Abstract
Purpose
Left ventricular (LV) mechanics by speckle-tracking echocardiography (STE) is prognostic in patients with cardiovascular diseases, but evidence related to community-dwelling individuals is uncertain. We therefore performed a systematic review and meta-analysis of STE as a predictor of adverse outcomes in the general population.
Methods
PRISMA guidelines were followed and MEDLINE and EMBASE were searched to identify eligible studies. Primary outcome was all-cause mortality and secondary outcomes were composite cardiac and cardiovascular end-point. Random effects meta-analysis was performed, and a modified Newcastle-Ottawa Assessment Scale was used for quality assessment.
Results
Eight papers matched the predefined criteria (total number of individuals studied=11,744). All publications assessed global longitudinal strain (GLS) by two-dimensional speckle-tracking echocardiography (2D-STE), one assessed circumferential, radial and transverse strains, and one assessed GLS-derived post-systolic shortening. None assessed LV rotational measures in association with outcomes. Two studies reported associations between GLS and all-cause mortality and composite cardiovascular end-point. Six papers reported an association between GLS and composite cardiac end-point, three of which were from the same study. Four papers were suitable for meta-analysis. GLS predicted all-cause mortality (pooled minimally adjusted HR per unit strain (%)=1.07 [95% CI 1.03–1.11], p=0.001), and composite cardiovascular (pooled maximally adjusted HR=1.18 [1.09–1.28], p<0.0001) and cardiac (HR=1.08 [1.02–1.14], p=0.006) end-points. GLS also predicted coronary heart disease (HR=1.15 [1.03–1.29], p=0.017) and heart failure (HR=1.07 [1.02–1.13], p=0.012). The quality of all studies was good.
Conclusions
This study provides some evidence that STE may have utility as a measure of cardiac function and risk in the general population. 2D-STE-based GLS predicts total mortality, major adverse cardiac and cardiovascular end-points in community-dwelling individuals in a limited number of studies. Despite this, this systematic review also highlights important knowledge gaps in the current literature and further evidence is needed regarding the prognostic value of LV mechanics in unselected older populations.
Registration number: CRD42018090302.
Keywords: community-dwelling individuals, mortality, cardiovascular disease, left ventricular strain
Introduction
Left ventricular systolic dysfunction (LVSD), measured as a reduction in left ventricular (LV) ejection fraction (LVEF), is prognostic of adverse outcomes, including all-cause mortality and heart failure (HF) in the general population.1 Nevertheless, LVEF has well-recognized limitations and LVSD may occur when LVEF is normal.2
Speckle-tracking echocardiography (STE) is a comparatively new tool that quantifies myocardial mechanics,3 and may detect LVSD when LVEF is still preserved.2 Alterations in STE-derived LV strain are associated with risk factors for cardiovascular disease (CVD), including diabetes mellitus (DM),4 hypertension5 and obesity,6 and lower global longitudinal strain (GLS) predicts unfavorable outcomes in aortic stenosis, HF and hypertrophic cardiomyopathy.7–10
While systematic reviews and meta-analyses of STE-based LV strain as a predictor of adverse outcomes have previously been conducted based on studies of patients with established11,12 or established plus suspected CVD,13 a systemic review has not been performed for community-dwelling individuals, who were not selected on the basis of disease or clinical status. This is important, since selecting samples based on disease status can distort associations between risk factors and outcomes – termed index event bias (collider bias).14 Also, community-dwelling individuals are at lower risk of CVD compared with selected diseased populations, and the utility of STE in this setting is uncertain but potentially of value.
We therefore conducted a systematic review and meta-analysis to examine whether STE is associated with risk of total and cardiovascular mortality and morbidity independent of conventional risk factors in community-dwelling individuals (ie, in the general population).
Materials and methods
This systematic review and meta-analysis was conducted according to a previously published protocol15 and conforms to the PRISMA guidance.16 The protocol was registered with the PROSPERO database (CRD42018090302).
Eligibility criteria
All longitudinal studies (including placebo arms of population-based clinical trials) that assessed the prospective association of any STE-derived parameter with at least one of the pre-specified outcomes in community-dwelling individuals (>18 years), who were not selected on the basis of disease or clinical status were eligible. Studies were included if they were reported in English, published in peer-reviewed journals and adhered to appropriate ethical standards. Abstracts, reviews, conference proceedings or letters to the editor were excluded.
Outcomes
The primary outcome was all-cause mortality. Secondary outcomes were 1) a composite cardiac end-point, including any combination of cardiovascular mortality, coronary heart disease (CHD) events (myocardial infarction, unstable angina, angina/ischemia requiring emergent hospitalization or revascularization), HF hospitalization, new-onset atrial fibrillation (AF), life-threatening arrhythmia, recorded automatic implantable cardioverter defibrillator shocks, or 2) composite cardiovascular end-points, including a composite cardiac end-point and stroke, transient-ischemic attacks or peripheral arterial disease with arterial revascularization procedure. Any individual secondary end-points included in composite cardiac or cardiovascular end-points were considered as tertiary outcomes.
Search strategy
Literature was searched in MEDLINE and EMBASE via OvidSP interface. Search strategies are shown in the Supplementary materials and data to be extracted were predefined.15 The last search was carried out on February 28, 2018. Additional papers could be identified by searching the reference lists of relevant articles and their citation metrics using Web of Science Core Collection.
Study selection and data extraction
Search results from each database were combined and duplicates were removed before screening. Initial title and abstract screening were performed and full texts of selected articles were retrieved and double screened for eligibility using a predefined eligibility form,15 and data extracted using a predefined form.15 Screening and extraction was performed by two researchers working independently (L.A. and C.P.). Discrepancies were reviewed and resolved through consensus.
Quality assessment
A modified version of the Newcastle-Ottawa Quality Assessment Scale of cohort studies17 was used to assess the quality of included papers.15 The total quality score was reported as the average of the two researchers’ scores ranging from 0 (lowest quality score) to 7 (highest quality score). Papers were included irrespective of the quality assessment score.
Statistical methods
All analyses were performed using Stata 15.1 (StataCorp LLC, USA). We used random effects meta-analysis to pool effect estimates and calculated the 95% CIs of the relevant HRs based on the expectation of heterogeneity between different studies. All HRs were rescaled to per unit strain (%). Results were presented graphically as forest plots and heterogeneity was assessed using Higgins Thompson I2 test and Cochran’s Q test.15
We planned to carry out a meta-analysis on a minimally adjusted model (ie, age, sex and ethnicity [if relevant]) and a maximally adjusted model, including cardiovascular risk factors and conventional echocardiographic measures. Meta-analyses were only possible for GLS. Endocardial strain was used for primary analyses, but we performed sensitivity analysis by repeating analyses replacing endocardial with midwall or epicardial strains when available.
We planned to assess potential sources of heterogeneity,15 but were unable to perform any subgroup analysis or meta-regression due to the limited number of identified studies per analysis. Similarly, it proved impossible to compare different software for STE analysis due to lack of relevant data.
Results
Search results and study selection
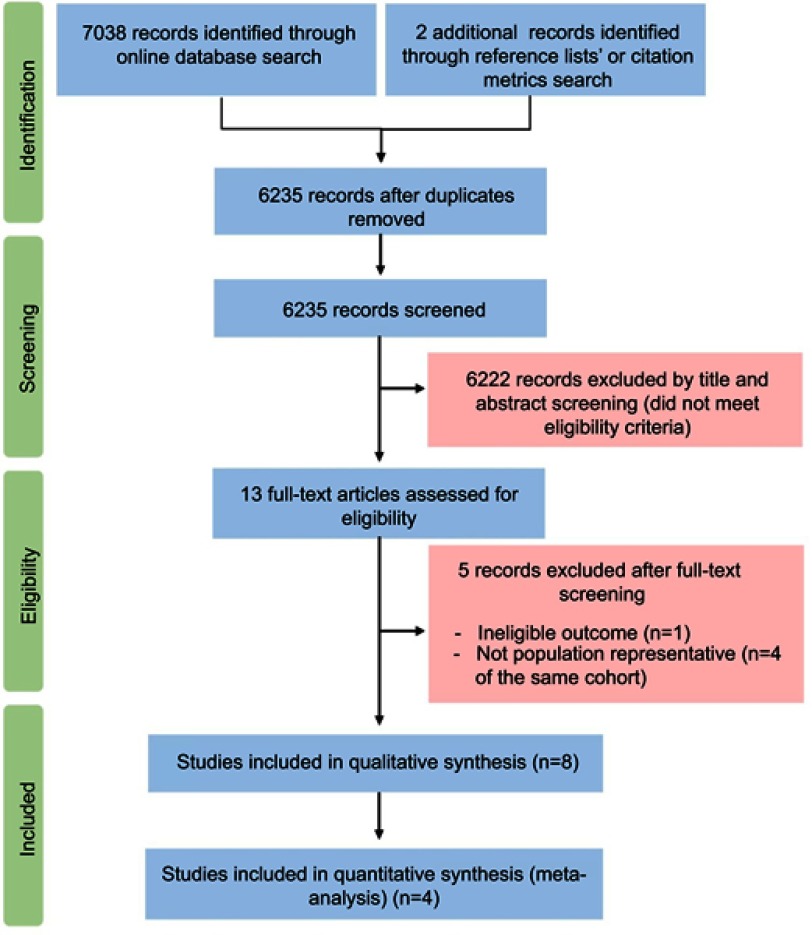
A PRISMA diagram is shown in Figure 1. A total of 7040 records were identified. After removing duplicates, 6222 records of 6235 were excluded by title and abstract. Thirteen full text articles were assessed for eligibility. Five did not meet the inclusion criteria (n=1: ineligible outcome;18 n=4: same cohort, deemed not population representative due to selection criteria19–22), the other eight papers from five studies were eligible (n=2 from Cardiovascular Abnormalities and Brain Lesion study,23,24 n=3 from Copenhagen City Heart Study25–27, and n=1 from Framingham Offspring Study and Framingham Omni Study,28 Flemish Study on Environment, Genes and Health Outcomes [FLEMENGHO],29 and Atherosclerosis Risk in Communities 30).
Figure 1.
PRISMA flow diagram illustrates different stages of this systematic review.
Characteristics of included papers
Characteristics of included papers are shown in Table 1 (additional information regarding the studies is included in Table S1). Most (4/8) were based on US samples (two papers from the same study).23,24,28,30 The remainder included one paper from Belgium29 and three papers (from the same study) from Denmark.25–27 The total number of participants was 11,744, participants in the five studies reported in the eight identified papers ranged between 675 and 6118. Follow-up ranged between 608 days (469–761) (median; IQR)30 and 12.5 years (9.4–12.8).27 One study recruited participants free of CVD at baseline,28 while others included participants with known CVD.
Table 1.
Brief characteristics of included studies
| Reference | Study name | Study design | Region | n | Age (years) | Female (%) | Ethnicity | F/U | HTN (%) | DM (%) | Dyslipidemia (%) | Smoking status (%) | Known CVD (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *Russo et al (2014)23 | Cardiovascular Abnormalities and Brain Lesion (CABL) study | Longitudinal (cohort) study | Manhattan, USA | 708 | 71±9 | 431 (61) | 66.8% Hispanics, 17.1% blacks, 14.1% whites, and 2% of other race-ethnicities. | 4.8±1.5 years (0.06, 7.38) |
548 (77.4) | 197 (27.8) | Hypercholesterolemia: 462 (65.2) | Smoking history: 374 (53) | CAD: 36 (5.08) AF: 41 (5.79 |
| Cheng et al (2015)28 | The Framingham Offspring Study and the Framingham Omni Study | Framingham, Massachusetts, USA | 2831 | 66±9 | 1613 (57) | 259 (9) non-white ethnicity | 6.0±1.2 years | 1679 (59) | 365 (13) | N/A | Current smoker: 236 (8) | 0 | |
| *Russo et al (2015)24 | CABL study | Manhattan, USA | 675 | 71±9 | 408 (60) | N/A | 63.6±18.7 months | 521 (77) | 187 (27.7) | Hypercholesterolemia: 443 (65.6) | N/A | CAD: 39 (5.7) Hx HF: 19 (2.8) |
|
| Kuznetsova et al (2016)29 | Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO) | Northern Belgium | 791 | 50.8±15.5 | 410 (51.8) | White Europeans | Median (5th–95th percentile): 7.9 years (3.7–9.6) | 326 (41.2) | 34 (4.3) | N/A | Current smokers: 167 (21.1) | 43 (5.4) | |
| †Biering-Sorensen et al (2017)25 | Copenhagen City Heart Study | Copenhagen, Denmark | 1296 | 57.0±16.2 | 747 (57.6) | Almost all white | Median (IQR): 11.0 years (9.9–11.2) | 489 (37.8) | 122 (9.4) | N/A | Never: 428 (33.3) Previous: 426 (33.2) Current: 430 (33.5) |
Previous IHD: 64 (4.9) | |
| †Brainin et al (2018)26 | Copenhagen City Heart Study | Copenhagen, Denmark | 1296 | 56.9±16.2 | 747 (57.6) | Almost all white | Median (IQR): 11.0 years (9.9–11.2) | 489 (37.7) | 122 (9.4) | 186 (14.3) | Never: 380 (29.3) Previous: 394 (30.4) Current: 401 (30.9) |
Previous IHD: 64 (4.9) | |
| †Modin et al (2018)27 | Copenhagen City Heart Study | Copenhagen, Denmark | 1294 | 57.0±16.2 | 744 (57.5) | N/A | Median (IQR): 12.5 years (9.4–12.8) | 489 (38.3) | 123 (9.5) | N/A | 406 (33.4) | IHD: 63 (4.9) Ischemic stroke: 25 (1.9) |
|
| Shah et al (2017)30 | Atherosclerosis Risk in Communities (ARIC) | USA | 6118 | Median (IQR): 75.3 (71.7, 79.7) | 3548 (58) | 22% black | Median (IQR): 608 days (469–761) | 5078 (83) | 2325 (38) | N/A | Ever: 3793 (62) Current smoker: 367 (6) |
CAD: 1040 (17) MI: 489 (8) PAD: 367 (6) Stroke: 245 (4) AF: 428 (7) |
Notes: *Studies are from the same cohort (CABL study). †Studies are from the same cohort (Copenhagen City Heart Study).
Abbreviations: AF, atrial fibrillation; CAD, coronary artery disease; CVD, cardiovascular disease; DM, diabetes mellitus; F/U, follow up; HTN, hypertension; HF, heart failure; Hx, history of; IHD, ischemic heart disease; MI, myocardial infarction; N/A, not reported; PAD, peripheral arterial disease.
Exposures and outcomes of included papers
Exposures and outcomes from included papers are shown in Table 2. All used two-dimensional speckle-tracking echocardiography (2D-STE). Two used Philips QLAB 8.1, two used TomTec CPA and four used EchoPac. All studies (n=8) assessed GLS. One study also assessed circumferential, radial and transverse strains28 and another assessed GLS-derived post-systolic shortening measures.26 None assessed LV rotational measures in association with the chosen outcomes. All papers except one27 provided data on exposure reliability (intra-observer,29,30 inter-observer24 reproducibility or both23,25,26,28).
Table 2.
Exposure and outcome characteristics of the included papers
| Exposure characteristics | Outcome characteristics | |||||||
|---|---|---|---|---|---|---|---|---|
| References |
|
|
Number of sonographers perform the analysis | Provided data on exposure reliability | Primary outcome | Secondary outcomes | Tertiary outcomes | |
| (All- cause mortality) | Composite CV end point | Composite cardiac end point |
||||||
| Russo et al (2014)23 |
|
|
|
Intra-observer reproducibility: ICC 0.82 (95% CI; 0.60–0.93, <0.01), mean difference (0.07±2.3%), and COV (SD/mean) 8.4%. Inter-observer reproducibility: ICC 0.85, mean difference (0.08±2.4%) and COV 9.2%. |
n=58 (included ischemic stroke [n=16], MI [n=10], and vascular death [n=32]) |
|||
| Cheng et al (2015)28 |
|
|
|
Intra-observer reproducibility: Average COV: <6% for global longitudinal and circumferential strain. <9% for global transvers and radial strain. Inter-observer reproducibility: Average COV: ≤4% for global longitudinal and circumferential strain. <8% for global transvers and radial strain. |
n=199 14= CHD, 8= cerebrovascular disease, and 13= other CVD causes. 164 death not attributable to a CVD cause. |
New-onset CHD: n=69 (comprising fatal or nonfatal MI, coronary insufficiency, and angina pectoris) |
New-onset CHD: n=69 (comprising fatal or nonfatal MI, coronary insufficiency, and angina pectoris) HF: n=71 |
|
| Russo et al (2015)24 |
|
|
|
Inter-observer reproducibility: ICC 0.85, mean difference (0.08±2.4%), and COV 0.09. |
AF: n=32 | |||
| Kuznetsova et al (2016)29 |
|
|
|
Intra-observer reproducibility: Absolute bias 0.47±0.55% and absolute limit of agreement ranged from 0.62% to 1.55% (reproducibility =1.1%). Relative bias −2.51±3.02% and the limits of agreement ranged from 8.44% to 3.41% (reproducibility=6.1%). |
n=96 (comprised cardiac end-points, stroke, transient ischemic attack, aortic aneurysm, arterial embolism, and revascularization of peripheral arteries) |
n=68 (Included coronary events, fatal and nonfatal HF, pulmonary heart disease, new-onset AF, and life-threatening arrhythmias) |
Coronary events: n=34 [included fatal and nonfatal MI, coronary revascularization, and new-onset angina (stable or unstable)] |
|
| Biering-Sorensen et al (2017)25 |
|
|
|
Intra-observer reproducibility: Mean difference ±1.96 SD (0.1±1.6%). Inter-observer reproducibility: Mean difference ±1.96 SD (−0.08±2.0%). |
n=149 (comprising AMI [n=43], HF [n=78], and CV death [n=74]) |
AMI: n=43 (3.3%) HF: n=78 (6.0%) CV death: n=74 (5.7%) |
||
| Brainin et al (2018)26 |
|
|
|
Intra-observer reproducibility: PSS: mean difference ±1.96 SD (0.2±0.95) PSI: mean difference ±1.96 SD (0.25±0.74) Inter-observer reproducibility: PSS: mean difference ±1.96 SD (−0.04±0.73) PSI: mean difference ±1.96 SD (0.06±0.56) |
n=236 (18.1%) | n=149 (11.5%) (composite of HF [n=78], MI [n=43], and CV death [n=74]) |
||
| Modin et al (2018)27 |
|
|
|
No | n=222 (17.2%) (Composite outcome of either IHD or HF) n=145 (65%) in hypertensive participants and n=77 (35%) in non-hypertensive individuals |
|||
| Shah et al (2017)30 |
|
|
|
Intra-observer reproducibility: Mean difference±SD (0.2±1.4% for LS in apical 4 chamber view; and 0.8±1.2% in apical 2-chamber view) and COV 7.7% for LS in apical 4-chamber view and 6.4% in apical 2-chamber view. |
n=194 (composite of deaths [n=145] and HF [n=113]) |
|||
Abbreviations: AF, atrial fibrillation; CV, cardiovascular; CVD, cardiovascular disease; CHD, coronary heart disease; COV, coefficient of variation; GLS, global longitudinal strain; HF, heart failure; ICC, intra-class correlation coefficient; IHD, ischemic heart disease; MI, myocardial infarction; PSI, post-systolic index; PSS, post-systolic shortening; 2D-STE, two-dimensional speckle-tracking echocardiography.
GLS and all-cause mortality
Two studies found associations between 2D-STE-derived measures and all-cause mortality Table (3).26,28 GLS was reported in both studies; however, only one28 provided both minimally and maximally adjusted estimates. Consequently, meta-analysis was only performed on the two minimally adjusted estimates; pooled HR=1.07 (1.03–1.11), p=0.001 (Figure 2A).
Table 3.
Results of studies assessed the association between two-dimensional speckle-tracking echocardiographic-derived measures and all-cause mortality, composite cardiovascular and cardiac end-points
| Citation | Exposure | Primary outcome | Secondary outcomes | Unit | ||||
|---|---|---|---|---|---|---|---|---|
| All- cause mortality | Composite CV end point | Composite cardiac end point | ||||||
| HRs, 95% CI, P | n (events) Adjustments, |
HRs, 95% CI, P | n (events) Adjustments, |
HRs, 95% CI, P | n (events) Adjustments, |
|||
| Russo et al (2014)23 | Global longitudinal strain (GLS) |
|
|
n = 58 |
|
|
Per unit decrease | |
| 1.24 (1.12, 1.37), <0.001 | None | |||||||
| 1.15 (1.03, 1.28), 0.012 | Age, sex, SBP, DBP, HTN, anti-hypertensive medications, DM, LVMi, relative wall thickness, LAVi, diastolic dysfunction, and AF (Model 1) | |||||||
| 1.15 (1.03, 1.28), 0.012 | Model 1+ LVEF | |||||||
| Cheng et al, (2015)28 | Global average longitudinal strain | n = 199 |
|
|
n = 69 | Per 1 SD change (SD=3.3%) | ||
| 1.31 (1.14, 1.52), 0.0002 | Age, sex and ethnicity (Model 1) | 1.37 (1.06, 1.76), 0.01 | Model 1 | |||||
| 1.24 (1.05, 1.46), 0.01 | Age, sex, ethnicity, BMI, SBP, DBP, anti-hypertensive treatment, total/HDL cholesterol, DM, smoking status, and HR (Model 2) | 1.36 (1.03, 1.79), 0.03 | Model 2 | |||||
| 1.21 (1.02, 1.44), 0.03 | age, sex, ethnicity, BMI, SBP, DBP, anti-hypertensive treatment, total/ HDL cholesterol, DM, smoking status, LV mass, LV fractional shortening, and HR (Model 3) | 1.29 (0.96, 1.74), 0.09 | Model 3 | |||||
| Global average circumferential strain | 1.3 (1.12, 1.52), 0.0007 | Model 1 | 1.1 (0.85, 1.42), 0.48 | Model 1 | Per 1 SD change (SD=5.8%) | |||
| 1.21 (1.04, 1.42), 0.02 | Model 2 | 1.14 (0.87, 1.48), 0.34 | Model 2 | |||||
| 1.11 (0.92, 1.34), 0.27 | Model 3 | 1.11 (0.81, 1.51), 0.53 | Model 3 | |||||
| Global average radial strain | 0.73 (0.61, 0.87), 0.0003 | Model 1 | 0.87 (0.67, 1.13), 0.3 | Model 1 | Per 1 SD change (SD=16.8%) | |||
| 0.76 (0.64, 0.91), 0.002 | Model 2 | 0.9 (0.68, 1.17), 0.43 | Model 2 | |||||
| 0.82 (0.68, 0.98), 0.03 | Model 3 | 0.95 (0.72, 1.26), 0.72 | Model 3 | |||||
| Global average transvers strain | 0.93 (0.81, 1.07), 0.32 | Model 1 | 1.02 (0.80, 1.29), 0.89 | Model 1 | Per 1 SD change (SD=7.1%) | |||
| 0.99 (0.85, 1.14), 0.85 | Model 2 | 1.02 (0.81, 1.29), 0.87 | Model 2 | |||||
| 1 (0.85, 1.17), 0.97 | Model 3 | 1.04 (0.81, 1.34), 0.75 | Model 3 | |||||
| Kuznetsova et al (2016)29 | GLS |
|
n = 68 | |||||
|
|
|
1.75 (1.39, 2.20), <0.0001 | Clinical model = Family clusters, sex, age, BMI, SBP, serum cholesterol, smoking, antihypertensive treatment, DM, and a history of cardiac disease. | 1.54 (1.21, 1.96), 0.0005 | Clinical model | Per 1 SD decrease (SD= 2.5%) |
|
| 1.75 (1.36, 2.20), <0.0001 | Clinical model + LVMi | 1.54 (1.21, 2.0), 0.0005 | Clinical model + LVMi | |||||
| 1.61 (1.27, 2.08), <0.0001 | Clinical model + TDI e′ | 1.45 (1.13, 1.85), 0.0045 | Clinical model + TDI e′ | |||||
| 1.61 (1.27, 2.08), <0.0001 | Clinical model + LVMI + TDI e′ | 1.45 (1.13, 1.89), 0.0041 | Clinical model + LVMI + TDI e′ | |||||
|
|
|
1.74 (1.35, 2.19), <0.0001 | Clinical model | 1.54 (1.22, 1.95), 0.0005 | Clinical model | Per 1 SD decrease (SD= 2.9%) | |
| 1.7 (1.35, 2.14), <0.0001 | Clinical model + LVMi | 1.54 (1.22, 1.95), 0.0005 | Clinical model + LVMi | |||||
| 1.62 (1.25, 2.05), <0.0001 | Clinical model + TDI e′ | 1.43 (1.12, 1.87), 0.0043 | Clinical model + TDI e′ | |||||
| 1.62 (1.25, 2.05), 0.0001 | Clinical model + LVMI + TDI e′ | 1.46 (1.12, 1.87), 0.0041 | Clinical model + LVMI + TDI e′ | |||||
|
|
|
1.66 (1.33, 2.10), <0.0001 | Clinical model | 1.49 (1.18, 1.90), 0.001 | Clinical model | Per 1 SD decrease (SD= 2.2%) | |
| 1.66 (1.31, 2.10), <0.0001 | Clinical model + LVMi | 1.49 (1.18, 1.90), 0.001 | Clinical model + LVMi | |||||
| 1.55 (1.23, 1.97), 0.0002 | Clinical model + TDI e′ | 1.41 (1.10, 1.81), 0.0067 | Clinical model + TDI e′ | |||||
| 1.55 (1.23, 1.97), 0.0002 | Clinical model + LVMI + TDI e′ | 1.41 (1.11, 1.81), 0.0062 | Clinical model + LVMI + TDI e′ | |||||
| Biering-Sorensen et al (2017)25 | GLS |
|
|
|
|
|
||
| 1.12 (1.08, 1.17), <0.001 | None | Per unit (1%) decrease | ||||||
| 1.08 (1.04, 1.13), <0.001 | Age and sex | |||||||
| 1.07 (1.01, 1.11), 0.013 | Clinical model = Age, sex, HR, HTN, DM, previous ischemic heart disease, SBP, and pro-BNP (>150 pmol/L) | |||||||
| 1.05 (1.0, 1.11), 0.045 | Clinical model + LVEF(<50%), LVMi, LV dimension, deceleration time, LA dimension, and E/e′ | |||||||
| Brainin et al (2018)26 |
|
|
|
|
||||
| GLS | 1.05 (1.02, 1.09), 0.004 | None | 1.12 (1.08, 1.17), <0.001 | None | Per unit (1%) decrease | |||
| Post systolic index | 1.33 (1.21, 1.47), <0.001 | None | 1.36 (1.20, 1.54), <0.001 | None | Per 1% increase | |||
| 1.14 (1.0, 1.30), 0.044 | Age, sex, HTN, HR, LVMi, LVEF, GLS, pro-B-type natriuretic peptide, previous ischemic heart disease, SBP, LAVi, e’, estimated glomerular filtration rate, and E/A | 1.22 (1.04, 1.43), 0.014 | Age, sex, HTN, HR, LVMi, LVEF, GLS, pro-B-type natriuretic peptide, previous ischemic heart disease, SBP, LAVi, e’, estimated glomerular filtration rate, and E/A | |||||
| Modin et al (2018)27 | GLS |
|
|
|
|
|
||
| 1.67 (1.41, 1.99), <0.001 | None | Per 5% decrease | ||||||
| 1.37 (1.14, 1.65), 0.001 | Clinical model = Age, sex, SBP, smoking status, DM and total cholesterol and HTN | |||||||
| 1.23 (0.99, 1.52), 0.06 | Clinical model + GLS, LVMi, LAVi, LVIDd/height, HR, E/e′, a′, prevalent IHD, and abnormal ECG. | |||||||
| Shah et al (2017)30 | GLS |
|
|
|
|
Results were not used for meta-analysis as GLS and LVEF were combined into a composite measure and used as a surrogate of LV systolic dysfunction | ||
Abbreviations: AF, atrial fibrillation; BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; ECG, electrocardiogram; GLS, global longitudinal strain; HTN, hypertension; HRs, hazard rations; HR, heart rate; IHD, ischemic heart disease; LAVi, left atrial volume index; LV, left ventricular; LVEF, left ventricular ejection fraction; LVMi, left ventricular mass index; LVIDd, left ventricular internal dimension in diastole; SBP, systolic blood pressure; SD, standard deviation; TDI, tissue Doppler Imaging.
Figure 2.

GLS as a predictor of all-cause mortality (A), composite cardiac end-point (B) and cardiovascular end-point (C). All-cause mortality HR estimates are from minimally adjusted (Cheng et al) and unadjusted (Brainin et al) models. Composite cardiovascular and cardiac end-points are based on maximally adjusted models (listed in the Supplementary materials). For Kuznetsova et al, endocardial-wall strain is shown. Hazard ratios are per unit change in strain value. The heterogeneity assessment including the I2 statistics and p-value of Q test is shown.
GLS and composite cardiovascular end-point
Two studies reported associations between GLS and a composite cardiovascular end-point, but neither provided a minimally adjusted estimate (Table 3).23,29 Random effect meta-analysis indicated that lower 2D-STE-based GLS was associated with higher risk of a composite cardiovascular end-point; pooled maximally adjusted HR=1.18 (1.09–1.28), p<0.0001 (Figure 2C). Substituting mid-wall or epicardial-wall strains for endocardial strain did not alter this finding (Figure S1).
GLS and composite cardiac end-point
Among six papers which assessed different 2D-STE-derived measures,25–30 only GLS or a GLS-derived measure (post-systolic index) was associated with a composite cardiac end-point (Table 3). Three of these papers were from the same study population;25–27 one study was not suitable for quantitative synthesis because the estimates provided combined GLS and LVEF, and were available only for a selected high-risk subset of the population (stage A and B HF);30 therefore, data from three papers25,28,29 were used for meta-analysis. Low GLS predicted higher HR of a composite cardiac end-point; pooled maximally adjusted HR=1.08 (1.02–1.14), p=0.006 (Figure 2B). A sensitivity analysis showed that replacing the endocardial with mid- or epicardial-wall strains29 had minimal effect (Figure S3). Analysis of minimally adjusted estimates is shown in Figure S2.
GLS and tertiary outcomes
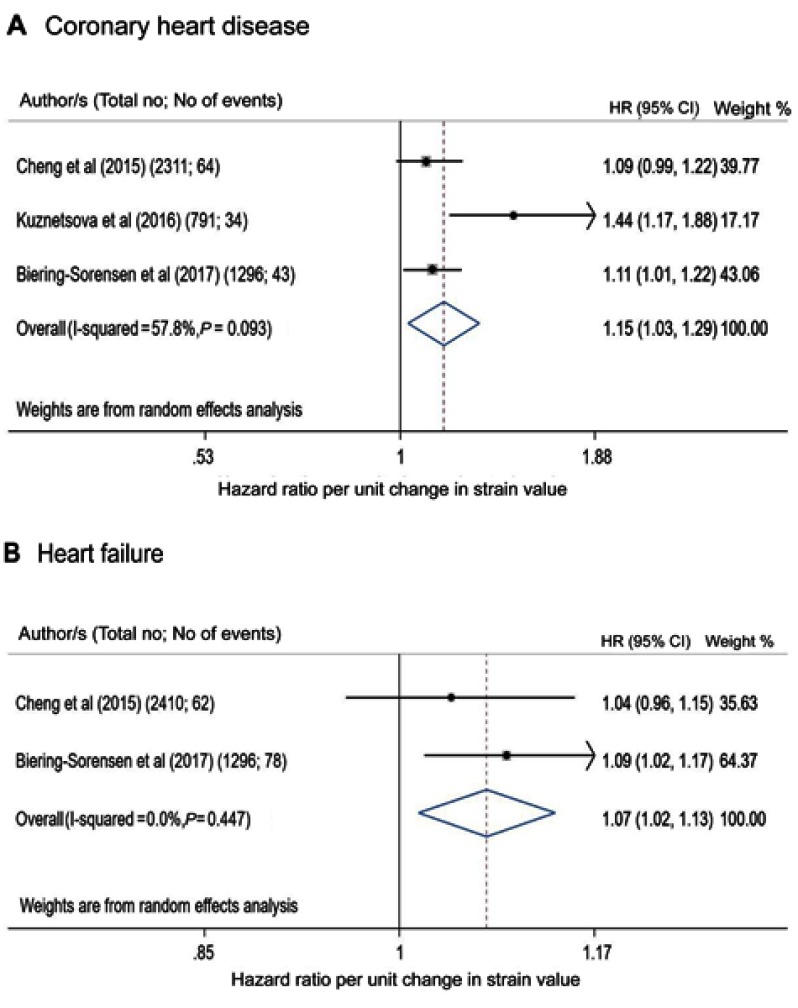
Four papers provided data on GLS in association with tertiary outcomes,24,25,28,29 one of which assessed circumferential, radial and transverse strains28 (Table 4). Three papers reported CHD,25,28,29 two HF,25,28 one AF24 and one cardiovascular death.25 GLS was associated with CHD, AF and HF, whereas circumferential strain was only associated with HF although this was assessed in only one study (Table 4). Meta-analysis showed that GLS was a predictor of CHD and HF (CHD maximally adjusted HR=1.15 [1.03–1.29], p=0.017; HF HR=1.07 [1.01–1.13], p=0.012; Figure 3). Meta-analysis based on minimally adjusted estimates is shown in Figure S4, and further sensitivity analysis was performed for CHD replacing the endocardial with mid- or epicardial-wall strains and results were hardly altered (Figure S5). Additional information from studies that reported Kaplan–Meier data related to various outcomes is shown in Table S1.
Table 4.
Results of studies assessed the association between two-dimensional speckle-tracking echocardiographic-derived measures and tertiary outcomes
| References | Tertiary outcomes (n) | Exposure | Results | Unit | |
|---|---|---|---|---|---|
| HRs, 95% CI, P | Adjustments | ||||
| Cheng et al (2015)28 | 1. Coronary heart disease (69) (comprising fatal or nonfatal myocardial infarction, coronary insufficiency, and angina pectoris) | Global average longitudinal strain | 1.37 (1.06, 1.76), 0.01 | Age, sex and ethnicity (Model 1) | Per 1 SD change (SD=3.3%) |
| 1.36 (1.03, 1.79), 0.03 | Age, sex, ethnicity, BMI, SBP, DBP, anti-hypertensive treatment, total/HDL cholesterol, DM, smoking status, and HR (Model 2) | ||||
| 1.29 (0.96, 1.74), 0.09 | age, sex, ethnicity, BMI, SBP, DBP, anti-hypertensive treatment, total/ HDL cholesterol, DM, smoking status, LV mass, LV fractional shortening, and HR (Model 3) | ||||
| Global average circumferential strain | 1.1 (0.85, 1.42), 0.48 | Model 1 | Per 1 SD change (SD=5.8%) | ||
| 1.14 (0.87, 1.48), 0.34 | Model 2 | ||||
| 1.11 (0.81, 1.51), 0.53 | Model 3 | ||||
| Global average radial strain | 0.87 (0.67, 1.13), 0.3 | Model 1 | Per 1 SD change (SD=16.8%) | ||
| 0.9 (0.68, 1.17), 0.43 | Model 2 | ||||
| 0.95 (0.72, 1.26), 0.72 | Model 3 | ||||
| Global average transvers strain | 1.02 (0.80, 1.29), 0.89 | Model 1 | Per 1 SD change (SD=7.1%) | ||
| 1.02 (0.81, 1.29), 0.87 | Model 2 | ||||
| 1.04 (0.81, 1.34), 0.75 | Model 3 | ||||
| 2. Heart failure (71) | Global average longitudinal strain | 1.45 (1.14, 1.84), 0.003 | Model 1 | Per 1 SD change (SD=3.3%) | |
| 1.29 (0.99, 1.69), 0.06 | Model 2 | ||||
| 1.14 (0.86, 1.50), 0.37 | Model 3 | ||||
| Global average circumferential strain | 1.7 (1.29, 2.25), 0.0002 | Model 1 | Per 1 SD change (SD=5.8%) | ||
| 1.59 (1.18, 2.14), 0.002 | Model 2 | ||||
| 1.41 (1.0, 2.0), 0.05 | Model 3 | ||||
| Global average radial strain | 0.64 (0.46, 0.88), 0.007 | Model 1 | Per 1 SD change (SD=16.8%) | ||
| 0.82 (0.59, 1.13), 0.22 | Model 2 | ||||
| 0.98 (0.72, 1.34), 0.92 | Model 3 | ||||
| Global average transvers strain | 0.73 (0.57, 0.93), 0.01 | Model 1 | Per 1 SD change (SD=7.1%) | ||
| 0.79 (0.61, 1.02), 0.07 | Model 2 | ||||
| 0.84 (0.65, 1.1), 0.21 | Model 3 | ||||
| Russo et al, (2015)24 | Atrial fibrillation (32) | Global average longitudinal strain | 1.2 (1.08, 1.34), 0.001 | None | Per unit (1%) decrease Death as a Competing Risk |
|
1.22 (1.04, 1.43), 0.015 |
Age, obesity, HTN, antihypertensive treatment, coronary artery disease, LVMi, relative wall thickness. |
||||
| Kuznetsova et al (2016)29 | Coronary heart disease (34) Comprising fatal and nonfatal myocardial infarction, coronary revascularization, and new-onset angina (stable or unstable). |
Global longitudinal strain | |||
|
2.45 (1.61, 3.66), <0.0001 | Clinical model = Family clusters, sex, age, BMI, SBP, serum cholesterol, smoking, antihypertensive treatment, DM, and a history of cardiac disease. | Per 1 SD decrease (SD= 2.5%) | ||
| 2.53 (1.68, 3.82), <0.0001 | Clinical model + LVMi | ||||
| 2.32 (1.51, 3.55), <0.0001 | Clinical model + TDI e′ | ||||
| 2.4 (1.54, 3.71), <0.0001 | Clinical model + LVMI + TDI e′ | ||||
|
2.34 (1.58, 3.50), <0.0001 | Clinical model | Per 1 SD decrease (SD= 2.9%) | ||
| 2.44 (1.62, 3.77), <0.0001 | Clinical model + LVMi | ||||
| 2.24 (1.46, 3.43), 0.0002 | Clinical model + TDI e′ | ||||
| 2.29 (1.50, 3.56), 0.0002 | Clinical model + LVMI + TDI e′ | ||||
|
2.3 (1.58, 3.38), <0.0001 | Clinical model | Per 1 SD decrease (SD= 2.2%) | ||
| 2.4 (1.61, 3.56), <0.0001 | Clinical model + LVMi | ||||
| 2.2 (1.47, 3.30), <0.0001 | Clinical model + TDI e′ | ||||
| 2.26 (1.49, 3.38), <0.0001 | Clinical model + LVMI + TDI e′ | ||||
| Biering-Sorensen et al (2017)25 | 1. Heart failure (78) | Global longitudinal strain | 1.16 (1.09, 1.23), <0.001 | None | Per unit (1%) decrease |
| 1.12 (1.05, 1.18), <0.001 | Age and sex (Model 1) | ||||
| 1.1 (1.03, 1.17), 0.003 | Age, sex, HR, HTN, DM, previous IHD, SBP, and pro-BNP (>150 pmol/L) (Model 2) | ||||
| 1.09 (1.02, 1.17), 0.016 | Age, sex, HR, HTN, DM, previous IHD, SBP, pro-BNP (>150 pmol/L), LVEF(<50%), LVMi, LV dimension, deceleration time, LA dimension, and E/e′ (Model 3) | ||||
| 2. Acute myocardial infarction (43) | 1.16 (1.08, 1.26), <0.001 | None | Per unit (1%) decrease | ||
| 1.13 (1.04, 1.22), 0.003 | Model 1 | ||||
| 1.1 (1.01, 1.19), 0.022 | Model 2 | ||||
| 1.11 (1.01, 1.22), 0.024 | Model 3 | ||||
| 3. Cardiovascular death (74) | 1.06 (1.0, 1.13), 0.059 | None | Per unit (1%) decrease | ||
| 1.02 (0.96, 1.08), 0.54 | Model 1 | ||||
| 0.99 (0.93, 1.06), 0.85 | Model 2 | ||||
| 0.98 (0.91, 1.06), 0.59 | Model 3 | ||||
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; HTN, hypertension; HRs, hazard rations; HR, heart rate; IHD, ischemic heart disease; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; LVMi, left ventricular mass index; SBP, systolic blood pressure; SD, standard deviation; TDI, tissue Doppler Imaging.
Figure 3.
GLS as a predictor of coronary heart disease (A) and heart failure (B) on maximally adjusted models (listed in the Supplementary materials). For Kuznetsova et al, endocardial-strain is shown. Hazard ratios are per unit change in strain value. The heterogeneity assessment including the I2 statistics and p-value of Q test is shown.
Publication bias and study quality
Assessment of publication bias was not possible due to the small number of identified studies. The quality of the studies was good. Seven scored a maximum 723–26,28–30 and one scored 627 (Table S2). The degree of heterogeneity indicated by I-square was small in most of the meta-analyses and was only large in two analyses.
Discussion
This systematic review and meta-analysis summarizes current evidence about the prognostic value of STE-derived measures in the general population. 2D-STE-derived GLS was the most studied measure and it predicted total mortality, major adverse cardiac and cardiovascular end-points in community-dwelling individuals in a limited number of studies that included a total of 11,744 participants. Although information on potential confounders was limited and inconsistent, there was some evidence that this was independent of conventional cardiovascular risk factors and other echocardiographic measures. There was insufficient evidence in relation to other myocardial deformation indices or 3D-STE-derived indices to draw conclusions with respect to outcomes. Therefore, this systematic review also highlights important knowledge gaps in the current literature regarding the possible utility of myocardial deformation indices in unselected older populations, and further evidence is still required, particularly regarding 3D-STE.
Risk assessment and management of patients with CVD are guided by the measurement of LV global systolic function.2 LVEF is considered the cornerstone in assessing LV systolic function,1 but LV strain imaging is attracting interest as an additional tool to improve risk assessment and guide management in diseased populations.2,12 Nevertheless, the evidence on the utility of STE as a measure of cardiac function and risk in community-dwelling individuals has been limited. We provide a synthesis of current evidence that provides some support for GLS as a useful risk measure but also highlights the need for more information regarding the utility of STE for risk assessment and diagnosis. Based on limited numbers of identified studies, GLS was a prognostic marker of cardiovascular mortality and morbidity independent of conventional risk factors. This is important because risk factors which potentially lead to CVDs such as aging, hypertension and DM are common characteristics of longitudinal population-based samples of elderly23–30 and are known to be associated with alterations in GLS even when LVEF is still normal.4,5,31,32
According to the disease progression, the layers of the myocardium as well as the various other contributors to cardiac mechanics can be affected differently.33 Disease affecting the subendocardial layer such as ischemia, hypertension or DM tends to impair longitudinal mechanics, while circumferential and twist mechanics remain preserved or even enhanced to preserve the overall LV systolic performance and LVEF.33,34 With more involvement of the mid-myocardial and subepicardial layers, both circumferential and twist mechanics will deteriorate leading to a reduction in LVEF.3,33 In unselected population without overt cardiac diseases, Russo et al characterized the relationship between multidirectional myocardial mechanics with radial thickening and LVEF.35 Radial strain was more influenced by circumferential strain than longitudinal strain explaining why radial thickening, and hence LVEF, is less sensitive than longitudinal function in detecting subclinical LVSD.35 This may contribute to the added prognostic value of GLS over LVEF especially when LVEF is still normal or mildly impaired.12
STE-based LV strain imaging allows comprehensive quantification of complex myocardial mechanics. While STE is increasingly used in clinical practice, it suffers from inherent technical limitations.31 High-quality images and adequate frame rates are crucial for accurate tracking. For 2D-STE, multiple views are required which is time-consuming to apply in large population-based studies. Indeed, among identified studies,23–30 only one study measured circumferential, radial and transverse strains,28 while none measured LV rotation.26 This could be due to analysis time required or the limited feasibility and reproducibility of these measurements. Nevertheless, circumferential strain, both MRI-based36 and 2D-STE based,28 was an independent prognostic marker for incident HF over and beyond traditional risk factors and conventional measures in subjects free of CVDs of community-dwelling individuals. Further, Cheng et al have suggested that distinct components of LV mechanics (ie, GLS, circumferential strain, etc.) are differently associated with individual CVD outcomes,28 but it was not possible to answer this question due to the limited number of studies. In the future, 3D imaging methods may overcome some of the limitations of 2D-STE and provide additional insight to the different relationship between individual components of LV mechanics and CVD outcomes.
Study limitations
A number of limitations of this study ought to be acknowledged. This systematic review was limited to English language publications, which may have introduced a selection bias. We identified only eight papers based on five different studies; all identified studies used 2D-STE and no study examined additive the prognostic value of 3D-STE-derived LV deformation indices in a general population. Once multiple publications from the same study were accounted for, meta-analyses were based on either two or three studies limiting the precision of estimates of between-study variance which could result in an underestimate of the width of the confidence intervals. Further, meta-analysis should be performed when results of at least ten relevant studies are available. The small number of studies also precluded sub-group analysis and meta-regression to explore sources of heterogeneity between studies. We assumed a priori that there would be heterogeneity between the various observational studies and consequently used random effects modeling, although there was not strong evidence of heterogeneity in the identified studies. Since estimates from random effects models behave like the fixed effect estimate as heterogeneity decreases, it is not likely that this will have introduced substantial error. The small number of studies also prevented us from employing formal assessment of publication bias (e.g. funnel plots), but this bias cannot be excluded. Analyses employed different vendor-specific software and possibly different software versions; both are factors which may introduce systematic differences between studies. For this reason, GLS analysis including LV segmentation is different between studies included in this meta-analysis (e.g. endocardial analysis of GLS from 12 LV-segments,28 transmural analysis of GLS from 18 LV-segments25 or GLS from only 6 LV-segments29). However, since we examined associations with outcomes in relation to a continuous exposure (GLS) the impact of this source of heterogeneity is likely to be small, consistent with our sensitivity analysis.
Conclusion
This study synthesized current evidence regarding STE-derived measures as prognostic indicators of mortality and cardiovascular events in community-dwelling individuals. Despite limited number of studies in this meta-analysis, LV GLS by 2D-STE showed prognostic value in this population and may add to conventional cardiovascular risk factors and other echocardiographic measures. However, our findings also highlight the limitations of the existing evidence base and identify important knowledge gaps in the current literature regarding the possible utility of myocardial deformation indices and 3D-STE in unselected older populations – these are issues where further evidence is needed.
Acknowledgments
LA is supported by a scholarship grant from Imam Abdulrahman Bin Faisal University for her postgraduate studies (PhD) at UCL. AH works in a unit that receives support from the UK Medical Research Council (Programme Code MC_UU_12019/1), he also receives support from the British Heart Foundation (PG/15/75/31748, CS/15/6/31468, CS/13/1/30327), and the National Institute for Health Research University College London Hospitals Biomedical Research Centre. CP receives support from the British Heart Foundation (CS/15/6/31468). RH is supported by the UK Medical Research Council (MC_UU_12019/2).
Abbreviation list
AF, atrial fibrillation; CHD, coronary heart disease; CVD, cardiovascular disease; DM, diabetes mellitus; GLS, global longitudinal strain; HF, heart failure; LV, left ventricular; LVEF, left ventricular ejection fraction; LVSD, left ventricular systolic dysfunction; MI, myocardial infarction; STE, speckle-tracking echocardiography.
Disclosure
This work was presented at the British Society of Echocardiography 2018 as a presentation with interim findings. Professor Alun Hughes reports grants from British Heart Foundation, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108(8):977–982. doi: 10.1161/01.CIR.0000085166.44904.79 [DOI] [PubMed] [Google Scholar]
- 2.Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11(2 Pt 1):260–274. [DOI] [PubMed] [Google Scholar]
- 3.Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese society of echocardiography. J Am Soc Echocardiogr. 2011;24(3):277–313. doi: 10.1016/j.echo.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 4.Jensen MT, Sogaard P, Andersen HU, et al. Global longitudinal strain is not impaired in type 1 diabetes patients without albuminuria: the Thousand & 1 study. JACC Cardiovasc Imaging. 2015;8(4):400–410. doi: 10.1016/j.jcmg.2014.12.020 [DOI] [PubMed] [Google Scholar]
- 5.Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE, Tighe DA. Cardiac mechanics in mild hypertensive heart disease: a speckle-strain imaging study. Circ Cardiovasc Imaging. 2009;2(5):382–390. doi: 10.1161/CIRCIMAGING.108.811620 [DOI] [PubMed] [Google Scholar]
- 6.Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110(19):3081–3087. doi: 10.1161/01.CIR.0000147184.13872.0F [DOI] [PubMed] [Google Scholar]
- 7.Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, Oh DJ. Global 2-dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol. 2009;54(7):618–624. doi: 10.1016/j.jacc.2009.04.061 [DOI] [PubMed] [Google Scholar]
- 8.Hung CL, Verma A, Uno H, et al. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol. 2010;56(22):1812–1822. doi: 10.1016/j.jacc.2010.06.044 [DOI] [PubMed] [Google Scholar]
- 9.Yingchoncharoen T, Gibby C, Rodriguez LL, Grimm RA, Marwick TH. Association of myocardial deformation with outcome in asymptomatic aortic stenosis with normal ejection fraction. Circ Cardiovasc Imaging. 2012;5(6):719–725. doi: 10.1161/CIRCIMAGING.112.977348 [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Pozios I, Haileselassie B, et al. Role of global longitudinal strain in predicting outcomes in hypertrophic cardiomyopathy. Am J Cardiol. 2017;120(4):670–675. doi: 10.1016/j.amjcard.2017.05.039 [DOI] [PubMed] [Google Scholar]
- 11.Shetye A, Nazir SA, Squire IB, McCann GP. Global myocardial strain assessment by different imaging modalities to predict outcomes after ST-elevation myocardial infarction: A systematic review. World J Cardiol. 2015;7(12):948–960. doi: 10.4330/wjc.v7.i12.948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100(21):1673–1680. doi: 10.1136/heartjnl-2013-304474 [DOI] [PubMed] [Google Scholar]
- 13.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2(5):356–364. doi: 10.1161/CIRCIMAGING.109.862334 [DOI] [PubMed] [Google Scholar]
- 14.Dahabreh IJ, Kent DM. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA. 2011;305(8):822–823. doi: 10.1001/jama.2011.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Saikhan L, Park C, Hardy R, Hughes A. Prognostic implications of left ventricular strain by speckle-tracking echocardiography in population-based studies: a systematic review protocol of the published literature. BMJ Open. 2018;8. doi: 10.1136/bmjopen-2018-023346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 17.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quailty of nonrandomised studies in meta-analyses Available from: http://www ohri ca/programs/clinical_epidemiology/oxford.asp. Accessed July 8, 2019. [Google Scholar]
- 18.Russo C, Jin Z, Homma S, et al. Subclinical left ventricular dysfunction and silent cerebrovascular disease: the Cardiovascular Abnormalities and Brain Lesions (CABL) study. Circulation. 2013;128(10):1105–1111. doi: 10.1161/CIRCULATIONAHA.113.001984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang H, Negishi K, Wang Y, Nolan M, Saito M, Marwick TH. Echocardiographic screening for non-ischaemic stage B heart failure in the community. Eur J Heart Fail. 2016;18(11):1331–1339. doi: 10.1002/ejhf.2016.18.issue-11 [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Marwick TH, Wang Y, et al. Association between electrocardiographic and echocardiographic markers of stage B heart failure and cardiovascular outcome. ESC Heart Fail. 2017;4(4):417–431. doi: 10.1002/ehf2.12151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H, Negishi K, Wang Y, Nolan M, Marwick TH. Imaging-guided cardioprotective treatment in a community elderly population of stage B heart failure. JACC Cardiovasc Imaging. 2017;10(3):217–226. doi: 10.1016/j.jcmg.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Yang H, Nolan M, Pathan F, Negishi K, Marwick TH. Variations in subclinical left ventricular dysfunction, functional capacity, and clinical outcomes in different heart failure aetiologies. ESC Heart Fail. 2018;05:05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo C, Jin Z, Elkind MS, et al. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail. 2014;16(12):1301–1309. doi: 10.1002/ejhf.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo C, Jin Z, Sera F, et al. Left ventricular systolic dysfunction by longitudinal strain is an independent predictor of incident atrial fibrillation: a community-based cohort study. Circ Cardiovasc Imaging. 2015;8(8):e003520. doi: 10.1161/CIRCIMAGING.115.003520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biering-Sorensen T, Biering-Sorensen SR, Olsen FJ, et al. Global longitudinal strain by echocardiography predicts long-term risk of cardiovascular morbidity and mortality in a low-risk general population: the copenhagen city heart study. Circ Cardiovasc Imaging. 2017;10(3). doi: 10.1161/CIRCIMAGING.116.005521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brainin P, Biering-Sorensen SR, Mogelvang R, Sogaard P, Jensen JS, Biering-Sorensen T. Postsystolic shortening by speckle tracking echocardiography is an independent predictor of cardiovascular events and mortality in the general population. J Am Heart Assoc. 2018;7(6). doi: 10.1161/JAHA.118.008528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modin D, Biering-Sorensen SR, Mogelvang R, Landler N, Jensen JS, Biering-Sorensen T. Prognostic value of echocardiography in hypertensive versus nonhypertensive participants from the general population. Hypertension. 2018;71(4):742–751. doi: 10.1161/HYPERTENSIONAHA.117.10674 [DOI] [PubMed] [Google Scholar]
- 28.Cheng S, McCabe EL, Larson MG, et al. Distinct aspects of left ventricular mechanical function are differentially associated with cardiovascular outcomes and all-cause mortality in the community. J Am Heart Assoc. 2015;4(10):e002071. doi: 10.1161/JAHA.115.002071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuznetsova T, Cauwenberghs N, Knez J, et al. Additive prognostic value of left ventricular systolic dysfunction in a population-based cohort. Circ Cardiovasc Imaging. 2016;9(7). doi: 10.1161/CIRCIMAGING.116.004661 [DOI] [PubMed] [Google Scholar]
- 30.Shah AM, Claggett B, Loehr LR, et al. Heart failure stages among older adults in the community: the atherosclerosis risk in communities study. Circulation. 2017;135(3):224–240. doi: 10.1161/CIRCULATIONAHA.117.027305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collier P, Phelan D, Klein AA. Test in context: myocardial strain measured by speckle-tracking echocardiography. J Am Coll Cardiol. 2017;69(8):1043–1056. doi: 10.1016/j.jacc.2016.11.026 [DOI] [PubMed] [Google Scholar]
- 32.Kuznetsova T, Herbots L, Richart T, et al. Left ventricular strain and strain rate in a general population. Eur Heart J. 2008;29(16):2014–2023. doi: 10.1093/eurheartj/ehn280 [DOI] [PubMed] [Google Scholar]
- 33.Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle. JACC Cardiovasc Imaging. 2008;1(3):366–376. [DOI] [PubMed] [Google Scholar]
- 34.Seo Y, Ishizu T, Atsumi A, Kawamura R, Aonuma K. Three-dimensional speckle tracking echocardiography. Circ J. 2014;78(6):1290–1301. doi: 10.1253/circj.CJ-14-0360 [DOI] [PubMed] [Google Scholar]
- 35.Russo C, Jin Z, Homma S, et al. Relationship of multidirectional myocardial strain with radial thickening and ejection fraction and impact of left ventricular hypertrophy: a study in a community-based cohort. Echocardiography. 2013;30(7):794–802. doi: 10.1111/echo.12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi EY, Rosen BD, Fernandes VR, et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the multi-ethnic study of atherosclerosis. Eur Heart J. 2013;34(30):2354–2361. doi: 10.1093/eurheartj/eht133 [DOI] [PMC free article] [PubMed] [Google Scholar]