TO THE EDITOR
A 68-year-old man was admitted to our clinic because of vomiting. He had no notable past history. He was a social drinker, and developed abdominal distension for a few weeks before admission. Ileus was found in the small intestine on computed tomography (CT) of the abdomen. An intestinal tube was inserted, but his symptom remained. Further examination revealed that the cause of ileus was a tumor mass in the small intestine. Laparoscopic-assisted partial excision of small intestine was performed. The pathological diagnosis of the small intestinal mass was diffuse large B-cell lymphoma (DLBCL), germinal center B-cell-like. He was referred to the hematological division for additional chemotherapy.
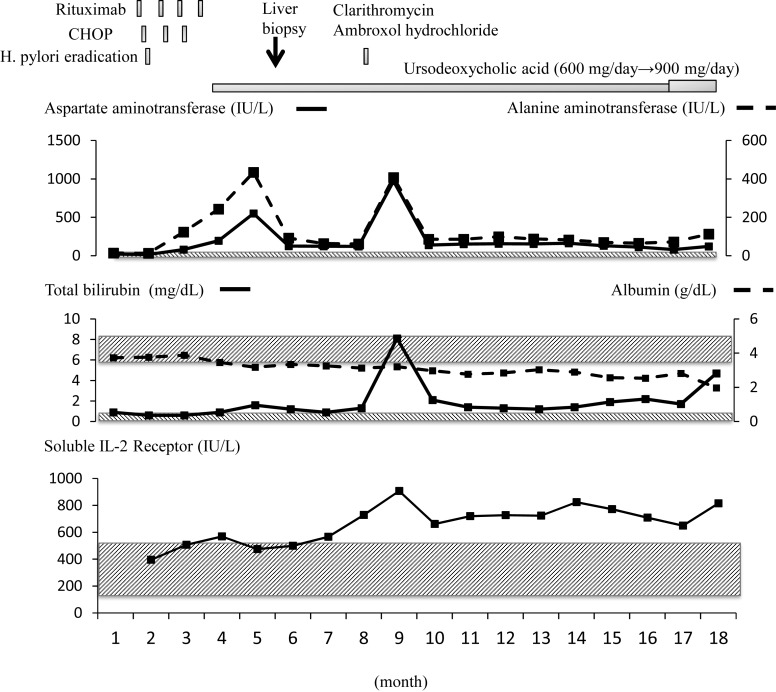
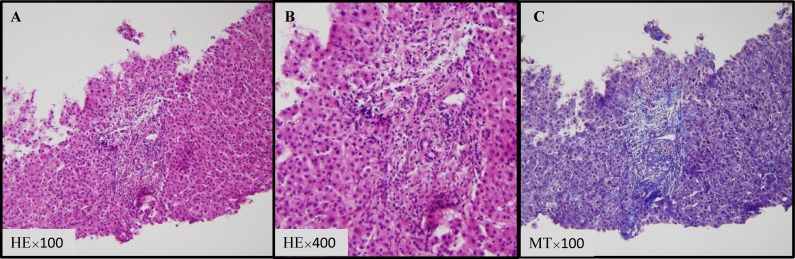
FDG-positron emission tomography (PET) was performed and uptake in the gastric lesion was observed. Upper endoscopy revealed concomitant MALT lymphoma. Amoxicillin, clarithromycin and omeprazole were prescribed for the treatment of Helicobacter pylori. Rituximab-combined CHOP (R-CHOP) therapy was started to treat stage I DLBCL. Trimethoprim-sulfamethoxazole, an H2 blocker, was administered to prevent Pneumocystis carinii pneumonia and gastric ulcer. No hepatotoxicity was observed during the first cycle of R-CHOP therapy. After 2 cycles, slight increases in liver enzymes were noted on laboratory testing; aspartate aminotransferase (AST) 80 IU/L (reference range: 5-40), alanine aminotransferase (ALT) 123 IU/L (reference range: 5-35) and γ-glutamyl transpeptidase (GGT) 22 IU/L (reference range: 8-64) (Fig. 1). As the patient exhibited no symptoms, R-CHOP therapy was continued with caution. After 3 cycles, AST, ALT and GGT levels increased to 195, 243 and 257 IU/L, respectively. Ultrasonography and CT of the abdomen demonstrated no abnormality in the liver. Serological tests for hepatitis A, B and C viruses, cytomegalovirus, Epstein-Barr virus, autoantibodies and anti-mitochondrial antibody were all negative. Hepatitis E virus was not assessed. At this point, drug-induced liver injury related to CHOP therapy was suspected. We discontinued CHOP therapy and switched to rituximab monotherapy. Ursodeoxycholic acid (600 mg/day) was added. Another cycle of rituximab was administered. Three weeks later, his AST, ALT and GGT levels increased to 550, 434 and 460 IU/L, respectively (Fig. 1). As rituximab was the only drug used at this point, we considered it to be the cause of liver injury and it was discontinued. At this time, the percentage of eosinophils slightly increased (0.3% to 3.2%). On liver biopsy, lymphoma cells were not detected in the specimen and no evidence of viral infection, such as intranuclear or cytoplasmic inclusion bodies, was observed (Fig. 2A-C). The pathological diagnosis was chronic hepatitis, A2-3, F1. Liver damage remained after discontinuation of rituximab therapy and his liver function gradually decreased. Five months later, clarithromycin and ambroxol hydrochloride was prescribed for sinusitis for 7 days. His AST, ALT and total bilirubin levels markedly increased to 973 IU/L, 407 IU/L and 8.1 mg/dL (reference range: 0.3-1), respectively, and he developed jaundice and ascites (Fig. 1). The patient also had anorexia and general malaise. Antibiotics were stopped and jaundice disappeared. PET-CT and CT were performed 5 and 11 months later, respectively, but there was no evidence of lymphoma recurrence or any other causes of liver damage. Twelve months later, jaundice developed again and ascites increased. He was treated using diuretics, and cell-free and concentrated ascites reinfusion therapy, but he died due to liver failure 14 months after the discontinuation of rituximab.
Fig. 1.
The clinical course and related laboratory data. The shadowed areas indicate the reference ranges of each parameter.
Fig. 2.
Histopathology of the liver specimen. The panels show (A) (B) hematoxylin-eosin (HE) staining and (C) Masson trichrome staining (MT) at the indicated magnification ×100 (A), ×400 (B) and ×100 (C).
Rituximab is a humanized anti-CD20 antibody. Its major side effects are related to infusion reactions. Recently, the reactivation of hepatitis B virus (HBV) infection has gained attention, particularly after immunosuppressive therapy, including rituximab therapy.1,2 HBV reactivation has been reported as a potentially fatal complication for rituximab-containing chemotherapy regimens.2 However in this case, the patient was negative for HBV virus. We found no other causes of liver failure such as other viruses, autoimmune diseases or malignancies, including lymphoma. Imaging studies were unable to detect any signs of relapse of lymphoma throughout the treatment period. The pathological diagnosis of the liver specimen was consistent with drug-induced liver injury. Based on these findings, we concluded rituximab to be the cause of liver injury, which led to death.
Several reports have described rituximab-induced liver injury.3-9 In most cases, rituximab was used in combination with other drugs that may induce liver injury, as in this case, and we were unable to conclude whether liver injury was solely due to rituximab therapy.3-6 In two cases, rituximab was administered to patients with concomitant liver damage, which suggests that rituximab can exacerbate liver dysfunction.6,7 In one case, rituximab led to death in a short period after hematopoietic stem cell transplantation.7 In this case, although the patient did not have concomitant liver disease or undergo transplantation before rituximab treatment, based on the clinical course, rituximab-induced liver injury increased the vulnerability to other potentially toxic drugs (clarithromycin or ambroxol hydrochloride), which had been used safely, thereby resulting in liver failure after long-term use. As the effects of rituximab remain for 6 to 9 months or longer, it is possible that it caused liver injury even though it was stopped after the second incidence of deteriorated liver function.10 This is the first case report of rituximab causing severe liver injury in combination with other drugs in a patient with normal liver function, which resulted in death. The limitation of this study is that we were unable to specify the mechanism of rituximab-induced fatal liver injury based on the second re-biopsy and final pathological findings of the liver.
COMPLIANCE WITH ETHICAL STANDARDS
Funding: This study was conducted without any financial support. Authors Yutaka Shimazu and Masaharu Nohgawa declare that they have no conflict of interest.
Ethical approval: All procedures performed in this study involving the patient were in accordance with the ethical standards of our institutional and national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was received from the patient.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT, American Gastroenterological Association Institute American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015; 148: 215-219. 10.1053/j.gastro.2014.10.039 [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Govindarajan S, Fong TL, Redeker AG. Spontaneous reactivation in chronic hepatitis B: patterns and natural history. J Clin Gastroenterol. 1990; 12: 562-568. 10.1097/00004836-199010000-00015 [DOI] [PubMed] [Google Scholar]
- 3.Smith SM, Pitcher BN, Jung SH, et al. Safety and tolerability of idelalisib, lenalidomide, and rituximab in relapsed and refractory lymphoma: the Alliance for Clinical Trials in Oncology A051201 and A051202 phase 1 trials. Lancet Haematol. 2017; 4: e176-e182. 10.1016/S2352-3026(17)30028-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jo T, Horio K. Severe liver damage and nonallergic bronchitis with eosinophilia in a patient with follicular lymphoma treated with bendamustine plus rituximab. Case Rep Oncol. 2014; 7: 497-502. 10.1159/000365566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latus J, Klein R, Koetter I, et al. Cholestatic liver disease after rituximab and adalimumab and the possible role of cross-reacting antibodies to Fab 2 fragments. PLoS One. 2013; 8: e78856. 10.1371/journal.pone.0078856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tajiri K, Tsuneyama K, Miyazono T, et al. A case of primary biliary cirrhosis that progressed rapidly after treatment involving rituximab. Case Rep Gastroenterol. 2013; 7: 195-201. 10.1159/000351173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qazilbash MH, Qu Z, Hosing C, et al. Rituximab-induced acute liver failure after an allogeneic transplantation for chronic myeloid leukemia. Am J Hematol. 2005; 80: 43-45. 10.1002/ajh.20413 [DOI] [PubMed] [Google Scholar]
- 8.Toprak SK, Karakuş S. Rituximab-related reversible hepatocellular damage. Turk J Haematol. 2012; 29: 422-424. 10.5505/tjh.2012.98853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Prete CJ, Cohen NS. A case of rituximab-induced hepatitis. Cancer Biother Radiopharm. 2010; 25: 747-748. 10.1089/cbr.2010.0806 [DOI] [PubMed] [Google Scholar]
- 10.Barth MJ, Goldman S, Smith L, et al. Rituximab pharmacokinetics in children and adolescents with de novo intermediate and advanced mature B-cell lymphoma/leukaemia: a Children’s Oncology Group report. Br J Haematol. 2013; 162: 678-683. 10.1111/bjh.12434 [DOI] [PMC free article] [PubMed] [Google Scholar]