Summary
Immunosuppression caused by parasitic infections represents the foremost way by which the parasites overcome or escape the host’s immune response. Glucan is a well-established natural immunomodulator with the ability to significantly improve immune system, from innate immunity to both branches of specific immunity. Our review is focused on the possible role of glucan’s action in antiparasite therapies and vaccine strategies. We concluded that the established action of glucan opens a new window in treatment and protection against parasitic infections.
Keywords: glucan, parasite, Toxoplasma, Leishmania, immunity
Background
Natural products that are useful in treating various diseases have been intensively sought after throughout the history of mankind. Almost than 40 years ago, β-glucan was described as biological response modifier (BRM) that could stimulate tumor rejection in mice (Yanagawa et al., 1984). As with many other BRM, it was classified as “nonspecific” because the cellular and molecular targets were unknown and its effects appeared to be highly pleiotropic and even more unpredictable. Despite long-term interest and research, the mechanism of how β-glucan affects various biological processes remained an enigma for a rather long time. Only in the last decade has extensive research by numerous scientific groups helped to reveal the extraordinary effects that β-glucan has on our immune system. A schematic representation of the basic molecular structure of β-glucan is presented in Figure 1.
Fig. 1.
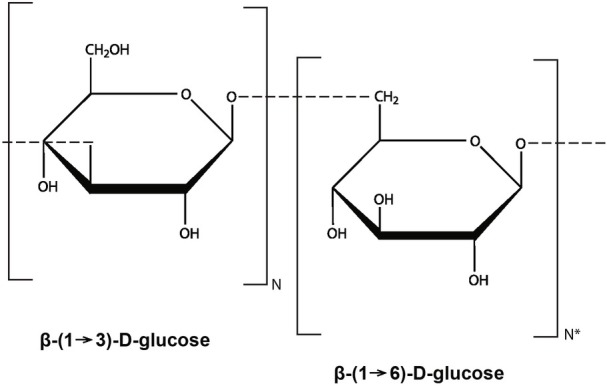
Schematic representation of the basic molecular structure of glucan molecule.
For a long time, β-glucan has been studied in infections. Using several experimental models, it has been well established that β-glucan protects against infection with both bacteria and protozoa, and enhances antibiotic efficacy in infections with antibiotic-resistant bacteria. The protective effect of β-glucan was shown in experimental infections with Candida albicans, Streptococcus suis, Plasmodium berghei, Staphylococcus aureus, and Escherichia coli; for review, see Vetvicka and Novak (2011).
Among the well-studied pleiotropic effects of β-glucan, we can mention stimulation of both humoral and cellular immunity (Novak and Vetvicka, 2008), metabolic control of diabetes (Wursch and Pi-Sunyer, 1997), stimulation of wound healing (Browder et al., 1988), stress reduction (Vetvicka and Vetvickova, 2014), attenuation of chronic fatigue syndrome (Vetvicka and Vetvickova, 2015), lowering cholesterol levels (Braaten et al., 1994), and inhibition of cancer (Sima et al., 2015). Readers seeking a summary of glucan actions can read a recent monography by Větvička (2013) or other additional excellent reviews (de Oliveira Silva et al., 2017; Vannucci et al., 2017; Bacha et al., 2017; Vetvicka et al., 2017; Alves da Cunha et al., 2017). A schematic view on the role of glucan in stimulation of immune reactions is shown in Figure 2. For more information on innate and adaptive immunity, see Netea et al. (2016).
Fig. 2.
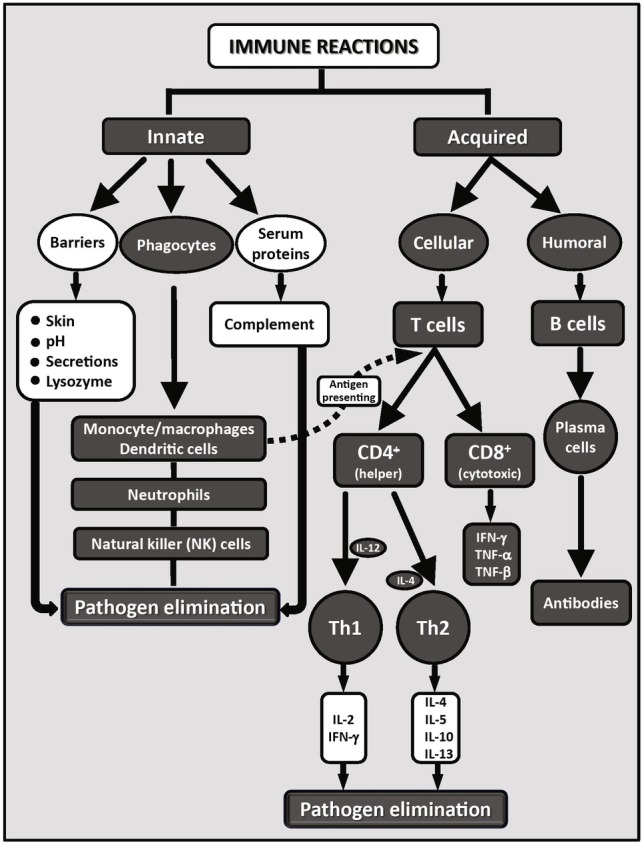
Various aspects of the both branches of immune reactions. Reaction known to be influenced by glucan are represented in black, reactions where glucan has no confirmed effects are shown in white.
Another advantage of glucan use is that it works in many species. Glucan has been found to be active in invertebrates, including earthworms (Beschin et al., 1998), bees (Mazzei et al., 2016), and shrimp (Duvic and Soderhall, 1990), and in vertebrates, including fish (Anderson, 1992), chickens (Vetvicka and Oliveira, 2014c), mice (Patchen and MacVittie, 1982), rats (Horvathova et al., 2008), hamsters (Wang et al., 1997), dogs (Vetvicka and Oliveira, 2014b), pigs (Vetvicka and Oliveira, 2014a), calves (Buddle et al., 1988), and monkeys (Reynolds et al., 1980), possibly making it the only immunomodulator active in every species tested. It is clear that with so many reports; several types of glucan were used, leading to the question if the same glucan will have the same results across species. So far only one study exists directly comparing effects of two different types of glucan in chicken, mice, dogs and pigs (De Oliveira et al, submitted). The study showed that these glucans had identical effects in all four different species. From these results, it is not surprising that glucan is intensively studied in humans, too (Kushner et al., 2014; Richter et al., 2014; Větvička, 2013).
Special role of glucan action has been established in invertebrates, representing one of the major defensive mechanisms. Glucan is involved in the prophenoloxidase system, and glucan-binding protein with a specific affinity to glucan plays an important role in protection of invertebrate animals, especially arthropods, against parasites and other invading pathogens; for review, see Vetvicka and Sima (2017) and Soderhall and Cerenius (1998).
Despite the fact that infections were one of the first studied actions of glucan in vertebrates, the question of glucan and parasites remains rather overlooked. At the same time, parasitic diseases are a major cause of morbidity and mortality, with more than three billion people infected worldwide (Bhutta et al., 2014, Torgeson et al., 2015). As most infections occur in developing countries, the need for a dependable and economical cure/prevention is particularly high. As the immune system of infected individuals seems to be particularly compromised (Samuel, 2016), an established immunostimulant, such as glucan, might be the ideal solution. The role of glucan in parasitic infection was intensively studied in the 1980s and, after three decades of neglect, the focus of glucan studies is slowly returning to this topic.
Mechanisms involved in glucan stimulation of immunological and inflammatory responses fall beyond the scope of this review. However, the most important action resulting in adequate stimulation is probably the way glucan interact with their receptors. The main glucan receptors are complement receptor 3 (CR3, CD11b/CD18) and Dectin-1. The first receptor belongs to the β2-integrin family and is found mostly on macrophages, leukocytes, and NK cells. Glucan bind to the lectin site of this receptor and the overlapping I-domain of CD11b. The stimulation of cells relies on simultaneous binding of glucan and iC3b-opsonized material (Xia et al., 1999). On the other hand, Dectin-1 is a type II transmembrane protein present on neutrophils, macrophages and dendritic cells. Upon binding of glucan, an immunoreceptor tyrosine-based activating motif is phosphorylated (Brown, 2006). In addition, stimulation of Dectin-1 receptor by glucan is mediated via Syk/NF-kB signaling axis (Fang et al., 2012). For a review dedicated to the molecular interaction of glucan with receptors, see (Legentil et al., 2015). The confusion regarding the effects of various route of administration was finally resolved by studies carefully comparing the effects after individual routs of administration and showing that the effects are the same (Vetvicka and Vetvickova, 2008; Vojtek et al., 2017).
The development of an entirely new class of antiparasitic drugs is rare and lately seems to be near impossible. At the same time, parasitic pathogens, particularly the intracellular pathogens, are as dangerous as ever. The question is – can glucan satisfy the need for a new drug?
Leishmania
One of the most studied parasites is Leishmania. Under normal conditions, the immune system cannot cope with this infection, so it is necessary to significantly boost immune reactions. An in vivo experiment used a genetically susceptible mouse strain infected with Leishmania major (Goldman and Jaffe, 1991) and showed that four intravenous injections of glucan resulted in significant suppression of infection, whereas intraperitoneal injections produced little or no effects. Older experiments showed not only protection using combination of glucan and killed Leishmania, but also the positive effects of adoptive transfer by spleen cells isolated from vaccinated animals (Jarecki-Black et al., 1985). Subsequent observation showed that four intraperitoneal injections prior to infection with Leishmania offered significant reduction in the amastigote proliferation (Al Tuwaijri et al., 1987).
In an in vitro experimental design, infected J-774A.1 macrophages stimulated with glucan showed elevated amounts of host-protective molecules such as nitric oxide and inflammatory cytokines. Even more interesting was the synergy of glucan with a standard drug, miltefosine (Shivahare et al., 2016).
Another interesting approach demonstrating the effectiveness of glucan was its use in an experimental vaccine based on L. donovani promastigotes. This vaccine offered significant protection regardless the route of application (Cook and Holbrook, 1983; Novak and Vetvicka, 2008). These data were based on older studies showing good effects of intravenous glucan injections and even stronger stimulation by glucan-promatostigotes combination (Cook and Holbrook, 1983). Similar data were obtained using a hamster model, where besides the effects of a glucan-prostigmatoses combination, significant protection was found after application of glucan alone, both in vivo and in vitro (Cook et al., 1982). The resistance caused by injection of glucan lasted up to 80 days (Holbrook et al., 1981b). Glucan alone offered protective effects in combination with every antigen fraction tested (Obaid et al., 1989). Similar results were obtained on a model of L. infantum (Lasarow et al., 1992). Glucan offered protection against L. amazonensis via stimulation of NK cell activities (Yatawara et al., 2009). It is important to note that whereas different authors used different routes of glucan administration, the effects of glucan were same application (Cook and Holbrook, 1983; Novak and Vetvicka, 2008), which further supports the fact that glucan is active via all ways of administration.
Another study found that a 45-day application of glucan eliminated the spleen and liver parasite burden in a model of visceral leishmaniasis. Detailed analysis suggested the importance of glucan-mediated production of interleukin-12 and interleukin-17 (Ghosh et al., 2013).
Other infections
A similar study evaluating the synergy between antihelmintic drug praziquantel and glucan used mice infected with Mesocestoids vogae tetrathyridia. The results showed that combined treatment resulted in suppression of fibrogenesis in the liver cell protection against oxidative damage, and possible stimulation of parenchyma regeneration (Velebny et al., 2008). The same group previously reported that a synergistic therapy with glucan and praziquantel increased macrophage activity and resulted in increased immunoglobulin levels to the secretory antigens, but decrease to the somatic antigens, probably caused by changes in antigen exposure (Hrckova et al., 2007).
Toxoplasma gondii is a common intracellular parasite, particularly dangerous for immunocompromised individuals, as the infection results in suppression of cell-mediated branch of immune reactions. The use of glucan stimulated the production of interleukin-10 in infected animals more than the standard drug, sulfadiazine (Picka et al., 2005), but the relevance to the potential treatment is unclear, as the study did not measure the possible changes in parasitic load.
Older studies showed 100 % protection against formalin-killed erythrocytic stages of Plasmodium berghei after simultaneous intravenous glucan injections (Holbrook et al., 1981a). Mushroom-derived lentinan was used during blood-stage infection with Plasmodium yoelii. When used as a prophylaxis, glucan strongly decreased parasitemia and increased overall survival rate. Stimulation of Th1 (subset of T helper lymphocytes) response was suggested due to the stimulation of nitric oxide, interleukin-12, and interferon-γ production. In addition, this study found stimulation of dendritic cell maturation and reduced Treg (regulatory T lymphocytes) action (Zhou et al., 2009). As this group used lentinan, glucan already approved for clinical use, the results have strong clinical potential.
In a study of Eimeria vermiformis infection, mice were first immunosuppressed with dexamethasone, then infected with oocysts of E. vermiformis, and finally treated with oat β-glucan by intragastric or subcutaneous routes (Yun et al., 1997). Fecal oocyst shedding was reduced in the glucan-treated groups compared to the control group. Immunosuppressed mice which received no glucan treatment showed more severe clinical signs of the disease and a 50 % mortality, while minimal clinical signs and no mortality were recorded in the glucan-treated groups. In addition, all classes of immunoglobulin showed elevated levels. Yun et al. (2003) later showed that oat glucan lowered fecal oocyst shedding by 40 %, probably via changes of lymphocyte populations in various lymphatic organs.
In aquaculture, glucan represents an important part of the supplements-driven stimulation of immune system. As infection with parasitic ciliate Ichthyiohthirius multifiliis is often fatal, it is not surprising that glucan-supplemented food was tested as possible protection. Comparing short- and long-term applications, the study confirmed the need for longer administration of glucan (Lauridsen and Buchmann, 2010). A similar study showed significant protection after longer application (Jaafar et al., 2011). In addition, glucan offered protection against Loma salmonae given either intraperitoneally or orally (Guselle et al., 2010), offering a new window for successful vaccination of commercially farmed fish. Later studies using common carp, however, did not confirm these results (Herczeg et al., 2017). For a summary on glucan-derived stimulation of immune reaction in case of L. salmonae infection, see Rodriguez-Tovar et al. (2011).
Glucan combined with zinc and porcine immunoglobulins significantly reduced the number of larvae in Toxocara canis infections (Soltys et al., 1996). As the authors never explained the reasons behind this particular combination, the results are difficult to interpret. Later experiments showed that glucan alone, when applied with a highly infective dose of T. canis eggs, showed strong stimulative and restoration effects (Boroskova et al., 1998).
Other aspects
Besides stimulating antiparasitic immunity and subsequently suppressing the parasitic infection, glucan can also be involved in a completely different role. In addition to helping to protect the host, glucan can also play a direct role in life of the parasite. Glucan is present in oocyst walls of Toxoplasma and Eimeria, where its fibers are part of trabecular scaffold in the inner layer of oocyst wall, but it is not a component of sporocyst and tissue cyst walls. This glucan might be targeted by drugs specific for glucan synthase (Bushkin et al., 2012). However, the absence of glucan in tissue cysts suggests that glucan receptors are not involved in human innate and acquired immune responses to Toxoplasma.
In the case of the intracellular pathogen Histoplasma capsulatum, the binding of the pathogen to the membrane of macrophages is mediated by the glucan present in the cell walls. In addition, alpha glucan is important for H. capsulatum virulence; whereas, glucan is antigenic and are involved in modulation of the host immune response (Gorocica et al., 2009). Unfortunately, no additional information is currently available.
Conclusion
In two waves of scientific interest, spanning three decades, glucan studies have consistently shown its ability to offer solid protection against parasitic infections. Despite positive effects, glucan treatment has, in general, been considered questionable, particularly due to the problems with obtaining the same glucan in subsequent batches (which is inherited problem to most natural molecules), and to the lack of knowledge of the mechanisms of action. Some of the confusing results originally reported might be contributed to the lack of high-quality glucan available at the time of those studies.
The overwhelming conclusion reached from this review is that, as an adjuvant, glucan can be as effective as, and at the same time safer than, conventional bacterial or other adjuvants (Roohvand et al., 2017; Li and Wang, 2015; De Smet et al., 2014). In the last decade, our knowledge of glucan and its mechanisms of action have improved tremendously. In addition, large companies are now able to produce large batches of glucan, allowing the researchers to work with identical glucan for many years. Our short review described the current knowledge of glucan action in various parasitic infections. We believe that glucan application might open a new window in treatment and protection against parasitic infections via development of vaccines.
Footnotes
Conflict of interest Authors declare no conflict of interest.
Reference
- Al tuwaijri A.S., Mahmoud A.A., Al mofleh I.A., Al khuwaitir S.A.. Effect of glucan on Leishmania major infection in BALB/c mice. J Med. Microbiol. 1987;23(4):363–365. doi: 10.1099/00222615-23-4-363. [DOI] [PubMed] [Google Scholar]
- Alves da cunha M.A., Albornoz S.L., Queiroz santos V.A., Sanchez W.N., Barbosa-dekker A.M., Dekker R.F.H. Atta-ur-rahman A.-U.-R. Studies in Natural Products Chemistry. Waltham, MA: Elsevier; 2017. Structure and biological functions of D-glucan and their applications; pp. 309–337. (Eds) (1st edition) [Google Scholar]
- Anderson D.P.. Immunostimulants, adjuvants, and vaccine carriers in fish: Applications to aquaculture. Annual Review of Fish Diseases. 1992;2:281–307. doi: 10.1016/0959-8030(92)90067-8. [DOI] [Google Scholar]
- Bacha U., Nasir M., Iqbal S., Anjum A.A.. Nutraceutical, anti-Inflammatory, and immune modulatory effects of beta-glucan isolated from yeast. Biomed Res. Int. 2017;8972678 doi: 10.1155/2017/8972678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beschin A., Bilej M., Hanssens F., Raymakers J., Van Dyck E., Revets H., Brys L., Gomez J., De Baetselier P., Timmermans M.. Identification and cloning of a glucan- and lipopolysaccharide-binding protein from Eisenia foetida earthworm involved in the activation of prophenoloxidase cascade. J. Biol. Chem. 1998;273(38):24948–24954. doi: 10.1074/jbc.273.38.24948. [DOI] [PubMed] [Google Scholar]
- Bhutta Z.A., Sommerfeld J., Lassi Z.S., Salam R.A., Das J.K.. Global burden, distribution, and interventions for infectious diseases of poverty. Infect. Dis. Poverty. 2014;3:21. doi: 10.1186/2049-9957-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroskova Z., Reiterova K., Dubinsky P., Tomasovicova O., Machnicka B.. Inhibition of lymphoproliferative response and its restoration with a glucan immunomodulator in mice with experimental larval toxocarosis. Folia Microbiol., (Praha) 1998;43(5):475–476. doi: 10.1007/BF02820794. [DOI] [PubMed] [Google Scholar]
- Braaten J.T., Wood P.J., Scott F.W., Wolynetz M.S., Lowe M.K., Bradley-white P., Collins M.W.. Oat beta-glucan reduces blood cholesterol concentration in hypercholesterolemic subjects. Eur. J. Clin. Nutr. 1994;48(7):465–474. [PubMed] [Google Scholar]
- Browder W., Williams D., Lucore P., Pretus H., Jones E., Mcnamee R.. Effect of enhanced macrophage function on early wound healing. Surgery. 1988;104(2):224–230. [PubMed] [Google Scholar]
- Brown G.D.. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006;6(1):33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- Buddle B.M., Pulford H.D., Ralston M.. Protective effect of glucan against experimentally induced staphylococcal mastitis in ewes. Vet. Microbiol. 1988;16(1):67–76. doi: 10.1016/03781135(88)90127-7. [DOI] [PubMed] [Google Scholar]
- Bushkin G.G., Motari E., Magnelli P., Gubbels M.J., Dubey J.P., Miska K.B., Bullitt E., Costello C.E., Robbins P.W., Samuelson J.. Beta-1,3-glucan, which can be targeted by drugs, forms a trabecular scaffold in the oocyst walls of Toxoplasma and Eimeria. M. Biol. 2012;3(5) doi: 10.1128/mBio.00258-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.A., Holbrook T.W.. Immunogenicity of soluble and particulate antigens from Leishmania donovani effect of glucan as an adjuvant. Infect. Immun. 1983;40(3):1038–1043. doi: 10.1128/iai.40.3.1038-1043.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.A., Holbrook T.W., Dougherty W.J.. Protective effect of glucan against visceral leishmaniasis in hamsters. Infect. Immun. 1982;37(3):1261–1269. doi: 10.1128/iai.37.3.1261-1269.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira C.A.F., Vetvicka V., Zanuzzo F.S. b-Glucan successfully stimulated the inmmune system in different jawed vertebrate species. Vet. Immunol. Immunopathol. 2018. (submitted) [DOI] [PubMed]
- De Oliveira Silva V., Oliveira De Moura N., Rodrigues De Oliveira L.J., Peconick A.P., Pereira L.J.. Promising effects of beta-glucan on metabolism and on the immune responses: Review article. Am. J. Immunol. 2017;13(1):62–72. doi: 10.3844/ajisp.2017.62.72. [DOI] [Google Scholar]
- De Smet R., Allais L., Cuvelier C.A.. Recent advances in oral vaccine development: yeast-derived beta-glucan particles. Hum. Vaccin. Immunother. 2014;10(5):1309–1318. doi: 10.4161/hv.28166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvic B., Soderhall K.. Purification and characterization of a beta-1,3-glucan binding protein from plasma of the crayfish Pacifastacus leniusculus. J. Biol. Chem. 1990;265(16):9327–9332. [PubMed] [Google Scholar]
- Fang J., Wang Y., Lv X., Shen X., Ni X., Ding K.. Structure of a beta-glucan from Grifola frondosa and its antitumor effect by activating Dectin-1/Syk/NF-kappaB signaling. Glycoconj J. 2012;29(5 – 6):365–377. doi: 10.1007/s10719-012-9416-z. [DOI] [PubMed] [Google Scholar]
- Ghosh K., Sharma G., Saha A., Kar S., Das P.K., Ukil A.. Successful therapy of visceral leishmaniasis with curdlan involves T-helper 17 cytokines. J. Infect. Dis. 2013;207(6):1016–1025. doi: 10.1093/infdis/jis771. [DOI] [PubMed] [Google Scholar]
- Goldman R., Jaffe C.L.. Administration of beta-glucan following Leishmania major infection suppresses disease progression in mice. Parasite Immunol. 1991;13(2):137–145. doi: 10.1111/j.1365-3024.1991.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Gorocica P., Taylor M.L., Alvarado-Vasquez N., Perez-Torres A., Lascurain R., Zenteno E.. The interaction between Histoplasma capsulatum cell wall carbohydrates and host components: relevance in the immunomodulatory role of histoplasmosis. Mem. Inst. Oswaldo Cruz. 2009;104(3):492–496. doi: 10.1590/S0074-02762009000300016. [DOI] [PubMed] [Google Scholar]
- Guselle N.J., Speare D.J., Markham R.J.F., Patelakis S.. Efficacy of intraperitoneally and orally administered ProVale, a yeast β-(1,3)/(1,6)-D-glucan product, in inhibiting xenoma formation by the microsporidian Loma salmonae on rainbow trout gills. N. Am. J. Aquac. 2010;72(1):65–72. doi: 10.1577/A09-017.1. [DOI] [Google Scholar]
- Herczeg D., Sipos D., Dan A., Loy C., Kallert D.M., Eszterbauer E.. The effect of dietary immunostimulants on the susceptibility of common carp Cyprinus carpio to the white spot parasite, Ichthyophthirius multifiliis. Acta. Vet. Hung. 2017;65(4):517–530. doi: 10.1556/004.2017.050. [DOI] [PubMed] [Google Scholar]
- Holbrook T.W., Cook J.A., Parker B.W.. Glucan-enhanced immunogenicity of killed erythrocyte stages of Plasmodium berghei. Infect. Immun. 1981a;32(2):542–546. doi: 10.1128/iai.32.2.542-546.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook T.W., Cook J.A., Parker B.W.. Immunization against Leishmania donovani glucan as an adjuvant with killed promastigotes. Am. J. Trop. Med. Hyg. 1981b;30(4):762–768. doi: 10.4269/ajtmh.1981.30.762. [DOI] [PubMed] [Google Scholar]
- Horvathova E., Eckl P.M., Bresgen N., Slamenova D.. Evaluation of genotoxic and cytotoxic effects of H2O2 and DMNQ on freshly isolated rat hepatocytes; protective effects of carboxymethyl chitin-glucan. Neuro Endocrinol Lett. 2008;29(5):644–648. [PubMed] [Google Scholar]
- Hrckova G., Velebny S., Kogan G.. Antibody response in mice infected with Mesocestoides vogae (syn Mesocestoides corti) tetrathyridia after treatment with praziquantel and liposomised glucan. Parasitol. Res. 2007;100(6):1351–1359. doi: 10.1007/s00436-006-0434-2. [DOI] [PubMed] [Google Scholar]
- Jaafar R.M., Skov J., Kania P.W., Buchmann K.. Dose dependent effects of dietary immunostimulants on rainbow trout immune parameters and susceptibility to the parasite Ichthyophthirius multifiliis. Aquaculture Res. Dev. 2011;S3:001. doi: 10.4172/2155-9546-S3-001. [DOI] [Google Scholar]
- Jarecki-Black J.C., Glassman A.B., James E.R.. Adoptive transfer of vaccine-induced resistance to Leishmania donovani. Am. J. Trop. Med. Hyg. 1985;34(6):1095–1097. doi: 10.4269/ajtmh.1985.34.1095. [DOI] [PubMed] [Google Scholar]
- Kushner B.H., Cheung I.Y., Modak S., Kramer K., Ragupathi G., Cheung N.K.. Phase I trial of a bivalent gangliosides vaccine in combination with beta-glucan for high-risk neuroblastoma in second or later remission. Clin. Cancer. Res. 2014;20(5):1375–1382. doi: 10.1158/1078-0432.CCR-13-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasarow R.M., Williams D.L., Theis J.H.. Humoral responses following immunization with Leishmania infantum (ex. Oklahoma): a comparison of adjuvant efficacy in the antibody responses of Balb-C mice. Int. J. Immunopharmacol. 1992;14(5):767–772. doi: 10.1016/0192-0561(92)90074-U. [DOI] [PubMed] [Google Scholar]
- Lauridsen J.H., Buchmann K.. Effects of short- and long-term glucan feeding of rainbow trout Salmonidae on the susceptibility to Ichthyophthirius multifiliis infections. Acta Ichthyol. Piscat. 2010;40(1):61–66. doi: 10.3750/Aip2010.40.1.08. [DOI] [Google Scholar]
- Legentil L., Paris F., Ballet C., Trouvelot S., Daire X., Vetvicka V., Ferrieres V.. Molecular interactions of beta-(1-->3)-glucan with their receptors. Molecules. 2015;20(6):9745–9766. doi: 10.3390/molecules20069745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Wang F.. Polysaccharides: Candidates of promising vaccine adjuvants. Drug Discov. Ther. 2015;9(2):88–93. doi: 10.5582/ddt.2015.01025. [DOI] [PubMed] [Google Scholar]
- Mazzei M., Fronte B., Sagona S., Carrozza M.L., Forzan M., Pizzurro F., Bibbiani C., Miragliotta V., Abramo F., Millanta F., Bagliacca M., Poli A., Felicioli A.. Effect of 1,3-1,6 beta-glucan on natural and experimental deformed wing virus infection in newly emerged honeybees Apis mellifera ligustica. PLoS One. 2016;11(11):e0166297. doi: 10.1371/journal.pone.0166297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M.G., Joosten L.A., Latz E., Mills K.H., Natoli G., Stunnenberg H.G., O’Neill L.A., Xavier R.J.. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352(6284) doi: 10.1126/science.aaf1098. aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak M., Vetvicka V.. Beta-glucan, history, and the present: immunomodulatory aspects and mechanisms of action. J. Immunotoxicol. 2008;5(1):47–57. doi: 10.1080/15476910802019045. [DOI] [PubMed] [Google Scholar]
- Obaid K.A., Ahmad S., Khan H.M., Mahdi A.A., Khanna R.. Protective effect of L. donovani antigens using glucan as an adjuvant. Int. J. Immunopharmacol. 1989;11(3):229–235. doi: 10.1016/0192-0561(89)90159-8. [DOI] [PubMed] [Google Scholar]
- Patchen M.L., Macvittie T.J.. Use of glucan to enhance hemopoietic recovery after exposure to cobalt-60 irradiation. Adv. Exp. Med. Biol. 1982;155:267–272. doi: 10.1007/978-1-4684-4394-3_27. [DOI] [PubMed] [Google Scholar]
- Picka M.C.M., Calvi S.A., Lima C.R.G., Santos I.A.T., Marcondes-Machado J.. Measurement of IL-10 serum levels in balb/c mice treated with beta-1, 3 polyglucose or sulfadiazine and acutely infected by Toxoplasma gondii. J. Venom Anim. Toxins Incl. Trop. Dis. 2005;11(4):540–556. doi: 10.1590/S1678-91992005000400012. [DOI] [Google Scholar]
- Reynolds J.A., Kastello M.D., Harrington D.G., Crabbs C.L., Peters C.J., Jemski J.V., Scott G.H., Di luzio N.R.. Glucan-induced enhancement of host resistance to selected infectious diseases. Infect. Immun. 1980;30(1):51–57. doi: 10.1128/iai.30.1.51-57.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J., Svozil V., Kral V., Rajnohova Dobiasova L., Stiborova I., Vetvicka V.. Clinical trials of yeast-derived beta-(1,3) glucan in children: effects on innate immunity. Ann. Transl. Med. 2014;2(2):15. doi: 10.3978/j.issn.2305-5839.2014.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Tovar L.E., Speare D.J., Markham R.J.. Fish microsporidia: immune response, immunomodulation and vaccination. Fish Shellfish Immunol. 2011;30(4 – 5):999–1006. doi: 10.1016/j.fsi.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Roohvand F., Shokri M., Abdollahpour-Alitappeh M., Ehsani P.. Biomedical applications of yeast- a patent view, part one: yeasts as workhorses for the production of therapeutics and vaccines. Expert Opin. Ther. Pat. 2017;27(8):929–951. doi: 10.1080/13543776.2017.1339789. [DOI] [PubMed] [Google Scholar]
- Samuel F.. Opportunistic parasitism: Parasitic association with the host that has compromised immune system. J. Bacteriol. Parasitol. 2016;7(1):1000261–1000264. doi: 10.4172/2155-9597.1000261. [DOI] [Google Scholar]
- Shivahare R., Ali W., Singh U.S., Natu S.M., Khattri S., Puri S.K., Gupta S.. Immunoprotective effect of lentinan in combination with miltefosine on Leishmania-infected J-774A.1 macrophages. Parasite Immunol. 2016;38(10):618–627. doi: 10.1111/pim.12346. [DOI] [PubMed] [Google Scholar]
- Sima P., Vannucci L., Vetvicka V.. Glucan and cancer: historical prospective. Canc. Transl. Med. 2015;1(6):209–214. [Google Scholar]
- Soderhall K., Cerenius L.. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998;10(1):23–28. doi: 10.1016/S0952-7915(98)80026-5. [DOI] [PubMed] [Google Scholar]
- Soltys J., Boroskova Z., Dubinsky P., Tomasovicova O., Auer H., Aspock H.. Effect of glucan immunomodulator on the immune response and larval burdens in mice with experimental toxocarosis. Appl. Parasitol. 1996;37(3):161–167. [PubMed] [Google Scholar]
- Torgerson P.R., Devieesschauwer B., Praet N., Speybroek N., Willingham A.L., Kasuga F., Rokni M.B., Zhou X.N., Fevre E.M., Sripa B., Gargouri N., Furst T., Budke C.M., Carabin H., Kirk M.D., Angulo F.J., Havelaar A., De Silva N.. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: A data synthesis. PLoS Med. 2015;12(12) doi: 10.1371/journal.pmed.1001920. e.1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci L., Sima P., Vetvicka V., Krizan J.. Lentinan properties in anticancer therapy: A review on the last 12-year literature. Am. J. Immunol. 2017;13(1):50–61. doi: 10.3844/ajisp.2017.50.61. [DOI] [Google Scholar]
- Velebny S., Hrckova G., Kogan G.. Impact of treatment with praziquantel, silymarin and/or beta-glucan on pathophysiological markers of liver damage and fibrosis in mice infected with Mesocestoides vogae Cestoda tetrathyridia. J. Helminthol. 2008;82(3):211–219. doi: 10.1017/S0022149X08960776. [DOI] [PubMed] [Google Scholar]
- Vetvicka V., Novak M.. Biological action of β-glucan. Vetvicka V., Novak M.. Biology and Chemistry of Beta Glucan Bentham Science. 2011;Vol. 1:10–18. (Eds) [Google Scholar]
- Vetvicka V., Oliveira C.. Beta(1-3)(1-6)-D-glucan modulate immune status in pigs: potential importance for efficiency of commercial farming. Ann. Transl. Med. 2014a;2(2):16. doi: 10.3978/j.issn.2305-5839.2014.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetvicka V., Oliveira C.. β(1-3)(1-6)D-glucan modulate immune status and blood glucose levels in dogs. Br. J. Pharmaceut Res. 2014b;4:981–991. doi: 10.9734/BJPR/2014/7862. [DOI] [Google Scholar]
- Vetvicka V., Oliveira C.. β(1,3)(1,6)-D-glucan with strong effects on immune status in chicken: potential importance of efficiency of commercial farming. J. Nutr. Health Science. 2014c;1:310–317. [Google Scholar]
- Vetvicka V., Sima P.. Various roles of β-glucan in invertebrates. Invertebrate Surviv. J. 2017;14:488–493. [Google Scholar]
- Vetvicka V., Sima P., Vannucci L. Holban A. M., Grumezescu A. M. Handbook of Food Bioengineering. Vol. 8. Academic Press; 2017. Beta glucan as therapeutic food; pp. 239–256. (Therapeutic Foods) [Google Scholar]
- Vetvicka V., Vetvickova J.. A comparison of injected and orally administered b-glucans. J. Am. Nutr. Assoc. 2008;11:42–49. [Google Scholar]
- Vetvicka V., Vetvickova J.. Anti-stress action of an orally-given combination of resveratrol, beta-glucan, and vitamin C. Molecules. 2014;19(9):13724–13734. doi: 10.3390/molecules190913724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetvicka V., Vetvickova J.. β-glucan attenuates chronic fatique syndrome in murine model. J. Nat. Sci. 2015;1:e112. [Google Scholar]
- Větvička V. β-glucan as Natural Biological Response Modifiers. New York: Nova Science Publishers, Inc; 2013. pp. 1–162. [Google Scholar]
- Vojtek B., Mojzisova J., Smrco P., Drazovska M.. Effects of orally administered b-1,3/1,6-glucan on vaccination responses and immunological parameters in dogs. Food Agricul. Immunol. 2017;28(6):993–1002. doi: 10.1080/09540105.2017.1324407. [DOI] [Google Scholar]
- Wang L., Behr S.R., Newman R.K., Newman C.W.. Comparative cholesterol-lowering effects of barley β-glucan and barley oil in golden syrian hamsters. Nutrition Research. 1997;17(1):77–88. doi: 10.1016/S0271-5317(96)00234-5. [DOI] [Google Scholar]
- Wursch P., Pi-Sunyer F.X.. The role of viscous soluble fiber in the metabolic control of diabetes. A review with special emphasis on cereals rich in beta-glucan. Diabetes Care. 1997;20(11):1774–1780. doi: 10.2337/diacare.20.11.1774. [DOI] [PubMed] [Google Scholar]
- Xia Y., Vetvicka V., Yan J., Hanikyrova M., Mayadas T., Ross G.D.. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J. Immunol. 1999;162(4):2281–2290. [PubMed] [Google Scholar]
- Yanagawa T., Oguro M., Takagi T., Takenaga K.. Direct antitumor activity of biologica response modifiers (B.R.M.) proven by an in vitro sensitivity test. Gan To Kagaku Ryoho. 1984;11(10):2155–2162. [PubMed] [Google Scholar]
- Yatawara L., Wickramasinghe S., Nagataki M., Takamoto M., Nomura H., Ikeue Y., Watanabe Y., Agatsuma T.. Aureobasidium-derived soluble branched (1,3-1,6) beta-glucan (Sophy beta-glucan) enhances natural killer activity in Leishmania amazonensis-infected mice. Korean J. Parasitol. 2009;47(4):345–351. doi: 10.3347/kjp.2009.47.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C.H., Estrada A., Van Kessel A., Gajadhar A.A., Redmond M.J., Laarveld B.. Beta-(1-->3, 1-->4) oat glucan enhances resistance to Eimeria vermiformis infection in immunosuppressed mice. Int. J. Parasitol. 1997;27(3):329–337. doi: 10.1016/S0020-7519(96)00178-6. [DOI] [PubMed] [Google Scholar]
- Yun C.H., Estrada A., Van Kessel A., Park B.C., Laarveld B.. Beta-glucan, extracted from oat, enhances disease resistance against bacterial and parasitic infections. FEMS Immunol. Med. Microbiol. 2003;35(1):67–75. doi: 10.1016/S0928-8244(02)00460-1. [DOI] [PubMed] [Google Scholar]
- Zhou L.D., Zhang Q.H., Zhang Y., Liu J., Cao Y.M.. The shiitake mushroom-derived immuno-stimulant lentinan protects against murine malaria blood-stage infection by evoking adaptive immune-responses. Int. Immunopharmacol. 2009;9(4):455–462. doi: 10.1016/j.intimp.2009.01.010. [DOI] [PubMed] [Google Scholar]


