TO THE EDITOR
Ibrutinib is a first-in-class, oral, once-daily Bruton’s tyrosine kinase inhibitor. A multicenter, phase II study (August 5, 2014 to December 22, 2016) assessed the efficacy and safety of single-agent ibrutinib in Japanese patients with relapsed/refractory mantle cell lymphoma (MCL) who had progressed after receiving 1 to 5 prior treatment regimens (NCT02169180; MCL2002). The primary analysis (up to April 30, 2015) was previously reported.1 The final analysis is presented here, with a median follow-up of 22.5 months (range, 3.0-27.6). Per protocol, the study ended following ibrutinib marketing approval in Japan.
The study was conducted at 11 sites in Japan, and enrolled 16 patients ≥ 20 years old with histologically confirmed MCL who had progressed after receiving 1 to 5 prior treatment regimens.1 Patients received oral ibrutinib 560 mg once daily in 28-day cycles (continuous, without interruption) until disease progression (or relapse if the patient achieved a complete response [CR]), unacceptable toxicity, or study end, whichever occurred first. The primary endpoint was overall response rate (ORR), assessed by independent review committee (IRC) until the primary analysis and by the investigator thereafter. Secondary endpoints included investigator-assessed duration of response (DOR), progression-free survival (PFS), and overall survival (OS). Safety endpoints included adverse events (AEs).
All patients received ibrutinib and were included in the response-evaluable and safety populations. Baseline demographics and characteristics were reported previously.1 The median duration of ibrutinib treatment was 9.9 months (range, 2.8-27.6), with 7 (43.8%) patients receiving ibrutinib for more than 12 months. The median number of cycles was 11.0 (range, 4.0-31.0). Median relative dose intensity was 98.2% (range, 47.5-100.0). Dose reduction due to ≥ 1 AE occurred in 2 (12.5%) patients (both at the primary analysis [1 with grade 2 rash and decreased platelet count; 1 with grade 3 stomatitis]). Nine (56.3%) patients had their dose interrupted for ≥ 7 continuous days. Ten patients discontinued study treatment due to disease progression (n = 6), AEs (n = 3), or consent withdrawal (n = 1); 6 patients remaining on ibrutinib at study termination were offered continued access to ibrutinib (all accepted).
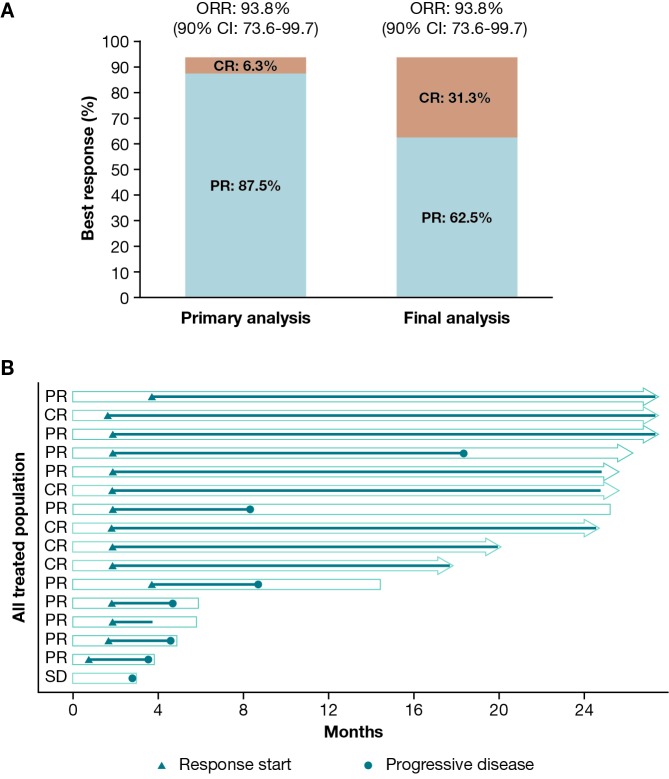
Table 1 and Figure 1 summarize the investigator-assessed efficacy endpoints and individual responses. The ORR was 93.8% (15/16 patients; 90% confidence interval [CI]: 73.6-99.7) at both the primary and final analyses, and comparable to the IRC-assessed ORR of 87.5% (90% CI: 65.6-97.7; 14/16 patients) in the primary analysis. However, there was a deepening of response, with the number of patients who achieved a CR increasing from 1 (6.3%) to 5 (31.3%) from the primary to the final analysis. DOR ranged from 1.8+ to 25.8+ months in the 15 patients who achieved CR or partial response (vs 1.1+ to 6.4+ months at primary analysis); in 2 patients, DOR was ≥ 2 years. PFS ranged from 2.8 to 27.6+ months (vs 2.8 to 8.0+ months at primary analysis); in 6 patients, PFS was ≥ 2 years. OS ranged from 3.0 to 27.6+ months (vs 3.0 to 8.3+ months at primary analysis). Median DOR, PFS, and OS could not be estimated.
Table 1. Investigator-assessed efficacy endpoints.
| Parameter | All patients (n = 16) |
|---|---|
| Best overall response, n (%) | |
| CR | 5 (31.3) |
| PR | 10 (62.5) |
| Stable disease | 1 (6.3) |
| Progressive disease | 0 |
| ORR (CR or PR), n (%) | 15 (93.8) |
| Exact 90% CI | (73.6-99.7) |
| DOR,* months | |
| Median (95% CI) | NE (2.92-NE) |
| Censored, n (%) | 9 (60.0) |
| Range | 1.8+ to 25.8+ |
| ≥ 18 months, n (%) | 7 (46.7) |
| ≥ 2 years, n (%) | 2 (13.3) |
| PFS, months | |
| Median (95% CI) | NE (4.67-NE) |
| Censored, n (%) | 9 (56.3) |
| Range | 2.8 to 27.6+ |
| ≥ 18 months, n (%) | 8 (50.0) |
| ≥ 2 years, n (%) | 6 (37.5) |
| OS | |
| Median (95% CI) | NE (5.85-NE) |
| Censored, n (%) | 9 (56.3) |
| Range | 3.0 to 27.6+ |
| ≥ 18 months, n (%) | 9 (56.3) |
| ≥ 2 years, n (%) | 8 (50.0) |
*n = 15.
+, censored observation; CI, confidence interval; CR, complete response; DOR, duration of response; NE, not estimable; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response.
Fig 1.
(A) Investigator-assessed best response and (B) individual responses.*
*Each bar represents 1 patient in the study. Right arrow cap indicates censored. Bars without an arrow indicate non-censored. Overall survival is represented by the total length of the bar. The section after the line has stopped indicates the length of time the patient was monitored. Progression-free survival is represented by the length of the bar from the left to the circle, or the full length of the bar if there is no circle denoting progressive disease. Duration of response is represented by the length of the line from the triangle to the circle, or the full length of the line if no circle.
CI, confidence interval; CR, complete response; ORR, overall response rate; PR, partial response; SD, stable disease.
Compared with AEs reported in the primary analysis,1 there were no remarkable changes in incidence and severity of AEs reported in the final analysis. All patients had ≥ 1 AE; the most commonly reported treatment-emergent AEs (reported in at least 4 patients) were stomatitis (50.0%), diarrhea (37.5%), nasopharyngitis and platelet count decreased (31.3% each), paronychia, upper respiratory tract infection, constipation, anemia, and thrombocytopenia (25.0% each) (Table 2). Although the incidences of stomatitis, nasopharyngitis, platelet count decreased, and paronychia in this study were higher than those reported in previous studies of ibrutinib (10.0% or less), the majority of these treatment-emergent AEs were grade 1 or 2 and the treatment was continued without changing the dose. Hemorrhagic AEs were reported in 8 (50.0%) patients compared with 5 (31.3%) patients in the primary analysis. Newly reported hemorrhagic AEs included a serious grade 3 gastric hemorrhage in 1 patient, which resolved after drug interruption. Cardiac arrhythmias were reported in 3 (18.8%) patients compared with 2 (12.5%) patients in the primary analysis.
Table 2. AEs* occurring in ≥ 15% of patients.
| All grades, n (%) | Grade ≥ 3, n (%) | |
|---|---|---|
| All AEs | 16 (100.0) | 13 (81.3) |
| Infections and infestations | 14 (87.5) | 1 (6.3) |
| Nasopharyngitis | 5 (31.3) | 0 |
| Paronychia | 4 (25.0) | 0 |
| Upper respiratory tract infection | 4 (25.0) | 0 |
| Skin infection | 3 (18.8) | 0 |
| Gastrointestinal disorders | 13 (81.3) | 2 (12.5) |
| Stomatitis | 8 (50.0) | 1 (6.3) |
| Diarrhea | 6 (37.5) | 0 |
| Constipation | 4 (25.0) | 0 |
| Dyspepsia | 3 (18.8) | 0 |
| Skin and subcutaneous tissue disorders | 11 (68.8) | 0 |
| Dry skin | 3 (18.8) | 0 |
| Purpura | 3 (18.8) | 0 |
| Blood and lymphatic system disorders | 10 (62.5) | 6 (37.5) |
| Anemia | 4 (25.0) | 1 (6.3) |
| Thrombocytopenia | 4 (25.0) | 1 (6.3) |
| General disorders and administration site conditions | 10 (62.5) | 3 (18.8) |
| Disease progression | 3 (18.8) | 3 (18.8) |
| Fatigue | 3 (18.8) | 0 |
| Investigations | 10 (62.5) | 3 (18.8) |
| Platelet count decreased | 5 (31.3) | 1 (6.3) |
| Alanine aminotransferase increased | 3 (18.8) | 0 |
| Aspartate aminotransferase increased | 3 (18.8) | 0 |
| Musculoskeletal and connective tissue disorders | 6 (37.5) | 0 |
| Muscle spasms | 3 (18.8) | 0 |
| Metabolism and nutrition disorders | 5 (31.3) | 2 (12.5) |
| Decreased appetite | 3 (18.8) | 0 |
| Nervous system disorders | 4 (25.0) | 0 |
| Headache | 3 (18.8) | 0 |
*Listed by Medical Dictionary for Regulatory Activities System Organ Class (Version 19.1) and Preferred Term.
AE, adverse event.
Grade ≥ 3 AEs were reported in 13 (81.3%) patients; the most common were disease progression (18.8%), lymphopenia, and neutropenia (12.5% each). Serious AEs were reported in 8 (50.0%) patients. Of these, 3 experienced fatal disease progression. The remaining 5 patients reporting serious AEs had: sepsis (grade 4; drug discontinued); hyperuricemia and leukocytosis (grade 4 and 3, respectively; drug discontinued); interstitial lung disease (grade 2; probably related to study treatment; drug discontinued); gastric hemorrhage and gastric ulcer (both grade 3; drug interrupted); and atrial fibrillation (grade 2 on day 485; drug interrupted).
AEs leading to treatment discontinuation were reported in 4 (25.0%) patients, including 1 each with grade 4 sepsis, grade 2 interstitial lung disease, and grade 2 pneumonitis (possibly related to study treatment and recovered/resolving from AE), and 1 with hyperuricemia and leukocytosis. These were serious AEs, except for the pneumonitis. Seven patients died during the study, each due to disease progression, with 3 deaths due to treatment-emergent disease progression AEs.
The final results of this phase II study were consistent with those for the primary analysis.1 Single-agent ibrutinib at 560 mg/day showed high and durable efficacy and a tolerable safety profile in Japanese patients with relapsed/refractory MCL, supporting ibrutinib as a reasonable treatment option in these patients.
ACKNOWLEDGMENTS
We thank the patients who participated in this trial and their families. This study was funded by Janssen Pharmaceutical K.K. Writing assistance was provided by Safeer Mughal, PhD, and Ian Phillips, PhD, of PAREXEL, and was funded by Janssen Global Services.
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Footnotes
CONFLICT OF INTEREST: Dai Maruyama has received honoraria from Janssen, and has received research funding from Janssen. Hirokazu Nagai has received honoraria from Eisai, Chugai, Mundipharma, and Takeda, and has received research funding from AbbVie, AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Janssen, Gilead, Mundipharma, Ono, Otsuka, and Takeda. Noriko Fukuhara has received research funding from AbbVie, Bayer, Eisai, Gilead, Janssen, Ono, and Takeda. Toshiyuki Kitano and Takayuki Ishikawa have no conflicts of interest to declare. Tomoaki Nishikawa is an employee of Janssen.
REFERENCE
- 1.Maruyama D, Nagai H, Fukuhara N, et al. Efficacy and safety of ibrutinib in Japanese patients with relapsed or refractory mantle cell lymphoma. Cancer Sci. 2016; 107: 1785-1790. 10.1111/cas.13076 [DOI] [PMC free article] [PubMed] [Google Scholar]