Abstract
Background
Troponin elevation occurs commonly in the setting of transcatheter aortic valve replacement (TAVR). There is a lack of information on the extent of troponin elevation post TAVR that is prognostically significant. We assessed the optimal cutoff for post‐TAVR troponin T elevation that correlates with long‐term mortality. We also examined the relationship between coronary artery disease (CAD) and prognostically significant myocardial injury in TAVR.
Methods and Results
This is a retrospective, observational single‐center study involving patients who underwent TAVR at Cleveland Clinic between 2010 and 2015. Five hundred ten patients were included (mean follow‐up of 2.6±1.3 years). Receiver operating characteristic analysis showed that troponin T elevation ≥3× upper limit of normal was the best predictor of long‐term mortality post TAVR with area under the curve of 0.57, with transapical TAVR patients excluded. Multivariate analyses confirmed that troponin T elevation ≥3× upper limit of normal was significantly associated with increased long‐term mortality post TAVR (hazard ratio 1.57, CI 1.04–2.38, P=0.03). The most common causes for the presence of unrevascularized CAD included the presence of chronic total occlusion in the native/graft vessels (49.7%) and diffuse/complex CAD unsuitable for PCI (24.6%). The presence of unrevascularized CAD and significant left main disease correlated with increased mortality, but not with the presence of prognostically significant myocardial injury.
Conclusions
Troponin T elevation of ≥3× upper limit of normal is associated with increased long‐term mortality after TAVR, except for the transapical approach. This prognostically significant myocardial injury does not appear to be secondary to severe CAD/unrevascularized CAD or left main disease, but rather is associated with other factors such as post‐TAVR complications.
Keywords: coronary artery disease, left main disease, mortality, myocardial injury, prognosis, transcatheter aortic valve implantation, troponin T
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Catheter-Based Coronary and Valvular Interventions, Diagnostic Testing
Clinical Perspective
What Is New?
Troponin T elevation of ≥3× upper limit of normal is associated with increased long‐term mortality after transcatheter aortic valve replacement, except for the transapical approach, thereby representing the threshold of myocardial injury in the setting of transcatheter aortic valve replacement that is prognostically significant.
Prognostically significant myocardial injury was noted to be independent of the severity of coronary artery disease, presence/extent of unrevascularized coronary artery disease, or significant left main disease in this study.
What Are the Clinical Implications?
Clinically, the study suggests that prompt recognition and aggressive management of post transcatheter aortic valve replacement complications may have a bigger impact on reducing the incidence of prognostically significant myocardial injury in the setting of transcatheter aortic valve replacement, rather than the completeness of revascularization, though this needs further scrutiny in randomized controlled clinical trials.
Introduction
Transcatheter aortic valve replacement (TAVR) is a well‐established intervention for inoperable and selected high‐risk surgical patients with severe aortic stenosis.1, 2 Recent studies have demonstrated that it may also be an alternative treatment for intermediate surgical risk patients with severe aortic stenosis, though long‐term outcomes of TAVR compared with surgical valve replacement in this population remain under scrutiny.3, 4 Perioperative myocardial infarction occurs in 0% to 15% of patients undergoing TAVR and has been linked with poor clinical outcomes.5, 6, 7, 8, 9, 10 The VARC‐2 (Valve Academic Research Consortium) consensus document defines myocardial infarction in patients undergoing TAVR as new ischemic symptoms or signs occurring within 72 hours of TAVR with rise in cardiac biomarkers (>15× upper limit of normal [ULN] elevation in troponin or >5× ULN increase in creatine kinase MB fraction).11 Myocardial infarction occurring in the setting of TAVR has been clearly linked with worse short‐ and long‐term mortality.12 However, multiple studies have shown that cardiac biomarker release alone (creatine kinase MB fraction or troponin elevation) in the absence of any additional signs or symptoms of cardiac ischemia on ECG, cardiac imaging, or coronary angiography (representing myocardial injury rather than infarction) also correlates with increased mortality post TAVR.7, 8, 10, 13 For instance, creatine kinase MB fraction elevation >2× ULN has previously been reported to correlate with poor clinical outcomes.7 Although previous studies have similarly shown that isolated troponin elevation by itself is related to poor clinical outcomes in the setting of TAVR, no specific cutoff /threshold of TnT elevation that correlates with poor clinical outcomes has been proposed. Paradis et al showed that cardiac biomarker elevation, lower than what would be considered diagnostic of MI, was associated with increased mortality, especially in patients undergoing transfemoral TAVR.8 Prior studies have demonstrated that cardiac biomarker elevation is poorly predictive of long‐term outcomes in patients undergoing transapical TAVR, possibly because of additional nonischemic injury to the myocardium from surgical manipulation.8, 9 Hence, the prognostic significance of biomarker elevation has been more reliably investigated and established in patients other that those undergoing transapical TAVR.
Many studies have scrutinized the link between severity of coronary disease, left main disease, or the extent of unrevascularized coronary disease and long‐term mortality post TAVR. The results have been conflicting.14 However, a recent meta‐analysis exploring the impact of coronary artery disease on long‐term mortality in TAVR concluded that patients with coronary artery disease have an increased risk of 1‐year mortality.14 None of the studies, however, have explored the potential mechanism underlying the increase in mortality in patients with coronary artery disease. We postulated that the deleterious effect of the severity/extent of coronary artery disease, presence of significant left main stem disease, or presence of unrevascularized coronary artery disease on long‐term mortality in TAVR patients must be secondary to increased risk of prognostically significant perioperative myocardial injury.
To investigate this hypothesis, we carried out a retrospective registry‐based observational analysis to examine (1) to what extent/cutoff of cardiac troponin T (TnT) elevation, if any, is best able to predict long‐term mortality post TAVR, thereby representing prognostically significant myocardial injury in this setting; and (2) to assess the impact of the extent of coronary artery disease, presence of left main stem (LMS) disease, and the presence /extent of unrevascularized coronary disease (expressed as Duke myocardial jeopardy score) on mortality. In addition, the study investigated whether the effect of coronary artery disease on mortality in the setting of TAVR was because of increased risk of prognostically significant myocardial injury.15, 16 This is a particularly important question to address, since revascularization of severe/critical coronary lesions, including severe LMS disease, is commonly done with a view to reduce risk of TAVR‐associated myocardial injury/infarction and improve clinical outcomes, without sufficient evidence to support this practice.
Methods
The study was approved by the Institutional Review Board, informed consent was waived, data were de‐identified, and the study complied with institutional guidelines for ethical research. The data that support the findings of this study are available from the corresponding author upon reasonable request. We retrospectively analyzed data from the Cleveland Clinic TAVR registry. We included all 510 patients who underwent TAVR at Cleveland Clinic between 2010 and 2015 for whom data for postoperative TnT levels were available. Mortality data were obtained via death recorded on Cleveland Clinic electronic health records for patients and the Social Security Death Index.
Significant coronary artery disease was defined as presence of >50% stenosis of the LMS coronary artery or in any pre‐existing coronary artery bypass grafts (treated or untreated). In the remainder of coronary arteries, significant stenosis was described as >70% narrowing of the respective coronary artery. Duke myocardial jeopardy score was calculated to measure the extent of unrevascularized coronary disease present before TAVR.15, 16
TnT was measured using troponin T STAT assay (fourth generation, Ref 04660307, Roche, ULN 0.03 ng/mL) at 6‐hourly intervals in the first 48 hours of TAVR (the values were normalized to either the ULN for the assay or the pre‐TAVR value with 24 hours of TAVR where available). The highest value in the first 48 hours was used in the analysis.
A receiver operating characteristic analytic tool was used to test various cutoffs (≥1×, ≥3×, ≥5×, ≥10×, ≥15×, and ≥20× ULN) for the extent of troponin elevation recorded post TAVR to identify the best cutoff that correlated with long‐term mortality following TAVR. The Delong test was used to compare the receiver operating characteristic curves in this part of the analysis.17 All statistical computation was implemented using an R software package, Version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria). All continuous variables are expressed as mean±SD unless specified. Various levels of TnT elevation (≥1×, ≥3×, ≥5×, ≥10×, ≥15×, and ≥20× ULN) were also assessed in a univariate analysis with log‐rank testing.
We used a multivariable weighted Cox regression model18 to assess whether the most optimal TnT elevation cutoff identified by receiver operating characteristic and univariate analysis was still an independent predictor of increased mortality post TAVR after adjusting for other clinical comorbidities. The traditional Cox regression model relies on the proportional hazards assumption. However, this assumption can be violated in practice and because of that, the coefficients may be over‐ or underestimated. Weighted Cox regression model is an alternative that supplies well‐interpretable average effects in case of nonproportional hazards. This part of the analysis was performed in 2 steps: in the first step, the model was developed based on all clinical variables of interest based on prior studies and the current hypothesis. Then the final model was developed only keeping the variables that were statistically significant at the 5% level of significance from the full model. This was particularly done to avoid potential bias in estimation, given the total number of events and the variables being considered in the study. Some of the categorical variables had multiple levels, with very few patients in their subgroups. To improve the model, we combined some of the groups within 2 of the variables of interest to reduce the number of variable levels in the final analysis. For instance, type of TAVR had 6 levels to start with. Three of the groups (transsubclavian, transcarotid, and transcaval TAVR) were combined with transaortic TAVR and analyzed together as other TAVR modalities. Similarly, severity of chronic lung disease, initially categorized into 4 categories based on forced expiratory volume in 1 second (FEV1) as: no chronic lung disease (FEV1 >75% of predicted), mild (FEV1 60%–75% of predicted), moderate (FEV1 50%–59% of predicted), and severe chronic lung disease (FEV1 <50% predicted) was analyzed at 2 levels: no/mild chronic lung disease versus moderate/severe lung disease.
Finally, binary multivariable logistic regression analysis was used to study the determinants of prognostically significant myocardial injury, in particular to assess the relationship between perioperative myocardial injury and the severity of coronary artery disease, the presence of LMS disease, as well as the presence/severity of unrevascularized coronary disease. Following the same logic as mentioned in the case of the Cox model (to avoid potential bias in estimation), we had followed a similar 2‐stage procedure. Only statistically significant variables from the full model, at the 5% level of significance, were retained to produce the final multivariable logistic model. For any hypothesis testing of interest, a P<5% was considered significant.
Results
In total, 747 patients underwent TAVR between 2010 and 2015 at Cleveland Clinic, for whom data on postoperative TnT levels were available for 510 patients. The mean follow‐up interval for the study population was 2.6±1.3 years. Overall mortality in patients post TAVR over this follow‐up period was 26.6%. There was no statistically significant difference in mortality between patients who had TnT measured post TAVR versus those who did not (20.6% versus 29.4%, P=0.3). Of the 510 patients included in the final analysis, 28% underwent transapical TAVR, 57.6% transfemoral TAVR, and 13.1% transaortic TAVR.
TnT ≥3× ULN was the best predictor of long‐term mortality using receiver operating characteristic analysis with area under the curve of 0.56 (95% CI 0.52–0.59) (Figure 1A), when used as a solitary variable. Since TnT elevation in transapical TAVR is known to correlate poorly with clinical outcomes, we repeated the analysis excluding transapical‐TAVR patients. Interestingly, ≥3× ULN TnT remained the best predictor of mortality even with transapical‐TAVR patients excluded, with a similar area under the curve of 0.57, though with a slightly broader CI possibly because of fewer patients in the analysis (95% CI: 0.51–0.61) (Figure 1B). Table 1 provides a summary of the characteristics of the study population, divided into those with ≥3× elevation in TnT versus those with <3× ULN TnT elevation post TAVR, based on the cutoff identified that best represented prognostically significant myocardial injury. As expected, troponin elevation was significantly higher in patients with transapical TAVR (≈3 times as much) compared with transfemoral TAVR or transaortic TAVR (mean value 0.56±0.55 ng/mL versus 0.19±0.45 ng/mL versus 0.20±0.16 ng/mL, P<0.001) (Figure 2).
Figure 1.
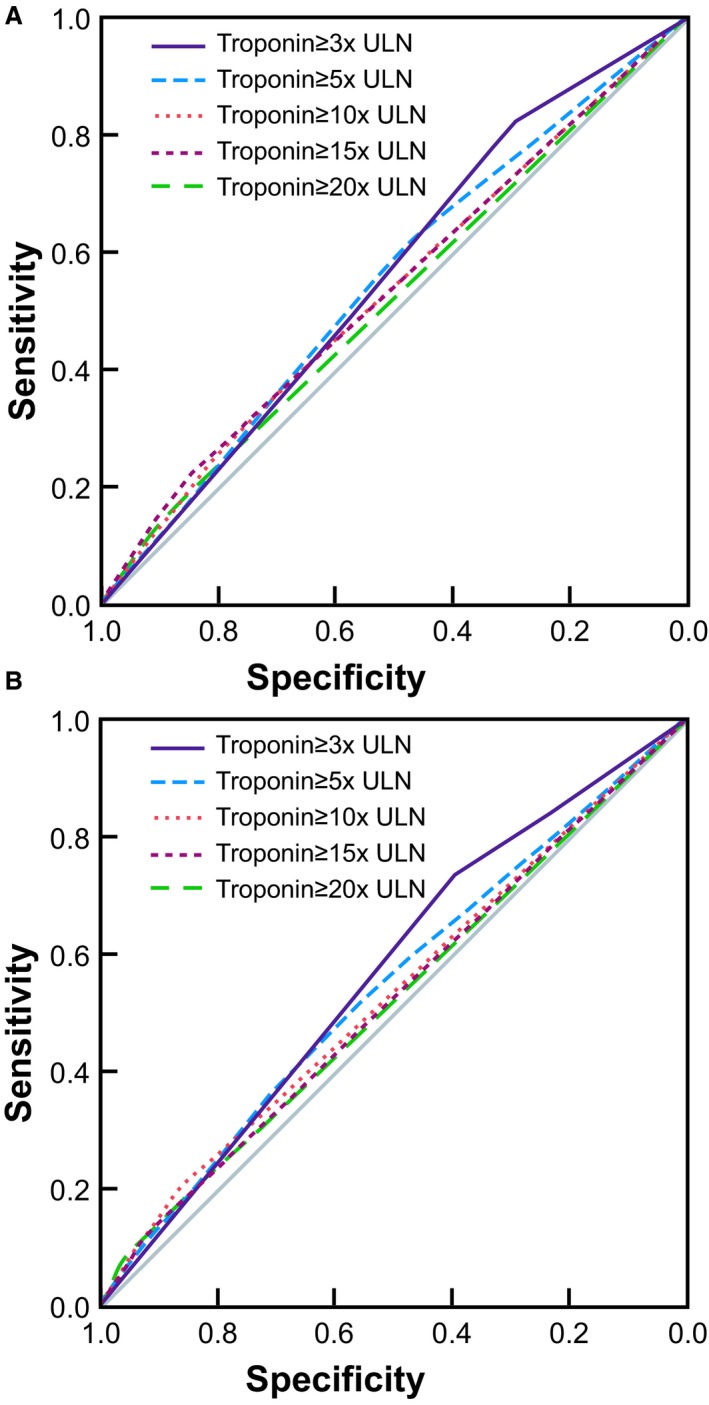
ROC analysis of various levels of TnT elevation (expressed as ratio of ULN) with regard to their ability to predict long‐term mortality following TAVR: ≥3× ULN was the best cutoff to predict long‐term mortality with all patients included (A), ≥3× ULN remained the best cutoff after transapical TAVR patients were excluded with better AUC (B). AUC indicates area under the curve; ROC, receiver operating characteristic; TnT, troponin T; TAVR, transcatheter aortic valve replacement; ULN, upper limit of normal.
Table 1.
Description of the TAVR Study Population, Split into 2 Groups Based on the Level of TnT Elevation Noted to Be Prognostically Significant in the Study (≥3× ULN vs <3× ULN), n=510
| Patient Characteristics (% of Total) | TnT<3× ULN (n=134) | TnT≥3× ULN (n=376) | Statistical Significance of Differences |
|---|---|---|---|
| Age, y | 81±9.7 | 81.2±8.4 | NS |
| Male | 60.4% | 54% | NS |
| Female | 39.6% | 46% | NS |
| BMI, kg/m2 | 30.09±7.5 | 28.1±6.2 | P=0.003* |
| Hypertension (94.7%) | 94% | 94.9% | NS |
| Diabetes mellitus present (44.1%) | 48.5% | 42.6% | NS |
| Dyslipidemia (91.2%) | 91.8% | 91% | NS |
| Approach for TAVR (% cases) | |||
| Transapical (28%) | 2.2% | 37.2% | P<0.001* |
| Transfemoral (57.6%) | 85.8% | 47.6% | |
| Transaortic (13.1%) | 11.9% | 13.6% | |
| Transsubclavian (0.6%) | 0% | 0.8% | |
| Transcarotid (0.4%) | 0% | 0.5% | |
| Transcaval (0.2%) | 0% | 0.3% | |
| CKD present (pre‐TAVR serum creatinine >2 mg/dL) (7.5%) | 5.3% | 8.9% | NS |
| Current smoker (4.3%) | 4.5% | 4.3% | NS |
| LVEF ≤40% (systolic heart failure history) vs >40% (19.8%) | 31.3% | 15.7% | P<0.001* |
| No–mild chronic lung disease (with FEV1 ≥60%) (70%) | 71.64% | 69.41% | NS |
| Moderate–severe chronic lung disease (with FEV1 <60%) (30%) | 28.36% | 30.59% | |
| Immediate post‐TAVR complications (composite of bleeding/valve dysfunction/stroke/prolonged ventilation/dialysis need /limb ischemia /postoperative PPM‐ICD need/cardiac arrest/ multisystem failure) (33.5%) | 19.4% | 38.6% | P<0.001* |
| Severity of coronary disease | |||
| 0‐vessel disease (35.5%) | 38.8% | 34.3% | NS |
| 1‐vessel disease (17.1%) | 20.1% | 16% | |
| 2‐vessel disease (13.9%) | 15.7% | 13.3% | |
| 3‐vessel disease (33.5%) | 25.4% | 36.4% | |
| Left main stem disease (12%) | 10.4% | 12.5% | NS |
| History of coronary artery disease (CABG/MI/PCI in past) (59%) | 47.8% | 63% | P=0.002* |
| Duke myocardial jeopardy score (mean score) | 0.84 | 0.89 | NS |
| Unrevascularized coronary disease present (27.5%) | 23.9% | 28.7% | NS |
| Mortality | 20.1% | 32.7% | P=0.006* |
| Follow‐up period | 2.9±1.3 years | 2.5±1.4 years | P=0.004* |
BMI indicates body mass index; CABG, coronary artery bypass graft; CKD, chronic kidney disease; FEV1, forced expiratory volume in 1 second; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NS, not significant; PPM‐ICD, permanent pacemaker–implantable cardioverter defibrillator; TAVR, transcatheter aortic valve replacement; TnT, troponin T; ULN, upper limit of normal.
indicates a statistically significant difference between the two groups.
Figure 2.
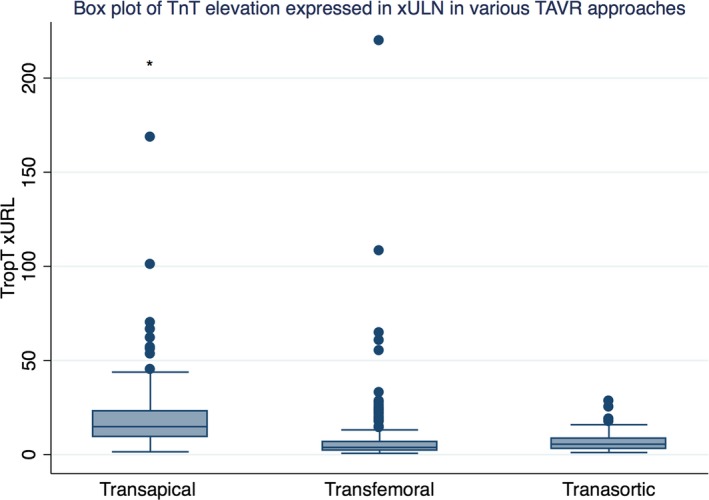
Box plot comparing extent of TnT elevation in various TAVR approaches: TA‐TAVR had significantly higher TnT elevation compared with TF‐TAVR and TAo‐TAVR. TA‐TAVR indicates transapical transcatheter aortic valve replacement; Tao‐TAVR, transaortic transcatheter aortic valve replacement; TF‐TAVR, transfemoral transcatheter aortic valve replacement; TnT, troponin T; TropT, troponin T rapid test; ULN, upper limit of normal.
TnT elevation ≥15× ULN, meeting VARC‐2 definition of myocardial infarction, was noted in 102/510 patients (20% of cases). This TnT elevation was present in 98.6% (71/72) of transapical‐TAVR patients, 7.7 % (21/273) of transfemoral‐TAVR, and 15.5% (9/58) of transaortic‐TAVR patients. Moreover, TnT≥3× ULN was noted in 73.7% (376/510) of all patients. There were 97.9% (140/143) patients with transapical‐TAVR, 60.8% (179/294) of patients with transfemoral‐TAVR, and 76.1% (51/67) of patients with transaortic TAVR. Pre‐TAVR TnT was measured in 98 patients and noted to be elevated >1× ULN in 58/98 (59.1%) patients.
In univariate analysis, using log‐rank test, TnT≥3× ULN was the most significantly associated with long‐term mortality for the whole group, regardless of the TAVR modality (P=0.003) and when patients with transapical‐TAVR were excluded (P=0.007) (Figure 3A and 3B). In comparison, in univariate survival analysis TnT≥15× ULN was not significantly associated with long‐term mortality for the study population as a whole, even when transapical‐TAVR patients were excluded (P=0.09). Also, in the multivariate analysis, TnT≥3× ULN elevation remained an independent predictor of long‐term mortality post TAVR (hazard ratio 1.57, CI 1.04–2.38, P=0.03) (Tables 2 and 3). Other clinical factors that correlated with increased mortality were age, severity of lung disease, presence of unrevascularized coronary disease, presence of LMS disease, and the presence of immediate post‐TAVR complications (composite of bleeding/valve dysfunction/stroke/prolonged ventilation/dialysis need/limb ischemia /need for permanent pacemaker–implantable cardioverter defibrillator /cardiac arrest/ multisystem failure) (Tables 2 and 3).
Figure 3.
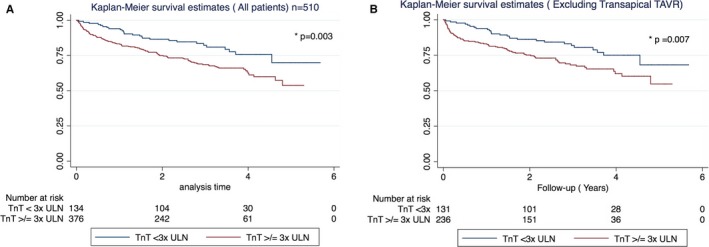
Troponin T elevation ≥3× ULN correlated with worse long‐term survival post TAVR in both univariate and multivariate analysis. A, All patients including TA‐TAVR (n=510). B, TA‐TAVR patients excluded. TA‐TAVR indicates transapical‐transcatheter aortic valve replacement; TAVR, transcatheter aortic valve replacement; TnT, troponin T; ULN, upper limit of normal.
Table 2.
Multivariate Cox Regression Showing that TnT≥3× ULN Remains a Significant Determinant of Long‐Term Mortality Following TAVR, Despite Adjusting for Other Relevant Clinical Factors (Full Cox Model With All Variables of Interest)
| HR | CI Lower 0.95 | CI Upper 0.95 | z Value | P Value | |
|---|---|---|---|---|---|
| TnT ≥3× ULN* | 1.6962 | 1.0756 | 2.6748 | 2.2737 | 0.0230 |
| Unrevascularized coronary artery disease present* | 1.9826 | 1.0305 | 3.8143 | 2.0500 | 0.0404 |
| Duke jeopardy score | 0.9413 | 0.7971 | 1.1117 | −0.7125 | 0.4762 |
| Type of TAVR (transapical vs transfemoral) | 1.1518 | 0.7363 | 1.8019 | 0.6190 | 0.5359 |
| Type of TAVR (transapical vs other TAVR modalities) | 1.1418 | 0.6003 | 2.1718 | 0.4042 | 0.6860 |
| Chronic kidney disease (S. Cr >2 mg/dL) | 1.4472 | 0.7857 | 2.6656 | 1.1860 | 0.2356 |
| Left main disease present* | 1.9694 | 1.1818 | 3.2820 | 2.6008 | 0.0093 |
| Severity of coronary disease (1 vessel vs no significant coronary disease) | 0.9460 | 0.5543 | 1.6145 | −0.2035 | 0.8388 |
| Severity of coronary disease (2 vessel vs no significant coronary disease) | 0.8657 | 0.4817 | 1.5557 | −0.4824 | 0.6296 |
| Severity of coronary disease (3 vessel vs no significant coronary disease) | 0.6167 | 0.3384 | 1.1239 | −1.5786 | 0.1144 |
| History of CABG/MI/PCI | 1.2814 | 0.9062 | 1.8120 | 1.4029 | 0.1606 |
| Age, y* | 1.0238 | 1.0015 | 1.0465 | 2.0962 | 0.0361 |
| BMI, kg/m2 | 0.9934 | 0.9665 | 1.0211 | −0.4695 | 0.6387 |
| History of hypertension | 1.2884 | 0.5415 | 3.0650 | 0.5730 | 0.5667 |
| History of diabetes mellitus | 0.9460 | 0.6387 | 1.4010 | −0.2772 | 0.7816 |
| Current smoker | 0.9619 | 0.3817 | 2.4240 | −0.0825 | 0.9343 |
| Dyslipidemia | 1.3734 | 0.7390 | 2.5524 | 1.0033 | 0.3157 |
| Heart failure (LVEF ≤40% vs >40%) | 1.4387 | 0.9243 | 2.2394 | 1.6111 | 0.1072 |
| Moderate–severe chronic lung disease (vs no–mild chronic lung disease)* | 1.8837 | 1.2980 | 2.7338 | 3.3323 | 0.0009 |
| Post TAVR complications* | 1.8048 | 1.2638 | 2.5774 | 3.2476 | 0.0012 |
BMI indicates body mass index; CABG, coronary artery bypass graft; HR, hazard ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction; S. Cr, serum creatinine; TAVR, transcatheter aortic valve replacement; TnT, troponin T; ULN, upper limit of normal.
Highlights the statistically significant variables.
Table 3.
Multivariate Cox Regression Showing That TnT ≥3× ULN Remains a Significant Determinant of Long‐Term Mortality Following TAVR, Despite Adjusting for Other Relevant Clinic Factors (Final Cox Model With Only Significant Variables From Table 2 in the Final Analysis)
| HR | CI Lower 0.95 | CI Upper 0.95 | z Value | P Value | |
|---|---|---|---|---|---|
| TnT ≥3× ULN | 1.5754 | 1.0414 | 2.3832 | 2.1522 | 0.0314 |
| Unrevascularized coronary artery disease | 1.4519 | 1.0188 | 2.0691 | 2.0629 | 0.0391 |
| Left main disease | 1.5196 | 0.9961 | 2.3182 | 1.9419 | 0.0522 |
| Age, y | 1.0277 | 1.0077 | 1.0481 | 2.7252 | 0.0064 |
| Moderate–severe chronic lung disease (vs no–mild chronic lung disease) | 1.9182 | 1.3299 | 2.7667 | 3.4856 | 0.0005 |
| Post TAVR complications | 1.8762 | 1.3444 | 2.6184 | 3.7000 | 0.0002 |
HR indicates hazard ratio; TAVR, transcatheter aortic valve replacement; TnT, troponin T; ULN, upper limit of normal.
Unrevascularized coronary disease was noted in 27.5% of the patients (distribution of unrevascularized disease was as follows: left anterior descending 14.19%, diagonal 16.04%, left circumflex 16.35%, obtuse marginal 18.8%, and right coronary artery 34.56%) (Figure 4). The most common reasons for unrevascularized coronary disease included the presence of chronic total occlusion in the native or graft vessels (49.7%), diffuse coronary disease or complex coronary anatomy not suitable for percutaneous coronary intervention (PCI) (24.6%), presence of collaterals or the coronary vessel supplied to a small coronary territory (20.6%), negative stress test (1.5%), absence of any clinical symptoms (1%), and failed PCI (2.5%) (Figure 5).
Figure 4.
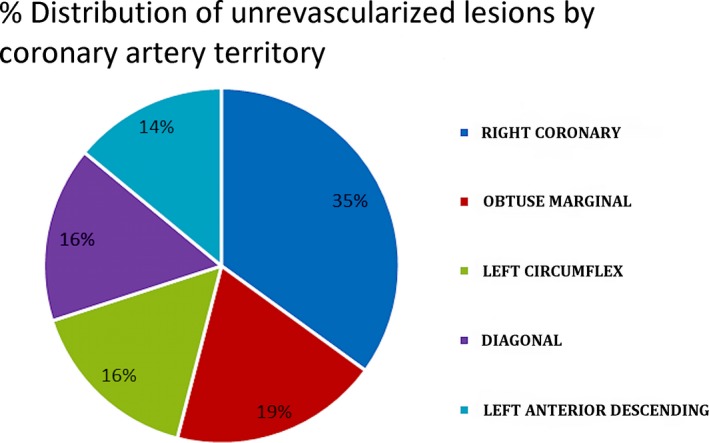
Percentage distribution of unrevascularized coronary disease based on the coronary artery involved.
Figure 5.
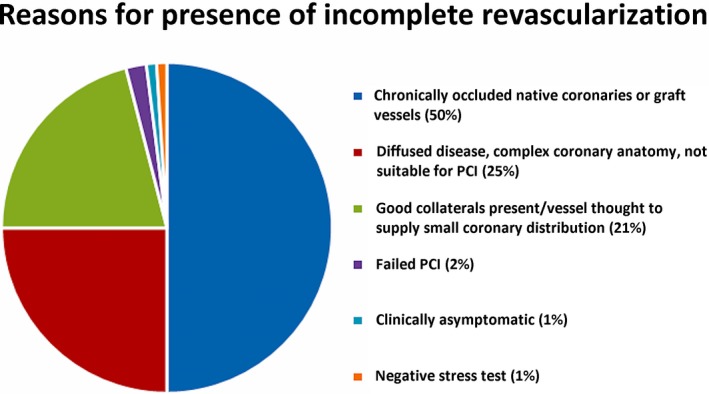
Percentage distribution of reasons for the presence of incomplete revascularization in the study population. PCI indicates percutaneous coronary intervention.
Interestingly, while unrevascularized coronary disease and LMS disease were both associated with an increase in long‐term mortality post‐TAVR, in binary logistic regression analysis (Tables 4 and 5), neither correlated with prognostically significant troponin elevation (even after adjusting for the type of TAVR). Rather, other factors such as post TAVR complications (composite of bleeding/valve dysfunction/stroke/prolonged ventilation/dialysis need/limb ischemia /postoperative permanent pacemaker–implantable cardioverter defibrillator need/cardiac arrest/multisystem failure), a prior history of coronary artery bypass graft/PCI/myocardial infarction, female sex, and lower body mass index were significantly associated with increased odds of prognostically relevant myocardial injury (Tables 4 and 5). Interestingly, patients with left ventricular ejection fraction ≤40% had lower odds of prognostically significant myocardial injury than those with left ventricular ejection fraction >40% (Tables 4 and 5). Furthermore, transfemoral and other TAVR modalities had significantly reduced odds of prognostically significant myocardial injury compared with transapical TAVR, as expected.
Table 4.
Binary Logistic Regression: Studying Association of the Severity of Coronary Artery Disease (Independent of Revascularization Status), Significant Left Main Stem Disease (Independent of Revascularization Status), and the Presence/Extent of Unrevascularized Coronary Disease With Prognostically Significant Myocardial Injury (in This Model Defined as ≥3× ULN Elevation in TnT) (Full Binary Logistic Regression Model With All Variables of Interest Included) (Full Binary Logistic Regression Model With All Variables of Interest Included)
| OR | z Value | P Value | 95% Lower CI | 95% Upper CI | |
|---|---|---|---|---|---|
| Unrevascularized coronary disease present | 1.2208 | 0.3570 | 0.7209 | 0.4118 | 3.7166 |
| Duke jeopardy score | 0.9461 | −0.3930 | 0.6941 | 0.7182 | 1.2508 |
| Type of TAVR (transapical vs transfemoral) | 0.0249 | −5.8210 | 0.0000 | 0.0057 | 0.0742 |
| Type of TAVR (transapical vs other TAVR modalities) | 0.0508 | −4.2330 | 0.0000 | 0.0106 | 0.1805 |
| Chronic kidney disease (S. Cr >2 mg/dL) | 2.2921 | 1.6120 | 0.1070 | 0.8731 | 6.6957 |
| Left main disease | 0.7754 | −0.5400 | 0.5889 | 0.3074 | 1.9663 |
| Severity of coronary disease (1 vessel) | 1.2237 | 0.5630 | 0.5733 | 0.6100 | 2.4952 |
| Severity of coronary disease (2 vessel) | 1.3236 | 0.6850 | 0.4933 | 0.5975 | 2.9881 |
| Severity of coronary disease (3 vessel) | 1.3753 | 0.7910 | 0.4290 | 0.6267 | 3.0544 |
| History of CABG/MI/PCI | 1.9671 | 2.8120 | 0.0049 | 1.2303 | 3.1643 |
| Age, y | 1.0138 | 0.9000 | 0.3680 | 0.9840 | 1.0445 |
| BMI, kg/m2 | 0.9582 | −2.1760 | 0.0295 | 0.9217 | 0.9956 |
| History of hypertension | 0.5176 | −1.2710 | 0.2036 | 0.1780 | 1.3820 |
| History of diabetes mellitus | 0.8784 | −0.4950 | 0.6206 | 0.5260 | 1.4717 |
| Current smoker | 0.6169 | −0.7700 | 0.4411 | 0.1852 | 2.2282 |
| Dyslipidemia | 0.6989 | −0.8350 | 0.4040 | 0.2908 | 1.5835 |
| Heart failure (LVEF ≤40% vs >40%) | 0.2797 | −4.1700 | 0.0000 | 0.1521 | 0.5059 |
| Moderate–severe chronic lung disease vs no–mild chronic lung disease | 1.6607 | 1.8020 | 0.0716 | 0.9636 | 2.9124 |
| Post TAVR complications | 2.0726 | 2.5830 | 0.0098 | 1.2039 | 3.6512 |
| Sex (male_1_female_0) | 0.5645 | −2.0970 | 0.0360 | 0.3289 | 0.9599 |
BMI indicates body mass index; CABG, coronary artery bypass graft; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; S. Cr, serum creatinine; TAVR, transcatheter aortic valve replacement; TnT, troponin T; ULN, upper limit of normal.
Table 5.
Binary Logistic Regression: Studying Association of the Severity of Coronary Artery Disease (Independent of Revascularization Status), Significant Left Main Stem Disease (Independent of Revascularization Status), and the Presence/Extent of Unrevascularized Coronary Disease With Prognostically Significant Myocardial Injury (in This Model Defined as ≥3× ULN Elevation in TnT) (Final Binary Logistic Regression Model With Only Significant Variables from Table 5 Included in Final Analysis)
| OR | z Value | P Value | 95% Lower CI | 95% Upper CI | |
|---|---|---|---|---|---|
| Type of TAVR (transapical vs transfemoral) | 0.0309 | −5.7290 | 0.0000 | 0.0074 | 0.0866 |
| Type of TAVR (transapical vs other TAVR modalities) | 0.0567 | −4.2820 | 0.0000 | 0.0124 | 0.187 |
| History of CABG/MI/PCI | 2.0547 | 3.0870 | 0.0020 | 1.3038 | 3.2586 |
| BMI, kg/m2 | 0.9486 | −3.1220 | 0.0018 | 0.9173 | 0.9804 |
| Heart failure, LVEF ≤40% vs >40% | 0.3364 | −3.9060 | 0.0001 | 0.1936 | 0.5794 |
| Post TAVR complications | 2.1404 | 2.7750 | 0.0055 | 1.2634 | 3.7143 |
| Sex (male_1 vs female_0) | 0.6541 | −1.7530 | 0.0797 | 0.4052 | 1.0492 |
BMI indicates body mass index; CABG, coronary artery bypass graft; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; TAVR, transcatheter aortic valve replacement; TnT, troponin T; ULN, upper limit of normal.
Discussion
One of the main findings from the study was that ≥3× ULN was the cutoff for TnT elevation that was most predictive of increased long‐term mortality following TAVR, particularly where transapical‐TAVR patients were excluded from analysis (Tables 2 and 3; Figures 1 and 3). In our study, transapical‐TAVR was associated with significantly higher mean increase in TnT compared with transfemoral TAVR and transaortic‐TAVR, similar to prior studies (Figure 2). No specific level of TnT elevation could be identified that correlated with increased mortality in transapical TAVR, when analyzed separately. That may be in part because of a small sample size and fewer events. We verified that ≥3× ULN TnT elevation remained an independent predictor of long‐term mortality in both univariate and multivariate analysis, even after adjusting for other patient comorbidities, including the severity/extent of coronary disease (expressed as number of coronary territories involved: 0 versus 1 versus 2 versus 3 vessel coronary disease, irrespective of revascularization status), presence /absence of LMS disease (irrespective of revascularization status), presence/absence of unrevascularized coronary disease and/or presence/extent of unrevascularized coronary disease (measured by the Duke myocardial jeopardy score) (Tables 2 and 3). Other variables in the analysis are listed in Tables 2 and 3.
Previous studies have shown a definite correlation between rise in cardiac biomarkers post TAVR and poor clinical outcomes, particularly in transfemoral TAVR.6, 7, 8, 9 The Fourth Universal Definition of myocardial makes a clear distinction between myocardial infarction and myocardial injury, defining myocardial injury as any elevation in cardiac troponin at least 1 value above the 99th percentile upper reference limit.19 Troponin elevation more than the 99th percentile of ULN occurs commonly in TAVR, particularly transapical‐TAVR. Although the cutoffs for prognostically relevant myocardial injury have been studied extensively in other settings such as coronary artery bypass graft,20 similar cutoffs to define prognostically significant myocardial injury in the setting of TAVR remain unknown. Paradis et al showed that cardiac biomarker elevation, lower than what would be considered diagnostic of myocardial infarction, was associated with increased mortality, especially in transfemoral TAVR.8 Studies have investigated the correlation between other cutoff of TnT elevation with mortality. For instance, TnT elevation to the extent that defines myocardial infarction (>15× ULN) per the VARC‐2 criteria has been linked with poor prognosis, though with conflicting results.12, 21 The cutoff for prognostically significant myocardial injury in TAVR in our study (≥3× ULN) was much lower than the VARC‐2 defined cutoff for myocardial infarction (15× ULN), similar to the findings by Paradis et al. However, myocardial injury should not be confused with myocardial infarction. While myocardial injury is defined by a rise in cardiac enzymes alone irrespective of additional evidence of myocardial ischemia, the diagnosis of myocardial infarction in the setting of TAVR is currently defined by the VARC‐2 criteria as >15× ULN rise in troponin post TAVR in the presence of additional evidence of myocardial ischemia. Our study was specifically focused at identifying the threshold for prognostically significant myocardial injury in TAVR patients and did not seek to investigate the best troponin cutoff for defining acute myocardial infarction in these patients.
Prior studies have examined the relationship between coronary artery disease and mortality post TAVR.5, 12, 14, 22, 23, 24, 25 In particular, more complex coronary disease has been linked with increased mortality post TAVR. However, the mechanism underlying this relationship remains unknown. Our study shows that the presence of LMS disease and unrevascularized coronary disease are both associated with increased risk of long‐term mortality (Tables 2 and 3). However, neither the Duke myocardial jeopardy score, nor the severity of coronary disease (defined in terms of 0 versus 1 versus 2 versus 3 vessel disease) was associated with increased mortality (Table 2). Also, none of the measures of severity/extent of coronary disease, presence of LMS disease, and/or presence/extent of unrevascularized coronary disease contributed to prognostically significant myocardial injury (Table 4). This was true even when transapical‐TAVR patients were removed from the analysis. Instead, post‐TAVR complications and prior history of coronary artery bypass graft/myocardial infarction/PCI (probably suggestive of a higher‐risk patient phenotype) were more important determinants of prognostically significant myocardial injury (prior history of coronary artery bypass graft/myocardial infarction/PCI may be indicative of higher‐risk patient profile) (Tables 4 and 5). Thus, there was no direct relationship between coronary disease and prognostically significant myocardial injury in TAVR in our study. We speculate that the presence of LMS disease and unrevascularized coronary disease may be markers of more diffuse vascular disease/higher‐risk patient profile, which may be the cause of the increased long‐term all‐cause mortality in these patients, rather than periprocedural myocardial injury. That may also be the reason why some studies have shown a link between more complex coronary disease and mortality. Indirectly, the above findings do not support the need for routine complete revascularization strategy before TAVR.
While TnT≥3× ULN was clearly linked with increased mortality post TAVR in our study, its clinical implications and utility are less certain. For instance, though TnT elevation ≥3× ULN was the best cutoff to predict long‐term mortality, overall the area under the curve for this cutoff was still fairly close to the chance performance diagonal line (Figure 1A and 1B). Furthermore, this level of TnT elevation was present in as many as 60.8% of transfemoral‐TAVR patients, thus being a common occurrence in the setting of TAVR. Also, we found that prognostically significant myocardial injury post TAVR was not associated with severe coronary disease/LMS disease or unrevascularized coronary disease in our study; hence, it is less likely to be representative of periprocedural coronary ischemia. This indicates that overall by itself, TnT elevation may be a poor predictor of long‐term outcomes in the setting of TAVR, and its clinical/prognostic utility may be limited. Likewise, it is unclear how the risk of prognostically significant myocardial injury may be reduced. More studies are needed to better understand the mechanism underlying TnT release in the setting of TAVR. However, in our study, a composite of major and minor post TAVR adverse events (including bleeding/valve dysfunction/stroke/prolonged ventilation/dialysis need/limb ischemia/postoperative permanent pacemaker–implantable cardioverter defibrillator need/cardiac arrest/multisystem failure) was a very significant determinant of both prognostically significant TnT elevation and mortality. Considering the strong association of post‐TAVR complications with both significant myocardial injury and with worse long‐term mortality, it can be hypothesized that recognizing any complications early and minimizing hemodynamic instability in the setting of TAVR may potentially reduce incidence/severity of myocardial injury and improve mortality.
Study Strengths and Limitations
The strengths of the study include a large sample size (n=510 patients) and a long follow‐up. However, our study had some obvious limitations, the most important being the observational nature of the study. Even though we adjusted for a variety of variables in the multivariate model, there is always the possibility that unknown confounders may have affected the overall results. The study included patients who had TnT measures perioperatively among all patients who had TAVR in this time frame. While the mortality in the patients who had TnT measured versus those who did not was not significantly different, the possibility of any selection bias cannot be eliminated. Our study could not identify any single cutoff of TnT elevation in the case of transapical‐TAVR that correlated with clinical outcomes, though this analysis was limited by a smaller number of patients/events when transapical‐TAVR patients were examined separately. Hence the specific level of TnT elevation that is prognostically significant, if any, for transapical‐TAVR remains to be determined. Furthermore, considering the overall low area under the curve for TnT elevation as a predictor of long‐term (all cause) mortality, its clinically utility in the setting of TAVR may be limited, as discussed above. Because of the small number of patients in our study with TnT measured at baseline, we did not specifically assess whether long‐term mortality was influenced by pre‐TAVR TnT elevation. Though pre‐TAVR TnT elevation has been linked with poor outcomes in other studies,12 further studies are needed to clarify the exact clinical significance and implications of elevated TnT pre‐TAVR. Finally, our study has utilized all‐cause mortality as the end point; however, using cardiovascular death as an end point in future studies may provide more insight and it is possible that prognostically significant myocardial injury, as defined by our study, may be able to better predict cardiovascular mortality compared with all‐cause mortality. It is important to recognize that the TnT threshold for prognostically significant myocardial injury in our study is specific to the TnT assay (troponin T STAT assay fourth generation) used in the study and may be different with other troponin assays currently available commercially.
Conclusion
Troponin elevation ≥3× ULN appears to be the best predictor of long‐term mortality among various cutoffs of TnT elevation post TAVR that were assessed (including ≥1×, ≥3×, ≥5×, ≥10×, ≥15×, and ≥20× ULN elevation), and appears to be the best representative of prognostically significant myocardial injury (defined in this instance purely in terms of elevation in TnT above the ULN), other than in patients with transapical‐TAVR. A cutoff for prognostically significant TnT elevation in transapical‐TAVR, if any, remains to be determined. The presence of LMS disease and the presence of unrevascularized disease were both associated with increased mortality in our study, but this was not secondary to increased risk of prognostically significant myocardial injury. Overall, though, our study established the cutoff of TnT elevation that significantly correlates with increased long‐term mortality post TAVR. The clinical and prognostic utility of TnT alone in TAVR appears to be limited and is at best a marker of other clinical factors linked to mortality in TAVR such as post‐TAVR complications and/or a higher‐risk phenotype. It is unclear how the risk of prognostically significant myocardial injury can be modified or how prognostically significant myocardial injury in the setting of TAVR should be managed; however, considering the highly significant association between post TAVR complications with both significant myocardial injury and long‐term mortality, it can be postulated that prompt recognition and management of any complications will be beneficial.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e011889 DOI: 10.1161/JAHA.118.011889.)
References
- 1. Beohar N, Kirtane AJ, Blackstone E, Waksman R, Holmes D Jr, Minha S, Alli O, Suri RM, Svensson LG, Leon M, Kodali S. Trends in complications and outcomes of patients undergoing transfemoral transcatheter aortic valve replacement: experience from the partner continued access registry. JACC Cardiovasc Interv. 2016;9:355–363. [DOI] [PubMed] [Google Scholar]
- 2. Svensson LG, Blackstone EH, Rajeswaran J, Brozzi N, Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Tuzcu EM, Webb JG, Kapadia S, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Thourani VH, Pichard AD, Bavaria JE, Herrmann HC, Williams MR, Babaliaros V, Genereux P, Akin JJ, PARTNER Trial Investigators . Comprehensive analysis of mortality among patients undergoing tavr: results of the partner trial. J Am Coll Cardiol. 2014;64:158–168. [DOI] [PubMed] [Google Scholar]
- 3. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG, PARTNER 2 Investigators . Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 4. Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V, Smalling R, Lim S, Malaisrie SC, Kapadia S, Szeto WY, Greason KL, Kereiakes D, Ailawadi G, Whisenant BK, Devireddy C, Leipsic J, Hahn RT, Pibarot P, Weissman NJ, Jaber WA, Cohen DJ, Suri R, Tuzcu EM, Svensson LG, Webb JG, Moses JW, Mack MJ, Miller DC, Smith CR, Alu MC, Parvataneni R, D'Agostino RB Jr, Leon MB. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate‐risk patients: a propensity score analysis. Lancet. 2016;387:2218–2225. [DOI] [PubMed] [Google Scholar]
- 5. Goel SS, Ige M, Tuzcu EM, Ellis SG, Stewart WJ, Svensson LG, Lytle BW, Kapadia SR. Severe aortic stenosis and coronary artery disease—implications for management in the transcatheter aortic valve replacement era: a comprehensive review. J Am Coll Cardiol. 2013;62:1–10. [DOI] [PubMed] [Google Scholar]
- 6. Rodes‐Cabau J, Gutierrez M, Bagur R, De Larochelliere R, Doyle D, Cote M, Villeneuve J, Bertrand OF, Larose E, Manazzoni J, Pibarot P, Dumont E. Incidence, predictive factors, and prognostic value of myocardial injury following uncomplicated transcatheter aortic valve implantation. J Am Coll Cardiol. 2011;57:1988–1999. [DOI] [PubMed] [Google Scholar]
- 7. Barbash IM, Dvir D, Ben‐Dor I, Badr S, Okubagzi P, Torguson R, Corso PJ, Xue Z, Satler LF, Pichard AD, Waksman R. Prevalence and effect of myocardial injury after transcatheter aortic valve replacement. Am J Cardiol. 2013;111:1337–1343. [DOI] [PubMed] [Google Scholar]
- 8. Paradis JM, Maniar HS, Lasala JM, Kodali S, Williams M, Lindman BR, Damiano RJ Jr, Moon MR, Makkar RR, Thourani VH, Babaliaros V, Xu K, Ayele GM, Svensson L, Leon MB, Zajarias A. Clinical and functional outcomes associated with myocardial injury after transfemoral and transapical transcatheter aortic valve replacement: a subanalysis from the partner trial (placement of aortic transcatheter valves). JACC Cardiovasc Interv. 2015;8:1468–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ribeiro HB, Dahou A, Urena M, Carrasco JL, Mohammadi S, Doyle D, Le Ven F, Allende R, Amat‐Santos I, Paradis JM, DeLarochelliere R, Puri R, Abdul‐Jawad Altisent O, del Trigo M, Campelo‐Parada F, Pibarot P, Dumont E, Rodes‐Cabau J. Myocardial injury after transaortic versus transapical transcatheter aortic valve replacement. Ann Thorac Surg. 2015;99:2001–2009. [DOI] [PubMed] [Google Scholar]
- 10. Ribeiro HB, Nombela‐Franco L, Munoz‐Garcia AJ, Lemos PA, Amat‐Santos I, Serra V, de Brito FS Jr, Abizaid A, Sarmento‐Leite R, Puri R, Cheema AN, Ruel M, Nietlispach F, Maisano F, Moris C, Del Valle R, Urena M, Abdul Jawad Altisent O, Del Trigo M, Campelo‐Parada F, Jimenez Quevedo P, Alonso‐Briales JH, Gutierrez H, Garcia Del Blanco B, Perin MA, Siqueira D, Bernardi G, Dumont E, Cote M, Pibarot P, Rodes‐Cabau J. Predictors and impact of myocardial injury after transcatheter aortic valve replacement: a multicenter registry. J Am Coll Cardiol. 2015;66:2075–2088. [DOI] [PubMed] [Google Scholar]
- 11. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodes‐Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB; Valve Academic Research Consortium‐2 . Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium‐2 consensus document. J Thorac Cardiovasc Surg. 2013;145:6–23. [DOI] [PubMed] [Google Scholar]
- 12. Koskinas KC, Stortecky S, Franzone A, O'Sullivan CJ, Praz F, Zuk K, Raber L, Pilgrim T, Moschovitis A, Fiedler GM, Juni P, Heg D, Wenaweser P, Windecker S. Post‐procedural troponin elevation and clinical outcomes following transcatheter aortic valve implantation. J Am Heart Assoc. 2016;5:e002430 DOI: 10.1161/JAHA.115.002430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yong ZY, Wiegerinck EM, Boerlage‐van Dijk K, Koch KT, Vis MM, Bouma BJ, Henriques JP, Cocchieri R, Piek JJ, de Mol BA, Baan J Jr. Predictors and prognostic value of myocardial injury during transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2012;5:415–423. [DOI] [PubMed] [Google Scholar]
- 14. Sankaramangalam K, Banerjee K, Kandregula K, Mohananey D, Parashar A, Jones BM, Jobanputra Y, Mick S, Krishnaswamy A, Svensson LG, Kapadia SR. Impact of coronary artery disease on 30‐day and 1‐year mortality in patients undergoing transcatheter aortic valve replacement: a meta‐analysis. J Am Heart Assoc. 2017;6:e006092 DOI: 10.1161/JAHA.117.006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Califf RM, Phillips HR III, Hindman MC, Mark DB, Lee KL, Behar VS, Johnson RA, Pryor DB, Rosati RA, Wagner GS. Prognostic value of a coronary artery jeopardy score. J Am Coll Cardiol. 1985;5:1055–1063. [DOI] [PubMed] [Google Scholar]
- 16. Dash H, Johnson RA, Dinsmore RE, Harthorne JW. Cardiomyopathic syndrome due to coronary artery disease. I: relation to angiographic extent of coronary disease and to remote myocardial infarction. Br Heart J. 1977;39:733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 18. Schemper M, Wakounig S, Heinze G. The estimation of average hazard ratios by weighted Cox regression. Stat Med. 2009;28:2473–2489. [DOI] [PubMed] [Google Scholar]
- 19. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology /American College of Cardiology /American Heart Association /World Heart Federation Task Force for the Universal Definition of Myocardial I . Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72:2231–2264. [DOI] [PubMed] [Google Scholar]
- 20. Thielmann M, Sharma V, Al‐Attar N, Bulluck H, Bisleri G, Jh Bunge J, Czerny M, Ferdinandy P, Frey UH, Heusch G, Holfeld J, Kleinbongard P, Kunst G, Lang I, Lentini S, Madonna R, Meybohm P, Muneretto C, Obadia JF, Perrino C, Prunier F, Sluijter JPG, Van Laake LW, Sousa‐Uva M, Hausenloy DJ. Esc joint working groups on cardiovascular surgery and the cellular biology of the heart position paper: perioperative myocardial injury and infarction in patients undergoing coronary artery bypass graft surgery. Eur Heart J. 2017;38:2392–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sinning JM, Hammerstingl C, Schueler R, Neugebauer A, Keul S, Ghanem A, Mellert F, Schiller W, Muller C, Vasa‐Nicotera M, Zur B, Welz A, Grube E, Nickenig G, Werner N. The prognostic value of acute and chronic troponin elevation after transcatheter aortic valve implantation. EuroIntervention. 2016;11:1522–1529. [DOI] [PubMed] [Google Scholar]
- 22. Paradis JM, White JM, Genereux P, Urena M, Doshi D, Nazif T, Hahn R, George I, Khalique O, Harjai K, Lasalle L, Labbe BM, DeLarochelliere R, Doyle D, Dumont E, Mohammadi S, Leon MB, Rodes‐Cabau J, Kodali S. Impact of coronary artery disease severity assessed with the syntax score on outcomes following transcatheter aortic valve replacement. J Am Heart Assoc. 2017;6:e005070 DOI: 10.1161/JAHA.116.005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khawaja MZ, Asrress KN, Haran H, Arri S, Nadra I, Bolter K, Wilson K, Clack L, Hancock J, Young CP, Bapat V, Thomas M, Redwood S. The effect of coronary artery disease defined by quantitative coronary angiography and syntax score upon outcome after transcatheter aortic valve implantation (TAVI) using the edwards bioprosthesis. EuroIntervention. 2015;11:450–455. [DOI] [PubMed] [Google Scholar]
- 24. Dewey TM, Brown DL, Herbert MA, Culica D, Smith CR, Leon MB, Svensson LG, Tuzcu M, Webb JG, Cribier A, Mack MJ. Effect of concomitant coronary artery disease on procedural and late outcomes of transcatheter aortic valve implantation. Ann Thorac Surg. 2010;89:758–767; discussion 767. [DOI] [PubMed] [Google Scholar]
- 25. D'Ascenzo F, Conrotto F, Giordana F, Moretti C, D'Amico M, Salizzoni S, Omede P, La Torre M, Thomas M, Khawaja Z, Hildick‐Smith D, Ussia G, Barbanti M, Tamburino C, Webb J, Schnabel RB, Seiffert M, Wilde S, Treede H, Gasparetto V, Napodano M, Tarantini G, Presbitero P, Mennuni M, Rossi ML, Gasparini M, Biondi Zoccai G, Lupo M, Rinaldi M, Gaita F, Marra S. Mid‐term prognostic value of coronary artery disease in patients undergoing transcatheter aortic valve implantation: a meta‐analysis of adjusted observational results. Int J Cardiol. 2013;168:2528–2532. [DOI] [PubMed] [Google Scholar]


