Abstract
Background
Preparticipation cardiovascular screening in athletes is fully endorsed by major medical societies, yet the most effective screening protocol remains debated. We prospectively compared the performance of the American Heart Association (AHA) 14‐point screening evaluation and a resting ECG for cardiovascular screening of high school athletes.
Methods and Results
Competitive athletes participating in organized high school or premier/select level sports underwent cardiovascular screening using the AHA 14‐point history and physical examination, and an ECG interpreted with the Seattle Criteria. A limited echocardiogram was performed for all screening abnormalities. The primary outcome measure was identification of a cardiovascular disorder associated with sudden cardiac death. From October 2014 to June 2017, 3620 high school athletes (median age, 16 years; range 13–19; 46.2% female; 78.6% white, 8.0% black) were screened. One or more positive responses to the AHA 14‐point questionnaire were present in 814 (22.5%) athletes. The most common history responses included chest pain (8.1%), family history of inheritable conditions (7.3%), and shortness of breath (6.4%). Abnormal physical examination was present in 356 (9.8%) athletes, and 103 (2.8%) athletes had an abnormal ECG. Sixteen (0.4%) athletes had conditions associated with sudden cardiac death. The sensitivity (18.8%), specificity (68.0%), and positive predictive value (0.3%) of the AHA 14‐point evaluation was substantially lower than the sensitivity (87.5%), specificity (97.5%), and positive predictive value (13.6%) of ECG.
Conclusions
The AHA 14‐point evaluation performs poorly compared with ECG for cardiovascular screening of high school athletes. The use of consensus‐derived history questionnaires as the primary tool for cardiovascular screening in athletes should be reevaluated.
Keywords: athlete, ECG, preparticipation, screening, sudden cardiac death
Subject Categories: Sudden Cardiac Death, Secondary Prevention, Cardiomyopathy, Electrocardiology (ECG)
Short abstract
See Editorial Maron et al
Clinical Perspective
What Is New?
The American Heart Association 14‐point evaluation for cardiovascular screening in athletes produces a high number of false‐positive results with a poor sensitivity and low positive predictive value.
ECG screening outperforms the American Heart Association 14‐point by all measures of statistical performance when interpreted by experienced clinicians.
What Are the Clinical Implications?
Cardiovascular screening using only the American Heart Association 14‐point evaluation will miss the majority of athletes with conditions at risk of sudden cardiac death.
Recommendations for the routine use of the American Heart Association 14‐point evaluation, or similar history‐based questionnaires, as the principal tool for preparticipation cardiovascular screening of young athletes should be reevaluated.
Introduction
Sudden cardiac death (SCD) is the leading cause of death in young athletes during sports.1, 2 Preparticipation cardiovascular screening aimed at the detection of disorders associated with SCD is universally supported by major medical societies; however, the best method for cardiovascular screening of young athletes remains controversial.3, 4, 5, 6, 7 At the center of the debate is the addition of a resting ECG to the traditional history and physical examination (H&P). Since 1996, the American Heart Association (AHA) has recommended a comprehensive personal and family H&P as the primary screening protocol and has opposed routine use of ECG during the preparticipation assessment.8, 9, 10 In contrast, a 2005 consensus guideline by the European Society of Cardiology recommended the systematic use of ECG in the cardiovascular screening of athletes.11
Cardiovascular screening by H&P or by ECG presents unique challenges and limitations. Several studies have documented the low sensitivity and high positive response rate of preparticipation history questionnaires.12, 13, 14, 15, 16 Likewise, concerns for inaccurate ECG interpretation, high false‐positive rates, and unnecessary testing or restriction from sports have been raised regarding ECG screening.10, 17, 18 In an attempt to improve the sensitivity of the H&P, the AHA in 2014 expanded its 12‐point evaluation by including 2 additional history questions.10 To date, no study has evaluated the performance of the AHA 14‐point evaluation for the cardiovascular screening of athletes. The purpose of this study was to examine the performance of the AHA 14‐point evaluation compared with an ECG for the cardiovascular screening of high school student athletes.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
This prospective study investigated high school student athletes undergoing voluntary cardiovascular screening. Student athletes participating in competitive sports or extracurricular physical activities at the high school or premier/select level were included in the study. A competitive athlete was defined as a young person participating in organized sports or extracurricular physical activities requiring regular practice and competitions. The extracurricular physical activities required a high level of physical conditioning such as dance, martial arts, and participation in the Reserve Officers’ Training Corps. Screenings were conducted by the Nick of Time Foundation (Mill Creek, Washington; https://nickoftimefoundation.org) in collaboration with the UW Medicine Center for Sports Cardiology at Seattle‐area high schools from October 2014 to June 2017. Student nonathletes and student athletes with preexisting cardiac conditions were excluded from the analysis (Figure).
Figure 1.
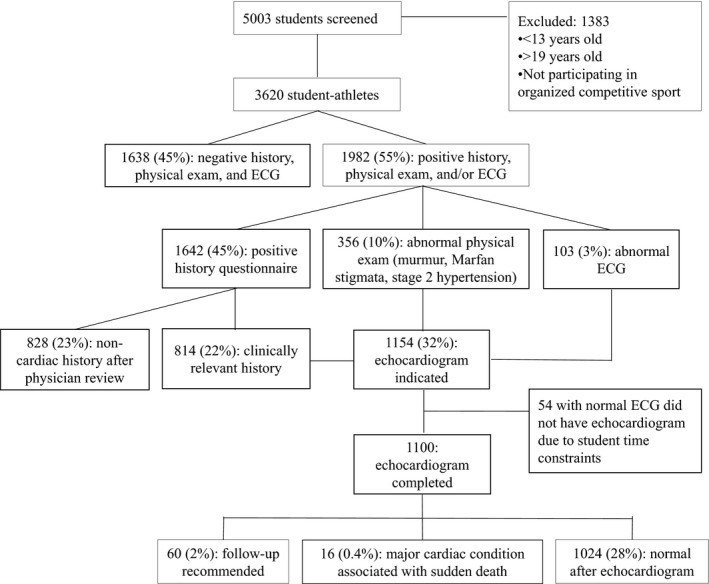
Flow diagram of study population.
Self‐reported demographic information, sports participation data, medications, and past medical history were collected via survey. The cardiovascular screening protocol for all subjects consisted of a personal and family history questionnaire per the AHA 14‐point recommendations; measurement of blood pressure, height, and weight; cardiac auscultation while standing, supine, and supine with Valsalva; examination for physical stigmata of Marfan syndrome; and a resting 12‐lead ECG. History questionnaires were provided to families before the screen, and parents and student athletes were encouraged to complete the questionnaire together. Physical examinations were performed by physicians and advance practice providers trained in primary care, sports medicine, and cardiology. Blood pressure measurements were repeated if the initial measurement was abnormal. ECGs were performed by medically trained volunteers using a standard 12‐lead placement and a portable ECG machine (CardeaScreen, Cardiac Insight Inc., Bellevue, WA). Each ECG was overread by a sports medicine physician, cardiologist, or electrophysiologist experienced in ECG interpretation in athletes using the Seattle Criteria.19
The questionnaires, physical examination, and ECG were reviewed with the student athlete by a sports medicine or cardiology physician. Each positive history response was further detailed with additional questions guided by the interviewing physician. All subjects with a screening abnormality on history, physical examination, and/or ECG were referred for a limited on‐site echocardiogram. Indications for an echocardiogram included clinically relevant history responses that could not be confidently classified as noncardiac in nature after physician review; any cardiac murmur regardless of quality; physical stigmata suggestive of Marfan syndrome; a systolic blood pressure ≥160 mm Hg or diastolic blood pressure ≥100 mm Hg on repeat measurement; and/or an abnormal ECG.
Echocardiograms were performed by licensed cardiac sonographers using portable ultrasound systems (Sonosite Edge II, Fujifilm SonoSite Inc., Bothell, WA). Pediatric and adult cardiologists familiar with athletic cardiac remodeling and disorders associated with SCD in young athletes supervised all image acquisition and interpretation. The limited echocardiogram protocol consisted of parasternal long axis and short axis and apical 4‐chamber views. Quantitative assessments included the end‐diastolic left ventricular chamber and wall thickness dimensions, fractional shortening, aortic diameters at the sinuses of Valsalva and the ascending aorta, and tricuspid regurgitant jet velocities for pulmonary artery pressure assessment using spectral Doppler. Valve function was assessed qualitatively using 2‐dimensional imaging and color Doppler, and attempts were made to identify the location of the right and left coronary artery ostia and left main bifurcation. The right ventricle was evaluated from parasternal and apical views as well as subcostal views if needed, with subjective assessment of size and function. Quantitative assessment of the right ventricle, including basal diameter and tricuspid annular plane systolic excursion, was performed when right ventricular abnormalities were suspected by ECG or initial echocardiographic images.
Statistical Analysis
The primary outcome measure was the identification of a cardiovascular disorder associated with SCD. Descriptive statistics such as proportions, means, and cross tabulations were used to analyze collected data. Sensitivity, specificity, positive and negative predictive values, and overall accuracy of the AHA 14‐point evaluation and ECG were calculated including 95% CIs. Findings on H&P or ECG not considered clinically relevant to the final diagnosis were not included in the statistical performance calculations.
This research involved the use of nonidentifiable data provided by the Nick of Time Foundation. Written informed consent, including the use of nonidentifiable data for research purposes, is required by the Nick of Time Foundation to participate in the screening program. Participants under 18 years of age must provide signed parental consent and participant assent forms. Each participant is assigned a unique identification number at the screening, and all data collected at the screening event are deidentified. The Nick of Time Foundation contacts families of students with abnormal findings referred for further cardiology evaluation to confirm the final diagnosis. The Nick of Time Foundation releases deidentified, coded data to University of Washington investigators for research purposes. The identity of the screening participants is confidential and available only to the Nick of Time Foundation. Deidentified data are maintained in a secure Research Electronic Data Capture database maintained by the Biomedical Informatics core of the University of Washington Institute for Translational Health Sciences. A Human Subjects Division review determination form for “Use of Nonidentifiable Specimen/Data” was completed and approved by regulatory advisors from the Institute for Translational Health Sciences. All authors had full access to all the data in the study and take responsibility for its integrity and the data analysis.
Results
Demographics
A total of 5003 high school students aged 13 to 19 years (median age, 16 years) were screened during the study period, including 3620 competitive student athletes included in this analysis (Figure). Of the student athletes, 46.2% were female, 78.6% white, 16.3% Asian/Pacific Islander, and 8.0% black (Table 1). Predominant sports included basketball (22.5%), soccer (22.3%), and track and field (20.1%).
Table 1.
Demographics of Study Population
| Student athletes | 3620 |
| Mean age, y (range) | 16 (13–19) |
| Sex (%)a | |
| Male | 1928 (53.3) |
| Female | 1673 (46.2) |
| Race/Ethnicity (%)b | |
| White | 2845 (78.6) |
| Black | 291 (8) |
| Asian/Pacific Islander | 590 (16.3) |
| Hispanic/Latino | 285 (7.9) |
| Other | 186 (5.3) |
| Sports (%)c | |
| Baseball | 348 (9.6) |
| Basketball | 816 (22.5) |
| Cross country | 442 (12.2) |
| Football | 590 (16.3) |
| Lacrosse | 228 (6.3) |
| Other | 456 (12.6) |
| Soccer | 809 (22.3) |
| Swimming/diving | 335 (9.3) |
| Tennis | 309 (8.5) |
| Track/field | 729 (20.1) |
| Volleyball | 289 (8.0) |
Sex unmarked for 19 student athletes.
Multiple student athletes listed >1 race/ethnicity.
Multiple student athletes participated in >1 sport.
AHA 14‐Point Questionnaire
Of 3620 student athletes, 1642 (45.4%) marked at least 1 positive history response on the questionnaire. After further review with a physician, 828 (50.4% of the positive history questionnaires) were deemed not clinically concerning for cardiac disease. The remaining 814 (22.5%) student athletes were considered to have a positive history questionnaire warranting further evaluation. Chest pain/discomfort/tightness/pressure related to exertion, excessive and unexplained shortness of breath/fatigue or palpitations associated with exercise, and a family history of inheritable cardiac conditions represented the most common positive history responses (Table 2).
Table 2.
Positive Response Before and After Physician Review to the American Heart Association 14‐Point History Questions
| Total Positive Responses Before Physician Review | Total Positive Responses After Physician Review | |
|---|---|---|
| Do you get chest pain/discomfort/tightness/pressure related to exertion? | 440 (12.2%) | 293 (8.1%) |
| Have you had unexplained syncope (passing out) or near‐syncope (nearly passing out)? | 320 (8.8%) | 202 (5.6%) |
| Do you get excessive and unexplained shortness of breath/fatigue or palpitations associated with exercise? | 346 (9.6%) | 233 (6.4%) |
| Have you been told you have a heart murmur? | 158 (4.4%) | 92 (2.5%) |
| Have you been told you have elevated blood pressure? | 86 (2.4%) | 36 (1.0%) |
| Have you been previously restricted from participation in sports? | 296 (8.2%) | 111 (3.1%) |
| Have you had prior testing for the heart, ordered by a physician? | 256 (7.1%) | 145 (4.0%) |
| Has one or more relatives had premature death (sudden and unexpected, or otherwise) before 50 years of age attributable to heart disease? | 238 (6.6%) | 160 (4.4%) |
| Has a close relative <50 years of age had disability from heart disease? | 308 (8.5%) | 185 (5.1%) |
| Does a family member have any of these heart conditions: hypertrophic or dilated cardiomyopathy, long‐QT syndrome, or other ion channelopathies, Marfan syndrome, or clinically significant arrhythmias; specific knowledge of genetic cardiac conditions in family members? | 485 (13.4%) | 266 (7.3%) |
| One or more positive history responses | 1642 (45.4%) | 814 (22.5%) |
Physical Examination
A total of 356 (9.8%) subjects had an abnormal physical examination. Abnormal findings included 280 (7.7%) athletes with a cardiac murmur, 35 (1.0%) with features possibly suggestive of Marfan syndrome, and 56 (1.5%) with an elevated blood pressure (≥160/100 mm Hg).
Electrocardiogram
Of 3620 ECGs, 103 (2.8%) were abnormal. The most common ECG abnormalities included pathologic Q‐waves, T‐wave inversion, premature ventricular contractions, and ventricular preexcitation/Wolff‐Parkinson‐White pattern (Table 3).
Table 3.
Electrocardiographic Abnormalities
| Normal ECG | 3517 (97.2%) |
| Abnormal ECG | 103 (2.8%) |
| T‐wave inversion | 20 |
| ST‐segment depression | 5 |
| Pathologic Q‐waves | 25 |
| Complete RBBB | 2 |
| Left atrial enlargement | 4 |
| Left axis deviation | 9 |
| Right atrial enlargement | 1 |
| Right ventricular hypertrophy | 2 |
| Ventricular preexcitation/WPW | 9 |
| Prolonged QTca | 7 |
| Ventricular arrhythmiab | 1 |
| Premature ventricular contractions | 11 |
| Sinus tachycardia ≥120 bpm | 2 |
| Otherc | 4 |
RBBB indicates right bundle branch block; WPW, Wolff‐Parkinson‐White.
Three with QTc ≥500 ms; 4 with QTc ≥470 ms (male) or ≥480 ms (female).
Bigeminy.
One each: ectopic beats, borderline Q‐waves, borderline T‐wave inversion, prolonged S‐wave upstroke in V2 and V3.
Echocardiogram
A limited echocardiogram was indicated for 1154 subjects. Fifty‐four subjects could not complete their echocardiogram on‐site because of student time constraints and were referred to their primary care physician for further evaluation. Of these 54 subjects, all had a normal ECG, 36 had a positive history questionnaire, 21 had an abnormal physical examination (3 with cardiac murmurs and 18 with elevated blood pressure), and 3 had both an abnormal H&P. All subjects with an abnormal ECG had an echocardiogram (Figure).
A total of 1100 echocardiograms were performed on‐site for a screening abnormality: 778 (70.7%) echocardiograms were performed for evaluation of a positive history questionnaire, 103 (9.4%) for an abnormal ECG, and 335 (30.5%) for an abnormal physical examination (277 for cardiac murmurs, 35 for Marfan stigmata, and 38 for elevated blood pressure). Of the 1100 echocardiograms performed, 979 had 1 indication, 110 had 2 indications, and 6 had 3 indications.
Major Cardiac Conditions
Sixteen (0.4%) athletes were identified with cardiac conditions associated with SCD (Table 4). Identified conditions included 9 athletes with Wolff‐Parkinson‐White, 3 with long QT syndrome, 2 with hypertrophic cardiomyopathy, 1 with a dilated aorta, and 1 with an anomalous origin of the right coronary artery. The AHA 14‐point evaluation was flagged as abnormal in 7 of 16 (43.8%) cases. The ECG was flagged as abnormal in 15 of 16 (93.8%) cases. No athlete was identified with a cardiac condition associated with SCD solely from an abnormal physical examination. If the AHA 14‐point evaluation was the only screening tool to trigger further cardiac testing, and provided all positive history questionnaires and all abnormal physical examination findings prompted additional testing with an ECG and echocardiogram, 9 of 16 (56.2%) cases would remain undetected including 3 Wolff‐Parkinson‐White, 3 long QT syndrome, 2 hypertrophic cardiomyopathy, and 1 dilated aorta. If ECG were the only screening tool to trigger further cardiac testing, only 1 of 16 (6.2%) cases would remain undetected (anomalous coronary artery). The statistical performance of the AHA 14‐point evaluation versus ECG is shown in Table 5.
Table 4.
Identified Cardiac Disorders Associated With Sudden Cardiac Death
| Cardiac Disorder | Age, Race, Sex, Sport | Sports Physical or Well‐Child Evaluation Within 12 Months | Positive Findings on History and Physical Examination | ECG Findings | Echocardiogram Findings |
|---|---|---|---|---|---|
| Anomalous coronary artery | 16 y/o black male; baseball, basketball, football, soccer | No |
History: SOB, h/o murmur, prior cardiac testing PE: murmur |
Normal | Anomalous origin of the right coronary artery from the left coronary cusp |
| Dilated aorta | 14 y/o white male; basketball, cross country | Yes | None | Pathologic Q‐wavesa | Ascending aorta 3.6 cm |
| HCM | 15 y/o white male; baseball, golf | Yes | PE: murmur | ST‐segment depression | IVSd thickness 2.0 cm |
| HCM | 15 y/o black male; baseball, basketball | No | PE: murmur | Ventricular preexcitation | IVSd thickness 1.7 cm |
| LQTS | 16 y/o Asian female; cross country, martial arts | Unknown | None | QTc 501 | Normal |
| LQTS | 16 y/o white female; dance | Unknown | None | QTc 556 | Normal |
| LQTS | 15 y/o white female; tennis, volleyball | Unknown | History: syncope | QTc 511 | Normal |
| WPW | 15 y/o white male; soccer | Yes |
History: FHx heart disease <50, genetic conditiona
PE: murmur |
Ventricular preexcitation | Normal |
| WPW | 17 y/o white male; ROTC | Yes | None | Ventricular preexcitation | Normal |
| WPW | 14 y/o Hispanic female; soccer | Unknown | History: CP, SOB, syncope | Ventricular pre‐excitation | Normal |
| WPW | 15 y/o white male; lacrosse, soccer | Unknown | None | Ventricular preexcitation | Normal |
| WPW | 16 y/o white male; baseball | Yes | None | Ventricular preexcitation | Normal |
| WPW | 15 y/o white male; soccer | Yes |
History: FHx heart disease <50, genetic conditiona
PE: murmur |
Ventricular preexcitation | Normal |
| WPW | 17 y/o white female; cross country, track/field | Unknown | History: syncope | Ventricular preexcitation | Normal |
| WPW | 16 y/o white female; golf | Yes | History: CP, SOB, prior cardiac testing, genetic condition | Ventricular preexcitation | Normal |
| WPW | 15 y/o white male; basketball, football, track/field | Yes | History: prior restriction from sport, FHx premature deatha | Ventricular preexcitation | Normal |
History: SOB=“excessive and unexplained shortness of breath/fatigue or palpitations associated with exercise”; CP=“chest pain/pressure/tightness/discomfort related to exertion”; syncope=“unexplained syncope (passing out) or near‐syncope (nearly passing out)”; FHx premature death=“one or more relatives had premature death (sudden and unexpected, or otherwise) before 50 years of age attributable to heart disease”; genetic condition=“family member [with] any of these heart conditions: hypertrophic or dilated cardiomyopathy, long‐QT syndrome, or other ion channelopathies, Marfan syndrome, or clinically significant arrhythmias; specific knowledge of genetic cardiac conditions in family members”; FHx heart disease <50=“close relative <50 years of age had disability from heart disease”. h/o indicates history of; HCM, hypertrophic cardiomyopathy; IVSd, interventricular septum thickness at end diastole; LQTS, long QT syndrome; PE, physical examination; ROTC, Reserve Officers’ Training Corps; WPW, Wolff‐Parkinson‐White; y/o, year old.
Findings on history and physical examination or on ECG not considered relevant to the diagnosis and therefore not included in the statistical performance calculations.
Table 5.
Statistical Performance of the AHA 14‐Point Evaluation and ECG
| AHA 14‐Point (95% CI) | ECG (95% CI) | |
|---|---|---|
| Sensitivity | 18.8% (4.1–45.7) | 87.5% (61.7–98.5) |
| Specificity | 75.1% (73.7–76.5) | 97.5% (97.0–98.0) |
| Positive predictive value | 0.3% (0.1–0.9) | 13.6% (10.7–17.2) |
| Negative predictive value | 99.5% (99.4–99.6) | 99.9% (96.9–98.0) |
| Accuracy | 74.9% (73.4–76.3) | 97.5% (96.9–97.9) |
AHA indicates American Heart Association.
Discussion
The purpose of cardiovascular screening as defined by the AHA is to “identify or raise suspicion of previously unrecognized and largely genetic or congenital cardiovascular diseases known to cause sudden cardiac arrest and sudden death in young people.”10 While limited outcomes‐based evidence is available from screening trials of young athletes, most experts believe that early detection of potentially lethal disorders in athletes can decrease cardiovascular morbidity and mortality through risk stratification, disease‐specific interventions, and/or exercise modifications.5, 20, 21, 22 Routine preparticipation cardiovascular screening by H&P has been endorsed by most major cardiology and primary care organizations in the United States for over 2 decades.4, 5, 7, 8, 9, 10 However, a growing body of evidence has revealed the limitations of screening history questionnaires used for the detection of cardiac conditions at risk for SCD.12, 13, 14, 15, 16
This is the first study to investigate the performance of the AHA 14‐point evaluation with a comparison to ECG screening. The data demonstrate that ECG screening, when performed by experienced clinicians, is superior to the AHA 14‐point evaluation for the detection of cardiovascular disorders at risk of SCD. The poor performance and high positive response rate of the AHA 14‐point evaluation is consistent with other studies examining history questionnaires employed for cardiovascular screening in young athletes. In a study of cardiovascular screening involving 1071 high school student athletes, 30% had at least 1 positive personal or family history response on the Pre‐participation Physical Evaluation Monograph (4th Edition).12 In a study of 2506 high school student athletes from Texas, 35.7% had a positive questionnaire based on the AHA 12‐point evaluation.14 In a study of 1596 high school, college, and professional athletes, 23.8% had at least 1 positive response to the AHA 12‐point personal and family history elements.13 Similarly, a multicenter study of 5258 college athletes found at least 1 positive cardiac symptom or family history response reported by 33.3% of athletes.16
The accuracy of a screening tool to detect the conditions of greatest concern without unnecessary additional testing for false‐positive results is an important criterion in the evaluation of screening modalities and a measure used previously to criticize ECG screening.9, 10 In a meta‐analysis of 15 studies and 47 137 athletes undergoing cardiovascular screening, the pooled sensitivity/specificity of a screening history, physical examination, and ECG was 20%/94%, 9%/97%, and 94%/93%, respectively.23 In 2 independent reports of cardiovascular screening in Division I college athletes, all 8 athletes identified with a disorder associated with SCD (hypertrophic cardiomyopathy, 3, long QT syndrome, 1, Wolff‐Parkinson‐White, 4) were detected by an abnormal screening ECG and would have been missed if screening with H&P alone.15, 24 While the AHA 14‐point recommendations attempted to improve the sensitivity of the H&P by expanding the language and number of history questions, it did not accomplish its intended goal and may have increased the chance of a false‐positive response.
The sensitivity of the AHA 14‐point evaluation in this study (18.8%), while low, is likely inflated compared with clinical practice, as every athlete with a positive history response may not receive additional testing with an ECG and echocardiogram as performed in this investigation. On the basis of positive predictive value (0.3%) of the AHA 14‐point evaluation, ≈300 athletes would need a positive H&P to identify 1 athlete with a cardiac condition at risk of SCD. The AHA estimated prevalence of cardiac conditions associated with SCD is also 0.3% (or 1 in 300 athletes),9, 10 suggesting that true‐positive findings may be random and that a positive H&P does not appear to identify a subgroup of athletes at higher risk of an underlying pathologic cardiac disorder. In contrast, this study suggests that 1 of every 7 athletes flagged with an abnormal ECG will have a condition associated with SCD.
In this study, ECG screening surpassed the AHA 14‐point evaluation in all statistical measures of performance. Importantly, the false‐positive rate for ECG, when interpreted by experienced clinicians using modern standards that distinguish physiologic cardiac adaptations from findings suggestive of cardiac pathology, was only 2.4%. The evolution and improved accuracy of ECG interpretation standards for athletes has readily improved specificity without compromising sensitivity.19, 25, 26, 27, 28, 29 The high number of false‐positive responses to the AHA 14‐point history questions creates a challenge for the examining clinician to determine which responses are clinically concerning and warrant additional investigation. In this study, experienced sports medicine and cardiology physicians determined that approximately half of the positive responses were noncardiac in nature. It is unclear in the broader clinical context how primary care providers react to these responses, if more information is gathered, if additional testing is ordered, or if a large number of positive history responses are simply ignored. Even if every athlete with a clinically relevant positive response undergoes an ECG as in this study, this still does not capture the majority of athletes with detectable conditions at risk for SCD.
Athletes may experience cardiovascular symptoms related to an underlying cardiac disorder.30 However, it is not evident that screening with a history questionnaire will effectively identify athletes with relevant warning signs or symptoms. In this study, the history questionnaire generated a large number of positive responses, with 45.4% of subjects marking at least 1 affirmative response and 22.5% having at least 1 positive response that could not be excluded as noncardiac after physician review. The most common history responses pertained to “chest pain/pressure/tightness/discomfort related to exertion,” “excessive and unexplained shortness of breath/fatigue or palpitations associated with exercise,” and a listing of genetic cardiac conditions. The wording of these symptom inquiries is broad and nonspecific and may overlap with common physiologic sensations during exercise. The listing of genetic cardiac conditions is likely unfamiliar to the athlete, and families may mistake common conditions for the genetic disorders listed. More research is needed to understand what questions, with what wording, with what prompts, and in what format (ie, written, video, or verbal) may better detect athletes of all ages with cardiovascular symptoms warranting more evaluation.
In 1996, the AHA screening guidelines acknowledged that “preparticipation screening by history and physical examination alone (without noninvasive testing) is not sufficient to guarantee detection of many critical cardiovascular abnormalities in large populations of young trained athletes.”8 Indeed, a 1996 review of 134 cases of SCD in competitive athletes found that only 18% of athletes who died of a cardiovascular condition had cardiovascular symptoms in the 3 years preceding death, and only 1 case (0.7%) was adequately detected by a preparticipation medical evaluation.31 As new data improves our understanding of cardiovascular screening tools in young athletes, it seems unjustified to continue emphasizing the use of a screening tool with poor performance, inconsistent application, and little chance of effectively identifying the majority of young athletes at risk of SCD. However, leaders of the AHA guidelines have recently advocated for legislation to mandate use of the AHA 14‐point during well child‐care evaluations, a position fully unsupported by science.32, 33
Acknowledging the limitations of preparticipation screening by H&P does not equate with a recommendation for universal ECG screening. It might, however, place a greater emphasis and additional resources toward research and education to advance preventive strategies, improve screening tools, and develop a larger physician workforce capable of more effective cardiovascular screening, especially in high‐risk athlete groups.
Limitations
This study involved ECG interpretation by physicians experienced in ECG screening and familiar with modern standards for ECG interpretation in athletes. Thus, ECG interpretation accuracy may not be reproducible in other settings or with clinicians with less experience. ECG interpretation was not blinded and occurred concurrently with review of the history and physical examination forms. While this is similar to clinical practice, it may have introduced potential bias. In addition, ECGs were interpreted using the Seattle Criteria before publication of the updated International Criteria for ECG interpretation in athletes, which may reduce the ECG false‐positive rate further.19, 27, 28, 29 Subjects with positive history responses or abnormalities on physical examination also underwent ECG screening and a limited echocardiogram. It is unlikely that this standard of additional testing is present in clinical practice, and therefore the reported sensitivity of the AHA 14‐point evaluation may be overestimated. In addition, subjects with a fully negative screen on history, physical examination, and ECG; subjects with a history response thought to be noncardiac in nature and a normal physical examination and ECG; and 54 subjects with an abnormal history or physical examination but normal ECG did not undergo an echocardiogram; thus, it is possible some structural disorders were missed. This study included mostly white high school student athletes and may not be applicable to other athlete populations, including college‐aged athletes or older. Larger studies are needed to examine racial differences in the performance of different assessment tools during preparticipation cardiovascular screening in athletes.
Conclusion
The AHA 14‐point evaluation produces a high number of false‐positive results with a poor sensitivity and very low positive predictive value. ECG screening outperforms the AHA 14‐point questionnaire by all measures of statistical performance when interpreted by experienced clinicians. Recommendations for the routine use of the AHA 14‐point evaluation or similar history‐based questionnaires as the principal tool for preparticipation cardiovascular screening of young athletes should be reevaluated.
Disclosures
Dr Drezner is the medical director, and Drs Pelto, Prutkin, Owens, Salerno, and Harmon are on the Medical Advisory Committee for the Nick of Time Foundation. The remaining authors have no disclosures to report.
Acknowledgments
The authors are grateful to the Nick of Time Foundation and its team of volunteers for making this research possible.
(J Am Heart Assoc. 2019;8:e012235 DOI: 10.1161/JAHA.119.012235.)
References
- 1. Harmon KG, Asif IM, Maleszewski JJ, Owens DS, Prutkin JM, Salerno JC, Zigman ML, Ellenbogen R, Rao AL, Ackerman MJ, Drezner JA. Incidence, cause, and comparative frequency of sudden cardiac death in National Collegiate Athletic Association athletes: a decade in review. Circulation. 2015;132:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation. 2009;119:1085–1092. [DOI] [PubMed] [Google Scholar]
- 3. Maron BJ, Friedman RA, Kligfield P, Levine BD, Viskin S, Chaitman BR, Okin PM, Saul JP, Salberg L, Van Hare GF, Soliman EZ, Chen J, Matherne GP, Bolling SF, Mitten MJ, Caplan A, Balady GJ, Thompson PD; American Heart Association Council on Clinical Cardiology Advocacy Coordinating Committee, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Surgery and Anesthesia, Council on Epidemiology and Prevention, Council on Functional Genomics and Translational Biology, Council on Quality of Care and Outcomes Research, and American College of Cardiology . Assessment of the 12‐lead ECG as a screening test for detection of cardiovascular disease in healthy general populations of young people (12‐25 years of age): a scientific statement from the American Heart Association and the American College of Cardiology. Circulation. 2014;130:1303–1334. [DOI] [PubMed] [Google Scholar]
- 4. American Academy of Family Physicians, American Academy of Pediatrics, American College of Sports Medicine, American Medical Society for Sports Medicine, American Orthopaedic Society for Sports Medicine and American Osteopathic Academy of Sports Medicine . Preparticipation Physical Evaluation. 4th ed Itasca, IL: American Academy of Pediatrics; 2010. [Google Scholar]
- 5. Drezner JA, O'Connor FG, Harmon KG, Fields KB, Asplund CA, Asif IM, Price DE, Dimeff RJ, Bernhardt DT, Roberts WO. AMSSM position statement on cardiovascular preparticipation screening in athletes: current evidence, knowledge gaps, recommendations and future directions. Br J Sports Med. 2017;51:153–167. [DOI] [PubMed] [Google Scholar]
- 6. Hainline B, Drezner JA, Baggish A, Harmon KG, Emery MS, Myerburg RJ, Sanchez E, Molossi S, Parsons JT, Thompson PD. Interassociation consensus statement on cardiovascular care of college student‐athletes. J Am Coll Cardiol. 2016;67:2981–2995. [DOI] [PubMed] [Google Scholar]
- 7. Maron BJ, Levine BD, Washington RL, Baggish AL, Kovacs RJ, Maron MS. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 2: preparticipation screening for cardiovascular disease in competitive athletes: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66:2356–2361. [DOI] [PubMed] [Google Scholar]
- 8. Maron BJ, Thompson PD, Puffer JC, McGrew CA, Strong WB, Douglas PS, Clark LT, Mitten MJ, Crawford MH, Atkins DL, Driscoll DJ, Epstein AE. Cardiovascular preparticipation screening of competitive athletes. A statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation. 1996;94:850–856. [DOI] [PubMed] [Google Scholar]
- 9. Maron BJ, Thompson PD, Ackerman MJ, Balady G, Berger S, Cohen D, Dimeff R, Douglas PS, Glover DW, Hutter AM Jr, Krauss MD, Maron MS, Mitten MJ, Roberts WO, Puffer JC. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2007;115:1643–1655. [DOI] [PubMed] [Google Scholar]
- 10. Maron BJ, Friedman RA, Kligfield P, Levine BD, Viskin S, Chaitman BR, Okin PM, Saul JP, Salberg L, Van Hare GF, Soliman EZ, Chen J, Matherne GP, Bolling SF, Mitten MJ, Caplan A, Balady GJ, Thompson PD. Assessment of the 12‐lead electrocardiogram as a screening test for detection of cardiovascular disease in healthy general populations of young people (12‐25 years of age): a scientific statement from the American Heart Association and the American College of Cardiology. J Am Coll Cardiol. 2014;64:1479–1514. [DOI] [PubMed] [Google Scholar]
- 11. Corrado D, Pelliccia A, Bjornstad HH, Vanhees L, Biffi A, Borjesson M, Panhuyzen‐Goedkoop N, Deligiannis A, Solberg E, Dugmore D, Mellwig KP, Assanelli D, Delise P, van‐Buuren F, Anastasakis A, Heidbuchel H, Hoffmann E, Fagard R, Priori SG, Basso C, Arbustini E, Blomstrom‐Lundqvist C, McKenna WJ, Thiene G. Cardiovascular pre‐participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:516–524. [DOI] [PubMed] [Google Scholar]
- 12. Fudge J, Harmon KG, Owens DS, Prutkin JM, Salerno JC, Asif IM, Haruta A, Pelto H, Rao AL, Toresdahl BG, Drezner JA. Cardiovascular screening in adolescents and young adults: a prospective study comparing the Pre‐participation Physical Evaluation Monograph 4th Edition and ECG. Br J Sports Med. 2014;48:1172–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dunn TP, Pickham D, Aggarwal S, Saini D, Kumar N, Wheeler MT, Perez M, Ashley E, Froelicher VF. Limitations of current AHA guidelines and proposal of new guidelines for the preparticipation examination of athletes. Clin J Sport Med. 2015;25:472–477. [DOI] [PubMed] [Google Scholar]
- 14. Zeltser I, Cannon B, Silvana L, Fenrich A, George J, Schleifer J, Garcia M, Barnes A, Rivenes S, Patt H, Rodgers G, Scott W. Lessons learned from preparticipation cardiovascular screening in a state funded program. Am J Cardiol. 2012;110:902–908. [DOI] [PubMed] [Google Scholar]
- 15. Drezner JA, Prutkin JM, Harmon KG, O'Kane JW, Pelto HF, Rao AL, Hassebrock JD, Petek BJ, Teteak C, Timonen M, Zigman M, Owens DS. Cardiovascular screening in college athletes. J Am Coll Cardiol. 2015;65:2353–2355. [DOI] [PubMed] [Google Scholar]
- 16. Drezner JA, Owens DS, Prutkin JM, Salerno JC, Harmon KG, Prosise S, Clark A, Asif IM. Electrocardiographic screening in National Collegiate Athletic Association athletes. Am J Cardiol. 2016;118:754–759. [DOI] [PubMed] [Google Scholar]
- 17. Chaitman BR. An electrocardiogram should not be included in routine preparticipation screening of young athletes. Circulation. 2007;116:2610–2614; discussion 2615. [DOI] [PubMed] [Google Scholar]
- 18. Thompson PD, Levine BD. Protecting athletes from sudden cardiac death. JAMA. 2006;296:1648–1650. [DOI] [PubMed] [Google Scholar]
- 19. Drezner JA, Ackerman MJ, Anderson J, Ashley E, Asplund CA, Baggish AL, Borjesson M, Cannon BC, Corrado D, DiFiori JP, Fischbach P, Froelicher V, Harmon KG, Heidbuchel H, Marek J, Owens DS, Paul S, Pelliccia A, Prutkin JM, Salerno JC, Schmied CM, Sharma S, Stein R, Vetter VL, Wilson MG. Electrocardiographic interpretation in athletes: the “Seattle criteria.” Br J Sports Med. 2013;47:122–124. [DOI] [PubMed] [Google Scholar]
- 20. Maron BJ, Zipes DP, Kovacs RJ. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: preamble, principles, and general considerations: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66:2343–2349. [DOI] [PubMed] [Google Scholar]
- 21. Malhotra A, Dhutia H, Finocchiaro G, Gati S, Beasley I, Clift P, Cowie C, Kenny A, Mayet J, Oxborough D, Patel K, Pieles G, Rakhit D, Ramsdale D, Shapiro L, Somauroo J, Stuart G, Varnava A, Walsh J, Yousef Z, Tome M, Papadakis M, Sharma S. Outcomes of cardiac screening in adolescent soccer players. N Engl J Med. 2018;379:524–534. [DOI] [PubMed] [Google Scholar]
- 22. Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. 2006;296:1593–1601. [DOI] [PubMed] [Google Scholar]
- 23. Harmon KG, Zigman M, Drezner JA. The effectiveness of screening history, physical exam, and ECG to detect potentially lethal cardiac disorders in athletes: a systematic review/meta‐analysis. J Electrocardiol. 2015;48:329–338. [DOI] [PubMed] [Google Scholar]
- 24. Fuller C, Scott C, Hug‐English C, Yang W, Pasternak A. Five‐year experience with screening electrocardiograms in National Collegiate Athletic Association division I athletes. Clin J Sport Med. 2016;26:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corrado D, Pelliccia A, Heidbuchel H, Sharma S, Link M, Basso C, Biffi A, Buja G, Delise P, Gussac I, Anastasakis A, Borjesson M, Bjornstad HH, Carre F, Deligiannis A, Dugmore D, Fagard R, Hoogsteen J, Mellwig KP, Panhuyzen‐Goedkoop N, Solberg E, Vanhees L, Drezner J, Estes NA III, Iliceto S, Maron BJ, Peidro R, Schwartz PJ, Stein R, Thiene G, Zeppilli P, McKenna WJ. Recommendations for interpretation of 12‐lead electrocardiogram in the athlete. Eur Heart J. 2010;31:243–259. [DOI] [PubMed] [Google Scholar]
- 26. Sheikh N, Papadakis M, Ghani S, Zaidi A, Gati S, Adami PE, Carre F, Schnell F, Wilson M, Avila P, McKenna W, Sharma S. Comparison of electrocardiographic criteria for the detection of cardiac abnormalities in elite black and white athletes. Circulation. 2014;129:1637–1649. [DOI] [PubMed] [Google Scholar]
- 27. Drezner JA, Sharma S, Baggish A, Papadakis M, Wilson MG, Prutkin JM, Gerche A, Ackerman MJ, Borjesson M, Salerno JC, Asif IM, Owens DS, Chung EH, Emery MS, Froelicher VF, Heidbuchel H, Adamuz C, Asplund CA, Cohen G, Harmon KG, Marek JC, Molossi S, Niebauer J, Pelto HF, Perez MV, Riding NR, Saarel T, Schmied CM, Shipon DM, Stein R, Vetter VL, Pelliccia A, Corrado D. International criteria for electrocardiographic interpretation in athletes: consensus statement. Br J Sports Med. 2017;51:704–731. [DOI] [PubMed] [Google Scholar]
- 28. Zorzi A, Calore C, Vio R, Pelliccia A, Corrado D. Accuracy of the ECG for differential diagnosis between hypertrophic cardiomyopathy and athlete's heart: comparison between the European Society of Cardiology (2010) and International (2017) criteria. Br J Sports Med. 2018;52:667–673. [DOI] [PubMed] [Google Scholar]
- 29. McClean G, Riding NR, Pieles G, Watt V, Adamuz C, Sharma S, George KP, Oxborough D, Wilson MG. Diagnostic accuracy and Bayesian analysis of new international ECG recommendations in paediatric athletes. Heart. 2019;105:152–159. [DOI] [PubMed] [Google Scholar]
- 30. Campbell RM, Berger S, Drezner J. Sudden cardiac arrest in children and young athletes: the importance of a detailed personal and family history in the pre‐participation evaluation. Br J Sports Med. 2009;43:336–341. [DOI] [PubMed] [Google Scholar]
- 31. Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996;276:199–204. [PubMed] [Google Scholar]
- 32. Maron BJ, Friedman RA, Caplan A. Ethics of preparticipation cardiovascular screening for athletes. Nat Rev Cardiol. 2015;12:375–378. [DOI] [PubMed] [Google Scholar]
- 33. Maron BJ, Estes NAM III, Maron MS. Is it fair to screen only competitive athletes for sudden death risk, or is it time to level the playing field? Am J Cardiol. 2018;121:1008–1010. [DOI] [PubMed] [Google Scholar]


