Abstract
Background
Excess transmission of pressure pulsatility caused by increased arterial stiffness may incur microcirculatory damage in end organs (target organ damage [TOD]) and, in turn, elevate risk for cardiovascular disease (CVD) events.
Methods and Results
We related arterial stiffness measures (carotid‐femoral pulse wave velocity, mean arterial pressure, central pulse pressure) to the prevalence and incidence of TOD (defined as albuminuria and/or echocardiographic left ventricular hypertrophy) in up to 6203 Framingham Study participants (mean age 50±15 years, 54% women). We then related presence of TOD to incident CVD in multivariable Cox regression models without and with adjustment for arterial stiffness measures. Cross‐sectionally, greater arterial stiffness was associated with a higher prevalence of TOD (adjusted odds ratios ranging from 1.23 to 1.54 per SD increment in arterial stiffness measure, P<0.01). Prospectively, increased carotid‐femoral pulse wave velocity was associated with incident albuminuria (odds ratio per SD 1.28, 95% CI, 1.02–1.61; P<0.05), whereas higher mean arterial pressure and central pulse pressure were associated with incident left ventricular hypertrophy (odds ratio per SD 1.37 and 1.45, respectively; P<0.01). On follow‐up, 297 of 5803 participants experienced a first CVD event. Presence of TOD was associated with a 33% greater hazard of incident CVD (95% CI, 0–77%; P<0.05), which was attenuated upon adjustment for baseline arterial stiffness measures by 5–21%.
Conclusions
Elevated arterial stiffness is associated with presence of TOD and may partially mediate the relations of TOD with incident CVD. Our observations in a large community‐based sample suggest that mitigating arterial stiffness may lower the burden of TOD and, in turn, clinical CVD.
Keywords: arterial stiffness, cardiovascular disease, epidemiology, pulse wave velocity, target organ damage
Subject Categories: Epidemiology, Cardiovascular Disease, Risk Factors
Clinical Perspective
What Is New?
We related arterial stiffness measures to the prevalence and incidence of target organ damage (defined as albuminuria and/or echocardiographic left ventricular hypertrophy), and then related the presence of target organ damage to incident cardiovascular disease in analyses without and with adjustment for arterial stiffness measures.
Greater arterial stiffness was associated with a greater prevalence and incidence of target organ damage, and the latter, in turn, was associated with a greater hazard of incident cardiovascular disease events.
What Are the Clinical Implications?
Elevated arterial stiffness promotes target organ damage and partially mediates the relations of the latter to incident cardiovascular disease, suggesting that mitigating arterial stiffness may lower the burden of both target organ damage and clinical cardiovascular disease.
Introduction
Numerous studies have demonstrated a strong association between high blood pressure (BP) and structural and functional changes in end organs (heart, brain, eyes, and kidneys), which are referred to as target organ damage (TOD).1 Evidence of TOD is frequently seen in individuals with more severe or long‐standing elevations in BP. With wider use of imaging and screening tests (which are also increasing in sensitivity), TOD is becoming more apparent among asymptomatic individuals with milder forms of BP elevation.1 Importantly, any presence of TOD confers significant risk for developing overt cardiovascular disease (CVD)1 and there is evidence to suggest that regression of TOD with BP lowering may mitigate this risk.2, 3, 4, 5 Thus, recent BP guidelines1 recommend screening for TOD in individuals with high BP. Such screening is accomplished typically by evaluating the retinal microcirculation, a spot urine specimen for presence of albuminuria, and assessment for ECG or echocardiographic evidence of left ventricular (LV) hypertrophy (LVH).1
In conjunction with the clinical guidelines’ emphasis on TOD screening, an emerging body of literature suggests that TOD may be caused by end‐organ microcirculatory injury caused by excess transmission of pressure pulsatility from arterial stiffening, above and beyond the effects of peripheral BP elevation.6, 7, 8, 9, 10, 11, 12 To date, however, a comprehensive understanding of the interrelations between central vascular hemodynamics, hypertensive TOD, and CVD outcomes has been lacking. Most prior studies have assessed the relations between peripheral (rather than central) BP and TOD in a single end organ, often using a cross‐sectional study design.13, 14, 15, 16, 17, 18, 19 Few studies have evaluated the relative contributions of steady state and pulsatile pressure (ie, mean arterial pressure [MAP] and pulse pressure [PP], respectively) and aortic stiffness (as reflected by carotid‐femoral pulse wave velocity [CFPWV]) to the occurrence of TOD assessed across multiple end organs and, thereafter, progression to CVD incidence.
We hypothesized that elevated arterial stiffness would be associated with TOD cross‐sectionally and with the incidence of TOD prospectively. We postulated that the association of arterial stiffness measures with CVD incidence would be mediated partly via the impact of steady state and pulsatile pressure hemodynamic components on TOD. We also posited that the association of TOD with CVD incidence would be partially attenuated when accounting for concomitant arterial stiffness (Figure 1). We tested these hypotheses in a community‐based sample with contemporaneous assessments of central hemodynamic and arterial stiffness measures and TOD.
Figure 1.
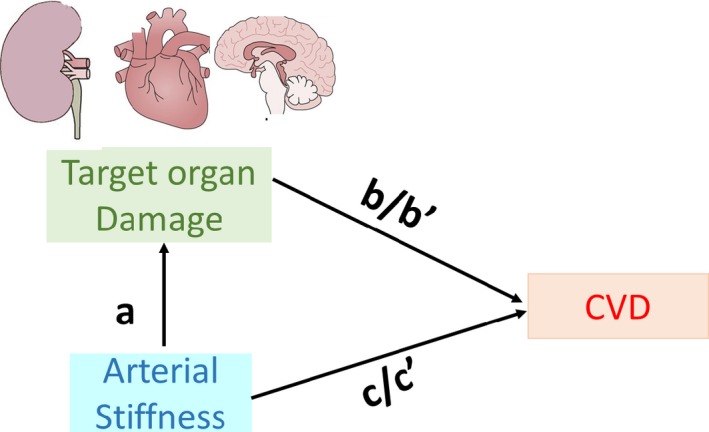
Conceptual framework for analyses. “a” refers to multivariable‐adjusted regression coefficient relating arterial stiffness measure to presence vs absence of target organ damage (TOD); it is the direct effect of arterial stiffness on TOD. “b” refers to multivariable‐adjusted regression coefficient relating TOD to the incidence of cardiovascular disease (CVD) (without arterial stiffness measures in the model); it is the overall effect of TOD on CVD corrected for confounders. “b’” refers to multivariable‐adjusted regression coefficient relating TOD to the incidence of CVD with additional adjustment for arterial stiffness measures; it is the direct effect of TOD on CVD incidence. “c” refers to multivariable‐adjusted regression coefficient relating arterial stiffness measures to the incidence of CVD; it is the overall effect of arterial stiffness on CVD incidence. “c’” refers to multivariable‐adjusted regression coefficient relating arterial stiffness measures to the incidence of CVD with additional adjustment for presence of TOD at baseline; it is the direct effect of arterial stiffness on CVD incidence.
Methods
Study Sample
The design and selection criteria of FHS (Framingham Heart Study) cohorts have been previously described. Beginning in 1948, approximately two thirds of the households in Framingham, MA, were enrolled in the Original Cohort (n=5209).20 Their offspring (and their spouses) and the children of the offspring were enrolled in the Offspring Cohort and the Generation 3 Cohort in 1971 and 2002, respectively, as detailed elsewhere.21, 22 In addition, 2 Omni cohorts (combined n=916) were recruited in 1994 and 2003 to reflect the increasingly diverse population of Framingham. These cohorts specifically targeted residents of Hispanic, Asian, Indian, African American, Pacific Islander, and Native American descent.23 The study protocol was approved by the institutional review board at the Boston Medical Center and all participants provided written informed consent. All data and materials have been made publicly available at the National Heart, Lung, and Blood Institute's data repository BioLINCC.24,25.
For the current investigation (Figure 2), we considered 7927 FHS participants who took part in Offspring Cohort examination 8 (2005–2008), Third Generation Cohort examination 1 (2002–2005), First Omni Cohort examination 3 (2007–2008), Second Omni Cohort examination 1 (2003–2005), or New Offspring Spouse examination 1 (2003–2005). These examinations are referred to as the baseline for the present analyses. Overall, 6203 individuals had data available for covariates, central hemodynamics, LVH, and albuminuria, and were included in analyses relating central hemodynamics with albuminuria and LVH (Figure 2). Of these participants, 3144 underwent brain magnetic resonance imaging (MRI) and were included in analyses relating hemodynamic measures with all types of organ damage (Figure 2). In total, 5803 individuals without history of CVD had no missing data for covariates, albuminuria, LVH, or central hemodynamics and were included in analyses on the association of albuminuria and LVH with incident cardiovascular outcomes (Figure 2). A subsample of 4215 individuals free of albuminuria (Offspring and Third Generation Cohort participants) and 1111 free of echocardiographic LVH (Offspring Cohort) attended the subsequent examination at which incidence of albuminuria and echocardiographic LVH was reassessed.
Figure 2.

Derivation of study samples. CVD indicates cardiovascular disease; LVH, left ventricular hypertrophy; MAP, mean arterial pressure; MRI, magnetic resonance imaging; PP, pulse pressure; PWV, pulse wave velocity; WMH, white matter hyperintensity.
Hemodynamic Assessment With Arterial Tonometry
Supine brachial systolic and diastolic BPs were obtained using an auscultatory device.26 Vascular stiffness was assessed using arterial applanation tonometry as previously described.26, 27, 28 Briefly, arterial tonometry with simultaneous ECG recordings was performed on the brachial, femoral, and carotid arteries on the right side of the body of participants. Transit distances for arterial pulse waves were assessed by body surface measurements from the suprasternal notch to the pulse‐recording sites. MAP was derived from integration of the brachial waveform, which was calibrated by using systolic and diastolic auscultatory BP at the time of tonometry. Diastolic BP and integrated MAP were used to calibrate carotid pressure tracings from which central aortic pressure was calculated. Details of signal analyses and data processing have been published.26, 27, 28 In our experience at FHS, MAP derived from applanation tonometry of the brachial artery is very highly correlated with that derived from a similarly calibrated and integrated signal‐averaged oscillometric brachial pressure waveform (r exceeds 0.96).
For the present investigation, we assessed 3 primary measures of arterial stiffness and central hemodynamics: (1) CFPWV, the current reference standard for aortic stiffness; (2) central PP (CPP), ie, the BP amplitude in the proximal aorta; and (3) central MAP, reflecting steady state pressure in the large arteries.
Target Organ Damage
Transthoracic echocardiography with Doppler color flow imaging was performed at the index FHS examinations using a standardized protocol. All echocardiograms were evaluated by an experienced sonographer or cardiologist and measured using a standardized reading protocol. Cardiac dimensions were quantified using digital images and the leading‐edge technique as recommended by the American Society of Echocardiography. LV mass was calculated according to American Society of Echocardiography guidelines.29 We defined LVH as LV mass index >95 g/m2 and >115 g/m2 for women and men, respectively.29 A subsequent measure of LV mass (obtained using the same imaging and measurement protocol) was available in the Offspring Cohort at their ninth examination cycle (2011–2014).
Urinary albumin‐creatinine ratio (UACR) was measured from spot morning urine samples obtained from participants during their FHS examinations. UACR is a reliable measure of urinary albumin excretion, and is highly correlated with albumin excretion rates obtained from 24‐hour collection.30 Urinary albumin concentration was measured using an immunoturbidimetry assay. Urinary creatinine was assessed using a modified Jaffé method. Albuminuria was defined using the sex‐specific cut points of UACR ≥17 mg/g (men) or ≥25 mg/g (women).31 Follow‐up measurements of UACR were available at the subsequent examination in a majority of the participants (see Figure 2).
Brain MRI was performed on a subset of FHS participants using methods that have been previously described.32, 33 The MRI markers of TOD were covert brain infarcts (CBIs) (ie, in the absence of a clinical stroke event or transient ischemic attack), and large white matter hyperintensities (WMHs). MRI acquisition, measurement techniques, and interrater reliability have been previously described.32, 33 Operators blinded to participants’ demographic, clinical, and biomarker data rated the images of interest. We determined the volume of WMH according to previously published methods,33 and defined extensive WMH where the natural log of the ratio of WMH volume to total cranial volume was >1 SD above the age‐adjusted mean value for this cohort.34 We manually characterized CBIs based on their size, location, and imaging characteristics, as previously described.34
Clinical Cardiovascular Outcomes
All FHS participants are under continuous surveillance for the incidence of CVD events and death. We obtained medical records for all hospitalizations and physician visits related to new‐onset CVD during follow‐up, which were reviewed by an adjudication panel consisting of 3 physician investigators using standardized criteria.35 For the present investigation, CVD was comprised of a composite of cardiovascular death, fatal or nonfatal myocardial infarction, stroke, angina pectoris, unstable angina (prolonged ischemic episode with documented reversible ST‐segment changes), transient ischemic attack, heart failure, and intermittent claudication. Criteria for these CVD events have been previously described.35
Statistical Analysis
The 5 different study samples (Figure 2) contributed to different sets of analyses. We used the largest sample (sample 1, N=6203) for cross‐sectional analyses relating each of the 3 arterial stiffness/hemodynamic measures (separate analyses for each measure) to the presence of TOD (echocardiographic LVH and albuminuria, modeled individually and conjointly) (Figure 1; a). A smaller sample (sample 2, N=3144) was used for relating arterial stiffness measures to prevalence of TOD as evidenced by brain MRI (CBI and WMH, modeled individually and conjointly with echocardiographic LVH and albuminuria). We used multivariable logistic regression to evaluate the cross‐sectional associations, with CFPWV, CPP, and MAP as the independent variables and TOD (albuminuria, LVH, CBI, and WMH) as the dependent variables, and fitting separate models for each combination of arterial stiffness measure and TOD. We also fit a model with presence versus absence of any TOD as the binary dependent variable. Regression models adjusted for age, sex, body mass index, diabetes mellitus, antihypertensive treatment, smoking, prevalent CVD, total cholesterol/high‐density lipoprotein cholesterol ratio, triglycerides, lipid‐lowering medications, heart rate, and estimated glomerular filtration rate. Given the correlation among the 3 arterial stiffness variables (see Results section below), they were not mutually adjusted for one another in the regression models in primary analyses.
In secondary analyses, we modeled LV mass and UACR as continuous variables using linear regression models adjusting for the covariates noted above. For all analyses, CFPWV was transformed by taking the inverse to reduce skewness of the distribution of the data, and multiplied by −1000 to retain the directionality of the variable, in which larger values are associated with worse outcomes.
In prospective analyses, we related each arterial stiffness measure individually to the incidence of TOD on follow‐up among individuals free of TOD at baseline using multivariable logistic regression models. Separate analyses were performed for incident albuminuria (sample 3, N=4215 participants free of baseline albuminuria who attended the next follow‐up examination) and incident echocardiographic LVH (sample 4, N=1111 individuals free of LVH at baseline who had repeated echocardiographic examination at the next follow‐up examination). Models were adjusted for the covariates listed above.
In additional prospective analyses, we related the presence of TOD at the baseline examination to the incidence of CVD in 5803 individuals without prevalent CVD (sample 5, N=5803) using Cox regression models after confirming that the assumption of proportionality of hazards was met. The models were adjusted for the aforementioned covariates besides prevalent CVD. In addition, to describe how associations between TOD and incident CVD may be mediated by arterial stiffness measures, we evaluated associations of TOD with CVD after adjusting for CFPWV, CPP, and MAP (in separate models) to assess the extent of attenuation of associations (Figure 1; b/b’).
Since the relations of TOD and arterial stiffness may be bidirectional, we performed additional analyses in which we related the 3 arterial stiffness measures individually to CVD incidence (sample 5, N=5803) using multivariable‐adjusted Cox regression models. We evaluated these relations with and without adjusting for presence versus absence of TOD (Figure 1; c/c’).
We considered a 2‐sided P<0.05 statistically significant across all analyses. The variance in all models was adjusted for familial structure, using generalized estimating equations for linear and logistic models, and the robust sandwich estimator for Cox proportional hazards models. We performed statistical analyses using SAS software version 9.4 (SAS Institute, Inc). Dr Vasan had access to all of the study data and takes responsibility for its integrity and that of the data analyses.
Results
The baseline characteristics of all participants included in any analyses and in subgroups by analysis type are presented in Table 1 (samples are as shown in Figure 2). Arterial stiffness measures were correlated with each other: age‐ and sex‐adjusted Pearson correlation coefficients of MAP were 0.50 and 0.47 in relation to CPP and PWV, respectively, and 0.28 between CPP and PWV (all P<0.0001).
Table 1.
Characteristics of Study Participants (According to Different Samplesa)
| Characteristic | Sample for Cross‐Sectional Analyses | Sample for Outcome Analyses | |||
|---|---|---|---|---|---|
| Larger Sample (Sample 1) | Sample With Brain MRI Measures (Sample 2) | Incident TOD | Incident CVD (Sample 5) | ||
| Incident Microalbuminuria (Sample 3) | Incident LVH (Sample 4) | ||||
| No. | 6203 | 3144 | 4215 | 1111 | 5803 |
| Age, y | 50±15 | 50±15 | 48±14 | 63±8 | 49±15 |
| Women, No. (%) | 3340 (53.8) | 1683 (53.5) | 2271 (53.9) | 633 (57.0) | 3176 (54.7) |
| Systolic BP, mm Hg | 121±16 | 120±16 | 119±15 | 125±16 | 120±16 |
| Diastolic BP, mm Hg | 74±10 | 74±10 | 75±9 | 74±9 | 75±10 |
| Hypertension, No. (%) | 1966 (31.7) | 915 (29.1) | 1058 (25.1) | 525 (47.3) | 1662 (28.6) |
| Duration, median (Q1–Q3)b | 10 (6–18) | 10 (6–18) | 10 (2–18) | 10 (2–18) | 10 (2–18) |
| BP‐lowering medication, No. (%) | 1452 (23.4) | 667 (21.2) | 731 (17.3) | 421 (37.9) | 1180 (20.3) |
| Duration, median (Q1–Q3)b | 6 (2–14) | 6 (2–14) | 6 (2–10) | 6 (2–10) | 6 (2–10) |
| Central PP, mm Hg | 58±19 | 57±19 | 55±16 | 65±19 | 57±18 |
| MAP, mm Hg | 93±12 | 92±12 | 91±11 | 96±11 | 92±12 |
| CFPWV, m/s | 8.3±3.0 | 8.2±2.8 | 7.8±2.3 | 9.5±2.8 | 8.1±2.7 |
| Heart rate, beats per min | 60±9 | 59±9 | 60±9 | 59±9 | 60±9 |
| BMI, kg/m2 | 26.9±5.0 | 26.8±4.8 | 26.7±4.9 | 27.5±4.7 | 26.8±4.9 |
| Prevalent CVD, No. (%) | 400 (6.4) | 142 (4.5) | 141 (3.3) | 95 (8.6) | … |
| Smoking, No. (%) | 779 (12.6) | 331 (10.5) | 513 (12.2) | 85 (7.7) | 735 (12.7) |
| Diabetes mellitus, No. (%) | 382 (6.2) | 168 (5.3) | 162 (3.8) | 85 (7.7) | 283 (4.9) |
| Duration, median (Q1–Q3)b | 6 (2–14) | 6 (2–14) | 6 (2–10) | 6 (2–10) | 6 (2–10) |
| Treatment for diabetes mellitus, No. (%) | 255 (4.1) | 108 (3.4) | 95 (2.3) | 52 (4.7) | 180 (3.1) |
| Duration, median (Q1–Q3)b | 6 (2–10) | 2 (2–10) | 2 (2–6) | 2 (2–8) | 2 (2–10) |
| Glycated hemoglobin,c % | 5.7±0.6 | 5.7±0.5 | 5.6±0.5 | 5.6±0.5 | 5.7±0.6 |
| TC/HDL cholesterol ratio | 3.6±1.3 | 3.6±1.3 | 3.6 ±1.3 | 3.5±1.0 | 3.6±1.3 |
| Triglycerides, mg/dL | 114±80 | 111±77 | 111±78 | 111±61 | 113±80 |
| Lipid medication, No. (%) | 1264 (20.4) | 603 (19.2) | 675 (16.0) | 392 (35.3) | 979 (16.9) |
| eGFR, mL/min per 1.73 m² | 94±19 | 94±18 | 96±17 | 81±14 | 95±18 |
| Cohort, No. (%) | |||||
| Offspring | 2308 (37.2) | 1269 (40.4) | 1414 (33.5) | 1111 (100.0) | 1983 (34.2) |
| Third Generation | 3657 (59.0) | 1875 (59.6) | 2801 (66.5) | 0 (0.0) | 3614 (62.3) |
| Omni 1 | 238 (3.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 206 (3.5) |
Summary statistics are mean±SD unless otherwise specified. BMI indicates body mass index; BP, blood pressure; CFPWV, carotid‐femoral pulse wave velocity; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; LVH, left ventricular hypertrophy; MAP, mean arterial pressure; MRI, magnetic resonance imaging; PP, pulse pressure; Q, quartile; TC/HDL, total cholesterol/high‐density lipoprotein cholesterol; TOD, target organ damage.
See Figure 2 for derivation of samples.
Among those with the relevant condition. Duration was measured in the Offspring Cohort only and was estimated based on number of examinations attended with the condition present.
Not measured in Generation 3.
In a sample of 6203 individuals, 476 (7.7%) and 602 (9.7%) had albuminuria and echocardiographic LVH, respectively, whereas 960 participants (15.5%) had ≥1 form of TOD (Table 2). The prevalence of TOD rose across tertiles of all 3 arterial stiffness measures (Figure 3). In cross‐sectional analyses, CPP, MAP, and PWV were all positively related to prevalent albuminuria and LVH with no marked differences in multivariable‐adjusted odds ratios for different arterial stiffness measures or across type of TOD (P<0.01 for all, Table 2). In additional analyses limited to the Offspring Cohort (in which data on the durations of hypertension and diabetes mellitus were available; see Table 1), we adjusted for the duration of both hypertension and diabetes mellitus in multivariable models. In these additional analyses, the association of arterial stiffness measures with the prevalence of TOD was maintained (Table 2, lower half). The associations of PWV with prevalent TOD were maintained even after additional adjustment for CPP or brachial PP (Table 3). Associations of MAP and PWV with TOD were also maintained when the corresponding regression models were adjusted for brachial systolic or diastolic BP (Table 3, lower half). In additional analyses modeling albuminuria and LV mass as continuous variables (natural logarithmically transformed to normalize their skewed distributions), these associations with arterial stiffness measures remained robust (Table 4).
Table 2.
Cross‐Sectional Relations of Pulsatile and Steady State Hemodynamics (Arterial Stiffness Measures) to TOD
| Characteristic | Sample 1 | Sample 2 (With Brain MRI Measures) | ||||
|---|---|---|---|---|---|---|
| Albuminuria | LVH | ≥1 Form of TOD (Albuminuria, LVH) | CBIs | WMH | ≥1 Form of TOD (Albuminuria, LVH, CBIs, or WMH) | |
| No. of participants | 6203 | 6203 | 6203 | 3144 | 3144 | 3144 |
| No. with TOD | 476 | 602 | 960 | 231 | 399 | 907 |
| Arterial stiffness measure | OR (95% CI) for TOD | |||||
| Central PP | 1.27 (1.16–1.39)* | 1.51 (1.38–1.66)* | 1.44 (1.33–1.56)* | 1.04 (0.90–1.20) | 1.15 (1.01–1.30)† | 1.32 (1.20–1.46)* |
| Central MAP | 1.23 (1.11–1.36)* | 1.38 (1.25–1.53)* | 1.36 (1.25–1.48)* | 1.17 (0.997–1.38) | 1.29 (1.15–1.45)* | 1.38 (1.26–1.52)* |
| CFPWV | 1.54 (1.31–1.80)* | 1.27 (1.09–1.47)‡ | 1.39 (1.22–1.57)* | 1.28 (1.02–1.61)† | 1.26 (1.03–1.54)† | 1.31 (1.14–1.51)* |
| Analyses with additional adjustment for duration of hypertension and diabetes mellitus (data for Offspring Cohort only) | ||||||
| No. of participants | 2307 | 2307 | 2307 | 1269 | 1269 | 1269 |
| No. with TOD | 295 | 380 | 580 | 161 | 194 | 517 |
| Arterial stiffness measure | OR (95% CI) for TOD | |||||
| Central PP | 1.17 (1.04–1.30)‡ | 1.38 (1.24–1.53)* | 1.30 (1.18–1.43)* | 1.00 (0.84–1.20) | 1.17 (1.01–1.35)† | 1.21 (1.08–1.36)‡ |
| Central MAP | 1.25 (1.09–1.44)‡ | 1.24 (1.11–1.40)* | 1.30 (1.16–1.45)* | 1.08 (0.88–1.31) | 1.28 (1.09–1.49)‡ | 1.29 (1.13–1.46)* |
| CFPWV | 1.38 (1.12–1.70)‡ | 1.08 (0.90–1.29) | 1.19 (1.01–1.40)† | 1.24 (0.95–1.62) | 1.35 (1.01–1.80)† | 1.21 (1.003–1.47)† |
Odds ratios (ORs) are reported for 1‐SD increase in arterial stiffness measure and are adjusted for age, sex, body mass index, diabetes mellitus, antihypertensive treatment, smoking, prevalent cardiovascular disease, total cholesterol/high‐density lipoprotein cholesterol ratio, triglycerides, lipid‐lowering medications, heart rate, estimated glomerular filtration rate. Duration of hypertension and diabetes mellitus was measured in the Offspring Cohort only and was estimated based on number of examintions attended with the condition present. CBIs indicates covert brain infarcts; CFPWV, carotid‐femoral pulse wave velocity; LVH, left ventricular hypertrophy; MAP, mean arterial pressure; PP, pulse pressure; TOD, target organ damage; WMH, large white matter hyperintensity on brain magnetic resonance imaging.
*P<0.001; † P<0.05; ‡ P<0.01.
Figure 3.
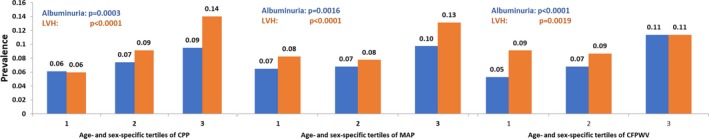
Prevalence of target organ damage (albuminuria and left ventricular hypertrophy [LVH]) according to tertile of arterial stiffness measures. P values indicate tests of trend across tertiles of arterial stiffness measure (A, central pulse pressure [CPP]; B, mean arterial pressure [MAP]; C, carotid‐femoral pulse wave velocity [CFPWV]).
Table 3.
Cross‐Sectional Relations of Pulsatile and Steady State Hemodynamics (Arterial Stiffness Measures) to TOD: Results Adjusting for Brachial BP Variables
| Characteristic | Sample 1 | Sample 2 |
|---|---|---|
| ≥1 Form of TOD (Albuminuria, LVH) | ≥1 Form of TOD (Albuminuria, LVH, CBIs, or WMH) | |
| No. of participants | 6203 | 3144 |
| No. with TOD | 960 | 907 |
| Arterial stiffness measure | OR (95% CI) for TOD | |
| Central PP | 1.44 (1.33–1.56)* | 1.32 (1.20–1.46)* |
| Central MAP | 1.36 (1.25–1.48)* | 1.38 (1.26–1.52)* |
| Adjusted for brachial systolic BP | 1.17 (1.05–1.30)† | 1.23 (1.08–1.39)† |
| Adjusted for brachial diastolic BP | 1.53 (1.38–1.68)* | 1.46 (1.30–1.64)* |
| CFPWV | 1.39 (1.22–1.57)* | 1.31 (1.14–1.51)* |
| Adjusted for central PP | 1.17 (1.02–1.33)‡ | 1.18 (1.02–1.37)‡ |
| Adjusted for brachial PP | 1.19 (1.05–1.35)† | 1.19 (1.03–1.38)‡ |
| Adjusted for brachial systolic BP | 1.16 (1.01–1.33)‡ | 1.12 (0.96–1.30) |
| Adjusted for brachial diastolic BP | 1.38 (1.21–1.57)* | 1.27 (1.10–1.46)† |
Odds ratios (ORs) are reported for 1‐SD increase in arterial stiffness measure and are adjusted for age, sex, body mass index, diabetes mellitus, antihypertensive treatment, smoking, prevalent cardiovascular disease, total cholesterol/high‐density lipoprotein cholesterol ratio, triglycerides, lipid‐lowering medications, heart rate, estimated glomerular filtration rate. Arterial stiffness measures are not adjusted for one another unless indicated otherwise. See Figure 2 for derivation of samples. BP indicates blood pressure; CBIs, covert brain infarcts; CFPWV, carotid‐femoral pulse wave velocity; LVH, left ventricular hypertrophy; MAP, mean arterial pressure; PP, pulse pressure; TOD, target organ damage; WMH, large white matter hyperintensity on brain magnetic resonance imaging.
*P<0.001; † P<0.01; ‡ P<0.05.
Table 4.
Relations of Pulsatile and Steady State Hemodynamics (Arterial Stiffness Measures) and UACR and LV Mass Modeled as Continuous Variables in Sample 1
| Arterial Stiffness Measure | Log UACR | Log LVMI | ||
|---|---|---|---|---|
| Beta (SE) | P Value | Beta (SE) | P Value | |
| Central PP | 0.134 (0.015) | <0.0001 | 0.037 (0.003) | <0.0001 |
| Central MAP | 0.071 (0.013) | <0.0001 | 0.026 (0.003) | <0.0001 |
| CFPWV | 0.144 (0.019) | <0.0001 | 0.018 (0.004) | <0.0001 |
Beta coefficients are per 1‐SD increase in arterial stiffness measure. Log urine albumin/creatinine ratio (UACR) and log left ventricular (LV) mass index (LVMI) are the dependent variables modeled as continuous variables. Models adjusted for the following covariates: age, sex, body mass index, diabetes mellitus, antihypertensive treatment, smoking, prevalent cardiovascular disease, total cholesterol/high‐density lipoprotein cholesterol ratio, triglycerides, lipid‐lowering medications, heart rate, estimate glomerular filtration rate. CFPWV indicates carotid‐femoral pulse wave velocity; MAP, mean arterial pressure; PP, pulse pressure; SE, standard error.
In a subsample of 3144 individuals with available brain MRI data at the baseline examinations, 231 (7.3%) and 399 (12.7%) had CBI and WMH, respectively, whereas 907 participants (28.8%) had ≥1 form of TOD (Table 2). The prevalence of WMH rose across tertiles of all 3 vascular stiffness measures (Figure 4). Higher PWV was related to greater odds of prevalent CBI (P<0.05), whereas this association was borderline significant for MAP and statistically nonsignificant for CPP (Table 2). All 3 arterial stiffness measures were associated positively with prevalent WMH in multivariable‐adjusted analyses (P<0.05; Table 2).
Figure 4.
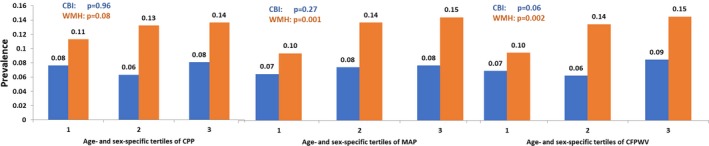
Prevalence of target organ damage (covert brain infarcts [CBIs] and large white matter intensities [WMHs]) according to tertile of arterial stiffness measures. P values indicate tests of trend across tertiles of arterial stiffness measure. CFPWV indicates carotid‐femoral pulse wave velocity; CPP, central pulse pressure; MAP, mean arterial pressure.
In prospective analyses, relating arterial stiffness measures to incident TOD (separate analyses for albuminuria and LVH), 224 of 4215 individuals (5.3%) developed new‐onset albuminuria and 118 of 1111 participants (10.6%) developed echocardiographic LVH (Table 5). In multivariable‐adjusted analyses, a 1‐SD increase in transformed PWV was associated with a 28% greater odds of incident albuminuria (P<0.05), whereas 1‐SD increases in MAP and CPP were associated with a 37% to 45% higher odds of incident LVH (P<0.01 for both, Table 5). The associations of PWV with incident albuminuria were maintained even after additional adjustment for CPP, brachial PP, or brachial systolic or diastolic BP (Table 5). The associations of MAP with incident LVH were also maintained when the corresponding regression models were adjusted for brachial diastolic BP but slightly attenuated upon adjustment for brachial systolic BP (Table 5). In analyses additionally adjusting for the durations of both hypertension and diabetes mellitus in the Offspring Cohort, the association of arterial stiffness measures with the incidence of TOD was maintained.
Table 5.
Relations of Pulsatile and Steady State Hemodynamics (Arterial Stiffness Measures) to Incident TOD
| Characteristic | Albuminuria | Echocardiographic LVHa |
|---|---|---|
| Analyses of Sample 3 and Sample 4 | ||
| No. of participants free of TOD at baseline with data at follow‐up | 4215 | 1111 |
| No. with TOD at follow‐up | 224 | 118 |
| Arterial stiffness measure | OR (95% CI) for incident TOD | |
| Central PP | 1.08 (0.91–1.27) | 1.45 (1.17–1.79)† |
| Central MAP | 1.07 (0.90–1.27) | 1.37 (1.10–1.69)‡ |
| Adjusted for brachial systolic BP | 1.07 (0.85–1.36) | 1.29 (0.97–1.71) |
| Adjusted for brachial diastolic BP | 1.08 (0.88–1.34) | 1.59 (1.19–2.12)‡ |
| CFPWV | 1.28 (1.02–1.61)§ | 1.26 (0.93–1.71) |
| Adjusted for central PP | 1.27 (1.003–1.61)§ | 1.04 (0.76–1.43) |
| Adjusted for brachial PP | 1.30 (1.01–1.66)§ | 1.14 (0.83–1.56) |
| Adjusted for brachial systolic BP | 1.31 (1.01–1.69)§ | 1.12 (0.80–1.56) |
| Adjusted for brachial diastolic BP | 1.29 (1.02–1.63)§ | 1.26 (0.92–1.72) |
| Analyses with additional adjustment for duration of hypertension and diabetes mellitus (data for Offspring participants only) | ||
| No. of participants free of TOD at baseline with data at follow‐up | 1413 | 1110 |
| No. with TOD at follow‐up | 126 | 118 |
| Arterial stiffness measure | OR (95% CI) for incident TOD | |
| Central PP | 0.94 (0.77–1.15) | 1.46 (1.18–1.80)† |
| Central MAP | 0.96 (0.79–1.18) | 1.39 (1.12–1.73)‡ |
| CFPWV | 1.29 (0.95–1.74) | 1.27 (0.94–1.73) |
Odds ratios (ORs) are reported for 1‐SD increase in arterial stiffness measure and are adjusted for age, sex, body mass index, diabetes mellitus, antihypertensive treatment, smoking, prevalent cardiovascular disease, total cholesterol/high‐density lipoprotein cholesterol ratio, triglycerides, lipid‐lowering medications, heart rate, and estimated glomerular filtration rate. Duration was measured in the Offspring Cohort only and was estimated based on the number of examinations attended with the condition present. BP indicates blood pressure; CFPWV, carotid‐femoral pulse wave velocity; MAP, mean arterial pressure; PP, pulse pressure; TOD, target organ damage.
Echocardiographic left ventricular hypertrophy (LVH) at follow‐up was measured in the Offspring Cohort only.
† P<0.001; ‡ P<0.01; § P<0.05.
On follow‐up (median 10.3 years, range 0.04–13.7), 297 of 5803 (5.1%) individuals free of prevalent CVD experienced a first CVD event, including 207 events in 5206 (4.0%) participants without TOD at baseline, and 90 events in 777 (11.6%) individuals with prevalent TOD, including 20 events in 68 (29.4%) participants with both forms of TOD. In multivariable models not adjusting for arterial stiffness, the presence of at least 1 form of organ damage was associated with a 33% increased risk of CVD, and the presence of 2 types of organ damage was associated with a 127% increased risk of CVD compared with the referent group without any TOD (Table 6). Additional adjustment for arterial stiffness variables attenuated these associations by 4% to 23%, with the greatest attenuation upon adjustment for CPP (Table 6). In additional analyses limited to the Offspring Cohort and adjusting for durations of hypertension and diabetes mellitus, the aforementioned associations were maintained (Table 6, lower part).
Table 6.
Relations of TOD to the Incidence of CVD on Follow‐Up
| TOD | No. of Participants | No. of Events | HR (95% CI) | |||
|---|---|---|---|---|---|---|
| Models adjusted for: | Only Covariates | Covariates+CPP | Covariates+MAP | Covariates+PWV | ||
| Analyses of Sample 5 | ||||||
| No. of TODs | ||||||
| 0 | 5026 | 207 | Referent | Referent | Referent | Referent |
| ≥1 | 777 | 90 | 1.33 (1.00–1.77)* | 1.26 (0.94–1.68) | 1.31 (0.98–1.74) | 1.32 (0.99–1.75) |
| % Attenuation of effect size for ≥1 TOD upon adjustment for arterial stiffness measure | 0.0 (referent) | 20.7 | 6.6 | 4.7 | ||
| No. of TODs | ||||||
| 0 | 5026 | 207 | Referent | Referent | Referent | Referent |
| 1 | 709 | 70 | 1.21 (0.89–1.63) | 1.17 (0.86–1.59) | 1.19 (0.88–1.62) | 1.20 (0.89–1.62) |
| 2 | 68 | 20 | 2.27 (1.39–3.69)† | 1.88 (1.12–3.16)* | 2.17 (1.31–3.59)† | 2.18 (1.33–3.58)† |
| P for trend | 0.007 | 0.04 | 0.01 | 0.01 | ||
| % Attenuation of effect size for TOD upon adjustment for arterial stiffness measure | ||||||
| 1 TOD | Referent | 17.7 | 5.3 | 3.8 | ||
| 2 TOD | Referent | 22.9 | 5.4 | 4.6 | ||
| Analyses with additional adjustment for duration of hypertension and diabetes mellitus (data for the Offspring Cohort only) | ||||||
| No. of TODs | ||||||
| 0 | 1557 | 135 | Referent | Referent | Referent | Referent |
| ≥1 | 425 | 73 | 1.28 (0.95–1.71) | 1.23 (0.91–1.66) | 1.27 (0.95–1.72) | 1.27 (0.95–1.71) |
| % Attenuation of effect size for ≥1 TOD upon adjustment for arterial stiffness measure | 0.0 (referent) | 14.8 | 0.4 | 1.3 | ||
| No. of TODs | ||||||
| 0 | 1557 | 135 | Referent | Referent | Referent | Referent |
| 1 | 377 | 55 | 1.15 (0.84–1.58) | 1.14 (0.83–1.56) | 1.15 (0.84–1.58) | 1.15 (0.84–1.57) |
| 2 | 48 | 18 | 2.14 (1.27–3.61)† | 1.85 (1.07–3.21)* | 2.17 (1.27–3.69)† | 2.13 (1.26–3.61)† |
| P for trend | 0.02 | 0.06 | 0.02 | 0.02 | ||
| % Attenuation of effect size for TOD upon adjustment for arterial stiffness measure | ||||||
| 1 TOD | Referent | 8.6 | No attenuation | 0.3 | ||
| 2 TOD | Referent | 19.4 | No attenuation | 0.8 | ||
Hazard ratios (HRs) are adjusted for age, sex, body mass index, diabetes mellitus, antihypertensive treatment, smoking, total cholesterol/high‐density lipoprotein cholesterol ratio, triglycerides, lipid‐lowering medications, heart rate, and estimated glomerular filtration rate. Duration of hypertension and diabetes mellitus was measured in the Offspring Cohort only and was estimated based on number of examinations attended with the condition present. CPP indicates central pulse pressure; CVD, cardiovascular disease; MAP, mean arterial pressure; PWV, pulse wave velocity; TOD, target organ damage.
*P<0.05; † P<0.01.
In analyses relating arterial stiffness measures to CVD incidence, a 1‐SD increase in CPP was associated with a 25% greater risk of CVD, an association that was attenuated 5% upon adjustment for the presence of TOD (Table 7). Figure 5 displays the greater cumulative incidence of CVD with presence of TOD and with rising tertile‐defined category of each arterial stiffness measure (P<0.01 for all).
Table 7.
Relations of Arterial Stiffness Measures to CVD Incidence
| Arterial Stiffness Measure | Model Adjusted for Covariates But Not for TOD | Model Adjusted for Covariates+Presence of TOD (Yes/No) | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Central PP | 1.25 (1.12–1.40) | <0.0001 | 1.24 (1.10–1.39) | 0.0003 |
| % Attenuation upon adjustment for TOD | Referent | 5.1% | ||
| Central MAP | 1.10 (0.97–1.23) | 0.14 | 1.08 (0.96–1.22) | 0.21 |
| CFPWV | 1.15 (0.95–1.39) | 0.16 | 1.13 (0.94–1.37) | 0.21 |
| No. of events/No. at risk | 297/5803 | |||
Hazard ratios (HRs) correspond to 1‐SD increase in the arterial stiffness measure and are adjusted for age, sex, body mass index, diabetes mellitus, antihypertensive treatment, smoking, total cholesterol/high‐density lipoprotein cholesterol ratio, triglycerides, lipid‐lowering medications, heart rate, and estimated glomerular filtration rate. CFPWV indicates carotid‐femoral pulse wave velocity; CVD, cardiovascular disease; MAP, mean arterial pressure; PP, pulse pressure; TOD, target organ damage.
Figure 5.
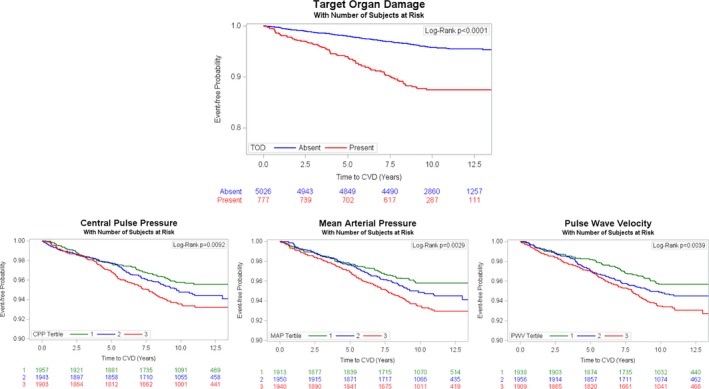
Incidence of cardiovascular disease (CVD) according to presence vs absence of target organ damage (TOD) (top panel) and according to tertile of arterial stiffness measure (bottom 3 panels).
Secondary analyses of the composite outcome of CVD or mortality yielded results essentially similar to the primary analyses (Tables 8 and 9). Presence of TOD and higher CPP were both independently associated with the composite outcome. Adjustment for arterial stiffness led to a 2% to 23% attenuation of the observed hazards ratio associated with presence of TOD. Conversely, adjustment for TOD attenuated the association of CPP with the outcome modestly.
Table 8.
Relations of TOD to the Incidence of CVD and Mortality (Composite Outcome)
| TOD | No. of Participants | No. of Events | HR (95% CI) | |||
|---|---|---|---|---|---|---|
| Models Adjusted For | Only Covariates | Covariates+CPP | Covariates+MAP | Covariates+PWV | ||
| No. of TODs | ||||||
| 0 | 5026 | 347 | Referent | Referent | Referent | Referent |
| ≥1 | 777 | 154 | 1.31 (1.06–1.61)* | 1.25 (1.02–1.54)* | 1.30 (1.05–1.60)* | 1.30 (1.06–1.60)* |
| % Attenuation of effect size for ≥1 TOD upon adjustment for arterial stiffness measure | 0.0 (referent) | 16.7 | 3.5 | 2.9 | ||
| No. of TODs | ||||||
| 0 | 5026 | 347 | Referent | Referent | Referent | Referent |
| 1 | 709 | 125 | 1.23 (0.99–1.53) | 1.20 (0.96–1.49) | 1.22 (0.98–1.52) | 1.22 (0.99–1.52) |
| 2 | 68 | 29 | 1.92 (1.28–2.88)† | 1.65 (1.08–2.51)* | 1.88 (1.25–2.84)† | 1.87 (1.24–2.82)† |
| P for trend | 0.002 | 0.013 | 0.003 | 0.002 | ||
| % Attenuation of effect size for TOD upon adjustment for arterial stiffness measure | ||||||
| 1 TOD | Referent | 12.5 | 2.3 | 2.0 | ||
| 2 TOD | Referent | 23.2 | 3.1 | 3.5 | ||
Hazard ratios (HRs) are adjusted for age, sex, body mass index, diabetes mellitus, antihypertensive treatment, smoking, total cholesterol/high‐density lipoprotein cholesterol ratio, triglycerides, lipid‐lowering medications, heart rate, and estimated glomerular filtration rate. CPP indicates central pulse pressure; CVD, cardiovascular disease; MAP, mean arterial pressure; PWV, pulse wave velocity; TOD, target organ damage.
*P<0.05; † P<0.01.
Table 9.
Relations of Arterial Stiffness to Composite Outcome of CVD Incidence or Death
| Arterial Stiffness Measure | Model Adjusted for Covariates But Not TOD | Model Adjusted for Covariates+Presence of TOD (Yes/No) | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| CPP | 1.20 (1.10–1.30) | <0.0001 | 1.18 (1.09–1.29) | 0.0001 |
| % Attenuation upon adjustment for TOD | Referent | 6.2% | ||
| Central MAP | 1.05 (0.96–1.16) | 0.30 | 1.04 (0.94–1.15) | 0.45 |
| CFPWV | 1.09 (0.94–1.27) | 0.24 | 1.08 (0.93–1.25) | 0.31 |
| No. of events/No. at risk | 501/5803 | |||
Hazard ratios (HRs) are per 1‐SD increment in arterial stiffness measure and are adjusted for age, sex, body mass index, diabetes mellitus, antihypertensive treatment, smoking, total cholesterol/high‐density lipoprotein cholesterol ratio, triglycerides, lipid‐lowering medications, heart rate, and estimated glomerular filtration rate. CFPWV indicates carotid‐femoral pulse wave velocity; CPP, central pulse pressure; CVD, cardiovascular disease; MAP, mean arterial pressure; TOD, target organ damage.
Discussion
Principal Findings
In our large community‐based sample, we investigated the associations of arterial stiffness measures with TOD and explored the relations of both sets of measures with incident CVD and mortality. Our principal findings were 3‐fold. First, higher arterial stiffness was associated with increased burden of TOD both in cross‐sectional and prospective analyses. These associations were consistent across the 3 arterial stiffness measures (CPP, MAP, and CFPWV) for different measures of TOD (albuminuria, LVH, and WMH) in cross‐sectional analyses; however, higher CFPWV was more strongly associated with higher odds of prevalent CBI compared with the other arterial stiffness measures. In prospective analyses of subsamples with repeated assessment of albuminuria and LVH at a follow‐up examination, greater CFPWV was associated with higher odds of incident albuminuria, whereas higher CPP and MAP were associated with incident LVH. The results of these cross‐sectional and prospective analyses relating arterial stiffness to prevalent and incident TOD and new‐onset CVD were maintained even upon adjustment for the durations of hypertension and diabetes mellitus in analyses limited to the Offspring Cohort in which such durations could be estimated. Overall, these associations seem intuitive, given that central hemodynamic factors (reflected by MAP and CPP) impose greater ventricular afterload and directly increase LV wall stress. CFPWV, on the other hand, may better capture the downstream impact of elevated aortic stiffness on the renal microcirculation and consequent albuminuria.
Second, presence of TOD was associated with incident CVD and the composite outcome of CVD or death. Additional adjustment for arterial stiffness measures modestly attenuated these relations; this attenuation was most evident upon adjustment for CPP. Since coronary heart disease, cerebrovascular events, and heart failure are key components of incident CVD, our observations may be intuitive as these events are more directly related to central hemodynamic factors. Overall, these observations are consistent with the notion that one key component of the vascular risk posed by the presence of TOD may be attributable to the association of TOD with elevated arterial stiffness.
Third, increased CPP was associated with greater incidence of CVD and the composite outcome of CVD or death. Additional adjustment for presence of TOD only modestly attenuated this association, confirming the conjoint and independent impacts of arterial stiffness and TOD on adverse outcomes. These observations suggest that only a modest component of the vascular risk posed by elevated CPP may be mediated by the association of the latter with presence of TOD, itself an antecedent of CVD.
Comparison With the Published Literature and Mechanisms
As noted earlier, several reports have related the presence of TOD to the incidence of CVD.1 A parallel set of investigations, including from our group,36, 37, 38, 39, 40, 41, 42 have underscored the relations of elevated arterial stiffness to CVD incidence. On the other hand, studies relating arterial stiffness or pulsatile hemodynamics to TOD have been predominantly cross‐sectional and typically focused on a single end organ.13, 14, 15, 16, 17, 18, 19, 43, 44, 45, 46, 47 To our knowledge, data are limited relating vascular stiffness measures to multiple measures of TOD prospectively and elucidating the 3‐way relations of these traits to incidence of CVD (Figure 1).
Our investigation establishes links between higher arterial stiffness and incident TOD in prospective analyses of individuals free of TOD at baseline. The strength of the association, presence of a dose‐response, temporality of the relations, and biological plausibility are all consistent with a possible causal relationship between measures of arterial stiffness and new‐onset TOD on follow‐up. Furthermore, we demonstrate distinctive and synergistic associations of both presence of TOD and greater vascular stiffness with incident CVD and the composite outcome of CVD or death. These observations raise the possibility that mitigation of arterial stiffness may reduce the burden of TOD. They confirm prior reports linking TOD to CVD and are consistent with data suggesting that the monitoring and prevention of TOD may reduce the incidence of CVD.2, 3, 4, 5
The associations of vascular stiffness with manifestations of TOD and, in turn, CVD may reflect underlying common molecular mechanisms that link microcirculatory endothelial dysfunction to increased transmission of pulsatile flow in end organs.6, 7, 8, 9, 10, 11 Substantial experimental evidence links arterial stiffness to endothelial dysfunction and the presence of TOD, invoking tissue‐level mechanisms of heightened inflammation and oxidative stress, and key cellular pathways (notably sirtuins, AMP kinase, m‐TOR, and klotho) as reviewed elsewhere.48
Strengths and Limitations
The large community‐based sample with the continuous surveillance for incidence of CVD strengthens our investigation. Three additional features of our investigation distinguish it from prior reports: (1) the comprehensive cross‐sectional analyses including multiple measures of arterial stiffness and multiple measures of TOD; (2) the prospective analyses assessing the relations of multiple measures of arterial stiffness to the incidence of new‐onset TOD; and (3) the longitudinal analyses relating presence of TOD to incidence of CVD and the exploration of attenuation of these relations by adjustment for arterial stiffness measures to gain insights into potential mediating influences of the latter measures on CVD.
Nonetheless, several limitations of our approach warrant acknowledgment. Select measures of TOD at baseline and follow‐up were available only on subsamples, thereby constraining the overall investigation. Additionally, the observational nature of the study precludes any causal inferences and limits the ability to draw mechanistic insights into the complex relations of arterial stiffness, TOD, and CVD. We did not correct for multiple testing, which underscores the importance of replicating some of our key findings in additional samples. Last, but not the least, the Framingham cohorts are overwhelmingly white, and relations of vascular stiffness to TOD may vary with race and ethnicity.
Conclusions
Our comprehensive evaluations of a large community‐based sample suggest that greater arterial stiffness is associated with a higher prevalence and incidence of TOD. We confirm the known association of TOD with incident CVD and demonstrate that the bidirectional relations of arterial stiffness and TOD may mediate, at least partially, the greater vascular risk associated with presence of TOD. Additional studies are warranted to confirm our findings in multiethnic samples and to elucidate whether lowering arterial stiffness will mitigate TOD and, in turn, lower risk for clinical CVD.
Sources of Funding
This work was supported by the National Heart, Lung, and Blood Institute's FHS (National Institutes of Health [NIH] contracts N01‐HC‐25195, HHSN268201500001I and 75N92019D00031) and NIH grants HL080124, HL071039, HL077447, HL107385, 1R01HL126136, 5R01HL107385, 1R01HL60040, 1RO1HL70100, R01HL131532, and R01HL134168. Dr Vasan is supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine.
Disclosures
Mitchell is owner of Cardiovascular Engineering, Inc, a company that designs and manufactures devices that measure arterial stiffness. The company uses these devices in clinical trials that evaluate the effects of diseases and interventions on vascular stiffness. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2019;8:e012141 DOI: 10.1161/JAHA.119.012141.)
References
- 1. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GY, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group . 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 2. Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris K, Aurup P, Dahlof B. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356. [DOI] [PubMed] [Google Scholar]
- 3. Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlof B, LIFE Study Investigators . Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349. [DOI] [PubMed] [Google Scholar]
- 4. Fagard RH, Celis H, Thijs L, Wouters S. Regression of left ventricular mass by antihypertensive treatment: a meta‐analysis of randomized comparative studies. Hypertension. 2009;54:1084–1091. [DOI] [PubMed] [Google Scholar]
- 5. Ibsen H, Olsen MH, Wachtell K, Borch‐Johnsen K, Lindholm LH, Mogensen CE, Dahlof B, Devereux RB, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Lederballe‐Pedersen O, Nieminen MS, Omvik P, Oparil S, Wan Y. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension. 2005;45:198–202. [DOI] [PubMed] [Google Scholar]
- 6. Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end‐organ damage. J Appl Physiol (1985). 2008;105:1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain. 2011;134:3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wahlin A, Ambarki K, Birgander R, Malm J, Eklund A. Intracranial pulsatility is associated with regional brain volume in elderly individuals. Neurobiol Aging. 2014;35:365–372. [DOI] [PubMed] [Google Scholar]
- 9. Hashimoto J, Ito S. Some mechanical aspects of arterial aging: physiological overview based on pulse wave analysis. Ther Adv Cardiovasc Dis. 2009;3:367–378. [DOI] [PubMed] [Google Scholar]
- 10. Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension. 2011;58:839–846. [DOI] [PubMed] [Google Scholar]
- 11. Henry‐Feugeas MC, Koskas P. Cerebral vascular aging: extending the concept of pulse wave encephalopathy through capillaries to the cerebral veins. Curr Aging Sci. 2012;5:157–167. [DOI] [PubMed] [Google Scholar]
- 12. Maillard P, Mitchell GF, Himali JJ, Beiser A, Tsao CW, Pase MP, Satizabal CL, Vasan RS, Seshadri S, DeCarli C. Effects of Arterial Stiffness on Brain Integrity in Young Adults From the Framingham Heart Study. Stroke. 2016;47:1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Cheng S, Aragam J, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Relations of central hemodynamics and aortic stiffness with left ventricular structure and function: the Framingham Heart Study. J Am Heart Assoc. 2016;5:e002693 DOI: 10.1161/JAHA.115.002693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Upadhyay A, Hwang SJ, Mitchell GF, Vasan RS, Vita JA, Stantchev PI, Meigs JB, Larson MG, Levy D, Benjamin EJ, Fox CS. Arterial stiffness in mild‐to‐moderate CKD. J Am Soc Nephrol. 2009;20:2044–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsao CW, Himali JJ, Beiser AS, Larson MG, DeCarli C, Vasan RS, Mitchell GF, Seshadri S. Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology. 2016;86:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peralta CA, Jacobs DR Jr, Katz R, Ix JH, Madero M, Duprez DA, Sarnak MJ, Criqui MH, Kramer HJ, Palmas W, Herrington D, Shlipak MG. Association of pulse pressure, arterial elasticity, and endothelial function with kidney function decline among adults with estimated GFR >60 mL/min/1.73 m(2): the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2012;59:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yano Y, Sato Y, Fujimoto S, Konta T, Iseki K, Moriyama T, Yamagata K, Tsuruya K, Yoshida H, Asahi K, Kurahashi I, Ohashi Y, Watanabe T. Association of high pulse pressure with proteinuria in subjects with diabetes, prediabetes, or normal glucose tolerance in a large Japanese general population sample. Diabetes Care. 2012;35:1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gutierrez J, Elkind MS, Cheung K, Rundek T, Sacco RL, Wright CB. Pulsatile and steady components of blood pressure and subclinical cerebrovascular disease: the Northern Manhattan Study. J Hypertens. 2015;33:2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verhaaren BF, Vernooij MW, de Boer R, Hofman A, Niessen WJ, van der Lugt A, Ikram MA. High blood pressure and cerebral white matter lesion progression in the general population. Hypertension. 2013;61:1354–1359. [DOI] [PubMed] [Google Scholar]
- 20. Dawber TR, Meadors GF, Moore FE Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 22. Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 23. Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O'Connor GT, Rapoport DM, Redline S, Robbins J, Samet JM, Wahl PW. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 24. National Heart, Lung, and Blood Institute . Framingham Heart Study (FHS) Offspring (OS) and OMNI 1 Cohorts. Available at: https://biolincc.nhlbi.nih.gov/studies/framoffspring/. Accessed June 8, 2019.
- 25. National Heart, Lung, and Blood Institute . Framingham Heart Study (FHS) Third Generation (Gen III), OMNI 2, and New Offspring (NOS) Cohorts. Available at: https://biolincc.nhlbi.nih.gov/studies/gen3/. Accessed June 8, 2019.
- 26. Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. [DOI] [PubMed] [Google Scholar]
- 28. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 30. Nathan DM, Rosenbaum C, Protasowicki VD. Single‐void urine samples can be used to estimate quantitative microalbuminuria. Diabetes Care. 1987;10:414–418. [DOI] [PubMed] [Google Scholar]
- 31. Mattix HJ, Hsu CY, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002;13:1034–1039. [DOI] [PubMed] [Google Scholar]
- 32. Romero JR, Preis SR, Beiser AS, DeCarli C, Lee DY, Viswanathan A, Benjamin EJ, Fontes J, Au R, Pikula A, Wang J, Kase CS, Wolf PA, Irrizary MC, Seshadri S. Lipoprotein phospholipase A2 and cerebral microbleeds in the Framingham Heart Study. Stroke. 2012;43:3091–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D'Agostino R, Wolf PA. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 34. Pikula A, Beiser AS, DeCarli C, Himali JJ, Debette S, Au R, Selhub J, Toffler GH, Wang TJ, Meigs JB, Kelly‐Hayes M, Kase CS, Wolf PA, Vasan RS, Seshadri S. Multiple biomarkers and risk of clinical and subclinical vascular brain injury: the Framingham Offspring Study. Circulation. 2012;125:2100–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kannel WB, Wolf PA, Garrison RJ. Section 34: some risk factors related to the annual incidence of cardiovascular disease and death in pooled repeated biennial measurements. Framingham Heart Study, 30 Year Follow‐Up. Bethesda, MD: US Department of Health and Human Services; 1987. [Google Scholar]
- 36. Mitchell GF, Hwang S‐J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events. Circulation. 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ohyama Y, Ambale‐Venkatesh B, Noda C, Kim JY, Tanami Y, Teixido‐Tura G, Chugh AR, Redheuil A, Liu CY, Wu CO, Hundley WG, Bluemke DA, Guallar E, Lima JAC. Aortic arch pulse wave velocity assessed by magnetic resonance imaging as a predictor of incident cardiovascular events: the MESA (Multi‐Ethnic Study of Atherosclerosis). Hypertension. 2017;70:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, Vita JA, Benjamin EJ, Mitchell GF, Vasan RS. Relation of Central Arterial Stiffness to Incident Heart Failure in the Community. J Am Heart Assoc. 2015;4:e002189 DOI: 10.1161/JAHA.115.002189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Sloten TT, Sedaghat S, Laurent S, London GM, Pannier B, Ikram MA, Kavousi M, Mattace‐Raso F, Franco OH, Boutouyrie P, Stehouwer CDA. Carotid stiffness is associated with incident stroke: a systematic review and individual participant data meta‐analysis. J Am Coll Cardiol. 2015;66:2116–2125. [DOI] [PubMed] [Google Scholar]
- 40. Takashima N, Turin TC, Matsui K, Rumana N, Nakamura Y, Kadota A, Saito Y, Sugihara H, Morita Y, Ichikawa M, Hirose K, Kawakani K, Hamajima N, Miura K, Ueshima H, Kita Y. The relationship of brachial‐ankle pulse wave velocity to future cardiovascular disease events in the general Japanese population: the Takashima Study. J Hum Hypertens. 2014;28:323–327. [DOI] [PubMed] [Google Scholar]
- 41. Chirinos JA, Kips JG, Jacobs DR Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am Coll Cardiol. 2012;60:2170–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mattace‐Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. [DOI] [PubMed] [Google Scholar]
- 43. Wu S, Chen D, Zeng X, Wen J, Zhou C, Xiao K, Hu P, Chen W. Arterial stiffness is associated with target organ damage in subjects with pre‐hypertension. Arch Med Sci. 2018;14:1374–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Andrikou E, Tsioufis C, Dimitriadis K, Flessas D, Chatzistamatiou V, Grassos C, Papavasiliou M, Papadopoulos D, Stefanadis C. Parallel deterioration of albuminuria, arterial stiffness and left ventricular mass in essential hypertension: integrating target organ damage. Nephron Clin Pract. 2011;119:c27–c34. [DOI] [PubMed] [Google Scholar]
- 45. Lu Y, Zhu M, Bai B, Chi C, Yu S, Teliewubai J, Xu H, Wang K, Xiong J, Zhou Y, Ji H, Fan X, Yu X, Li J, Blacher J, Zhang Y, Xu Y. Comparison of carotid‐femoral and brachial‐ankle pulse‐wave velocity in association with target organ damage in the community‐dwelling elderly Chinese: the Northern Shanghai Study. J Am Heart Assoc. 2017;6:e004168 DOI: 10.1161/JAHA.116.004168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fan X, Zhu M, Chi C, Yu S, Xiong J, Lu Y, Bai B, Xu Y, Zhang Y. Association of arteriosclerosis and/or atherosclerosis with hypertensive target organ damage in the community‐dwelling elderly Chinese: the Northern Shanghai Study. Clin Interv Aging. 2017;12:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim ED, Tanaka H, Ballew SH, Sang Y, Heiss G, Coresh J, Matsushita K. Associations between kidney disease measures and regional pulse wave velocity in a large community‐based cohort: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2018;72:682–690. [DOI] [PubMed] [Google Scholar]
- 48. Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age‐related disease. Circ Res. 2018;123:825–848. [DOI] [PMC free article] [PubMed] [Google Scholar]


