Abstract
Background
Evidence accumulated that some glucose‐lowering medications protect against cardiovascular events (CVEs) in patients with type 2 diabetes mellitus (T2DM) and established cardiovascular disease. The present study evaluated if and how glucose‐lowering medication prescription pattern changes in T2DM after a CVE.
Methods and Results
DATAFILE (Diabetes Therapy After a Cardiovascular Event) was a retrospective multicenter study conducted at 12 diabetes mellitus specialist outpatient clinics in Italy. We identified T2DM patients with an incident CVE for whom a follow‐up visit was available after the event. We selected control T2DM patients without an incident CVE, who were matched with cases for age, sex, known diabetes mellitus duration, baseline hemoglobin A1c, kidney function, and follow‐up time. We extracted clinical variables and compared prescribed therapies at baseline and follow‐up. We included 563 patients with and 497 matched patients without an incident CVE. As expected, patients with a subsequent CVE had a higher baseline prevalence of ischemic heart disease. After a median of 9.5 months, in patients with versus those without a CVE, there was a significant increase in the prescription of beta‐blockers, loop diuretics, dual antiplatelet therapy, and, among glucose‐lowering medications, a significant decrease in metformin. Hemoglobin A1c marginally declined only in the control group, whereas low‐density lipoprotein cholesterol decreased only in patients with CVE.
Conclusions
This study highlights that occurrence of a CVE in T2DM patients did not prime the prescription of glucose‐lowering medications provided with cardiovascular protective effects, even though glucose control remained poor. These data emphasize the need to optimize the therapeutic regimen of T2DM patients with established cardiovascular disease, according to updated guidelines.
Keywords: appropriateness, diabetes mellitus, pharmacology
Subject Categories: Diabetes, Type 2; Epidemiology; Secondary Prevention; Complications; Quality and Outcomes
Clinical Perspective
What Is New?
Recent treatment algorithms recognize that some glucose‐lowering medications protect from adverse cardiovascular outcomes in patients with type 2 diabetes mellitus with established cardiovascular disease.
In this study, we found that prescription of diabetes mellitus drugs did not change substantially in patients who experienced a cardiovascular event compared with those who did not, while prescription of drugs for the control of risk factors significantly increased.
What Are the Clinical Implications?
Our findings that, after a cardiovascular event, there seems to be more focus on the control of blood pressure, lipids, and platelet aggregation than on diabetes mellitus drugs that have the potential to reduce the risk of event recurrence suggest that it will be important to actively promote the treatment paradigm shift bestowed by national and international guidelines, moving from the “treat to target” to the “treat to benefit” approach.
Introduction
The therapeutic armamentarium for the treatment of type 2 diabetes mellitus (T2DM) has dramatically expanded in the past 10 years. With several classes of glucose‐lowering medications (GLMs) available and many different drugs for each class, the modern management of T2DM requires such an extensive knowledge of drug characteristics that it should be more appropriately delivered by diabetes mellitus specialists.1
Because cardiovascular disease (CVD) is the leading cause of death in patients with T2DM, prevention of CVD has become a major goal of T2DM management.2 On the basis of mode of action, preclinical findings, and pathophysiological studies in humans, many GLM classes were expected to exert protection against cardiovascular complications. Various degrees of evidence were available for pioglitazone,3 dipeptidyl peptidase‐4 inhibitors (DPP‐4i),4 glucagon‐like peptide‐1 receptor agonists (GLP‐1RA),5 and sodium‐glucose cotransporter‐2 inhibitors (SGLT2i).6 Conversely, observational studies suggested that use of sulfonylureas may worsen cardiovascular outcomes.7, 8 While such concern has never been confirmed in dedicated randomized trials, cardiovascular outcome trials (CVOTs) completed since 2013 have shown reasonable cardiovascular safety but no cardiovascular protection by DPP‐4i.9, 10, 11, 12 Initial findings raised concerns that DPP‐4i might increase the risk of hospitalization for heart failure (HHF),12 which has been subsequently ruled out.13 While consistently increasing hospitalization for heart failure risk in predisposed individuals, pioglitazone was shown to be provided with potential pleiotropic effects against cardiovascular events (CVEs) attributable to progression of atherosclerosis.14 CVOTs on SGLT2i performed mostly on patients with T2DM with established CVD cumulatively showed significant benefits against CVE, especially hospitalization for heart failure, and cardiovascular death.15, 16, 17 Finally, although with some differences among the various molecules, CVOTs on GLP‐1RA showed protection from CVE in patients with established CVD.18, 19, 20
Despite strong evidence that SGLT2i and GLP‐1RA improve cardiovascular outcomes of patients with T2DM with established CVD, the use of these GLMs in routine clinical practice remains relatively low, with a preference for sulfonylureas and DPP‐4i. Based on results of CVOTs and on the new consensus report on the management of T2DM,1 the prescription of SGLT2i and GLP‐1RA should increase in patients with T2DM and CVD. On the other side, several studies support a link between hypoglycemia, especially severe, and adverse cardiovascular outcomes.21 In addition, risk of hypoglycemia appears to increase after a CVE,22, 23 suggesting that, especially in aged patients with T2DM with CVD, use of a GLM associated with a higher risk of hypoglycemia should be avoided whenever possible.
Because application of evidence to clinical practice can encounter limitations and take time, we herein wished to survey whether occurrence of a CVE in patients with T2DM primed changes in the prescription patterns by diabetes mellitus specialists.
Methods
The data sets analyzed during the current study are not publicly available because of Society policy but are available from the corresponding author on reasonable request.
Study Design
DATAFILE (Diabetes Therapy After a Cardiovascular Event) was a retrospective multicenter study conducted at 12 diabetes mellitus specialist outpatient clinics in Italy. The study was promoted and supported by the Italian Diabetes Society as an audit of therapeutic appropriateness at participating centers. The protocol was approved by the local ethical committee of the coordinated center (University Hospital of Padova) and notified to the ethical committees of participating centers, in agreement with national regulations on observational retrospective studies. Patients’ data were anonymized such that it was impossible to recall the identity of patients whose data composed the database. In this condition, according to national regulations, requirement for informed consent was waived.
Data Source and Extraction
Anonymized patients’ data were extracted from the same electronic chart system at all centers (MyStar Connect, Me.te.da, Italy). A dedicated software was developed to interrogate the electronic chart and extract data in a clinical research form without manual intervention. We identified all cases of patients in each center with a diagnosis of T2DM who, between January 1, 2010, and December 31, 2018, experienced a CVE, as registered in the electronic chart. Patients were included in the analysis if they had at least 1 visit at the diabetes mellitus clinic before the event and at least 1 visit at the same diabetes mellitus clinic after the event, as recorded in the electronic database. The following CVEs were considered: acute myocardial infarction, stroke, or transient ischemic attack; new diagnosis of ischemic heart disease; new diagnosis of heart failure; and revascularization procedures (coronary, cerebral, or peripheral). In parallel, we identified control patients with a diagnosis of T2DM who, during the same period and in the same center as each case, did not experience a CVE, as reported in the chart. At the time of data extraction, for each case at each center, a matched control was searched in the center electronic chart based on the following variables and tolerance: same sex; age ±3 years; time since diabetes mellitus diagnosis ±3 years; hemoglobin A1c (HbA1c) ±0.5%; serum creatinine ±10%; and follow‐up time ±50%. When a match was not found, the case was retained in the study database. Because unpaired tests were used to compare the 2 groups (see below), the different sample size did not impact between‐group comparison. To evaluate to what extent missing matches affected the balance between groups, we performed a sensitivity analysis wherein cases without a control match were excluded.
The primary objective was to compare the percent change in prescription of various GLM classes between the group of cases with CVE and controls without CVE. Secondary objectives were to compare (1) the change in the prescription of cardiovascular medications and (2) the change in parameters of cardiovascular risk factors and HbA1c between the 2 groups. We performed a sensitivity analysis restricting the time window to the period when SGLT2i became available in Italy and favorable CVOTs were published (from January 2015 to December 2018). The electronic chart system records only visits at the diabetes mellitus outpatient clinics, thereby assuring that the prescriptions were made by the diabetologist or, if made by other specialists who followed the patients at time of the CVE, they were validated by the diabetologist at the follow‐up visit.
The following parameters were extracted at both visits for all patients: age, sex, known diabetes mellitus duration, current smoking status, body weight, height, body mass index, waist circumference, systolic and diastolic blood pressure, heart rate, fasting plasma glucose, HbA1c, total cholesterol, high‐density lipoprotein cholesterol, triglycerides (low‐density lipoprotein [LDL] was calculated using the Friedewald equation),24 liver enzymes, serum creatinine (estimated glomerular filtration rate [eGFR] was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation),25 albumin excretion rate (as albumin/creatinine ratio in milligrams per gram). At baseline, we also collected information on chronic diabetic complications as reported in the electronic chart, including retinopathy and macular edema, peripheral or autonomic neuropathy, peripheral arterial disease and previous peripheral revascularization, diabetic foot, stroke or transient ischemic attack, previous cerebral revascularization, ischemic heart disease, or previous coronary revascularization. Detailed information on prescribed medications for the treatment of T2DM and concomitant risk factors were retrieved. Drug dosages were not available, nor was information on whether the patients actually took the drugs.
Statistical Analysis
Data are expressed as mean±standard deviation for continuous variables or as percentage for categorical variables. Normality was checked using the Kolmogorov–Smirnov test and nonnormal variables were transformed into their logarithm before analysis with parametric tests. Comparison between 2 groups was performed using the unpaired 2‐tailed Student t test for continuous variables or with chi‐square for categorical variables. In addition to P values, the standardized difference was calculated to compare the balance between the 2 matched groups: a value of standardized difference >0.10 was considered indicative of a clinically meaningful imbalance. Intragroup changes were analyzed using the paired 2‐tailed Student t test for continuous variables, such as HbA1c, or using the Wilcoxon rank‐sum test for categorical variables. To adjust for baseline differences between the 2 groups, exploratory multivariable linear regression analyses were performed, with all covariates entered as a single block in the model. Statistical significance was conventionally accepted at P<0.05. SPSS version 23 was used.
Results
Patient Characteristics
We identified 563 patients with a CVE: The majority were new diagnoses of ischemic heart disease (47.8%) and revascularization procedures (49.4%), followed by acute myocardial infarction (25.0%) and new diagnoses of heart failure (15.8%). On average, each patient had 2.1 diagnoses, the most common combinations being myocardial infarction, ischemic heart disease, and/or coronary revascularization. The matching procedure identified 497 control patients without a CVE, while 66 cases (11.7%) did not have a match on the basis of prespecified matching criteria.
As shown in Table 1, the 2 groups were well balanced for several clinical characteristics, including age (69 years), sex (73% males), time since diabetes mellitus diagnosis (14 years) and HbA1c (7.7%). Although body mass index was well balanced, waist circumference was slightly higher in patients with than in those without a CVE. In patients with CVE, total cholesterol and LDL cholesterol were significantly lower, reflecting more frequent use of statins, and heart or peripheral arterial disease were more prevalent at baseline. Most common GLMs at baseline were metformin, sulfonylureas, DPP‐4i, and insulin. Except for subtle differences in diet alone and metformin use, the GLM prescription pattern was similar between cases and controls. Rather, cases with CVE had a higher prescription of cardiovascular medications than controls without CVE, reflecting the higher baseline cardiovascular risk.
Table 1.
Clinical Characteristics of Patients in the Two Groups
| Cases With CVE (n=563) | Controls Without CVE (n=497) | Comparison | ||||
|---|---|---|---|---|---|---|
| % | Value | % | Value | P Value | STD | |
| Age, y | 100.0 | 69.1±9.4 | 100.0 | 69.2±9.1 | 0.840 | 0.012 |
| Sex, male, % | 100.0 | 73.5 | 100.0 | 73.0 | 0.856 | 0.011 |
| Current smoking, % | 25.8 | 17.2 | 73.2 | 17.6 | 0.639 | 0.009 |
| Time since diagnosis, y | 99.6 | 14.3±10.3 | 99.8 | 13.9±9.9 | 0.484 | 0.043 |
| Body weight, kg | 100.0 | 83.6±16.7 | 99.0 | 81.9±16.0 | 0.087 | 0.106 |
| Height, cm | 99.8 | 167.1±11.5 | 99.0 | 167.2±9.0 | 0.935 | 0.005 |
| BMI, kg/m2 | 99.8 | 29.8±5.1 | 99.0 | 29.3±5.2 | 0.161 | 0.087 |
| Waist circumference, cm | 51.7 | 106.7±12.6 | 82.1 | 104.5±12.6 | 0.023 | 0.175 |
| Systolic blood pressure, mm Hg | 100.0 | 139.7±19.8 | 97.8 | 140.7±20.5 | 0.417 | 0.050 |
| Diastolic blood pressure, mm Hg | 100.0 | 77.4±23.7 | 97.8 | 77.2±10.7 | 0.820 | 0.014 |
| Heart rate, bpm | 40.7 | 73.9±12.6 | 50.3 | 76.0±12.3 | 0.057 | 0.174 |
| Fasting plasma glucose, mg/dL | 92.5 | 156.4±50.5 | 96.4 | 155.7±51.9 | 0.849 | 0.012 |
| HbA1c, % | 99.8 | 7.7±1.3 | 98.2 | 7.7±1.4 | 0.822 | 0.014 |
| Total cholesterol, mg/dL | 99.5 | 163.7±40.2 | 93.6 | 172.5±38.3 | <0.001 | 0.222* |
| HDL cholesterol, mg/dL | 99.5 | 45.5±13.0 | 92.4 | 48.3±13.9 | 0.001 | 0.209* |
| Triglycerides, mg/dL | 99.5 | 155.7±109.3 | 92.8 | 146.1±82.9 | 0.119 | 0.099 |
| LDL cholesterol, mg/dL | 99.5 | 87.1±34.3 | 92.0 | 94.3±33.2 | 0.001 | 0.214* |
| AST, U/L | 72.3 | 24.4±13.6 | 86.5 | 23.8±11.8 | 0.507 | 0.046 |
| ALT, U/TL | 67.1 | 26.1±19.7 | 85.5 | 26.1±17.4 | 0.952 | 0.004 |
| Serum creatinine, mg/L | 98.6 | 1.1±0.4 | 94.6 | 1.2±3.8 | 0.398 | 0.051 |
| eGFR, mL/min per 1.73 m2 | 98.6 | 69.7±22.7 | 94.6 | 70.0±20.9 | 0.803 | 0.016 |
| UACR, mg/g | 57.9 | 186.9±592.1 | 74.6 | 146.7±571.3 | 0.362 | 0.069 |
| Complications | ||||||
| CKD stage III or higher, % | 100.0 | 33.7 | 100.0 | 28.8 | 0.072 | 0.024 |
| Elevated albuminuria, % | 57.9 | 42.3 | 74.6 | 38.3 | 0.276 | 0.083 |
| Retinopathy, % | 83.1 | 23.5 | 85.7 | 21.1 | 0.395 | 0.057 |
| Macular edema, % | 83.1 | 5.3 | 85.7 | 4.7 | 0.659 | 0.030 |
| Neuropathy (peripheral or autonomic) | 40.5 | 28.1 | 43.1 | 29.0 | 0.834 | 0.020 |
| Peripheral arterial disease | 45.5 | 41.0 | ||||
| Arteriosclerosis obliterans, % | 31.3 | 21.1 | 0.014 | 0.233* | ||
| Revascularization, % | 5.1 | 3.4 | 0.391 | 0.082 | ||
| Diabetic foot, % | 41.0 | 20.3 | 42.3 | 16.2 | 0.261 | 0.108 |
| Cerebrovascular disease | 67.3 | 70.8 | ||||
| Stroke/TIA, % | 3.7 | 4.3 | 0.695 | 0.029 | ||
| Cerebral revascularization, % | 5.1 | 3.4 | 0.391 | 0.009 | ||
| Heart disease | 79.0 | 80.9 | ||||
| Ischemic cardiomyopathy, % | 29.9 | 21.1 | 0.004 | 0.202* | ||
| Revascularization, % | 5.1 | 3.4 | 0.391 | 0.067 | ||
| GLMs | 100.0 | 100.0 | ||||
| Diet alone, % | 4.1 | 9.1 | <0.001 | 0.202* | ||
| Metformin, % | 59.7 | 59.0 | 0.810 | 0.014 | ||
| Sulfonylurea/repaglinide, % | 31.3 | 30.0 | 0.652 | 0.028 | ||
| DPP‐4i, % | 15.5 | 15.9 | 0.843 | 0.011 | ||
| GLP‐1RA, % | 3.9 | 2.2 | 0.113 | 0.099 | ||
| Pioglitazone, % | 5.9 | 6.6 | 0.601 | 0.029 | ||
| SGLT2i, % | 1.8 | 1.4 | 0.634 | 0.032 | ||
| Insulin, % | 47.6 | 40.0 | 0.013 | 0.154* | ||
| Cardiovascular medications | 100.0 | 100.0 | ||||
| Statins, % | 70.5 | 54.7 | <0.001 | 0.331* | ||
| Ezetimibe, % | 6.9 | 6.2 | 0.652 | 0.028 | ||
| ACEi/ARBs, % | 70.5 | 63.8 | 0.020 | 0.143* | ||
| Beta‐blockers, % | 41.6 | 26.6 | <0.001 | 0.320* | ||
| Calcium‐channel blockers, % | 29.1 | 27.4 | 0.524 | 0.038 | ||
| Loop diuretics, % | 26.6 | 16.5 | <0.001 | 0.248* | ||
| Other diuretic, % | 24.0 | 29.6 | 0.040 | 0.127* | ||
| Antiplatelet agents, % | 67.3 | 53.1 | <0.001 | 0.293* | ||
| Dual antiplatelet therapy, % | 8.7 | 7.2 | 0.382 | 0.055 | ||
| Anticoagulants, % | 9.9 | 6.4 | 0.039 | 0.128* | ||
The percentage (%) of available data is shown for each variable. For the comparison between groups, standardized differences (STDs) are shown in addition to P values. *Significant imbalance is observed when STD is >0.10 and P value is <0.05. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CKD, chronic kidney disease; CVE, cardiovascular event; DPP‐4i, dipeptidyl peptidase 4 inhibitor; eGFR, estimated glomerular filtration rate; GLP‐1RA, glucagon‐like peptide 1 receptor agonist; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SGLT2i, sodium glucose cotransporter 2 inhibitor; TIA, transient ischemic attack; UACR, urinary albumin creatinine ratio.
Changes in GLMs
The mean and median follow‐up time was 9.5 months in both groups. In the group of patients with CVE, prescription of metformin declined by 5.3%, while prescription of DPP‐4i (+3.7%) and insulin (+2.8%) increased. Overall, the use of sulfonylureas or repaglinide did not change, but that of glibenclamide (glyburide) modestly decreased (−1.4%). In the control group without CVE, prescription of sulfonylurea/repaglinide significantly decreased (−2.6%), whereas that of DPP4i significantly increased (+7.0%). In the comparison between the 2 groups, only the prescription of metformin was significantly different, with a decline in cases with CVE (Figure 1). Since the 2 groups differed from some baseline variables, we performed multivariable regression analyses: the between‐group difference in the change of metformin prescription remained significant after adjusting for lipid profile (−4.7%; P=0.041) or for the prevalence of ischemic heart disease (−5.1%; P=0.036), but was no longer significant after adjusting for baseline therapy with diet alone (P=0.109).
Figure 1.
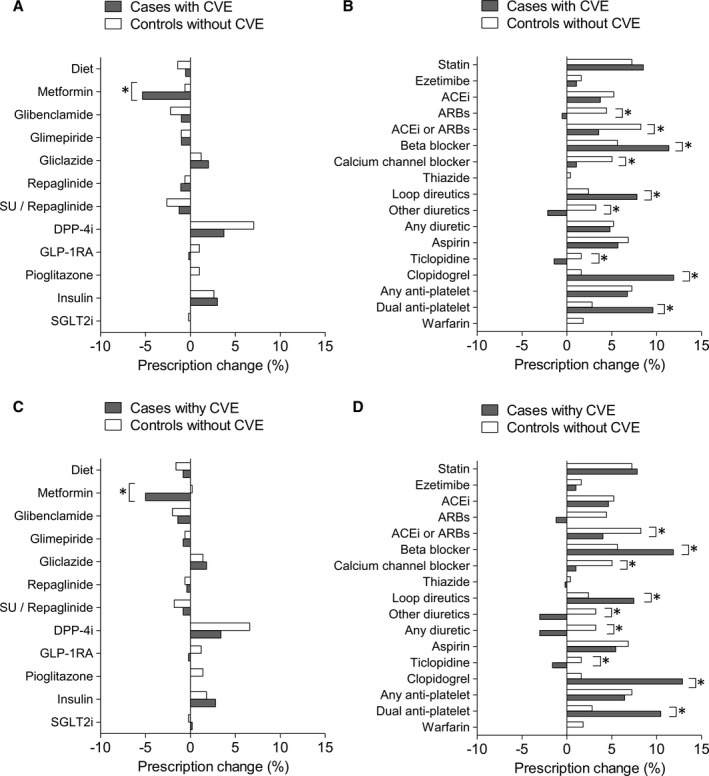
Change in prescription patterns. A and B, Change in the prescription pattern for glucose lowering medications (A) and other medications (B) in the complete data set of patients with CVE (n=563) and controls without CVE (n=497). C and D, Change in the prescription pattern for glucose‐lowering medications (C) and other medications (D) in the data set of matched patients (n=497/group). Data are reported as net percent (positive or negative) of patients with a change in prescription for each medication. *P<0.05 between groups. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CVE, cardiovascular event; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; GLP‐1RA, glucagon‐like peptide 1 receptor agonists; SGLT2i, sodium glucose cotransporter 2 inhibitors; SU, sulfonylurea.
We thus focused on patients who were withdrawn from metformin after a CVE (n=51). Patients no longer prescribed metformin at follow‐up versus those staying on metformin (n=272) had a more marked reduction in eGFR (−5.1 [from 68.1 to 63.0] versus −0.5 [from 68.2 to 67.7] mL/min per 1.73 m2; P=0.009) and a strikingly higher new prescription of insulin (+25.5% [from 41.2% to 66.7%] versus +1.2% [from 48.3% to 49.5%]; P<0.001).
Overall, the number of GLM classes prescribed per individual remained unchanged in patients with CVE (from 1.7±0.8 to 1.7±0.8; P=0.954) and increased marginally in controls (from 1.5±0.9 to 1.6±0.9; P=0.007).
Restricting the analysis to the period from 2015 to 2018 yielded 129 cases and 125 controls with a similar balance of baseline characteristics as in the entire cohort. In this subanalysis, there was a slight decrease in the prescription of pioglitazone in patients with CVE versus controls (not shown). When focusing only on patients with poor glycemic control at baseline (HbA1c >8.0%; n=185 cases and 154 controls), there was still a deprescription of metformin and an increase in insulin among cases and a decrease of SGLT2i among controls (not shown).
Changes in Cardiovascular Medications
In both groups, there were significant changes in the prescription of cardiovascular medications, with increases in statins, angiotensin‐converting enzyme inhibitors, diuretics, and antiplatelet agents. In the comparison between the 2 groups, patients with CVE had a significant increase in the prescription of loop diuretics, beta‐blockers, and dual antiplatelet therapy as compared with controls without CVE.
Changes in Parameters of Risk Factor Control
In patients with CVE, there were significant improvements in diastolic blood pressure, total and LDL cholesterol, and triglycerides, and a decline in eGFR. In controls without CVE, there were significant improvements in HbA1c and triglycerides and a decline in eGFR. In the comparison between groups, systolic blood pressure and total and LDL cholesterol declined more in patients with CVE than in controls (Table 2).
Table 2.
Change in Glucose Control and Risk Factors
| Cases With CVE | Controls Without CVE | Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow‐Up | Change | P Value | Baseline | Follow‐Up | Change | P Value | P Value | |
| Body weight, kg | 83.6±16.7 | 84.5±33.9 | 0.9±29.9 | 0.473 | 81.8±16.1 | 81.6±16.4 | −0.3±5.6 | 0.289 | 0.397 |
| SBP, mm Hg | 139.7±19.8 | 138.2±19.7 | −1.5±22.8 | 0.131 | 140.8±20.6 | 142.1±21.0 | 1.3±21.1 | 0.181 | 0.046 |
| DBP, mm Hg | 77.4±23.7 | 75.3±10.3 | −2.1±23.5 | 0.032 | 77.1±10.7 | 77.1±11.1 | 0.0±11.7 | 0.953 | 0.072 |
| HbA1c, % | 7.7±1.3 | 7.6±1.4 | −0.1±1.2 | 0.094 | 7.7±1.4 | 7.5±1.2 | −0.2±1.4 | 0.002 | 0.204 |
| Total cholesterol, mg/dL | 163.8±40.3 | 156.3±36.6 | −7.5±40.4 | <0.001 | 172.5±38.3 | 169.8±39.0 | −2.7±31.5 | 0.089 | 0.045 |
| HDL cholesterol, mg/dL | 45.5±13.0 | 45.5±12.4 | 0.0±8.7 | 0.946 | 48.1±13.7 | 48.2±14.3 | 0.1±10.8 | 0.914 | 0.958 |
| LDL cholesterol, mg/dL | 87.1±34.4 | 81.6±31.4 | −5.5±34.6 | 0.000 | 94.8±33.5 | 93.9±32.7 | −0.9±27.3 | 0.505 | 0.030 |
| Triglycerides, mg/dL | 156.1±109.8 | 146.3±89.5 | −9.8±91.3 | 0.012 | 146.5±85.0 | 136.6±81.0 | −9.9±70.7 | 0.005 | 0.980 |
| eGFR, mL/min per 1.73 m2 | 69.2±21.9 | 67.3±22.2 | −1.9±11.6 | <0.001 | 70.0±20.9 | 68.5±20.4 | −1.4±11.9 | 0.009 | 0.574 |
| UACR, mg/g | 189.5±630.7 | 198.4±658.4 | 8.9±440.2 | 0.743 | 146.4±615.5 | 149.5±554.4 | 3.0±211.7 | 0.813 | 0.840 |
P values are shown for each intragroup comparisons and for between‐group comparisons. CVE, cardiovascular event; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure; UACR, urinary albumin/creatinine ratio.
Sensitivity Analysis
After excluding 66 CVE cases not having a match among controls without CVE, the database was composed of 497 patients/group. The overall balance between groups did not improve as compared with the primary analysis (Figure 2). The changes in the prescription of GLMs and of other therapies were essentially superimposable to those in the primary analysis (Figure 1C and 1D).
Figure 2.
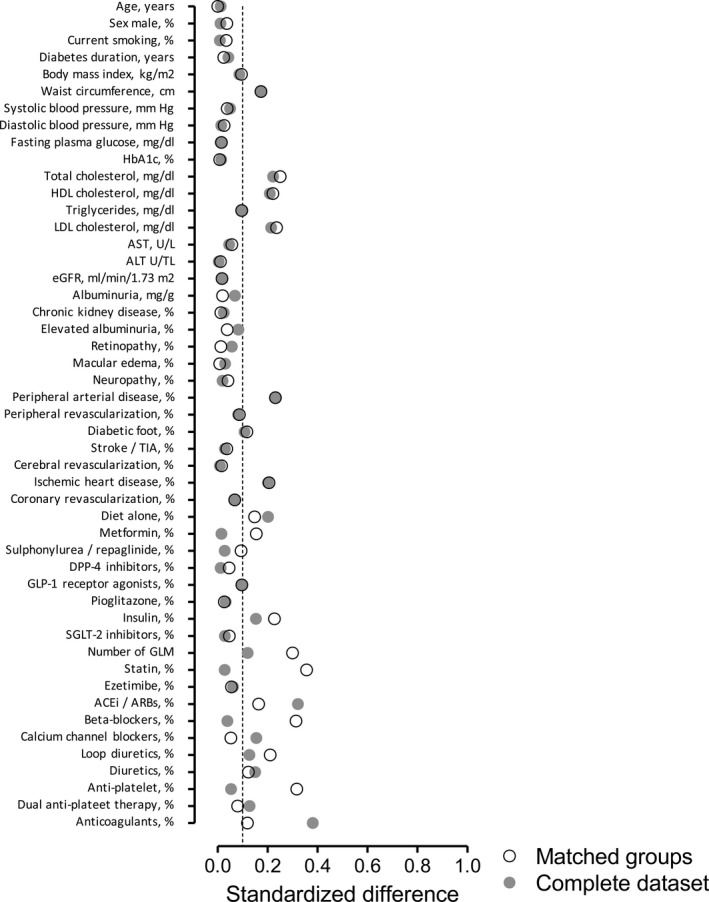
Balance of clinical characteristics between the 2 groups. Absolute standardized mean difference (STD) is presented for each variable in the comparison between the 2 groups for the entire data set (gray) and for matched groups (transparent), separately. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; GLM, glucose‐lowering medication; GLP‐1, glucagon‐like peptide 1; LDL, low‐density lipoprotein; SGLT2, sodium glucose cotransporter 2.
Discussion
In this analysis of therapeutic appropriateness in the real world, we found that occurrence of a CVE in patients with T2DM prompted marginal changes in the prescription of GLMs, with no increase in drugs provided with protective cardiovascular effects and no decrease in drugs with a high risk of hypoglycemia. Rather, there was a significant change in the prescription pattern of typical cardiovascular medications, suggestive of a more intensive control of risk factors. Consistently, patients with CVE showed an improvement in blood pressure and lipid profile but no reduction in HbA1c, which remained suboptimal.
Positive results of CVOTs published since 2015 are now being incorporated into guidelines1 and medicine labels. With some very recent exceptions, such studies have been conducted mostly in T2DM patients with established CVD, and there is consensus that results cannot be automatically extrapolated to patients without CVD.26 Therefore, one would expect prescriptions of SGLT2i and GLP‐1RA to increase in patients with T2DM and CVD, for whom a clear benefit in the prevention of CVE has been shown. We designed the DATAFILE study to evaluate whether and to what extent occurrence of a CVE drove modifications in the prescription of drugs for the treatment of T2DM. The study was performed at diabetes mellitus specialist clinics, as general practitioners were not allowed to initiate prescription of DPP‐4i, GLP‐1RA, and SGLT2i in Italy during the study period. Extracting data from the databases of diabetes mellitus outpatient clinics assured that prescriptions were made or at least validated by diabetologists, and were not simply those made by the specialist(s) who cared for the patients during the CVE. Notably, we observed minimal changes in GLM prescriptions and none going in the direction of improved appropriateness. The significant deprescription of metformin may be attributable to the fact that some patients developed contraindications to metformin, such as renal impairment or respiratory failure during or after the CVE.27, 28 This is suggested by the eGFR reduction observed in patients no longer prescribed metformin, although there was no patient with an eGFR decline below 30 mL/min per 1.73 m2. Because a wide range of observational data suggest that metformin can be safely used in patients with CVD and with CKD up to an eGFR of 30 mL/min per 1.73 m2,29, 30, 31 metformin deprescription seems to reflect an inappropriate lack of confidence in the safety of this drug.
Internal validity of the analysis was demonstrated by the increased prescription of several cardiovascular medications in patients after a CVE, which was expected. When the analysis was restricted to the period when SGLT2i were available and CVOTs on SGLT2i and GLP‐1RA were being published, still no increase in cardioprotective GLMs was observed. These results suggest that, while intensive control of risk factors is pursued in clinical practice, optimization of the GLM regimen is an unmet need in patients with T2DM after a CVE in a diabetes mellitus specialist care setting.
Some limitations of this study are intrinsic to its retrospective nature. There was no prospective data recording, the electronic chart was not linked to administrative data, and CVEs were recorded as reported by physicians. Thus, an eventual underreporting would imply that not all patients with incident CVEs were identified (eg, for peripheral revascularization, having the highest percentage of absence) and that some controls without a recorded CVE may actually have experienced an event. This may have caused an underestimation of the treatment difference between the 2 groups. Yet, even within the group of patients with a reported CVE, changes in GLM prescription were marginal, suggesting that underreporting of CVEs was not a major cause of the negative results of our analysis. Nonetheless, as typically occurring in retrospective studies on data routinely collected for clinical purposes, there was a substantial absence of data for other important variables. Although it was impossible to evaluate whether patients actually took the prescribed medications, the study focused on diabetologists’ prescribing attitudes, thereby being unrelated to information on drug dispensation and compliance. Finally, we collected data only at 3 to 12 months after the event, and long‐term information was not available. This is important because changes in medications for the control of risk factors (eg, LDL cholesterol) may be prioritized over changes in GLMs soon after an acute CVE. Furthermore, it should be mentioned that only 2 CVOTs enrolled patients soon (3–6 months) after an acute event, and they showed no cardiovascular protection by the GLP‐1RA lixisenatide32 and the DPP‐4i alogliptin,11 thereby making the urgency of changing the GLM prescription questionable. Nonetheless, therapeutic inertia has been clearly documented to worsen outcomes of patients with diabetes mellitus,33, 34 which is important, especially in view of the fact that HbA1c remained high.
The reasons why HbA1c did not decline in the CVE group are likely manifold and possibly include the choice of less ambitious HbA1c targets because of (1) concomitant use of drugs with a high hypoglycemia risk (sulfonylureas and insulin), (2) comorbidities (mainly chronic kidney disease and CVD), and (3) patients being considered more fragile after a CVE. Altogether, a cautious approach in the treatment of these patients emerges along with a limited awareness of the beneficial cardiovascular effects of SGLT2i and GLP1‐RA. It should be noted that some reimbursement restrictions imposed on GLP‐1RA and SGLT2i during the study period may have prevented the prescription of these GLMs to patients who could have benefited from protection against a CVE and death.
The study also has remarkable strengths: Patients were well characterized in their clinical profile, with detailed clinical information that is not available in registries or administrative databases. In addition, the match between cases and controls was good, with only variables directly related to CVE being unbalanced at baseline.
The present study advocates a call to action for stakeholders involved in diabetes mellitus care. Results of CVOTs available since 2015 should drive a paradigm shift in the treatment of T2DM, with more focus on cardiovascular prevention. We acknowledge that such a paradigm shift, bestowed by national and international guidelines, is still under way, and we will resurvey the situation in the future to monitor this unmet need.
Author Contributions
Study design: Fadini, Perseghin, Bonora, Avogaro. Data collection and analysis: Fadini, Frison, Lapolla, Gatti, Bossi, Del Buono, Fornengo, Gottardo, Laudato, Perseghin, and Bonora. Manuscript writing: Fadini, Avogaro. Manuscript revision: Frison, Simioni, Lapolla, Gatti, Bossi, Del Buono, Fornengo, Gottardo, Laudato, Perseghin, and Bonora. All authors approved the final version of the manuscript.
Sources of Funding
The study was promoted and supported by the Italian Diabetes Society.
Disclosures
Dr Fadini received grant support and lecture or advisory board fees from AstraZeneca, Boehringer‐Ingelheim, Eli Lilly, NovoNordisk, Sanofi, Genzyme, Abbott, Novartis, and Merck Sharp & Dohme. Dr Frison served as consultant for NovoNordisk. Dr Simioni received lecture or consultancy fees from Astra‐Zeneca, Boehringer‐Lilly, Novartis, NovoNordisk, Sanofi‐Aventis, Takeda, Merck Sharp & Dohme, and Abbott and research support from NovoNordisk. Dr Lapolla received grant support and lectures or advisory board fees from Novo Nordisk, Sanofi, Abbott, and Eli Lilly. Dr Bossi received grants or personal fees from Lilly, NovoNordisk, Johnson & Johnson, Boehringer‐Ingelheim, Artsana, Takeda, Bayer, Sanofi, and AstraZeneca. Dr Fornengo received lecture or advisory board fees from Astra‐Zeneca, Boehringher‐Ingelheim, Lilly, Sanofi, NovoNordisk, Takeda, and Merck Sharp & Dohme. Dr Perseghin received grant support and lecture or advisory board fees from Abbott, AstraZeneca, Boehringer‐Ingelheim, Eli Lilly, Janssen, Menarini Diagnostics, Merck Sharp & Dohme, NovoNordisk, Sanofi, Servier, and Takeda. Dr Bonora has served as an advisory board member for Abbott, AstraZeneca, Boehringer‐Ingelheim, Bristol‐Myers‐Squibb, Bruno Farmaceutici, Janssen, Johnson & Johnson, Lilly, Merck Sharp & Dohme, Mundipharma, Novartis, NovoNordisk, Roche, Sanofi, Servier, and Takeda, and received research grants from AstraZeneca, Genzyme, Menarini Diagnostics, NovoNordisk, Roche, and Takeda. Dr Avogaro received research grants and lecture or advisory board fees from Merck Sharp & Dome, AstraZeneca, Novartis, Boeringher‐Ingelheim, Sanofi, Mediolanum, Janssen, and NovoNordisk. The remaining authors have no disclosures to report.
Acknowledgments
We thank Alessia Russo for the invaluable technical support.
(J Am Heart Assoc. 2019;8:e012244 DOI: 10.1161/JAHA.119.012244.)
References
- 1. Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. 9. Cardiovascular disease and risk management: standards of medical care in diabetes—2018. Diabetes Care. 2018;41:S86–S104. [DOI] [PubMed] [Google Scholar]
- 3. Loke YK, Kwok CS, Singh S. Comparative cardiovascular effects of thiazolidinediones: systematic review and meta‐analysis of observational studies. BMJ. 2011;342:d1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fadini GP, Avogaro A. Cardiovascular effects of DPP‐4 inhibition: beyond GLP‐1. Vascul Pharmacol. 2011;55:10–16. [DOI] [PubMed] [Google Scholar]
- 5. Ussher JR, Drucker DJ. Cardiovascular actions of incretin‐based therapies. Circ Res. 2014;114:1788–1803. [DOI] [PubMed] [Google Scholar]
- 6. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495–502. [DOI] [PubMed] [Google Scholar]
- 7. Phung OJ, Schwartzman E, Allen RW, Engel SS, Rajpathak SN. Sulphonylureas and risk of cardiovascular disease: systematic review and meta‐analysis. Diabet Med. 2013;30:1160–1171. [DOI] [PubMed] [Google Scholar]
- 8. Tzoulaki I, Molokhia M, Curcin V, Little MP, Millett CJ, Ng A, Hughes RI, Khunti K, Wilkins MR, Majeed A, Elliott P. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ. 2009;339:b4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, Alexander JH, Pencina M, Toto RD, Wanner C, Zinman B, Woerle HJ, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT, von Eynatten M, McGuire DK. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. [DOI] [PubMed] [Google Scholar]
- 11. White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. [DOI] [PubMed] [Google Scholar]
- 12. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. [DOI] [PubMed] [Google Scholar]
- 13. Zannad F, Rossignol P. Dipeptidyl peptidase‐4 inhibitors and the risk of HF: regression to the truth? Circulation. 2019;139:362–365. [DOI] [PubMed] [Google Scholar]
- 14. Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta‐analysis of randomized trials. JAMA. 2007;298:1180–1188. [DOI] [PubMed] [Google Scholar]
- 15. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 16. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 17. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 18. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Ohman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marso SP, Daniels GH, Brown‐Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, Pagidipati NJ, Chan JC, Gustavson SM, Iqbal N, Maggioni AP, Ohman P, Poulter NR, Ramachandran A, Zinman B, Hernandez AF, Holman RR. Cardiovascular outcomes with glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a meta‐analysis. Lancet Diabetes Endocrinol. 2018;6:105–113. [DOI] [PubMed] [Google Scholar]
- 21. Zinman B, Marso SP, Christiansen E, Calanna S, Rasmussen S, Buse JB. Hypoglycemia, cardiovascular outcomes, and death: the leader experience. Diabetes Care. 2018;41:1783–1791. [DOI] [PubMed] [Google Scholar]
- 22. Standl E, Stevens SR, Armstrong PW, Buse JB, Chan JCN, Green JB, Lachin JM, Scheen A, Travert F, Van de Werf F, Peterson ED, Holman RR. Increased risk of severe hypoglycemic events before and after cardiovascular outcomes in TECOS suggests an at‐risk type 2 diabetes frail patient phenotype. Diabetes Care. 2018;41:596–603. [DOI] [PubMed] [Google Scholar]
- 23. Pieber TR, Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pratley RE, Woo V, Heller S, Lange M, Brown‐Frandsen K, Moses A, Barner Lekdorf J, Lehmann L, Kvist K, Buse JB. DEVOTE 3: temporal relationships between severe hypoglycaemia, cardiovascular outcomes and mortality. Diabetologia. 2018;61:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Warnick GR, Knopp RH, Fitzpatrick V, Branson L. Estimating low‐density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin Chem. 1990;36:15–19. [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Avogaro A, Fadini GP, Sesti G, Bonora E, Del Prato S. Continued efforts to translate diabetes cardiovascular outcome trials into clinical practice. Cardiovasc Diabetol. 2016;15:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lipska KJ. Metformin use in patients with historical contraindications. Ann Intern Med. 2017;166:225–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crowley MJ, Diamantidis CJ, McDuffie JR, Cameron CB, Stanifer JW, Mock CK, Wang X, Tang S, Nagi A, Kosinski AS, Williams JW Jr. Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic review. Ann Intern Med. 2017;166:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ekstrom N, Schioler L, Svensson AM, Eeg‐Olofsson K, Miao Jonasson J, Zethelius B, Cederholm J, Eliasson B, Gudbjornsdottir S. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open. 2012;2:e001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lalau JD, Kajbaf F, Bennis Y, Hurtel‐Lemaire AS, Belpaire F, De Broe ME. Metformin treatment in patients with type 2 diabetes and chronic kidney disease stages 3A, 3B, or 4. Diabetes Care. 2018;41:547–553. [DOI] [PubMed] [Google Scholar]
- 31. Lazarus B, Wu A, Shin JI, Sang Y, Alexander GC, Secora A, Inker LA, Coresh J, Chang AR, Grams ME. Association of metformin use with risk of lactic acidosis across the range of kidney function: a community‐based cohort study. JAMA Intern Med. 2018;178:903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257. [DOI] [PubMed] [Google Scholar]
- 33. Khunti K, Kosiborod M, Ray KK. Legacy benefits of blood glucose, blood pressure and lipid control in individuals with diabetes and cardiovascular disease: time to overcome multifactorial therapeutic inertia? Diabetes Obes Metab. 2018;20:1337–1341. [DOI] [PubMed] [Google Scholar]
- 34. Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]


