Summary
The serotoninergic and FMRFamidergic nervous system of the attachment organs of trematodes were examined using immunocytochemical techniques and confocal scanning laser microscopy. Adult trematodes from eight families as well as cercariae and metacercariae from ten families were studied. TRITC-conjugated phalloidin was used to stain the muscle fibres. The serotonin- and FMRFamide-immunoreactive (IR) nerve cells and fibres were revealed to be near the muscle fibres of the oral and ventral suckers of the trematodes and their larvae. The results indicate the important role of neurotransmitters, serotonin and neuropeptide FMRFamide in the regulation of muscle activity in the attachment organs of trematodes and can be considered in perspective for the development of new anthelmintic drugs, which can interrupt the function of the attachment organs of the parasites.
Keywords: Trematodes, attachment organs, neurotransmitters, serotonin, FMRFamide, nervous system
Introduction
The parasitic flatworms, trematodes have well-developed adhesive organs represented by oral and ventral suckers. These allow parasites to adhere to the substrate (i.e., to the host organs and tissues) and play an important role in their feeding and, in some cases, their locomotion. The musculature of its adhesive organs consists of longitudinal, circular and radial muscle fibres. Contractions of longitudinal (meridionally oriented) muscles open the sucker while contractions of circularly arranged fibres, in conjunction with the radial muscles that run between the inner and outer surfaces of the sucker close it (Mair et al., 1998; Halton & Maule, 2004; Yastrebov & Yastrebova, 2014; Krupenko & Dobrovolskij, 2015).
It is well known that diseases caused by parasitic flatworms are of medical and agricultural problem as they are harmful to human health as well as they are producing great economic losses in agriculture. That is why studying this animal group is important, not only for solving fundamental medical problems, but also for having great practical significance.
The nervous system of trematodes is well differentiated, consisting of central and peripheral parts, which participate in the regulation of many functions including feeding, locomotion, reproduction and migration. Data on the innervations of trematodes’ fixation organs are limited but allowing us to assume on an important role of nervous system in the mechanisms of adhesion of parasites to the host tissues (McKay et al., 1990, 1991; Niewiadomska et al., 1996a,b; McVeigh et al., 2009; Leksanboon et al., 2012).
Several neuronal signal substances, including serotonin and FMRFamide-related peptides (FaRPs) have been identified in central and peripheral nervous systems of flatworm parasites indicating the neurochemical complexity of their nervous system (Gustafsson 1987; Magee et al., 1993; Terenina et al., 2006). Serotonin (5-hydroxytryptamine, 5-HT) appears to be the dominant biogenic amine in Platyhelminthes. In trematodes 5-HT was identified by number of biochemical methods in crude extracts of Schistosoma haematobium, S. japonicum, S. mansoni (Bennett et al., 1969; Chou et al., 1972), Haplometra cylindracea, Opisthorchis felineus, Azygia lucii, Codonocephalus urnigerus (Terenina & Gustafsson, 2003). Immunocytochemical studies have verified the presence of serotonin in all trematode species examined so far. 5-HT immunoreactivity has been demonstrated in their central and peripheral nervous systems: in cerebral ganglia, lateral nerve cords, transversal commissures, in subepithelial and submuscular nerve plexuses.
The FMRFamide is a member of the neuropeptides FaRPs family and was firstly isolated from the mollusks Macrocalista nimbosa (Price & Greenberg, 1977). So far the only four authentic FaRPs have been isolated from flatworms: YIRFamide (from turbellarian Bdelloura candida); GYIRFamide (from turbellarian B. candida, Dugesia tigrina); RYIRFamide (from turbellarian Artioposthia triangulate), GNFFRFamide (from cestode Moniesia expansa) (see McVeight et al, 2009 for references). None of FaRPs was isolated from trematodes, but numerous immunocytochemical investigations using different antibodies (such as anti-RF, anti-GYIRF and anti-FMRF) indicate that FaRPs are broadly expressed in their nervous system (Gustafsson et al., 2002). Endogenous FMRF-like peptides are remarkably potent in parasitic worms (Day & Maule, 1999) and there are reasons to believe that peptidergic signalling could be an attractive target for new anthelmintic drugs developing to treat the infections (Mousley et al., 2005). In this context, the attachment organs of parasites may be a convenient model for drug investigations which will interfere with the function of suckers and thus entire parasite’s adhesion to the host tissues.
Current research is focusing on the innervations of the musculature of the oral and ventral suckers of trematodes and its larvae (cercariae and metacercariae) with serotoninergic and peptidergic (FMRFamidergic) structures. The results obtained in this study allowed to extend our knowledge about nervous system of attachment organs in selected trematode species, their potential functions and could stimulate further research in this field.
Materials and Methods
Trematodes and fixation procedure
Specimens of adult trematodes (from eight families) and larvae (cercariae and metacercariae) from ten families, collected in various regions in Russia and Belarus were used in the study (see Table 1).
Table 1.
The investigated species of trematodes.
| Adults |
| Opisthorchis felineus Rivolta, 1884 (Opisthorchiidae Loos, 1899) |
| Allocreadium isoporum Looss, 1984 (Allocreadiidae Looss, 1902) |
| Plagiorchis laricola Skrjabin, 1924 (Plagiorchiidae Lühe, 1901) |
| Opisthioglyphe ranae Frölich, 1791 (Plagiorchiidae Lühe, 1901) |
| Gorgodera cygnoides Zeder, 1800 (Gorgoderidae Looss, 1899) |
| G. loossi Sinitzin, 1905 (Gorgoderidae Looss, 1899) |
| Paramphistomum cervi Zeder, 1790 |
| (Paramphistomidae Fischoeder, 1901) |
| Aspidogaster conchicola K.Baer, 1827 |
| (Aspidogastridae Poche, 1907) |
| Cercariae |
| Cercaria parvicaudata Stunkard and Shaw, 1931 |
| (Renicolidae Dollfus, 1939) |
| Plagiorchis elegans Rudolphi, 1802 (Plagiorchiidae Lühe, 1901) |
| Cryptocotyle lingua Creplin, 1825 (Heterophyidae Leiper, 1914) |
| Trichobilharzia szidati Neuhaus, 1952 |
| (Sсhistosomatidae Stiles and Hassall, 1898) |
| Bilharziella polonica Kowalewski, 1895 |
| (Sсhistosomatidae Stiles and Hassall, 1898) |
| Sphaerostomum globiporum Rudolphi, 1802 |
| (Opecoelidae Ozaki, 1925) |
| Moliniella anceps Molin, 1859 (Echinostomatidae Looss, 1899) |
| Himasthla elongata Mehlis, 1831 (Echinostomatidae Looss, 1899) |
| Metacercariae |
| Leucochloridiomorpha lutea von Baer, 1826 |
| (Leucochloridiomorphidae Yamaguti, 1958) |
| Cotylurus sp. (Strigeidae Railliet, 1919) |
The material was fixed in 4 % paraformaldehyde (PFA) in 0.1 M phosphate buffer (PBS, Sigma) at 4 °С and at a рH of 7.4. For storage, it was transferred to the PBS buffer with 10 % sucrose. Part of the samples (adult trematodes) was embedded in Tissue-Tek, frozen and sectioned at 20μm on a Bright cryostat. The sections were collected on chrom-alum-gelatine-coated glass slides, dried for approximately two hours at room temperature, and were either stained directly or stored at -20 °C. Other samples (cercariae and metacercariae) were stained as whole mounts.
Immunocytochemistry
Whole mounts and cryostat sections of worms were stained with rabbit anti-5-HT (Instar, USA) (1:500) or rabbit anti-FMRFamide (Peninsula, Belmont, CA, USA) (1:500) primary antibodies in PBS containing 1 % (v/v) Triton X 100 (Sigma) (PBS-T) according to the method described by Coons et al. (1955). The whole mounts (cercariae and metacercariae) were incubated with the primary antibody for five days at 4 °C, and with the secondary goat anti-rabbit Alexa 488 (Molecular Probes, USA) (1:400) antibodies in PBS-T over five days at 4 °C. The sections were incubated with the primary antibody for two days and with the secondary antibody for three hours. Controls included omission of the primary antibody and the substitution of the primary antibody with non-immune rabbit serum.
Staining of musculature with TRITC-conjugated phalloidin
In order to study the relationship between the patterns of the FMRFamide-IR and 5-HT-IR nervous elements and the musculature, staining with TRITC-conjugated phalloidin (Sigma, USA) (1:200) was performed according to the method described by Wahlberg (1998).
Confocal scanning laser microscopy
The specimens stained with anti-5HT, anti-FMRFamide and TRITC-labelled phalloidin were examined using a fluorescent microscope Leica DM 1000 (А.N. Severtsov Institute of Ecology and Evolution of Russian Academy of Sciences, Center of Parasitology), a Leica TCS SP5 confocal scanning laser microscope (The Pushchino Scientific Сenter of Russian Academy of Sciences) and
a Leica TCS 4D confocal scanning laser microscope coupled to a LeitzAristoplan fluorescence microscope (The Department of Biology, Abo Akademi, Finland).
Ethical Approval and/or Informed Consent
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Results
Adults
Serotonin (5-HT)- and FMRFamide-immunoreactive (IR) nerve cells and fibres were found among the muscle fibres of the oral and the ventral suckers of the adult trematodes. The positive 5-HT-IR and FMRFamide-IR was also discovered in cells and nerve fibres located near the attachment organs. 5-HT- and FMRFamide-IR fibres extending from the main nerve cord towards the ventral sucker were found in Opisthorchis felineus (Fig. 1a b). Inside the ventral sucker, a network of 5-HT-IR nerve fibres can be seen (Fig. 1a) 5-HT- and FMRFamide-IR nerve cells and fibres were also found in the oral sucker of Allocreadium isoporum (Fig. 1c) and in the ventral sucker of Plagiorchis laricola (Fig. 1d) Gorgodera cygnoides (Fig. 1e) and Paramphistomum cervi (Fig. 1f) 5-HT-IR nerve cells and fibres can be seen near the muscles of the oral (Fig. 2a) and ventral (Fig. 2b) suckers of Opisthiogliphe ranae. Thin, 5-HT-IR fibres innervate the ventral sucker of Gorgodera loossi (Fig. 2c) The adhesive disc located on the ventral surface of Aspidogaster conchicola, a representative of the ancient trematode group, is strongly innervated by 5-HT-IR nerve fibres (Fig. 2d)
Fig. 1.

(a – f). The serotoninergic and FMRFamidergic components of the nervous system in the attachment organs of adult trematodes. (а, b) Opisthorchis felineus: (а) 5-HT-immunoreactive (IR) nerve cells and fibres located near and inside the ventral sucker (arrows), scale bar 50μm; (b) FMRFamide-IR fibres (thin arrows) extending from the main nerve cords towards the ventral sucker (thick arrow) are indicated, scale bar 50μm; (c) Allocreadium isoporum. 5-HT-(IR) nerve cell and fibres located near and inside of the oral sucker (arrows), scale bar 100μm; (d) Plagiorchis laricola. FMRFamide-IR nerve cells (short arrows) and fibres (long arrows) located close to the ventral sucker, scale bar 30μm. Inset: the pattern of TRITC-phalloidin labeled F-actin in the ventral sucker; (e) Gorgodera cygnoides. Max projection shows the pattern of 5-HT-IR nerve fibres (arrows) among the muscle fibres of the ventral sucker staining with TRITC-phalloidin, scale bar 50μm; (f) Paramphistomum cervi. 5-HT-IR nerve fibres located among the muscles of the ventral sucker (arrows), scale bar 100μm.
Fig. 2.
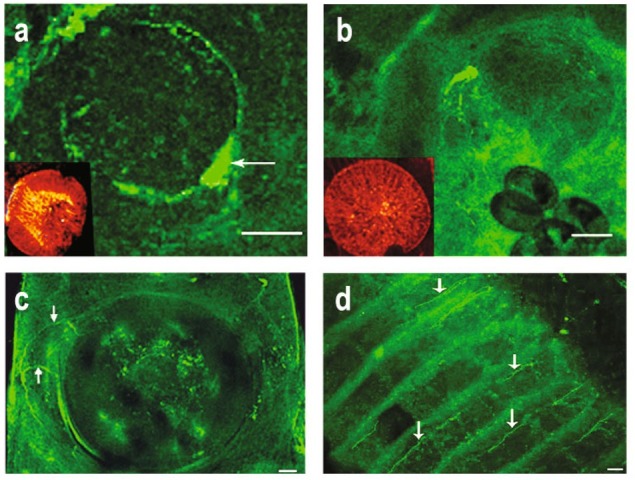
(a – d). The serotoninergic and FMRFamidergic nerve elements in the attachment organs of adult trematodes. (a, b) Opisthiogliphe ranae. 5-HT-IR nerve cells and fibres located near the oral (a) (large arrow) and ventral (b) suckers, scale bar 30μm. Inset: the pattern of TRITC-phalloidin labeled F-actin in the suckers. (c) Gorgodera loossi. 5-HT-IR nerve cell located near the ventral sucker. Note the 5-HT-IR nerve fibres extending to the musculature of the ventral sucker (arrows), scale bar 50μm; (d) Aspidogaster conchicola. 5-HT-IR nerve fibres observed in the adhesive disc situated on the ventral body surface (arrows), scale bar 20μm.
Cercariae
The 5HT- and FMRFamide-ergic nerve structures have been found in attachment organs of trematode larvae – cercariae and metacercariae. Figs. 3 and 4 show the FMRFamide-IR fibres running to the ventral and oral suckers of Cercaria parvicaudata (Fig. 3a, b) and Plagiorchis elegans (Fig. 3c, d) the ventral sucker of Сryptocotyle lingua (Fig. 3e) and the oral sucker of Moliniella anceps (Fig. 4e) and Himasthla elongata (Fig. 4h)
Fig. 3.

(a – f). The serotoninergic and FMRFamidergic components of the nervous system in the attachment organs of cercariae. (a, b) Cercaria parvicaudata. FMRFamide-IR nerve fibres extended to the ventral (a) and oral (b) suckers (arrows). The brain commissure is marked with a thick arrow (b). Scale bar 20μm; (c, d) Plagiorchis elegans. FMRFamide-IR nerve fibres located near the ventral (c) and oral (d) suckers (long arrows). The main nerve cords are marked with a thick arrows (с), scale bar 20μm; (e, f) Cryptocotyle lingua. FMRFamide-IR(e) and 5-HT-IR(f) fibres near the ventral sucker (long arrows). The main nerve cord is marked with a thick arrow (f), scale bar on (e) - 15μm; on (f) -20μm.
Fig. 4.

(a – h). The serotoninergic and FMRFamidergic components in the attachment organs of cercariae of trematodes. (a, b) Trichobilharzia szidati. 5-HT-IR fibres in the oral (a) and ventral (b) suckers (arrows); The main nerve cord is marked with a thick arrow (b), scale bar 10μm; (c) Bilharziella polonica. 5-HT-IR fibres extending towards the ventral sucker (thin arrows), scale bar 10μm. Note the nerve cells near the ventral sucker (thick arrows). Inset: the pattern of TRITC-phalloidin labeled F-actin in the ventral sucker; (d) Sphaerostomum globiporum. 5-HT-IR cells near the ventral sucker (arrow), scale bar 10μm; ( e, f) Moliniella anceps. FMRFamide-IR (e) and 5-HT-IR (f) fibres in the oral sucker (thin arrows), scale bar 20μm. The nerve cells are marked by thick arrows (f); ( g, h) Himasthla elongata. (g) 5-HT-IR fibres extended to the ventral sucker (arrows). Inset: the pattern of TRITC phalloidin labeled F-actin in the ventral sucker. (h) FMRFamide-immunoreactivity in the oral sucker (arrow), scale bar 20μm.
Positive 5-HT-immunoreactivity has been revealed in the nerve fibres running to the ventral sucker of cercariae of Сryptocotyle lingua (Fig. 3f) the oral and ventral suckers of Trichobilharzia szidati (Fig. 4a, b) the ventral sucker of Bilharziella polonica (Fig. 4c) Sphaerostomum globiporum (Fig. 4d) and H. elongata (Fig. 4g) and the oral sucker of M. anceps (Fig. 4f)
Metacercariae
The ventral sucker of Leucochloridiomorpha lutea metacercariae is strongly innervated with 5-HT-IR and FMRFamide-IR nervous fibres extending from the main longitudinal nerve cords (Fig. 5a, b) The innervations of the ventral sucker with 5-HTergic fibres have been observed in the metacercariae of Cotylurus sp. (Fig. 5c) The summary data relating to the identification of 5-HT- and FMRFamide-IR components of the nervous systems in the oral and ventral suckers of trematodes are presented in Table 2.
Fig. 5.

(a – c). The serotoninergic and FMRFamidergic components of the nervous system in the attachment organs of metacercariae. (a, b) Leucochloridiomorpha lutea. 5-HT-IR (a) and FMRFamide-IR (b) nerve fibres in the ventral sucker (thin arrows). The main nerve cords are marked with thick arrows. Scale bar 50 μm; (c) Cotylurus sp. 5-HT-IR nerve fibres extending towards the ventral sucker (thin arrows) from the main nerve cord (thick arrow), scale bar 10μm.
Table 2.
Serotonin and neuropeptide FMRFamide in the nervous system of the attachment organs of trematodes.
| Species | 5-HT | FMRFamide | References |
|---|---|---|---|
| Adults | |||
| Bucephaloides gracilescens | + | + | Stewart et al., 2003a |
| Haplometra cylindracea | + | + | McKay et al., 1990 |
| Fasciola hepatica | + | - | Fhairweather et al., 1987 |
| - « - | - | + | Magee et al., 1989 |
| Schistosoma mansoni | + | + | Mair et al., 2000 |
| Opisthorchis felineus | + | + | Tolstenkov et al., 2010 |
| Opisthorchis felineus | + | + | our data |
| Allocreadium isoporum | + | - | - « - |
| Plagiorchis laricola | - | + | - « - |
| Gorgodera cygnoides | + | - | - « - |
| G. loossi | + | - | - « - |
| Paramphistomum cervi | + | - | - « - |
| Aspidogaster conchicola | + | - | - « - |
| Cercariae | |||
| Echinostoma caproni | + | + | Šebelova et al., 2004 |
| Cercaria emasculans | + | - | Pan et al., 1994 |
| Neoastiotrema trituri | + | + | Tolstenkov et al., 2012a,b |
| Cathaemasia hians | + | - | - « - |
| Echinostoma revolutum | + | - | - « - |
| Paramphistomum cervi | + | - | - « - |
| Psilohasmus oxyurus | - | + | - « - |
| Opisthorchis felineus | - | + | Tolstenkov et al., 2010 |
| Moliniella anceps | + | - | our data, Tolstenkov et al., 2012a |
| Bilharziella polonica | + | - | - « - |
| Trichobilharzia szidati | + | - | - « - |
| Plagiorchis elegans | - | + | our data |
| Himasthla elongata | + | + | - « - |
| Cryptocotyle lingua | + | + | - « - |
| Cercaria parvicaudata | + | + | our data, Tolstenkov et al., 2011 |
| Neoastiotrema trituri | + | + | Tolstenkov et al., 2012a,b |
| Cathaemasia hians | + | - | - « - |
| Echinostoma revolutum | + | - | - « - |
| Paramphistomum cervi | + | - | - « - |
| Psilohasmus oxyurus | - | + | - « - |
| Metacercariae | |||
| Diplostomum sp. | + | + | Barton et al., 1993 |
| Cotylurus erraticus | + | + | - « - |
| Opisthorchis viverini | - | + | Lecsanboon et al., 2012 |
| Bucephaloides gracilesctns | + | + | Stewart et al., 2003a |
| Apatemon cobitidis proterorhini | + | + | Stewart et al., 2003b |
| Leucochloridiomorpha lutea | + | + | our data |
| Cotylurus sp. | + | - | - « - |
+ substance detected; - substance not detected
Discussion
Due to well-developed muscle elements of the oral and ventral suckers, trematodes are able to attach themselves securely to the host organs and tissues. The innervations of the attachment organs are not always mentioned in the description of a general morphology of the trematodes nervous system . Only a few studies pointed out the presence of nerve structures in trematodes’ oral and ventral suckers. Serotoninergic and peptidergic nerve fibres have been found in the oral suckers of the adults of Bucephaloides gracilescens and in the oral and ventral suckers of Haplometra cylindracea, Schistosoma mansoni, Fasciola hepatica and Opisthorchis felineus (Stewart et al., 2003а; McKay et al., 1990,
1991; Mair et al., 2000; Fairweather et al., 1987; Gustafsson et al., 1987; Magee et al., 1989; Tolstenkov et al., 2010). There are also data indicating the existence of nerve fibres containing these neurotransmitters in the attachment organs of the larvae (cercariae and metacercariae) of Echinostoma caproni, Cercaria emasculans, Neoastiotrema trituri, Echinostoma revolutum, Cathaemasia hians, Paramphistomum cervi, Psilohasmus oxyurus, Cercaria parvicaudata, Diplostomum sp., Cotylurus erraticus, Apatemon cobitidis proterorhin, B. gracilescens, O. viverini, M. anceps and O. felineus (Šebelova et al., 2004; Pan et al., 1994; Barton et al., 1993; Lecsanboon et al., 2012; Stewart et al., 2003a, b; Tolstenkov et al., 2010, 2012а, b).
Our study was performed on adults and larval stages of different trematode species and confirmed the presence of 5-HT- and FMRFamid-ergic nerve structures in the oral and ventral suckers of adults (eight species) and larvae (ten species) from various taxonomic groups, including the most ancient subclass, Aspidogastrea. The results not only revealed for the first time the innervations of the attachment organs in trematode species not studied before, but also confirmed and expanded the data already available on this issue for several species (M. anceps, B. polonica, T. szidati, C. parvicaudata, O. felineus). It can be concluded that the presence of studied neuromediators in the innervations of the attachment organs is characteristic for phylum Trematoda.
We found that FMRF-immunoreactivity in the fixation organs of some trematodes studied herein was more intensive than 5-HT-immunoreactivity. This is true for Opisthorchis felineus (adults), metacercariae of Leucochloridiomorpha lutea and cercariae of Cryptocotyle lingua. A question on possible differences in localization pattern of each neurotransmitter between the adult trematodes and their larvae is an interesting one. Based on own data and existing literature it is difficult to perform such comparative analysis as it requires simultaneous staining and investigation of the different life stages in one trematode species, which were not available at the same time. In some cases the immunoreactivity to serotonin or FMRFamide was not enough pronounced (or even absent) in attachment organs, what could be due to a limited quality of samples. However, the presence of nerve structures could not be ruled out.
In general, in flatworms serotonin acts as an excitatory neurotransmitter. The exogenous 5-HT can induce or enhance the motility of muscle strips or contractions of individual musle fibres prepared from monogeneans, trematodes, and cestodes (reviewed by Halton & Maule 2004). FaRPs have also been shown to be myoexcitatory in a concentration-dependent manner when were applied exogenously to isolated muscle fibers or muscle-strips from free-living Bdelloura candida and Procerodes littoralis (Johnston et al., 1996; Moneypenny et al., 2001) and parasitic flatworms Schistosoma mansomi, Fasciola hepatica, Mesocestoides corti (Day et al., 1994; Marks et al., 1997; Hrčkova et al., 2002). It was showed that FaRPs are acting through different types of receptors involving different second messenger pathways than was shown for serotonin (Zamanian et al. 2011; Patocka et al. 2014). The innervations of the copulatory organ and genital tracts by FMRF-IP nerve fibres have also suggested a role of FaRPs in the reproductive system of platyhelminths (Gustafsson et al. 2002).
In summary, present study revealed the pattern of innervations of the attachment organs with serotoninergic and peptidergic nerve structures in adults and larvae of trematodes from various taxonomic groups, which have different life cycles, hosts and localization within them. The innervations of the oral and ventral suckers in trematodes imply an important role of the nervous system, namely its serotoninergic and peptidergic components in the regulation of the function of trematodes’ adhesive organs. Based on our observations and similar studies on various trematode species, we can conclude that the innervations of trematode fixative organs with serotoninergic and peptidergic (FaRPs) neurotransmitters are widely represented (if not universal) characteristic of this class of parasitic flatworms.
Acknowledgements
This study was supported by grant from Russian Foundation for Basic Research № 18-04-00349а.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Barton CH.L., Halton D.V., Shaw C., Maule A.G., Johnston C.F.. An immunocytochemical study of putative neurotransmitters in the metacercariae of two strigeoid trematodes from rainbow trout Oncorhynchus mykiss. Parasitol. Res. 1993;79:389–396. doi: 10.1007/BF00931828. [DOI] [PubMed] [Google Scholar]
- Bennett J., Bueding E., Timms A.R., Engstrom R.G.. Occurrence and levels of 5-hydroxytryptamine in Schistosoma mansoni. Mol. Pharmacol. 1969;5:542–545. [PubMed] [Google Scholar]
- Chou T.C., Bennett J., Bueding E.J.. Occurrence and concentrations of biogenic amines in trematodes. J. Parasitol. 1972;58:1098–1102. [PubMed] [Google Scholar]
- Coons A.H., Leduc E.H., Connolly J.M.. Studies of antibody production. I. A method for the histochemical demonstration of specific antibody and its application to a study for the hiperimmune rabbit. J. Exper. Med. 1955;102(1):49–60. doi: 10.1084/jem.102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T.A., Maule A.G.. Parasitic peptides! The structure and function of neuropeptides in parasitic worms. Peptides. 1999;20(8):999–1019. doi: 10.1016/S0196-9781(99)00093-5. [DOI] [PubMed] [Google Scholar]
- Day T.A., Maule A.G., Shaw C, Halton D.W., Moore S., Bennett J.L., Pax R.A.. Platyhelminth FMRFamide-related peptides (FaRPs) contract Schistosoma mansoni (Trematoda Digenea) muscle fibres in vitro. Parasitology. 1994;109:455–459. doi: 10.1017/s0031182000080707. [DOI] [PubMed] [Google Scholar]
- Fairweather I., Maule A.G., Mitchel S.H., Johnston C.F., Halton D.W.. Immunocytochemical demonstration of 5-hydroxytryptamine (serotonin) in the nervous system of the liver fluke, Fasciola hepatica (Trematoda, Digenea) Parasitol. Res. 1987;73(3):255–258. doi: 10.1007/BF00578514. [DOI] [PubMed] [Google Scholar]
- Gustafsson M.K.S.. Immunocytochemical demonstration of neuropeptides and serotonin in the nervous system of adult Schistosoma mansoni. Parasitol. Res. 1987;74(2):168–174. doi: 10.1007/BF00536029. [DOI] [PubMed] [Google Scholar]
- Gustafsson M.K.S., Halton D.W., Kreshchenko N.D., Movsessian S.O., Raikova O.I., Reuter M., Terenina N.B.. Neuropeptides in flatworms. Peptides. 2002;23(11):2053–2061. doi: 10.1016/S0196-9781(02)00193-6. [DOI] [PubMed] [Google Scholar]
- Halton D.W., Maule A.G.. Flatworm nerve-muscle: structural and functional analysis. Can. J. Zool. 2004;82(2):316–333. doi: 10.1139/z03-221. [DOI] [Google Scholar]
- Hrčková G., Velebný S., Halton D.W., Maule A.G.. Mesocestoides corti (syn. M. vogae modulation of larval motility by neuropeptides, serotonin and acetylcholine. Parasitology. 2002;24:409– 421. doi: 10.1017/s0031182001001329. [DOI] [PubMed] [Google Scholar]
- Johnston R.N., Shaw C, Halton D.W., Verhaer T P., Blair K.L., Brennan G.P., Price D.A., Anderson P.A.V.. Isolation localization, and bioactivity of the FMRFamide-related neuropeptides GYIRFamide and YIRFamide from the marine turbellarian Bdelloura candida. J. Neurochem. 1996;67:814–821. doi: 10.1046/j.1471-4159.1996.67020814.x. [DOI] [PubMed] [Google Scholar]
- Krupenko D.Y., Dobrovolskij A.A.. Somatic musculature in trematode hermaphroditic generation. BMC Evol. Biol. 2015;15:189. doi: 10.1186/s12862-015-0468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leksomboon R., Chaijaroonkhanarak W., Arunyanart CH., Umka J., Jones M.K., Sripa B.. Organization of the nervous system in Opisthorchis viverrini investigated by histochemical and immunohistochemical study. Parasitol. Int. 2012;61(1):107–111. doi: 10.1016/j.parint.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Magee R.M., Fairweather I, Johnston C.F., Halton D.W., Shaw C.. Immunocytochemical demonstration of neuropeptides in the nervous system of the liver fluke, Fasciola hepatica (Trematoda, Digenea) Parasitology. 1989;98(2):227–238. doi: 10.1017/S0031182000062132. [DOI] [PubMed] [Google Scholar]
- Magee C.A., Cahir M., Halton D.W., Johnston C.F., Shaw C.. Cytochemical observation on the nervous system of adult Corrigia vitta. J. Helminthol. 1993;67(3):189–199. doi: 10.1017/S0022149X00013122. [DOI] [PubMed] [Google Scholar]
- Mair G.R., Maule A.G., Show C., Halton D.W.. Muscling in on parasitic flatworms. Parasitol. Today. 1998;14(2):73–76. doi: 10.1016/S0169-4758(97)01182-4. [DOI] [PubMed] [Google Scholar]
- Mair G.R., Maule A.G., Day T.A., Halton D.W.. A confocal microscopical study of the musculature of adult Schistosoma mansoni. Parasitology. 2000;121(2):163–170. doi: 10.1017/S0031182099006174. [DOI] [PubMed] [Google Scholar]
- Marks N.J., Jonhson S., Halton D.W., Shaw C., Geary T.G., Moore S., Thompson D.P.. Physiological effects of platyhelminth RFamide peptides on muscle strip preparations of Fasciola hepatica (Trematoda, Digenea) Parasitology. 1996;113:394–401. [Google Scholar]
- Mckay D.M., Halton D.W., Johnston C.F., Fairweather J, Shaw C.. Occurrence and distribution of putative neurotransmitters in the frog-lung parasite Haplometra cylindracea (Trematoda: Digenea) Parasitol. Res. 1990;76(6):509–517. doi: 10.1007/BF00931056. [DOI] [PubMed] [Google Scholar]
- Mckay D.M., Halton D.W., Maule A.G., Johnston C.F., Fairweather J., Shaw C.. Cytochemical demonstration of cholinergic, serotoninergic and peptidergic nerve elements in Gorgoderina vitelliloba (Trematoda: Digenea) Int. J. Parasitol. 1991;21(1):71–80. doi: 10.1016/0020-7519(91)90122-N. [DOI] [PubMed] [Google Scholar]
- Mcveigh P., Mair G.R., Atkinson L., Ladurner P., Zamanian M., Novozhilova E., Marks N.J., Day T.A., Maule A.G.. Discovery of multiple neuropeptide families in the phylum Platyhelminthes. Int. J. Parasitol. 2009;39:1243–1252. doi: 10.1016/j.ijpara.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moneypenny C.G., Kreshchenko N.D., Day T.A., Moffett C., Halton D.W., Maule A.G.. Physiological effects of FMR-Famide-related peptides and classical transmitters on dispersed muscle fibres of the turbellarian, Procerodes littoralis. Parasitology. 2001;122:447–455. doi: 10.1017/S0031182001007508. [DOI] [PubMed] [Google Scholar]
- Mousley A., Maule A.G., Halton D.W., Marks N.J.. Inter-phyla studies on neuropeptides: the potential for broad-spectrum anthelmintic and/or endectocide discovery. Parasitology. 2005;131:143–167. doi: 10.1017/S0031182005008553. [DOI] [PubMed] [Google Scholar]
- Niewiadomska K., Czubaj A., Moczon T.. Cholinergic and aminergic nervous systems in developing cercariae and metacercariae of Diplostomum pseudospathaceum Niewiadomska, 1984 (Diginea) Int. J. Parasitol. 1996a;26(2):161–168. doi: 10.1016/0020-7519(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Niewiadomska K., Moczon T., Czubaj A., Kiseliene V.. Cholinergic and аminergic nervous systems of Cercaria globocaudata U. Szidat, 1940 (Strigeida, Digenea) Acta Parasitol. 1996b;41(2):84–89. doi: 10.1016/0020-7519(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Pan J.Z, Halton D.W., Shaw C.A., Maule G., Johnston C.F.. Serotonin and neuropeptide immunoreactivities in the intramolluscan stages of three marine trematode parasites. Parasitology. 1994;80(5):388–395. doi: 10.1007/BF00932376. [DOI] [PubMed] [Google Scholar]
- Patocka N., Sharma N., Rashid M., Ribeiro P.. Serotonin signaling in Schistosoma mansoni: serotonin–activated G protein-coupled controls parasite movement. PLOS. Patogens. 2014;10:e1003878. doi: 10.1371/journal.ppat.1003878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šebelova Š, Stewart M., Mousley A., Fried B., Marks N., Halton D. The muscularture and associated innervation of adult and intramolluscan stages of Echinostoma caproni (Trematoda) visualized by confocal microscopy. Parasitol. Res. 2004;93(3):196–206. doi: 10.1007/s00436-004-1120-x. [DOI] [PubMed] [Google Scholar]
- Skuce P.J, Johnston C.F, Fairweather I., Halton D.W, Shaw C.. A confocal scanning laser microscope study of the peptidergic and serotoninergic components of the nervous system in larval Schistosoma mansoni. Parasitology. 1990;101(2):227–234. doi: 10.1017/S0031182000063277. [DOI] [PubMed] [Google Scholar]
- Stewart M.T., Marks N.J., Halton D.W.. Neuroactive substance and associated major muscle system in Bucephaloides gracilescens (Trematoda: Digenea) metacercaria and adult. Parasitol. Res. 2003a;91(1):12–21. doi: 10.1007/s00436-003-0896-4. [DOI] [PubMed] [Google Scholar]
- Stewart M.T., Mousley A., Koubková B., Šebelová Š., Marks N.J., Halton D.W. Gross anatomy of the muscle systems and associated innervation of Apatemon cobitidis proterorhini metacercariae (Trematoda: Strigeidea), as visualised by confocal microscopy. Parasitology. 2003b;126(3):273–282. doi: 10.1017/S0031182002002780. [DOI] [PubMed] [Google Scholar]
- Terenina N.B., Gustafsson M.K.S. Neurotransmitters in helminths. Moscow: Science Publishing; 2003. p. 176. pp (In Russian. [Google Scholar]
- Terenina N.B., Tolstenkov O.O, Fagerholm H.P, Serbina E.A., Vodjanitskaja S.N., Gustafsson M.K.S.. The spacial relationship between the musculature and the NADPH-diaphorase activity of 5-HT and FMRF amide immunoreactivities in redia, cercaria and adult of Echinoparyphium aconiatum (Digenea) Tissue Cell. 2006;38(2):151–157. doi: 10.1016/j.tice.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Terenina N.B., Tolstenkov O.O., Gustafsson M.K.S., Osipova О.S., Kuklin V.V., Kuklina M.M.. Nervous and muscle system in cercariae and adults of trematodes Cryptocotyle lingua и Cryptocotyle concavum (Heterophyidae) Russian Parasitol. J. 2010;1:22–29. (In Russian) [Google Scholar]
- Tolstenkov O.O., Terenina N.B., Serbina E.A., Gustafsson M.K.S.. The spatial relationship between the musculature and the 5-HT and FMRFamide immunoreactivities in cercaria, metacercaria and adult Opisthorchis felineus (Digenea) Acta Parasitol. 2010;55(2):123–132. doi: 10.2478/s11686-010-0024-4. [DOI] [Google Scholar]
- Tolstenkov O.O., Prokofiev V.V., Terenina N.B., Gustafsson M.K.S.. The neuromuscular system in cercaria with different patterns of locomotion. Parasitol. Res. 2011;108(5):1219–1227. doi: 10.1007/s00436-010-2166-6. [DOI] [PubMed] [Google Scholar]
- Tolstenkov O.O., Akimova L., Terenina N. B., Gustafsson M.K.S.. The neuromuscular system in continuously swimming cercariae from Belarus. II. Echinostomata, Gymnocephala and Amphistomata. Parasitol. Res. 2012a;111(6):2301–2309. doi: 10.1007/s00436-012-3084-6. [DOI] [PubMed] [Google Scholar]
- Tolstenkov O.O., Akimova L.N., Terenina N. B., Gustafsson M.K.S.. The neuromuscular system in continuously swimming cercariae from Belarus. I Xiphidiocercariae. Parasitol. Res. 2012b;111(5):1977–1983. doi: 10.1007/s00436-012-3044-1. [DOI] [PubMed] [Google Scholar]
- Wahlberg M. H.. The distribution of F-actin during the development of Diphyllobothrium dendriticum (Cestoda) Cell Tissue Res. 1998;291(3):561–570. doi: 10.1007/s004410051025. [DOI] [PubMed] [Google Scholar]
- Yastrebov M.V., Yastrebova I.V. Muscular system of trematodes. Мoscow: KMK Scientifi c Press Ltd; 2014. p. 343. pp (In Rusian) [Google Scholar]
- Zamanian M., Kimber M.J., Mcveigh P., Carlson S.A., Maule A.G., Day T.A.. The repertoire of G protein-coupled receptors in the human parasite Schistosoma mansoni and the model organism Schmidtea mediterranea. BMC Genomics. 2011;12:596. doi: 10.1186/1471-2164-12-596. [DOI] [PMC free article] [PubMed] [Google Scholar]


