Abstract
Other iatrogenic immunodeficiency-associated lymphoproliferative disorders (OIIA-LPD), a category of immunodeficiency-associated LPD according to the World Health Organization classification, is associated with immunosuppressive drugs (ISDs). Several factors, including autoimmune disease (AID) activity, Epstein-Barr virus (EBV) infection, ISD usage, and aging, influence the development of OIIA-LPD, resulting in complicated clinical courses and outcomes. Most OIIA-LPD develops in patients with rheumatoid arthritis using methotrexate (MTX-LPD). The management of MTX-LPD is based on the clinical course, i.e., with/without regression, with/without relapse/regrowth event (RRE), LPD subtype, and ISDs for AIDs after LPD development. There are three clinical courses after ISD withdrawal: regressive LPD without relapse/regrowth (R-G), regressive LPD with RRE (R/R-G), and persistent LPD (P-G). The majority of EBV+ diffuse large B-cell lymphomas are classified in R-G, whereas classic Hodgkin lymphoma is generally classified in R/R-G. Polymorphic LPD (P-LPD) in MTX-LPD develops with heterogeneous pathological features similar to monomorphic LPD. Chemotherapy for MTX-LPD is selected according to that for de novo LPD, although the strategy for aggressive P-LPD and non-specific LPD is not well established. The absolute lymphocyte count in the peripheral blood has been suggested as a candidate marker for MTX-LPD development and RRE. Several clinical issues, including correct diagnosis among overlapping clinicopathological features in MTX-LPD and clinical management of LPD by ISDs other than MTX, require further investigation.
Keywords: : clinical management, iatrogenic immunodeficiency-associated lymphoproliferative disorders, methotrexate, rheumatoid arthritis
OTHER IATROGENIC IMMUNODEFICIENCY-ASSOCIATED LYMPHOPROLIFERATIVE DISORDERS (OIIA-LPD)
Immune deficiency is an important factor in the pathogenesis of LPD among a number of influencing events. According to the World Health Organization (WHO) classification, immunodeficiency-associated LPD (IA-LPD) is classified into four subtypes; LPD associated with primary immune disorders, lymphomas associated with HIV infection, post-transplant LPD (PTLD), and OIIA-LPD.1,2 OIIA-LPD is defined as lymphoid proliferations or lymphomas that develop in patients receiving immunosuppressive drugs (ISDs) for autoimmune diseases (AIDs) or conditions other than the post-transplant setting in the 2008 WHO classification.1 However, the ISD-mediated LPD category remains controversial because of the difficulty in confirming a direct effect of ISDs based on the high risk of LPD development in patients with AIDs as a natural clinical course. The standardized incidence ratio (SIR) of rheumatoid arthritis (RA) is 2-20-fold that in the control healthy population.3-12 In particular, the incidence rate is high in Japan. For example, the SIR in the NinJa study,6 SUCURE study,8 and IORRA cohort10 in Japan was 3.43, 6.18, and 4.61, respectively. In addition, the disease severity and accumulated inflammatory activity of RA may be related to LPD development.13 Although different AIDs are treated using different ISDs in OIIA-LPD, there is no direct evidence of the pathogenesis of LPD, except for LPD regression after ISD withdrawal. Methotrexate (MTX) is recognized as the ISD associated with LPD in the WHO classification (2001),14 categorized as the MTX-LPD disease entity. The category of OIIA-LPD was defined in the 2008 and 2017 WHO classifications1,2 to replace the concept of MTX-LPD. Among ISDs, MTX and anti-tumor necrosis factor inhibitors (TNFi) are candidates initiating LPD.1,2
OIIA-LPD originates from many cell types, including B, T, and natural killer (NK) cells.1,2,14 The major types of monomorphic LPD (M-LPD) are diffuse large B cell lymphoma (DLBCL) and classic Hodgkin lymphoma (CHL), comprising 58% and 15.3% of 274 cases of OIIA-LPD, respectively, in the 2017 WHO classification.1 Rare subtypes of LPD include polymorphic features, such as polymorphic/lymphoplasmacytic infiltrates (P/L-I), Hodgkin-like lesions (HLL), and Epstein-Barr virus-positive mucocutaneous ulcer (EBVMCU), which comprise 9.9%, 2.2%, and 3.3%, respectively.1 Although non-destructive LPD (ND-LPD), defined as PTLD in the WHO classification,1,2 is not described in OIIA-LPD, it accounts for 10-15% of MTX-LPD according to previous reports.15,16 Although ISDs other than MTX promote LPD development based on previous reports, assessment of such non-MTX-LPD is difficult because of the small number of cases. Thus, this article focuses on the management of MTX-LPD in OIIA-LPD. In addition, although the WHO classification does not separate DLBCL subtypes, such as EBV+DLBCL and DLBCL-NOS, in OIIA-LPD,1,2 we analyzed them specifically focusing on the differences between de novo DLBCL and MTX-DLBCL.
LPD REGRESSION AFTER ISD WITHDRAWAL
LPD regression after ISD withdrawal is one of the unique characteristics, suggesting a direct influence on the pathogenesis of LPD development. Although this phenomenon is also described in other disorders like PTLD,17 it mainly develops in patients with MTX-LPD. LPD regression after MTX withdrawal is observed in 20-70% of patients. In the 2017 WHO classification, 40.4% of 188 patients with OIIA-LPD demonstrated LPD regression.1 Among 545 patients with MTX-LPD collected in a study by Pfizer, Japan, LPD regression after MTX withdrawal comprised 86.2% of the 302 evaluable events.18 As more than 50% of the patients without relapse/regrowth events (RRE) after LPD regression do not require additional chemotherapy and have a superior overall survival (OS),16,19 watchful wait therapy (WW) is recommended initially when MTX-LPD develops. To clarify the clinical management of MTX-LPD, which includes avoiding additional therapy for regressive LPD, we propose three clinical courses after ISD withdrawal: LPD regression after ISD withdrawal without RRE (regressive group, R-G), LPD regression after ISD withdrawal with RRE (relapse/regrowth group, R/R-G), and persistent LPD after ISD withdrawal (persistent group, P-G) (Figure 1).16,19 In these reports, the ratio of the three groups was similar. In R-G and R/R-G, but not P-G, the direct influence of ISDs on the pathogenesis of LPD development was suggested because they exhibited LPD regression phenomenon. In P-G, de novo LDP and irreversible MTX-LPD due to ISDs are involved, although it is difficult to discriminate between them. Regarding this point, the differences between de novo LPD and MTX-LPD have been investigated in several studies.20-23 Carreras et al. demonstrated differences in the phenotype and genetic characteristics of MTX-LPD-DLBCL and de novo DLBCL such as a genomic profile with 3q and 12 gains, 13q loss, different expression levels of relevant pathogenic biomarkers, and a microenvironment with high numbers of cytotoxic T lymphocytes and M2 macrophages.21 In other studies, most MTX-DLBCL cases had an activated-B-cell immunophenotype, especially Epstein-Barr virus (EBV) - positive cases, and MTX-EBV+DLBCL commonly expressed CD30.22,23 Ejima-Yamada et al. found that EBV infection in MTX-B cell-LPD in patients with RA is associated with a lower incidence of CpG island methylator phenotype and B-cell lymphoma 2 expression, resulting in LPD regression after MTX withdrawal and improved prognoses.24
Fig. 1.
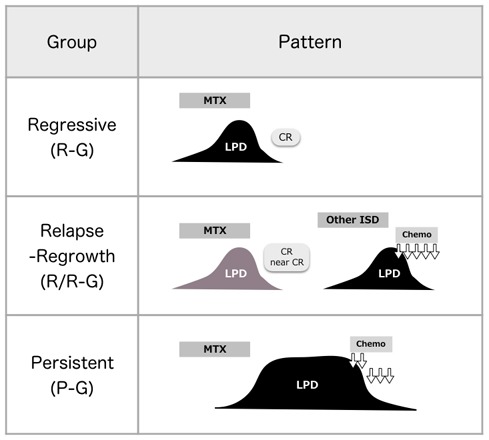
Three clinical courses of methotrexate-associated lymphoproliferative disorders (MTX-LPD)19
Three clinical courses are observed in patients with MTX-LPD after immunosuppressive drug (ISD) withdrawal: regressive LPD without relapse/regrowth event (RRE, regressive group, R-G), regressive LPD with RRE (R/R-G), and persistent LPD (P-G). CR, complete response; Chemo, chemotherapy.
The three clinical groups have different trends in clinical parameters: serum C-reactive protein (CRP) and serum soluble interleukin-2 receptor (sIL-2R) are increased in R/R-G, whereas serum lactate dehydrogenase (LDH), CRP, and sIL-2R are increased in P-G.16,19 Furthermore, the OS for R-G is significantly better than that for the other two groups.16 Thus, RRE is an important event among patients with regressive LPD after ISD withdrawal. According to previous reports, RRE develops within 2-3 years after LPD regression.15,16,19,25 In our study, the rate of regressive LPD (R-G and R/R-G) was different for each LPD of MTX-LPD; a higher rate was observed for MTX-EBV+DLBCL, MTX-P-LPD, MTX-EBVMCU and MTX-ND-LPD, whereas MTX-DLBCL-NOS had a lower rate.
Recent studies have suggested that lymphocytes play an important role in the pathogenesis of LPD regression after ISD.26-30 Inui et al. reported recovery of lymphocytes in peripheral blood (PB) in patients with regressive LPD after MTX, although not in patients with P-G.26 Saito et al. confirmed this finding among patients with MTX-LPD, and reported that the recovered lymphocytes were composed of CD8+ T cells and natural killer (NK) cells.27 They also demonstrated a decrease in the absolute lymphocyte count (ALC) in PB towards the time of LPD development in patients with regressive LPD, suggesting a key role of lymphocytes in the pathogenesis of MTX-LPD.27 Furthermore, the change in lymphocytes may influence RRE. Among 43 patients with MTX-LPD, the ALC gradually decreased toward RRE in R/R-G, but not in R-G.30 Regarding prognosis factors, an ALC of < 600/µL in PB is one of the prognostic factors for de novo CHL.31 The value of ALC as a prognostic factor has also been confirmed for several lymphomas.32-35 Taken together, lymphocytes in PB are suggested to be one of the triggers in the development of MTX-LPD.
CLINICAL MANAGEMENT OF MTX-LPD
The clinical management of MTX-LPD is illustrated in Figure 2, according to the previous reports in clinical practice. After WW following cessation of ISDs, patients without RRE do not require additional therapy (R-G, [1]), whereas additional chemotherapy is administered at the time of RRE (R/R-G, [2]). Chemotherapy is also considered for patients with non-regressive LPD under WW (P-G, [3]). On the other hand, the evaluation of WW has been considered for OIIA-LPD since around 2010, and it is not suitable for patients with aggressive LPD with severe organ damage. Thus, chemotherapy is initiated without confirmation of the regressive phase under these conditions (chemotherapy group, Ch-G, [4]). Furthermore, chemotherapy is initiated if the LPD has an inadequate response under LPD regression (included in P-G, [5]). Based on reports of M-LPD in MTX-LPD, including DLBCL and CHL, chemotherapy is selected according to the strategy of de novo LPD. In contrast, little information is available about patients with aggressive polymorphic LPD (P-LPD), such as P/L-I, HLL, and EBVMCU, in MTX-LPD. Based on previous reports, chemotherapy for P-LPD is selected according to the similar pathological features to DLBCL or CHL, as described in later reports of MTX-P-LPD and MTX- EBVMCU. Although the clinical outcome of patients with ND-LPD is as good as that of patients with P-LPD, aggressive ND-LPD is observed.15,16 Similar to P-LPD, chemotherapy for such aggressive cases is based on the diagnosis of similar pathological features in M-LPD (described in the MTX-ND-LPD section). However, the relevance of this concept of aggressive LPD in P-LPD and ND-LPD has yet to be evaluated.
Fig. 2.
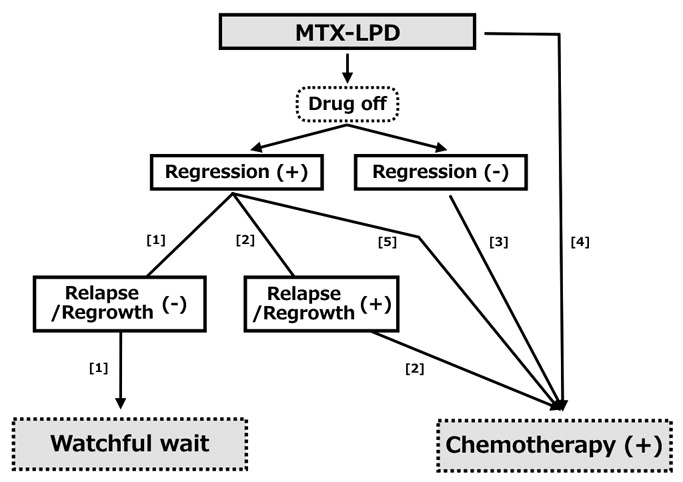
Clinical management of methotrexate-associated lymphoproliferative disorders (MTX-LPD)
The first step after MTX-LPD development is watchful waiting (WW) following the cessation of immunosuppressive drugs (ISDs). Additional therapy is not required for patients without relapse/regrowth event (RRE, R-G, [1]), but chemotherapy is administered when RRE develops (R/R-G, [2]). Chemotherapy is also considered for patients with non-regressive LPD under WW (persistent group, P-G, [3]). On the other hand, WW therapy is not suitable for patients with aggressive LPD, and chemotherapy is initiated without confirmation of the regressive phase (chemotherapy group, Ch-G, [4]). Furthermore, chemotherapy is initiated if the LPD remains in partial response or exhibits a low response during regression (included in P-G, [5]). The details are also described in the text.
Regarding the administration of ISDs at the time of LPD development, all ISDs are basically withdrawn because identifying the drug responsible for LPD is difficult. The majority of OIIA-LPD is MTX-LPD in RA. Although LPD associated with ISDs, except MTX (non-MTX-LPD), may develop, our understanding of non-MTX-LPD is limited because of the small number of patients with combinations of AIDs and ISDs. In addition, the history of MTX administration is carefully assessed among non-MTX-LPD patients because RRE occurs within several years after the withdrawal of MTX. In this article, the clinical features of MTX-LPD are listed based on data summarized from previous reports that described the clinical information such as the clinical patterns of R-G, R/R-G, P-G, and Ch-G.
MTX-DLBCL
The common LPD in OIIA-LPD is DLBCL (30-60%). According to the WHO classification, DLBCL comprises several subtypes, including not-otherwise-specified (NOS) and EBV positive (EBV+) subtypes.1 In this article, we analyzed the clinical features of MTX-DLBCL, including subtypes such as MTX-EBV+ DLBCL and MTX-DLBCL-NOS, and highlighted the clinical aspects in Tables 1 and 2.16,25,36-55 Although the WHO classification described DLBCL as DLBCL-type including DLBCL and LPDs similar to the pathological features of DLBCL in OIIA,25 in our study demonstrated DLBCLs as MTX-EBV+ DLBCL and MTX-DLBCL-NOS. The number of patients with MTX-EBV+DLBCL and MTX-DLBCL-NOS were 66 and 50, respectively (Tables 2, 3). Thus, the rate of EBV positivity was 57% among patients with MTX-DLBCL, although this value varies in previous studies. For example, EBV positivity in 24.1% of 29 patients,56 45% of 42 patients,57 and 82% of 34 patients25 was reported. The incidence of de novo EBV+ DLBCL among all the DLBCL cases was <5-15% of Asian and Latin American patients and <5% of Western patients.1,2 Thus, the rate of EBV positivity is higher in MTX-DLBCL. Although MTX-EBV+DLBCL is female dominant (71%), MTX-DLBCL-NOS has a lower incidence rate in females (59%). Mean ages at the development of MTX-DLBCL are similar between MTX-EBV+DLBCL and MTX-DLBCL-NOS (66 and 68 years, respectively, Table 1), which is similar to de novo DLBCL.58 In both types of MTX-DLBCL, the most common AID was RA, and the duration of AID and MTX administration was over 10 and 5 years, respectively. Two-thirds of the patients had clinical stage (CS) 3 or 4 MTX-DLBCL (Table 1). Regarding the laboratory data for MTX-DLBCL in our previous study,16 the median serum levels of LDH, CRP, and sIL-2R in EBV+DLBCL and DLBCL-NOS were 240 IU/L, 1.4 mg/dL, 1440 U/mL, and 208 IU/L, 4.7 mg/dL, and 1525 U/mL, respectively, demonstrating similar trends between the two types of MTX-DLBCL. The EBV latency in MTX-EBV+DLBCL was type II dominant.58
Table 1. Clinical features of diffuse large B cell lymphoma (DLBCL) in methotrexate associated lymphoproliferative disorders (MTX-LPDs).
| Subtype | Author | Year | N | Sex | Age | Basal disease | MTX duration (y) | Clinical State | Ref | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Types | Duration (y) | |||||||||||||||||||||||
| M | F | ND | Mean | Range | RA | Other | Mean | Range | Mean | Range | I | II | III | IV | E | |||||||||
| MTX- EBV+ DLBCL |
Kojima | 2006 | 1 | 1 | 0 | 0 | 64 | - | 1 | - | 14 | - | 7 | - | 1 | 0 | 0 | 0 | 0 | 36 | ||||
| Miyazaki | 2007 | 3 | 0 | 3 | 0 | 69 | 68-71 | 3 | - | 12.9 | 7.3-19.3 | 6.1 | 2.8-8.3 | 1 | 2 | 0 | 0 | 0 | 37 | |||||
| Jois | 2007 | 1 | 0 | 1 | 0 | 48 | 0 | CD | 1.3 | - | 1.3 | - | 0 | 0 | 0 | 1 | 0 | 38 | ||||||
| Ogasawara | 2007 | 1 | 1 | 0 | 0 | 71 | - | 1 | - | 20 | - | ND | - | 0 | 0 | 0 | 1 | 0 | 39 | |||||
| Rizzi | 2009 | 4 | 0 | 4 | 0 | 63 | 46-86 | 4 | - | 18.5 | 11-24 | 1.5 | 0.9-3 | 0 | 0 | 2 | 2 | 0 | 40 | |||||
| Ishida | 2013 | 1 | 0 | 1 | 0 | 76 | - | 1 | - | 10 | - | 10 | - | 0 | 0 | 0 | 1 | 0 | 41 | |||||
| Yamakawa | 2014 | 2 | 0 | 2 | 0 | 64 | 61-67 | 2 | - | NR | 8.7 | 1.3-16 | 1 | 0 | 1 | 0 | 0 | 42 | ||||||
| Kameda | 2014 | 2 | 1 | 1 | 0 | 66 | 61-71 | 2 | - | 15.5 | 14-17 | 10.5 | 9-12 | 2 | 0 | 0 | 0 | 0 | 43 | |||||
| Kawano | 2014 | 1 | 0 | 1 | 0 | 74 | - | 1 | - | 10 | - | 4.1 | - | 0 | 0 | 0 | 1 | 0 | 44 | |||||
| Kudoh | 2014 | 1 | 0 | 1 | 0 | 75 | - | 1 | - | 5 | - | 5 | - | 0 | 0 | 0 | 1 | 0 | 45 | |||||
| Shimizu | 2015 | 2 | 1 | 1 | 0 | 69 | 63, 75 | 1 | DM+SjS | 9 | ND, 9 | 6.5 | 4, 9 | 0 | 0 | 0 | 0 | 2 | 46 | |||||
| Ikeda | 2016 | 1 | 0 | 1 | 0 | 78 | - | 1 | - | 18 | - | 2 | - | 1 | 0 | 0 | 0 | 0 | 47 | |||||
| Matsumoto | 2016 | 1 | 1 | 0 | 0 | 63 | - | 1 | - | 10 | - | 10 | - | 0 | 0 | 0 | 1 | 0 | 48 | |||||
| Hatano | 2017 | 1 | 1 | 0 | 0 | 61 | - | 1 | - | 7 | - | 6 | - | 0 | 0 | 0 | 1 | 0 | 49 | |||||
| Takei | 2017 | 1 | 0 | 1 | 0 | 65 | - | 1 | - | ND | - | 7 | - | 0 | 0 | 0 | 1 | 0 | 50 | |||||
| Gion | 2017 | 27 | 8 | 19 | 0 | 69.2 | 55-82 | 27 | - | NR | NR | 4 | 4 | 2 | 17 | 0 | 25 | |||||||
| Oebisu | 2018 | 1 | 0 | 1 | 0 | 46 | - | 1 | - | 5 | - | 5 | - | 0 | 0 | 0 | 1 | 0 | 51 | |||||
| Suzuki | 2018 | 1 | 0 | 1 | 0 | 66 | - | 1 | - | 7 | - | 7 | - | 0 | 0 | 0 | 1 | 0 | 52 | |||||
| Tokuhira | 2018 | 14 | 5 | 9 | 0 | 70.7 | 54-88 | 13 | PV1 | 15.3 | 1.3-32.4 | 6.7 | 1.1-15.1 | 2 | 1 | 1 | 10 | 0 | 16 | |||||
| Total | 66 | 19 | 47 | 0 | 68 | 64 | 3 | 13.5 | 6.1 | 12 | 7 | 6 | 39 | 2 | ||||||||||
| MTX- DLBCL- NOS |
Ebeo | 2003 | 1 | 1 | 0 | 0 | 54 | - | 1 | - | 12 | - | 5 | - | 0 | 0 | 0 | 1 | 0 | 53 | ||||
| Kojima | 2006 | 3 | 0 | 3 | 0 | 66 | 65-68 | 3 | - | 13.7 | 1-35 | 2 | 1-3 | 0 | 0 | 0 | 3 | 0 | 36 | |||||
| Miyazaki | 2007 | 6 | 3 | 3 | 0 | 65 | 53-77 | 6 | - | 3.2 | 1.6-13.4 | 4.3 | 0.8-9.5 | 0 | 1 | 3 | 2 | 0 | 37 | |||||
| Rizzi | 2009 | 4 | 2 | 1 | 1 | 63 | 58-69 | 2 | PV2 | 12 | 5-20 | 5.5 | 3-7 | 2 | 0 | 0 | 0 | 2 | 40 | |||||
| Yamakawa | 2014 | 7 | 3 | 4 | 0 | 68 | 52-74 | 7 | - | ND | 4.1 | 0.6-8.2 | 1 | 4 | 0 | 2 | 0 | 42 | ||||||
| Kameda | 2014 | 4 | 2 | 2 | 0 | 70 | 61-80 | 4 | - | 17.5 | 10-34 | 10.8 | 7-16 | 2 | 0 | 1 | 1 | 0 | 43 | |||||
| Kawano | 2014 | 4 | 3 | 1 | 0 | 65.5 | 60-76 | 4 | - | 8.8 | 3-15 | 4.8 | 3-7 | 1 | 0 | 1 | 2 | 0 | 44 | |||||
| Kawahara | 2015 | 1 | 1 | 0 | 0 | 64 | - | 1 | - | 2 | - | 2 | - | 0 | 0 | 0 | 1 | 0 | 54 | |||||
| Miyagawa | 2015 | 1 | 0 | 1 | 0 | 56 | - | 1 | - | 7 | - | 2 | - | 0 | 0 | 0 | 1 | 0 | 55 | |||||
| Gion | 2017 | 6 | 2 | 4 | 0 | 66 | 58-72 | 6 | - | NR | NR | 0 | 1 | 0 | 5 | 0 | 25 | |||||||
| Tokuhira | 2018 | 13 | 3 | 10 | 0 | 67 | 41-77 | 12 | SLE1 | 8.4 | 3.3-19.1 | 4.2 | 1.1-8.4 | 0 | 2 | 1 | 10 | 0 | 16 | |||||
| Total | 50 | 20 | 29 | 1 | 66 | 46 | 3 | 11.1 | 5.4 | 6 | 8 | 6 | 28 | 2 | ||||||||||
M, men; F, female, EBV, Epstein-Barr virus; NOS, not otherwise specified; NR, not recorded; RA, rheumatoid arthritis; CD, Crohn disease; DM, dermatomyositis; SjS, Sjögren syndrome; PV, psoriasis vulgaris; SLE, systemic lupus erythematosus; Ref, reference.
Table 2. Clinical outcome of diffuse large B cell lymphoma (DLBCL) in methotrexate (MTX) associated lymphoproliferative disorders.
| Subtype | Author | Year | N | Pattern | Chemotherapy | Alive/Dead | Alive | Dead | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pattern | Duration (M) | Pattern | Duration (M) | ||||||||||||||||||||||||||||||
| R | R/R | P | Ch | Other | A | D | NR | R | R/R | P | Ch | R | RR | P | Chemo | R | R/R | P | Ch | Other | R | RR | P | Chemo | |||||||||
| MTX- EBV+ DLBCL |
Kojima | 2006 | 1 | 0 | 0 | 0 | 1* | 0 | * NR | 1 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | 4 | 0 | 0 | 0 | 0 | - | - | - | - | - | ||||
| Miyazaki | 2007 | 3 | 2 | 0 | 1* | 0 | 0 | * R-CHOP | 3 | 0 | 0 | 2 | 0 | 1 | 0 | 109, 18 | - | 26 | - | 0 | 0 | 0 | 0 | - | - | - | - | - | |||||
| Jois | 2007 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 8 | - | - | - | 0 | 0 | 0 | 0 | - | - | - | - | - | ||||||
| Ogasawara | 2007 | 1 | 0 | 0 | 0 | 1* | 0 | * CHOP | 1 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | 24 | 0 | 0 | 0 | 0 | - | - | - | - | - | |||||
| Rizzi | 2009 | 4 | 4 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 4 | 0 | 0 | 0 | 16, 48, 11, 30 | - | - | - | 0 | 0 | 0 | 0 | - | - | - | - | - | ||||||
| Ishida | 2013 | 1 | 0 | 0 | 0 | 1* | 0 | * R-THP-COP | 1 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | 30 | 0 | 0 | 0 | 0 | - | - | - | - | - | |||||
| Yamakawa | 2014 | 2 | 0 | 0 | 0 | 2* | 0 | * R-CHOP | 1 | 1 | 0 | 0 | 0 | 0 | 1 | - | - | - | 45 | 0 | 0 | 0 | 1 | - | - | - | - | 90 | |||||
| Kameda | 2014 | 2 | 1 | 0 | 0 | 1* | 0 | * R-CHOP | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 15 | - | - | 6 | 0 | 0 | 0 | 0 | - | - | - | - | - | |||||
| Kawano | 2014 | 1 | 0 | 0 | 1* | 0 | 0 | * Radiation | 1 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | ND | - | 0 | 0 | 0 | 0 | - | - | - | - | - | |||||
| Kudoh | 2014 | 1 | 0 | 0 | 0 | 1* | 0 | * R-CHOP | 1 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | 48 | 0 | 0 | 0 | 0 | - | - | - | - | - | |||||
| Shimizu | 2015 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 96 | - | - | - | 1 | 0 | 0 | 0 | - | 2 | - | - | - | ||||||
| Ikeda | 2016 | 1 | 0 | 0 | 1* | 0 | 0 | * R-CHOP | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 12 | - | - | - | 0 | 0 | 0 | 0 | - | - | - | - | - | |||||
| Matsumoto | 2016 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 7 | - | - | - | 0 | 0 | 0 | 0 | - | - | - | - | - | ||||||
| Hatano | 2017 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 24 | - | - | - | 0 | 0 | 0 | 0 | - | - | - | - | - | ||||||
| Takei | 2017 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 12 | - | - | - | 0 | 0 | 0 | 0 | - | - | - | - | - | ||||||
| Gion | 2017 | 27 | 20 | 0 | 2* | 4** | 1*** | * R-CHOP, ** R-THP-CO 1, R-CHOP 3, *** found on autopsy |
27 | 0 | 0 | 20 | 1 | 2 | 4 | Mean 30.8 (2.9-78) | 5.2 | 6, 30 | 3.3, 10, 4, 3.6 | 0 | 0 | 0 | 0 | - | - | - | - | - | |||||
| Oebisu | 2018 | 1 | 0 | 1* | 0 | 0 | 0 | * R-CHOP+AlloSCT | 1 | 0 | 0 | 0 | 1 | 0 | 0 | - | ND | - | - | 0 | 0 | 0 | 0 | - | - | - | - | - | |||||
| Suzuki | 2018 | 1 | 0 | 0 | 1* | 0 | 0 | * Steroid pulse | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1.5 | - | - | - | 0 | 0 | 0 | 0 | - | - | - | - | - | |||||
| Tokuhira | 2018 | 14 | 8 | 1* | 5** | 0 | 0 | * R-CHOP, ** CHOP3, PSL 2 |
10 | 4 | 0 | 7 | 1 | 2 | 0 | Mean 70 (22-145) | 24 | 17, 208 | - | 1 | 0 | 3 | 0 | - | 70 | - | 1, 1, 11 | - | |||||
| Total | 66 | 41 | 2 | 11 | 11 | 1 | 60 | 6 | 0 | 39 | 3 | 8 | 10 | 2 | 0 | 3 | 1 | 0 | |||||||||||||||
| MTX- DLBCL- NOS |
Ebeo | 2003 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 24 | 0 | 0 | 0 | 0 | - | - | - | - | - | ||||||||
| Kojima | 2006 | 3 | 0 | 0 | 0 | 3* | 0 | * NR | 1 | 2 | 0 | 0 | 0 | 0 | 1 | - | - | - | 48 | 0 | 0 | 0 | 2 | - | - | - | - | 12, 4 | |||||
| Miyazaki | 2007 | 6 | 0 | 0 | 6* | 0 | 0 | * R-CHOP 5, CyclOBEAP 1 | 6 | 0 | 0 | 0 | 0 | 6 | 0 | - | - | 9, 58, 43, 37, 3, 5 | - | 0 | 0 | 0 | 0 | - | - | - | - | - | |||||
| Rizzi | 2009 | 4 | 1 | 0 | 0 | 1* | 2** | * CHOP **NR | 2 | 0 | 2 | 1 | 0 | 0 | 1 | 6 | - | - | 15 | 0 | 0 | 0 | 0 | - | - | - | - | - | |||||
| Yamakawa | 2014 | 7 | 2 | 0 | 0 | 5* | 0 | * Rituximab 1, R-CHOP 5 | 6 | 1 | 0 | 2 | 0 | 0 | 4 | 32, 14 | - | - | 48, 84, 8, 15 |
0 | 0 | 0 | 1 | - | - | - | - | 27 | |||||
| Kameda | 2014 | 4 | 1 | 0 | 0 | 3* | 0 | * R-CHOP 3 | 2 | 2 | 0 | 1 | 0 | 0 | 1 | 15 | - | - | 30 | 0 | 0 | 0 | 2 | - | - | - | - | ND, 15 | |||||
| Kawano | 2014 | 4 | 1 | 1* | 2** | 0 | 0 | * R-CHOP, ** Rituximab, R-CHOP |
4 | 0 | 0 | 1 | 1 | 2 | 0 | 35 | 31 | 10, 23 | - | 0 | 0 | 0 | 0 | - | - | - | - | - | |||||
| Kawahara | 2015 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 24 | - | - | - | 0 | 0 | 0 | 0 | - | - | - | - | - | ||||||
| Miyagawa | 2015 | 1 | 0 | 0 | 1* | 0 | 0 | * R-CHOP | 1 | 0 | 0 | 1 | 0 | 0 | 0 | - | - | 6 | - | 0 | 0 | 0 | 0 | - | - | - | - | - | |||||
| Gion | 2017 | 6 | 2 | 0 | 2* | 1** | 1*** | * R-CHOP, CHOP, ** R-CHOP, *** found on autopsy |
3 | 3 | 0 | 2 | 0 | 1 | 0 | 1.8, 29 | - | 10 | - | 0 | 0 | 1 | 1 | 1 | - | - | 22 | 78 | |||||
| Tokuhira | 2018 | 13 | 4 | 2* | 7** | 0 | 0 | * CHOP 2, ** PSL 2, R-CHOP 5 | 8 | 5 | 0 | 4 | 1 | 3 | 0 | 25, 30, 33, 68 | 41 | 21, 21, 24 | - | 0 | 1 | 4 | 0 | - | - | 18 | 1, 2, 3, 55 | - | |||||
| Total | 50 | 13 | 3 | 18 | 13 | 3 | 35 | 13 | 2 | 13 | 2 | 13 | 7 | 0 | 1 | 5 | 6 | 1 | |||||||||||||||
EBV, Epstein-Barr virus; N NOS, not otherwise specified; NR, not recorded; R, regressive group; R/R, relapse/regrowth group; P. persistent group; Ch, chemotherapy-G; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone; R-THP-COP, rituximab, cyclophosphamide, pirarubicin, vincristine and prednisolone; alloSCT, allogenic stem cell transplantation; CyclOBEAP, cyclophosphamide, vincristine, bleomycin, etoposide, doxorubicin, and prednisolone; A, alive; D, dead.
Table 3. Clinical features of classical Hodgkin lymphoma (CHL), polymorphic lymphoproliferative disorders (P-LPD), and non-specific LPD (NS-LPD).
| Subtype | Author | Year | N | Sex | Age | Basal disease | MTX duration | Clinical State | EBV | Comment | Ref | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Types | Duration | Ratio | Latency | ||||||||||||||||||||||||||||
| M | F | Mean | Range | RA | Other | Mean | Range | Mean | Range | I | II | III | IV | + | - | I | II | III | |||||||||||||
| MTX- CHL |
Kojima | 2006 | 3 | 1 | 2 | 37 | 52-63 | 3 | 14.7 | 9-28 | 8.3 | 7-9 | 1 | 0 | 2 | 0 | 2 | 1 | NR | 36 | |||||||||||
| Rizzi | 2009 | 7 | 1 | 6 | 45 | 6-70 | 5 | JRA 2 | 10 | 1-24 | 2.9 | 0.7-10 | 1 | 2 | 2 | 2 | 5 | 1 | NR | 40 | |||||||||||
| Loo | 2013 | 6 | 1 | 5 | 53 | 25-77 | 5 | PM 1 | NR | NR | 1 | 1 | 1 | 3 | 4 | 2 | NR | 72 | |||||||||||||
| Yamakawa | 2014 | 3 | 1 | 2 | 69 | 65-76 | 3 | NR | 3.9 | 2.3-5.5 | 0 | 2 | 1 | 0 | 1 | 2 | 0 | 2 | 0 | 42 | |||||||||||
| Kameda | 2014 | 2 | 1 | 1 | 72 | 65-79 | 2 | 10 | 8-12 | 11.5 | 15-18 | 0 | 0 | 0 | 2 | 1 | 1 | NR | 43 | ||||||||||||
| Takasumi | 2015 | 1 | 0 | 1 | 68 | - | 1 | - | 14 | - | 7 | - | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 73 | |||||||||
| Tsukazaki | 2017 | 1 | 0 | 1 | 88 | - | 1 | - | 14 | - | 6 | - | 0 | 0 | 0 | 1 | 1 | 0 | NR | 74 | |||||||||||
| Gion | 2017 | 17 | 4 | 13 | 65 | 84 | 17 | - | NR | NR | 4 | 0 | 8 | 5 | 14 | 3 | NR | 25 | |||||||||||||
| Eso | 2018 | 1 | 1 | 0 | 83 | - | 1 | - | 6 | - | 6 | - | 0 | 0 | 0 | 1 | 1 | 0 | NR | 75 | |||||||||||
| Tokuhira | 2018 | 10 | 3 | 7 | 65 | 53-79 | 10 | - | 15.5 | 5.6-39 | 5.8 | 2.8-5.6 | 0 | 1 | 1 | 8 | 8 | 2 | NR | 16 | |||||||||||
| Total | 51 | 13 | 38 | 62 | 48 | 3 | 13 | 5.6 | 7 | 6 | 15 | 23 | 38 | 12 | 0 | 3 | 0 | ||||||||||||||
| MTX-polymorphic LPD | Kojima | 2006 | 3 | 1 | 2 | 63 | 57-73 | 3 | - | NR | 3.3 | 2-5 | 3 | 0 | 0 | 0 | 3 | 0 | NR | P-LPD | 36 | ||||||||||
| Komatsuda | 2008 | 1 | 0 | 1 | 63 | - | 1 | - | 14 | - | 14 | - | 0 | 0 | 0 | 1 | 1 | 0 | NR | HLL | 81 | ||||||||||
| Rizzi | 2009 | 2 | 1 | 1 | 40 | 33-47 | 2 | - | 8 | 2.9-13 | 5 | 4.9-5 | 0 | 0 | 0 | 1 | 2 | 0 | NR | HLL | 40 | ||||||||||
| Tokuhira | 2012 | 1 | 1 | 0 | 69 | - | 1 | - | - | 1.1 | - | 0 | 0 | 1 | 0 | 1 | 0 | HLL | 19 | ||||||||||||
| Yamakawa | 2014 | 2 | 1 | 1 | 75 | 71-79 | 2 | - | NR | 8 | 8-8 | 1 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 1 | P-LPD | 42 | |||||||||
| Kawano | 2014 | 1 | 0 | 1 | 56 | - | 1 | - | 5 | - | 2.5 | - | 0 | 0 | 0 | 1 | ND | HLL | 44 | ||||||||||||
| Ohkura | 2015 | 1 | 1 | 0 | 70 | - | 1 | - | 20 | - | 8 | - | 1 | 0 | 0 | 0 | 1 | 0 | NR | HLL | 82 | ||||||||||
| Yamakawa | 2016 | 1 | 1 | 0 | 78 | - | 1 | - | 12 | - | 4 | - | 0 | 0 | 0 | 1 | 1 | 0 | NR | P-LPD | 83 | ||||||||||
| Tsukui | 2018 | 5 | 0 | 5 | 72 | 63-78 | 5 | - | NR | 10 | 2-15 | ND | 3 | 4 | 0 | NR | P-LPD | 84 | |||||||||||||
| Total | 17 | 6 | 11 | 65 | 17 | 0 | 11 | 7.2 | 5 | 0 | 2 | 7 | 15 | 0 | 1 | 0 | 1 | ||||||||||||||
| MTX- NS- LPD |
Tokuhira | 2018 | 9 | 4 | 5 | 65 | 45-80 | 8 | PV 1 | 16 | 5.4-37.8 | 8.9 | 4.2-16.3 | 3 | 3 | 0 | 3 | 4 | 3 | NR | 16 | ||||||||||
EBV, Epstein-Barr virus; NR, not recorded; RA, rheumatoid arthritis; JRA, juvenile rheumatoid arthritis; PM, polymyositis; PV, psoriasis vulgaris: Ref, reference.
The clinical outcomes of MTX-DLBCL are listed in Table 2. The ratios of R-G, R/R-G, P-G, and Ch-G in MTX-EBV+DLBCL and MTX-DLBCL-NOS were 62%, 3%, 17%, 17%, and 26%, 6%, 36%, 26%, respectively. The rate of regressive LPD (R-G+R/R-G) was higher in MTX-EBV+DLBCL than in MTX-DLBCL-NOS (65% and 32%, respectively), supporting the previous report.58 The ratio of patients with RRE in MTX-EBV+DLBCL and MTX-DLBCL-NOS was low (4% and 6%, respectively). The survival rate was higher for MTX-EBV+DLBCL than for MTX-DLBCL-NOS (91% and 60%, respectively) (Table 2). In addition, poorer survival rates were noted for P-G and Ch-G of MTX-DLBCL-NOS (72% and 53%, respectively), whereas higher survival rates were found for R-G of MTX-EBV+DLBCL and MTX-DLBCL-NOS (95% and 100%, respectively). As de novo EBV+DLBCL in elderly patients has a poor prognosis,59-61 the clinical outcome of MTX-EBV+DLBCL reflects a superior OS. Regarding the pathological heterogeneity of OIIA-LPD, LPD resembling DLBCL includes P-LPD, such as EBVMCU, P/L-I, and HLL.1 Furthermore, the definition of EBVMCU includes the clinical manifestations, such as ulceration with EBV-positive cells, whereas P-LPD has similar pathological features to EBVMCU without the clinical manifestation of skin or mucosal ulceration. In addition, the OS for P-LPD was as good as that for EBVMCU. Thus, discriminative clinicopathological diagnosis of EBV+LPD, including EBV+DLBCL, P-LPD, and EBVMCU, in OIIA-LPD remains vague. Previous reports found a similar OS between MTX-EBV+DLBCL and MTX-DLBCL-NOS.16,57 In our series, several patients with MTX-EBV+DLBCL or MTX-DLBCL-NOS in P-G died due to progressive disease.16 Due to the delay of diagnosis of MTX-DLBCL when the disease entity of MTX-LPD was not well recognized, insufficient chemotherapy, such as prednisolone monotherapy, was consequently given to the patients, resulting in poor outcomes. Therefore, withdrawal of ISDs should be first performed to prevent LPD aggravation when manifestations resembling MTX-LPD, such as fever, lymphoadenopathy, or respiratory symptoms, and laboratory abnormalities, such as increased serum LDH, CRP, and sIL-2R, develop (described in “Clinical-MTX-LPD”). The standard therapy for MTX-DLBCL patients with P-G or Ch-G is R-CHOP-based chemotherapy (Table 2). Rituximab monotherapy is occasionally given to MTX-DLBCL patients considering the LPD and AID activity in clinical practice.44,62 Although rituximab monotherapy can be effective against AIDs, especially RA, it is not suitable to cure DLBCL. Other MTX-DLBCL cases are also reported.24,56-59,63-71
MTX-CHL
Fifty-one cases of MTX-CHL from 11 reports are summarized in Tables 3 and 4.16,25,36,40,42,43,72-75 Although one study described the pathological diagnosis as CHL-type,25 such cases were categorized into MTX-CHL in this article. Thirty-eight of the patients were female, and the mean age was 62 years, which is older than that for de novo CHL.58 Ninety-four percent of patients (48/51) had RA or other AIDs, such as juvenile RA (JRA, N=1), and polymyositis (PM, N=1). The mean duration of AID and MTX administration was 13 years and 5.6 years, respectively. The rate of CS 3 and 4 was 75% (41/55). EBV positivity was noted in 76% (38/51). Among patients with MTX-CHL in our series, the median serum LDH, CRP, and sIL-2R levels were 214 IU/L, 12.3 mg/dL, and 3110 U/mL, respectively.16 The CRP level was significantly higher in CHL than in DLBCL.16 Clinical outcomes of MTX-CHL are shown in Table 4. The incidence rates of R-G, R/R-G, P-G, and Ch-G were 14%, 40%, 24%, and 21%, respectively. A lower incidence rate of R-G (14%) and a higher rate of R/R-G (40%) were noted for MTX-CHL than for DLBCL, suggesting frequent RRE in MTX-CHL. Chemotherapy was administered to 86% of patients with MTX-CHL (Table 4). Kuita et al. compared 26 patients with MTX-CHL among 219 patients with MTX-LPD, and all of them received chemotherapy with a median progression-free survival of 5 months.15 Chemotherapy was based on ABVD, although R-CHOP was also given (Table 4). Regarding OS, 78% of MTX-CHL patients (39/50) survived, although the survival rate differs among studies. For example, MTX-CHL patients in our series with a relatively long observation period (median, 4.4 years) had a poorer OS (5-year OS, 33%)16 than those in other studies.15,25 The survival rate in R-G, R/R-G, P-G, and Ch-G was 100%, 65%, 67%, and 100%, respectively (Table 4). As the observation duration of 4 MTX-CHL patients in R-G was shorter than 2 years, the ratio in R-G may further decrease during the long-follow up. The pathological features of P-LPD, including HLL and EBVMCU, often resemble those of CHL, with Reed-Sternberg-like cells, explaining the difficulty in diagnosis of MTX-CHL. In contrast, the lower rate of R-G in MTX-CHL may be because P-LPD cases are not categorized as MTX-CHL.
Table 4. Clinical outcome of classical Hodgkin lymphoma (CHL), polymorphic lymphoproliferative disorders (P-LPD), and non-specific LPD (NS-LPD).
| Subtype | Author | Year | N | Pattern | Therapy | Alive/Dead | Alive | Dead | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pattern | Duration (M) | Pattern | Duration (M) | |||||||||||||||||||||||||||||
| R | R/R | P | Ch | ND | A | D | ND | R | R/R | P | Ch | R | RR | P | Chemo | R | R/R | P | Ch | R | RR | P | Chemo | |||||||||
| MTX-CHL | Kojima | 2006 | 3 | 0 | 0 | 0 | 3* | 0 | * NR | 3 | 0 | 0 | 0 | 0 | 0 | 3 | - | - | - | 66, 74, 24 | 0 | 0 | 0 | 0 | - | - | - | - | ||||
| Rizzi | 2009 | 6 | 2 | 1* | 2* | 1* | 0 | *NR | 6 | 0 | 0 | 2 | 1 | 2 | 1 | 12, 12 | 12 | NR | 18 | 0 | 0 | 0 | 0 | - | - | - | - | |||||
| Loo | 2013 | 6 | 0 | 0 | 0 | 5* | 1 | * ABVD 3, CHOP 1, COPP 1 | 5 | 0 | 1 | 0 | 0 | 0 | 5 | - | - | - | 72, 12, 27, 72, 72 | 0 | 0 | 0 | 0 | - | - | - | - | |||||
| Yamakawa | 2014 | 3 | 1 | 2 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 79 | - | - | 0 | 1 | 0 | 0 | - | 17 | - | - | ||||||
| Kameda | 2014 | 2 | 0 | 1* | 0 | 1* | 0 | *ABVD | 2 | 0 | 0 | 0 | 1 | 0 | 1 | - | 15 | - | 90 | 0 | 0 | 0 | 0 | - | - | - | - | |||||
| Takasumi | 2015 | 1 | 0 | 0 | 1* | 0 | 0 | * No therapy due to PD | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | 0 | 0 | 1 | 0 | - | 1 | - | - | |||||
| Tsukazaki | 2017 | 1 | 0 | 0 | 0 | 1* | 0 | * ABVD | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 24 | - | - | - | 0 | 0 | 0 | 0 | - | - | - | - | |||||
| Gion | 2017 | 17 | 4 | 7* | 6** | 0 | 0 | * ABVD, ** ABVD, 6, R-CHOP base 1 | 16 | 1 | 0 | 4 | 7 | 5 | 0 | - | - | - | - | 0 | 0 | 1 | 0 | - | - | - | - | |||||
| Eso | 2018 | 1 | 0 | 0 | 1* | 0 | 0 | *rituximab→brentuximab vedotin→nivolumab | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | 0 | 0 | 1 | 0 | - | - | ND | - | |||||
| Nakazato | 2018 | 1 | 0 | 0 | 1* | 0 | 0 | * ABVD→brentuximab vedotin | 1 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | 12 | - | 0 | 0 | 0 | 0 | - | - | - | - | |||||
| Tokuhira | 2018 | 10 | 0 | 9* | 1* | 0 | 0 | * ABVD | 3 | 7 | 0 | 0 | 3 | 0 | 0 | - | 61, 67, 177 | - | - | 0 | 6 | 1 | 0 | - | Mean 28 (10-58) | 24 | - | |||||
| Total | 51 | 7 | 20 | 12 | 11 | 1 | 39 | 11 | 1 | 7 | 13 | 8 | 11 | 0 | 7 | 4 | 0 | |||||||||||||||
| MTX-polymorphic LPD | Kojima | 2006 | 3 | 2 | 1* | 0 | 0 | 0 | * NR | 2 | 1 | 0 | 2 | 0 | 0 | 0 | 3, 1 | - | - | - | 0 | 1 | 0 | 0 | - | 12 | - | - | ||||
| Komatsuda | 2008 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 5 | - | - | - | 0 | 0 | 0 | 0 | - | - | - | - | ||||||
| Rizzi | 2009 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 9, ND | - | - | - | 0 | 0 | 0 | 0 | - | - | - | - | ||||||
| Tokuhira | 2012 | 1 | 0 | 1* | 0 | 0 | 0 | * ABVD, C-MOPP, CHOP | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | 0 | 1 | 0 | 0 | - | 7 | - | - | |||||
| Yamakawa | 2014 | 2 | 1 | 0 | 0 | 1* | 0 | * R-CHOP | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 37 | - | - | 9 | 0 | 0 | 0 | 0 | - | - | - | - | |||||
| Kawano | 2014 | 1 | 0 | 1* | 0 | 0 | 0 | * ABVD | 1 | 0 | 0 | 0 | 1 | 0 | 0 | - | 21 | - | - | 0 | 0 | 0 | 0 | - | - | - | - | |||||
| Ohkura | 2015 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 12 | - | - | - | 0 | 0 | 0 | 0 | - | - | - | - | ||||||
| Yamakawa | 2016 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | - | - | - | 0 | 0 | 0 | 0 | - | - | - | - | ||||||
| Tsukui | 2018 | 5 | 5 | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 4 | 0 | 0 | 0 | ND | - | - | - | 1 | 0 | 0 | 0 | ND | - | - | - | ||||||
| Total | 17 | 13 | 3 | 0 | 1 | 0 | 14 | 3 | 0 | 12 | 1 | 0 | 1 | 1 | 2 | 0 | 0 | |||||||||||||||
| MTX- NS- LPD |
Tokuhira | 2018 | 9 | 8 | 1* | 0 | 0 | 0 | * PSL (poor PS) | 8 | 1 | 0 | 8 | 1 | 0 | 0 | Mean 67 (22-194) | - | - | - | 0 | 1 | 0 | 0 | - | 24 | - | - | ||||
EBV, Epstein-Barr virus; NR, not recorded; ND-LPD, non-distractive LPD; R, regressive group; R/R, relapse/regrowth group; P. persistent group; ABVD, doxorubicin, bleomycin, vinblastine and dacarbazine; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisolone; COPP, clophosphamide, vincristine, procarbazine, and prednisolone; PD, progressive disease; R-COP, rituximab, cyclophosphamide, vincristine and prednisolone; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone.; C-MOPP, cyclophosphamide, vincristine, procarbazine, prednisolone; PD, progression disease; A, alive; D, dead.
Regarding the clinical management, the OS is poorer for MTX-CHL patients in R/R-G (65%) than those in Ch-G (100%) (Table 4). If RRE occurs at a higher rate in MTX-CHL, as found in this analysis, chemotherapy without WW or during WW is one of the options to improve OS, but such a strategy may be less advantageous for patients in R-G (14%). Several studies have investigated predictive surrogate markers for RRE; one such marker is decreased ACL in PB at the time of LPD development or RRE, as mentioned for LPD regression.26-29 In our report, an ALC < 1000/µL at the time of LPD development is predictive of RRE.30 Regarding other therapy options, several drugs, such as anti-PD-1 antibody and anti-CD30 antibody, can be applied for de novo CHL.75,76 Eso et al. described a MTX-CHL patient with oral ulceration receiving rituximab, brentuximab vedotin (BV), and nivolumab during the clinical course.75 The response was insufficient and the patient died due to disease progression. In another case report, Nakazato et al. reported a patient with RA-MTX-CHL who relapsed after ABVD administration, and she achieved complete response (CR) and remission of RA by BV administration as salvage therapy.76 Other cases of MTX-CHL have been previously reported.37,44,57,65,66,77-79 Rizz et al.40 and Miranda et al.80 also reviewed cases of CHL in OIIA-LPD.
MTX-P-LPD
B-LPD among IA-LPD usually exhibits EBV-related lesions, including hyperplasia, P-LPD, aggressive lymphomas, and rarely, indolent lymphomas.1 The subtypes of PTLD, commonly EBV-mediated, are categorized into 3 types: ND-LPD, P-LPD, and M-LPD.1 Although the former 2 subtypes are not clearly defined in OIIA-LPD, the subtypes of polymorphic/lymphoplasmacytic-LPD (the 2008 WHO classification)1 or P/L-I (the 2017 WHO classification),2 and HLL have consistent features with P-LPD.1 To clarify this issue in OIIA-LPD, Kurita et al. proposed 3 categories of OIIA-LPD as defined for PTLD: reactive hyperplasia LPD (RH-LPD) as ND-LPD, P-LPD, and M-LPD.15 In their analysis, P-LPD, consisting of P/L-I, HLL, and other p-LPD, accounted for 15% of 219 patients with MTX-LPD. The clinical data for P-MTX-LPD based on the previous reports are shown in Tables 3 and 4.19,36,40,42,44,81-84 Among 17 patients with MTX-P-LPD, 11 and 6 were consistent with P-LPD and HLL, respectively. There were six male and 11 female patients. The mean age was 65 years, and all patients had RA. The mean duration of RA and MTX was 11 years and 7.2 years, respectively. Regarding tumor involvement, 41% of the patients had extranodal lesions, whereas 29% had localized lesions (Table 3). EBV positivity, based on EBER-1 staining, was 100% among 14 patients with P-LPD for whom EBER-1 staining was performed. Thirteen of the 17 MTX-P-LPD patients (76%) were in R-G, whereas 3 patients and 1 patient were in R/R-G and Ch-G, respectively (Table 4). Patients in R/R-G and Ch-G received ABVD and R-CHOP, respectively. The survival rate of the 17 patients with MTX-P-LPD was 82.3% (14/17). Among the patients with MTX-P-LPD, the patients with ulceration or localized mucosal lesions were categorized into EBVMCU, although the diagnosis was based on the original article in this list. The recommended chemotherapy for aggressive or relapsed P-LPD has yet to be defined, although some strategies, such as ABVD for HLL and R-CHOP for P-LPD, have been successful. Patients with MTX-P-LPD are also described in several reports.57,85,86
MTX-EBVMCU
EBVMCU is a newly recognized clinicopathological entity in the 2017 WHO classification occurring in elderly patients or due to ISD administration.1 However, our understanding of this disease is limited because of its broad clinical and pathological features. Data for 32 cases of MTX-EBVMCU are presented in Table 5.78,87-97 Twenty-seven female and five male patients were included. The mean age (70 years) was higher than that for other subtypes. Twenty-eight patients had RA, and they all had dermatomyositis, PM, Crohn's disease, and Sjögren’s syndrome. The oral mucosa was the principal site, and cases involving the tongue, skin, eyelid, lip, nose, buccal mucosa, colon, rectum, or lung were also reported. Among 32 cases, 26 cases were in R-G, whereas 1 case, 4 cases, and 1 case were in R/R-G, P-G and Ch-G, respectively. Regarding chemotherapy, R-CHOP and ABVD were used for patients in P-G, and ABVD was used for patients in R/R-G (Table 5). The survival rate was 97% (31/32), but one patient died due to disease progression. Daroontum et al. reported a patient with MTX-EBVMCU who developed EBV+ P-LPD and EBV+DLBCL within 3 years.95 Similar to MTX-P-LPD, chemotherapy for aggressive MTX-EBVMCU is decided according to the pathological features of M-LPD such as DLBCL and CHL. In this analysis, such chemotherapy was found to be effective for aggressive MTX-EBVMCU; however, whether the strategy of chemotherapy based on pathological features is suitable for MTX-EBVMCU requires further investigation. Other MTX-EBVMCU cases have been reported.22,65,78,99-111 Sinit et al. reviewed 100 patients with EBVMCU separated by age, ISD, and primary/acquired immunodeficiency.112
Table 5. Clinical features and outcome of Epsiten-Barr virus-positive mucocutaneous ulcer (EBVMCU).
| Subtype | Author | Year | N | Sex | Age | Basal disease | MTX duration | Tumor sites | Pattern | LPD therapy | Alive /Dead |
OS (M) | Ref | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Types | Duration | ||||||||||||||||||||||||||||||||||
| M | F | Mean | Range | RA | Other | Mean | Range | Mean | Range | oral | skin | tongue | eyelid | other | R | R/R | P | Ch | A | D | Mean | Range | |||||||||||||
| MTX-EBVMCU | Satoh | 2009 | 1 | 0 | 1 | 73 | - | 1 | - | NR | 5 | - | 0 | 0 | 0 | 0 | Gastric | 1 | 0 | 0 | 0 | 1 | 0 | 8 | - | 87 | |||||||||
| Dojcinov | 2010 | 1 | 0 | 1 | 80 | - | 1 | - | NR | NR | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 60 | - | 78 | ||||||||||||
| Attard | 2012 | 1 | 0 | 1 | 81 | - | 1 | NR | NR | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 12 | - | 88 | |||||||||||||
| Hashizume | 2012 | 1 | 0 | 1 | 62 | - | 0 | DM 1 | NR | 7 | - | 0 | 0 | 0 | 1 | (+Lip, Nose) | 1 | 0 | 0 | 0 | 1 | 0 | 1 | - | 89 | ||||||||||
| Yamakawa | 2014 | 5 | 0 | 5 | 63 | 56-76 | 4 | PM 1 | NR | 4.8 | 0.3-10 | 0 | 2 | 0 | 2 | Bucca Mucosa | 4 | 0 | 0 | 1* | * R-CHOP | 4 | 0 | 24 | 1-37 | 42 | |||||||||
| Sadasivam | 2014 | 1 | 0 | 1 | 66 | - | 1 | NR | NR | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 19 | - | 90 | |||||||||||||
| Moran | 2015 | 1 | 0 | 1 | 53 | - | 0 | CD 1 | NR | 1 | - | 0 | 0 | 0 | 0 | Colon, Rectum | 0 | 1* | 0 | 0 | * ABVD | 1 | 0 | 24 | - | 91 | |||||||||
| Hashimoto | 2015 | 2 | 0 | 2 | 74 | 74, 74 | 2 | - | 3.2 | 0.5, 6 | 2.7 | 0.5, 5 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 30 | 12-48 | 92 | ||||||||||
| Nakauyaca | 2016 | 1 | 0 | 1 | 67 | - | 1 | - | 20 | - | 20 | - | 0 | 0 | 1 | 0 | 0 | 0 | 1* | 0 | * No therapy due to death | 0 | 1 | 0 | - | 93 | |||||||||
| Chen | 2017 | 1 | 0 | 1 | 58 | - | 1 | - | NR | NR | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 9 | - | 94 | ||||||||||||
| Furudate | 2017 | 1 | 1 | 0 | 74 | - | 1 | - | NR | 7 | - | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 24 | - | 95 | |||||||||||
| Daroontum | 2018 | 7 | 0 | 7 | 75 | 61-85 | 6 | SjS 1 | 10 | 2-22 | 4 | 2-10 | 4 | 2 | 1 | 0 | 6 | 0 | 1* | 0 | * R-CHOP | 7 | 0 | 40 | 31-81 | 96 | |||||||||
| Satou | 2018 | 9 | 4 | 5 | 72 | 48-87 | 9 | - | NR | 8.3 | 4-19 | 8 | 0 | 1 | 0 | 7 | 0 | 2* | 0 | * R-CHOP, ABVD | 9 | 0 | 45 | 8-139 | 97 | ||||||||||
| Total | 32 | 5 | 27 | 70 | 28 | 4 | 9.4 | 6.3 | 17 | 5 | 4 | 3 | 3 | 26 | 1 | 4 | 1 | 31 | 1 | 32 | |||||||||||||||
ND, not described; RA, rheumatoid arthritis; PM, polymyositis; PV, psoriasis vulgaris. EBV, Epstein-Barr virus; ND, not described; DM, dermatomyositis; PM, polymyositis; CD, Crohn disease; R, regressive group; R/R, relapse/regrowth group; P. persistent group; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone; ABVD, doxorubicin, bleomycin, vinblastine and dacarbazine; A, alive; D, dead: OS ,overall survival; Ref, reference.
MTX-ND / RH / non-specific (NS) - LPD
ND-LPD in PTLD is categorized into three subtypes, plasmacytic hyperplasia, infectious mononucleosis, and florid follicular hyperplasia.1 In contrast, it is not defined for OIIA-LPD in the WHO classification, although it is common. Several cases of MTX-ND-LPD as RH or NS have been reported.15,16,57 In our study, nine patients with MTX-NS-LPD were detected among 62 patients with MTX-LPD (14.5%), with the third highest incidence following DLBCL and CHL (Table 3).16 The mean age was 65 years, and the AIDs included 8 cases of RA and 1 case of psoriasis vulgaris. The mean duration of RA and MTX administration was 16 years and 8.9 years, respectively. The CS of 6 patients was 1 or 2, and 3 patients had CS 4. Four of 7 (57%) patients were EBV+. Eight patients were categorized into R-G, whereas one patient who died due to EBV-LPD after RRE was categorized into R/R-G (Table 4). In our series, the median serum LDH, CRP, and sIL-2R in these 9 patients were 243 (range, 170-414) U/L, 2.0 (range, 0.3-9) mg/dL, and 1810 (range, 716-4510) U/L, respectively.16 In a report by Kurita et al., all 28 MTX-RH-LPD patients with ND-LPD survived without chemotherapy, although 3 patients (11%) had stage IV disease.15
Regarding the management of MTX-ND/RH/NS-LPD, WW is useful because the major clinical course of this subtype is R-G. Similar to P-LPD, the strategy for patients with aggressive or relapsed MTX-ND/RH/NS-LPD is not defined. In previous reports, a number of other cases were demonstrated to be MTX-LPD.110-125 We treated several patients with MTX-ND-LPD that changed to CHL during the clinical course; one patient developed severe EBV infection, and 2 diagnosed with ND-MTX-LPD developed CHL during WW.126 The remaining issues, such as the definition of this category and management of aggressive MTX-ND/RH/NS-LPD, along with other issues specific to each type of MTX-LPD require further investigation.
Other MTX-LPD
Several other subtypes of MTX-LPD, including low grade B cell lymphomas, such as follicular lymphoma (FL),1,2,19,57,65,71 mucosa-associated lymphoid tissue (MALT) lymphoma,1,2,19,36,57,65,66,71,127-129 Burkitt lymphoma (BL),1,2,57,130 intravascular lymphoma (IVL),131,132 lymphoplasmacytic lymphoma,65 plasmablastic lymphoma,19,71 adult T-cell leukemia/lymphoma (ATLL),133 angioimmunoblastic T-cell lymphoma (AITL),1,2,134,135 anaplastic large cell lymphoma,1,2,65,136,137 subcutaneous panniculitis-like T-cell lymphoma (SPLTCL),138 peripheral T-cell lymphoma (PLCL),1,2,19,36,37,57,139-141 and extranodal natural killer/T (NK/T)-cell lymphoma,1,2,66,71,142-144 have been reported. Their clinical patterns vary. Regressive LPD after MTX withdrawal develops in FL, MALT lymphoma, ATLL, NK/T-cell lymphoma, SPLTCL, and PLTL, whereas some diseases relapse. In contrast, some patients with BL, IVL, or NK/T-cell lymphoma were classified into P-G. EBV was detected in MTX-low-grade lymphomas, which is commonly negative in de novo low-grade lymphomas. For example, EBV + FL1 and MALT lymphoma cases in R-G that are EBV+ have been reported.129 Regarding LPD regression, EBV-negative LPD, including NK/T-lymphoma and PTCL, also have a regressive phase.66,141 Due to the small number of patients and the limited information on each subtype, assessment of optimal clinical management, such as WW or chemotherapy when LPD develops, requires further investigation in a larger patient population.
NON-MTX-LPD
A number of studies described non-MTX-LPD in comparison with MTX-LPD, although non-MTX-LPD-to-MTX-LPD ratios vary.23,24,57,65,66,71,128 For example, Yamada et al. reviewed the background of OIIA-B-LPD,23 and described that 25% of OIIA-LPD is associated with non-MTX drugs. Ichikawa et al. reported 17 patients with non-MTX-associated LPD among 102 patients with OIIA-LPD.57 Furthermore, it is well established that AIDs other than RA develop in LPD patients during ISD treatment.145-153 In addition, many cases of AIDs in regressive LPD patients after the withdrawal of non-MTX ISDs have been reported, suggesting the immunosuppressive effects leading to LPD development.81,154-161 The history of MTX administration should be carefully assessed in patients with non-MTX-LPD because RRE occurs within several years after the withdrawal of MTX, especially in patients with RA.
CLINICAL MTX-LPD
In clinical practice, symptoms resembling LPD, and laboratory data, such as fever, lymphadenopathy, respiratory symptoms, and increased serum LDH/CRP/sIL-2R levels, are not rare. These manifestations may also develop in the pathogeneses of viral infection and AID activity. As a first step, drug withdrawal is tentatively attempted when such manifestations develop, similar to MTX-LPD. In addition, tissue biopsy should be performed to confirm the diagnosis; however, biopsy may fail for patients with rapid LPD regression after MTX withdrawal.44 We analyzed such patients as the clinical MTX-LPD (c-MTX-LPD) group, composed of suspicious-LPD and proven-LPD (Figure 3).162 MTX was withdrawn at the time of suspicious-LPD, and some of those with suspicious-LPD developed MTX-LPD, as confirmed by tissue biopsy (proven-LPD). Among the 28 patients with c-MTX-LPD, 7 developed proven-LPD after suspicious-LPD regression, 6 of whom had CHL and one who had plasmablastic lymphoma. Four of the 7 patients with proven-LPD(+) died, whereas all patients with proven-LPD(-) survived. The clinical manifestations included fever, increased serum CRP (>5 mg/dL), and sIL-2R level >4000 U/L at the time of recurrence, resembling LPD symptoms, suggesting the development of proven-LPD.162 Although the reason is unclear, the subtypes, including EBV+ DLBCL, DLBCL -NOS, P-LPD, and EBVMCU, did not develop under clinical management with prompt MTX withdrawal. As the incidence of R/R-G among these subtypes was low (Tables 2, 4, and 5), such LPD may include suspicious c-MTX-LPD.
Fig. 3.
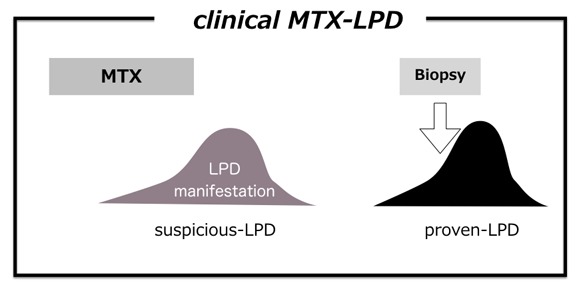
Clinical methotrexate-associated lymphoproliferative disorders (c-MTX-LPD)162
Under MTX administration, MTX withdrawal is attempted when LPD or LPD-like manifestations occur (suspicious-LPD). However, MTX withdrawal often induces prompt regression of suspicious-LPD, making tissue biopsy impossible. After regression of suspicious-LPD, LPD occasionally recurs as relapse/regrowth event, resulting in the LPD diagnosis by tissue biopsy (proven-LPD).
Anti-AID therapy after the development of MTX-LPD
AID activity commonly flares within one year after AID withdrawal in R-G or after chemotherapy in R/R-G and P-G. Thus, safe ISD usage without inducing LPD after OIIA-LPD development has been investigated. The guidelines of the American College of Rheumatology (2015) described several points regarding the usage of AIDs after OIIA-LPD development: very low quality evidence of ISD usage after OIIA-LPD, a possible increased risk of lymphoma associated with TNFi, and no evidence of increased risk by abatacept (ABT) or tocilizumab (TCZ).163 The guidelines of the Japan College of Rheumatology state that ISDs should be avoided as much as possible, and MTX and TNFi are not generally recommended.164,165 As many factors, such as type of AID, LPD subtype, ISD dose and duration, EBV activity, ALC in PB, and age, may be related to the development or relapse of OIIA-LPD, a complete understanding of AID usage after OIIA-LPD is difficult. In the analysis of c-MTX-LPD, seven ISDs, tacrolimus (FK), bucillamine (BUC), salazosulfapyridine (SSZ), infliximab (IFX), ABT, iguratimod (IGU), and adalimumab, were combined with MTX.162 Thirteen types of ISDs were administered after suspicious-LPD, and 21 patients without proven-LPD received 12 ISDs, MTX, FK, BUC, SSZ, IFX, ABT, IGU, etanercept (ETN), golimumab, TCZ, certolizumab pegol, or tofacitinib, after suspicious-LPD regression. On the other hand, 7 patients with proven-LPD received FK, BUC, SSZ, or ETN, which were also administered to patients without proven-LPD. For patients with RA-MTX-LPD in CR, a difference between AIDs before and after MTX-LPD development was not observed.162 From these data, it is difficult to identify the roles of specific ISDs in MTX-LPD development after MTX withdrawal.
PREDICTION OF MTX-LPD
Although the prediction of MTX-LPD is essential to prevent a poor prognosis due to AIDs, no concrete assessment is available. As mentioned earlier, a decrease in ALC in PB is one candidate, although this method is difficult to apply to P-G.27,29 Based on the higher incidence of OIIA-LPD in Japan, several studies have focused on human leukocyte antigen (HLA) analysis. Yamakawa et al. analyzed 20 Japanese patients with MTX-LPD, and found that the allele B*15:11 is closely related to EBV infection.42 Another HLA study on 25 patients with MTX-LPD identified 11 alleles, including A*2402 and DRB1*0405, that were significantly more commonly detected than in the general population.16 However, data on HLA alleles are inconsistent between these two studies. Regarding PTLD, several studies suggested the possibility of prediction of LPD by measuring the EBV viral load166-169; however, standardization of the EBV assay and cut-off values for positive selection are of concern. Impairment due to EBV, as indicated by impaired T-cell response against EBV and increased EBV viral load after MTX administration, has been reported in patients with RA,170-175 and several reports demonstrated the association between EBV viral road and disease activity in patients with juvenile idiopathic arthritis who developed MTX-LPD.176 In contrast, several recent studies reported that the EBV viral load does not increase under the administration of ISDs such as MTX, TNFi, and ABT.177-179
SUMMARY
The data presented in this article are summarized in Table 6, and the specific features of MTX-LPD based on the previous data, including this review, are listed in Table 7. The proposal for clinical management of MTX-LPD is presented in Figure 4. When c-MTX-LPD develops, the first step is the withdrawal of MTX, in addition to differential diagnosis by annual health checks, laboratory investigations, such as serum LDH, CRP, sIL-2R levels, and other examinations for viral infections, basal disease activity, and other considered diseases. Imaging, including computer tomography and positron-emission tomography, is also used for the differential diagnosis. Examinations of bone marrow aspiration/biopsy and the measurement of EBV viral load are performed if necessary. To make a definite diagnosis of LPD, tissue biopsy is required at any time during the clinical course. After withdrawal of MTX, chemotherapy is administered to patients with non-regressive or aggressive LPD (P-G), whereas patients achieving complete response (CR) are followed-up without additional chemotherapy. When LPD relapses or recurs, chemotherapy is initiated (R/R-G). In contrast, the duration of WW is an important issue among patients with residual tumor burden under LPD regression. The management of patients with responses less than CR state after MTX withdrawal is dependent on the physician’s decision whether chemotherapy is required under such situation. In our report, the recovered ALC in PB lasted for at least 2 years in patients with MTX-LPD in R-G, and it gradually decreased after 6 months of MTX withdrawal toward RRE in R/R-G.30 Because the pathogenesis of LPD regression is complexed on the basis of various factors, including LPD subtypes, LDP tumor burden, EBV activation, types or combination of ISDs, and ALC in PB, the definite answer for the duration of WW is not concluded yet. Regarding non-MTX-LPD, the patient accumulation is required to conclude the precious assessment, although the management of non-MTX-LPD might be based on that of MTX-LPD.
Table 6. Summary of methotrexate associated lymphoproliferative disorders (MTX-LPDs).
| MTX-LPD | N | Female (%) | Age* (y) | Basal disease* (y) | MTX duration* (y) | CS 3 or 4 |
EBV+ (%) |
Clinical course (%) | Survival rate (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R-G | R/R-G | P-G | Ch-G | All | R-G | R/R-G | P-G | Ch-G | |||||||||
| EBV+DLBCL | 66 | 71 | 68 | 13.5 | 6.1 | 62 | 100 | 62 | 3 | 17 | 17 | 91 | 95 | 100 | 72 | 90 | |
| DLBCL-NOS | 50 | 59 | 66 | 11.1 | 5.4 | 60 | 0 | 26 | 6 | 36 | 26 | 60 | 100 | 67 | 72 | 53 | |
| CHL | 51 | 74 | 62 | 13 | 5.6 | 75 | 76 | 14 | 40 | 24 | 21 | 78 | 100 | 65 | 67 | 100 | |
| P-LPD | 17 | 64 | 65 | 11 | 7.2 | 64 | 100 | 76 | 18 | 0 | 6 | 82 | 92 | 33 | - | 0 | |
| EBVMUCU | 32 | 84 | 70 | 9.4 | 6.3 | -** | 100 | 81 | 3 | 13 | 3 | 97 | 100 | 100 | 75 | 100 | |
| NS-LPD | 9 | 56 | 65 | 16 | 8.9 | 33 | 57 | 89 | 11 | 0 | 0 | 89 | 100 | 0 | - | - | |
| Total | 225 | 70 | 66 | 12.5 | 6.1 | 54.7 | 70.6 | 48 | 13.5 | 20.3 | 16.4 | 81.3 | 97.2 | 67.7 | 67.2 | 81 | |
EBV, Epstein-Barr virus; DLBCL, diffuse large B cell lymphoma; NOS, not otherwise specified; CHL, classical Hodgkin lymphoma; P-LPD, polymorphic LPD; EBVMCU, EEBV-positive mucocutaneous ulcer; CS, clinical stage; R-G, regression group; R/R-G, relapse/regrowth group; P-G, persistent group; Ch-G, chemotherapy group.
* indicated mean values. ** involved sites were mainly oral cavity and/or skin.
Table 7. Clinicopathological features of methotrexate-associated lymphoproliferative disorders (MTX-LPDs).
| 1. General features - autoimmune disease (majority; rheumatoid arthritis) - female dominant - 5-10-times higher incidence in Japan - median age: 65-70 years at the development of LPD - basal disease duration:10-15 years - MTX duration: 5-10 years |
| 2. Pathological features - MTX-LPDs from numerous cell origins such as B, T, and NK cells - classified into ND/RH/NS-LPD, P-LPD, and M-LPD - P-LPD: HLL and P/L-I - EBVMCU also develops - major M-LPD; DLBCL (30-40%) and CHL (10-20%) - EBV positivity in CHL: 70-80% |
| 3. Clinical features - clinical state 3 or 4: over 50% of MTX-LPD - LPD regression after MTX: 50-70% - three clinical patterns after LPD regression with MTX withdrawal: R-G, R/R-G, and P-G - LPD regression: related to recovery of lymphocytes in peripheral blood (PB) - population of lymphocytes in PB: CD8+T cells and NK cells - lower rate of DLBCL and higher rate of CHL in R/R-G - aggressive LPD is also seen in ND/RH/NS-LPD, P-LPD, and EBVMCU |
| 4. Prognosis - OS of MTX-LPD: 70-85% survived - R-G and EBVMUC: better OS - elderly (>70 years): poor OS |
| 5. Others - usage of ISDs after LPD development: under investigation - prediction of LPD development: under investigation for ALC in PB, HLA alleles, and EBV viral load. |
NK, natural killer; ND, non-destructive; RH, reactive hyperplasia; NS, non-specific; P-LPD, polymorphic LPD; M-LPD, monomorphic LPD; HLL, Hodgkin-like lesions; P/L-I; polymorphic/lymphoplasmacytic infiltrates; EBVMCU; Epstein-Barr virus-positive mucocutaneous ulcer; DLBCL, diffuse large B cell lymphoma; CHL, classical Hodgkin lymphoma; R-G, regressive group; R/R-G, relapse/regrowth group; P-G. persistent group; ISD, immunosuppressive drug; ALC, absolute lymphocyte count; HLA, human leukocyte antigen.
Fig. 4.
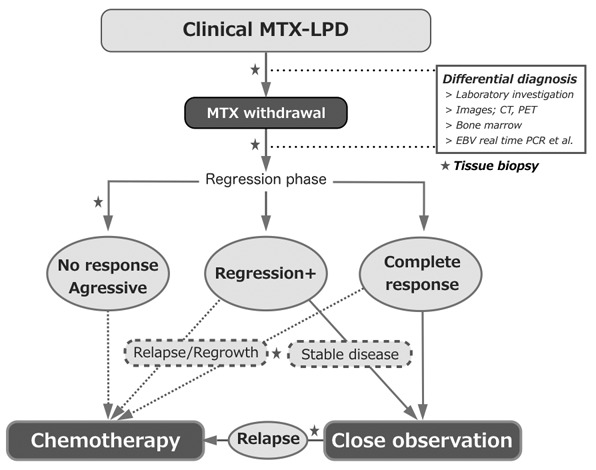
Clinical management of methotrexate-associated lymphoproliferative disorders (MTX-LPD)
When clinical-MTX-LPD develops, MTX withdrawal is the first procedure. In addition to watchful waiting, the differential diagnosis is performed by laboratory investigation and imaging. Bone marrow aspiration/biopsy and the measurement of EBV viral load are considered according to the patient’s clinicopathogenesis. To diagnose LPD, tissue biopsy is required at any time during the clinical course. After MTX withdrawal, chemotherapy is considered for patients with non-regressive LPD or aggressive LPD, and relapse/regrowth after the regressive phase. Among patients with residual LPD under LPD regression, the decision for chemotherapy is based on the physician’s assessment.
The pathogenesis of MTX-LPD and related factors are illustrated in Figure 5. Impairment by EBV, the chronic inflammation process in AIDs, immune abnormality, including genetic background, such as HLA restriction, and aging may underlie IA-LPD. ISD administration accelerates the impairment of anti-LPD immunity, and consequently, LPD develops. The duration between ISD administration and LPD development ranges widely from a few months to over 30 years; therefore, triggers, including a decrease in ALC in PB, may occur at the time of LPD development. After ISD withdrawal, LPD regresses in R-G and R/R-G, but not in P-G. Lymphocytes, such as CD8+ T cells and NK cells, play important roles in LPD regression. When ISDs are administered for AID activity after LPD development, no RRE occurs if the potent immunity against LPD remains (R-G), whereas loosing anti-LPD immunity is categorized in R/R-G. After MTX-LPD development, several factors, such as the subtypes of MTX-LPD, decrease in ALC in PB, EBV reactivation, and impairment of anti-LPD immunity mediated by ISDs, may influence RRE. Although the disease entity of MTX-LPD is still controversial, the phenomenon of regression after withdrawing ISDs suggests the direct influence of MTX on LPD development. MTX-LPD is an important adverse event in AIDs, resulting in a poor outcome.
Fig. 5.
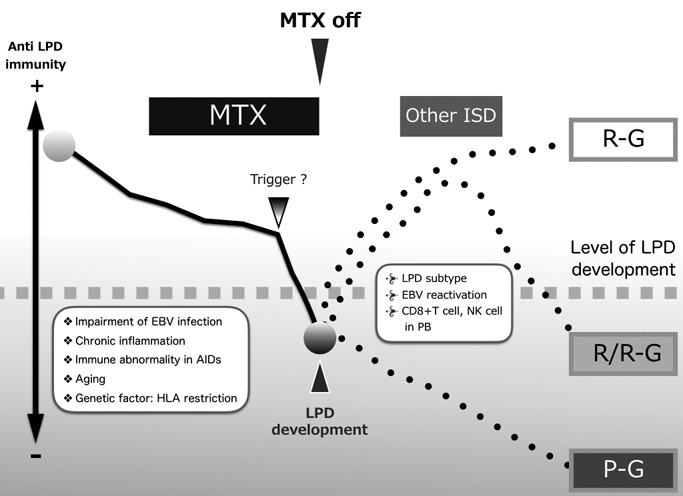
Pathogenesis of methotrexate-associated lymphoproliferative disorders (MTX-LPD)
Regarding the development of MTX-LPD, impairment by EBV, the chronic inflammation process in autoimmune diseases (AIDs), immune abnormality in AIDs, including genetic background, such as human leukocyte antigen (HLA) restriction, may be underlying factors. Immune suppressive drugs (ISDs), including methotrexate (MTX), accelerate the impairment of anti-LPD immunity, and consequently, LPD develops. Triggers, including a decrease in the absolute lymphocyte count (ALC) in peripheral blood (PB), may occur at the time of LPD development. After ISD withdrawal, LPD regression occurs in the regressive group (R-G) and relapse/regrowth group (R/R-G), but not in the persistent group (P-G). Lymphocytes, such as CD8+ T cells and natural killer (NK) cells, play important roles in LPD regression. When ISDs are administered for AID activity after LPD development, no relapse/regrowth event (RRE) occurs if the potent immunity against LPD remains (R-G), whereas the loss of anti-LPD immunity is categorized in R/R-G. After MTX-LPD development, several factors, such as the subtypes of MTX-LPD, decrease in ALC in PB, EBV reactivation, and impairment of anti-LPD immunity mediated by ISDs, may influence RRE.
On the other hand, many ISDs, including MTX and TNFi, have significantly improved the quality of life and OS of patients with AIDs, especially RA. To establish a safe AIDs treatment for patients developing MTX-LPD, issues, such as a standard therapy for each subtype of MTX-LPD, the validation of predictive factors, including ALC, HLA alleles, EBV viral load, and the estimation of OS under long-term observation, need to be assessed to discriminate among MTX-LPD subtypes. In addition, the clinical assessment of non-MTX-LPD is another important issue in OIIA-LPD. As several clinical questions regarding OIIA-LPD, such as whether the management of non-MTX-LPD is similar to that of MTX-LPD, remain, further analyses will be performed for their clarification.
Footnotes
CONFLICT OF INTEREST: TJ received honoraria from Takeda Pharmaceutical Company Limited. The remaining authors declare no competing financial interests.
REFERENCES
- 1.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Immunodeficiency-associated lymphoproliferative disorders. revised 4th ed, IARC publications. 2017; pp. 443-464. [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Immunodeficiency-associated lymphoproliferative disorders. 4th ed, IARC publications. 2008. pp. 335-351. [Google Scholar]
- 3.Harigai M. Lymphoproliferative disorders in patients with rheumatoid arthritis in the era of widespread use of methotrexate: A review of the literature and current perspective. Mod Rheumatol. 2018; 28: 1-8. 10.1080/14397595.2017.1352477 [DOI] [PubMed] [Google Scholar]
- 4.Balandraud N, Guis S, Baptiste Meynard J, et al. Long-term treatment with methotrexate or tumor necrosis factor α inhibitors does not increase epstein-barr virus load in patients with rheumatoid arthritis. Arthritis Rheum. 2007; 57: 762-767. 10.1002/art.22783 [DOI] [PubMed] [Google Scholar]
- 5.Yoshida Y, Takahashi Y, Yamashita H, et al. Clinical characteristics and incidence of methotrexate-related lymphoproliferative disorders of patients with rheumatoid arthritis. Mod Rheumatol. 2014; 24: 763-765. 10.3109/14397595.2013.878016 [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto A, Chiba N, Tsuno H, et al. Incidence of malignancy and the risk of lymphoma in Japanese patients with rheumatoid arthritis compared to the general population. J Rheumatol. 2015; 42: 564-571. 10.3899/jrheum.140533 [DOI] [PubMed] [Google Scholar]
- 7.Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015; 17: 212. 10.1186/s13075-015-0728-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harigai M, Nanki T, Koike R, et al. Risk for malignancy in rheumatoid arthritis patients treated with biological disease-modifying antirheumatic drugs compared to the general population: A nationwide cohort study in Japan. Mod Rheumatol. 2016; 26: 642-650. 10.3109/14397595.2016.1141740 [DOI] [PubMed] [Google Scholar]
- 9.Hellgren K, Baecklund E, Backlin C, et al. Rheumatoid arthritis and risk of malignant lymphoma: is the risk still increased? Arthritis Rheumatol. 2017; 69: 700-708. 10.1002/art.40017 [DOI] [PubMed] [Google Scholar]
- 10.Shimizu Y, Nakajima A, Inoue E, et al. Characteristics and risk factors of lymphoproliferative disorders among patients with rheumatoid arthritis concurrently treated with methotrexate: a nested case-control study of the IORRA cohort. Clin Rheumatol. 2017; 36: 1237-1245. 10.1007/s10067-017-3634-5 [DOI] [PubMed] [Google Scholar]
- 11.Chisaki Y, Aoji S, Yano Y. Analysis of adverse drug reaction risk in elderly patients using the Japanese adverse drug event report (JADER) Database. Biol Pharm Bull. 2017; 40: 824-829. 10.1248/bpb.b16-00930 [DOI] [PubMed] [Google Scholar]
- 12.Mercer LK, Regierer AC, Mariette X, et al. Spectrum of lymphomas across different drug treatment groups in rheumatoid arthritis: a European registries collaborative project. Ann Rheum Dis. 2017; 76: 2025-2030. 10.1136/annrheumdis-2017-211623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baecklund E, Iliadou A, Askling J, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006; 54: 692-701. 10.1002/art.21675 [DOI] [PubMed] [Google Scholar]
- 14.Jaffe ES, Stein H, Vardiman JW. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Immunodeficiency-associated lymphoproliferative disorders. 3rd ed, IARC publications. 2001; pp. 256-271. [Google Scholar]
- 15.Kurita D, Ichikawa A, Sasaki Y, et al. Clinicopathological Study of Methotrexate-Associated Lymphoproliferative Disorders in Rheumatoid Arthritis Patients: Histological Classification and Predictive Factors for Disease Progression. ASH meeting. Atlanta, #4168, 2017. [Google Scholar]
- 16.Tokuhira M, Saito S, Okuyama A, et al. Clinicopathologic investigation of methotrexate-induced lymphoproliferative disorders, with a focus on regression. Leuk Lymphoma. 2018; 59: 1143-1152. 10.1080/10428194.2017.1369073 [DOI] [PubMed] [Google Scholar]
- 17.DeStefano CB, Desai SH, Shenoy AG, Catlett JP. Management of post-transplant lymphoproliferative disorders. Br J Haematol. 2018; 182: 330-343. 10.1111/bjh.15263 [DOI] [PubMed] [Google Scholar]
- 18.Pfizer-Japan-Inc: Rheumatrex Proper Use Information. In: vol 21, Tokyo. 2014.
- 19.Tokuhira M, Watanabe R, Nemoto T, et al. Clinicopathological analyses in patients with other iatrogenic immunodeficiency-associated lymphoproliferative diseases and rheumatoid arthritis. Leuk Lymphoma. 2012; 53: 616-623. 10.3109/10428194.2011.625101 [DOI] [PubMed] [Google Scholar]
- 20.Ando M, Sato Y, Takata K, et al. A20 (TNFAIP3) deletion in Epstein-Barr virus-associated lymphoproliferative disorders/lymphomas. PLoS One. 2013; 8: e56741. 10.1371/journal.pone.0056741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carreras J, Yukie Kikuti Y, Miyaoka M, et al. Genomic profile and pathologic features of diffuse large B-cell lymphoma subtype of methotrexate-associated lymphoproliferative disorder in rheumatoid arthritis patients. Am J Surg Pathol. 2018; 42: 936-950. 10.1097/PAS.0000000000001071 [DOI] [PubMed] [Google Scholar]
- 22.Koens L, Senff NJ, Vermeer MH, Willemze R, Jansen PM. Methotrexate-associated B-cell lymphoproliferative disorders presenting in the skin: A clinicopathologic and immunophenotypical study of 10 cases. Am J Surg Pathol. 2014; 38: 999-1006. 10.1097/PAS.0000000000000225 [DOI] [PubMed] [Google Scholar]
- 23.Yamada K, Oshiro Y, Okamura S, et al. Clinicopathological characteristics and rituximab addition to cytotoxic therapies in patients with rheumatoid arthritis and methotrexate-associated large B lymphoproliferative disorders. Histopathology. 2015; 67: 70-80. 10.1111/his.12627 [DOI] [PubMed] [Google Scholar]
- 24.Ejima-Yamada K, Oshiro Y, Okamura S, et al. Epstein–Barr virus infection and gene promoter hypermethylation in rheumatoid arthritis patients with methotrexate-associated B cell lymphoproliferative disorders. Virchows Arch. 2017; 470: 205-215. 10.1007/s00428-016-2030-x [DOI] [PubMed] [Google Scholar]
- 25.Gion Y, Iwaki N, Takata K, et al. Clinicopathological analysis of methotrexate-associated lymphoproliferative disorders: comparison of diffuse large B-cell lymphoma and classical Hodgkin lymphoma types. Cancer Sci. 2017; 108: 1271-1280. 10.1111/cas.13249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inui Y, Matsuoka H, Yakushijin K, et al. Methotrexate-associated lymphoproliferative disorders: management by watchful waiting and observation of early lymphocyte recovery after methotrexate withdrawal. Leuk Lymphoma. 2015; 56: 3045-3051. 10.3109/10428194.2015.1022769 [DOI] [PubMed] [Google Scholar]
- 27.Saito S, Suzuki K, Yoshimoto K, et al. Restoration of decreased T helper 1 and CD8+ T cell subsets is associated with regression of lymphoproliferative disorders developed during methotrexate treatment. Front Immunol. 2018; 9: 621. 10.3389/fimmu.2018.00621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takanashi S, Aisa Y, Ito C, et al. Clinical characteristics of methotrexate-associated lymphoproliferative disorders: relationship between absolute lymphocyte count recovery and spontaneous regression. Rheumatol Int. 2017; 37: 1629-1633. 10.1007/s00296-017-3764-8 [DOI] [PubMed] [Google Scholar]
- 29.Saito S, Kaneko Y, Yamaoka K, Tokuhira M, Takeuchi T. Distinct patterns of lymphocyte count transition in lymphoproliferative disorder in patients with rheumatoid arthritis treated with methotrexate. Rheumatology. 2017; 56: 940-946. 10.1093/rheumatology/kex002 [DOI] [PubMed] [Google Scholar]
- 30.Tokuhira M, Tanaka Y, Takahashi Y, et al. The Absolute Count of Lymphocytes Is a Strong Predictive Factor for Relapse/Regrowth Events and Outcomes in Patients with Regressive Methotrexate-Associated Lymphoproliferative Disorders ASH meeting. San Diego, #1589, 2018. [Google Scholar]
- 31.Hasenclever D, Diehl V, Armitage JO, et al. A prognostic score for advanced Hodgkin’s disease. International prognostic factors project on advanced Hodgkin’s disease. N Engl J Med. 1998; 339: 1506-1514. 10.1056/NEJM199811193392104 [DOI] [PubMed] [Google Scholar]
- 32.Ho CL, Lu CS, Chen JH, et al. Neutrophil/lymphocyte ratio, lymphocyte/monocyte ratio, and absolute lymphocyte count/absolute monocyte count prognostic score in diffuse large B-cell lymphoma: useful prognostic tools in the rituximab era. Medicine (Baltimore). 2015; 94: e993. 10.1097/MD.0000000000000993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vassilakopoulos TP, Dimopoulou MN, Angelopoulou MK, et al. Prognostic implication of the absolute lymphocyte to absolute monocyte count ratio in patients with classical Hodgkin lymphoma treated with doxorubicin, bleomycin, vinblastine, and dacarbazine or equivalent regimens. Oncologist. 2016; 21: 343-353. 10.1634/theoncologist.2015-0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keane C, Tobin J, Talaulikar D, et al. A high LDH to absolute lymphocyte count ratio in patients with DLBCL predicts for a poor intratumoral immune response and inferior survival. Oncotarget. 2018; 9: 23620-23627. 10.18632/oncotarget.25306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li N, Zhang L, Song HL, et al. Prognostic impact of absolute lymphocyte count/absolute monocyte count ratio and prognostic score in patients with nasal-type, extranodal natural killer/T-cell lymphoma. Tumour Biol. 2017; 39: 1010428317705503. [DOI] [PubMed] [Google Scholar]
- 36.Kojima M, Itoh H, Hirabayashi K, et al. Methtrexate-associated lymphoproliferative disorders. A clinicopathological study of 13 Japanese cases. Pathol Res Pract. 2006; 202: 679-685. 10.1016/j.prp.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki T, Fujimaki K, Shirasugi Y, et al. Remission of lymphoma after withdrawal of methotrexate in rheumatoid arthritis: relationship with type of latent Epstein-Barr virus infection. Am J Hematol. 2007; 82: 1106-1109. 10.1002/ajh.21003 [DOI] [PubMed] [Google Scholar]
- 38.Jois RN, Gaffney K, Cane P, Nicholson AG, Wotherspoon AC. Methotrexate-associated lymphoproliferative disorder masquerading as interstitial lung disease. Histopathology. 2007; 51: 709-712. 10.1111/j.1365-2559.2007.02830.x [DOI] [PubMed] [Google Scholar]
- 39.Ogasawara T, Kimura A, Yasuyama M, et al. [2-year complete remission after withdrawal of methotrexate in a rectal methotrexate-associated diffuse large B-cell lymphoma]. Rinsho Ketsueki. 2007; 48: 485-490. [PubMed] [Google Scholar]
- 40.Rizzi R, Curci P, Delia M, et al. Spontaneous remission of “methotrexate-associated lymphoproliferative disorders” after discontinuation of immunosuppressive treatment for autoimmune disease. Review of the literature. Med Oncol. 2009; 26: 1-9. 10.1007/s12032-008-9069-8 [DOI] [PubMed] [Google Scholar]
- 41.Ishida M, Hodohara K, Yoshii M, et al. Methotrexate-related Epstein-Barr virus-associated lymphoproliferative disorder occurring in the gingiva of a patient with rheumatoid arthritis. Int J Clin Exp Pathol. 2013; 6: 2237-2241. [PMC free article] [PubMed] [Google Scholar]
- 42.Yamakawa N, Fujimoto M, Kawabata D, et al. A clinical, pathological, and genetic characterization of methotrexate-associated lymphoproliferative disorders. J Rheumatol. 2014; 41: 293-299. 10.3899/jrheum.130270 [DOI] [PubMed] [Google Scholar]
- 43.Kameda T, Dobashi H, Miyatake N, et al. Association of higher methotrexate dose with lymphoproliferative disease onset in rheumatoid arthritis patients. Arthritis Care Res. 2014; 66: 1302-1309. 10.1002/acr.22306 [DOI] [PubMed] [Google Scholar]
- 44.Kawano N, Ono N, Kawano S, et al. Clinical features and outcomes of 9 patients with immunodeficiency-associated lymphoproliferative disorders treated at a single institution. J Clin Exp Hematop. 2014; 54: 187-196. 10.3960/jslrt.54.187 [DOI] [PubMed] [Google Scholar]
- 45.Kudoh M, Harada H, Matsumoto K, et al. Methotrexate-associated lymphoproliferative disorder arising in the retromolar triangle and lung of a patient with rheumatoid arthritis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014; 118: e105-e110. 10.1016/j.oooo.2014.02.029 [DOI] [PubMed] [Google Scholar]
- 46.Shimizu S, Inokuma D, Murata J, et al. Cutaneous manifestations of methotrexate-associated lymphoproliferative disorders: report of two cases and a review of the literature. Acta Derm Venereol. 2015; 95: 366-367. 10.2340/00015555-1951 [DOI] [PubMed] [Google Scholar]
- 47.Ikeda K, Nakamura T, Kinoshita T, et al. Methotrexate-related lymphoproliferative disorder of the stomach in a patient with rheumatoid arthritis: a case of disease regression after methotrexate cessation. Clin J Gastroenterol. 2016; 9: 17-21. 10.1007/s12328-015-0624-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsumoto R, Numata K, Doba N, et al. A case of multiple hepatic lesions associated with methotrexate-associated lymphoproliferative disorder. J Med Ultrason. 2016; 43: 545-551. 10.1007/s10396-016-0740-y [DOI] [PubMed] [Google Scholar]
- 49.Hatano T, Ohishi M, Yoshimoto G, et al. Methotrexate-related lymphoproliferative disorder presenting with severe swelling of the elbow joint: A case report. JBJS Case Connect. 2017; 7: e65. 10.2106/JBJS.CC.17.00002 [DOI] [PubMed] [Google Scholar]
- 50.Takei D, Abe T, Amano H, et al. Methotrexate-associated primary hepatic malignant lymphoma following hepatectomy: A case report. Int J Surg Case Rep. 2017; 31: 5-9. 10.1016/j.ijscr.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oebisu N, Hoshi M, Ieguchi M, et al. Lymphoproliferative disorder with pathological fracture of the femur in a patient with rheumatoid arthritis treated with methotrexate: A case report. Mol Clin Oncol. 2018; 9: 187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki E, Kanno T, Kimura S, et al. Methotrexate-associated lymphoproliferative disorder complicated by severe acute respiratory failure and ileal perforation:a case report. Fukushima J Med Sci. 2018; 64: 82-88. 10.5387/fms.2017-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ebeo CT, Girish MR, Byrd RP, Roy TM, Mehta JB. Methotrexate-induced pulmonary lymphoma. Chest. 2003; 123: 2150-2153. 10.1378/chest.123.6.2150 [DOI] [PubMed] [Google Scholar]
- 54.Kawahara A, Tsukada J, Yamaguchi T, Katsuragi T, Higashi T. Reversible methotrexate-associated lymphoma of the liver in rheumatoid arthritis: a unique case of primary hepatic lymphoma. Biomark Res. 2015; 3: 10. 10.1186/s40364-015-0035-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyagawa K, Shibata M, Noguchi H, et al. Methotrexate-related primary hepatic lymphoma in a patient with rheumatoid arthritis. Intern Med. 2015; 54: 401-405. 10.2169/internalmedicine.54.3361 [DOI] [PubMed] [Google Scholar]
- 56.Niitsu N, Okamoto M, Nakamine H, Hirano M. Clinicopathologic correlations of diffuse large B-cell lymphoma in rheumatoid arthritis patients treated with methotrexate. Cancer Sci. 2010; 101: 1309-1313. 10.1111/j.1349-7006.2010.01517.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ichikawa A, Arakawa F, Kiyasu J, et al. Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: histology, Epstein-Barr virus, and clonality are important predictors of disease progression and regression. Eur J Haematol. 2013; 91: 20-28. 10.1111/ejh.12116 [DOI] [PubMed] [Google Scholar]
- 58.Muto R, Miyoshi H, Sato K, et al. Epidemiology and secular trends of malignant lymphoma in Japan: analysis of 9426 cases according to the World Health Organization classification. Cancer Med. 2018; 7: 5843-5858. 10.1002/cam4.1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dojcinov SD, Venkataraman G, Pittaluga S, et al. Age-related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood. 2011; 117: 4726-4735. 10.1182/blood-2010-12-323238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Castillo JJ, Beltran BE, Miranda RN, et al. EBV-positive diffuse large B-cell lymphoma, not otherwise specified: 2018 update on diagnosis, risk-stratification and management. Am J Hematol. 2018; 93: 953-962. 10.1002/ajh.25112 [DOI] [PubMed] [Google Scholar]
- 61.Oyama T, Yamamoto K, Asano N, et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res. 2007; 13: 5124-5132. 10.1158/1078-0432.CCR-06-2823 [DOI] [PubMed] [Google Scholar]
- 62.Kawano N, Ono N, Yoshida S, et al. Successful treatment of immunodeficiency-associated EBV-negative lymphoproliferative disorders in rheumatoid arthritis by methotrexate withdrawal and prevention of its relapse by rituximab administration. J Clin Exp Hematop. 2012; 52: 193-198. 10.3960/jslrt.52.193 [DOI] [PubMed] [Google Scholar]
- 63.Boulanger E, Rieux-Laucat F, Picard C, et al. Diffuse large B-cell non-Hodgkin’s lymphoma in a patient with autoimmune lymphoproliferative syndrome. Br J Haematol. 2001; 113: 432-434. 10.1046/j.1365-2141.2001.02749.x [DOI] [PubMed] [Google Scholar]
- 64.Lim IG, Bertouch JV. Remission of lymphoma after drug withdrawal in rheumatoid arthritis. Med J Aust. 2002; 177: 500-501. [DOI] [PubMed] [Google Scholar]
- 65.Hasserjian RP, Chen S, Perkins SL, et al. Immunomodulator agent-related lymphoproliferative disorders. Mod Pathol. 2009; 22: 1532-1540. 10.1038/modpathol.2009.131 [DOI] [PubMed] [Google Scholar]
- 66.Kondo S, Tanimoto K, Yamada K, et al. Mature T/NK-cell lymphoproliferative disease and Epstein–Barr virus infection are more frequent in patients with rheumatoid arthritis treated with methotrexate. Virchows Arch. 2013; 462: 399-407. 10.1007/s00428-013-1389-1 [DOI] [PubMed] [Google Scholar]
- 67.Tatsumi G, Ukyo N, Hirata H, Tsudo M. Primary hepatic lymphoma in a patient with rheumatoid arthritis treated with methotrexate. Case Rep Hematol. 2014; 2014: 1-5. 10.1155/2014/460574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horie N, Kawano R, Kaneko T, Shimoyama T. Methotrexate-related lymphoproliferative disorder arising in the gingiva of a patient with rheumatoid arthritis. Aust Dent J. 2015; 60: 408-411. 10.1111/adj.12235 [DOI] [PubMed] [Google Scholar]
- 69.Chang MD, Markham MJ, Liu X. Epstein-Barr virus-positive diffuse large B-cell lymphoma involving the colon in a patient with ulcerative pancolitis and polymyositis on long-term methotrexate therapy. Gastroenterol Res. 2016; 9: 83-86. 10.14740/gr720e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tokuyama K, Okada F, Matsumoto S, et al. EBV-positive MTX-diffuse large B cell lymphoma in a rheumatoid arthritis patient. Jpn J Radiol. 2014; 32: 183-187. 10.1007/s11604-013-0280-y [DOI] [PubMed] [Google Scholar]
- 71.Jeon YW, Yoon JH, Lee SE, et al. Clinical manifestations of autoimmune disease-related non-Hodgkin lymphoma: a Korean single-center, retrospective clinical study. Korean J Intern Med. 2016; 31: 944-952. 10.3904/kjim.2015.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loo EY, Medeiros LJ, Aladily TN, et al. Classical Hodgkin lymphoma arising in the setting of iatrogenic immunodeficiency: a clinicopathologic study of 10 cases. Am J Surg Pathol. 2013; 37: 1290-1297. 10.1097/PAS.0b013e31828e6564 [DOI] [PubMed] [Google Scholar]
- 73.Takasumi M, Okai K, Asano T, et al. [A case of methotrexate-associated lymphoproliferative disorder diagnosed by liver biopsy]. Nippon Shokakibyo Gakkai Zasshi. 2015; 112: 115-122. [DOI] [PubMed] [Google Scholar]
- 74.Tsukazaki Y, Shinohara T, Tanaka K, et al. Hepatosplenic Hodgkin lymphoma without lymphadenopathy following reversible methotrexate-associated lymphoproliferative disorder. Mod Rheumatol. 2017; 27: 372-375. 10.3109/14397595.2014.977513 [DOI] [PubMed] [Google Scholar]
- 75.Eso Y, Uza N, Shirakawa K, et al. Choledochoduodenal fistula during chemotherapy with brentuximab vedotin for methotrexate-associated lymphoproliferative disorder. Intern Med. 2018; 57: 2203-2207. 10.2169/internalmedicine.0557-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakazato T, Takanashi S, Hirano M, et al. Brentuximab vedotin is effective for rheumatoid arthritis in a patient with relapsed methotrexate-associated Hodgkin lymphoma. Ann Hematol. 2018; 97: 1489-1491. 10.1007/s00277-018-3279-8 [DOI] [PubMed] [Google Scholar]
- 77.Svensson AM, Jacobson ER, Ospina D, Tindle BH. Reversible Epstein-Barr virus-negative lymphadenopathy and bone marrow involved by Hodgkin’s lymphoma in a rheumatoid arthritis patient undergoing long-term treatment with low-dose methotrexate: a case report and review of the literature. Int J Hematol. 2006; 83: 47-50. 10.1532/IJH97.NA0503 [DOI] [PubMed] [Google Scholar]
- 78.Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. EBV positive mucocutaneous ulcer--a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010; 34: 405-417. 10.1097/PAS.0b013e3181cf8622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carbone A, Spina M, Gloghini A, Tirelli U. Classical Hodgkin’s lymphoma arising in different host’s conditions: pathobiology parameters, therapeutic options, and outcome. Am J Hematol. 2011; 86: 170-179. 10.1002/ajh.21910 [DOI] [PubMed] [Google Scholar]
- 80.Miranda RN, Loo E, Medeiros LJ. Iatrogenic immunodeficiency-associated classical hodgkin lymphoma: clinicopathologic features of 54 cases reported in the literature. Am J Surg Pathol. 2013; 37: 1895-1897. 10.1097/PAS.0000000000000095 [DOI] [PubMed] [Google Scholar]
- 81.Komatsuda A, Wakui H, Nimura T, Sawada K. Reversible infliximab-related lymphoproliferative disorder associated with Epstein-Barr virus in a patient with rheumatoid arthritis. Mod Rheumatol. 2008; 18: 315-318. 10.3109/s10165-008-0053-0 [DOI] [PubMed] [Google Scholar]
- 82.Ohkura Y, Shindoh J, Haruta S, et al. Primary adrenal lymphoma possibly associated with Epstein-Barr virus reactivation due to immunosuppression under methotrexate therapy. Medicine (Baltimore). 2015; 94: e1270. 10.1097/MD.0000000000001270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamakawa H, Yoshida M, Katagi H, et al. Pulmonary and retroperitoneal lesions induced by methotrexate-associated lymphoproliferative disorder in a patient with rheumatoid arthritis. Mod Rheumatol. 2016; 26: 441-444. [DOI] [PubMed] [Google Scholar]
- 84.Tsukui D, Kanda H, Shinozaki-Ushiku A, et al. Polymorphic lymphoproliferative disorders in patients with rheumatoid arthritis are associated with a better clinical outcome. Mod Rheumatol. 2018; 28: 621-625. 10.1080/14397595.2017.1387223 [DOI] [PubMed] [Google Scholar]
- 85.Kikuchi K, Miyazaki Y, Tanaka A, et al. Methotrexate-related Epstein-Barr Virus (EBV)-associated lymphoproliferative disorder--so-called “Hodgkin-like lesion”--of the oral cavity in a patient with rheumatoid arthritis. Head Neck Pathol. 2010; 4: 305-311. 10.1007/s12105-010-0202-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takemori N, Kaneko H, Nakamura M, Kojima M. Complete remission of methotrexate-related Epstein-Barr-virus-associated Hodgkin-like lymphoma following withdrawal of MTX coupled with clarithromycin administration. Case Rep Hematol. 2012; 2012: 1-6. 10.1155/2012/658745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Satoh K, Yoshida N, Imaizumi K, et al. Reversible methotrexate-associated lymphoproliferative disorder resembling advanced gastric cancer in a patient with rheumatoid arthritis. Am J Med Sci. 2009; 338: 334-335. 10.1097/MAJ.0b013e3181acbb49 [DOI] [PubMed] [Google Scholar]
- 88.Attard AA, Praveen P, Dunn PJS, James GJ. Epstein-Barr virus–positive mucocutaneous ulcer of the oral cavity: the importance of having a detailed clinical history to reach a correct diagnosis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 114: e37-e39. 10.1016/j.oooo.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 89.Hashizume H, Uchiyama I, Kawamura T, et al. Epstein-Barr virus-positive mucocutaneous ulcers as a manifestation of methotrexate-associated B-cell lymphoproliferative disorders. Acta Derm Venereol. 2012; 92: 276-277. 10.2340/00015555-1274 [DOI] [PubMed] [Google Scholar]
- 90.Sadasivam N, Johnson RJ, Owen RG. Resolution of methotrexate-induced Epstein-Barr virus-associated mucocutaneous ulcer. Br J Haematol. 2014; 165: 584. 10.1111/bjh.12743 [DOI] [PubMed] [Google Scholar]
- 91.Moran NR, Webster B, Lee KM, et al. Epstein Barr virus-positive mucocutaneous ulcer of the colon associated Hodgkin lymphoma in Crohn’s disease. World J Gastroenterol. 2015; 21: 6072-6076. 10.3748/wjg.v21.i19.6072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hashimoto K, Nagao T, Saito T, Kinoshita H. Methotrexate-associated lymphoproliferative disorders of the tongue developing in patients with rheumatoid arthritis: a report of 2 cases and a review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015; 119: e1-e5. 10.1016/j.oooo.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 93.Nakauyaca A, Kalro A, Donaldson E, Patel H. Fatal outcome of an Epstein-Barr virus positive mucocutaneous ulcer secondary to methotrexate. Intern Med J. 2016; 46: 1226-1228. 10.1111/imj.13219 [DOI] [PubMed] [Google Scholar]
- 94.Chen BJ, Fang CL, Chuang SS. Epstein-Barr virus-positive mucocutaneous ulcer. Kaohsiung J Med Sci. 2017; 33: 50-51. 10.1016/j.kjms.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 95.Furudate K, Satake A, Narita N, Kobayashi W. Methotrexate-related lymphoproliferative disorder in patients with osteonecrosis of the jaw: A 3-case report and literature review. J Oral Maxillofac Surg. 2018; 76: 97-111. 10.1016/j.joms.2017.05.027 [DOI] [PubMed] [Google Scholar]
- 96.Daroontum T, Kohno K, Eladl AE, et al. Comparison of Epstein-Barr virus-positive mucocutaneous ulcer associated with treated lymphoma or methotrexate in Japan. Histopathology. 2018; 72: 1115-1127. 10.1111/his.13464 [DOI] [PubMed] [Google Scholar]
- 97.Satou A, Banno S, Hanamura I, et al. EBV-positive mucocutaneous ulcer arising in rheumatoid arthritis patients treated with methotrexate: single center series of nine cases. Pathol Int. 2019; 69: 21-28. 10.1111/pin.12745 [DOI] [PubMed] [Google Scholar]
- 98.Daroontum T, Kohno K, Inaguma Y, et al. Epstein-Barr virus (EBV)-positive diffuse large B-cell lymphoma arising in patient with a history of EBV-positive mucocutaneous ulcer and EBV-positive nodal polymorphous B-lymphoproliferative disorder. Pathol Int. 2019; 69: 37-41. 10.1111/pin.12738 [DOI] [PubMed] [Google Scholar]
- 99.Clarke LE, Junkins-Hopkins J, Seykora JT, Adler DJ, Elenitsas R. Methotrexate-associated lymphoproliferative disorder in a patient with rheumatoid arthritis presenting in the skin. J Am Acad Dermatol. 2007; 56: 686-690. 10.1016/j.jaad.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 100.Tanaka A, Shigematsu H, Kojima M, Sakashita H, Kusama K. Methotrexate-associated lymphoproliferative disorder arising in a patient with adult Still’s disease. J Oral Maxillofac Surg. 2008; 66: 1492-1495. 10.1016/j.joms.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 101.Roberts TK, Chen X, Liao JJ. Diagnostic and therapeutic challenges of EBV-positive mucocutaneous ulcer: a case report and systematic review of the literature. Exp Hematol Oncol. 2015; 5: 13. 10.1186/s40164-016-0042-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Makino Y, Tani C, Miyokawa N, et al. Multiple intestinal ulcers associated with primary Epstein-Barr virus infection in a patient with rheumatoid arthritis undergoing methotrexate therapy. Intern Med. 2015; 54: 2851-2855. 10.2169/internalmedicine.54.4735 [DOI] [PubMed] [Google Scholar]
- 103.Au JK, Said JW, Sepahdari AR, St. John MA. Head and neck Epstein-Barr virus mucocutaneous ulcer: case report and literature review. Laryngoscope. 2016; 126: 2500-2504. 10.1002/lary.26009 [DOI] [PubMed] [Google Scholar]
- 104.Saleh JZ, Lee LH, Schieke SM, Hosking PR, Hwang ST. Methotrexate-induced CD30+ T-cell lymphoproliferative disorder of the oral cavity. JAAD Case Rep. 2016; 2: 354-356. 10.1016/j.jdcr.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mishima S, Takahashi K, Tomioka T, Bessho K. Numb chin syndrome as initial manifestation of bisphosphonate-related osteomyelitis of the jaw and methotrexate-associated lymphoproliferative disorders: a rare case. Br J Oral Maxillofac Surg. 2016; 54: 114-115. 10.1016/j.bjoms.2015.10.031 [DOI] [PubMed] [Google Scholar]
- 106.Hujoel IA, Rubio-Tapia A, Dao LN, Porrata LF, Kane SV. Epstein-Barr virus-positive mucocutaneous ulcer in an immunosuppressed patient. ACG Case Rep J. 2018; 5: e32. 10.14309/crj.2018.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee YM, Kim JM. Epstein-Barr virus-positive mucocutaneous ulcer colliding with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (malt lymphoma) that developed in the palatine tonsils of an immunocompetent patient after gastric lymphoma relapse. Blood Res. 2018; 53: 329-332. 10.5045/br.2018.53.4.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McCormack C, Huang Q. EBV + mucocutaneous ulcer: a new entity of WHO 2017. Blood. 2018; 131: 1993. 10.1182/blood-2018-01-825570 [DOI] [PubMed] [Google Scholar]
- 109.Namiki T, Sone Y, Miura K, Tanaka M, Yokozeki H. Methotrexate-associated lymphoproliferative disorder: dermoscopic features. Case Rep Dermatol. 2018; 10: 149-153. 10.1159/000489694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Manipadam MT, Ravi PY, Sigamani E, Jeelani Y. Methotrexate-associated Epstein–Barr virus mucocutaneous ulcer: A case report and review of literature. Indian J Pathol Microbiol. 2018; 61: 255-257. 10.4103/IJPM.IJPM_135_16 [DOI] [PubMed] [Google Scholar]
- 111.Schwartz Z, Bowe RB, Coleman M, Magro CM. Pediatric oral Epstein-Barr virus associated self-remitting CD30+ lymphoproliferative disorder: A distinct entity. Ann Diagn Pathol. 2018; 37: 57-61. 10.1016/j.anndiagpath.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 112.Sinit RB, Horan KL, Dorer RK, Aboulafia DM. Epstein-Barr virus-positive mucocutaneous ulcer: case report and review of the first 100 published cases. Clin Lymphoma Myeloma Leuk. 2019; 19: e81-e92. 10.1016/j.clml.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 113.Kojima M, Motoori T, Hosomura Y, et al. Atypical lymphoplasmacytic and immunoblastic proliferation from rheumatoid arthritis: A case report. Pathol Res Pract. 2006; 202: 51-54. 10.1016/j.prp.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 114.Nagai S, Izutsu K, Watanabe T, Kurokawa M. Simultaneous appearance of methotrexate-associated lymphoproliferative disorder and tuberculous meningitis demonstrating the definitive role of immunosuppression. Ann Hematol. 2009; 88: 589-590. 10.1007/s00277-008-0619-0 [DOI] [PubMed] [Google Scholar]
- 115.Joannides AJ, El-Shaboury H, Guirguis R, Fasler JJ, Rajagopal V. Methotrexate-associated lymphoproliferative disorder presenting as disseminated malignancy. Rheumatology. 2011; 50: 1927-1928. 10.1093/rheumatology/ker214 [DOI] [PubMed] [Google Scholar]
- 116.Fujita T, Tanabe M, Iida E, Okimoto T, Matsunaga N. Multi-modality imaging findings of methotrexate-related Epstein–Barr virus-associated hepatic tumor. Clin Imaging. 2013; 37: 962-964. 10.1016/j.clinimag.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 117.Fukushima M, Katayama Y, Yokose N, et al. Primary central nervous system malignant lymphoma in a patient with rheumatoid arthritis receiving low-dose methotrexate treatment. Br J Neurosurg. 2013; 27: 824-826. 10.3109/02688697.2013.798857 [DOI] [PubMed] [Google Scholar]
- 118.Suemori K, Hasegawa H, Ishizaki J, et al. Methotrexate-associated lymphoproliferative disease with multiple pulmonary nodules in a patient with rheumatoid arthritis. Intern Med. 2015; 54: 1421-1425. 10.2169/internalmedicine.54.3542 [DOI] [PubMed] [Google Scholar]
- 119.Katsoulas N, Chrysomali E, Piperi E, Levidou G, Sklavounou-Andrikopoulou A. Atypical methotrexate ulcerative stomatitis with features of lymphoproliferative like disorder: report of a rare ciprofloxacin-induced case and review of the literature. J Clin Exp Dent. 2016; 8: e629-e633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nakamura H, Shibata Y, Takeda T. Sternoclavicular joint swelling in a patient with rheumatoid arthritis. J Rheumatol. 2016; 43: 2074-2075. 10.3899/jrheum.160401 [DOI] [PubMed] [Google Scholar]
- 121.Kobayashi Y, Kimura K, Fujitsu Y, et al. Methotrexate-associated orbital lymphoproliferative disorder in a patient with rheumatoid arthritis: a case report. Jpn J Ophthalmol. 2016; 60: 212-218. 10.1007/s10384-016-0439-z [DOI] [PubMed] [Google Scholar]
- 122.Furukawa S, Oobu K, Moriyama M, et al. Oral methotrexate-related lymphoproliferative disease presenting with severe osteonecrosis of the jaw: A case report and literature review. Intern Med. 2018; 57: 575-581. 10.2169/internalmedicine.8946-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lai WF, Chin YP, Liu CW, Tsai CY. Methotrexate-associated lymphoproliferative disease with multiple pulmonary nodules in a patient with rheumatoid arthritis. BMJ Case Rep. 2017; 2017. [DOI] [PMC free article] [PubMed]
- 124.Sato N, Toide N, Kizawa M, et al. Methotrexate-associated lymphoproliferative disorder in a patient with neuromyelitis optica spectrum disorder: an implication for pathogenesis mediated by Epstein-Barr virus. J Neurol Sci. 2017; 379: 219-221. 10.1016/j.jns.2017.06.020 [DOI] [PubMed] [Google Scholar]
- 125.Hashimoto K, Akagi M. Lymphoproliferative disorder in an elderly rheumatoid arthritis patient after longterm oral methotrexate administration: A case report. Mol Clin Oncol. 2018; 9: 293-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tokuhira M, Tabayashi T, Tanaka Y, et al. The aggressive clinical courses of Hodgkin lymphoma primarily diagnosed as methotrexate-induced non-specific lymphoproliferative disorder in patients with rheumatoid arthritis. J Clin Exp Hematop. 2017; 56: 165-169. 10.3960/jslrt.56.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baird RD, Nellis van Zyl-Smit R, Dilke T, Scott SE, Rassam SMB. Spontaneous remission of low-grade B-cell non-Hodgkin’s lymphoma following withdrawal of methotrexate in a patient with rheumatoid arthritis: case report and review of the literature. Br J Haematol. 2002; 118: 567-568. 10.1046/j.1365-2141.2002.03619.x [DOI] [PubMed] [Google Scholar]
- 128.Hoshida Y, Tomita Y, Zhiming D, et al. Lymphoproliferative disorders in autoimmune diseases in Japan: analysis of clinicopathological features and Epstein-Barr virus infection. Int J Cancer. 2004; 108: 443-449. 10.1002/ijc.11582 [DOI] [PubMed] [Google Scholar]
- 129.Ishigaki S, Masaoka T, Kameyama H, et al. Methotrexate-associated lymphoproliferative disorder of the stomach presumed to be mucosa-associated lymphoid tissue lymphoma. Intern Med. 2018; 57: 3249-3254. 10.2169/internalmedicine.0737-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Katsuragi T, Iwashige A, Tsukada J. [Immunodeficiency-associated Burkitt lymphoma developed in a patient receiving a long-term methotrexate therapy for rheumatoid arthritis]. Rinsho Ketsueki. 2016; 57: 9-14. [DOI] [PubMed] [Google Scholar]
- 131.Kikuchi J, Kaneko Y, Kasahara H, et al. Methotrexate-associated intravascular large B-cell lymphoma in a patient with rheumatoid arthritis. Intern Med. 2016; 55: 1661-1665. 10.2169/internalmedicine.55.6943 [DOI] [PubMed] [Google Scholar]
- 132.Hagihara M, Mese T, Ohara S, et al. Methotrexate-associated intravascular large B-cell lymphoma in a patient with rheumatoid arthritis: A very rare case. Intern Med. 2018; 57: 3001-3005. 10.2169/internalmedicine.0875-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Takajo I, Umekita K, Ikei Y, Oshima K, Okayama A. Adult T-cell leukemia/lymphoma as a methotrexate-associated lymphoproliferative disorder in a patient with rheumatoid arthritis. Intern Med. 2018; 57: 2071-2075. 10.2169/internalmedicine.0308-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hatanaka K, Nakamura N, Kojima M, et al. Methotrexate-associated lymphoproliferative disorders mimicking angioimmunoblastic T-cell lymphoma. Pathol Res Pract. 2010; 206: 9-13. 10.1016/j.prp.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 135.Kiyasu J, Arakawa F, Haji S, et al. Methotrexate-associated lymphoproliferative disorders with angioimmunoblastic T-cell lymphoma-like features accompanied by gamma-heavy chain disease in a patient with rheumatoid arthritis. Pathol Int. 2018; 68: 485-490; Epub ahead of print. 10.1111/pin.12703 [DOI] [PubMed] [Google Scholar]
- 136.Singh BK, Antony PT, Verma SK, Basu D, Negi VS. Anaplastic large cell lymphoma in a patient with systemic onset juvenile arthritis: case report and review of literature. Int J Rheum Dis. 2014; 17: 573-577. [DOI] [PubMed] [Google Scholar]
- 137.Cornejo CM, Novoa RA, Krisch RE, Kim EJ. Low-dose radiotherapy for primary cutaneous anaplastic large-cell lymphoma while on low-dose methotrexate. Cutis. 2016; 98: 253-256. [PubMed] [Google Scholar]
- 138.Nemoto Y, Taniguchi A, Kamioka M, et al. Epstein–Barr virus-infected subcutaneous panniculitis-like T-cell lymphoma associated with methotrexate treatment. Int J Hematol. 2010; 92: 364-368. 10.1007/s12185-010-0642-5 [DOI] [PubMed] [Google Scholar]
- 139.Hatachi S, Kunitomi A, Aozasa K, Yagita M. CD8 + T-cell lymphoproliferative disorder associated with Epstein–Barr virus in a patient with rheumatoid arthritis during methotrexate therapy. Mod Rheumatol. 2010; 20: 500-505. 10.3109/s10165-010-0300-z [DOI] [PubMed] [Google Scholar]
- 140.Koji H, Yazawa T, Nakabayashi K, et al. CD8-positive T-cell lymphoproliferative disorder associated with Epstein-Barr virus-infected B-cells in a rheumatoid arthritis patient under methotrexate treatment. Mod Rheumatol. 2016; 26: 271-275. 10.3109/14397595.2013.850613 [DOI] [PubMed] [Google Scholar]
- 141.Kamiya Y, Toyoshima M, Suda T. Endobronchial involvement in methotrexate-associated lymphoproliferative disease. Am J Respir Crit Care Med. 2016; 193: 1304-1306. 10.1164/rccm.201512-2338IM [DOI] [PubMed] [Google Scholar]
- 142.Terroso G, Aleixo J, Bernardes M, et al. Nasal type extranodal NK/T cell lymphoma diagnosed in a patient with rheumatoid arthritis under methotrexate. Acta Reumatol Port. 2014; 39: 77-81. [PubMed] [Google Scholar]
- 143.Tajima S, Takanashi Y, Koda K, Fukayama M. Methotrexate-associated lymphoproliferative disorder presenting as extranodal NK/T-cell lymphoma arising in the lungs. Pathol Int. 2015; 65: 661-665. 10.1111/pin.12346 [DOI] [PubMed] [Google Scholar]
- 144.Uchida T, Inoue M, Hua J, et al. Iatrogenic immunodeficiency-associated Epstein-Barr virus (EBV) -negative natural killer cell lymphoproliferative disorder in a patient undergoing rheumatoid arthritis therapy. Rinsho Ketsueki. 2017; 58: 624-629. [DOI] [PubMed] [Google Scholar]
- 145.Sliesoraitis S, Khan R, Rothman J. Methotrexate-induced Hodgkin disease in a patient with systemic lupus erythematosus. J Am Osteopath Assoc. 2009; 109: 325-328. [PubMed] [Google Scholar]
- 146.Dong L, Chen Y, Masaki Y, Okazaki T, Umehara H. Possible mechanisms of lymphoma development in Sjogren’s syndrome. Curr Immunol Rev. 2013; 9: 13-22. 10.2174/1573395511309010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tarella C, Gueli A, Ruella M, Cignetti A. Lymphocyte transformation and autoimmune disorders. Autoimmun Rev. 2013; 12: 802-813. 10.1016/j.autrev.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 148.Lam GY, Halloran BP, Peters AC, Fedorak RN. Lymphoproliferative disorders in inflammatory bowel disease patients on immunosuppression: lessons from other inflammatory disorders. World J Gastrointest Pathophysiol. 2015; 6: 181-192. 10.4291/wjgp.v6.i4.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pereira VPL, Robazzi TCMV. Biological therapy and development of neoplastic disease in patients with juvenile rheumatic disease: a systematic review. Rev Bras Reumatol Engl Ed. 2017; 57: 174-181. 10.1016/j.rbre.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 150.Smedby KE, Ponzoni M. The aetiology of B-cell lymphoid malignancies with a focus on chronic inflammation and infections. J Intern Med. 2017; 282: 360-370. 10.1111/joim.12684 [DOI] [PubMed] [Google Scholar]
- 151.Yang C, Huang J, Huang X, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with anti-tumour necrosis factor alpha agents: A systematic review and meta-analysis. J Crohn’s Colitis. 2018; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 152.Romão VC, Talarico R, Scirè CA, et al. Sjögren’s syndrome: state of the art on clinical practice guidelines. RMD Open. 2018; 4(suppl 1): e000789. 10.1136/rmdopen-2018-000789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Klein A, Polliack A, Gafter-Gvili A. Systemic lupus erythematosus and lymphoma: Incidence, pathogenesis and biology. Leuk Res. 2018; 75: 45-49. 10.1016/j.leukres.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 154.Lelièvre JD, Sacre K, Adle-Biassette H, et al. Epstein-Barr virus–associated lymphoproliferative disease after long-standing cyclosporine therapy for psoriasis: A case of spontaneous regression. J Am Acad Dermatol. 2005; 52(suppl 1): S24-S27. 10.1016/j.jaad.2004.06.043 [DOI] [PubMed] [Google Scholar]
- 155.Dubey S, Adebajo AO. Lymphoproliferative disorder due to sulphasalazine. BMJ Case Rep. 2009; 2009. [DOI] [PMC free article] [PubMed]
- 156.Ikeda T, Toyama S, Ogasawara M, et al. Rheumatoid arthritis complicated with immunodeficiency-associated lymphoproliferative disorders during treatment with adalimumab. Mod Rheumatol. 2012; 22: 458-462. 10.3109/s10165-011-0501-0 [DOI] [PubMed] [Google Scholar]
- 157.McGinness JL, Spicknall KE, Mutasim DF. Azathioprine-induced EBV-positive mucocutaneous ulcer. J Cutan Pathol. 2012; 39: 377-381. 10.1111/j.1600-0560.2011.01829.x [DOI] [PubMed] [Google Scholar]
- 158.Oda K, Minata M. Drug free remission after steroid-dependent disappearance of lymphoproliferative disorder in rheumatoid arthritis patient treated with TNF-alpha blockade: case study. Springerplus. 2015; 4: 41. 10.1186/s40064-015-0798-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nosotti L, Baiocchini A, Bonifati C, et al. Unusual case of B cell lymphoma after immunosuppressive treatment for psoriasis. World J Hepatol. 2015; 7: 814-818. 10.4254/wjh.v7.i5.814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.D’Haens G, Reinisch W, Colombel JF, et al. ENCORE investigators Five-year safety data from ENCORE, a European observational safety registry for adults with Crohn’s disease treated with infliximab [Remicade(R)] or conventional therapy. J Crohn’s Colitis. 2017; 11: 680-689. [DOI] [PubMed] [Google Scholar]
- 161.Okada K, Asakura S, Yano T, Kishimoto T. EBV-positive PEL-like lymphoma that developed in the course of antisynthetase syndrome treated with tacrolimus. Int J Hematol. 2018; 108: 329-334. 10.1007/s12185-018-2426-2 [DOI] [PubMed] [Google Scholar]
- 162.Tokuhira M, Saito S, Suzuki K, et al. Clinicopathological features of clinical methotrexate-related lymphoproliferative disorders. Leuk Lymphoma. 2019; 5: 1-8. 10.1080/10428194.2019.1585841 [DOI] [PubMed] [Google Scholar]
- 163.Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016; 68: 1-26. 10.1002/art.39480 [DOI] [PubMed] [Google Scholar]
- 164.https://www.ryumachi-jp.com/publication/pdf/MTX2016kanni.pdf.
- 165.Kameda H, Fujii T, Nakajima A, et al. Japan college of rheumatology guideline for the use of methotrexate in patients with rheumastoid arthritis. Mod Rheumatol. 2018; 24: 1-10. [DOI] [PubMed] [Google Scholar]
- 166.Stevens SJC, Verschuuren EA, Pronk I, et al. Frequent monitoring of Epstein-Barr virus DNA load in unfractionated whole blood is essential for early detection of posttransplant lymphoproliferative disease in high-risk patients. Blood. 2001; 97: 1165-1171. 10.1182/blood.V97.5.1165 [DOI] [PubMed] [Google Scholar]
- 167.Wagner HJ, Wessel M, Jabs W, et al. Patients at risk for development of posttransplant lymphoproliferative disorder: plasma versus peripheral blood mononuclear cells as material for quantification of Epstein-Barr viral load by using real-time quantitative polymerase chain reaction. Transplantation. 2001; 72: 1012-1019. 10.1097/00007890-200109270-00006 [DOI] [PubMed] [Google Scholar]
- 168.Tsai DE, Douglas L, Andreadis C, et al. EBV PCR in the diagnosis and monitoring of posttransplant lymphoproliferative disorder: results of a two-arm prospective trial. Am J Transplant. 2008; 8: 1016-1024. 10.1111/j.1600-6143.2008.02183.x [DOI] [PubMed] [Google Scholar]
- 169.Luskin MR, Heil DS, Tan KS, et al. The impact of EBV status on characteristics and outcomes of posttransplantation lymphoproliferative disorder. Am J Transplant. 2015; 15: 2665-2673. 10.1111/ajt.13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tosato G, Steinberg AD, Blaese RM. Defective EBV-specific suppressor T-cell function in rheumatoid arthritis. N Engl J Med. 1981; 305: 1238-1243. 10.1056/NEJM198111193052102 [DOI] [PubMed] [Google Scholar]
- 171.Toussirot E, Wendling D, Tiberghien P, Luka J, Roudier J. Decreased T cell precursor frequencies to Epstein-Barr virus glycoprotein gp110 in peripheral blood correlate with disease activity and severity in patients with rheumatoid arthritis. Ann Rheum Dis. 2000; 59: 533-538. 10.1136/ard.59.7.533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Balandraud N, Meynard JB, Auger I, et al. Epstein-Barr virus load in the peripheral blood of patients with rheumatoid arthritis: accurate quantification using real-time polymerase chain reaction. Arthritis Rheum. 2003; 48: 1223-1228. 10.1002/art.10933 [DOI] [PubMed] [Google Scholar]
- 173.Feng W, Cohen JI, Fischer S, et al. Reactivation of latent Epstein-Barr virus by methotrexate: a potential contributor to methotrexate-associated lymphomas. JNCI Journal of the National Cancer Institute. 2004; 96: 1691-1702. 10.1093/jnci/djh313 [DOI] [PubMed] [Google Scholar]
- 174.Balandraud N, Roudier J. Epstein-Barr virus and rheumatoid arthritis. Joint Bone Spine. 2018; 85: 165-170. 10.1016/j.jbspin.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 175.Kuusela E, Kouri VP, Olkkonen J, et al. Serum Epstein-Barr virus DNA, detected by droplet digital PCR, correlates with disease activity in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2018; 36: 778-784. [PubMed] [Google Scholar]
- 176.Kawada J, Iwata N, Kitagawa Y, Kimura H, Ito Y. Prospective monitoring of Epstein–Barr virus and other herpesviruses in patients with juvenile idiopathic arthritis treated with methotrexate and tocilizumab. Mod Rheumatol. 2012; 22: 565-570. 10.3109/s10165-011-0552-2 [DOI] [PubMed] [Google Scholar]
- 177.Miceli-Richard C, Gestermann N, Amiel C, et al. Effect of methotrexate and anti-TNF on Epstein-Barr virus T-cell response and viral load in patients with rheumatoid arthritis or spondylarthropathies. Arthritis Res Ther. 2009; 11: R77. 10.1186/ar2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Balandraud N, Texier G, Massy E, et al. Long term treatment with abatacept or tocilizumab does not increase Epstein-Barr virus load in patients with rheumatoid arthritis - A three years retrospective study. PLoS One. 2017; 12: e0171623. 10.1371/journal.pone.0171623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fujieda M, Tsuruga K, Sato T, et al. Monitoring of Epstein–Barr virus load and killer T cells in patients with juvenile idiopathic arthritis treated with methotrexate or tocilizumab. Mod Rheumatol. 2017; 27: 66-71. 10.1080/14397595.2016.1177247 [DOI] [PubMed] [Google Scholar]


