory, such as TNF-α, IL-1β, IL-12, and IL-23;[15, 16] M2 macrophages, on the other hand, are known to secrete anti-inflammatory cytokines and growth factors, such as IL-10 and TGFβ.[17] Interestingly, both M1 and M2 macrophages produce cytokine IL-6 that has both proinflammatory and anti-inflammatory functions[18] and is known to promote insulin secretion[19] or skeletal muscle tissue repair, depending on specific conditions.[20] M1 macrophages also release reactive molecules such as nitric oxide (NO) and reactive oxygen species (ROS),[21, 22] which impose profound impacts on molecular functions and are critically involved in obesity-associated pathology. [23, 24, 25, 26] In addition, recent studies suggest extracellular RNAs secreted by macrophages regulate tissue functions: cultured macrophages release microRNAs (miRNAs) in response to pro-inflammatory stimuli[27] and transfer miR-142 and miR-223 to co-cultured hepato-carcinoma cells;[28] and ATMs from obese mice release miR-155 and cause insulin resistance in insulin target cells.[29] However, the role of extracellular RNAs in regulating macrophage activation and tissue function is still largely unknown and requires further investigation.
Within the past decade, researchers have elucidated several key regulators that drive heterogeneous macrophage activation, including the signal transducer and activator of transcription (STAT) family, PPARγ/LXR, CREB-C/EBP, and interferon regulatory factors (IRFs). Interferons are one of the first cytokines identified as activators of the pro-inflammatory macrophage phenotype.[30, 31, 32] Interferon regulatory factors (IRFs) have long been known to control the activity of interferons (IFN), and increasing evidence supports their important role in regulating macrophage activation.[33] Of these, IRF1, 2, 5, and 6 drive macrophages toward the M1 pro-inflammatory type. Pro-inflammatory activation of murine macrophages elicited by LPS or IFNγ are inhibited by knockout of IRF1 or IRF2.[34] IRF5 promotes “M1-like” activation of human peripheral blood macrophages and inhibits expression of M2-associated genes.[35] IRF6 was recently found to suppress “M2-like” activation of murine bone marrow-derived macrophage (BMDM) by inhibiting PPARγ gene transcription.[36] In contrast, some other members of the IRF family, such as IRF3, 4, and 9, mediate anti-inflammatory signaling through type I Interferon responses. In human microglia, IRF3 mediates anti-inflammatory responses by activating the PI3K/Akt pathway and promoting “M2-type” cell activation.[37] Induction of IRF4 by IL4 contributes to “M2-like” alternative macrophage priming in murine BMDMs. In addition, during the “M2-like” activation of murine macrophages triggered by parasites or fungi, researchers observed significant Lysine Demethylase 6B (KDM6B, or Jmjd3)-mediated histone demethylation at the locus of the Irf4 gene, further implicating its involvement in the activation process.[38] Another member of this family, IRF9, is believed to mediate interferon tau-induced anti-inflammatory responses and M2 activation of murine BMDMs.[39]
The Janus Kinase-STAT pathway can drive M1 activation. IFNγ is thought to induce expression of M1-associated genes by triggering dimerization of STAT1,[40] and mice bearing STAT1 deficiency fail to respond to IFNγ and IFNα.[41] In addition, LPS-induced IFNβ activation enhances the formation of STAT1-STAT2 heterodimers to mediate the induction of M1-associated genes by forming the IFN-stimulated gene factor 3 complex.[42] Another member of the STAT family, STAT6, is associated with M2 macrophage activation. IL-4 and IL-13 are considered two critical M2 inducers, as suggested by several in vitro and in vivo studies,[43, 44, 45] and STAT6 can mediate IL-4a signaling and regulate expression of M2 signature genes.[43, 46] STAT6 signaling is further mediated by monocyte chemoattractant protein-1-induced protein (MCPIP), which induces M2-promoting ROS, endoplasmic reticulum stress, and autophagy.[47]
Another important group of macrophage activation regulators are in the CCAAT-enhancer-binding proteins (C/EBP) family, in particular, C/EBPα, β, and σ. C/EBPβ mediates signaling of toll-like receptor (TLR) and cAMP-responsive element-binding protein (CREB) that induce expression of arginase 1 (ARG1), an M2 marker protein in mice. C/EBPβ also promotes the expression of another M2 signature gene, mannose receptor c-type 1 (Mrc1), upon induction by CREB. Deletion of CREB binding sites in the promoter region of C/EBPβ consistently abolishes muscle tissue repair (an M2 macrophage-mediated function in mice) and inhibits the expression of numerous M2 signature genes, including macrophage scavenger receptor 1 (Msr1), IL-10, IL-13 receptor subunit, receptor α1 (Il13ra1), and Arg1 within macrophages; deletion of these binding sites does not alter the levels of pro-inflammatory M1 signature genes.[48] Further, CREB exerts the negative feedback regulation of pro-inflammatory TLR signaling mediated by MSK1/2 kinases through the induction of IL-10 production and dual specificity protein phosphatase 1 to limit inflammation.[49, 50] In contrast to C/EBPα, C/EBPσ induces M1 pro-inflammatory responses in murine BMDMs.[51] Another member of the C/EBP family, C/EBPα, is believed to be necessary for both M1 and “M2 activation of murine macrophages.[52] All these studies have suggested a critical role for CREB-C/EBP signaling in regulating heterogeneous activation of macrophages.
The lipid metabolism regulator Peroxisome Proliferator-Activated Receptor gamma (PPARγ) can negatively regulate numerous pro-inflammatory genes.[53, 54] Knockout of PPARγ in murine myeloid cells can abolish their “M2-like” activation and enhance susceptibility to inflammation-associated health issues including obesity, insulin resistance, and glucose-intolerance.[55] In addition, in murine thioglycollate-elicited macrophages and human peripheral blood monocytes, PPARγ is thought to mediate the signaling of IL-4 and IL-13, which are well-known inducers of “M2-like” phenotypes.[56] In cultured murine macrophages, PPARγ interacts with STAT6 as a cofactor to facilitate the induction of genes under the regulation of PPARγ.[57] Similarly, liver X receptors (LXRs; nuclear transcription factors that heterodimerize with the retinoid X receptor RXRα) contribute to reduced pro-inflammatory signaling.[58, 59, 60] Indeed, LXRs have long been recognized for their role in the amelioration of autoimmune diseases and regression of atherosclerotic plaques, which are extensively orchestrated by M2 macrophages.[61, 62]
In recent years, increasing evidence supports the critical roles of miRNAs in regulating macrophage activation by targeting key regulators. PPARγ directly binds upstream of miR-223 and mediates anti-inflammatory signaling by inhibiting the expression of Nuclear Factor of Activated T-Cells 5 (NFATt5) and RAS p21 Protein Activator 1 (RASA1), thereby promoting “M2-like” activation. Deletion of miR-223 can abolish PPARγ-regulated M2 activation of murine macrophages in vivo and ex vivo.[63] In addition, miR-223 inhibits the expression of PBX/Knotted 1 Homeobox 1 (Pknox1), thus suppressing NFκB/JNK signaling and “M1-like” pro-inflammation activation.[64] miR-223 also inhibits the pro-inflammatory differentiation of murine intestinal macrophages by targeting C/EBPβ,[65] and blunts the transition of THP-1 cells and peripheral human blood monocytes toward inflammatory macrophages.[66]
In contrast to miR-223, miR-155 regulates pro-inflammatory “M1-like” activation. TLR agonists (LPS, hypomethylated DNA, or Pam3CSK4) and pro-inflammatory cytokines (TNF-α, IFNβ, or IFNγ) increase miR-155 expression in cultured murine macrophages.[67, 68] In alcoholic liver disease, miR-155 mediates an NF-κB-regulated pro-inflammatory response by stabilizing TNF-α mRNA, thus promoting its synthesis.[69] In addition to directly boosting pro-inflammatory factors, miR-155 also contributes to inflammation by suppressing signaling on the anti-inflammatory side, targets of which include suppressor of cytokine signaling 1 (SOCS1), phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase 1 (SHIP1), [68, 70] and IL13RA1/STAT6.[71] Interestingly, despite extensive support for a pro-inflammatory role of miR-155, it also reduces the production of pro-inflammatory cytokine in murine BMDMs by targeting TGF-β Activated Kinase 1/MAP3K7 Binding Protein 2 (TAB2),[72] suggesting dual roles, each of which are likely dependent on specific conditions.
Inhibiting the expression of key transcription factors is an important mechanism by which microRNAs manipulate macrophage functions, and is exemplified by the action of miR-125 and Let7c. miR-125 mediates pro-inflammatory signaling of IFNγ by suppressing the expression of the M2-promoting transcription factor IRF4, and thus contributes to an “M1-like” phenotype;[73] it can, however, also suppress pro-inflammatory signaling in other macrophage types.[74] In contrast to miR-125, Let-7c reduces the levels of inflammation-associated factors such as C/EBPσ, IL-12, and major histocompatibility complex (MHC) class II, and is required for “M2-like” phenotypes of macrophages.[51] In addition, other miRNAs have also been reported to manipulate macrophage activation, including the M1-promoting miR-9, miR-127, miR-124,[75, 76, 77] and the M2-promoting miR-132, and miR-146a.[78, 79] Interestingly, miR-21 contributes to both M1 and M2 activation, although each under different conditions in separate studies.[80, 81]
The profound impact of miRNAs on activation of macrophages that critically orchestrate obesity-associated adipose tissue pathogenesis suggests their potential as promising therapeutic targets. However, despite intensive research on miRNA involvement in diseases, most studies focused on cancer-related conditions, and therefore, less is known about their role in adipose tissue of healthy and obese subjects. Another challenge of addressing miRNA-regulated macrophage activation in obesity originates from the complex nature of macrophage heterogeneity: despite the widely adopted M1/M2 polarization system, increasing studies suggest that this model largely built using in vitro cell cultures is too simple to depict the multi-faceted and dynamic activation states of tissue-residing macrophages. This is not surprising considering the highly complex and diverse microenvironments in healthy or diseased tissues. In recent years, the accumulating evidence [82, 83, 84] suggests macrophage activation states display spectrum-like patterns across a variety of tissues and species (Figure 1); however, a systemic model that comprehensively annotates complex macrophage features under different conditions is still lacking.
Figure 1.
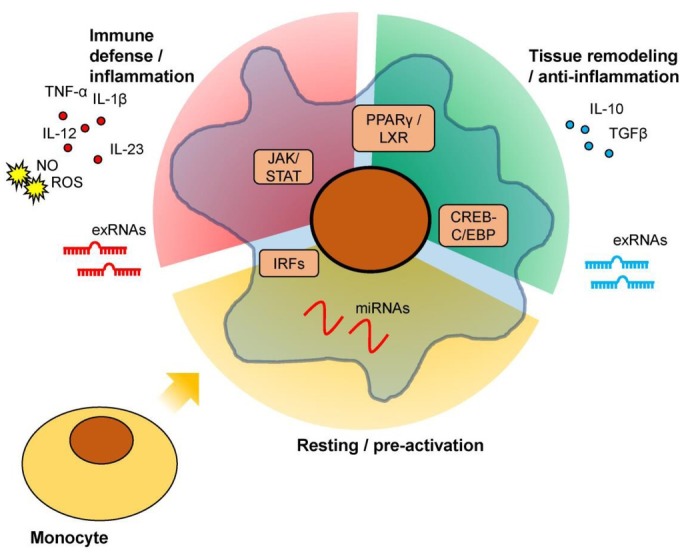
Schematic description of multi-faced macrophage activation. Upon monocyte differentiation into macrophages, dependent on specific antigens or stimuli they encounter and controlled by intracellular regulators, macrophages may adopt different phenotypes that 1) orchestrate immune defense and/or tissue inflammation; 2) conduct anti-inflammation and/or tissue remodeling functions; 3) maintain a resting/pre-activation state until further stimulation; or other functions dependent on the specific micro-environment of the tissue loci. Secretory factors, including cytokines, reactive chemicals, and exRNAs, are an important approach for macrophages to impact tissue functions.
Advances in high-throughput sequencing and single-cell technologies allow in-depth analyses of cell populations to identify distinct subsets and dissect regulatory mechanisms underlying cell function. However, currently available algorithms are not tailored to depict macrophage activation and often result in ambiguous characterization of dynamic activation state changes in vivo. To address this major knowledge gap, we generated single-cell transcriptome data of ATMs from healthy and obese mice and primary bone marrow-derived monocytes and macrophages to develop new high-resolution algorithms. The outcome was the creation of a two-index platform, MacSpectrum (https://macspectrum.uconn.edu) that enables comprehensive high-resolution mapping of monocyte/macrophage activation states from diverse mixed cell populations. The capability of MacSpectrum to dissect macrophage heterogeneity was well-supported by its performance on the samples from human and murine species, under in vitro and in vivo conditions, and in bulk and single-cell sequencing formats. Importantly, MacSpectrum revealed an unprecedented sequential activation pattern of monocytes/macrophages in obesity and unique cell programs under the regulation of miRNAs. The performance of MacSpectrum suggests that novel bioinformatic algorithms tailored to macrophage study could provide promising strategies to address the challenges of investigating miRNA-regulated activation in obesity and facilitate more focused therapeutic development through sub-population separation, functional annotation, and signature gene identification.
Acknowledgement
We are grateful to Dr. Christopher Bonin at UConn Health for his diligent support for this article.
Footnotes
Conflict of Interest
Conflict of Interests: Authors declare no conflict of interests.
Source of Foundation
The work was supported by National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK RO1DK098662 to B. Zhou).
Reference
- 1.Sharma AM, Staels B. Review: Peroxisome proliferator-activated receptor gamma and adipose tissue--understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab. 2007;92:386. doi: 10.1210/jc.2006-1268. –. [DOI] [PubMed] [Google Scholar]
- 2.Schipper HS, Prakken B, Kalkhoven E, Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol Metab. 2012;23:407. doi: 10.1016/j.tem.2012.05.011. –. [DOI] [PubMed] [Google Scholar]
- 3.Hubler MJ, Peterson KR, Hasty AH. Iron homeostasis: a new job for macrophages in adipose tissue? Trends Endocrinol Metab. 2015;26:101. doi: 10.1016/j.tem.2014.12.005. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796. doi: 10.1172/JCI19246. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59:879. doi: 10.1007/s00125-016-3904-9. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347. doi: 10.1194/jlr.M500294-JLR200. et al. –. [DOI] [PubMed] [Google Scholar]
- 7.Dalmas E, Clement K, Guerre-Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011;32:307. doi: 10.1016/j.it.2011.04.008. –. [DOI] [PubMed] [Google Scholar]
- 8.Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 2011;60:2474. doi: 10.2337/db11-0194. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology. 2007;148:868. doi: 10.1210/en.2006-0687. –. [DOI] [PubMed] [Google Scholar]
- 10.Constant VA, Gagnon A, Yarmo M, Sorisky A. The antiadipogenic effect of macrophage-conditioned medium depends on ERK1/2 activation. Metabolism. 2008;57:465. doi: 10.1016/j.metabol.2007.11.005. –. [DOI] [PubMed] [Google Scholar]
- 11.Maumus M, Sengenes C, Decaunes P, Zakaroff-Girard A, Bourlier V, Lafontan M. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: influence of fat mass microenvironment and growth. J Clin Endocrinol Metab. 2008;93:4098. doi: 10.1210/jc.2008-0044. et al. –. [DOI] [PubMed] [Google Scholar]
- 12.Zaragosi LE, Wdziekonski B, Villageois P, Keophiphath M, Maumus M, Tchkonia T. Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes. 2010;59:2513. doi: 10.2337/db10-0013. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu ZG. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23:898. doi: 10.1038/cr.2013.75. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castoldi A, Naffah de Souza C, Camara NO, Moraes-Vieira PM. The Macrophage Switch in Obesity Development. Front Immunol. 2015;6:637. doi: 10.3389/fimmu.2015.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goerdt S, Politz O, Schledzewski K, Birk R, Gratchev A, Guillot P. Alternative versus classical activation of macrophages. Pathobiology. 1999;67:222. doi: 10.1159/000028096. et al. –. [DOI] [PubMed] [Google Scholar]
- 16.Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohlson SS, O’Conner SD, Hulsebus HJ, Ho MM, Fraser DA. Complement, c1q, and c1q-related molecules regulate macrophage polarization. Front Immunol. 2014;5:402. doi: 10.3389/fimmu.2014.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez-Menocal L, Faridi MH, Martinez L, Shehadeh LA, Duque JC, Wei Y. Macrophage-derived IL-18 and increased fibrinogen deposition are age-related inflammatory signatures of vascular remodeling. Am J Physiol Heart Circ Physiol. 2014;306:H641. doi: 10.1152/ajpheart.00641.2013. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17:1481. doi: 10.1038/nm.2513. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Li Y, Wu Y, Wang L, Wang X, Du J. Interleukin-6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J Biol Chem. 2013;288:1489. doi: 10.1074/jbc.M112.419788. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold CE, Whyte CS, Gordon P, Barker RN, Rees AJ, Wilson HM. A critical role for suppressor of cytokine signalling 3 in promoting M1 macrophage activation and function in vitro and in vivo. Immunology. 2014;141:96. doi: 10.1111/imm.12173. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brune B, Dehne N, Grossmann N, Jung M, Namgaladze D, Schmid T. Redox control of inflammation in macrophages. Antioxid Redox Signal. 2013;19:595. doi: 10.1089/ars.2012.4785. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattos RT, Medeiros NI, Menezes CA, Fares RC, Franco EP, Dutra WO. Chronic Low-Grade Inflammation in Childhood Obesity Is Associated with Decreased IL-10 Expression by Monocyte Subsets. PLoS One. 2016;11:e0168610. doi: 10.1371/journal.pone.0168610. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzanavari T, Giannogonas P, Karalis KP. TNF-alpha and obesity. Curr Dir Autoimmun. 2010;11:145. doi: 10.1159/000289203. –. [DOI] [PubMed] [Google Scholar]
- 25.Aroor AR, DeMarco VG. Oxidative stress and obesity: the chicken or the egg? Diabetes. 2014;63:2216. doi: 10.2337/db14-0424. –. [DOI] [PubMed] [Google Scholar]
- 26.Litvinova L, Atochin DN, Fattakhov N, Vasilenko M, Zatolokin P, Kirienkova E. Nitric oxide and mitochondria in metabolic syndrome. Front Physiol. 2015;6:20. doi: 10.3389/fphys.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortega FJ, Moreno M, Mercader JM, Moreno-Navarrete JM, Fuentes-Batllevell N, Sabater M. Inflammation triggers specific microRNA profiles in human adipocytes and macrophages and in their supernatants. Clin Epigenetics. 2015;7:49. doi: 10.1186/s13148-015-0083-3. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J Immunol. 2013;191:6250. doi: 10.4049/jimmunol.1301728. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell. 2017;171:372. doi: 10.1016/j.cell.2017.08.035. et al. –. [DOI] [PubMed] [Google Scholar]
- 30.Murray HW, Rubin BY, Rothermel CD. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983;72:1506. doi: 10.1172/JCI111107. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brummer E, Morrison CJ, Stevens DA. Recombinant and natural gamma-interferon activation of macrophages in vitro: different dose requirements for induction of killing activity against phagocytizable and nonphagocytizable fungi. Infect Immun. 1985;49:724. doi: 10.1128/iai.49.3.724-730.1985. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pace JL, Russell SW, Torres BA, Johnson HM, Gray PW. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983;130:2011. –. [PubMed] [Google Scholar]
- 33.Savitsky D, Tamura T, Yanai H, Taniguchi T. Regulation of immunity and oncogenesis by the IRF transcription factor family. Cancer Immunol Immunother. 2010;59:489. doi: 10.1007/s00262-009-0804-6. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salkowski CA, Kopydlowski K, Blanco J, Cody MJ, McNally R, Vogel SN. IL-12 is dysregulated in macrophages from IRF-1 and IRF-2 knockout mice. J Immunol. 1999;163:1529. –. [PubMed] [Google Scholar]
- 35.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231. doi: 10.1038/ni.1990. et al. –. [DOI] [PubMed] [Google Scholar]
- 36.Li C, Ying W, Huang Z, Brehm T, Morin A, Vella AT. IRF6 regulates alternative activation by suppressing PPARgamma in male murine macrophages. Endocrinology. 2017;158:2837. doi: 10.1210/en.2017-00053. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarassishin L, Suh HS, Lee SC. Interferon regulatory factor 3 plays an anti-inflammatory role in microglia by activating the PI3K/Akt pathway. J Neuroinflammation. 2011;8:187. doi: 10.1186/1742-2094-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936. doi: 10.1038/ni.1920. et al. –. [DOI] [PubMed] [Google Scholar]
- 39.Ying W, Kanameni S, Chang CA, Nair V, Safe S, Bazer FW. Interferon tau alleviates obesity-induced adipose tissue inflammation and insulin resistance by regulating macrophage polarization. PLoS One. 2014;9:e98835. doi: 10.1371/journal.pone.0098835. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415. doi: 10.1126/science.8197455. –. [DOI] [PubMed] [Google Scholar]
- 41.Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431. doi: 10.1016/s0092-8674(00)81288-x. et al. –. [DOI] [PubMed] [Google Scholar]
- 42.Wienerroither S, Shukla P, Farlik M, Majoros A, Stych B, Vogl C. Cooperative Transcriptional Activation of Antimicrobial Genes by STAT and NF-kappaB Pathways by Concerted Recruitment of the Mediator Complex. Cell Rep. 2015;12:300. doi: 10.1016/j.celrep.2015.06.021. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451. doi: 10.1146/annurev.immunol.021908.132532. –. [DOI] [PubMed] [Google Scholar]
- 44.Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwan-ska M. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623. doi: 10.1016/s1074-7613(04)00107-4. et al. –. [DOI] [PubMed] [Google Scholar]
- 45.Brombacher F, Arendse B, Peterson R, Holscher A, Holscher C. Analyzing classical and alternative macrophage activation in macrophage/neutrophil-specific IL-4 receptor-alpha-deficient mice. Methods Mol Biol. 2009;531:225. doi: 10.1007/978-1-59745-396-7_15. –. [DOI] [PubMed] [Google Scholar]
- 46.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627. doi: 10.1038/380627a0. et al. –. [DOI] [PubMed] [Google Scholar]
- 47.Kapoor N, Niu J, Saad Y, Kumar S, Sirakova T, Becerra E. Transcription factors STAT6 and KLF4 implement macrophage polarization via the dual catalytic powers of MCPIP. J Immunol. 2015;194:6011. doi: 10.4049/jimmunol.1402797. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, Rosenthal N. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci U S A. 2009;106:17475. doi: 10.1073/pnas.0908641106. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim C, Wilcox-Adelman S, Sano Y, Tang WJ, Collier RJ, Park JM. Antiinflammatory cAMP signaling and cell migration genes co-opted by the anthrax bacillus. Proc Natl Acad Sci US A. 2008;105:6150. doi: 10.1073/pnas.0800105105. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ananieva O, Darragh J, Johansen C, Carr JM, McIlrath J, Park JM. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat Immunol. 2008;9:1028. doi: 10.1038/ni.1644. et al. –. [DOI] [PubMed] [Google Scholar]
- 51.Banerjee S, Xie N, Cui H, Tan Z, Yang S, Icyuz M. MicroRNA let-7c regulates macrophage polarization. J Immunol. 2013;190:6542. doi: 10.4049/jimmunol.1202496. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee B, Qiao L, Lu M, Yoo HS, Cheung W, Mak R. C/EBPalpha regulates macrophage activation and systemic metabolism. Am J Physiol Endocrinol Metab. 2014;306:E1144. doi: 10.1152/ajpendo.00002.2014. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759. doi: 10.1038/nature03988. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137. doi: 10.1016/j.cmet.2007.06.010. et al. –. [DOI] [PubMed] [Google Scholar]
- 55.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116. doi: 10.1038/nature05894. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378. doi: 10.1038/22572. et al. –. [DOI] [PubMed] [Google Scholar]
- 57.Szanto A, Balint BL, Nagy ZS, Barta E, Dezso B, Pap A. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699. doi: 10.1016/j.immuni.2010.11.009. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161. doi: 10.1016/s1097-2765(01)00164-2. et al. –. [DOI] [PubMed] [Google Scholar]
- 59.Bensinger N AG, Hong SJ, Beceiro C, Bradley S, Zelcer N MN. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245. doi: 10.1016/j.immuni.2009.06.018. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szanto A, Roszer T. Nuclear receptors in macrophages: a link between metabolism and inflammation. FEBS Lett. 2008;582:106. doi: 10.1016/j.febslet.2007.11.020. –. [DOI] [PubMed] [Google Scholar]
- 61.Weng Q, Wang J, Wang J, Wang J, Sattar F, Zhang Z. Lenalidomide regulates CNS autoimmunity by promoting M2 macrophages polarization. Cell Death Dis. 2018;9:251. doi: 10.1038/s41419-018-0290-x. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rahman K, Vengrenyuk Y, Ramsey SA, Vila NR, Girgis NM, Liu J. Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. J Clin Invest. 2017;127:2904. doi: 10.1172/JCI75005. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ying W, Tseng A, Chang RC, Morin A, Brehm T, Triff K. Micro-RNA-223 is a crucial mediator of PPARgamma-regulated alternative macrophage activation. J Clin Invest. 2015;125:4149. doi: 10.1172/JCI81656. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125:2892. doi: 10.1161/CIRCULATIONAHA.111.087817. et al. –. [DOI] [PubMed] [Google Scholar]
- 65.Zhou H, Xiao J, Wu N, Liu C, Xu J, Liu F. MicroRNA-223 Regulates the Differentiation and Function of Intestinal Dendritic Cells and Macrophages by Targeting C/EBPbeta. Cell Rep. 2015;13:1149. doi: 10.1016/j.celrep.2015.09.073. et al. –. [DOI] [PubMed] [Google Scholar]
- 66.Ismail N, Wang Y, Dakhlallah D, Moldovan L, Agarwal K, Batte K. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013;121:984. doi: 10.1182/blood-2011-08-374793. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci US A. 2005;102:3627. doi: 10.1073/pnas.0500613102. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci U S A. 2009;106:7113. doi: 10.1073/pnas.0902636106. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bala S, Marcos M, Kodys K, Csak T, Catalano D, Mandrekar P. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor {alpha} (TNF{alpha}) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem. 2011;286:1436. doi: 10.1074/jbc.M110.145870. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31:220. doi: 10.1016/j.immuni.2009.06.024. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martinez-Nunez RT, Louafi F, Sanchez-Elsner T. The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor alpha1 (IL13Ralpha1) J Biol Chem. 2011;286:1786. doi: 10.1074/jbc.M110.169367. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ceppi M, Pereira PM, Dunand-Sauthier I, Barras E, Reith W, Santos MA. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci US A. 2009;106:2735. doi: 10.1073/pnas.0811073106. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chaudhuri AA, So AY, Sinha N, Gibson WS, Taganov KD, O’Connell RM. MicroRNA-125b potentiates macrophage activation. J Immunol. 2011;187:5062. doi: 10.4049/jimmunol.1102001. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082. doi: 10.4049/jimmunol.179.8.5082. et al. –. [DOI] [PubMed] [Google Scholar]
- 75.Thulin P, Wei T, Werngren O, Cheung L, Fisher RM, Grander D. MicroRNA-9 regulates the expression of peroxisome proliferator-activated receptor delta in human monocytes during the inflammatory response. Int J Mol Med. 2013;31:1003. doi: 10.3892/ijmm.2013.1311. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ying H, Kang Y, Zhang H, Zhao D, Xia J, Lu Z. MiR-127 modulates macrophage polarization and promotes lung inflammation and injury by activating the JNK pathway. J Immunol. 2015;194:1239. doi: 10.4049/jimmunol.1402088. et al. –. [DOI] [PubMed] [Google Scholar]
- 77.Sun Y, Li Q, Gui H, Xu DP, Yang YL, Su DF. MicroRNA-124 mediates the cholinergic anti-inflammatory action through inhibiting the production of pro-inflammatory cytokines. Cell Res. 2013;23:1270. doi: 10.1038/cr.2013.116. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu F, Li Y, Jiang R, Nie C, Zeng Z, Zhao N. miR-132 inhibits lipopolysaccharide-induced inflammation in alveolar macrophages by the cholinergic anti-inflammatory pathway. Exp Lung Res. 2015;41:261. doi: 10.3109/01902148.2015.1004206. et al. –. [DOI] [PubMed] [Google Scholar]
- 79.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481. doi: 10.1073/pnas.0605298103. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caescu CI, Guo X, Tesfa L, Bhagat TD, Verma A, Zheng D. Colony stimulating factor-1 receptor signaling networks inhibit mouse macrophage inflammatory responses by induction of microRNA-21. Blood. 2015;125:e1. doi: 10.1182/blood-2014-10-608000. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Z, Brandt S, Medeiros A, Wang S, Wu H, Dent A. Micro-RNA 21 is a homeostatic regulator of macrophage polarization and prevents prostaglandin E2-mediated M2 generation. PLoS One. 2015;10:e0115855. doi: 10.1371/journal.pone.0115855. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958. doi: 10.1038/nri2448. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889. doi: 10.1038/ni.1937. –. [DOI] [PubMed] [Google Scholar]
- 84.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274. doi: 10.1016/j.immuni.2014.01.006. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]


