Abstract
Gynecologic carcinomas, including cervical cancer, present a significant burden on low- and middle-income countries (LMICs). Brachytherapy plays an integral role in the treatment of gynecologic carcinomas, as it is essential for both curative and palliative treatment. However, there are numerous geographic and economic barriers to providing brachytherapy to cancer patients in LMICs. This article examines the role and delivery of brachytherapy in gynecological cancer treatment; brachytherapy capacity in LMICs, including infrastructure, equipment, and human resources considerations; commissioning, training, and clinical implementation of brachytherapy in LMICs; other challenges, and strategies for improvement in brachytherapy delivery in LMICs, including innovation and current and upcoming international initiatives.
Introduction
Cervical carcinoma, although relatively rare in most developed countries, is a significant global health problem. According to the World Health Organization (WHO) 528,000 women are diagnosed each year with cervical carcinoma and 266,000 women succumb to the disease each year, 90% of them in low- to middle-income countries.1 In these areas the incidence: mortality ratio exceeds 50%. This is the equivalent to 1 life lost every 2 minutes, which approximates the number of women who die annually in pregnancy and childbirth. Most of these women are raising their children, and contributing to the economic well-being of their families and communities. Even more alarming, although incidence and death rates are decreasing in high-income countries, cervical cancer deaths are projected to increase by 25% over the next 10 years in low-income countries. The highest rates are in Central and Southern America, East Africa, South and Southeast Asia, and the Western Pacific. In 55 countries, it is the number one cancer killer and most common cancer among women in 45 countries. In 2013, at the World Health Assembly, an action plan for the prevention and control of noncommunicable diseases was agreed upon and cervical cancer control was considered a priority.
Many efforts to eradicate this disease focus on screening and prevention. The cause has been well-established, with the human papilloma virus (HPV) subtypes 16 and 18 causing 70% of cases worldwide. There are 3 vaccines currently available—Gardasil (quadrivalent), Gardasil 9 (nonavalent) and Cervarix. All 3 prevent infections with HPV types 16 and 18 but Gardasil also protects again HPV types 6 and 11, which cause 90% of genital warts.2 Gardasil 9 also adds HPV types 31, 33, 45, 52, and 58. Since the vaccines do not treat pre-existing HPV infection or disease, the vaccination is recommended before sexual activity begins. The vaccine is currently not available in many countries. Other efforts that focus on prevention emphasize screening to detect precancerous disease. Screening in developed countries consists of a Papanicolaou smear and HPV testing. In most low- to middle-income countries, where these techniques may not be available, the cervix is treated with acetic acid and abnormal areas are treated with cryotherapy, a “screen and treat” approach.
Not only does prevention and treatment of noninvasive precursor lesions affect this alarming incidence:mortality ratio, it is also profoundly affected by the lack of adequate treatment once the diagnosis is made. The challenges are innumerable including lack of epidemiologic data in some areas to convince local leaders of the severity of the problem; lack of funding for supplies, equipment and infrastructure; lack of an effective referral system and inequality of access to healthcare for women. Access to linear accelerators (LINACS) in most of the developing world is poor and disparate. For example, Tanzania, with a population of 47.4 million is served by only 2 old cobalt machines, although the charity organization Radiating Hope (www.radiatinghope.org) partnered with the Foundation for Cancer Care in Tanzania to provide a LINACS. For comparison, in the United States (US), there is 1 radiation machine per 100,000 people.3 Guatemala, with a population of 15.8 million, has only 5 radiation centers and 11 radiation machines, or 1 radiation machine per 1.4 million people. There are several clinics (HOPE Radio-therapy Center, International Institute of Advanced Therapies, and Hospital el Pilar) that offer radiation therapy and brachytherapy in Guatemala.4 Although there are numerous studies proving the survival benefit with the addition of brachytherapy to external beam therapy in the primary treatment of cervical carcinoma,5,6 many countries face unique challenges and struggle to offer this modality as a routine part of gynecologic cancer care.5,6 For example, in Guatemala, there are 4 brachytherapy delivery systems, but no high-dose-rate brachytherapy equipment.7
In this issue, we would discuss the role and delivery of brachytherapy for carcinoma, the capacity in low- to middle-income countries, challenges with implementation of a brachytherapy program and strategies for improvement.
The Role and Delivery of Brachytherapy in Treatment of Gynecologic Cancers
Brachytherapy plays an integral role in the treatment of gynecologic carcinomas. Placing a brachytherapy source in close proximity to tumor allows a high dose of radiation to the target and minimizes the dose to nearby sensitive organs, making it both safer and more effective than external beam alone.8 For early endometrial carcinoma, cylinder brachytherapy is used to treat the vaginal cuff following primary surgical therapy. However, for the medically inoperable patient with primary uterine carcinoma, brachytherapy may serve as the only treatment or can be combined with external therapy. In vaginal carcinoma, brachytherapy is often combined with external therapy and the boost dose to the primary tumor may be accomplished with intracavitary therapy. However, for advanced vaginal disease, more complex treatments with interstitial needles are necessary for eradication of residual disease following external beam therapy. For cervical carcinoma, the disease that leads to the most gynecologic cancer deaths worldwide, brachytherapy is an essential part of definitive therapy. Brachytherapy delivers almost half the target dose while relatively sparing the normal tissues compared to external beam therapy. Not only is brachytherapy an essential component of the treatment of cervical carcinoma but also precise well-timed delivery is vital for tumor eradication.9
Beginning in the early 1900s, brachytherapy was delivered by the low-dose rate (LDR) method. Instruments or needles were placed in the operating room and the patient was confined to bed while dose was delivered at approximately 50 cGy/h over several days. This method exposed ancillary personnel to radiation and given the immobility of the patients, put them at risk for deep venous thrombosis, myocardial infarction, and pulmonary emboli. In more recent years, LDR has been replaced with either pulsed-dose rate (PDR) or high-dose rate (HDR). Both of these methods allow more convenient, expeditious delivery (1–3 Gy/min although with PDR this is “pulsed” over every hour). Furthermore, the ancillary staff is not exposed to radiation, making it safer for both the patients and the health professionals. Although safer and more convenient, an HDR source, 192Ir, has a much shorter half-life than 137Cs, an LDR source.10 This, as well as the necessary site-specific equipment, can serve as a barrier to offering HDR therapy.
Brachytherapy Capacity in Low-and Middle-Income Countries
Brachytherapy offers a viable method of treating certain cancers in regions of the world where geographic and economic barriers would otherwise prevent cancer patients from receiving fractionated courses of external beam radiotherapy. For cervical cancer, which accounted for 7.5% of cancer-related deaths in women in developing countries in 2012, the combination of external beam radiotherapy and brachytherapy is essential to cure locally advanced cases.11
Low- and middle-income countries (LMICs) are ill-equipped to address this growing burden of cancer. More than 50% and up to 90% of cancer patients requiring radiation therapy do not have access to it.12 Dozens of countries lack radiotherapy altogether. A correlation between gross national income and availability of radiotherapy resources has been reported, and less than 5% of global spending on cancer occurs in the developing world. Accurately characterizing radiotherapy infrastructure in LMICs is difficult owing to the absence of robust published data. Quantification of brachytherapy capacity is even more limited. Currently, most information regarding radiotherapy capacity originates from the Directory of Radiotherapy Centres (DIRAC).13 DIRAC is a centralized, comprehensive database maintained by the International Atomic Energy Agency (IAEA) that contains information about teletherapy and brachytherapy equipment, imaging, treatment planning software, and personnel.
Though the database captures 90% of existing radiotherapy facilities and continuously updates information, DIRAC has limitations. DIRAC gathers surveys from institutions that self-report and has no mechanism to verify equipment status or fluctuations in personnel. Discordance exists among other sources of brachytherapy capacity data. The shortage of brachytherapy services is perhaps more urgent as brachytherapy afterloaders are not uniformly distributed between various LMICs.12 Additionally, there is a paucity of professionals to commission, use, and maintain safe and effective brachytherapy practices in developing countries. There is an inadequate number of regional and national training programs to train the physicians, medical physicists, therapists, nurses, and engineers to fill this void of human capital.14
The following is an overview of the brachytherapy capacity of 3 regions encompassing most of LMICs: Africa, Asia, and Latin America and the Caribbean.
Africa
Of the 54 countries considered part of the African continent, 52 are low and middle income based on current World Bank criteria.15 Equatorial Guinea and Seychelles are considered high income although neither have radiotherapy services.13 This continent has the lowest access to radiotherapy among the 3 regions with 28 countries lacking radiotherapy services altogether. Less than 30% of radiation needs are currently met in Africa. Radiation resources are concentrated in Northern Africa and South Africa. This uneven distribution obscures an even greater scarcity of services in the other African countries. Brachytherapy is only available in 20 African countries.16 As with teletherapy machines, most of brachytherapy facilities are located in the more economically developed regions of South Africa and Northern Africa.
In addition to insufficient brachytherapy equipment, the dearth of human capital remains a major barrier to increasing access to brachytherapy in Africa. The delivery of safe and efficacious brachytherapy demands specialized education and training. However, there are insufficient training centers for physicians, medical physicists, therapists, nurses, and engineers in Africa. Only 10 African countries offer radiation oncology training programs.17
Despite these challenges, some African countries are implementing new brachytherapy programs and others are enhancing existing brachytherapy infrastructure. In 2013, a team of physicians and physicists from the US and Canada partnered with Radiating Hope to install an HDR afterloader at Institut Joliot-Curie Cancer Center in Dakar, Senegal.18 This cancer center is the only center offering radiotherapy in Senegal and serves many surrounding West African nations. Before 2013, brachytherapy was not available in the country. Considering time and imaging limitations in Senegal, the team used fixed-geometry applicators and established a library of preplans. There is hope that this successful model can be reproduced in other countries lacking brachytherapy. In addition, Zimbabwe is expanding capacity, including a center in Bulawayo that is commissioning 2 LINACS and an HDR unit.19
Asia
Unique to Asia is the high population with high density in rural regions where cancer burden, especially cervical cancer, is high. There are 30 LMICs in Asia where only 34%−45% of radiation needs are currently met.12 As with other regions, there is a vast gap between radiotherapy capacity, and the oncologic needs with an estimated 1.5 million people for every teletherapy machine. In Southeast Asia alone, an estimated 2230 teletherapy machines would be needed to meet the recommended 4 MV machines per million people.20 It has been 15 years since the IAEA published results of a survey on the status of radiotherapy in 17 countries in the Asia and Pacific region.21 This report devoted significant attention to an analysis of brachytherapy. The authors created an index of brachytherapy capacity (brtx-cap) that defined the number of cervical cancer patients that could be treated with existing equipment. The brtx-cap demonstrated significant variation between countries in their ability to address the burden of cervical cancer. Less comprehensive but more current data exists for select countries and regions. India, the second most populous country in the world, has over 240 HDR and 6 LDR systems within the country (personal communication with Atomic Energy Regulatory Board, India). Most of equipped institutions only deliver intracavitary brachytherapy. One of the biggest barriers to more accessible brachytherapy in India is the lack of an adequately trained workforce that calls for thousands of radiation oncologists, physicists, and therapists to be trained. Postgraduate courses and training sessions in brachytherapy are being offered to meet this demand in India.22 Additionally, India is seeking greater self-sufficiency and working to develop HDR afterloaders and treatment software that can be produced within the country.3 In neighboring Bangladesh, a severe shortage of equipment and personnel exists but the government is making a concerted effort to increase radiotherapy capacity. The Bangladesh government would nearly double its brachytherapy capacity through procuring 5 HDR brachytherapy systems. Additionally, multiple organizations are partnering to increase radiation human resources in Bangladesh through both domestic and international training opportunities.19
Latin America and the Caribbean
There are 24 LMICs in this region, 7 of which lack radio-therapy all together. These countries are concentrated within the Caribbean but also include Belize and Suriname. Guyana, Nicaragua, Jamaica, and Paraguay have teletherapy machines but lack brachytherapy.13 Although there is more equipment per capita in this region than Africa, sufficient information regarding brachytherapy infrastructure remains elusive.12 In 2008 and 2009, the IAEA conducted a patterns of care study, sending online surveys about brachytherapy practices, facilities, and staffing to radiotherapy centers in 17 Latin American countries.23 Just over half the survey respondents reported performing brachytherapy. Brachytherapy was largely employed to treat gynecologic cancers with very limited use in the management of breast, prostate, gastrointestinal, or other malignancies. Although most used some form of image guidance, there was a split between centers using LDR and HDR sources. Unfortunately, only 20% of centers completed the survey and no responses were received from Brazil, the most populous country in Latin America. A more recent publication reported that there were 114 high-dose-rate devices in Brazil as of 2014.19 Radiotherapy services, including brachytherapy, are concentrated in large, urban areas, which translates into limited access and long wait times for cancer patients in rural areas.24 Further, radiation oncology training is only offered in 12 countries in this region, with even fewer offering formal medical physics training.25
Commissioning, Training, and Clinical Implementation of Brachytherapy in LMICs
The choice of brachytherapy source, whether LDR or HDR, and the platform to be used, is a complex decision and beyond the scope of this article. However, HDR has a number of advantages including ability to perform it in an outpatient setting, smaller source size and applicators, ability to perform dose optimization, and an increase in patient comfort and decreased risk of thromboembolic events. There are disadvantages and possible risks with HDR brachytherapy including expense, requirement for frequent source changes, the need for a reliable power supply, and requirement of much more specific training and knowledge of the afterloader’s components. This is particularly relevant in LMICs, where reliable power supply and technical expertise is not always available. Adequate training, commissioning of the afterloader, and a framework for clinical implementation are important because of the large potential for serious therapeutic misadventure with HDR. Here we describe general guidelines for commissioning, training, and clinical implementation of brachytherapy in LMICs.
Commissioning and Quality Assurance
Simple treatment methods may be established in LMICs to facilitate delivery of HDR treatment without complex treatment planning. However, given the complexity of HDR afterloaders and the potential pitfalls with their use, there is no way to simplify commissioning and quality assurance in LMICs. It is much safer and more efficient to follow a previously developed and accepted commissioning procedure than to develop an institution-specific procedure. Both the IAEA and the American Association of Physicists in Medicine have accepted procedures.26,27 These procedures entail con-firming proper mechanical and electrical operation of the afterloader and active interlocks, integrity and activity of the radioactive source, integrity and geometry of the applicators, and accurate operation of the treatment planning computer system. When possible, the commissioning documents should be reviewed by someone external to the project.28
Ongoing quality assurance is also required before treatment. This includes verification of source strength, verification of source positional accuracy including source wire positioning as well as guide tube measurements, verification of source temporal accuracy, and a second check system for dose verification. These requirements are not unique to LMICs, but require expertise that may not always be available in such settings.
Training
Training is a critical and often neglected step in implementing any new technology. Most of errors in brachytherapy treatment delivery are the result of human error, miscommunications, or misunderstanding of equipment operation rather than failure of devices.27 Therefore, it is imperative that all members of the brachytherapy team are adequately trained. The AAPM Task Group-59 report recommends that the treatment team consists of a radiation oncologist with special expertise in brachytherapy, preferably HDR; a medical physicist with expertise in brachytherapy who receives at least vendor-supported on-site training on the treatment machine and treatment planning system; and a treatment-unit operator who could be either the physician, physicist, dosimetrist, or a radiation therapist.29 In LMICs, radiation oncologists frequently have training in brachytherapy, but not typically in HDR. Therefore, the procedure for applicator placement for a typical tandem and ovoid implant for cervical cancer may come quite easily for them, whereas knowledge of the radiobiology of conversion from LDR to HDR doses may not. Physicists in LMICs may or may not have expertise in brachytherapy. Sufficient on-site training in brachytherapy dosimetry, afterloader procedures, and continuous quality assurance are required. For this reason, it may be best to implement a simple treatment planning method for gynecological brachytherapy using fixed-geometry applicators, a library of treatment plans, and isodose overlays to confirm adequate dosimetry until the physicist is experienced enough to perform more complex treatment planning.30 The entire treatment team should receive training in radiation safety, HDR emergency procedures, and monitoring the patient and treatment unit during delivery. We also strongly recommend that clinics in LMICs implement new technology partnerships with clinics that have expertise in these areas to ensure ongoing support and peer review for their processes.
Clinical Implementation
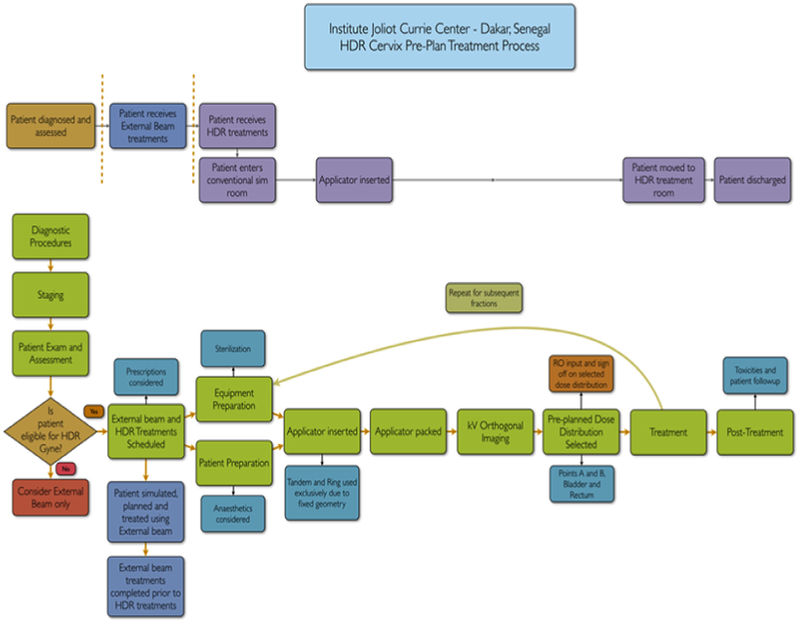
Before HDR treatment delivery, a number of additional steps are needed to assure smooth and safe treatment delivery. A written step-by-step description of the procedure with a flow diagram and check-list should be approved by all members of the treatment team. The Figure shows an example of a flow diagram used for implementation of HDR treatment. We also recommend a multidisciplinary prospective risk assessment be undertaken by the team involved in the commissioning and training process. This is an opportunity to fine-tune the treatment process and to clarify misunderstandings between team members. The goal should be to identify potential ways that the established treatment technique could fail and then to avoid those failures by adding quality assurance or second checks to the treatment process.31 Every step from patient preparation, applicator placement, imaging for treatment planning, treatment planning, transfer of the patient to the treatment room, and delivery of treatment should be discussed and practiced as a dry-run before delivery of the first treatment.
Figure.
Flow diagram for treatment delivery at the Institut Curie in Dakar Senegal. RO, radiation oncology; kV, kilovolt; HDR, high-dose rate.
HDR brachytherapy is a high-risk but high-gain procedure that is a valuable addition to clinics in LMICs. Implementing the technology requires a comprehensive plan for commissioning, training, and clinical implementation for the safety of treatment personnel and patients.
Other Challenges
Establishing a sustainable pathway for equipment repair and maintenance presents another challenge. In sub-Saharan Africa, up to 70% of medical equipment lies idle because of errors made during acquisition or installation and a lack of adequate training and technical support.32 Of the equipment in use, 25%−35% is not operable because of equipment malfunction, and a lack of capacity in the local environment for repair. An advantage for most LDR techniques is the relative simplicity of the applicators. However, for HDR techniques, the electromechanical components of both the afterloader and the associated vault require repair and maintenance capacity, as well as a regular supply of the relevant isotope. HDR and PDR sources also require a higher level of security compared to LDR sources.33 Implementing and maintaining these necessary processes require a significant, sustained commitment from the involved parties.
Another consideration is that many patients needing brachytherapy in resource-limited locations must often travel significant distances to receive treatment. These patients may not have resources to support themselves during their treatment and are effectively homeless during their treatment course. For example, a portion of the those under treatment in Mulago hospital in Uganda come from neighboring countries, such as Rwanda, which has no radiotherapy capacity.34 The mechanism of appropriately selecting and supporting these patients must be considered.
Strategies for Improvement in Brachytherapy Delivery in LMICs
Local (at Country Level) Engagement
At the country level, recognizing the need for expanding training in brachytherapy and developing strategies simultaneously to target the multiple issues described above is crucial.
Strategic planning, appropriate resource allocation for infrastructure development in a phased manner with strict achievable goals are required at the national level. Simultaneously, brachytherapy personnel team training, including problem-oriented/practical learning initiatives and development of standard operating procedures and roadmaps for successful implementation of brachytherapy program is vital. In India, many regional cancer centers like TATA Memorial center in Mumbai and All India institute of Medical Sciences, New Delhi, are improving cancer treatment facilities by development of centers in rural areas, providing greater access to brachytherapy. Recognized regional cancer centers also offer short- and long-term national and international training programs including brachytherapy. Apart from these, cancer organizations like the Association of Radiation Oncologist of India (AROI) and the Indian Brachytherapy Society (IBS) engage in training workshops to promote brachytherapy practice.
Industry Engagement
Industry partners in radiation oncology such as Elekta and Varian recognize the need for further brachytherapy capacity building and have developed initiatives toward that goal. Elekta has developed a program called BrachyAcademy, which aims to advance the use of brachytherapy worldwide.35 BrachyAcademy is comprised of a peer-to-peer education program, clinical workshops and training visits for sites in LMICs. Similarly, Varian is playing an active role in Cervix Cancer Research Network (CCRN), a subsidiary of the Gynecologic Cancer InterGroup (GCIG). The GCIG is an organization of international cooperative group, which is focused on increasing access to clinical trials to improve outcomes for women with gynecologic cancers in LMICs.36 In addition to the initiatives ongoing, industry partners can further contribute to successful implementation of brachytherapy in LMICs through rigorous on-site training, which brings together international and local experts in each country when brachytherapy is initiated. In addition, ongoing support from industry is essential for successful implementation after the initial training for success of any new brachytherapy program. Finally, an official certification from industry documenting steps of successful implementation of the brachytherapy program demonstrated by a review of measurable outcomes would be beneficial to replicate the training model in other centers in LMICs.
International Initiatives
The American Brachytherapy Society (ABS) has embraced international relationships with an increasing number of brachytherapy societies around the globe. The ABS recognizes the dearth of brachytherapy capacity and training within LMICs. Concurrently, ABS is mindful of the growing enthusiasm of radiation oncology trainees and brachytherapists to address this gap in oncologic care through sharing their time and expertise with practitioners and patients beyond the US. Through its International Committee, ABS has cultivated relationships with counterparts in developing regions, such as the Indian Brachytherapy Society (IBS) and Asociación Latinoamericana de Terapia Radiante Oncológica (ALATRO). Expert brachytherapists from the US have been invited to present and lead educational sessions at international meetings. ABS has strengthened ties with other professional groups, societies, and nonprofit organizations in high-income countries including American Society of Therapeutic Radiation Oncology (ASTRO), The Groupe Européen de Curiethérapie (GEC) and the European Society for Radiotherapy & Oncology (ESTRO), IAEA, American Radiation Oncology Resident Organization (ARRO), International Cancer Expert Corps (ICEC), and Radiating Hope. All parties acknowledge the importance of enhancing existing brachytherapy programs and implementing new ones to address the cancer burden in the developing world and are working to incorporate the expertise of ABS members into existing and future initiatives. Brachytherapy leaders in the US have worked to build and sustain meaningful international research collaborations.
The ABS realizes the importance of the next generation of brachytherapists, their zeal for global oncology, and their potential ability to ameliorate disparity in brachytherapy access. In addition to the Brachytherapy Scholarship Program, geared toward enhancing brachytherapy skills of residents and junior physician members of ABS, ABS has been a crucial supporter of the ARRO Global Health Rotation Initiative. This Initiative was created by US and Canadian radiation oncology residents to provide a continually updated database of radiation oncology centers willing to host trainees from high-, middle-, and low-income countries for educational exchanges. To date, there are 16 sites that have agreed to participate. Although these trainee rotation locations would undoubtedly increase, there is still a need to provide observerships for practicing radiation oncologist who seek additional instruction in brachytherapy. A collection of observership sites for practicing radiation oncologists, modeled after the ARRO Global Health Rotation Initiative, would provide an invaluable resource. Sharing of educational materials through web casts and other forms of distance learning and peer review supported by individual physicians, societies, and vendors also become more important. Site visits to new and established centers by teams of physicians, therapists, nurses, and physicists are helpful in assessing need and implementing new technology. Sustaining these beneficial relationships and exciting endeavors require considerable effort from all involved parties.
In addition to education and training, a coordinated effort among various international groups is needed in promoting brachytherapy and gynecological oncology research. Although CCRN has been leading gynecological clinical trials around the world, it has been challenging to engage LMICs in these projects. We need to identify champions of gynecological research in LMICs and work with them to develop the infrastructure for research in brachytherapy and gynecological cancers in these settings.
Conclusion
Cervical cancer is the fourth most common cancer affecting women worldwide, ~90% of deaths occurring in LMICs.37 In the developing world, cervical cancer is the leading cause of cancer-related deaths in women.38 Thus, much of the global burden of cervical cancer falls on LMICs.
Brachytherapy plays an integral role in the treatment of gynecologic carcinomas.11 Brachytherapy is essential for both the curative and palliative treatment of cervical cancer. However, there are numerous geographic and economic barriers to providing brachytherapy to cancer patients in LMICs. More than 50% and up to 90% of cancer patients requiring radiation therapy do not have access to it.11 Lack of funding for expensive supplies and equipment; poor infrastructure support including needed equipment and unreliable sources of power, and a shortage of well-trained radiation oncologists, medical physicists, and allied health professionals in these countries contribute to the ongoing shortfall. More importantly, radiation oncologists in LMIC must embrace the value of brachytherapy, realize the benefits of brachytherapy and realize that they are part of a global network of radiation oncologists working together.
Significant efforts are being made to combat the global disparity in access to brachytherapy. International initiatives have been established to provide training and expertise to professionals in LMICs. It is essential to maintain these relationships and continually assess the shifting needs in cancer treatment. Although major human and financial resources are ultimately needed to solve these disparities, these efforts begin at the local level, both here and abroad.
Footnotes
Conflict of interest: none.
References
- 1. http://www.who.int/gho/publications/world_health_statistics/2015/en/
- 2.Koutsky LA, Ault KA, Wheeler CM, et al. : A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med 347(21):1645–1651, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Banerjee S, Mahantshetty U, Shrivastava S: Brachytherapy in India—A long road ahead. J Contemp Brachytherapy 6:331–335, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. www.medicaltourismguatemala.com/hospitals.
- 5.Coia L, Won M, Lanciano R, et al. : The patterns of care outcome study for cancer of the uterine cervix. Results fo the second national practice survey. Cancer 66:2451–2456, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Han K, Milosevic M, Fyles A, et al. : Trends in the utiltization of brachyterhapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys 87:111–119, 2013 [DOI] [PubMed] [Google Scholar]
- 7. http://www.radiatinghope.org/guatemala-radiation-site.html.
- 8.Georg D, Kirisits C, Hillbrand M, et al. : In J Radiat Oncol Biol Phys 71:1272–1278, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Shaverdian N, Gondi V, Sklenar KL, et al. : Effects of treatment duration during concomitatnt chemoradiation therapy for cervical cancer. Int J Radiat Oncol Biol Phys 86:562–568, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Viswanathan V, Thomadsen B: American Brachytherapy Society consensus guidelines for advanced carcinoma of the cervix. Part I: General principles. Brachytherapy 11:33–46, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Cervical Cancer: Estimated incidence, mortality and prevalence worldwide in 2012. International Agency for Research on Cancer and World Health Organization. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed 21 March, 2016 [Google Scholar]
- 12.Zubizarreta EH, Fidarova E, Healy B, et al. : Need for radiotherapy in low and middle income countries—The silent crisis continues. Clin Oncol (R Coll Radiol) 27:107–114, 2015 [DOI] [PubMed] [Google Scholar]
- 13.DIRAC (directory of radiotherapy centres): International Atomic Energy Agency. http://www-naweb.iaea.org/nahu/dirac/default.asp. Accessed 21 February, 2016
- 14.Hanna TP: Radiation oncology in the developing world In: Halperin EC, Wazer DE, Perez CA, et al. (eds): Principles and Practice of Radiation Oncology, ed 6 Philadelphia, Lippincott Williams & Wilkins, 552–559, 2013
- 15.Data: Country and Lending Groups. The World Bank, 2016. http://data.worldbank.org/about/country-and-lending-groups. Accessed 21 February, 2016 [Google Scholar]
- 16.Abdel-Wahab M, Bourque J-M, Pynda Y, et al. : Status of radiotherapy resources in Africa: An international atomic energy agency analysis. Lancet Oncol 14:e168–e175, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Efstathiou JA, Heunis M, Karumekayi T, et al. : Establishing and delivering quality radiation therapy in resource-constrained settings: The story of Botswana. J Clin Oncol 34:27–35, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Einck JP, Hudson A, Shulman AC, et al. : Implementation of a high-dose-rate brachytherapy program for carcinoma of the cervix in Senegal: A pragmatic model for the developing world. Int J Radiat Oncol Biol Phys 89:462–467, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Atun R, Jaffray DA, Barton MB, et al. : Expanding global access to radiotherapy. Lancet Oncol 16:1153–1186, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Weng FK: Radiotherapy in southeast Asia: Room to grow. Lancet Oncol 16:1149–1150, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Tatsuzaki H, Levin CV: Quantitative status of resources for radiation therapy in Asia and Pacific region. Radiother Oncol 60:81–89, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Delaney GP, Barton MB: Evidence-based estimates of the demand for radiotherapy. Clin Oncol (R Coll Radiol) 27:70–76, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Guedea F, Ventura M, Londres B, et al. : Overview of brachytherapy resources in Latin America: A patterns-of-care survey. Brachytherapy 10:363–368, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Goss PE, Lee BL, Badovinac-Crnjevic T, et al. : Planning cancer control in Latin America and the Caribbean. Lancet Oncol 14:391–436, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Grover S, Xu MJ, Yeager A, et al. : A systematic review of radiotherapy capacity in low- and middle-income countries. Front Oncol 4:380, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.International Atomic Energy Agency: Human Health Campus, Medical Physics, Radiotherapy. Vienna: International Atomic Energy Agency, 2013 [Google Scholar]
- 27.Nath R, Anderson L, Meli J, et al. : Code of practice for brachytherapy physics: Report of the AAPM radiation therapy committee task group No.56. Med Phys 24(10):1557–1598, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Brown D, Shulman A, Hudson A, et al. : A framework for implementation of new radiation therapy technologies and treatment techniques in low-income countries. Med Phys 30(7):791–798, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Kubo D, Glasgow G, Pethel T, et al. : High dose-rate brachytherapy treatment delivery: Report of the AAPM Radiation Therapy Committee Task Group No. 59. Med Phys 25(4):375–403, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Einck J, Hudson A, Shulman A, et al. : Implentation of a high-dose-rate brachytherapy for carcinoma of the cervix in Senegal: A pragmatic model for the developing world. Int J Radiat Oncol Biol Phys 89(3):462–467, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Ortiz Lopez P: Tools for risk assessment in radiation therapy. Ann ICRP 41(3–4):197–207, 2012 [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization: Barriers to Innovation in the Field of Medical Devices. August 2010. Report No. 6
- 33.International Atomic Energy Agency: Security of Radioactive Sources: Implementing Guide. Vienna, 2009 [Google Scholar]
- 34.Israel Luutu: personal communication, 11.03.15.
- 35.BrachyAcademy: 2014. Available from: https://www.brachyacademy.com/ Cited April 15, 2016 [Google Scholar]
- 36.David Gaffney: What is the Cervix Cancer Research Network? Gynecologic Cancer InterGroup Cervix Cancer Research Network. Presented at Cervix Cancer Education Symposium; January, 2016; Bangkok, Thailand https://gciggroup.com/system/files/1%20Gaffney%20SPEAKER%20IntrotoCCRN%20%5BAutosaved%5D.pdf [Google Scholar]
- 37.GLOBOCAN, 2012. Available from: http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp
- 38.Forouzanfar MH, Foreman KJ, Delossantos AM, et al. : Breast and cervical cancer in 187 countries between 1980 and 2010: A systematic analysis. Lancet 378(9801):1474–1547, 2011 [DOI] [PubMed] [Google Scholar]