Abstract
Balance problems are common after a traumatic brain injury (TBI). Symptoms of dizziness, unsteadiness, or imbalance have been most frequently attributed to sensory organization problems involving the use of visual, proprioceptive, and/or vestibular information for postural control. These problems can be assessed with the Sensory Organization Test (SOT). However, as head trauma can affect any brain region, areas responsible for voluntary control of movements involved in dynamic balance tasks, such as the motor cortex and its projections, could also be compromised, which would likely affect one's limits of stability. The Limits of Stability (LOS) balance test has received little attention in TBI. In the present study, we compared the prevalence of SOT versus LOS abnormalities in a cohort of 48 patients, the majority classified as having mild or moderate chronic TBI. Compared with a normative database provided by the balance testing manufacturer, a larger portion of our cohort presented abnormalities in the LOS test. Dizziness Handicap Inventory (DHI) results indicated mild disability, with the five activities most frequently endorsed as problematic being: looking up, performing quick head movements, performing ambitious such as sports or dancing activities, feeling frustrated, and performing strenuous house/yard work. Although regression analysis revealed that both tests significantly predicted subjective scores on the DHI, more LOS than SOT testing variables were important predictors of DHI results indicating disability. These results suggest that the LOS test is an informative tool that should be included in any objective balance evaluations that screen TBI patients with balance complaints.
Keywords: balance, limits of stability, posture, sensory organization, symptoms, TBI
Introduction
Individuals who have sustained a traumatic brain injury (TBI) often experience balance-related symptoms such as dizziness, unsteadiness, or imbalance.1–5 These symptoms can become chronic, and have most frequently been attributed to damage to the vestibular system or related brain pathways, thus leading to deficits in postural control.6–12
The Sensory Organization Test (SOT) is currently the gold standard for objective assessment of balance related to the use of vestibular, as well as somatosensory and visual, inputs in postural control.13 This is a static balance test in which postural sway is measured during quiet stance on a force plate under a series of conditions, some of which have absent or inaccurate sensory input, allowing for an isolated evaluation of each system that controls posture. In TBI, several studies have used the SOT to detect sensory abnormalities of a vestibular14–16 and, to a lesser extent, visual nature, acutely and chronically post-injury.14,17
In addition to sensory inputs, balance control also depends on appropriate functioning of other systems, such as anticipatory postural adjustments and automatic postural reactions, as well as biomechanical and neuropsychological factors.18–20 Having a cone of stability within the normal range is yet another important component of postural control.21,22 Our cone of stability, or the extent to which we can move our body over our base of support without changing the support or having to take a step, can be objectively measured with a test named the Limits of Stability (LOS) test. The most commonly used force plate based device for the LOS test is the NeuroCom system (Natus Medical Inc., Seattle, WA).13 Rather than using a quiet stance, this is a test of dynamic balance in which maximal excursions of the center of gravity are measured when the subject voluntarily leans toward directions all around the body (forward, backward, lateral, and diagonal), while keeping a straight posture, leaning only from the ankles. Reduced limits of stability can affect one's ability to safely perform activities of daily life that involve leaning, bending over, or reaching,13 and has been associated with increased fall risk in several groups of patients, including the elderly23,24 and individuals with neurological conditions such as stroke,25 Parkinson's disease,22 and progressive supranuclear palsy.26 However, very little has been reported using the LOS test in patients with TBI.
As head trauma can affect any brain region, not only the vestibular system and its projections, but also areas responsible for voluntary control of balance, such as the motor cortex and its projections, could be compromised. It is therefore possible that a TBI can cause deficits in voluntary postural control, which the LOS test would show as reduced limits of stability. The first goal of this study was to quantify and compare the prevalence of abnormalities in the LOS test compared with abnormalities in the SOT in a group of individuals with a diagnosis of TBI.
In addition to objective measures of sensory organization and limits of stability in TBI, another interest of the present study was to explore the subjective aspect of balance deficits in our participants. We believe that objective measures of postural control become more clinically relevant when they can be related to how patients experience postural balance challenges in their daily lives. To our knowledge, no studies have investigated relationships between LOS and subjective complaints of balance problems in TBI, either alone or in comparison with the SOT. Therefore, the second goal of this study was to determine if patient-reported balance symptoms, as rated by the Dizziness Handicap Inventory (DHI),27 could be predicted based on their scores on the LOS and SOT tests.
Methods
Participants
This cross-sectional study investigated adults with non-penetrating TBI. As this was an exploratory analysis, our sample was extracted from a large natural history study, which is still ongoing, at the National Institutes of Health (NIH). Recruitment for the natural history study was done through the NIH Patient Recruitment and Public Liaison Office, the Center for Neuroscience and Regenerative Medicine Recruitment Core, and advertisements displayed in the community. Selection criteria for the larger study included a diagnosis of non-penetrating TBI within 5 years of onset, and the ability to walk independently without assistance and to provide informed consent. Exclusion criteria were contraindication to magnetic resonance imaging scanning, inability to read or speak English, and any significant medical or psychological symptoms that would preclude subjects from being able to complete the study requirements.
All subjects selected to participate in the natural history study received imaging, neuropsychology, vocational work, and gait and balance evaluations; however, not all subjects qualified for this cross-sectional analysis. Additional exclusion criteria for this sub-study were those with known confounders to balance performance, such as presence of non-TBI-related neurological disorders, lower-extremity orthopedic surgery within 6 months, symptomatic and/or untreated lower extremity or trunk musculoskeletal conditions, uncorrected visual disturbances, history of postural hypotension, history of or current alcohol abuse (Alcohol Use Disorders Identification Test >8), recreational drug use within 24 h prior to testing, severe depression (Beck Depression Inventory (BDI) >29), history of psychosis or untreated psychopathology, and invalid neuropsychological testing (attributed to practice effects, lack of effort, or questionable validity). Of the 131 participants enrolled in the natural history study between August, 2011 and August, 2015, 48 met the specific criteria for this analysis.
Procedures
History, including trauma data, and physical examination findings, were collected by our study physician. TBI severity was determined as per the Department of Veterans Affairs and Department of Defense.28 Participants were classified as medication users if they were taking any of the following psychotropic drugs regularly: pain relievers, benzodiazepines, sleep medications, antiepilepsy medications, antidepressants, or neurostimulants. In addition, depression severity was reported using the BDI. Participants were also asked to report the total number of brain injuries that they had sustained in the past, as well as any falls since their most recent TBI.
Balance testing with the NeuroCom SMART Balance Master (Natus Medical Inc., Seattle, WA) included the SOT and LOS assessments.13,29 Briefly, the SOT measures how well one uses sensory information for postural control. Subjects are required to stand quietly and still on a dynamically responsive force plate while equilibrium scores are generated reflecting the extent of each individual's sway. Higher scores reflect less body sway (i.e., better balance), with a maximum score of 100 indicating perfect balance and a score of 0 representing a fall. Data on a total of 6 conditions were collected, each with three 20 sec trials. All trials and conditions were included in the calculation of the following measurements reported in this study: Somatosensory Score (SOM), Visual Score (VIS), Vestibular Score (VEST), Preference Score (PREF), which reflects the extent to which an individual over-compensates through visual integration, and Equilibrium Composite Score (Comp).14,30,31 Scores were visually inspected for aphysiological responses, and no related data were excluded. Other measures also collected as part of the SOT were strategy analysis and center of gravity alignment, but these were not of interest in this study; therefore, they were not reported.
The LOS measures how far people can voluntarily lean their body in any direction keeping a straight posture before they lose their balance or take a step. Subjects are required to stand facing a computer screen that displays a stick figure, providing live-time visual feedback representative of the subject's dynamic center of pressure (COP); upon COP displacement, the on-screen figure moves accordingly. The on-screen figure is encircled by eight target boxes, each of which represents the subject's theoretical maximal cone of stability. Upon an audible cue, subjects are asked to lean as quickly and directly as possible toward the target highlighted on screen, and to hold this lean at the “limit” of his/her balance stability until another audible cue indicates the end of the trial. This task is intended to emulate the movements of an inverse pendulum; with feet planted maintaining an entirely straight posture, and flexion only at the ankles. One practice trial was performed before the test was conducted. All trials were included in the calculation of the following measurements, which are composite (eight directions/trials averaged) scores:
-
1.
Reaction time (RT): time (seconds) between the start of trial (audible cue) and patient's first movement toward the target
-
2.
Movement velocity (MVL): velocity of lean from initial stance to end-point excursion (EPE) (degrees of ankle flexion per second)
-
3.
EPE: greatest center of pressure displacement upon initial lean toward target (% of maximal LOS)
-
4.
Maximum excursion (MXE): greatest center of pressure displacement throughout entire 8 sec trial (% of maximal LOS)
-
5.
Directional control (DCL): extent to which direct target lean/COP displacement is executed, measured in % of straightness of COP displacement trajectory.
SOT and LOS measurements were statistically compared with an age-referenced normative data set provided by the manufacturer (personal communication with Daniel Dubiel at NeuroCom Balance Systems Customer Support, Natus Medical Inc., Seattle, WA) and thereby categorized as normal (NL) or abnormal (ABNL); >1.645 standard deviations of the normative mean. SOT normative data included 112 individuals in the age range of 20–59 years. LOS normative data included 47 individuals in the age range of 40–59 years. All subjects in the normative data set were reported to have no current or past diagnosis or injury affecting balance, be taking no medications affecting the central nervous system or known to affect balance or coordination, and have no symptoms of dizziness or lightheadedness, no symptoms suggestive of vestibular or neurological disorders, no psychological disorders including depression, no history of two or more unexplained falls within the past 6 months, and normal vision with or without glasses.
Subjective assessment
The DHI is a 25-item questionnaire that quantifies self-perceived deficits on everyday balance activities.27 It was originally designed for subjects with a vestibular abnormality;27,32 however, it has also been studied in TBI.5,29,33 Three domains are assessed: physical, emotional, and functional. The majority of questions inquire whether or not specified actions enhance “your problem [of dizziness],” warranting a response of “no,” “sometimes,” or “yes”; graded 0, 2, and 4, respectively. Because subjects with TBI may report balance symptoms other than dizziness alone,34 we adapted this tool for our cohort and oriented participants to answer each question in relation to any balance-related symptoms they might be experiencing (e.g., unsteadiness, light-headedness, imbalance), not necessarily those solely restricted to dizziness. Scores range from 0 to 100, which indicates maximum perceived disability.
Statistical analysis
One sample t tests were performed comparing the TBI cohort with normative data provided by the manufacturer (NeuroCom) on each balance variable. This analysis was conducted with IBM SPSS software version 19 (IBM, Somers, NY). A least-angle regression (LARS) analysis was used to identify SOT and LOS variables that could serve as predictors for DHI total score. In this analysis, three models were estimated. Each model was initially fit using all possible covariates (age, total number of brain injuries, counts on use of psychotropic medications, number of days since injury, and depression severity). In addition to the covariates, the first model included both SOT and LOS variables, whereas the second used only SOT and the third only used LOS variables. The LARS algorithm sequentially builds models by identifying the variable with the greatest correlation with the residuals, and increasing its coefficient in absolute value until another variable is more highly correlated to the adjusted residuals. Mallows' Cp was used to determine the best-fitting subset of variables for each of the three LARS models.35 The best-fit subset was defined as the model step in LARS that minimized Cp, subject to Cp ≥ P, where P is the number of predictor variables in the model in that step. After the best fit model was determined, bootstrap 95% confidence intervals for its model parameters were determined by taking 3000 bootstrap replications. Analyses were conducted in Stata 14 and R version 3.4.3. Significance was established at p < 0.05.
Results
Group characteristics
Neurological and musculoskeletal examination findings were normal for all participants in this study. Table 1 shows the characteristics of our TBI cohort. As can be seen, their time post-injury of ∼1 year indicates they were in the chronic injury stages. The majority were classified as having mild to moderate TBI. Nineteen out of 42 participants reported regular use of psychotropic medication; data were missing for 6 participants. BDI scores indicated minimal depression however, 11 participants scored as mildly depressed, and 6 scored as moderately depressed. Patients reported, on average, approximately three brain injuries; data were missing for eight subjects. Only one subject reported a fall, which was while roller-skating.
Table 1.
Characteristics of the TBI Cohort (n = 48), Displayed in Continuous Variables as “Average ± Standard Deviation”
| Age, years | 47.49 ± 16.12 |
| Time post-injury, days | 381.54 ± 416.90 |
| Medication use (yes / no) | 19 / 23 |
| Classification (m / cm / mo / se) | 20 / 9 / 12 / 7 |
| BDI Total score | 7.35 ± 7.85 |
| Total number of brain injuries | 3.35 ± 1.99 |
Continuous variables are displayed as “Ave ± SD,” and other variables are displayed as counts. Age ranged from 17 years to 80 years. Time post-injury ranged from 27 days to 1633 days. BDI score ranges and counts for minimal, mild, moderate, and severe depression classifications were “(range, n)”: (0–9, 31), (10–18, 11), (19–29, 6), and (30–63, 0), respectively. Number of brain injuries ranged from 1 to 8.
TBI, traumatic brain injury; m, mild; cm, complicated mild; mo, moderate; se, severe; BDI, Beck Depression Inventory.
Objective balance tests
Balance results for our sample, compared with the normative database are presented in Table 2. Our cohort scored significantly worse than controls on all variables, except VIS and DCL. The SOT results indicate significant sensory deficits overall (Comp scores) and, more specifically, in using proprioception (SOM) and vestibular (VEST) information to control balance, with an over reliance on visual inputs (PREF) even when those were misleading. The LOS results indicate significant slowness of reaction time and leaning movement, as well a reduced end-point and maximal excursions.
Table 2.
SOT and LOS Test Results: Comparison between the TBI Cohort and Normative Values Provided by Manufacturer
| TBI cohort (AVE ± SD) | Normative (AVE ± SD) | p value | |
|---|---|---|---|
| SOT | |||
| SOM | 94 ± 6 | 98 ± 5 | 0.001 |
| VIS | 88 ± 9 | 88 ± 8 | 0.705 |
| VEST | 54 ± 26 | 74 ± 11 | <0.001 |
| PREF | 105 ± 15 | 98 ± 7 | 0.003 |
| Comp | 72 ± 12 | 80 ± 6 | <0.001 |
| LOS | |||
| RT | 0.9 ± 0.2 | 0.7 ± 0.2 | <0.001 |
| MVL | 3.7 ± 1.6 | 5.0 ± 1.5 | <0.001 |
| EPE | 68.6 ± 16.2 | 84.9 ± 8.3 | <0.001 |
| MXE | 81.3 ± 13.9 | 98.0 ± 5.9 | <0.001 |
| DCL | 76.5 ± 6.8 | 75.2 ± 6.0 | 0.197 |
Significant p values at <0.05 are indicated in bold.
SOT, Sensory Organization Test; LOS, Limits of Stability; TBI, traumatic brain injury; AVE, average; SD, standard deviation, SOM, Somatosensory Score; VIS, Visual Score; VEST, Vestibular Score; PREF, Preference Score; Comp, Equilibrium Composite Score; RT, Reaction Time; MVL, Movement Velocity; EPE, end-point excursion, MXE, maximum excursion; DCL, directional control.
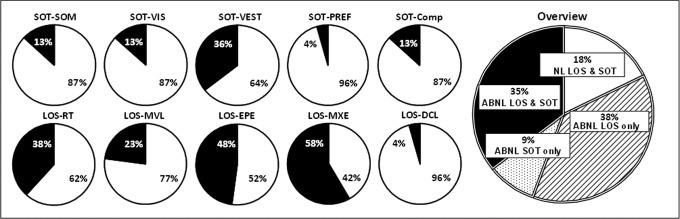
Counts of NL and ABNL scores show a greater percentage of participants scoring abnormally on the LOS (73%) than on the SOT (44%) (Fig. 1). The largest subgroup (38%) scored abnormally on the LOS test alone. This was followed closely by 35% with combined LOS and SOT abnormalities. A much smaller percentage (9%) presented with SOT deficits alone. Finally, 18% had no balance problems.
FIG. 1.
Pie charts showing balance classification of traumatic brain injury (TBI) cohort (n = 48) as normal (NL) (white on the charts) or abnormal (ABNL) (black or patterns on the charts) based on comparisons with normative data. Left, small pie charts: Subject scores within 1.6 standard deviations (SD) of the normative mean were categorized as NL (white on the charts); scores >1.6 SD were categorized as ABNL (black on the charts). Notice higher percentages for Vestibular Score (VES) on the Sensory Organization Test (SOT), and maximum excursion (MXE) on the Limits of Stability (LOS) test. Right, big pie chart showing an overview: participants were grouped into one of four mutually exclusive categories, which were cited here in order of magnitude: 1) ABNL LOS and NL SOT (ABNL LOS only), 2) ABNL balance on both tests (ABNL LOS and SOT), 3) NL balance on both tests (NL LOS and SOT), and 4) ABNL SOT and NL LOS (ABNL SOT only). Notice higher percentages for LOS abnormality.
Pertaining to the SOT specifically, we found that although the most common deficit in our cohort was related to vestibular integration (36% or 16/45 participants with attainable VEST data), some also showed somatosensory (13% or 6/46) and visual (13% or 6/45) integration deficits (Fig. 1). Pertaining to the LOS, the greatest percentage of abnormalities were in excursion variables (MXE 58% or 28/48, and EPE 48% or 23/48), followed by reaction time (38% or 18/47) and movement velocity (23% or 11/48). Very few had directional control problems (4% or 2/47).
Subjective assessment of balance-related disability
Only 31 participants had complete data sets for the regression analysis (9 did not complete the DHI and 8 did not the report number of brain injuries prior to the TBI that brought them to the study). The DHI total score was 15.2 ± 21.3 (range 0–72), indicating mild disability. Regression analysis results showed the first model (Model 1, Table 3) fitted by LARS, including SOT and LOS variables and covariates was able to predict 65% of variance in DHI responses. As expected, Model 1 explained more of the variability in DHI scores than the other two models. However, Models 2 and 3 (Table 3) more specifically revealed which variable(s) best predicted subjective symptoms within each test. Visual integration was the only relevant predictor of DHI among the SOT variables (Model 2: 55% of variance predicted); whereas all but one LOS variable (DCL) were relevant predictors of DHI (Model 3) with a similar degree of predicted variance (57.7%). All covariates were significant in all models, except for age, which was not a predictor in Model 2. Finally, the majority of regression coefficients were positive, except for the number of brain injuries, RT, VIS, and PREF, which were found to have negative coefficients because of their negative correlations with DHI after controlling for other variables in their respective models.
Table 3.
Regression Analysis to Predict DHI Scores from Covariates, SOT and LOS
| Model 1 (SOT & LOS) | Model 2 (SOT) | Model 3 (LOS) | |
|---|---|---|---|
| Statistical analysis | |||
| R2 | 0.65 | 0.55 | 0.58 |
| Cp | 12.25 | 5.38 | 9.02 |
| Cp - P |
0.25 |
0.38 |
0.02 |
| |
Beta (CI) |
Beta (CI) |
Beta (CI) |
| Predictor Variables | |||
| Covariates | |||
| Age | −0.09 (−0.67, 0.43) | — | −0.01 (−0.48, 0.46) |
| Time post-injury | 0.01 (−0.02, 0.04) | 0.001 (−0.003, 0.006) | 0.003 (−0.02, 0.03) |
| Medication use | 13.27 (−3.58, 37.90) | 12.59 (1.25, 28.25) | 16.73 (2.08, 35.00) |
| BDI | 1.24 (−0.08, 2.51) | 1.07 (0.20, 2.04) | 1.13 (0.05, 2.23) |
| Total no. of HI | −3.82 (−9.79, 1.72) | −2.34 (−5.48, −0.17) | −2.76 (−7.27, 1.31) |
| SOT | |||
| SOM | — | — | |
| VIS | −0.48 (−1.74, 0.86) | −0.15 (−0.66, 0.41) | |
| VEST | −0.17 (−0.80, 0.36) | — | |
| PREF | −0.39 (−1.41, 0.51) | — | |
| Comp | — | — | |
| LOS | |||
| RT | −31.70 (−95.46, 17.50) | −7.67 (−49.30, 33.71) | |
| MVL | −0.30 (−8.97, 6.67) | 0.70 (−6.24, 7.46) | |
| EPE | −0.08 (−1.08, 0.96) | 0.01 (−0.98, 1.03) | |
| MXE | — | −0.24 (−1.25, 1.12) | |
| DCL | 0.40 (−1.21, 2.00) | — | |
Best-fit models were built using different subsets of variables. Model 1 was fit on all possible covariates and predictors, Model 2 was fit on covariates and SOT, and Model 3 was fit on all covariates and LOS. Variables not included by least angle regression in the best-fit models are marked.
Bold values indicate equivalence to a p-value.
DHI, Dizziness Handicap Inventory; SOT, Sensory Organization Test; LOS, Limits of Stability; CI, confidence interval; BDI, Beck Depression Inventory; HI, head injuries; SOM, Somatosensory Score; VIS, Visual Score; VEST, Vestibular Score; PREF, Preference Score; Comp, Equilibrium Composite Score; RT, Reaction Time Composite Score; MVL, Movement Velocity Composite Score; EPE, End-Point Excursion Composite Score; MXE, Maximum Excursion Composite Score; DCL, directional control.
To identify which DHI items were more frequently associated with abnormal SOT and LOS findings, a map was created showing the overlapping counts between reported difficulties on DHI items and ABNL balance scores (Table 4). Each DHI item response was categorized as “deficit endorsed” (“Yes” response) or “deficit not endorsed” (“Sometimes” or “No”). For every DHI-balance combination in which a participant was categorized as both DHI “deficit endorsed” and SOT or LOS “abnormal,” one match was counted. These matches were summed cumulatively in a numerator, taken into the total number of “abnormal” counts per balance measure in the denominator, expressed via the percentages displayed. For example, the upper-leftmost cell would be described as: “Of the 14 participants with an abnormal Composite SOT score, 21.4% endorsed deficits when looking up.”
Table 4.
Percentage of Participants Whose Balance Parameter Normal/Abnormal (NL/ABNL) Categorizations Mapped onto DHI Responses
| SOTC | SOM | VIS | VEST | PREF | RT | MVL | EPE | MXE | DCL | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total count ABNL | 14 | 6 | 6 | 16 | 2 | 18 | 11 | 23 | 28 | 2 | Ave | ||
| P1 | Looking up | 16 | 21.4% | 50.0% | 50.0% | 18.8% | 0.0% | 27.8% | 27.3% | 43.5% | 32.1% | 0.0% | 27.1% |
| E2 | Feeling frustrated | 13 | 21.4% | 50.0% | 50.0% | 18.8% | 0.0% | 27.8% | 27.3% | 34.8% | 28.6% | 0.0% | 25.9% |
| F3 | Travel for business or recreation | 8 | 7.1% | 33.3% | 0.0% | 6.3% | 0.0% | 22.2% | 18.2% | 26.1% | 21.4% | 0.0% | 13.5% |
| P4 | Supermarket aisle | 3 | 0.0% | 16.7% | 0.0% | 0.0% | 0.0% | 11.1% | 9.1% | 13.0% | 10.7% | 0.0% | 6.1% |
| F5 | Getting into or out of bed | 8 | 14.3% | 50.0% | 16.7% | 12.5% | 0.0% | 16.7% | 18.2% | 26.1% | 21.4% | 0.0% | 17.6% |
| F6 | Social activities | 6 | 7.1% | 33.3% | 16.7% | 6.3% | 0.0% | 16.7% | 9.1% | 21.7% | 17.9% | 0.0% | 12.9% |
| F7 | Reading | 9 | 14.3% | 33.3% | 16.7% | 12.5% | 0.0% | 16.7% | 9.1% | 34.8% | 28.6% | 0.0% | 16.6% |
| P8 | Ambitious activities | 14 | 21.4% | 50.0% | 33.3% | 18.8% | 0.0% | 33.3% | 36.4% | 43.5% | 35.7% | 0.0% | 27.2% |
| E9 | Afraid to leave home alone | 5 | 14.3% | 33.3% | 16.7% | 12.5% | 0.0% | 16.7% | 9.1% | 17.4% | 14.3% | 0.0% | 13.4% |
| E10 | Embarrassed in public | 6 | 7.1% | 33.3% | 16.7% | 6.3% | 0.0% | 16.7% | 18.2% | 21.7% | 17.9% | 0.0% | 13.8% |
| P11 | Quick head movements | 16 | 28.6% | 66.7% | 50.0% | 25.0% | 0.0% | 33.3% | 36.4% | 43.5% | 35.7% | 0.0% | 31.9% |
| F12 | Avoiding heights | 9 | 21.4% | 66.7% | 33.3% | 18.8% | 0.0% | 22.2% | 18.2% | 30.4% | 25.0% | 0.0% | 23.6% |
| P13 | Turning over in bed | 8 | 14.3% | 33.3% | 16.7% | 12.5% | 0.0% | 16.7% | 18.2% | 26.1% | 17.9% | 0.0% | 15.6% |
| F14 | Strenuous house/yardwork | 11 | 7.1% | 50.0% | 0.0% | 0.0% | 0.0% | 16.7% | 18.2% | 26.1% | 10.7% | 0.0% | 12.9% |
| E15 | Avoiding driving in daytime | 7 | 7.1% | 33.3% | 0.0% | 6.3% | 0.0% | 22.2% | 18.2% | 21.7% | 17.9% | 0.0% | 12.7% |
| F16 | Appearing intoxicated | 6 | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 11.1% | 9.1% | 26.1% | 21.4% | 0.0% | 6.8% |
| P17 | Walking alone | 5 | 7.1% | 16.7% | 0.0% | 6.3% | 0.0% | 11.1% | 9.1% | 13.0% | 10.7% | 0.0% | 7.4% |
| E18 | Sidewalk | 5 | 7.1% | 33.3% | 0.0% | 6.3% | 0.0% | 11.1% | 18.2% | 21.7% | 17.9% | 0.0% | 11.6% |
| F19 | Concentrating | 10 | 7.1% | 33.3% | 0.0% | 6.3% | 0.0% | 22.2% | 18.2% | 21.7% | 17.9% | 0.0% | 12.7% |
| E20 | Walking around in dark | 10 | 14.3% | 50.0% | 16.7% | 12.5% | 0.0% | 22.2% | 36.4% | 34.8% | 28.6% | 0.0% | 21.5% |
| E21 | Afraid to stay home alone | 1 | 7.1% | 16.7% | 0.0% | 6.3% | 0.0% | 5.6% | 9.1% | 4.3% | 3.6% | 0.0% | 5.3% |
| E22 | Feeling handicapped | 4 | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 11.1% | 0.0% | 13.0% | 10.7% | 0.0% | 3.5% |
| E23 | Avoiding driving in dark | 7 | 7.1% | 33.3% | 0.0% | 6.3% | 0.0% | 22.2% | 18.2% | 21.7% | 17.9% | 0.0% | 12.7% |
| F24 | Stress on relationships | 4 | 0.0% | 16.7% | 0.0% | 0.0% | 0.0% | 11.1% | 9.1% | 17.4% | 14.3% | 0.0% | 6.9% |
| P25 | Being depressed | 5 | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 11.1% | 0.0% | 17.4% | 14.3% | 0.0% | 4.3% |
| Ave | 10.3% | 33.3% | 13.3% | 8.8% | 0.0% | 18.2% | 16.7% | 24.9% | 19.7% | 0.0% |
Each cell's percentage demonstrates “of this balance parameter's total ABNL participant count, __% also endorsed a deficit on this DHI item.“
DHI, Dizziness Handicap Inventory; SOTC, Sensory Organization Test Equilibrium Composite Score; SOM, Somatosensory Score; VIS, Visual Score; VEST, Vestibular Score; PREF, Preference Score; RT, Reaction Time Composite Score on the Limits of Stability Test; MVL, Movement Velocity Composite Score on the Limits of Stability Test; EPE, End-Point Excursion Composite Score on the Limits of Stability Test; MXE, Maximum Excursion Composite Score on the Limits of Stability Test; DCL, directional control; Ave, average.
The five activities most frequently checked off as being problematic, in order of frequency, were: looking up, performing quick head movements and ambitious such as sports or dancing activities, feeling frustrated, and performing strenuous house/yard work (Table 4). Also, when observing the shading patterns in Table 4, more diffuse and widespread shading can be seen for the LOS (right) than for the SOT (left) variables. Finally, although more matches were observed for the SOM variable, only six individuals scored abnormally (top row showing total count ABNL) in comparison with the larger numbers of participants with LOS abnormalities.
Discussion
This study identified deficits in the LOS and SOT tests among our cohort, who were predominantly classified with mild to moderate TBI, and were found to be at chronic stages post-injury. Sensory integration deficits included abnormal use of vestibular and somatosensory information for postural control as well as inadequate visual preference (Table 2). The most common sensory deficit was vestibular, with 36% scoring in the abnormal range (Fig. 1). Surprisingly, in comparison with SOT, more participants were found to have LOS problems, which included a reduced cone of stability in more than half (LOS MXE 58%), increased reaction time (38%), and decreased movement velocity (23%). Some, but not all, deficits were shown to be significantly associated with self-reported balance problems listed in the DHI. Our regression analysis revealed more variables with the LOS test as important predictors of self-reported balance difficulties than with the SOT. Importantly these relationships between objective and subjective balance problems were significantly influenced by the number of brain injuries, use of psychotropic medication, time since injury, and depression severity among participants.
To our knowledge, this is the first study to report the predominance of abnormalities in the NeuroCom LOS test in subjects with mild to moderate TBI in comparison with the SOT. Our results on the SOT not only confirm previous reports of vestibular deficits,17,31 but also suggest potential somatosensory involvement post-TBI and abnormal visual preference (Table 2). More surprisingly, LOS deficits were more prevalent than sensory organization problems in our cohort (Fig. 1). Similar deficits in the LOS test have been reported in the elderly and in those with various neurological disorders;22–26 however, not in TBI. To our knowledge, the only investigation on LOS reported in TBI focused on reliability as opposed to the extent of deficits.33 Although the SOT has been traditionally recommended as a key objective balance assessment for TBI,14,15,36 our study suggests that LOS abnormalities may be even more common, and therefore warrants consideration of this test as an additional means of characterizing balance abnormalities in TBI.
This study also evaluated relationships between objective (SOT and LOS) and subjective (DHI) measurements. In regards to the SOT regression results (Model 2, Table 3), our findings differed from what we expected. Based on previous research in a group of 10 patients with TBI, which reported condition 6 of the SOT to be significantly associated with the physical component of their DHI,17 we expected vestibular integration to be a significant predictor in our analysis. However, the only significant SOT predictor we found was visual integration. It is possible that because our regression analysis included more subjects and controlled for more covariates than the previous investigation, our results differed in that particular aspect of the SOT. Nevertheless, our findings are in line with previous research by confirming an association between the SOT and DHI in patients with TBI.
Remarkably, when only LOS variables were included in the regression model and covariates were controlled for, all but one measure (DCL) were significant DHI predictors (Model 3, Tables 3 and 4). Although Models 2 and 3 reached similar shared variance (∼55%), more LOS variables predicted DHI, showing that this test is more broadly related to subjective symptoms than SOT, which showed only one predictive variable. The only comparable study we were able to find investigated associations between static (quiet stance) and dynamic balance tests (timed up and go, tandem gait, and the dynamic gait index) and DHI scores.37 Their findings showed even stronger associations between dynamic tests and DHI than between static tests and DHI in those with balance problems from multiple disorders including TBI.37 Our sample was restricted to TBI, and therefore provides higher generalizability to this specific disorder.
The problems most frequently mentioned by our participants were looking up, performing quick head movements and ambitious such as sports or dancing activities, feeling frustrated, and performing strenuous house/yard work (Table 4). Any activities that involve leaning, bending over, or reaching can become more difficult for those who have reduced limits of stability.13 Therefore, it is not surprising that the problems listed, in particular performing ambitious and strenuous activities, were found to be strongly related to deficits of timing and excursion in the LOS test. In the elderly, a reduced cone of stability has serious implications, because it has been linked to increased fall risk.24 In our study, with the exception of only one individual, falls were not reported as an issue, but our cohort did report difficulty with high level physical daily life activities and frustration related to their balance problems, which may have a negative impact on their quality of life. As shown by Maskell and coworkers,34 dizziness, along with anxiety and depression, commonly has a profound impact on quality of life. Therefore, our findings reinforce the need to take into account measures of emotional status when investigating subjective symptoms and their relationship with objective balance measures in TBI.
The clinical implications here are the recognition that even those with TBI who are quite functional and not falling may still experience problems with daily activities consequent to impaired balance, perhaps especially dynamic balance. Therefore, if individuals with TBI complain of balance-related problems, we recommend that they be evaluated not only with the SOT but also with the LOS test, and treated accordingly. Because we used strict selection criteria in this analysis, and excluded anyone with confounding factors, we believe that our results can be likely attributed to the TBI event itself and can be generalized to those with mild to moderate classifications.
One limitation of this study was missing data on some of the demographic information and some of the assessments. Nine individuals did not complete the DHI, data on the total number of brain injuries were not recorded for eight, and medication information was not recorded for six. Missing data are not unexpected in large trials because participants may freely choose not to complete questionnaires, and researchers may fail to collect certain measures because of time constraints, patient fatigue, or other scheduling issues. With the smaller sample size resulting from missing data, the best-fit LARS regression models may have included more predictors (overfit) than may have been included in a larger sample. The reason we included many covariates in our models was that they were all clinically important and relevant to our research question. as they have been frequently explored in the TBI literature. Further, we purposely selected the LARS methods combined with model selection by Mallow's Cp statistic over simpler methods of regression modeling, as LARS is known to be more resistant to biased estimates from partial correlations between predictors. Another limitation is that our data collection of repetitive brain injuries was subject to recall bias based on patient self-report of their past history, and may not be as reliable as tracking a brain injury prospectively with a validated instrument.
Conclusion
In conclusion, this study provides evidence that abnormal scores on the LOS may be more predominant than abnormalities on the SOT in chronic mild to moderate TBI. Additionally, the LOS seemed to be a better predictor of subjective symptoms in our cohort than the SOT. These findings support the recommendation of the LOS test as an additional assessment in the evaluation of individuals who report chronic balance difficulties after a mild to moderate TBI.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health (Protocol #10-CC-0118), and by the Center for Neuroscience and Regenerative Medicine (CNRM). We thank Andre van der Merwe, at the Center for Neuroscience and Regenerative Medicine, for coordinating this project.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Tuohimaa P. (1978). Vestibular disturbances after acute mild head injury. Acta Otolaryngol. Suppl. 359, 3–67 [PubMed] [Google Scholar]
- 2. Berman J.M., and Fredrickson J.M. (1978). Vertigo after head injury—a five year follow-up. J. Otolaryngol. 7, 237–245 [PubMed] [Google Scholar]
- 3. Binder L.M. (1997). A review of mild head trauma. Part II: clinical implications. J. Clin. Exp. Neuropsychol. 19, 432–457 [DOI] [PubMed] [Google Scholar]
- 4. Cicerone K.D., and Kalmar K. (1995). Persistent postconcussion syndrome: the structure of subjective complaints after mild traumatic brain injury. J. Head Trauma Rehabil. 10, 1–17 [Google Scholar]
- 5. Alsalaheen B.A., Mucha A., Morris L.O., Whitney S.L., Furman J.M., Camiolo-Reddy C., Collins M., Lovell M., and Sparto P. (2010). Vestibular rehabilitation for dizziness and balance disorders after concussion. J. Neurol. Phys. Ther. 34, 87–93 [DOI] [PubMed] [Google Scholar]
- 6. Gurr B., and Moffat N. (2001). Psychological consequences of vertigo and the effectiveness of vestibular rehabilitation for brain injury patients. Brain Inj. 15,387–400 [DOI] [PubMed] [Google Scholar]
- 7. Umphred D.A. (2000). Neurological Rehabilitation. Mosby: St. Louis [Google Scholar]
- 8. Fife T.D., and Giza C. (2013). Posttraumatic vertigo and dizziness. Semin. Neurol. 33,238–243 [DOI] [PubMed] [Google Scholar]
- 9. Gurley J.M., Hujsak B.D., and Kelly J.L. (2013). Vestibular rehabilitation following mild traumatic brain injury. NeuroRehabil. 32, 519–528 [DOI] [PubMed] [Google Scholar]
- 10. Kleffelgaard I., Soberg H.L., Bruusgaard K.A., Tamber A.L., and Langhammer B. (2016). Vestibular rehabilitation after traumatic brain injury: case series. Phys. Ther. 96,1–6 [DOI] [PubMed] [Google Scholar]
- 11. Herdman S.J., and Clendaniel R.A., (2014). Vestibular Rehabilitation. Davis Company: Philadelphia [Google Scholar]
- 12. Kolev O.I., and Sergeeva M. (2016). Vestibular disorders following different types of head and neck trauma. Funct. Neurol. 31(2),75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobson GP, Newman CW, and Kartush JM. (1977). Handbook of Balance Function Testing. Singular Publishing Group: San Diego [Google Scholar]
- 14. Guskiewicz K.M. (2001). Postural stability assessment following concussion: one piece of the puzzle. Clin. J. Sport Med. 11,182–189 [DOI] [PubMed] [Google Scholar]
- 15. Pickett T.C., Radfar-Baublitz L.S., McDonald S.D., Walker W.C., and Cifu D.X. (2007). Objectively assessing balance deficits after TBI: role of computerized posturography. J. Rehabil. Res. Dev. 44,983–990 [DOI] [PubMed] [Google Scholar]
- 16. Scherer M.R., Burrows H., Pinto R., Littlefield P., French L.M., Tarbett A.K., and Schubert M.C.(2011). Evidence of central and peripheral vestibular pathology in blast-related traumatic brain injury. Otol. Neurotol. 32,571–80 [DOI] [PubMed] [Google Scholar]
- 17. Kaufman K.R., Brey R.H., Chou L.S., Rabatin A., Brown A.W., and Basford J.R. (2006). Comparison of subjective and objective measurements of balance disorders following traumatic brain injury. Med. Eng. Phys. 28,234–239 [DOI] [PubMed] [Google Scholar]
- 18. Horak F.B., Wrisley D.M., and Frank J. (2009). The balance evaluation systems test (BESTest) to differentiate balance deficits. Phys. Ther. 89, 484–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mancini M., and Horak F.B. (2010).The relevance of clinical balance assessment tools to differentiate balance deficits. Eur. J. Phys. Rehabil. Med. 46, 239–248 [PMC free article] [PubMed] [Google Scholar]
- 20. Shumway-Cook A., and Olmscheid R. (1990). A systems analysis of postural dyscontrol in traumatically brain-injured patients. J. Head Trauma Rehabil. 5, 51–62 [Google Scholar]
- 21. Faraldo-Garcia A., Santos-Perez S., Rossi-Izquierdo M., Lirola-Delgado A., Vaamonde-Sanchez-Andrade I., Del-Rio-Valeiras M., and Soto-Varela A. (2016). Posturographic limits of stability can predict the increased risk of falls in elderly patients with instability? Acta Otol. 136,1125–1129 [DOI] [PubMed] [Google Scholar]
- 22. Jessop R.T., Horowicz C., and Dibble L.E. (2006). Motor learning and Parkinson disease: Refinement of movement velocity and endpoint excursion in a limits of stability balance task. Neurorehabil. Neural Repair 20,459–467 [DOI] [PubMed] [Google Scholar]
- 23. Clark S., and Rose D.J. (2001). Evaluation of dynamic balance among community-dwelling older adult fallers: a generalizability study of the limits of stability test. Arch. Phys. Med. Rehabil. 82,468–474 [DOI] [PubMed] [Google Scholar]
- 24. Melzer I., Benjuya N., and Kaplanski J. (2004). Postural stability in the elderly: a comparison between fallers and non-fallers. Age Ageing 33,602–607 [DOI] [PubMed] [Google Scholar]
- 25. Chien C.W., Hu M.H., Tang P.F., Sheu C.F., and Hsieh C.L. (2007). A comparison of psychometric properties of the smart balance master system and the postural assessment scale for stroke in people who have had mild stroke. Arch. Phys. Med. Rehabil 88,374–380 [DOI] [PubMed] [Google Scholar]
- 26. Ganesan M., Pasha S.A., Pal P.K., Yadav R., and Gupta A. (2012). Direction specific preserved limits of stability in early progressive supranuclear palsy: a dynamic posturographic study. Gait. Pos. 35(4),625–629 [DOI] [PubMed] [Google Scholar]
- 27. Jacobson G.P., and Newman C.W. (1990). The development of the dizziness handicap inventory. Arch. Otolaryngol. Head Neck Surg. 116, 424–427 [DOI] [PubMed] [Google Scholar]
- 28. The Management of Concussion/mTBI Working Group. (2009) VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. J. Rehabil. Res. Dev. 46, CP1–68 [PubMed] [Google Scholar]
- 29. Faraldo-Garcia A., Santos-Perez S., Crujeiras R., and Soto-Varela A. (2016). Postural changes associated with ageing on the sensory organization test and the limits of stability in healthy subjects. Auris. Nasus. Larynx 43,149–154 [DOI] [PubMed] [Google Scholar]
- 30. Newton R. (1989). Review of tests of standing balance abilities. Brain Inj. 3,335–343 [DOI] [PubMed] [Google Scholar]
- 31. Basford J.R., Chou L.S., Kaufman K.R., Brey R.H., Walker A., Malec J.F., Moessner A.M., and Brown A.W. (2003). An assessment of gait and balance deficits after traumatic brain injury. Arch. Phys. Med. Rehabil. 84,343–349 [DOI] [PubMed] [Google Scholar]
- 32. Gottshall K., Drake A., Gray N., McDonald E., and Hoffer M.E. (2003). Objective vestibular tests as outcome measures in head injury patients. Laryngoscope 113,1746–1750 [DOI] [PubMed] [Google Scholar]
- 33. Newstead A.H., Hinman M.R., and Tomberlin J.A. (2005). Reliability of the Berg Balance Scale and balance master limits of stability tests for individuals with brain injury. J. Neurol. Phys. Ther. 29,18–23 [DOI] [PubMed] [Google Scholar]
- 34. Maskell F., Chiarelli P., and Isles R. (2006). Dizziness after traumatic brain injury: overview and measurement in the clinical setting. Brain Inj. 20, 293–305 [DOI] [PubMed] [Google Scholar]
- 35. Efron B., Hastie T., Johnstone I., and Tibshirani R. (2004). Least angle regression. Ann. Stat. 32,407–499 [Google Scholar]
- 36. Academy of Neurologic Physical Therapy and APTA Task Force on TBI. TBI EDGE outcome measures for research (2013). http://www.neuropt.org/professional-resources/neurology-section-outcome-measures-recommendations/traumatic-brain-injury (Last accessed September7, 2017)
- 37. Vereeck L., Truijen S., Wuyts F.L., Van de Heyning P.H. (2007). The dizziness handicap inventory and its relationship with functional balance performance. Otol. Neurotol. 28,87–93 [DOI] [PubMed] [Google Scholar]