Abstract
Background
Cardiovascular disease is a major cause of morbidity and mortality in children with chronic kidney disease. We sought to determine the prevalence of cardiovascular risk factors in children with glomerular disease and to describe current practice patterns regarding risk factor identification and management.
Methods and Results
Seven‐hundred sixty‐one children aged 0 to 17 years with any of 4 biopsy‐confirmed primary glomerular diseases (minimal change disease, focal segmental glomerulosclerosis, membranous nephropathy, and IgA nephropathy/vasculitis) were enrolled at a median of 16 months from glomerular disease diagnosis in the multicenter prospective Cure Glomerulonephropathy Network study. Prevalence of traditional (hypertension, hypercholesterolemia, and obesity) and novel (proteinuria, prematurity, and passive smoke exposure) cardiovascular risk factors were determined at enrollment and compared across glomerular disease subtypes. Frequency of screening for dyslipidemia and prescribing of lipid‐lowering or antihypertensive medications were compared across glomerular disease subtype, steroid exposure, and remission status groups. Compared with the general population, all traditional risk factors were more frequent: among those screened, 21% had hypertension, 51% were overweight or obese, and 71% had dyslipidemia. Children who were not in remission at enrollment were more likely to have hypertension and hypercholesterolemia. Fourteen percent of hypertensive children were not receiving antihypertensives. Only 49% underwent screening for dyslipidemia and only 9% of those with confirmed dyslipidemia received lipid‐lowering medications.
Conclusions
Children with primary glomerular diseases exhibit a high frequency of modifiable cardiovascular risk factors, particularly untreated dyslipidemia. Lipid panels should be routinely measured to better define the burden of dyslipidemia in this population. Current approaches to screening for and treating cardiovascular risk factors are not uniform, highlighting a need for evidence‐based, disease‐specific guidelines.
Keywords: cardiovascular disease risk factors, chronic kidney disease, high blood pressure, hypercholesterolemia, hypertension, pediatrics
Subject Categories: Cardiovascular Disease, Pediatrics, Risk Factors, Hypertension, Nephrology and Kidney
Clinical Perspective
What Is New?
Children with primary glomerular diseases have a high burden of cardiovascular disease risk factors relative to the general pediatric population.
The high burden of cardiovascular disease risk factors in these children is seen irrespective of remission status or steroid exposure duration.
Screening and treatment practice patterns in these children, particularly for dyslipidemia, are not uniform, and only 9% of children with glomerular disease and dyslipidemia in this cohort received lipid‐lowering treatment.
What Are the Clinical Implications?
Opportunities exist to target modifiable cardiovascular disease risk factors (hypertension, dyslipidemia, and obesity) in children with primary glomerular diseases.
Consistent uniform screening strategies and treatment guidelines, particularly for dyslipidemia, are urgently needed.
Introduction
Cardiovascular disease (CVD) is a major cause of morbidity and mortality in children with chronic kidney disease (CKD).1, 2 In children on dialysis, cardiovascular mortality rates are far higher than in the general pediatric population, and CVD accounts for 33% to 36% of all deaths.3, 4 As children with CKD become young adults, CVD prevalence increases further.5, 6 Accordingly, The American Heart Association's Expert Panel on Population and Prevention Science and the Council for Cardiovascular Disease in the Young included children with CKD in the highest‐risk group for CVD development.7
Childhood glomerular diseases are most often primary kidney disorders and less frequently secondary to systemic diseases such as lupus or chronic infections such as hepatitis B and C, and HIV infection. Primary glomerular diseases may be caused by known genetic mutations affecting glomerular basement membrane proteins, though the exact mechanism for most cases remains incompletely understood and attributed to immune‐related mechanisms.8 Their incidence ranges from 0.1 to 2 cases per 100 000 children per year.9 Despite their relative overall low frequency, they are a common cause of childhood CKD, accounting for 29% of all CKD cases in the 854 children enrolled in the CKiD (Chronic Kidney Disease in Children) study,10 and ≈31% of all end‐stage renal disease cases in 11 186 index kidney transplant recipients from the North American Pediatric Trials and Collaborative Studies.11 Many glomerular diseases—including minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), membranous nephropathy (MN) and, to a lesser extent, IgA nephropathy/IgA vasculitis (IgAN/IgAV)—are characterized by the presence of nephrotic syndrome. Nephrotic syndrome is associated with hypertension,12 dyslipidemias,13 and often prolonged exposure to corticosteroids leading to obesity and diabetes mellitus. However, the prevalence of traditional and nontraditional CVD risk factors in children with glomerular disease is not well described. Even less is known about current practice patterns regarding CVD risk factor evaluation and treatment in this population.
The Cure Glomerulonephropathy Network (CureGN) is a prospective multicenter cohort study of children and adults with glomerular disease funded by the National Institute of Diabetes and Digestive and Kidney Diseases.14 We hypothesized that children with glomerular disease, particularly those with active nephrosis, have the highest prevalence of CVD risk factors and are likely not receiving comprehensive screening and treatment at a level commensurate with their risk profile. Therefore, we sought to describe the prevalence of CVD risk factors in those children and to evaluate current practice patterns regarding identification and management of their CVD risk profile.
Methods
The supporting data are available at the National Institute of Diabetes and Digestive and Kidney Diseases central data repository.15
Study Sample
We performed a cross‐sectional examination of all participants younger than 18 years old at the time of consent who were enrolled in CureGN between December 2014 and January 2018. CureGN enrolls participants from multiple clinical sites in North America and Europe. Participants with a diagnostic kidney biopsy within 5 years before enrollment that meets pathologic criteria for MCD, FSGS, MN, or IgAN/IgAV are eligible for inclusion. Those with end‐stage renal disease at the time of screening or any of the following at the time of first kidney biopsy are excluded: systemic lupus erythematosus, HIV infection, diabetes mellitus, active hepatitis B/C, active malignancy, or prior solid organ/bone marrow transplant. The study protocol was approved by the Institutional Review Board at each participating center and all participants provided written, informed consent or assent at enrollment.
Data Collection
Demographic and clinical characteristics collected at study enrollment included age, sex, race, ethnicity, glomerular disease subtype, disease duration, medication use at or before enrollment, and treatment responsiveness (ie, steroid responsive, steroid‐resistant, or multidrug resistant, as noted in medical records by treating providers).
Other variables of interest included estimated glomerular filtration rate (eGFR), serum albumin, and urine protein‐to‐creatinine ratio (UPCR). The central CureGN laboratory utilized a Randox RX Daytona chemistry analyzer to measure serum and urine creatinine using the Jaffe method, and urine protein with a colorimetric method. When central measurements were not available (74% serum creatinine, 80% UPCR), serum creatinine and UPCR measurements performed in local laboratories within 60 days before and after enrollment were extracted from clinical records. Estimated GFR was calculated using the modified Schwartz formula.16
CVD Risk Factors Definitions
We assessed the prevalence at enrollment of pre‐existing CVD or any of the following traditional (hypertension, obesity, and dyslipidemia) or nontraditional (proteinuria, prematurity, and second‐hand smoke exposure) CVD risk factors. Three seated casual blood pressure measurements were obtained after 5 minutes of rest using a calibrated site‐dependent oscillometric device at the enrollment visit. Consensus recommendations for cuff size selection and patient preparation were followed at all sites.17 Hypertension was defined as a self‐reported history of hypertension. The second and third seated blood pressure measurements were averaged and used to stratify blood pressure level at enrollment. Patients were considered to have a hypertensive‐level casual blood pressure reading at enrollment if the average blood pressure measurement exceeded the 95th percentile for age, sex, and height.17 Participants were considered to have a prehypertensive level casual blood pressure at enrollment if the average blood pressure reading was between the 90th and 95th percentile for age, sex, and height. Weight and height were measured at enrollment visit and body mass index (BMI) was calculated using the Centers for Disease Control and Prevention BMI calculator. Weight was classified using age‐ and sex‐specific BMI percentiles from the Centers for Disease Control and Prevention growth chart as normal (BMI between the 5th percentile to less than the 85th percentile), overweight (BMI from 85th to less than the 95th percentile), or obese (BMI greater than or equal to the 95th percentile).18 Lipid panels performed in local laboratories were extracted from clinical records and were included if performed before and up to 7 days following enrollment. Fasting status information was not available. Dyslipidemia at enrollment was defined per the National Heart, Lung and Blood Institute and the Kidney Disease: Improving Global Outcomes clinical practice guidelines, and included a total cholesterol ≥200 mg/dL, low‐density lipoprotein ≥130 mg/dL, high‐density lipoprotein <40 mg/dL, or triglycerides ≥100 mg/dL (age 0–9 years) or ≥130 mg/dL (age 10–18 years).19 Significant proteinuria was defined as UPCR >2 quantified by a 24‐hour urine collection, a first morning void, or random spot urine. History of prematurity (birth before 37 weeks gestation), self‐reported smoking or exposure to second‐hand smoke, and pre‐existing CVD diagnoses including coronary artery disease, heart failure, heart arrhythmia, stroke, peripheral vascular disease, aortic aneurysm, and valvular heart disease were obtained from participant interviews at enrollment visit. Participants were considered in complete remission at enrollment if they had a UPCR <0.3 and serum albumin >3 g/dL.
CVD Risk Factor Screening and Treatment Practice Patterns
The following variables were extracted from patients’ local medical records using a standardized data collection form: lipid profile measurement (yes/no), prescription of lipid‐lowering medication (yes/no), type of lipid‐lowering medication, prescription of any antihypertensive medication, and prescription of renin–angiotensin–aldosterone system (RAAS) blockers specifically. Screening and treatment frequencies were compared across the 4 glomerular disease types, and across prespecified groups of interest (ie, participants with versus without nephrotic range proteinuria, and those prescribed versus not prescribed RAAS blockers).
Statistical Methods
Continuous variables were summarized as medians with interquartile ranges (IQRs) and categorical variables as frequencies. Demographic, clinical, medication, and practice pattern data were compared across MCD, FSGS, MN, and IgAN/IgAV groups, and practice pattern data were also compared across proteinuria and RAAS blockade groups, using a χ2 or Fisher exact test for categorical variables and the Kruskal–Wallis test for continuous variables. To compare the prevalence of CVD risk factors between disease groups, using IgAN/IgAV as the reference group, prevalence ratios were estimated using a modified Poisson regression model with a sandwich estimator.20 Modified Poisson rather than logistic models were used because of our interest in prevalence ratios rather than odds ratios, more robust standard error estimation, and precision and consistency of effect estimates with adjustment covariates.16, 21 Models to estimate prevalence ratios for hypertension, obesity, and hypercholesterolemia in MCD, FSGS, and MN versus IgAN/IgAV participants were adjusted for age, sex, race, and eGFR. We compared the prevalence of CVD risk factors in the CureGN pediatric cohort to prevalence reported in the US general pediatric population using 1‐sample binomial proportion tests.22, 23, 24, 25, 26 All analyses were conducted using SAS software, Version 9.4 (2013; SAS Institute Inc., Cary, NC).
Results
Cohort Description
A total of 761 children were included in this study: 283 (37%) with MCD, 177 (23%) with FSGS, 36 (5%) with MN, and 265 (35%) with IgAN/IgAV (Table 1). The median age at disease diagnosis was 9 years (IQR 5–13). Participants were enrolled at a median of 16 months (IQR 5–37) since diagnosis and 11 months (IQR 3–29) since their kidney biopsy. Most participants were white (71%), and the remaining were 18% black and 6% Asian. Hispanic or Latino ethnicity was reported by 12%. Among white children, IgAN/IgAV predominated (43%), 34% had MCD, and 19% had FSGS. Among black children, MCD predominated (45%), 41% had FSGS, and only 8% had IgAN/IgAV. Children with MCD had longer disease duration before enrollment, at 27 months versus 6 to 14 months in the other groups.
Table 1.
Pediatric CureGN Subjects
| Median (IQR) or n (%)a | Overall (n=761) | MCD (n=283) | FSGS (n=177) | MN (n=36) | IgAN (n=265) |
|---|---|---|---|---|---|
| Age at consent, y | 11 (7–15) | 8 (6–12) | 13 (8–16) | 15 (12–17) | 12 (9–15) |
| Disease duration (mo since diagnosis) | 16 (5–37) | 27 (10–51) | 14 (6–36) | 6 (2–22) | 11 (3–28) |
| Racec | |||||
| White | 510 (71) | 171 (65) | 98 (58) | 21 (64) | 220 (87) |
| Black | 132 (18) | 59 (22) | 54 (32) | 9 (27) | 10 (4) |
| Asian | 43 (6) | 21 (8) | 6 (4) | 1 (3) | 15 (6) |
| Other | 34 (5) | 13 (5) | 10 (6) | 2 (6) | 9 (4) |
| Hispanic/Latinob | 94 (12) | 35 (12) | 20 (11) | 8 (22) | 31 (12) |
| Male | 449 (59) | 174 (61) | 95 (54) | 16 (44) | 164 (62) |
| Disease classification | |||||
| Steroid/multi‐drug‐resistant | 134 (18) | 56 (20) | 59 (33) | 8 (22) | 11 (4) |
| Steroid sensitive | 256 (34) | 163 (58) | 41 (23) | 5 (14) | 47 (18) |
| Untreated | 101 (13) | 22 (8) | 28 (16) | 5 (14) | 46 (17) |
| Unknown/not applicable | 270 (35) | 42 (15) | 49 (28) | 18 (50) | 161 (61) |
| eGFR, mL/min per 1.73 m²c | 103 (86–124) | 113 (94–134) | 93 (69–114) | 103 (89–129) | 100 (86–119) |
| Serum albumin, g/dLd | 3.8 (3.0–4.2) | 3.7 (2.8–4.2) | 3.6 (2.6–4.2) | 3.2 (2.2–4.0) | 4.0 (3.6–4.2) |
| UPCRd | 0.5 (0.1–2.4) | 0.2 (0.1–2.6) | 1.2 (0.2–3.9) | 2.0 (0.9–7.0) | 0.4 (0.2–1.2) |
| Birth weight, kgd | 3.3 (3.0–3.7) | 3.3 (2.9–3.7) | 3.4 (2.9–3.7) | 3.3 (3.0–3.5) | 3.4 (3.1–3.7) |
CureGN indicates Cure Glomerulonephropathy Network; EGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; IgAN, IgA nephropathy; IQR, interquartile range; MCD, minimal change disease; MN, membranous nephropathy; UPCR, urine protein‐to‐creatinine ratio (measured on 24–hour urine, first morning void, or spot/random urine in hierarchy as available).
Percent reported among nonmissing observations.
≤1% missing.
5% to 10% missing.
10% to 20% missing.
At enrollment, median eGFR was 103 mL/min per 1.73 m2 overall and exceeded 90 mL/min per 1.73 m2 in all disease subgroups. Median serum albumin was normal at 3.8 g/dL (IQR 3.0–4.2) and median UPCR was mildly elevated at 0.5 mg/mg (IQR 0.1–2.4). In 521 children with serum albumin and UPCR measurements at enrollment, only 200 (38%) were in complete remission.
Immunosuppressive medication burden at enrollment was high with 421 (55%) of children receiving either steroids or a calcineurin inhibitor (Table 2). Steroid exposure duration exceeded 6 months in 43% of participants who were ever prescribed them (Table 3).
Table 2.
Immunosuppression at Enrollment
| Overall (n=761) | MCD (n=283) | FSGS (n=177) | MN (n=36) | IgAN/IgAV (n=265) | |
|---|---|---|---|---|---|
| Steroids, all | 297 (39%) | 138 (49%) | 57 (32%) | 11 (31%) | 91 (35%) |
| CNI, all | 232 (31%) | 120 (43%) | 84 (48%) | 16 (44%) | 12 (5%) |
| Steroids+CNI | 108 (14%) | 60 (21%) | 34 (19%) | 6 (17%) | 8 (2%) |
CNI indicates calcineurin inhibitor; FSGS, focal segmental glomerulosclerosis; IgAN, IgA nephropathy; IgAV, IgA vasculitis; MCD, minimal change disease; MN, membranous nephropathy.
Table 3.
Steroid Exposure From Diagnosis to Enrollment
| Overall (n=297) | MCD (n=138) | FSGS (n=57) | MN (n=11) | IgAN/IgAV (n=91) | |
|---|---|---|---|---|---|
| <6 mo | 133 (45%) | 42 (30%) | 28 (49%) | 6 (55%) | 57 (63%) |
| 6–12 mo | 45 (15%) | 19 (14%) | 11 (19%) | 0 (0%) | 15 (16%) |
| 12–24 mo | 43 (14%) | 28 (20%) | 3 (5%) | 2 (18%) | 10 (11%) |
| 24+ mo | 39 (13%) | 31 (22%) | 7 (12%) | 0 (0%) | 1 (1%) |
| Unknown | 37 (12%) | 18 (13%) | 8 (14%) | 3 (27%) | 8 (9%) |
FSGS indicates focal segmental glomerulosclerosis; IgAN, IgA nephropathy; IgAV, IgA vasculitis; MCD, minimal change disease; MN, membranous nephropathy.
CVD Risk Factors
Traditional risk factors
Hypertension
Hypertension was frequently observed (Table 4). Twenty‐one percent of participants self‐reported a history of hypertension, and 22% had a hypertensive‐level casual clinic blood pressure reading at enrollment. Hypertensive‐level blood pressure readings were noted in 34% of those with a known diagnosis of hypertension and 19% of normotensive children. After adjusting for between‐group differences in age and sex, a higher prevalence of hypertension or hypertensive‐level blood pressure was evident among participants with MN versus IgAN/IgAV; the association persisted even after adjusting for race and eGFR (Table 5). The prevalence of hypertension or hypertensive‐level blood pressure was similar among those with (38%) versus without (42%) at least 6 months of steroid exposure (Table 6). However, there was a significant difference in the prevalence of hypertension or hypertensive‐level blood pressure among those who were (29%) versus were not (44%) in complete remission at enrollment, P<0.001 (Table 7).
Table 4.
Cardiovascular Disease Burden in Pediatric CureGN Subjects at Enrollment
| n (%)a | Overall (n=761) | MCD (n=283) | FSGS (n=177) | MN (n=36) | IgAN/IgAV (n=265) | P Value |
|---|---|---|---|---|---|---|
| Hypertensionb , c | 161 (21) | 63 (22) | 46 (26) | 10 (28) | 42 (16) | 0.04 |
| Blood pressure level at enrollmentd | ||||||
| Normal | 460 (64) | 164 (61) | 95 (58) | 15 (46) | 186 (74) | 0.002 |
| Prehypertensive | 99 (14) | 39 (15) | 31 (19) | 6 (18) | 23 (9) | |
| Hypertensive | 160 (22) | 66 (25) | 38 (23) | 12 (36) | 44 (17) | |
| Lipid screen collected | 372 (49) | 172 (61) | 94 (53) | 22 (61) | 84 (32) | <0.001 |
| Dyslipidemia (any type), n/N (%) | 265/372 (71) | 132/172 (77) | 67/94 (71) | 20/22 (91) | 46/84 (55) | <0.001 |
| Total cholesterol ≥200 mg/dL, n/N (%) | 224/362 (62) | 120/168 (71) | 54/92 (59) | 18/22 (82) | 32/80 (40) | <0.001 |
| Triglycerides ≥100 mg/dL (age 0–9) or ≥130 mg/dL (age 10–18), n/N (%) | 158/257 (62) | 82/114 (72) | 41/63 (65) | 13/19 (68) | 22/61 (36) | <0.001 |
| HDL cholesterol <40 mg/dL, n/N (%) | 44/198 (22) | 14/90 (16) | 13/51 (26) | 4/13 (31) | 13/44 (30) | 0.17 |
| LDL cholesterol ≥130 mg/dL, n/N (%) | 104/178 (58) | 58/81 (72) | 20/45 (44) | 9/13 (69) | 17/39 (44) | 0.004 |
| Weight status at enrollmente | ||||||
| Underweight | 17 (2) | 7 (3) | 6 (4) | 0 (0) | 4 (2) | 0.16 |
| Normal | 346 (46) | 125 (45) | 73 (43) | 11 (31) | 137 (52) | |
| Overweight | 143 (19) | 61 (22) | 28 (17) | 9 (26) | 45 (17) | |
| Obese | 239 (32) | 86 (31) | 62 (37) | 15 (43) | 76 (29) | |
| Exposure to firsthand smoke | 12 (2) | 0 (0) | 4 (2) | 1 (3) | 7 (3) | 0.01 |
| Exposure to secondhand smokee | 176 (24) | 73 (26) | 35 (21) | 11 (32) | 57 (23) | 0.39 |
| Prematurity (<37 wks)e | 87 (12) | 30 (11) | 22 (13) | 3 (9) | 32 (13) | 0.84 |
| Pre‐existing CVDc , f | 14 (2) | 6 (2) | 4 (2) | 1 (3) | 3 (1) | 0.58 |
CureGN indicates Cure Glomerulonephropathy Network; CVD, cardiovascular disease; FSGS, focal segmental glomerulosclerosis; HDL, high‐density lipoproteins; IgAN, IgA nephropathy; IgAV, IgA vasculitis; LDL, low‐density lipoprotein; MCD, minimal change disease; MN, membranous nephropathy.
Percent reported among nonmissing observations.
Self‐reported history of hypertension.
<1% missing.
5% to 10% missing.
1% to 5% missing.
Heart arrhythmia (n=8), valvular heart disease (n=4), peripheral vascular disease (n=2), aortic aneurysm (n=1), coronary artery disease (n=1), heart failure (n=1), stroke (n=1).
Table 5.
Prevalence Ratios for Cardiovascular Risk Factors, by Disease
| Model 1: Unadjusted | Model 2: Adjusted for Age, Sex | Model 3: Model 2+Race | Model 4: Model 3+Enrollment eGFR | |
|---|---|---|---|---|
| Hypertension or hypertensive‐level blood pressurea | ||||
| MCD | 1.4 (1.1–1.8) | 1.3 (1.0–1.7) | 1.2 (1.0–1.6) | 1.3 (1.0–1.7) |
| FSGS | 1.5 (1.1–1.9) | 1.5 (1.1–1.9) | 1.3 (1.0–1.7) | 1.3 (1.0–1.8) |
| MN | 2.0 (1.4–2.8) | 2.1 (1.4–3.0) | 1.9 (1.3–2.8) | 1.9 (1.3–2.8) |
| IgAN/IgAV | Ref. | Ref. | Ref. | Ref. |
| Hypercholesterolemia | ||||
| MCD | 1.8 (1.3–2.4) | 1.7 (1.2–2.2) | 1.6 (1.2–2.1) | 1.6 (1.2–2.1) |
| FSGS | 1.5 (1.1–2.0) | 1.4 (1.0–1.9) | 1.3 (1.0–1.8) | 1.4 (1.0–1.9) |
| MN | 2.0 (1.5–2.9) | 2.1 (1.5–2.9) | 2.1 (1.5–2.9) | 2.1 (1.5–2.9) |
| IgAN/IgAV | Ref. | Ref. | Ref. | Ref. |
| Obesity | ||||
| MCD | 1.1 (0.8–1.4) | 1.0 (0.8–1.3) | 1.0 (0.7–1.3) | 0.9 (0.7–1.3) |
| FSGS | 1.3 (1.0–1.7) | 1.3 (1.0–1.7) | 1.2 (0.9–1.6) | 1.1 (0.8–1.5) |
| MN | 1.5 (1.0–2.3) | 1.6 (1.0–2.4) | 1.5 (1.0–2.4) | 1.4 (0.9–2.2) |
| IgAN/IgAV | Ref. | Ref. | Ref. | Ref. |
eGFR indicates estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; IgAN, IgA nephropathy; IgAV, IgA vasculitis; MCD, minimal change disease; MN, membranous nephropathy; ref, reference.
Self‐reported history or enrollment systolic and/or diastolic blood pressure >95th percentile.
Table 6.
Cardiovascular Risk Factors, by Steroids Exposure at Enrollment
| Steroid Exposure from Diagnosis to Enrollment | ||
|---|---|---|
| <6 Months (n=133) | 6+ Months (n=127) | |
| Hypertension or hypertensive‐level blood pressurea | 56 (42%) | 48 (38%) |
| Hypercholesterolemia | ||
| TC measured | 56 (42%) | 76 (60%) |
| TC >200 | 42/56 (75%) | 53/76 (70%) |
| Obesity | 47 (35%) | 44 (35%) |
TC indicates total cholesterol.
Self‐reported history or enrollment systolic and/or diastolic blood pressure >95th percentile.
Table 7.
Cardiovascular Risk Factors, by Remission Status at Enrollment
| In Remission at Enrollmenta | ||
|---|---|---|
| Yes (n=200) | No (n=321) | |
| Hypertension or hypertensive‐level blood pressureb | 58 (29%) | 141 (44%) |
| Hypercholesterolemia | ||
| TC measured | 110 (55%) | 160 (50%) |
| TC >200 | 47/110 (43%) | 115/160 (72%) |
| Obesity | 57 (29%) | 111 (35%) |
TC indicates total cholesterol.
Remission=serum albumin >3 g/dL and urine protein‐to‐creatinine ratio <0.3.
Self‐reported history or enrollment systolic and/or diastolic blood pressure >95th percentile.
Twenty‐six percent of participants with hypertension or hypertensive‐level blood pressure were not receiving any antihypertensive medication at enrollment, and only 68% were treated with RAAS‐blocking medications (Table 8). RAAS blockers were prescribed most frequently for hypertensive participants with FSGS at 84%. The proportion of hypertensive participants receiving treatment with any antihypertensive agent or with a RAAS blocker specifically was similar regardless of the presence or absence of nephrotic‐range proteinuria (89% and 79% in those with UPCR >2 versus 85% and 79% in those with UPCR≤2, P=0.5 and P=1.0, respectively).
Table 8.
Screening and Treatment Practice Patterns for CVD Disease Risk Factors in Pediatric CureGN Subjects
| n/N (%) | Overall (n=761) | MCD (n=283) | FSGS (n=177) | MN (n=36) | IgAN/IgAV (n=265) | P value |
|---|---|---|---|---|---|---|
| Patients with any hypertensiona, whole cohortb | 268/761 (35) | 108/283 (38) | 70/177 (40) | 19/36 (53) | 71/265 (27) | 0.001 |
| Number on any antihypertensive medication | 197/268 (74) | 74/108 (69) | 59/70 (84) | 14/19 (74) | 50/71 (70) | 0.12 |
| Number on RAAS blocker | 181/268 (68) | 68/108 (63) | 54/70 (77) | 14/19 (74) | 45/71 (63) | 0.18 |
| Patients on RAAS blocker, whole cohortb | 444/761 (58) | 120/283 (42) | 130/177 (73) | 24/36 (67) | 170/265 (64) | <0.001 |
| Among those with UPCR >2 | 98/168 (58) | 30/62 (48) | 35/51 (69) | 9/15 (60) | 24/40 (60) | 0.19 |
| Patients prescribed lipid‐ lowering medication, whole cohortb | 78/761 (10) | 9/283 (3) | 17/177 (10) | 6/36 (17) | 46/265 (17) | <0.001 |
| Among patients with lipid profile measured | 35/372 (9) | 5/172 (3) | 13/94 (14) | 6/22 (27) | 11/84 (13) | <0.001 |
| Among patients with TC >200 mg/dLc | 19/224 (9) | 4/120 (3) | 8/54 (15) | 6/18 (33) | 1/32 (3) | <0.001 |
| Among patients with dyslipidemia of any type | 25/265 (9) | 4/132 (3) | 9/67 (13) | 6/20 (30) | 6/46 (13) | <0.001 |
CureGN indicates Cure Glomerulonephropathy Network; CVD, cardiovascular disease; FSGS, focal segmental glomerulosclerosis; IgAN, IgA nephropathy; IgAV, IgA vasculitis; MCD, minimal change disease; MN, membranous nephropathy; RAAS blocker, renin‐angiotensin‐aldosterone system blocker; TC, total cholesterol; UPCR, urine protein‐to‐creatinine ratio.
Self‐reported history or enrollment systolic and/or diastolic blood pressure >95th percentile.
<1% missing.
Total cholesterol: MCD missing 41%, FSGS missing 48%, MN missing 39%, IgAN missing 70%.
Dyslipidemia
Only 49% of our study population had evidence of lipid screening. Among those with lipids measured, 265 (71%) had dyslipidemia of any type, and 224 (62%) had hypercholesterolemia. Hypercholesterolemia was most frequent in MN (82%) followed by MCD (71%), and least frequent in IgAN/IgAV (40%) (Table 4). Hypercholesterolemia was frequently observed regardless of steroid exposure duration (Table 6). Among screened participants in complete remission, 43% had hypercholesterolemia compared with 72% of those not in remission (P<0.001) (Table 7).
Only 25 children with dyslipidemia (9%) were prescribed a lipid‐lowering medication, which included statins, fish oil, or bile acid sequestrants. Forty‐three of the 78 children treated with lipid‐lowering agents lacked a lipid profile measurement, of whom 33 received fish oil. Normal lipid profiles were noted in 10 children treated for dyslipidemia. The frequency of lipid‐lowering medications differed across glomerular disease causes (Table 8). Overall, participants with MCD were least likely to receive lipid‐lowering treatment, whereas those with MN or IgAN/IgAV were more likely. The proportion of those treated with lipid‐lowering medications was also almost 2 times higher in participants with nephrotic‐range proteinuria (UPCR >2) compared with those without (16% versus 9%, P=0.02). Finally, lipid‐lowering medication use was almost twice as high in RAAS blocker users at 13% versus 6% in nonusers.
Obesity
Based on BMI percentile at study enrollment, 51% were either overweight (19%) or obese (32%); only 2% and 5% of overweight and obese participants, respectively, had moderate or severe edema noted on their enrollment visit physical examination with 78% and 79%, respectively, having no edema at all. Relative to participants with IgAN/IgAV, the prevalence ratio of obesity was slightly higher among patients with MN, although CIs were wide (Table 5). There was no difference in the proportion of obese participants in relation to steroid exposure duration (Table 6). The proportion of obese participants was lower in those in complete remission, though not significantly different from those who were not (Table 7).
Nontraditional risk factors
Proteinuria
Overall, the median UPCR was subnephrotic at enrollment (Table 1). The highest median UPCR was observed in the MN group at 2.0 (IQR 0.9–7.0). RAAS blockers were frequently prescribed (Table 8) with use in 58% of all children including 58% of those with nephrotic range proteinuria (UPCR >2), 71% of those with a UPCR of 0.3≤ and ≤2, and 52% in those with UPCR <0.3. The highest proportion of participants on RAAS blockers were those with FSGS (73%), and the lowest in participants with MCD (42%). RAAS blocker use was more frequent in children with steroid/multi‐drug‐resistant glomerular disease compared with steroid‐sensitive disease (67% versus 48%), P<0.001.
Other factors
Exposure to firsthand smoke was limited to 12 participants, while 24% reported exposure to secondhand smoke at home. Twelve percent were born prematurely.
Comparison to the general pediatric population
Except for prematurity, all risk factors were more frequent among children with glomerular disease (Figure).
Figure 1.
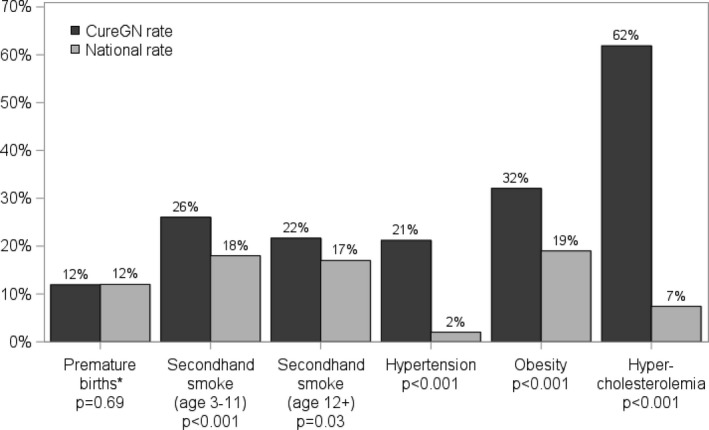
Cardiovascular risk disease burden in CureGN patients vs general pediatric population. *2004 National preterm birthrate, median year of birth for CureGN patients.22, 23, 24, 25, 26 CureGN indicates Cure Glomerulonephropathy Network.
Discussion
Our study examines the cardiovascular risk profile in the pediatric CureGN cohort, which represents the largest cohort of children with 4 common and biopsy‐confirmed glomerular diseases assembled to address these questions, and provides a contemporary description of practitioners’ practice patterns in screening and treatment of CVD risk factors in this high‐risk pediatric population in North America and Europe. As hypothesized, our findings confirm the high prevalence of CVD risk factors in children with glomerular disease and highlight potential areas for improvement in screening and treatment of these risk factors. Despite the relatively mild degree of CKD at enrollment (median eGFR exceeded 90 mL/min per 1.73 m2 in all subgroups), hypertension was reported by almost one fourth of patients, with a similar percentage exhibiting a hypertensive‐level blood pressure reading at their initial visit. Similarly, more than half were either overweight or obese, and in those who underwent lipid screening more than two thirds had dyslipidemia. Another concerning finding is the presence of these risk factors at a high prevalence regardless of cumulative steroid exposure duration, and even in the minority (38%) that were in complete remission at the time of enrollment.
In comparison, the prevalence of systolic hypertension, overweight/obesity, and dyslipidemia in the CKiD cohort was 14%, 24%, and 45%, respectively.27, 28 The Cardiovascular Comorbidity in Children with Chronic Kidney Disease (4C) study reported that 26% of patients had uncontrolled hypertension and 23% were overweight/obese.29 The NEPTUNE (Nephrotic Syndrome Study Network) study reported that 42% and 57% patients had uncontrolled hypertension and were overweight/obese, respectively.30 In CureGN, the prevalence of hypertensive‐level blood pressure at enrollment of 22% is lower than the similar NEPTUNE cohort, which may reflect a longer disease duration before the enrollment visit in CureGN, offering an opportunity to achieve better blood pressure control. We also report a higher prevalence of overweight/obesity (51%) compared with CKiD (24%) or 4C (23%), but comparable to the NEPTUNE cohort (57%). This may be secondary to side effects of steroids or other medications used to treat glomerular diseases, or elevated weights because of edema in participants with nephrotic syndrome, although we noted that moderate or severe edema was present in only 5% of obese participants. For children in the CureGN cohort who underwent lipid screening, the prevalence of dyslipidemia (71%) is higher than previously reported in the CKiD study (45%). This may be because of increased prevalence of dyslipidemia in children with proteinuria or nephrotic syndrome related to change in cholesterol metabolism, or because of a selected higher‐risk population receiving cholesterol screening in the CureGN cohort. This is suggested by the higher prevalence ratios for hypercholesterolemia and to a lesser extent hypertension in children with MCD and MN relative to their counterparts with IgAN/IgAV (Table 5). The former 2 conditions almost universally present with nephrotic syndrome and nephrotic‐range proteinuria, whereas the latter typically presents with subnephrotic range proteinuria, and hematuria.8 Most concerning, though, was the much higher prevalence of traditional CVD risk factors compared with the general pediatric population (Figure), as these potentially modifiable risk factors are present in children early in their glomerular disease course (median of 16 months since diagnosis), placing them at increased risk of developing overt cardiovascular disease as they age.
Our study highlights deficiencies and opportunities for improvement in screening and treatment patterns. It is apparent from our findings that screening for and treatment of CVD risk factors is not universal. For example, if patients were hypertensive, antihypertensives were prescribed in only 86% of patients. Similarly, RAAS blockers were prescribed in only 58% of those with nephrotic‐range proteinuria. Perhaps the most notable finding is the lack of universal screening for lipid abnormalities in children who would be considered at high risk for dyslipidemia because of their underlying disease. Our study also highlights a reluctance to prescribe lipid‐lowering medications, likely reflecting a lack of consensus regarding the relative risks and benefits of pharmacologic treatment of dyslipidemia in children in general.31, 32 We hypothesize that the remitting–relapsing nature of childhood nephrotic syndrome may dissuade providers from aggressively screening for and treating CVD risk factors because of the perceived “transient” risk; however, only 38% of patients were in complete remission at a median of 12 months following diagnosis, and the prevalence of hypertension, obesity, and hypercholesterolemia remained high even among children who were in remission. Therapeutic nihilism might therefore result in a missed opportunity to improve the long‐term cardiovascular risk profile.
This study has several limitations. We were limited to a cross‐sectional analysis of the enrollment visit dataset for the purposes of this report and did not have access to longitudinal follow‐up data, which are currently being accrued. The focus of future analyses will be on longitudinal data examining the relationship between baseline cardiovascular risk factors and future events including cardiovascular events, hospitalizations, and progression to end‐stage renal disease in both children and adults. Despite this limitation, the exclusive focus on children with 4 common biopsy‐confirmed glomerular diseases is a major strength of our report. Previous similar cohort studies (CKiD, 4C Study) had limited numbers of children with glomerular disease (22% and 9%, respectively) and the NEPTUNE cohort had only 147 children with nephrotic syndrome enrolled at the time of kidney biopsy.28, 29, 30 Despite the prospective design of CureGN, some clinical data for this cohort were collected retrospectively. Therefore, some data may be missing because of transition of care or other lapses, which might account for the low prevalence of lipid screening. However, the higher frequency of complete data available for other parameters such as eGFR, serum albumin, and UPCR suggests that the low prevalence of lipid screening is reflective of a clinical practice gap. For the available lipid measurements, we were not able to determine the fasting or postprandial nature of the collections. While this may interfere with the accuracy of triglyceride measurements, the high prevalence of hypercholesterolemia (62%) should not be significantly affected. Centralized laboratory measurements for serum creatinine and UPCR were completed for approximately one quarter of participants by the time of reporting; therefore, we relied on local laboratory data extracted from clinical records for most participants, which may introduce technique‐based variability. The lack of temporal relationship of certain factors, such as availability of UPCR at time of lipid measurements, limits the ability to accurately investigate risk factor interactions. Additionally, biomarkers related to glucose metabolism such as glycated hemoglobin, which is very relevant for this population given the high prevalence of overweight and obesity as well as the potential diabetogenic side effects of the medications commonly used to treat nephrotic syndrome, are not collected or centrally measured as part of the current study protocol. It is an important clinical question that could be the focus of an ancillary study proposal by our group or any interested member of the larger scientific community. Finally, the designation of hypertensive and prehypertensive‐level enrollment blood pressure readings followed the normative data introduced in the Fourth Report, as it was the reference blood pressure practice guideline at the time of the patients’ diagnosis and enrollment. The 2017 American Academy of Pediatrics Clinical Practice Guideline for screening and management of high blood pressure in children and adolescents has since replaced the Fourth Report.33 The exclusion of obese children from the reference group utilized to determine blood pressure percentile cutoffs in the 2017 guideline33 essentially leads to more strict cutoffs, which would further increase the prevalence of hypertensive‐level readings in our cohort had they been analyzed utilizing the new guideline cutoffs.
In conclusion, there is a high prevalence of potentially modifiable CVD risk factors in a large cohort of children with primary glomerular diseases. There appear to be ample opportunities for process improvement and targeted efforts to improve identification and treatment of those risk factors in high‐risk patient populations, particularly children with clinically complicated nephrotic syndrome course. Future efforts need to better align clinical practice with existing treatment guidelines, facilitate the conduct of interventional studies aiming to determine best practice, and ultimately the development of evidence‐based, disease‐specific clinical practice guidelines.
Appendix
The CureGN Consortium members (from within the 4 Participating Clinical Center networks and the Data Coordinating Center; authors above have been removed from this list). CureGN Principal Investigators are noted (*).
Columbia University: Wooin Ahn, Columbia; G.B. Appel, Columbia; R. Babayev, Columbia; I. Batal, Columbia; A.S. Bomback, Columbia; E. Brown, Columbia; E.S. Campenot, Columbia; P. Canetta, Columbia; B. Chan, Columbia; D. Chatterjee, Columbia; V.D. D'Agati, Columbia; E. Delbarba, Columbia; H. Fernandez, Columbia; B. Foroncewicz, University of Warsaw, Poland; A.G. Gharavi, Columbia*; G. M. Ghiggeri, Gaslini Children's Hospital, Italy; W.H. Hines, Columbia; N.G. Jain, Columbia; B.H. Kil, Columbia; K. Kiryluk, Columbia; W.L. Lau, Columbia; F. Lin, Columbia; F. Lugani, Gaslini Children's Hospital, Italy; M. Marasa, Columbia; G. Markowitz, Columbia; S. Mohan, Columbia; X. Mu, Columbia; K. Mucha, University of Warsaw, Poland; T.L. Nickolas, Columbia; S. Piva, Columbia; J. Radhakrishnan, Columbia; M.K. Rao, Columbia; S. Sanna‐Cherchi, Columbia; D. Santoriello, Columbia; M.B. Stokes, Columbia; N. Yu, Columbia; A.M. Valeri, Columbia; R. Zviti, Columbia.
Midwest Pediatric Nephrology Consortium (MWPNC): Larry A. Greenbaum*, Emory University; W.E. Smoyer*, Nationwide Children's; Amira Al‐Uzri Oregon Health & Science University; I. Ashoor, Louisiana State University Health Sciences Center; D. Aviles, Louisiana State University Health Sciences Center; R. Baracco, Children's Hospital of Michigan; J. Barcia, University of Virginia; S. Bartosh, University of Wisconsin; C. Belsha, Saint Louis University/Cardinal Glennon; C. Bowers, Nationwide Children's Hospital; M.C. Braun, Baylor College of Medicine/Texas Children's Hospital; A. Chishti, University of Kentucky; D. Claes, Cincinnati Children's Hospital; C. Cramer, Mayo Clinic; K. Davis, Washington University in St. Louis; E. Erkan, Cincinnati Children's Hospital Medical Center; D. Feig, University of Alabama, Birmingham; M. Freundlich, University of Miami/Holtz Children's Hospital; R. Gbadegesin, Duke University Medical Center; M. Hanna, Children's Colorado/University of Colorado; G. Hidalgo, East Carolina University; T.E. Hunley, Monroe Carell Jr Children's Hospital at Vanderbilt University Medical Center; A. Jain, Children's Hospital of Michigan; M. Khalid, JW Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, IN; M. Kallash, Nationwide Children's Hospital; J.C. Lane, Feinberg School of Medicine, Northwestern University; J. Mahan, Nationwide Children's; N. Mathews, University of Oklahoma Health Sciences Center; C. Nester, University of Iowa Stead Family Children's Hospital; C. Pan, Medical College of Wisconsin; L. Patterson, Children's National Health System; H. Patel, Nationwide Children's Hospital; A. Revell, Nationwide Children's Hospital; M.N. Rheault, University of Minnesota Masonic Children's Hospital; C. Silva, Connecticut Children's Medical Center; R. Sreedharan, Medical College of Wisconsin; T. Srivastava, Children's Mercy Hospital; J. Steinke, Helen DeVos Children's Hospital; K. Twombley, Medical University of South Carolina; S.E. Wenderfer, Baylor College of Medicine/Texas Children's Hospital; T.L. Vasylyeva, Texas Tech University Health Sciences Center; D.J. Weaver, Levine Children's Hospital at Carolinas Medical Center; C.S. Wong, University of New Mexico Health Sciences Center.
The University of North Carolina (UNC): Anand Achanti, Medical University of South Carolina (MUSC); S. Almaani, The Ohio State University (OSU); I. Ayoub, OSU; M. Budisavljevic, MUSC; V. Derebail, UNC; R. Falk, UNC*; H. Fatima, The University of Alabama at Birmingham (UAB); A. Fogo, Vanderbilt; T. Gehr, Virginia Commonwealth University (VCU); K. Gibson, UNC; D. Glenn, UNC; R. Harris, Vanderbilt; S. Hogan, UNC Koyal Jain, UNC; J. Charles Jennette, UNC; B. Julian, UAB; J. Kidd, VCU; L.‐P. Laurin, Hôpital Maisonneuve‐Rosemont (HMR) Montreal, H. Davis Massey, VCU; A. Mottl, UNC; P. Nachman, UNC; T. Nadasdy, MUSC; J. Novak, UAB; S. Parikh, OSU; V. Pichette, HMR Montreal; T.B. Powell, Columbia Nephrology Associates; M. Renfrow, UAB; D. Rizk, UNC; B. Rovin, OSU; V. Royal, HMR Montreal; M. Saha, UNC; N. Sanghani, Vanderbilt, S. Self, MUSC.
University of Pennsylvania (UPENN): Sharon Adler, Los Angeles Biomedical Research Institute at Harbor, University of California Los Angeles (UCLA); C. Alpers, University of Washington; R.B. Matar, Cleveland Clinic; E. Brown, University of Texas (UT) Southwestern Medical Center; D. Cattran, University of Toronto; M. Choi, Johns Hopkins; K.M. Dell, Case Western/Cleveland Clinic; R. Dukkipati, Los Angeles Biomedical Research Institute at Harbor UCLA; F.C. Fervenza, Mayo Clinic; A. Fornoni, University of Miami; C. Gadegbeku, Temple University; P. Gipson, University of Michigan; L. Hasely, University of Washington; S. Hingorani, Seattle Children's Hospital; M. Hladunewich, University of Toronto/Sunnybrook; J. Hogan, University of Pennsylvania; L.B. Holzman, University of Pennsylvania*; J. Ashley Jefferson, University of Washington; K. Jhaveri, North Shore University Hospital; D.B. Johnstone, Temple University; F. Kaskel, Montefiore Medical Center; A. Kogan, CHOP; J. Kopp, NIDDK Intramural Research Program; R. Lafayette, Stanford; K.V. Lemley, Children's Hospital of Los Angeles; L. Malaga‐Dieguez, NYU; Kevin Meyers, Children's Hospital of Pennsylvania; A. Neu, Johns Hopkins; M.M. O'Shaughnessy, Stanford; J.F. O'Toole, Case Western/Cleveland Clinic; R. Parekh, University Health; Network, Hospital for Sick Children; H. Reich, University Health Network, University of Toronto, Toronto, CAN; K. Reidy, Montefiore Medical Center; H. Rondon, UPMC; K.K. Sambandam, UT Southwestern; J.R. Sedor, Case Western/Cleveland Clinic; D.T. Selewski, University of Michigan; C.B. Sethna, Cohen Children's Medical Center‐North Shore Long Island Jewish (LIJ) Health System; J. Schelling, Case Western Reserve University; J.C. Sperati, Johns Hopkins; A. Swiatecka‐Urban, Children's Hospital of Pittsburgh; H. Trachtman, New York University; K.R. Tuttle, Spokane Providence Medical Center; J. Weisstuch, New York University; O. Zhdanova, New York University.
Data Coordinating Center (DCC): Laura Barisoni, University of Miami; B. Gillespie*, University of Michigan; D.S. Gipson*, University of Michigan; M. Helmuth, Arbor Research Collaborative for Health; M. Kretzler*, University of Michigan; S. Mansfield, Arbor Research Collaborative for Health; L. Mariani, University of Michigan; C.C. Nast, Cedars‐Sinai Medical Center; B.M. Robinson*, Arbor Research Collaborative for Health; M. Wladkowski, Arbor Research Collaborative for Health; J. Zee, Arbor Research Collaborative for Health.
Steering Committee Chair: Lisa M. Guay‐Woodford, Children's National Health System.
Sources of Funding
Funding for the CureGN consortium is provided by UM1DK100845, UM1DK100846, UM1DK100876, UM1DK100866, and UM1DK100867 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Patient recruitment is supported by NephCure Kidney International.
Disclosures
Parekh reports support from the NIH, NIDDK, CIHR/Canadian Kidney Foundation, and the University of Toronto. Sethna reports support from the American Heart Association and NIH. Fernandez reports support from TRANSFORM KL2, RO1 Research Supplement, and M. Irene Ferrer Scholar Award in Gender‐specific Medicine. Rheault reports support from Reata, Retrophin, Regulus, and Novartis. Vasylyeva reports speaker bureaus with Sanofi. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2019;8:e012143 DOI: 10.1161/JAHA.119.012143.)
Contributor Information
Isa F. Ashoor, Email: iashoo@lsuhsc.edu.
the CureGN Consortium:
M. Kallash, P. Gipson, L. Malaga‐Dieguez, B. Gillespie, D.S. Gipson, M. Kretzler, and B.M. Robinson
References
- 1. Chavers BM, Herzog CA. The spectrum of cardiovascular disease in children with predialysis chronic kidney disease. Adv Chronic Kidney Dis. 2004;11:319–327. [DOI] [PubMed] [Google Scholar]
- 2. Mitsnefes MM. Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol. 2012;23:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chavers BM, Molony JT, Solid CA, Rheault MN, Collins AJ. One‐year mortality rates in US children with end‐stage renal disease. Am J Nephrol. 2015;41:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mitsnefes MM, Laskin BL, Dahhou M, Zhang X, Foster BJ. Mortality risk among children initially treated with dialysis for end‐stage kidney disease, 1990–2010. JAMA. 2013;309:1921–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Groothoff JW, Gruppen MP, Offringa M, de Groot E, Stok W, Bos WJ, Davin JC, Lilien MR, Van de Kar NC, Wolff ED, Heymans HS. Increased arterial stiffness in young adults with end‐stage renal disease since childhood. J Am Soc Nephrol. 2002;13:2953–2961. [DOI] [PubMed] [Google Scholar]
- 6. Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F. Advanced coronary and carotid arteriopathy in young adults with childhood‐onset chronic renal failure. Circulation. 2002;106:100–105. [DOI] [PubMed] [Google Scholar]
- 7. Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J; American Heart Association Expert Panel on Population and Prevention Science, American Heart Association Council on Cardiovascular Disease in the Young, American Heart Association Council on Epidemiology and Prevention, American Heart Association Council on Nutrition, Physical Activity and Metabolism, American Heart Association Council on High Blood Pressure Research, American Heart Association Council on Cardiovascular Nursing, American Heart Association Council on the Kidney in Heart Disease, Interdisciplinary Working Group on Quality of Care and Outcomes Research . Cardiovascular risk reduction in high‐risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–2738. [DOI] [PubMed] [Google Scholar]
- 8. Wenderfer SE, Gaut JP. Glomerular diseases in children. Adv Chronic Kidney Dis. 2017;24:364–371. [DOI] [PubMed] [Google Scholar]
- 9. McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26:414–430. [DOI] [PubMed] [Google Scholar]
- 10. Ku E, Kopple JD, McCulloch CE, Warady BA, Furth SL, Mak RH, Grimes BA, Mitsnefes M. Associations between weight loss, kidney function decline, and risk of ESRD in the Chronic Kidney Disease in Children (CKiD) cohort study. Am J Kidney Dis. 2017;71:648–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) . 2014 Annual Transplant Report. 2014. Available at: https://web.emmes.com/study/ped/annlrept/annualrept2014.pdf. Accessed March 29, 2018.
- 12. Ray EC, Rondon‐Berrios H, Boyd CR, Kleyman TR. Sodium retention and volume expansion in nephrotic syndrome: implications for hypertension. Adv Chronic Kidney Dis. 2015;22:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Appel GB, Blum CB, Chien S, Kunis CL, Appel AS. The hyperlipidemia of the nephrotic syndrome. Relation to plasma albumin concentration, oncotic pressure, and viscosity. N Engl J Med 1985;312:1544–1548. [DOI] [PubMed] [Google Scholar]
- 14. Mariani LH, Bomback AS, Canetta PA, Flessner MF, Helmuth M, Hladunewich MA, Hogan JJ, Kiryluk K, Nachman PH, Nast CC, Rheault MN, Rizk DV, Trachtman H, Wenderfer SE, Bowers C, Hill‐Callahan P, Marasa M, Poulton CJ, Revell A, Vento S, Barisoni L, Cattran D, D'Agati V, Jennette JC, Klein JB, Laurin LP, Twombley K, Falk RJ, Gharavi AG, Gillespie BW, Gipson DS, Greenbaum LA, Holzman LB, Kretzler M, Robinson B, Smoyer WE, Guay‐Woodford LM, CureGN Consortium. CureGN study rationale, design, and methods: establishing a large prospective observational study of glomerular disease. Am J Kidney Dis. 2019;73:218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) . Central Repository. Available at: https://repository.niddk.nih.gov/home/. Accessed November 2, 2018.
- 16. Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention (CDC) . BMI Percentile Calculator for Child and Teen English Version. 2017. Available at: https://www.cdc.gov/healthyweight/bmi/calculator.html. Accessed on March 29, 2018.
- 19. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011;128(suppl 5):S213–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 21. Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22:661–670. [DOI] [PubMed] [Google Scholar]
- 22. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: final data for 2004. Natl Vital Stat Rep. 2006;55:1–101. [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention (CDC) . Vital signs: nonsmokers’ exposure to secondhand smoke — United States, 1999‐2008. MMWR Morb Mortal Wkly Rep 2010;59:1141–1146. [PubMed] [Google Scholar]
- 24. Xi B, Zhang T, Zhang M, Liu F, Zong X, Zhao M, Wang Y. Trends in elevated blood pressure among US children and adolescents: 1999–2012. Am J Hypertens. 2016;29:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief 2017;288:1–8. [PubMed] [Google Scholar]
- 26. Nguyen D, Kit B, Carroll M. Abnormal cholesterol among children and adolescents in the United States, 2011–2014. NCHS Data Brief. 2015;228:1–8. [PubMed] [Google Scholar]
- 27. Saland JM, Pierce CB, Mitsnefes MM, Flynn JT, Goebel J, Kupferman JC, Warady BA, Furth SL, CKiD Investigators . Dyslipidemia in children with chronic kidney disease. Kidney Int. 2010;78:1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Furth SL, Abraham AG, Jerry‐Fluker J, Schwartz GJ, Benfield M, Kaskel F, Wong C, Mak RH, Moxey‐Mims M, Warady BA. Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2132–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schaefer F, Doyon A, Azukaitis K, Bayazit A, Canpolat N, Duzova A, Niemirska A, Sozeri B, Thurn D, Anarat A, Ranchin B, Litwin M, Caliskan S, Candan C, Baskin E, Yilmaz E, Mir S, Kirchner M, Sander A, Haffner D, Melk A, Wuhl E, Shroff R, Querfeld U; 4C Study Consortium . Cardiovascular phenotypes in children with CKD: the 4C study. Clin J Am Soc Nephrol 2017;12:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sethna CB, Meyers KEC, Mariani LH, Psoter KJ, Gadegbeku CA, Gibson KL, Srivastava T, Kretzler M, Brady TM. Blood pressure and visit‐to‐visit blood pressure variability among individuals with primary proteinuric glomerulopathies. Hypertension. 2017;70:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members . KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int 2014;85:1303–1309. [DOI] [PubMed] [Google Scholar]
- 32. Lozano P, Henrikson NB, Morrison CC, Dunn J, Nguyen M, Blasi P, Whitlock EP. Lipid Screening in Childhood for Detection of Multifactorial Dyslipidemia: A Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality (US); 2016. Aug. Report No.: 14‐05204‐EF‐1. [PubMed] [Google Scholar]
- 33. Flynn JT, Kaelber DC, Baker‐Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM. Subcommittee on screening and management of high blood pressure in children. clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. [DOI] [PubMed] [Google Scholar]


