Abstract
Objectives: The purpose of this pilot study was to evaluate changes in adiposity, carotid intima media thickness (CIMT), and hepatic fat content measured via magnetic resonance imaging-estimated hepatic proton density fat fraction (PDFF) in antipsychotic (AP)-treated youth versus nonpsychiatric (NP) participants during participation in a 16-week behavioral weight loss (BWL) intervention.
Subjects/Methods: Overweight/obese AP-treated youth (n = 26) were randomized 2:1 to weekly treatment versus recommended care (RC) over 16 weeks. NP controls (n = 21) were assigned to weekly treatment. Dual-energy X-ray absorptiometry (DEXA)-measured adiposity, CIMT, and PDFF were measured at baseline and 16 weeks. Analyses assessed group differences in the effect of BWL on adiposity, CIMT, and PDFF.
Results: BWL was well tolerated in both AP-treated and NP groups. DEXA-measured fat decreased significantly in the NP group (F[1,16] = 11.81, p = 0.003), with modest improvements in adiposity and hepatic fat in the AP-treated group, while an increase in adiposity was observed in the RC group. Significant differences in endpoint DEXA total fat (F[2,34] = 4.81, p = 0.01) and PDFF (F[2,30] = 3.60, p = 0.04) occurred across treatment groups, explained by larger improvements in NP versus RC youth in DEXA total fat (p = 0.03) and PDFF (p = 0.04).
Conclusions: Intensive, family-based BWL treatment can improve whole-body adiposity and liver fat in obese youth, with decreases or attenuation of additional fat gain observed in AP-treated youth.
Keywords: obesity, antipsychotic, weight loss, child psychiatry
Introduction
Persons with major mental illnesses, including childhood-onset illnesses such as attention-deficit/hyperactivity disorder (ADHD), lose a mean of 15–20 years of potential life expectancy compared with the general population, primarily due to chronic obesity-related conditions (Colton and Manderscheid 2006; Nordentoft et al. 2013). Populations at increased risk for developing obesity include children and adolescents with common conditions such as ADHD and autism spectrum disorders (ASDs), where rates of overweight and obesity are higher than in the general population (Hill et al. 2015; Cortese et al. 2016).
Childhood- versus adult-onset obesity-related conditions in general lead to early onset of costly medical conditions such as cardiovascular disease (Morrison et al. 2007). Indeed, health care utilization in adults with ASD and ADHD is higher than in the general population, primarily related to treatment for chronic obesity-related illnesses such as diabetes and hyperlipidemia (Birnbaum et al. 2005; Ousseny et al. 2018). Thus, obesity prevention and treatment in this population are critical to preserving longevity, as well as for health care cost-containment.
Higher chronic disease burden in mentally ill individuals is multifactorial, associated with patient-, provider-, and system-level variables (Walker et al. 2015). As public health interest in managing cardiometabolic risk in this population grows, attention has begun to shift toward prevention in the particularly high-risk population of children with mental health conditions who receive early exposure to obesogenic psychotropic drugs, such as antipsychotic medications. In the early 1990s–2000s, a significant increase in the rate of antipsychotic prescriptions in youth was observed (Olfson et al. 2006), primarily for off-label treatment of irritability and aggression associated with disruptive behavior disorders (DBDs) or ADHD (Connor et al. 2006; Crystal et al. 2009; Pathak et al. 2010).
While antipsychotic treatment can significantly improve irritability and aggression in autism and other pediatric-onset disorders, treatment-related effects on adiposity, insulin sensitivity, and lipid profiles have been described (Calarge et al. 2009; Correll et al. 2009) and associated with adverse cardiometabolic outcomes (McIntyre and Jerrell 2008; Morrato et al. 2010). In previously antipsychotic naive children assigned to 12 weeks of randomized antipsychotic treatment, gold standard measures have been used to identify rapid-onset adverse treatment effects on whole-body and abdominal adiposity as well as tissue-specific insulin sensitivity (Nicol et al. 2018). In that study, combined rates of overweight and obesity rose from a general population norm of ∼30% to 47% in just 12 weeks.
Although differential weight gain effects among atypical agents have been observed (Newcomer 2007; Yoon et al. 2016), this difference does not appear to have a significant effect when compared across treatment studies—particularly in antipsychotic naive younger patients (Zhang et al. 2016). Although a small amount of metabolic effect may be weight independent (Birkenaes et al. 2008), the level of adiposity achieved during antipsychotic treatment, rather than the antipsychotic treatment itself, appears to be prominently correlated with direct measures of metabolic risk such as intrahepatic fat content (Nicol et al. 2015). Thus, addressing excess adiposity in this population is a primary risk prevention target.
Behavioral weight loss (BWL) interventions have been successfully used in adult mentally ill populations (Daumit et al. 2013; Erickson et al. 2016), and are considered first-line treatment for pediatric obesity (Quattrin and Wilfley 2017). However, unadapted BWL interventions may have attenuated efficacy in psychiatrically ill youth (Detke et al. 2016; Nicol et al. 2016b). Intensive family-based weight management interventions have received significant study in both pediatric obesity (Wilfley et al. 2007, 2017) and eating disorders (Le Grange et al. 2016), but have received no study in antipsychotic (AP)-treated youth.
We therefore aimed to pilot-test a family-based BWL intervention—initially developed for use in nonpsychiatric (NP) youth (Wilfley et al. 2007)—in mentally ill AP-treated youth, using validated biomarkers of cardiometabolic disease risk, including dual-energy X-ray absorptiometry (DEXA)-measured body fat, hepatic triglyceride content using magnetic resonance (MR) imaging-estimated proton density fat fraction (PDFF), and carotid intima media thickness (CIMT), evaluated as an early marker of cardiovascular disease risk in obese adolescents (Oren et al. 2003) and in youth with high-risk conditions such as hypertension (Baroncini et al. 2017). We hypothesized beneficial treatment-related changes in adiposity and other biomarkers of risk with BWL treatment compared with a recommended care (RC) health education control condition.
Materials/Subjects and Methods
Youth with overweight and obesity (body mass index [BMI] percentile of 85–94 and ≥95, respectively), ages 6–18, were assigned to interventions as follows: AP-treated youth were randomized 2:1 to weekly 16-week BWL or to the RC control condition consisting of 4 once-per-month health education sessions: NP participants received the 16-week BWL. AP-treated participants had documented clinically significant weight gain (e.g., weight gain over at least 6 months of antipsychotic treatment that exceeds the expected 5–6 lbs typically experienced in 1 year during peak adolescent growth) (Tanner 1989; Rogol et al. 2000), and documented psychiatric stability before enrollment. Active suicidality and estimated or documented intelligent quotient <70, treatment with weight loss agents [other than stable, concurrent stimulant medication at <1 mg/(kg·d) equivalent of methylphenidate], or prior diagnosis of an eating disorder was exclusionary.
Overweight or obese NP youth were recruited from primary care offices in the area. Eligibility based on height, weight, and BMI percentile was confirmed with medical records. NP participants could not be taking psychotropic medications or medications associated with weight loss. An adult guardian/primary caregiver provided written consent, and participants provided written assent for study participation. This study was conducted between July 2011 and March 2017 at Washington University School of Medicine in St. Louis, MO. The Washington University Institutional Review Board approved this study.
Study assessments
Consensus diagnoses were determined by review of medical records and the semistructured Mini International Neuropsychiatric Interview for Children (MINI-Kid). The Aberrant Behavior Checklist (ABC) (Aman and Singh 1994) was used to assess irritability and aggression, symptoms commonly treated with antipsychotic medications.
DEXA-measured whole-body total (kg) and percent fat mass, as well as whole-body lean (kg) mass, was measured with a Delphi W densitometer (Hologic, Waltham, MA). PDFF was determined by proton MR spectroscopy using a 1.5T scanner (MAGNETOM Sonata; Siemens, Erlangen, Germany). CIMT was measured by 9- to 13-MHz B-mode carotid artery ultrasonography (Sequoia; Acuson-Siemens, Mountain View, CA). A single sonographer acquired B-mode images of carotid arteries in the longitudinal axis bilaterally. A 1 cm region of the common carotid artery proximal to the bifurcation was identified and exported for off-line analysis with ProSolv software (FUJIFILM Medical Systems USA, Stamford, CT). CIMT measures were obtained by taking the average of far-wall intima media thickness from both left and right common carotid arteries. Each site represents the average of three measures of the maximum CIMT, obtained by two separate blinded raters. The interobserver intraclass correlation coefficient was 0.70.
Height and weight at baseline and 16 weeks used in primary analyses were performed on a calibrated, wall-mounted stadiometer (Seca® 240) and scale (Seca 684), respectively, by a trained research nurse. Fasting plasma lipids (total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride) and glucose were also obtained at baseline and 16 weeks. Weekly treatment weights were measured on a digital office-based scale (Health o meter; Sunbeam Products, Inc., Boca Raton, FL, 2010) to guide content of treatment sessions and to track progress.
Physical activity was estimated using parent or adult caregiver report of metabolic equivalent of task minutes per week using the International Physical Activity Questionnaire (IPAQ) (Hagstromer et al. 2008) at baseline and 16 weeks. Fidelity to BWL and RC treatment protocols was assessed via weekly interventionist self-report, using a measure developed for other pediatric BWL studies conducted by our group (TODAY Study Group 2010). Fidelity was also monitored in weekly team supervision sessions lead by the principal investigator (PI), incorporating analysis of video recorded treatment sessions. Treatment adherence was measured by in-person session attendance and interventionist assessment of homework completion and quality by a scale used in previous weight loss studies (TODAY Study Group 2010). See Supplementary Data for additional details on study assessments and procedures.
Randomization and treatment description
Randomization was generated in blocks of 10 and implemented by the study coordinator, with open-label treatment assignment. Group composition was monitored for gender, ethnicity, race, and age.
Family-based social facilitation treatment is a previously established BWL based on the Traffic Light Plan (Epstein and Squires 1988), adjusted to a 16-week duration for use in this study. The Stoplight Plan classifies both nutritional values of foods and physical activity intensity as RED for “stop and think” to indicate the least healthy options, YELLOW for “caution” to indicate moderately healthy options, and GREEN for “go” to indicate the healthiest options. Parents or adult guardians were required to attend sessions, ensure study-related homework was completed, and promote health behavior changes in the home, but were not considered study participants.
The target weight loss goal range is 0.5–2 lbs/week, in line with evidence-based childhood obesity treatment (Epstein et al. 2004) and recommendations for healthy child development and weight management (Barlow 2007; Rao 2008). Expected annual weight gain during normal growth and development varies based on age and gender, and can range from 2 to 3 lbs in pre- and postpubertal youth to 8–12 lbs/year during prepubertal and pubertal growth stages, with weight gain higher in prepubertal females than in males, and higher weight gain in males during puberty (Tanner 1989; Rogol et al. 2000). For individuals who are already high weight, the range of 0.5–2 lbs/week is a safe weight loss range for all ages and stages of growth.
Core components of effective BWL strategies were incorporated within a socioecological framework (Wilfley et al. 2007), along with other behaviors relevant to weight management (e.g., self-monitoring of activity and food intake, sleep) (Nicol et al. 2016b). Participants met with interventionists weekly to review goals and homework, to problem solve and plan. Weights were measured at each in-person session on an office-based scale so that participants could track progress. While treatment adaptation was not the goal of the present study (e.g., we did not aim to change the treatment content or treatment targets), some simple modifications were made to the method of treatment delivery to decrease cognitive load for participants.
Self-monitoring was simplified to reduce cognitive load by using a log with visual cues rather than written lists. Weekly eating and activity goals were adjusted to meet families at their current level of ability and readiness for change, with flexibility to repeat concepts not mastered from previous sessions if needed. A weekly “taste log” was used to encourage systematic exposures to new “Green” foods and reduce selectivity for “Red” foods. Adult caregivers were required to supervise completion of treatment-related homework and weekly parenting goals were set to facilitate uptake of new health behaviors. Caregivers were encouraged to adopt health behaviors identified in treatment sessions, but adult weight loss was not required or specifically tracked.
The RC condition consisted of monthly visits with a study interventionist, and included education about energy balance using the Stoplight Plan described above. Each monthly visit consisted of a weigh-in and check-in regarding successes and challenges experienced in the prior month regarding efforts to decrease caloric intake, increase fruit and vegetable consumption, and increase healthy physical activity, followed by identification of target areas for problem-solving. Self-monitoring and specific goal setting were not part of the control condition.
Adverse event monitoring
All participants provided consent to share medical information with their primary care providers (PCPs) and mental health providers (when applicable). All PCPs provided written clearance for their patients to participate in the intervention. Monitoring for development of adverse events, including excessive weight loss, development of acute suicidality, or exacerbation of psychiatric symptoms, was conducted at each study visit and reported to the patient's PCP or mental health provider to determine the appropriateness of ongoing study participation on a case-by-case basis. Laboratory, imaging, and anthropomorphic study assessments conducted at baseline and 16 weeks were reviewed for abnormal results, which were reported to the participant's PCP for additional follow-up.
Analytic strategy
In the present study, we aimed to evaluate DEXA-measured total fat, CIMT, and percent PDFF following 16 weeks of weekly, family-based, weight loss treatment or monthly health education in AP-treated youth, compared with NP youth also undergoing weekly, family-based, weight loss treatment. Primary analyses for each outcome were conducted using a modified intent-to-treat analysis (IBM SPSS, Armonk, NY), including participants with baseline and any subsequent measurements. Means and standard deviations (SDs) were used to describe the center and spread of all variables. The primary analytic model used analysis of covariance to test the main effect of treatment group (independent, fixed three-level factor) on week 16 values of each primary and secondary outcome (dependent variables), using each outcome's baseline value as the covariate term. Gender, age (≤12 years), and stimulant treatment (Y/N) were individually introduced into the models to determine any potential impact each variable had on the primary finding, if any. In cases where a significant main effect of treatment group was observed, post hoc pairwise comparisons were performed to evaluate differences in the endpoint variable after adjusting for baseline. Exploratory analyses applied a marginal model with an unstructured variance/covariance matrix to evaluate for main effects of treatment fidelity and adherence. Using a marginal model, we evaluated the effects of time and treatment group on fidelity and adherence separately. We then evaluated the main effect of time on change in DEXA fat, with fidelity and adherence inserted separately as covariates. This final model was run with and without treatment group inserted as a factor.
Results
Participant characteristics
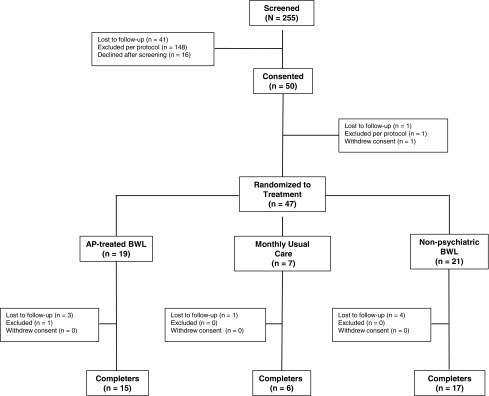
Participant disposition is detailed in Figure 1. Fifty participants were consented and enrolled in the study, with 47 receiving baseline evaluations. The mean age in the pooled sample was 13.3 years (SD = 2.43). The ratio of male:female participants was higher in the AP-treated groups, reflecting the higher prevalence of ASD, ADHD, and DBDs in boys (Merikangas et al. 2010). Groups were otherwise balanced on baseline characteristics (Table 1).
FIG. 1.
Participant disposition.
Table 1.
Baseline Participant Characteristics
| AP-treated (n = 19) | RC (n = 7) | NP (n = 21) | |
|---|---|---|---|
| Study population demographics | |||
| Age (in years), mean (SD) | 13.35 (2.57) | 12.80 (2.21) | 13.42 (2.46) |
| 0–11 Years of age, n (%) | 8 (42.11) | 2 (28.57) | 6 (28.57) |
| 12+ Years of age, n (%) | 11 (57.89) | 5 (71.43) | 15 (71.43) |
| Female, n (%) | 5 (26.32) | 1 (14.29) | 15 (71.43) |
| Nonwhite, n (%) | 4 (21.05) | 0 (100.00) | 9 (42.86) |
| Concurrent stimulant use, n (%) | 6 (31.58) | 3 (42.86) | 0 (0.00) |
| Primary outcomes, mean (SD) | |||
| DEXA total fat (kg) | 32.40 (17.68) | 31.94 (6.16) | 35.97 (11.67) |
| PDFF (%) | 6.52 (8.21) | 12.01 (7.82) | 4.72 (8.61) |
| CIMT (cm) | 0.0451 (0.0074) | 0.0494 (0.0058) | 0.0504 (0.0065) |
| Psychiatric diagnoses, n (%) | |||
| Neurodevelopmental disordersa | |||
| Primary | 11 (57.89) | 5 (71.43) | — |
| Secondary | 1 (5.26) | 1 (14.29) | — |
| Tertiary | 3 (15.79) | 0 (0.00) | — |
| ADHD | |||
| Primary | 4 (21.05) | 1 (14.29) | — |
| Secondary | 10 (52.63) | 5 (71.43) | — |
| Tertiary | 1 (5.26) | 0 (0.00) | — |
| Mood disorderb | |||
| Primary | 2 (10.53) | 1 (14.29) | — |
| Secondary | 4 (21.05) | 0 (0.00) | — |
| Tertiary | 3 (15.79) | 0 (0.00) | — |
| Disruptive behavior disorderc | |||
| Primary | 0 (0.00) | 0 (0.00) | — |
| Secondary | 0 (0.00) | 0 (0.00) | — |
| Tertiary | 1 (5.26) | 2 (28.57) | — |
| PTSD | |||
| Primary | 0 (0.00) | 0 (0.00) | — |
| Secondary | 2 (10.53) | 0 (0.00) | — |
| Tertiary | 0 (0.00) | 0 (0.00) | — |
| Anxiety disorderd | |||
| Primary | 1 (5.26) | 0 (0.00) | — |
| Secondary | 0 (0.00) | 1 (14.29) | — |
| Tertiary | 2 (10.53) | 2 (28.57) | — |
| Tourette's disorder | |||
| Primary | 0 (0.00) | 0 (0.00) | — |
| Secondary | 0 (0.00) | 0 (0.00) | — |
| Tertiary | 0 (0.00) | 1 (14.29) | — |
| OCD | |||
| Primary | 0 (0.00) | 0 (0.00) | — |
| Secondary | 0 (0.00) | 0 (0.00) | — |
| Tertiary | 1 (5.26) | 0 (0.00) | — |
| Psychotic disordere | |||
| Primary | 1 (5.26) | 0 (0.00) | — |
| Secondary | 2 (10.53) | 0 (0.00) | — |
| Tertiary | 0 (0.00) | 0 (0.00) | — |
Consists of autism spectrum disorder, learning disabilities, and intellectual disabilities.
Consists of major depressive disorder and bipolar disorder.
Consists of conduct disorder and oppositional defiant disorder.
Consists of generalized anxiety disorder, specific phobias, and panic disorder.
Consists of schizophrenia and schizophreniform disorder.
ADHD, attention-deficit/hyperactivity disorder; AP-treated, antipsychotic-treated youth undergoing weekly weight loss treatment; CIMT, carotid intima media thickness; DEXA, dual-energy X-ray absorptiometry; NP, nonpsychiatric youth undergoing weekly weight loss treatment; OCD, obsessive compulsive disorder; PDFF, proton density fat fraction; PTSD, posttraumatic stress disorder; RC, antipsychotic-treated youth undergoing monthly recommended care; SD, standard deviation.
AP-treated groups were diagnostically heterogeneous, with the mean number of psychiatric diagnoses being 2.65 (0.56). The majority of the AP-treated population had a primary or secondary diagnosis of an ASD (18), with ADHD being the second most common primary or secondary diagnosis (19). Three participants met the criteria for DBD diagnoses, including oppositional defiant disorder (ODD, 2) and conduct disorder (CD, 1; Supplementary Fig. S1).
AP treatment varied but was inclusive of commonly used antipsychotic agents, and doses were consistent with typical pediatric dosing: aripiprazole (n = 12, mean dose 9 ± 6.4 mg); lurasidone (n = 1, 40 mg); olanzapine (n = 2, 12.5 ± 10.6 mg); paliperidone (n = 1, 9 mg); quetiapine (n = 4, 225 ± 132.2 mg); risperidone (n = 4, 1.8 ± 0.9 mg); ziprasidone (n = 4, 5544.3 mg).
Session attendance was similar across treatment groups; the AP-BWL group attended 10.26 (5.56) of 16 sessions (64%), the NP group attended 11.91 (5.11) of 16 sessions (74%), and the monthly RC group attended 3.14 (1.46) of 4 sessions (79%; F[2,44] = 0.92, p = 0.41).
Primary and secondary outcomes
Whole-body adiposity
The mean change in DEXA-measured total fat by treatment group can be seen in Table 1. Significant differences in endpoint DEXA total fat, controlling for baseline (F[2,34] = 4.81, p = 0.01), occurred across groups, explained by larger improvements observed in NP versus RC (p = 0.03). Significant reductions from baseline in DEXA total fat were observed for the NP group only (F[1,16] = 11.81, p = 0.003). By contrast, BMI percentile, a clinical surrogate for body composition, decreased in all three treatment groups: −1.38 (2.09) in the NP group, −1.92 (3.45) in the AP group, and −0.32 (0.70) in the RC group (details are included in Supplementary Tables S1 and S2).
Hepatic fat content
Significant differences in endpoint PDFF values were observed between groups (F[2,30] = 3.60, p = 0.04), explained by larger beneficial changes in PDFF in NP-BWL versus usual care (UC) youth (p = 0.04) (Table 2). No significant decreases in PDFF from baseline were detected in any treatment group.
Table 2.
Change in Primary Outcome Measures by Treatment Group
| AP-treated | RC | NP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable (units) | Week 0 | Week 16 | Δ (SD), [CI] | Cohen's d | Week 0 | Week 16 | Δ (SD), [CI] | Cohen's d | Week 0 | Week 16 | Δ (SD), [CI] | Cohen's d | Main effect of treatment group |
| Primary outcome variables | |||||||||||||
| DEXA total fat (kg) | 33.37 (17.99) | 32.94 (19.99) | −0.43 (3.13) [−2.16 to 1.31] | −0.14 | 30.51 (5.33) | 31.27 (4.62) | 0.76 (2.05) [−1.39 to 2.91] | 0.37 | 37.76 (11.56) | 34.96 (11.84) | −2.79 (3.35) [−4.52 to −1.07] | −0.83 | F[2,34] = 4.81, p = 0.01a |
| PDFF (%) | 6.71 (8.97) | 6.30 (7.22) | −0.41 (4.95) [−3.55 to 2.73] | −0.08 | 13.26 (7.77) | 12.64 (7.52) | −0.62 (1.57) [−2.26 to 1.02] | −0.39 | 5.66 (9.44) | 3.91 (5.13) | −1.75 (4.93) [−4.38 to 0.87] | −0.35 | F[2,30] = 3.60, p = 0.04b |
| CIMT (cm) | 0.0439 (0.0075) | 0.0471 (0.0079) | 0.0032 (0.0098) [−0.0038 to 0.0101] | 0.33 | 0.0481 (0.0052) | 0.0467 (0.0054) | −0.0014 (0.0045) [−0.0061 to 0.0033] | −0.31 | 0.0497 (0.0070) | 0.0501 (0.0083) | 0.0004 (0.0064) [−0.0029 to 0.0037] | 0.06 | F[2,29] = 0.37, p = 0.70 |
p = 0.03, RC vs NP.
p = 0.04, RC vs NP.
AP-treated, antipsychotic-treated youth undergoing weekly weight loss treatment; CI, confidence interval; CIMT, carotid intima media thickness; DEXA, dual-energy X-ray absorptiometry; NP, nonpsychiatric youth undergoing weekly weight loss treatment; RC, antipsychotic-treated youth undergoing monthly recommended care; SD, standard deviation.
Carotid intima media thickness
No significant differences in endpoint CIMT values were observed across groups, with no consistent pattern of group-related change, and no detected significant improvements from baseline in any group.
Exploratory analyses
A significant relationship was observed between change in DEXA total fat and change in percent PDFF (r = 0.49, p = 0.004) (Fig. 2). Treatment-related changes in clinical variables and anthropomorphic measures are provided in Supplementary Data. There was no main effect of time or treatment group on adherence. No main effect of treatment group or adherence was detected in a mixed model evaluating the main effect of time on DEXA fat. Similarly, there was no main effect of time or treatment group on fidelity. No main effect of treatment group or fidelity was detected in a marginal model evaluating the main effect of time on DEXA fat. The introduction of age group, stimulant use, or gender into the primary model did not change the significance of reported results.
FIG. 2.
Relationship between change in DEXA-measured adiposity and change in percent PDFF. DEXA, dual-energy X-ray absorptiometry; PDFF, proton density fat fraction.
Adverse events
The behavioral treatment was well tolerated, and no treatment-related adverse behavioral or medical events were observed or reported.
Discussion
In this pilot randomized controlled trial, we set out to apply an unadapted, evidence-based, BWL intervention for reducing total body fat and associated markers of cardiometabolic risk. To our knowledge, this is the first study to apply an evidence-based, intensive, family-based, BWL intervention in AP-treated youth, using gold standard measures of cardiometabolic risk to evaluate treatment effects. Greater improvements in total body fat and hepatic fat were associated with BWL treatment of NP overweight/obese youth, relative to one or both comparison groups.
Both DEXA-measured fat and PDFF, which are proximal indicators of risk for type 2 diabetes and hepatic steatosis, changed differentially across treatment groups—namely, greater decreases were observed in weekly treatment groups as opposed to the RC group. While changes from baseline were statistically significant only for DEXA fat change in the NP group, the clear attenuation of what would otherwise be an ongoing fat gain in AP-treated youth undergoing RC (e.g., nutritional and health behavior change education) suggests possible utility of the intervention for attenuation of ongoing fat gain.
In a prior cross-sectional study comparing chronically AP-treated youth to age- and BMI percentile-matched NP youth, we demonstrated that total body adiposity and liver fat were similar in both treatment groups across a range of BMI percentile (Nicol et al. 2015), suggesting that total adiposity and associated cardiometabolic risk (e.g., liver steatosis) can be explained by the BMI percentile achieved during treatment. Although not primary outcomes of the present study, we detected numeric decreases in both weight and BMI percentile in both weekly treatment groups. By contrast, the RC group gained weight and had nominal change in BMI percentile during the course of the study.
Reductions in total body and liver fat observed during weekly treatment in NP youth were attenuated in AP-treated youth despite similar session attendance. The tolerability of weekly treatment was also similar in NP and AP-treated youth. This suggests that factors other than treatment adherence, a previously reported predictor of short-term treatment response (Theim et al. 2013), might contribute to greater effects on whole-body fat in NP versus AP-treated youth. A large proportion of AP-treated youth in this study were diagnosed with an ASD with prominent irritability, a condition for which certain antipsychotic treatments are approved. Prior survey of parents of overweight/obese AP-treated children with reported ASD indicated that difficulties changing health behaviors were observed during previous efforts at weight loss treatment. Specifically, aversion to physical activity and taste selectivity for homogeneous and energy-dense foods commonly seen in children with ASDs were reported as specific targets for treatment optimization (Nicol et al. 2016a). These and other challenges may offer targets for further adaptation of BWL to optimize response in the AP-treated population.
This pilot study is subject to limitations. First, our sample size was small with numeric differences in variances across groups. Furthermore, we included participants across a wide age range. These factors together with the short length of treatment may have reduced signal detection on primary outcomes, most specifically in CIMT. Although correlated with obesity and cardiovascular disease in adults (Koskinen et al. 2014), CIMT may be less sensitive in children than biomarkers such as PDFF (Nicol et al. 2015). In addition, substantial weight loss over 6–12 months may be required to demonstrate change in this marker of risk (Wunsch et al. 2006). Finally, our CIMT measurement method used the use of far-wall measurement only, which likely impacted the precision and reproducibility of the results. Finally, while changes in physical activity and dietary content were a primary focus of the BWL treatment used, these variables were not formally measured in this pilot study. Together, these factors may limit the interpretation of our findings.
Conclusion
Overall, the results confirm that BWL interventions can favorably impact early measures of cardiometabolic risk in youth. Effects were attenuated in the high-risk population of AP-treated youth, which may be attributed to unique behavioral characteristics and needs (Nicol et al. 2016a). Youth with AP-induced obesity may benefit from unadapted BWL interventions. However, treatment modifications may be needed to optimize results in the challenging population of AP-treated youth. Future targeted adaptation efforts will be needed to maximize effectiveness in these individuals. Clinical Trials Registration: NCT01222494.
Clinical Significance
These results should be interpreted with caution. While antipsychotic-treated youth demonstrated weight attenuation and modest metabolic benefit from an intensive BWL intervention, future studies testing optimized BWL interventions in this and other vulnerable populations are needed to determine how best to maximize treatment efficacy in high-risk youth.
Supplementary Material
Acknowledgments
The authors thank Lucy Zee, LCSW, Monica Mills, LPC, and Katie Keenoy, MS, who conducted treatment visits with study participants and assisted in modifying the BWL treatment.
Authors' Contributions
G.E.N. was the PI for this study, overseeing all study conduct, data collection, and analysis, and is the primary author of the article. R.K. assisted in creating modifications to the BWL treatment, provided treatment as a study interventionist, and was involved in data collection, analysis, interpretation, and writing of the methods, results, and conclusions of the article. E.J.L. is an expert in the conduct of clinical trials, both for pharmacologic and behavioral interventions, in psychiatric populations, and provided mentorship regarding study conduct and contributed to data analysis and interpretation of results. M.D.Y. assisted with data collection and management throughout the conduct of the study, performed all statistical analyses, and created figures and tables for the article and Supplementary Data. J.P.M. is the senior statistician on the project, and provided oversight regarding statistical analysis, interpretation, and presentation of results. A.R.R. served as the coordinator for the study, and contributed to data collection, management, and analysis efforts in addition to contributing to the Methods section of the article. J.A.S. is the laboratory manager who oversaw study conduct, regulatory adherence, and data management procedures, and also assisted in data collection, as well as providing assistance in generating methods materials for the article and Supplementary Data. R.L.F. served as a comentor on the project, providing expertise in the conduct of clinical research in the population of interest, analysis of data, and interpretation of results. D.W., an expert in the treatment of pediatric obesity, provided comentorship regarding delivery of the BWL treatment, data analysis, and interpretation of results. J.W.N. was the primary mentor for the project, providing guidance regarding all aspects of study conduct, data collection, data analysis, interpretation of results, and article preparation.
Disclaimer
Funding sources for this study had no role in the design or conduct of the research, including in the collection, management, analysis, and interpretation of the data, nor in the preparation, review, or approval of the manuscript, or in the decision to submit the manuscript for publication.
Disclosures
G.E.N. receives or has received research support from the National Institutes of Mental Health (NIMH), the Sidney R. Baer, Jr. Foundation, the Center for Brain Research in Mood Disorders at Washington University, and Otsuka America, Inc. for investigator-initiated research studies. She has served as principal or coinvestigator on research supported by NIH, Patient Centered Outcomes Research Institute (PCORI), and for industry-sponsored clinical trials funded by Alkermes, Takeda, and Shire. She serves as a consultant for Alkermes, Supernus, and Sunovion. EJL receives or has received support from NIH, the Food and Drug Administration, Takeda, Lundbeck, Janssen, Alkermes, the Taylor Family Institute for Innovative Psychiatric Research, the McKnight Brain Research Foundation, the Barnes/Jewish Foundation, and PCORI. R.L.F. receives or has received research support, acted as a consultant, and/or served on a speaker's bureau for Actavis, Akili, Alcobra, American Academy of Child & Adolescent Psychiatry, American Psychiatric Press, Bracket, CogCubed, Cognition Group, Coronado Biosciences, Elsevier, ePharmaSolutions, Forest, Genentech, GlaxoSmithKline, Guilford Press, Ironshore, Johns Hopkins University Press, KemPharm, Lundbeck, Medgenics, Merck, NIH, Neurim, Novartis, Otsuka, PCORI, Pfizer, Physicians Postgraduate Press, Purdue, Rhodes Pharmaceuticals, Roche, Sage, Shire, Sunovion, Supernus Pharmaceuticals, Syneurx, Takeda, Teva, TouchPoint, Tris, Validus, and WebMD. He receives or has received research support from NIH and Otsuka America, Inc. J.W.N. has served on a data safety monitoring board for Amgen and acted as a consultant for Indivior, Alkermes, Otsuka, Auris, Sunovion and for litigation, and has received honoraria for speaking (continuing medical education) from the American Society for Clinical Psychopharmacology and the American Diabetes Association. All other authors declare no conflicts of interest.
Supplementary Material
References
- Aman M, Singh N: Aberrant Behavior Checklist (ABC)—Community Supplementary Manual. East Aurora, NY, Slosson Educational Publications, 1994 [Google Scholar]
- Barlow SE: Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 120(Suppl 4):S164–S192, 2007 [DOI] [PubMed] [Google Scholar]
- Baroncini LAV, Sylvestre LC, Baroncini CV, Pecoits RF: Assessment of carotid intima-media thickness as an early marker of vascular damage in hypertensive children. Arq Bras Cardiol 108:452–457, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenaes AB, Birkeland KI, Engh JA, Faerden A, Jonsdottir H, Ringen PA, Friis S, Opjordsmoen S, Andreassen OA: Dyslipidemia independent of body mass in antipsychotic-treated patients under real-life conditions. J Clin Psychopharmacol 28:132–137, 2008 [DOI] [PubMed] [Google Scholar]
- Birnbaum HG, Kessler RC, Lowe SW, Secnik K, Greenberg PE, Leong SA, Swensen AR: Costs of attention deficit-hyperactivity disorder (ADHD) in the US: Excess costs of persons with ADHD and their family members in 2000. Curr Med Res Opin 21:195–206, 2005 [DOI] [PubMed] [Google Scholar]
- Calarge CA, Acion L, Kuperman S, Tansey M, Schlechte JA: Weight gain and metabolic abnormalities during extended risperidone treatment in children and adolescents. J Child Adolesc Psychopharmacol 19:101–109, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CW, Manderscheid RW: Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis 3:A42, 2006 [PMC free article] [PubMed] [Google Scholar]
- Connor DF, Carlson GA, Chang KD, Daniolos PT, Ferziger R, Findling RL, Hutchinson JG, Malone RP, Halperin JM, Plattner B, Post RM, Reynolds DL, Rogers KM, Saxena K, Steiner H: Juvenile maladaptive aggression: A review of prevention, treatment, and service configuration and a proposed research agenda. J Clin Psychiatry 67:808–820, 2006 [PubMed] [Google Scholar]
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK: Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 302:1765–1773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Moreira-Maia CR, St Fleur D, Morcillo-Penalver C, Rohde LA, Faraone SV: Association between ADHD and obesity: A systematic review and meta-analysis. Am J Psychiatry 173:34–43, 2016 [DOI] [PubMed] [Google Scholar]
- Crystal S, Olfson M, Huang C, Pincus H, Gerhard T: Broadened use of atypical antipsychotics: Safety, effectiveness, and policy challenges. Health Aff (Millwood) 28:w770–w781, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumit GL, Dickerson FB, Wang NY, Dalcin A, Jerome GJ, Anderson CA, Young DR, Frick KD, Yu A, Gennusa 3rd JV, Oefinger M, Crum RM, Charleston J, Casagrande SS, Guellar E, Goldberg RW, Campbell LM, Appel LJ: A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med 368:1594–1602, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke HC, DelBello MP, Landry J, Hoffmann VP, Heinloth A, Dittmann RW: A 52-week study of olanzapine with a randomized behavioral weight counseling intervention in adolescents with schizophrenia or bipolar I disorder. J Child Adolesc Psychopharmacol 26:922–934, 2016 [DOI] [PubMed] [Google Scholar]
- Epstein LH, Paluch RA, Kilanowski CK, Raynor HA: The effect of reinforcement or stimulus control to reduce sedentary behavior in the treatment of pediatric obesity. Health Psychol 23:371–380, 2004 [DOI] [PubMed] [Google Scholar]
- Epstein LH, Squires S: The Stoplight Eating Program for Children and Parents. Boston (Massachusetts), Little, Brown and Co., 1988 [Google Scholar]
- Erickson ZD, Mena SJ, Pierre JM, Blum LH, Martin E, Helleman GS, Aragaki DR, Firestone L, Lee C, Lee P, Kunkel CF, Ames D: Behavioral interventions for antipsychotic medication-associated obesity: A randomized, controlled clinical trial. J Clin Psychiatry 77:e183–e189, 2016 [DOI] [PubMed] [Google Scholar]
- Hagstromer M, Bergman P, De Bourdeaudhuij I, Ortega FB, Ruiz JR, Manios Y, Rey-Lopez JP, Philipp K, von Berlepsch J, Sjostrom M; HELENA Study Group: Concurrent validity of a modified version of the International Physical Activity Questionnaire (IPAQ-A) in European adolescents: The HELENA Study. Int J Obes (Lond) 32(Suppl 5):S42–S48, 2008 [DOI] [PubMed] [Google Scholar]
- Hill AP, Zuckerman KE, Fombonne E: Obesity and autism. Pediatrics 136:1051–1061, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen J, Magnussen CG, Sabin MA, Kahonen M, Hutri-Kahonen N, Laitinen T, Taittonen L, Jokinen E, Lehtimaki T, Viikari JS, Raitakari OT, Juonala M: Youth overweight and metabolic disturbances in predicting carotid intima-media thickness, type 2 diabetes, and metabolic syndrome in adulthood: The Cardiovascular Risk in Young Finns study. Diabetes Care 37:1870–1877, 2014 [DOI] [PubMed] [Google Scholar]
- Le Grange D, Hughes EK, Court A, Yeo M, Crosby RD, Sawyer SM: Randomized Clinical trial of parent-focused treatment and family-based treatment for adolescent anorexia nervosa. J Am Acad Child Adolesc Psychiatry 55:683–692, 2016 [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Jerrell JM: Metabolic and cardiovascular adverse events associated with antipsychotic treatment in children and adolescents. Arch Pediatr Adolesc Med 162:929–935, 2008 [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Brody D, Fisher PW, Bourdon K, Koretz DS: Prevalence and treatment of mental disorders among US children in the 2001–2004 NHANES. Pediatrics 125:75–81, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrato EH, Nicol GE, Maahs D, Druss BG, Hartung DM, Valuck RJ, Campanga E, Newcomer JW: Metabolic screening in children receiving antipsychotic drug treatment. Arch Pediatr Adolesc Med 164:344–351, 2010 [DOI] [PubMed] [Google Scholar]
- Morrison JA, Friedman LA, Gray-McGuire C: Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: The Princeton Lipid Research Clinics Follow-up Study. Pediatrics 120:340–345, 2007 [DOI] [PubMed] [Google Scholar]
- Newcomer JW: Metabolic considerations in the use of antipsychotic medications: A review of recent evidence. J Clin Psychiatry 68(Suppl 1):20–27, 2007 [PubMed] [Google Scholar]
- Nicol G, Worsham E, Haire-Joshu D, Duncan A, Schweiger J, Yinging M, Lenze E: Getting to more effective weight management in antipsychotic-treated youth: A survey of barriers and preferences. Child Obes 12:70–76, 2016a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol GE, de Las Fuentes L, Riek AE, Bernal-Mizrachi C, Lenze EJ, Miller JP, Schweiger JA, Yingling MD, Huang VJ, Dixon DJ, Hennekens CH, Newcomer JW: Adiposity and cardiometabolic risk in children with and without antipsychotic drug treatment. J Clin Endocrinol Metab 100:3418–3426, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol GE, Kolko RP, Mills M, Gunnarsdottir T, Yingling MD, Schweiger JA, Lenze EJ, Newcomer JW, Wilfley D: Behavioral weight loss treatment for youth treated with antipsychotic medications. Scand J Child Adolesc Psychiatry Psychol 4:96–104, 2016b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol GE, Yingling MD, Flavin KS, Schweiger JA, Patterson BW, Schechtman KB, Newcomer JW: Metabolic effects of antipsychotics on adiposity and insulin sensitivity in youths: A randomized clinical trial. JAMA Psychiatry 75:788–796, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordentoft M, Wahlbeck K, Hallgren J, Westman J, Osby U, Alinaghizadeh H, Gissler M, Laursen TM: Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS One 8:e55176, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Liu L, Moreno C, Laje G: National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry 63:679–685, 2006 [DOI] [PubMed] [Google Scholar]
- Oren A, Vos LE, Uiterwaal CS, Gorissen WH, Grobbee DE, Bots ML: Change in body mass index from adolescence to young adulthood and increased carotid intima-media thickness at 28 years of age: The Atherosclerosis Risk in Young Adults study. Int J Obes Relat Metab Disord 27:1383–1390, 2003 [DOI] [PubMed] [Google Scholar]
- Ousseny Z, Yinge Q, Thomas R, Steve S, Steve R, Massolo M, Croen LA: Healthcare service utilization and cost among adults with autism spectrum disorders in a U.S. integrated healthcare system. Autism Adulthood 1, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak P, West D, Martin BC, Helm ME, Henderson C: Evidence-based use of second-generation antipsychotics in a state medicaid pediatric population, 2001–2005. Psychiatr Serv 61:123–129, 2010 [DOI] [PubMed] [Google Scholar]
- Quattrin T, Wilfley DE: The promise and opportunities for screening and treating childhood obesity: USPSTF recommendation statement. JAMA Pediatr 171:733–735, 2017 [DOI] [PubMed] [Google Scholar]
- Rao G: Childhood obesity: Highlights of AMA Expert Committee recommendations. Am Fam Physician 78:56–63, 2008 [PubMed] [Google Scholar]
- Rogol AD, Clark PA, Roemmich JN: Growth and pubertal development in children and adolescents: Effects of diet and physical activity. Am J Clin Nutr 72(2 Suppl):521S–528S, 2000 [DOI] [PubMed] [Google Scholar]
- Tanner JM: Fetus into Man: Physical Growth Form Conception to Maturity, revised edition. Cambridge (Massachusetts), Harvard University Press, 1989 [Google Scholar]
- Theim KR, Sinton MM, Goldschmidt AB, Van Buren DJ, Doyle AC, Saelens BE, Stein RI, Epstein LH, Wilfley DE: Adherence to behavioral targets and treatment attendance during a pediatric weight control trial. Obesity (Silver Spring) 21:394–397, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODAY Study Group: Design of a family-based lifestyle intervention for youth with type 2 diabetes: The TODAY study. Int J Obes (Lond) 34:217–226, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ER, McGee RE, Druss BG: Mortality in mental disorders and global disease burden implications: A systematic review and meta-analysis. JAMA Psychiatry 72:334–341, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfley DE, Saelens BE, Stein RI, Best JR, Kolko RP, Schechtman KB, Wallendorf M, Welch RR, Perri MG, Epstein LH: Dose, content, and mediators of family-based treatment for childhood obesity: A multisite randomized clinical trial. JAMA Pediatr 171:1151–1159, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfley DE, Stein RI, Saelens BE, Mockus DS, Matt GE, Hayden-Wade HA, Welch RR, Schechtman KB, Thompson PA, Epstein LH: Efficacy of maintenance treatment approaches for childhood overweight: A randomized controlled trial. JAMA 298:1661–1673, 2007 [DOI] [PubMed] [Google Scholar]
- Wunsch R, de Sousa G, Toschke AM, Reinehr T: Intima-media thickness in obese children before and after weight loss. Pediatrics 118:2334–2340, 2006 [DOI] [PubMed] [Google Scholar]
- Yoon Y, Wink LK, Pedapati EV, Horn PS, Erickson CA: Weight gain effects of second-generation antipsychotic treatment in autism spectrum disorder. J Child Adolesc Psychopharmacol 26:822–827, 2016 [DOI] [PubMed] [Google Scholar]
- Zhang JP, Lencz T, Zhang RX, Mitta M, Maayan L, John M, Robinson DG, Fleischhacker WW, Kahn RS, Ophoff RA, Kane JM, Malhotra AK, Correll CU: Pharmacogenetic associations of antipsychotic drug-related weight gain: A systematic review and meta-analysis. Schizophr Bull 42:1418–1437, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.